Abstract
Background:
Routine surveillance protocols rely heavily on endomyocardial biopsy (EMB) for detection of rejection in pediatric heart transplant recipients. More sensitive echocardiographic tools to assess rejection may help limit the number of EMBs. This study compared changes in left ventricular (LV) strain in patients who had rejection versus those who did not.
Methods:
A single center retrospective review was conducted between 2013 and 2020. Patients were categorized based on rejection history. Echocardiograms were evaluated at the time of 2 consecutive EMBs; in the rejection group, the second echocardiogram was collected at the time of a rejection episode. Conventional measures of LV function and speckle-tracking echocardiography-derived longitudinal (LS) and circumferential strain (CS) were measured.
Results:
17 patients were in the non-rejection group and 17 were in the rejection group (30 total rejection episodes). The rejection group was older at the time of transplant (12.5 vs. 1.3 years, p = .01). A decline in CS was seen in the rejection group at the second echocardiogram [−18.5 (IQR −21.5, −14.6) to −15.7 (IQR −19.8, −13.2)] while CS improved in the non-rejection group [−20.8 (IQR −23.9, −17.8) to −23.9 (IQR −24.9, −20.1)]. This difference in change reached significance (p = .02). A similar pattern was seen in LS that neared significance (p = .06). There was no significant difference in ejection fraction change (p = .24).
Conclusions:
Patients in the non-rejection group displayed improvement in CS between echocardiograms while patients in the rejection group showed subsequent decline. Worsening of LV CS may help identify acute rejection in the early post-transplant period.
Keywords: acute rejection, biopsy, cardiac transplantation, echocardiography, pediatric transplantation, pediatrics
1 |. INTRODUCTION
Cardiac transplantation provides hope for an improved quality and increased quantity of life for many children with end-stage heart disease. However, these patients are committed to the adverse effects of lifelong immunosuppression, as well as a multitude of invasive procedures from regular blood draws to cardiac catheterizations with endomyocardial biopsy (EMB). Although biopsy remains the gold standard for diagnosing rejection, it is limited by sampling error and carries the risks of anesthesia, arrhythmias, cardiac perforation, and tricuspid valve injury.1 Moreover, the majority of EMBs are performed in asymptomatic patients as part of routine surveillance protocols to which there is no consensus, and substantial variation exists between transplant centers.2
Conventional echocardiography measurements of systolic function, including ejection fraction (EF), are regularly employed to monitor allograft function. While combined scoring algorithms of these measurements have shown promise in predicting cellular rejection in pediatric patients, they are limited in their sensitivity when detecting early/subclinical rejection episodes.3 Newer technology utilizing two-dimensional speckle-tracking echocardiography (2DSTE) has been shown to detect subclinical changes in ventricular function that precede changes in EF, for example, in adolescents receiving anthracycline chemotherapy.4 These measures have the potential to detect early subclinical rejection prior to a decrease in ejection fraction. This study aimed to compare changes in 2DSTE left ventricular (LV) strain during rejection and non-rejection states in pediatric heart transplant recipients. We hypothesized that there would be a decline in strain measurements during acute episodes of rejection without a significant change in EF.
2 |. METHODS
This was a single center retrospective study of pediatric heart transplant recipients less than 21 years of age at the time of transplant between April 2013 and April 2020. The study was approved by the institutional review board at the Medical University of South Carolina. Patients were included in the rejection group if they had one or more episodes of rejection at any time up to three years post-transplant. Patients were not excluded based on a known history of coronary artery vasculopathy (CAV). Heart transplant recipients with no history of rejection that met the above criteria were included in the non-rejection group. Baseline data including demographics such as age, sex, race, underlying diagnosis, and blood type were collected. Peri-operative factors such as need for mechanical support, dialysis, and panel reactive antibodies (PRA), ischemic time, cross match results, and blood product usage were recorded. Echocardiographic and catheterization data were recorded from consecutive EMB pairs. All patients were hemodynamically stable prior to catheterization. The first set of data included data from a non-rejection episode in both groups. The second set of data included data from a rejection episode in the rejection group and a non-rejection episode in the non-rejection group. Patients with more than one episode of rejection were included in the study if they had recovered with an interval echo and biopsy pair with no evidence of rejection. Immunosuppression regimens as well as interventions for rejection were collected.
Patients at our institution are stratified based on perceived risk of rejection (Supplemental Table S1) and are scheduled for routine right heart catheterizations with EMBs starting one to two weeks from the date of transplantation to three years post-transplant at which time biopsies are no longer obtained routinely. Within the first three years post-transplant, high risk patients underwent at least 14 isolated right heart catheterizations with biopsies and 3 right and left catheterizations with biopsy and coronary angiography. Comparatively, low risk patients had 7 isolated right heart catheterizations with biopsies and 3 combined right and left heart catheterizations, respectively. Additional biopsies were performed if there were specific concerns for rejection. Left heart catheterizations and coronary angiography were performed annually. For both groups the greatest biopsy burden was during the first-year’s surveillance, with gradually increasing time intervals between biopsies.
2.1 |. Rejection classification
Rejection was classified as antibody-mediated, cellular, or combined. Antibody-mediated rejection (AMR) was defined when at least 2 of the following 3 criteria were met: presence of circulating donor specific antibody (DSA), histologic and/or immunopathologic evidence of rejection on biopsy (complement deposition and/or endothelial activation) and/or any clinical concerns prompting enhanced immunosuppression and/or targeted therapies.5 Acute cellular rejection (ACR) was determined by International Society of Heart and Lung Transplantation (ISHLT) biopsy grade of 2R or grade 2 in conjunction with a clinical diagnosis of rejection treated with enhanced immunosuppression.6 A patient was considered recovered from rejection with improvement in clinical condition and resolution of pathology on biopsy.
2.2 |. Echocardiographic and catheterization data
Using either Phillips IE 33 or GE E95 machines, echocardiograms were obtained within 24 h of endomyocardial biopsy. Sedation status and anesthetic usage were matched for the baseline and rejection echocardiograms. Conventional echocardiographic measurements were collected retrospectively after being analyzed in the clinical environment using Xcelera© (Phillips Healthcare) by non-invasive imaging pediatric cardiologists in accordance with the American Society of Echocardiography recommendations.7,8 Circumferential and longitudinal strain were prospectively measured by a single observer from parasternal short axis at the mid-papillary level and apical 4-chamber views, respectively, using Cardiac Performance Analysis v. 3.0 (TOMTEC). Strain is reported as a negative value with a more negative value signifying better ventricular deformation. For this study’s purpose, a less negative value is described as a decrease in strain and a more negative value an increase or improvement in strain.
Hemodynamic data and coronary angiography from invasive catheterization were captured. Additionally, EKG intervals and laboratory values assessing end organ function (creatinine, liver enzymes, and brain natriuretic peptide), immunosuppression levels, DSA, and leukocyte gene expression profiling (Allomap™) were recorded when available at the time of a biopsy/echo pair.
2.3 |. Statistical analysis
Baseline differences between groups were assessed using the Mann Whitney U test or Fisher’s Exact test where appropriate. Differences in echocardiographic and catheterization measures between the two EMBs within groups was assessed using Wilcoxon signed-rank test. To determine if changes in echocardiographic and catheterization between groups were assessed using a generalized linear model with the variable as interest as the dependent variable, an indicator variable for “rejection” or “non-rejection” group, timepoint, and their interaction in the model. Random effect included for the intercept for each subject. Kenward Roger method used for computing the denominator degrees of freedom for the tests of fixed effects. A p-value of <.05 was considered statistically significant. SPSS v. 27 (IBM) was used for analysis.
3 |. RESULTS
During the study duration, there were 39 patients transplanted at our institution, 34 of which were included in this study. From the remaining 5 patients that were not included: one was a dual organ transplant, 1 was found to have rejection at the time of their first 2 biopsies without an interval normal biopsy, 1 that passed away on post-operative day 1, 1 with limited follow-up due to transfer to another program, and 1 that did not have a paired biopsy at the time of data collection.
There were 30 pairs of rejection data in 17 patients that made up the rejection cohort, and of these 7 were obtained outside of the routine schedule because of additional clinical concerns. Each rejection episode and treatment strategy are outlined in Table 1. The non-rejection group included 17 pairs of data in patients with no known history of rejection. Demographic and perioperative data are displayed in Table 2. The rejection cohort was significantly older than the control cohort with a median of 12.5 versus 1.3 years (p = .01). Echocardiogram and biopsy pairs in the rejection cohort were closer to the time of transplant than the non-rejection cohort with a median of 4.2 (0.5, 36.7) versus 7.5 (4.6, 18.5) months (p = .03). There was no significant difference in the time intervals between paired echocardiograms in the rejection and non-rejection cohorts (2.7 vs. 3.8 months, p = .12). Nine patients had more than one episode of rejection with interval periods of “non-rejection” states. There was no statistically significant difference between the two groups when comparing underlying diagnosis, race, gender, PRA, ischemic time, and additional comorbidities including inotropic and ventilator dependence at the time of transplant. Interestingly, the rejection cohort took longer to reach therapeutic tacrolimus levels and had significantly more blood product exposure, in particular platelets, than the non-rejection group. There was a strong association between history of rejection and mortality (71% of patients in the rejection group survived versus 100% of the non-rejection group, p = .02).
TABLE 1.
Rejection episode characteristics and treatment
| ACR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rejection episode no. | Months from transplant | Months from last normal Bx | > Grade 2 | > Grade 2R | AMR | DSA (+) | CAV | Other concerns | Enhanced immunosuppression |
| 1 | 0.5 | 0.3 | pAMR(I+) | x | Decrease in EF | PO steroids | |||
|
| |||||||||
| 2 | 0.7 | 0.2 | x | PO steroids | |||||
|
| |||||||||
| 3a | 12.7 | 0.7 | x | x | pAMR2 | x | Medication Noncompliance |
PP, ATG, BZ, RX, IV steroids | |
|
| |||||||||
| 4 | 0.8 | 0.2 | pAMR(I+) | x | Medication Noncompliance, Decrease in EF, Decrease in CI |
PO steroids, PP, ATG, BZ, RX | |||
|
| |||||||||
| 5 | 6.3 | 2.1 | Focal C3d | Decrease in CI | PO steroids | ||||
|
| |||||||||
| 6a | 21.5 | 9.8 | x | Decrease in EF | IV steroids | ||||
|
| |||||||||
| 7 | 2.9 | 0.9 | x | x | IvIg | ||||
|
| |||||||||
| 8 | 1.0 | 0.7 | x | Decrease in EF | IV steroids | ||||
|
| |||||||||
| 9 | 11.1 | 2.4 | x | x | History CAV1 | PO steroids | |||
|
| |||||||||
| 10 | 15.1 | 3.5 | x | History CAV1 | Decrease in EF | PO steroids | |||
|
| |||||||||
| 11 | 9.3 | 2.7 | x | Medication Noncompliance, Decrease in EF |
PO steroids | ||||
|
| |||||||||
| 12 | 6.0 | 5.4 | pAMR(I+) | PO steroids | |||||
|
| |||||||||
| 13 | 12.7 | 4.2 | x | Increase TAC goal | |||||
|
| |||||||||
| 14 | 7.5 | 3.3 | x | PO steroids | |||||
|
| |||||||||
| 15 | 24.4 | 11.9 | x | PO steroids | |||||
|
| |||||||||
| 16 | 4.4 | 1.5 | x | IV steroids | |||||
|
| |||||||||
| 17a | 18.9 | 6.7 | x | pAMR2 | x | History CAV1 | Decrease in EF, Decrease in CI | PO steroids, PP, ATG, BZ, RX | |
|
| |||||||||
| 18 | 23.1 | 1.6 | pAMR2 | x | History CAV2 | Medication Noncompliance |
IVIG, PP | ||
|
| |||||||||
| 19 | 24.8 | 6.9 | x | PO steroids, increase TAC goal | |||||
|
| |||||||||
| 20 | 6.9 | 1.6 | pAMR2 | x | Medication Noncompliance, Decrease in EF, Decrease in CI |
IVIG, IV steroids, PP | |||
|
| |||||||||
| 21a | 9.3 | 1.2 | pAMR(I+) | x | New CAV 2 | Medication Noncompliance, Decrease in EF Decrease in CI |
IV steroids, PP, ATG, BZ, RX | ||
|
| |||||||||
| 22 | 9.1 | 2.8 | x | IV steroids, IVIG | |||||
|
| |||||||||
| 23 | 10.8 | 1.0 | “Quilty phenomenon” | x | Elevated Allomap | IV steroids, PP, ATG, BZ, RX | |||
|
| |||||||||
| 24 | 16.2 | 4.0 | Trace C4 | x | History CAV2 | None | |||
|
| |||||||||
| 25a | 36.7 | 6.3 | pAMR(I+) | x | History CAV2 | Medication Noncompliance, Decrease in EF, Decrease in CI |
IV steroids, PP, ATG | ||
|
| |||||||||
| 26 | 6.4 | 2.4 | x | History CAV3 | Increase TAC goal | ||||
|
| |||||||||
| 27a | 17.6 | 3.6 | pAMR(H+) | x | History CAV3 | Decrease in EF, Decrease in CI | PO steroids, IvIG | ||
|
| |||||||||
| 28a | 24.7 | 6.1 | pAMR2 | History CAV3 | Increase TAC goal | ||||
|
| |||||||||
| 29 | 26.6 | 5.7 | pAMR(H+) | x | IV steroids | ||||
|
| |||||||||
| 30 | 18.2 | 6.1 | pAMR (H+) | x | Medication Noncompliance | PO steroids | |||
Abbreviations: ACR, acute cellular rejection; AMR, antibody-mediated rejection; ATG, thymoglobulin; Bx, biopsy; BZ, bortezomib; CAV, coronary artery vasculopathy; DSA, donor specific antibodies; IV, intravenous; IVIG, intravenous immunoglobulin; PO, oral; PP, plasmapheresis; RX, rituximab; TAC, tacrolimus.
Denotes a biopsy outside of routine biopsy protocol.
Decrease in EF ≥5%; Decrease in CI ≥0.5.
TABLE 2.
Patient demographics and pre-transplant characteristics
| Rejection (n = 17) | Non-rejection (n = 17) | p-value | |
|---|---|---|---|
| Male sex, n (%) | 13 (77) | 9 (53) | .28 |
|
| |||
| Race, n (%) | |||
| White | 9 (53) | 9 (53) | 1.00 |
| AA | 8 (47) | 7 (41) | |
| Multiracial | 0 | 1 (6) | |
|
| |||
| Ethnicity, n (%) Hispanic | 1 (6) | 1 (6) | 1.00 |
|
| |||
| Known genetic syndrome, n (%) | 3 (18) | 0 (0) | .23 |
|
| |||
| Significant comorbidities, n (%) | 5 (29) | 3 (18) | .69 |
|
| |||
| History of CHD, n (%) | 10 (59) | 10 (59) | .64 |
|
| |||
| History of blood transfusions, n (%) | 14 (82) | 14 (82) | 1.00 |
|
| |||
| PRA, n (%) | |||
| 0–10% | 3 (18) | 8 (47) | .12 |
| 10–50% | 6 (35) | 5 (29) | |
| >50% | 8 (47) | 4 (24) | |
|
| |||
| Blood type, n (%) | |||
| A | 6 (35) | 6 (35) | .12 |
| B | 7 (41) | 2 (12) | |
| O | 4 (24) | 9 (53) | |
|
| |||
| Alive at time of study, n (%) | 12 (71) | 17 (100) | .02a |
|
| |||
| Listed ABOi, n (%) | 1 (6) | 3 (18) | .58 |
|
| |||
| Cross match results, n (%) | |||
| Positive | 1 (5) | 0 | .23 |
| Weakly positive | 5 (30) | 2 (12) | |
| Negative | 11 (65) | 15 (88) | |
|
| |||
| Inotropic support at TOT, n (%) | 14 (82) | 15 (88) | .33 |
| Milrinone | 13 (77) | 13 (77) | |
| Dopamine | 1 (6) | 1 (6) | 1.00 |
| 2 or more inotropes | 0 | 1 (6) | |
|
| |||
| Dialysis at TOT, n (%) | 0 | 1 (7) | .48 |
|
| |||
| Mechanically ventilated at TOT | 1 (6) | 2 (12) | .50 |
|
| |||
| Pre-operative mechanical support, n (%) | 8 (47) | 9 (53) | .50 |
| LVAD/RVAD | 6 (35) | 7 (41) | |
| ECMO | 0 | 0 | 1.00 |
| BIVAD | 1 (6) | 0 | |
| ECMO to VAD | 1 (6) | 2 (12) | |
|
| |||
| Received thymoglobulin at TOT, n (%) | 11 (64) | 7 (41) | .15 |
|
| |||
| Total no of biopsiesb from TOT to 1/2021 | 12 (11, 15) | 10 (9, 12) | .01a |
|
| |||
| Age at TOT, (years) | 12.5 (2.1, 17.4) | 1.3 (0.6, 5.7) | .01a |
|
| |||
| Time on wait list, (days) | 107 (59, 300) | 129 (79, 269) | .61 |
|
| |||
| Total ischemic time, (min) | 256 (219, 299) | 265 (227, 288) | .76 |
|
| |||
| No of days until TAC initiation | 2 (2, 4) | 2 (2, 3) | .59 |
|
| |||
| No of days until two consecutive therapeutic levels of tacrolimus | 10 (8, 19) | 8 (4, 10) | .02a |
|
| |||
| Total no of blood products at the time of transplant, (units) | 10 (6, 18) | 6 (5, 8) | .03a |
| No of PRBC | 2 (2, 6) | 2 (1, 3) | .13 |
| No of platelets | 4 (2, 5) | 2 (1, 3) | .03a |
| No of cryoprecipitate | 2 (1, 3) | 1 (1, 2) | .07 |
| No of fresh frozen plasma | 2 (1, 7) | 1 (0, 2) | .06 |
|
| |||
| Months between paired echoes | 2.7 (1.2, 5.7) | 3.8 (3.1, 5.7) | .12 |
|
| |||
| Months from transplant at time of biopsy | 4.2 (0.6, 9.3) | 7.5 (6.2, 9.2) | .03a |
Abbreviations: AA, African American; ABOi, ABO incompatible; BIVAD, biventricular assist device; ECMO, extracorporeal membrane oxygenation; LVAD, left ventricular assist device; PRA, panel reactive antibodies; PRBC, packed red blood cells; RVAD, right ventricular; TOT, time of transplant; VAD, ventricular assist device.
Denotes statistical significance.
Continuous variables reported as medians (interquartile ranges).
3.1 |. Rejection episodes and immunosuppressive strategies
There were 11 episodes of ACR, 16 episodes of AMR, and 3 episodes of combined ACR and AMR. Two episodes of AMR were based on positive DSA and additional clinical information in the absence of biopsy changes. There were 4 ACR episodes of Grade 2R or greater rejection and one patient with a known diagnosis of CAV 3, which was present prior to his rejection episode. Two patients developed severe hemodynamic compromise with induction of anesthesia at the time of catheterization. By far the most common immunosuppression regimens at the time of rejection included tacrolimus (n = 28) and mycophenolate mofetil (n = 24) in combination with oral steroids (n = 11). Azathioprine (n = 4), sirolimus (n = 2), and cyclosporine (n = 2) were used alternatively. Two patients received outpatient subcutaneous immunoglobulins for previous history of AMR. There was concern for noncompliance at the time of 8 biopsies. Oral and intravenous steroids formed the basis of treatment for ACR, while AMR was treated utilizing combinations of steroids, intravenous immunoglobulin, bortezomib, rituximab, plasmapheresis, and thymoglobulin.
3.2 |. Changes in echocardiographic and catheterization data
Changes in echocardiographic and catheterization data within groups and between groups can be found in Table 3. All echocardiograms that were assessed had had adequate apical 4-chamber and short-axis clips to obtain strain. Change in LV volumes and in ejection fraction were not statistically significant between rejection and non-rejection groups. Changes in other left ventricular 2D, spectral Doppler and tissue Doppler measurements did not reach statistical significance between groups. Tricuspid annular plane systolic excursion (TAPSE), an assessment of right ventricle (RV) function, improved in the non-rejection group’s follow-up echocardiograms [0.8 (0.7, 1.1) to 0.9 (0.8, 1.2) cm], while the rejection cohort witnessed a decrease in TAPSE [0.8 (0.7, 1.0) to 0.7 (0.6, 1.0) cm], p = .02. There was a decline in circumferential strain in the rejection group at the second echocardiogram [−18.5 (−21.5, −14.6) to −15.7 (−19.8, −13.2)] while circumferential strain improved in the non-rejection group [−20.8 (−23.9, −17.8) to −23.9 (−24.9, −20.1)] representing a significant difference in change in circumferential strain between groups (p = .02) (Figures 1 and 2). Similarly, there was an observed worsening in longitudinal strain in the rejection cohort, and an improvement in longitudinal strain in the non-rejection group that neared statistical significance (p = .06).
TABLE 3.
Changes in variables analyzed with and without rejection
| Rejection |
Non-rejection |
||||||
|---|---|---|---|---|---|---|---|
| Baseline | Rejection state | p-value (within rejection group) | Baseline | Non-rejection state | p-value (within non-rejection group) | p-value (differences in changes between rejection and non-rejection groups) | |
| EF (%) | 57 (51, 62) | 57 (49, 63) | .43 | 61 (58, 64) | 61 (57, 65) | .59 | .24 |
|
| |||||||
| FS (%) | 35 (28, 40) | 32 (24, 35) | .02a | 38.5 (34, 40) | 35.9 (34, 39) | .86 | .04b |
|
| |||||||
| TAPSE (cm) | 0.8 (0.7, 1.0) | 0.7 (0.6, 1.0) | .41 | 0.8 (0.7, 1.1) | 0.9 (0.8, 1.2) | .06 | .02b |
|
| |||||||
| LV EDV (ml) | 79.4 (30.4, 120.6) | 83.7 (34.2, 106.6) | .33 | 35.1 (23.6, 48.4) | 31.4 (22.8, 67.7) | .18 | .71 |
|
| |||||||
| LV ESV (ml) | 29.7 (12.5, 54.1) | 36.7 (14.2, 55.8) | .23 | 13.3 (9.15, 19.3) | 12.4 (8.5, 28.2) | .19 | .56 |
|
| |||||||
| LS (%) | −13.9 (−15.2, −11.2) | −13.0 (−16.3, −8.9) | .37 | −15.5 (−17.3, −12.8) | −16.9 (−18.8, −14.7) | .06 | .06b |
|
| |||||||
| CS (%) | −18.5 (−21.5, −14.6) | −15.7 (−19.9, −13.2) | .14 | −20.8 (−23.9, −17.8) | −23.9 (−24.9, −20.1) | .10 | .02b |
|
| |||||||
| CI (L/min/m2) | 2.7 (2.2, 3.4) | 2.4 (2, 3.6) | .71 | 3.7 (3, 4) | 3.7 (2.6, 4.3) | .31 | .66 |
|
| |||||||
| Mixed Venous O2 Sat. (%) | 69 (66, 72) | 67 (60, 72) | .16 | 69 (64, 74) | 70 (67, 75) | .18 | .04b |
|
| |||||||
| AVO2 difference | 29 (26, 31) | 30 (26, 35) | .19 | 27 (25, 32.5) | 28 (23.3, 30.8) | .33 | .48 |
|
| |||||||
| PVRi (wood units/m2) | 2.8 (2.1, 3.5) | 2.4 (1.9, 4) | .89 | 2.5 (2.1, 2.8) | 2.7 (1.9, 3.7) | .62 | .69 |
|
| |||||||
| Mean PAP (mmHg) | 20 (18, 22) | 22 (20, 28) | .01b | 20 (17, 23) | 20 (18, 23.5) | .19 | .2 |
|
| |||||||
| RVEDP (mmHg) | 9 (6, 11) | 10 (7, 13) | .01b | 7 (5, 10) | 7 (6.5, 8) | .84 | .04b |
|
| |||||||
| PCWP (mmHg) | 12 (10, 14) | 13 (10, 18) | .02b | 10 (10, 13) | 12 (10, 12) | .60 | .1 |
|
| |||||||
| Heart rate (bpm) | 108 (85, 113) | 104 (86, 116) | .15 | 109 (98, 120) | 106 (85, 119) | <.01b | .47 |
Abbreviations: AVO2, arteriovenous oxygen difference; CI, cardiac index; CS, circumferential strain; EF, ejection fraction; FS, fractional shortening; LV EDV, left ventricular end diastolic volume; LV ESV, left ventricular end systolic volume, LS, longitudinal strain; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RVEDP, right ventricular end diastolic pressure; Sat, saturation; TAPSE, tricuspid annular plane systolic excursion.
Denotes statistical significance.
Continuous variables reported as medians (interquartile ranges).
FIGURE 1.
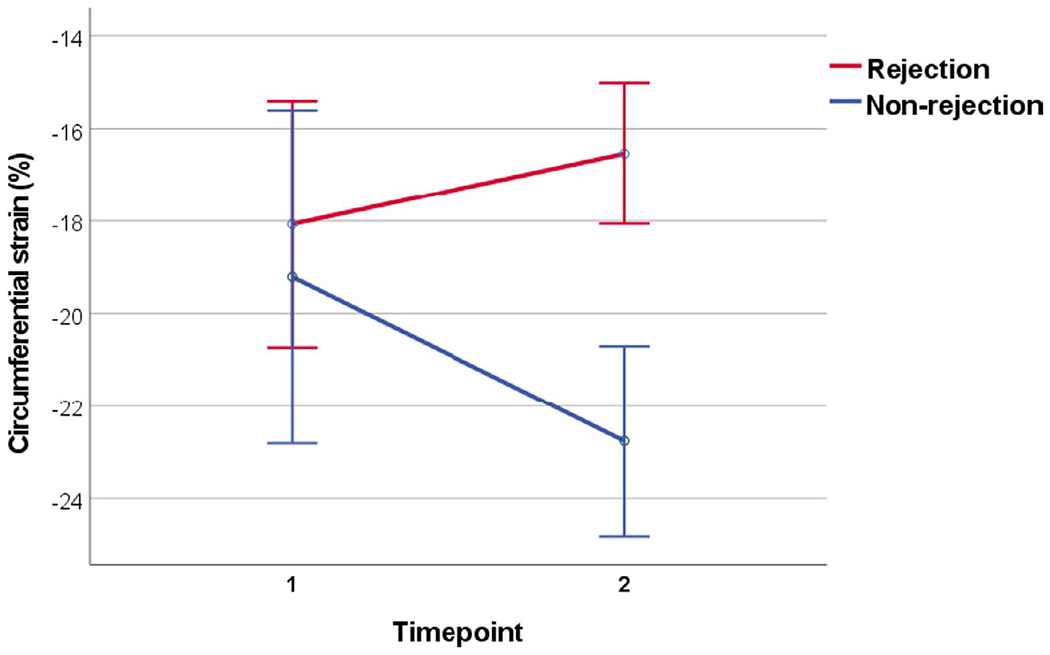
Difference in change in circumferential strain between the rejection and non-rejection group
FIGURE 2.
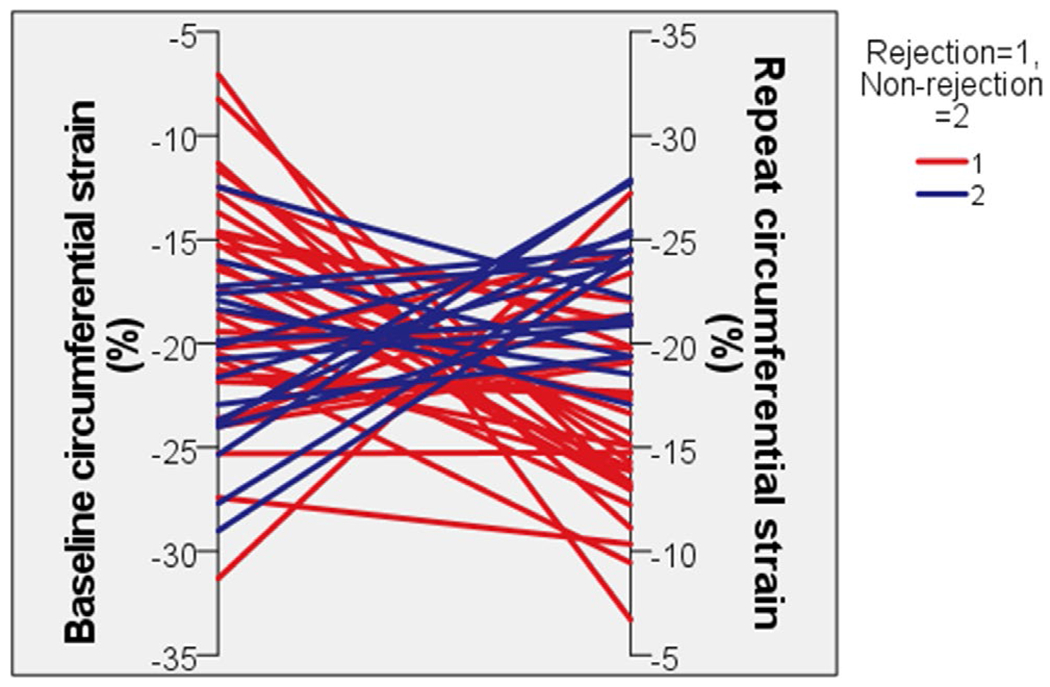
Changes in circumferential strain for individual patients
The only measurements obtained invasively by catheterization that showed a significant change between groups was mixed venous oxygen saturation (p = .04) and RV end diastolic pressure (p = .04). Changes in laboratory values assessing end organ function, Allomap™ score, and EKG data did not show a significant association with rejection.
4 |. DISCUSSION
In this study, we compared changes in echocardiographic measures of ventricular function between pediatric heart transplant recipients with and without rejection to identify candidate measures that may be useful in patients at high risk for rejection. The main finding of this study is that patients in the non-rejection group displayed improvement in LV CS between interval EMBs while patients in the rejection group displayed worsening LV CS between EMBs at the time of a rejection episode. Decreases in CS are counter to the natural history early after heart transplantation and appear to be a marker of rejection in pediatric heart transplant recipients even in the face of an unchanged EF.
The ability of 2DSTE to detect subclinical rejection episodes appear feasible based on previous animal and adult studies. Changes in strain by speckle tracking in a rat transplant model have proven useful in detecting subclinical LV dysfunction during histologically proven rejection when conventional echocardiographic measures failed to do so.9 Several studies in adult populations have shown significant reductions in global LS during episodes of moderate acute cellular rejection, even in the absence of changes in EF.10–12 Similarly, a cut off value of −17.6% for global CS was found to have sensitivity and specificity of 82% and 68%, for its detection of acute rejection despite preserved EF in adults.13
The literature for the utility of 2DSTE derived strain is less robust in the pediatric population but remains promising. A small, single center retrospective study showed a statistically significant decline in LS in 15 episodes of rejection in transplant patients one year after transplant.14 Engelhardt et al. identified a 33% decrease in global LS during episodes of acute clinical rejection in a small prospective study.15 The latter study only included rejection episodes that occurred at least three months from the time of transplant. Our results are in line with these previous studies and add the potential usefulness of circumferential strain in this population, particularly in the immediate post-transplant period.
Our results identified the natural history of LV strain in non-rejection patients. Early after transplant, pediatric patients experienced an improvement in circumferential strain. Previous studies have shown significant reductions in global longitudinal strain in pediatric patients one year post cardiac transplant when compared to healthy normal subjects, with the most significant difference noted in the early post-transplant recovery period.16 In a single retrospective review, these changes normalized by 1 year and remained relatively stable up to 5 years post-transplant in the absence of known rejection.17 These results are in line with a previous longitudinal study of 44 pediatric heart transplant recipients in which Lunze et al. utilized serial tissue Doppler velocities to assess cardiac function over 7-time intervals in the first-year post-transplant. Their findings again confirmed early biventricular dysfunction with gradual improvement in LV systolic function 1 year after transplant.18 Interestingly, Mahle et al. showed similar findings with slow improvements in tissue Doppler indices over a 6 month period post-transplant.19
There were a few notable differences between our rejection and non-rejection groups. The rejection cohort was much older than the non-rejection cohort at the time of transplant with a median age of 12.5 compared to 1.3 years of age. This is not completely surprising as older age at transplantation is an established risk factor for late rejection.20 The rejection cohort also had a much higher incidence of mortality (5 vs. 0 deaths, p = .02), which is consistent with a recent analysis of both the ISHLT registry and the Pediatric Heart Transplant Study, which showed a significant decrease in long-term survival following rejection in the first-year post-transplant.21 Rejection echo and biopsy pairs were closer in proximity to the time of transplant than the paired non-rejection data. The risk of rejection is highest within the first-year following transplant, with the greatest period of risk during the first month post-transplant.22 We included rejection episodes in this early period to increase generalizability; however, this strategy hindered the ability to compare baseline strain between the rejection and non-rejection groups. Differences within groups and changes between groups could still be compared as patients in the primary analysis served as their own controls.
Patients in the rejection cohort took longer to reach two consecutive therapeutic tacrolimus levels than the non-rejection cohort and received more blood products at the time of transplant. This suggests an important immunologic window in the immediate post-operative period where one should consider early optimization of immunosuppression and minimization of blood product exposure, especially platelets, which are known to be rich in HLA molecules, when able to do so.
4.1 |. Limitations
Due to the improved immunosuppressive therapies, acute rejection occurs relatively infrequently. This, in addition to the single center design, limited our sample size, which precluded us from performing additional analyses based on biopsy severity. Data were collected retrospectively and although there is a clinical post-transplant echocardiogram protocol, aside from strain, some measurements were not always obtained. Over the study duration there were also several different echocardiogram readers, and while they were unaware of the biopsy results at the time of the echo, they were not blinded. The age difference between groups may be a confounding factor, as older patients may not exhibit the same degrees of improvement observed in younger patients. It is also possible for a type II error to have occurred with some variables that were not consistently collected at every biopsy, such as Allomap™ and BNP levels. This study included “mild” cases of ACR, which may result in less pronounced changes in myocardial deformation. Despite this, our findings remained statistically significant, giving further credibility to the utility of echocardiography screening, specifically strain.
5 |. CONCLUSION
This study demonstrates a decline in 2DSTE LV circumferential strain is contrary to the natural history early after pediatric heart transplantation. Even in the very early transplant period, worsening of circumferential strain should be perceived as significant and prompt additional evaluation. Regular assessments of circumferential strain in this patient population may allow for early identification of rejection prior to the development of clinical symptoms or changes in ejection fraction. While the goal is to use these measures to transition from a post-transplant management strategy reliant on routine EMBs to one of EMB “For-Cause,” larger, prospective studies investigating 2DSTE strain are still needed prior to significant practice change.
Supplementary Material
Funding information
No funding was provided for this study, and no honorarium or other form of payment was provided to anyone to produce the manuscript.
Abbreviations:
- 2DSTE
two-dimensional speckle-tracking echocardiography
- ACR
acute cellular rejection
- AMR
antibody-mediated rejection
- CS
circumferential strain
- DSA
donor specific antibodies
- EF
ejection fraction
- EMB
endomyocardial biopsy
- ISHLT
International Society of Heart and Lung Transplantation
- LS
longitudinal strain
- LV
left ventricle
- PRA
panel reactive antibodies
- RV
right ventricle
- TAPSE
tricuspid annular plane systolic excursion
Footnotes
CONFLICTS OF INTEREST
None of the authors have conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.
REFERENCES
- 1.Pophal SG, Sigfusson G, Booth KL, et al. Complications of endomyocardial biopsy in children. J Am Coll Cardiol. 1999;34:2105–2110. [DOI] [PubMed] [Google Scholar]
- 2.Zinn MD, Wallendorf MJ, Simpson KE, Osborne AD, Kirklin JK, Canter CE. Impact of routine surveillance biopsy intensity on the diagnosis of moderate to severe cellular rejection and survival after pediatric heart transplantation. Pediatr Transplant. 2018;22:e13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Putzer GJ, Cooper D, Keehn C, Asante-Korang A, Boucek MM, Boucek RJ Jr. An improved echocardiographic rejection-surveillance strategy following pediatric heart transplantation. J Heart Lung Transplant. 2000;19:1166–1174. [DOI] [PubMed] [Google Scholar]
- 4.Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25:733–740. [DOI] [PubMed] [Google Scholar]
- 5.Colvin MM, Cook JL, Chang P, et al. Antibody-mediated rejection in cardiac transplantation: emerging knowledge in diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2015;131:1608–1639. [DOI] [PubMed] [Google Scholar]
- 6.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. [DOI] [PubMed] [Google Scholar]
- 7.Lai WW, Geva T, Shirali GS, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19:1413–1430. [DOI] [PubMed] [Google Scholar]
- 8.Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495;quiz 576–7. [DOI] [PubMed] [Google Scholar]
- 9.Pieper GM, Shah A, Harmann L, Cooley BC, Ionova IA, Migrino RQ. Speckle-tracking 2-dimensional strain echocardiography: a new noninvasive imaging tool to evaluate acute rejection in cardiac transplantation. J Heart Lung Transplant. 2010;29:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemmensen TS, Logstrup BB, Eiskjaer H, Poulsen SH. Changes in longitudinal myocardial deformation during acute cardiac rejection: the clinical role of two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2015;28:330–339. [DOI] [PubMed] [Google Scholar]
- 11.Mingo-Santos S, Monivas-Palomero V, Garcia-Lunar I, et al. Usefulness of two-dimensional strain parameters to diagnose acute rejection after heart transplantation. J Am Soc Echocardiogr. 2015;28:1149–1156. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Kato TS, Komamura K, et al. Utility of left ventricular systolic torsion derived from 2-dimensional speckle-tracking echocardiography in monitoring acute cellular rejection in heart transplant recipients. J Heart Lung Transplant. 2011;30:536–543. [DOI] [PubMed] [Google Scholar]
- 13.Tseng AS, Gorsi US, Barros-Gomes S, et al. Use of speckle-tracking echocardiography-derived strain and systolic strain rate measurements to predict rejection in transplant hearts with preserved ejection fraction. BMC Cardiovasc Disord. 2018;18:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehgal S, Blake JM, Sommerfield J, Aggarwal S. Strain and strain rate imaging using speckle tracking in acute allograft rejection in children with heart transplantation. Pediatr Transplant. 2015;19:188–195. [DOI] [PubMed] [Google Scholar]
- 15.Engelhardt K, Das B, Sorensen M, Malik S, Zellers T, Lemler M. Two-dimensional systolic speckle tracking echocardiography provides a noninvasive aid in the identification of acute pediatric heart transplant rejection. Echocardiography. 2019;36:1876–1883. [DOI] [PubMed] [Google Scholar]
- 16.Kailin JA, Miyamoto SD, Younoszai AK, Landeck BF. Longitudinal myocardial deformation is selectively decreased after pediatric cardiac transplantation: a comparison of children 1 year after transplantation with normal subjects using velocity vector imaging. Pediatr Cardiol. 2012;33:749–756. [DOI] [PubMed] [Google Scholar]
- 17.Godown J, Dodd DA, Stanley M, et al. Changes in left ventricular strain parameters following pediatric heart transplantation. Pediatr Transplant. 2018;22:e13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunze FI, Colan SD, Gauvreau K, et al. Cardiac allograft function during the first year after transplantation in rejection-free children and young adults. Circ Cardiovasc Imaging. 2012;5:756–764. [DOI] [PubMed] [Google Scholar]
- 19.Mahle WT, Cardis BM, Ketchum D, Vincent RN, Kanter KR, Fyfe DA. Reduction in initial ventricular systolic and diastolic velocities after heart transplantation in children: improvement over time identified by tissue Doppler imaging. J Heart Lung Transplant. 2006;25:1290–1296. [DOI] [PubMed] [Google Scholar]
- 20.Webber SA, Naftel DC, Parker J, et al. Late rejection episodes more than 1 year after pediatric heart transplantation: risk factors and outcomes. J Heart Lung Transplant. 2003;22:869–875. [DOI] [PubMed] [Google Scholar]
- 21.Dipchand AI. Current state of pediatric cardiac transplantation. Ann Cardiothorac Surg. 2018;7:31–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirklin JK, Naftel DC, Bourge RC, et al. Rejection after cardiac transplantation. A time-related risk factor analysis. Circulation. 1992;86:II236–II241. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.


