Abstract
We have recently established that the facultative phototrophic bacterium Rhodobacter sphaeroides, like the closely related Rhodobacter capsulatus species, contains both the previously characterized mobile electron carrier cytochrome c2 (cyt c2) and the more recently discovered membrane-anchored cyt cy. However, R. sphaeroides cyt cy, unlike that of R. capsulatus, is unable to function as an efficient electron carrier between the photochemical reaction center and the cyt bc1 complex during photosynthetic growth. Nonetheless, R. sphaeroides cyt cy can act at least in R. capsulatus as an electron carrier between the cyt bc1 complex and the cbb3-type cyt c oxidase (cbb3-Cox) to support respiratory growth. Since R. sphaeroides harbors both a cbb3-Cox and an aa3-type cyt c oxidase (aa3-Cox), we examined whether R. sphaeroides cyt cy can act as an electron carrier to either or both of these respiratory terminal oxidases. R. sphaeroides mutants which lacked either cyt c2 or cyt cy and either the aa3-Cox or the cbb3-Cox were obtained. These double mutants contained linear respiratory electron transport pathways between the cyt bc1 complex and the cyt c oxidases. They were characterized with respect to growth phenotypes, contents of a-, b-, and c-type cytochromes, cyt c oxidase activities, and kinetics of electron transfer mediated by cyt c2 or cyt cy. The findings demonstrated that both cyt c2 and cyt cy are able to carry electrons efficiently from the cyt bc1 complex to either the cbb3-Cox or the aa3-Cox. Thus, no dedicated electron carrier for either of the cyt c oxidases is present in R. sphaeroides. However, under semiaerobic growth conditions, a larger portion of the electron flow out of the cyt bc1 complex appears to be mediated via the cyt c2-to-cbb3-Cox and cyt cy-to-cbb3-Cox subbranches. The presence of multiple electron carriers and cyt c oxidases with different properties that can operate concurrently reveals that the respiratory electron transport pathways of R. sphaeroides are more complex than those of R. capsulatus.
The purple nonsulfur facultative phototrophic bacteria of Rhodobacter species, Rhodobacter capsulatus and its close relative Rhodobacter sphaeroides, are excellent model organisms for studying cellular energy transduction (46). They have versatile growth modes and are capable of both anoxygenic phototrophic (Ps) and vigorous respiratory (Res) growth in the presence of oxygen. They contain a photochemical reaction center (RC) (43, 44), a ubihydroquinone:cytochrome c (cyt c) oxidoreductase (cyt bc1 complex) (6, 45), and a c-type cytochrome as a mobile electron carrier (7, 8), but their Ps and Res electron transport chains are different (46, 47).
During the Ps growth of R. capsulatus, electrons are conveyed from the cyt bc1 complex to the RC either by the well-studied mobile electron carrier cyt c2 (7) or the more recently discovered membrane-anchored electron carrier cyt cy (17), encoded by cycA and cycY, respectively. On the other hand, in R. sphaeroides, the Ps electron transport pathway can be mediated only by a mobile electron carrier like cyt c2 (8) or its functional analogues such as isocyt c2 (33) (Fig. 1). Although Ps-grown cells of R. sphaeroides also harbor a membrane-anchored cyt cy, this electron carrier is not able to support Ps growth (27). Yet the Ps growth inability of R. sphaeroides mutants lacking cyt c2 can be restored readily by genetic introduction of R. capsulatus cyt cy (17, 18). These findings indicate that R. sphaeroides cyt cy is the culprit for the Ps growth inability of this species in the absence of cyt c2 (27), but the molecular basis of this observation remains unknown.
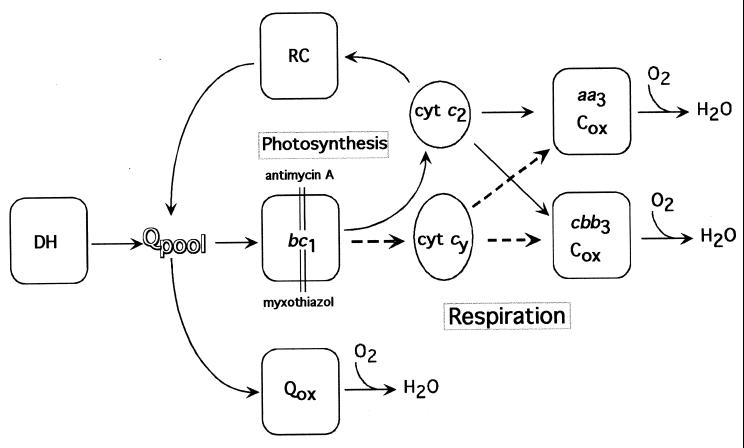
FIG. 1.
Photosynthetic and respiratory electron transport pathways of R. sphaeroides. DH, Qpool, bc1, RC, Qox, cyt c2, cyt cy, aa3-Cox, and cbb3-Cox correspond to the respiratory NADH and succinate dehydrogenases, membrane quinone pool, cyt bc1 complex, photochemical reaction center, hydroquinone oxidase, soluble electron carrier cyt c2, membrane-attached electron carrier cyt cy, aa3-type cyt c oxidase, and cbb3-type cyt c oxidase, respectively. Dotted arrows highlight the electron transfer pathways catalyzed by cyt cy. Antimycin A (Ant A) and myxothiazol (Myx) are inhibitors of the cyt bc1 complex.
Respiratory electron transport pathways of R. capsulatus and R. sphaeroides are also similar but not identical (46) (Fig. 1). Both species contain a respiratory electron transport pathway that is branched after the quinone pool. One of these branches contains hydroquinone oxidases (Qox) commonly found in microbes, while the other one harbors a mitochondrial-like cyt c oxidase(s) (Cox) converting oxygen to water (13). While R. capsulatus contains only one cbb3-type Cox (cbb3-Cox) (14, 19), both an aa3-type Cox (aa3-Cox) (16, 36), and a cbb3-Cox (12, 42) are present in R. sphaeroides. In the latter species, the cbb3-Cox is predominant in microaerobic or Ps-grown cells, while the aa3-Cox becomes a major component under high O2 tension (37). In both species, the nature of the Qox is not well known. Recent works including genome sequences (4, 11) suggest the presence of one Qox in R. capsulatus (21, 48; K. Zhang and F. Daldal, unpublished data) and possibly two Qox in R. sphaeroides.
Recently, we have established that R. sphaeroides cyt cy can function as an electron carrier between the cyt bc1 complex and the cbb3-Cox in R. capsulatus (27). This finding raised the issue of whether it can also act as an electron carrier during the Res growth of R. sphaeroides and, if so, whether it can donate electrons to both the aa3-Cox and the cbb3-Cox. Obviously, the presence of two c-type electron carriers and two cyt c oxidases complicates this analysis. To facilitate it, appropriate mutations inactivating either cyt c2 or cyt cy were introduced into R. sphaeroides strains lacking either cbb3-Cox or aa3-Cox activity. The double mutants thus obtained converted the crisscrossed respiratory electron transport pathways into simpler linear pathways (Fig. 1) and allowed estimation of their efficiencies. The data demonstrated for the first time that in R. sphaeroides either cyt c2 or cyt cy can act as an electron carrier between the cyt bc1 complex and either the cbb3-Cox or the aa3-Cox. Furthermore, they indicated that electron flow via the cyt c2 → cbb3-Cox and cyt cy → cbb3-Cox subbranches appears to be greater under semiaerobic growth conditions. During these studies, it was noted that in the absence of the latter respiratory pathway, light-harvesting protein complexes, which are usually absent in the presence of oxygen, were also produced.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli strains were grown on Luria broth (LB medium) supplemented with kanamycin, spectinomycin, or tetracycline at a final concentration of 50, 50, or 12.5 μg per ml, respectively. R. sphaeroides strains were grown in Sistrom's minimal medium (Med A) (40) supplemented as needed with 10, 10, or 2.5 μg of spectinomycin, kanamycin, or tetracycline, respectively, per ml. For semiaerobic growth, 2-liter flasks were filled with 1 liter of growth medium and shaken at 150 rpm with a rotary shaker (New Brunswick Scientific Co. Inc., Rutherford, N.J.). Photo- or chemoheterotrophic growth phenotypes and ability to catalyze the Nadi reaction (N′,N′-dimethyl-p-phenylenediamine + α-naphthol → indophenol blue + H2O) (24) were determined on solid media using cells grown in anaerobic jars containing H2- and CO2-generating gas packs (BBL) or an aerobic dark incubator at 35°C in the presence of atmospheric oxygen. Growth rates of various strains were determined using liquid cultures inoculated with freshly grown cells at appropriate dilutions. As needed, cultures were first incubated overnight at ambient temperature without shaking and then transferred to a rotary shaker at 35°C, and the turbidity was monitored using side-arm flasks and a Klett-Summerson colorimeter. All strains and plasmids used are listed in Table 1. The parental R. sphaeroides strain Ga (40) is referred to as wild type with respect to its cytochrome profile and its Ps and Res growth properties.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Genotype | Relevant phenotype | Source or reference |
|---|---|---|---|
| E. coli | |||
| HB101 | F proA2 hsdS20 recA1 ara-14 lacY1 galK2 rpsL20 supE44 xyl-5 mtl-1 | rB− mB− | Stratagene |
| S17.1 | thi pro hsdR recA ara RP4::2-Tc::Mu::kan:Tn7 integrated into chromosome | rB− mB+ Tpr Smr | 39 |
| R. sphaeroides | |||
| Ga | crt | Wild type | 40 |
| Gadc2 | crt Δ(cycA::spe) | Cyt c2− | 3 |
| Gadcy | crt Δ(cycY::kan) | Cyt cy− | 17 |
| Gadc2cy | crt, Δ(cycA::spe) Δ(cycY::kan) | Cyt c2− Cyt cy− | 17 |
| MT001 | crt Δ(ccoNO::kan) | cbb3-Cox− | 42 |
| KD02 | crt Δ(cycA::spe) Δ(ccoNO::kan) | Cyt c2−cbb3-Cox− | This work |
| KD04 | crt Δ(cycY::spe) Δ(ccoNO::kan) | Cyt cy−cbb3-Cox− | This work |
| JS100 | crt Δ(ctaD::spe) | aa3-Cox− | 36 |
| KD03 | crt Δ(cycY::kan) Δ(ctaD::spe) | Cyt cy−aa3-Cox− | This work |
| KD05 | crt Δ(cycA::kan) Δ(ctaD::spe) | Cyt c2−aa3-Cox− | This work |
| ME127 | crt Δ(ctaD::spe) Δ(ccoNO::kan) | cbb3-Cox− aa3-Cox− | 42 |
| BC17 | crt Δ(petABC(fbcFBC)::kan) | Cyt bc1− | 45 |
| Plasmids | |||
| pBSII | pBlueScript II KS(+) | Ampr | Stratagene |
| pHP45Ω | spe cartridge | Sper | 31 |
| pHP45Ω-Km | kan cartridge | Kanr | 9 |
| pSup202 | Suicide plasmid | Tetr Cmr Ampr | 39 |
| p4.2 | cycY::kan in PstI site of pSup202 | This work | |
| pcycA::spe | cycA::spe in PstI site of pSup202 | This work | |
| pKD10.2 | 2-kb spe cartridge of pHP45Ω in BamHI site of cycY carried by pU2000 (in opposite orientation of cycY) | This work | |
| pU2000 | 1.8-kb BglII-XbaI R. sphaeroides chromosomal DNA carrying cycY in pBSII KS(+) | 51 | |
| pKD11 | 3 kb cycY::spe fragment in EcoRI site of pSup202 | This work | |
| pSupC2 P2.71::kan | Δ(cycA::kan) at StuI site of cycA carried at PstI site of pSup202 | 8 |
Molecular genetic techniques and construction of various R. sphaeroides mutants.
Two different insertion-deletion alleles of cycA and cycY, carrying either spectinomycin resistance (Sper) or kanamycin resistance (Kanr)-conferring gene cartridges, were necessary for constructing various double mutants lacking cyt c2 or cyt cy and cbb3-Cox or aa3-Cox. Thus, a cycY::kan allele was constructed similarly to cycY::spe described previously (27), except that a Kanr-conferring cartridge instead of a Sper-conferring cartridge from pHP45Ω-K (9) was used. The desired allele was transferred into the suicide plasmid pSup202, conferring tetracycline resistance (Tetr), and via the E. coli donor strain S17.1 (39) conjugated into the appropriate R. sphaeroides strains, selecting for Sper or Kanr as required. Among the transconjugants, those that were Tets, and hence carrying a chromosomal allele replacement via a double-crossover event, were retained for further analyses (3). Introduction of appropriate cycA and cycY alleles into the R. sphaeroides Δ(ccoNO::kan) (MT001) and Δ(ctaD::spe) (JS100) mutants yielded the Δ(cycA::spe) Δ(ccoNO::kan) (KD02) and Δ(cycY::spe) Δ(ccoNO::kan) (KD04) and the Δ(cycY::kan) Δ(ctaD::spe) (KD03) and Δ(cycA::kan) Δ(ctaD::spe) (KD05) double mutants, respectively (Table 1).
Biochemical and spectroscopic techniques.
R. sphaeroides chromatophore membranes from washed cells grown semiaerobically in Med A were prepared using a French pressure cell at 18,000 lb/in2 and ultracentrifugation either in 50 mM 3-(N-morpholino)propanesulfonic acid (MOPS) buffer (pH 7.0) containing 1 mM KCl, EDTA, and phenylmethylsulfonyl fluoride for polyacrylamide gel electrophoresis (PAGE) analyses (18, 20) or in 50 mM MOPS buffer (pH 7.2) containing 5 mM MgCl2 for spectroscopic studies (50). Membrane fragments were washed twice, resuspended at a known protein concentration in the same buffer, and stored frozen at −80°C until further use. Protein content of the samples was determined by the method of Bradford (2) or Lowry et al. (22), using bovine serum albumin as a standard. Bacteriochlorophyll concentration was measured by the optical absorption of acetone-methanol (7:2, vol/vol) extracts, using an extinction coefficient ɛ775 of 75 mM−1 cm−1 (5). Sodium dodecyl sulfate (SDS)-PAGE was performed using 16.5% acrylamide Tris-Tricine gels as described by Schägger and von Jagow (35). Samples were denatured for 5 min at 37°C in SDS loading buffer prior to electrophoresis, and gels were stained with Coomassie brilliant blue to visualize the polypeptides. The c-type cytochromes were revealed via the intrinsic peroxidase activity of their heme group, using 3,3′,5,5′-tetramethylbenzidine (TMBZ) and H2O2 (41). The amounts of cytochromes in chromatophore membranes were estimated by recording at room temperature reduced (with ascorbate or dithionite)-minus-oxidized (with ferricyanide) optical difference spectra. Either a Hitachi 3400 or Jasco 7800 spectrophotometer and the extinction coefficients ɛ604–630 of 23 mM−1 cm−1, ɛ561–575 of 22 mM−1 cm−1, and ɛ551–540 of 29 mM−1 cm−1 were used for a-, b-, and c-type cytochromes, respectively.
Cyt c oxidase activities in membrane fragments were determined using either reduced horse heart cyt c as a substrate or by monitoring O2 consumption. In the first case, immediately prior to the assay, horse heart cyt c was reduced by addition of sodium dithionite, excess of which was eliminated via chromatography through a small column containing Sephadex G-10 as a matrix (14, 20). Oxidation of cyt c was monitored at 550 nm in a stirred cuvette, thermostated at 25°C, containing 50 mM Tris (pH 8.0) buffer supplemented with 0.1 mg of dodecyl maltoside per ml, 5 μM myxothiazol (Myx), and 40 μM reduced cyt c. As needed, 2 mM potassium cyanide (KCN) was used as an inhibitor (34). In the second case, respiratory rates were measured by polarography at 28°C with a Clark-type oxygen electrode (Yellow Springs Instruments Inc., Yellow Springs, Ohio). These assays used 0.25 to 0.30 mg of membrane fragments per ml resuspended in 50 mM MOPS buffer (pH 7.2) containing 5 mM MgCl2, 2 mM sodium ascorbate, and 200 μM N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) or 50 μM horse heart cyt c (15, 48). As needed, 100 μM KCN was used to determine the amount of Cox-independent O2 consumption during the assay, and enzymatic activities were expressed in moles of substrate consumed per minute per milligram of protein. TMPD was also substituted with 2,3,5,6-tetramethyl-1,4-phenylenediamine (DAD) or 3,6-dichlorophenol indophenol (DCIP) as an electron donor and KCN with NaN3 as an inhibitor (34). Kinetic changes in the absorption of c-type cytochromes were monitored at 551 minus 540 nm at 28°C, using a Jasco V-550 dual-wavelength spectrophotometer equipped with a Jasco ETC-505T rapid-mixing and temperature control apparatus (15).
To estimate amounts of the light-harvesting complexes in membrane fragments of various R. sphaeroides mutants, 50-ml cultures were grown aerobically with vigorous shaking in 250-ml flasks to an optical density at 630 nm of less than 0.4. Cells were collected by centrifugation and resuspended in 50 mM potassium phosphate buffer (pH 7.0) containing 10 mM EDTA and leupeptin, pepstatin A, and Pefabloc SC (Roche Inc., Indianapolis, Ind.) as protease inhibitors at concentrations suggested by the supplier. After addition of 1/50 volume of lysozyme (10 mg/ml) and 1 h of incubation on ice, cells were frozen at −80°C for 10 min, thawed, and sonicated on ice for 5 min at a 40% relative output in a sonicator (model 150; Dynatech Laboratories Inc., Farmingdale, N.Y.). Cell debris was removed by centrifugation at 13,000 rpm for 30 min at 4°C, and the supernatants were centrifuged at 150,000 × g for 1 h. Membrane fragments thus obtained were washed with 50 mM potassium phosphate (pH 7.0) buffer containing 1 mM EDTA, resuspended in the same buffer, and stored at −80°C until further use. Optical absorption spectra were recorded between the wavelengths of 380 and 1,000 nm with a Beckman DU640 spectrophotometer, using an amount of R. sphaeroides membranes corresponding to a total protein content of 0.2 mg per ml in 10 mM potassium phosphate (pH 7.2) buffer containing 1 mM EDTA.
Chemicals.
All chemicals, including the redox mediators, were of analytical grade and obtained from commercial sources as described previously (15). n-Dodecyl β-d-maltoside was from Anatrace; horse heart cyt c (type VI) and TMPD were from Sigma-Aldrich Chemical Co. (St. Louis, Mo.).
RESULTS
R. sphaeroides strain Ga cells grown under semiaerobic conditions contain both cbb3-Cox and aa3-Cox activities.
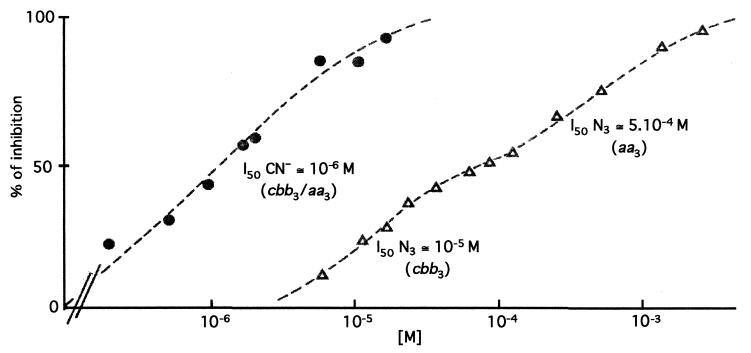
Both cbb3-Cox and aa3-Cox activities in the wild-type R. sphaeroides strain Ga can be fully inhibited by cyanide (CN−) and azide (N3−) anions. However, while CN− affects both Cox activities similarly, N3− inhibits them differently (34). Thus, to assess whether cells grown under semiaerobic conditions contained both of these enzymes, ascorbate/cyt c-dependent Cox activity was measured in the presence of increasing concentration of these inhibitors, using membranes of wild-type strain Ga (Fig. 2). The cbb3-Cox activity was totally inhibited by 100 μM N3−, whereas the aa3-Cox activity required 2 to 3 mM N3− for full inhibition. Moreover, the inhibition by CN− was monophasic (90% inhibition at 20 μM KCN), while that by N3− was biphasic, with a decrease in slope around 100 μM NaN3, corresponding to about 60% inhibition of total activity. Thus, the R. sphaeroides cells used in this study contained both of the Cox activities simultaneously.
FIG. 2.
Inhibition of the cyt c oxidase activity found in R. sphaeroides membranes. Inhibitory effects of CN− (circles) and N3− (triangles) on ascorbate and cyt c oxidase activities found in membrane fragments of R. sphaeroides wild-type strain Ga were determined as described in Materials and Methods. aa3 and cbb3 correspond to the aa3-Cox and cbb3-Cox, respectively. See the text for details.
R. sphaeroides mutants with linear, c-type cytochrome-dependent respiratory electron transport pathways.
In R. sphaeroides, c-type cytochromes containing branches of the respiratory electron transport pathways are complicated due to the presence of the cyt c2, cyt cy, cbb3-Cox, and aa3-Cox (Fig. 1). To simplify these crisscrossed pathways, four double mutants containing only one electron carrier (cyt c2 or cyt cy) and one Cox (cbb3-Cox or aa3-Cox) were obtained (Table 1). Growth phenotypes of these mutants confirmed that among the electron transport components mutated, only cyt c2 was required for Ps growth (8) (Table 2). Their doubling times under Res growth conditions (about 100 to 120 min on Med A at 35°C) were similar to those of their parents, with the exception of the cbb3-Cox− aa3-Cox− double mutant (ME127), which grew slightly slower (doubling time of approximately 150 min). In particular, Res growth of the cyt bc1− (BC17), cyt c2− cyt cy− (Gadc2cy), and cbb3-Cox− aa3-Cox− (ME127) mutants were consistent with the presence of an alternate respiratory pathway, involving a Qox (13). It was also noted that mutants lacking the cbb3-Cox (MT001, KD02, and especially KD04) formed highly pigmented colonies under respiratory growth conditions.
TABLE 2.
Electron transport chain components of various R. sphaeroides mutants, and their cytochrome contents in membranesa
| Strain | Phenotype | Electron carrier(s)/cyt c oxidase(s) | Cyt c profile
|
Cyt a | Cyt b | Cyt c | Cyt b/Cyt c ratio | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| cp | c1 | co | cy | c2 | |||||||
| Ga | Ps+ Res+ Nadi+ | Cyt c2 + cyt cy/cbb3-Cox + aa3-Cox | + | + | + | + | + | 0.8 | 2.2 | 3.8 | 0.57 |
| Gadc2 | Ps− Res+ Nadi+ | Cyt cy/cbb3-Cox + aa3-Cox | + | + | + | + | − | 0.34 | 1.0 | 1.3 | 0.77 |
| Gadcy | Ps+ Res+ Nadi+ | Cyt c2/cbb3-Cox + aa3-Cox | + | + | + | − | + | 0.37 | 1.0 | 1.45 | 0.68 |
| Gadc2cy | Ps− Res+ Nadi+ | None/cbb3-Cox + aa3-Cox | + | + | + | − | − | 0.24 | 1.0 | 0.95 | 1.05 |
| MT001 | Ps+ Res+ Nadislow | Cyt c2 + cyt cy/aa3-Cox | − | + | − | + | + | 0.48 | 1.15 | 1.14 | 1.0 |
| KD02 | Ps− Res+ Nadislow | Cyt cy/aa3-Cox | − | + | − | + | − | 0.42 | 0.93 | 0.7 | 1.3 |
| KD04 | Ps+ Res+ Nadislow | Cyt c2/aa3-Cox | − | + | − | − | + | 0.42 | 0.87 | 0.69 | 1.26 |
| JS100 | Ps+ Res+ Nadi+ | Cyt c2 + cyt cy/cbb3-Cox | + | + | + | + | + | NDb | 1.2 | 1.9 | 0.63 |
| KD03 | Ps+ Res+ Nadi+ | Cyt cy/cbb3-Cox | + | + | + | − | + | ND | 1.42 | 1.65 | 0.86 |
| KD05 | Ps− Res+ Nadi+ | Cyt cy/cbb3-Cox | + | + | + | + | − | ND | 2.2 | 1.56 | 0.87 |
| ME127 | Ps+ Res+ Nadi− | Cyt c2 + cyt cy/none | − | + | − | + | + | ND | 1.28 | 1.06 | 1.2 |
| BC17 | Ps− Res+ Nadi+ | Cyt c2 + cyt cy/cbb3-Cox + aa3-Cox | + | − | + | + | + | 0.5 | 1.05 | 2.05 | 0.5 |
Cyt a, cyt b, and cyt c values are expressed as nanomoles of heme per milligram of protein, using appropriate extinction coefficients, as described in Materials and Methods. Cyt c profiles are tabulated from the data in Fig. 4.
ND, not detected.
Next, the ability of all strains to perform the Nadi reaction (24), described in Materials and Methods, was tested as an indication of their Cox activities. Among them, only the cbb3-Cox− aa3-Cox− mutant (ME127) exhibited a complete Nadi− phenotype (i.e., no blue color formation after more than 15 min of staining) (Table 2). The cbb3-Cox− mutant (MT001) and its cyt c2−cbb3-Cox− (KD02) and cyt cy− cbb3-Cox− (KD04) derivatives showed various degrees of Nadislow phenotypes (i.e., formation of a bluish color within a few minutes of staining), with KD04 being the most defective in this respect. The remaining mutants were Nadi+ (i.e., formed a blue color in less than 1 min of staining), like the wild-type R. sphaeroides strain Ga. These observations indicated that absence of either the aa3-Cox (JS100), cyt c2 (Gadc2), cyt cy (Gadcy), or their combination (Gadc2cy, KD03, and KD05) did not affect the Nadi phenotype. Thus, in R. sphaeroides, the Nadi+ phenotype correlated better with the presence of an active cbb3-Cox rather than an aa3-Cox. It was also noted that the Nadi phenotype of various mutants was affected by the presence or absence of the electron carriers cyt c2 and cyt cy and also by the growth conditions used. For example, the cyt cy− cbb3-Cox− mutant KD04 exhibited a stronger Nadi+ phenotype if younger colonies were stained, but conversely, the cyt c2− cbb3-Cox− mutant KD02 became as Nadi− as the cbb3-Cox− aa3-Cox− mutant ME127 when grown in enriched YCC medium (30).
Cytochrome contents of various R. sphaeroides mutants.
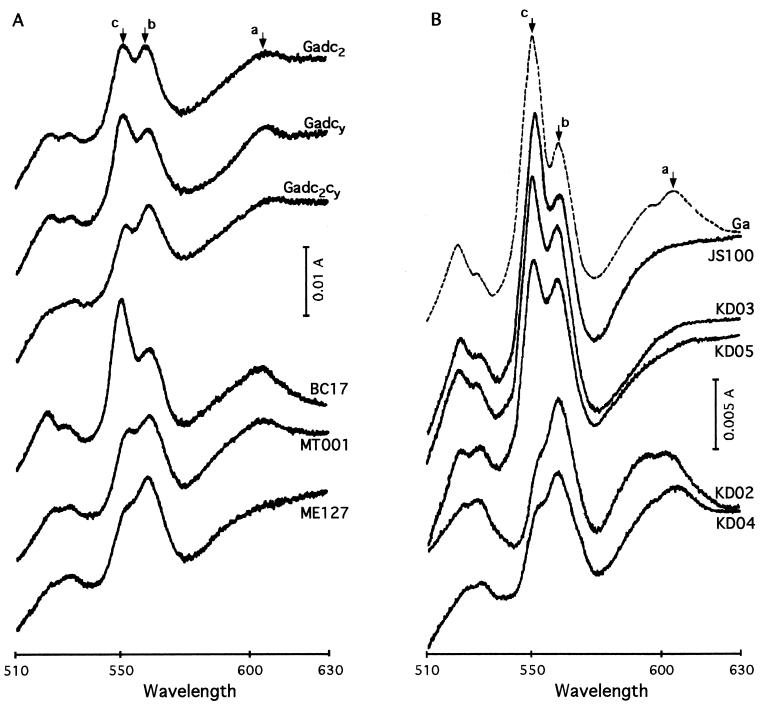
The contents of the a-, b-, and c-type cytochromes in chromatophore membranes, and in membrane supernatants, of various mutants grown by respiration in Med A were estimated using reduced-minus-oxidized optical absorption difference spectra (Fig. 3 and Table 2). Comparison of the reduced-minus-oxidized spectra of the cbb3-Cox− (MT001) and cbb3-Cox− aa3-Cox− (ME127) mutants with other strains revealed significant decreases in the amounts of the c-, b-, and a-type cytochromes (peaks around 550, 560, and 605 nm, respectively) (Fig. 3A). In addition, contribution of the b- and c-type cyt subunits of the cyt bc1 complex to total cytochrome contents of membranes was readily visible in the 550- to 560-nm region when the cyt bc1− mutant BC17 was compared to other mutants. Importantly, comparison of the cyt c2− cbb3-Cox− (KD02), cyt cy− aa3-Cox− (KD03), cyt cy− cbb3-Cox− (KD04), and cyt c2− aa3-Cox− (KD05) double mutants with their respective parents (cbb3-Cox− [MT001] and aa3-Cox− [JS100]) and with the wild-type strain Ga revealed that the 605-nm broad peak was absent in mutants carrying ctaD::spe and that the 550-nm peak was decreased significantly in those containing ccoNO::kan, as expected based on their genotypes (Fig. 3B).
FIG. 3.
Reduced-minus-oxidized optical absorption difference spectra of various R. sphaeroides mutants. The spectra were recorded between 510 and 630 nm at 25°C, using membrane fragments as described in Materials and Methods. (A) Spectra obtained with the cyt c2− (Gadc2), cyt cy− (Gadcy), cyt c2− cyt cy− (Gadc2cy), cyt bc1− (BC17), cbb3-Cox− (MT001), and cbb3-Cox− aa3-Cox− (ME127) mutants; (B) those obtained with the wild-type strain Ga and the aa3-Cox− (JS100), cyt cy−, aa3-Cox− (KD03), cyt c2− aa3-Cox− (KD05), cyt c2− cbb3-Cox− (KD02), and cyt cy− cbb3-Cox− (KD04) mutants. Absorption peaks corresponding to the a-, b-, and c-type cytochromes are indicated by arrows. Note that the absorbance scale used in panel A is twice as large as that in panel B.
In all mutants, the amount of a-type cytochromes was lower (e.g., as low as 0.24 ± 0.01 nmol/mg of protein in the cyt c2− cyt cy− mutant Gadc2cy) than in the wild-type strain Ga (0.8 ± 0.04 nmol/mg of protein) (Table 2). The b-type heme content was also low in almost all mutants (around 1.2 ± 0.3 nmol/mg of protein); an exception was the amount in the cyt cy− aa3-Cox− (KD03) mutant, which was similar to that in Ga (2.2 ± 0.1 nmol/mg of protein). Further, the amounts of c-type cytochromes dropped from 3.8 ± 0.2 nmol/mg of protein in Ga to 0.7 ± 0.02 nmol/mg of protein in cyt c2− cbb3-Cox− (KD02) and cyt cy− cbb3-Cox− (KD04) mutants as a result of mutations eliminating various c-type cytochromes. Elimination of the cbb3-Cox decreased the total amount of the b-type cytochromes by about one-half and that of the c-type cytochromes by more than two-thirds. Consequently, the cyt b-to-cyt c ratios increased from 0.5 ± 0.04 in membranes of the cyt bc1− mutant (BC17) to 1.3 ± 0.1 in those of the cyt c2− cbb3-Cox− mutant (KD02). Thus, clearly the contents of the a-, b-, and c-type cytochromes in membrane fragments from various strains varied as a function of the missing components.
The c-type cytochrome contents of chromatophore membrane supernatants of the mutants were also determined using ascorbate-reduced-minus-ferricyanide-oxidized optical difference spectra (not shown). The data indicated that R. sphaeroides mutants carrying a cycA knockout allele had about 1/10 of the 550-nm absorption peak found in a wild-type strain. The absorption maximum of the remaining ascorbate-reducible c-type cyt was shifted to 552 nm, consistent with it being isocyt c2 (33). Moreover, supernatants of the cyt bc1− mutant BC17 contained about threefold more cyt c2 than those of the wild-type strain Ga. The presence of increased amounts of cyt c2 in supernatants of mutants lacking the cyt bc1 complex has been observed previously in R. capsulatus (32).
Cyt c profile of mutants lacking components of the c-type cytochrome-dependent respiratory branch of R. sphaeroides.
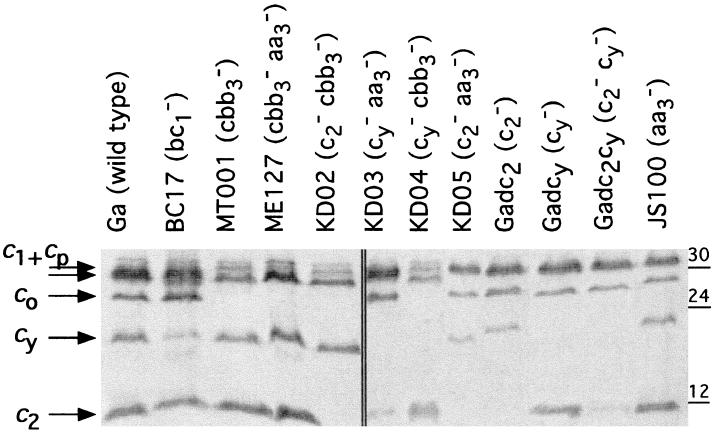
Chromatophore membrane proteins of the mutants were further analyzed using TMBZ–SDS-PAGE to determine the profiles of their c-type cytochromes. At least five major TMBZ-stained bands of molecular masses ranging from approximately 30 to 12 kDa were discernible in the wild-type strain Ga (Fig. 4). Under the separation conditions used here, the cyt cp subunit of the cbb3-Cox and cyt c1 subunit of the cyt bc1 complex run together as an unresolved doublet, which was followed by the cyt co subunit of the cbb3-Cox and cyt cy. Cyt c2 ran ahead of the other c-type cytochromes, but not being membrane anchored, its amount varied in different samples. Additional c-type cytochromes of unidentified nature running behind cyt cp were also visible, and one of them is likely to correspond to the dimethyl sulfoxide-inducible dorC product (25, 38). As expected, cyt cy was absent in Gadcy, Gadc2cy, KD03, and KD04; similarly, Gadc2, Gadc2cy, KD02, and KD05 (Table 1) lacked cyt c2 (18, 27). Equally, cyt co was missing in ME127 (cbb3-Cox− aa3-Cox−) and in MT001 (cbb3-Cox−) and its derivatives. On the other hand, JS100 (aa3-Cox−) was like the wild-type strain Ga with respect to its c-type cytochrome content. The overall spectral and TMBZ–SDS-PAGE data established that the cyt c profiles of various mutants were in agreement with their genotypes.
FIG. 4.
c-type cytochromes detected in various R. sphaeroides mutants. Membrane fragments of various mutants were analyzed by SDS-PAGE, and c-type cytochromes were revealed via their peroxidase activities using TMBZ as described in Materials and Methods. c1+cp, co, cy, and c2 correspond to the cyt c1 and cp subunits of the cyt bc1 complex and the cbb3-Cox, cyt co subunit of the cbb3-Cox, membrane-attached electron carrier cyt cy, and soluble electron carrier cyt c2, respectively. Additional unidentified c-type cytochromes running behind the doublet corresponding to cyt cp+c1 are also visible.
Respiratory electron flow and Cox activities in various R. sphaeroides mutants.
Using the above-described mutant, we probed first for the presence of the Cox activity in chromatophore membranes of various mutants, using reduced horse heart cyt c as a substrate. As expected, in semiaerobically grown cells of all strains except the cbb3-Cox− aa3-Cox− mutant ME127, substantial amounts of Cox activity were detected, although the cbb3-Cox− mutants exhibited lower levels (data not shown). Next, a detailed characterization of the electron flow through the different components of the respiratory chain was undertaken by using specific substrates and inhibitors. It is known that while addition of NADH or succinate activates electron transport via the respiratory dehydrogenases, inhibitors like antimycin A (Ant A) or Myx block it at the level of the cyt bc1 complex (Fig. 1) (46). Respiratory capabilities of various mutants studied here were assessed directly by monitoring the rates of O2 consumption depending on NADH or succinate, and in the presence or absence of exogenous cyt c, as performed previously with various R. capsulatus mutants (15).
Membrane fragments of the wild-type strain Ga rapidly oxidized various substrates, and the ensuing O2 uptake was sensitive to Myx, dropping from 20.6 to 5.8 μmol of O2 consumed/mg of protein/min in the presence of 2 μM of Myx (Table 3). Addition of 50 μM horse heart cyt c stimulated the rate of NADH oxidation to 30.9 μmol of O2 consumed/mg of protein/min. This suggested that exogenous cyt c substituted well for the shortage of endogenous cyt c2, which could have been depleted during cell disruption and membrane fragments preparation. Exogenous cyt c also greatly increased the rate of NADH oxidation (from 0.9 to 2.6 μmol of O2 consumed/mg of protein/min) or ascorbate-Cox activity (from 6.6 to 23.4 μmol of O2 consumed/mg of protein/min) in the cyt c2− cyt cy− (Gadc2cy), cyt cy− aa3-Cox− (KD03), and cyt cy− cbb3-Cox− (KD04) mutants lacking the membrane-attached cyt cy (Table 3). Moreover, pairwise comparison of various strains containing or lacking cyt cy (i.e., Gadc2 versus Gadcy, KD02 versus KD04, and KD05 versus KD03) indicated that the presence of cyt cy led almost in all cases to higher O2 consumption activities. Thus, cyt cy plays an important role as an electron carrier in the respiratory electron transport of R. sphaeroides, for in its absence the respiratory activities become more dependent on exogenous cyt c.
TABLE 3.
Respiratory activities in membranes of various R. sphaeroides mutants grown by respirationa
| Strain | Activity (μmol of O2 consumed/mg of protein/min)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| NADH
|
Succinate
|
Ascorbate
|
||||||
| Alone | +Cyt c | +Myx | +Cyt c, +CN | Alone | +Cyt c | +DCIP | +Cyt c | |
| Ga | 20.6 | 30.9 | 5.8 | 6.2 | 6.9 | 8.3 | 56.0 | 57.0 |
| KD02 | 5.2 | 7.7 | 2.2 | 2.3 | 4.7 | 7.5 | 19.3 | 34.0 |
| KD04 | 2.3 | 4.9 | 1.1 | 1.2 | 2.0 | 6.0 | 3.6 | 43.0 |
| KD03 | 4.8 | 12.0 | 4.1 | 4.4 | 4.8 | 8.8 | 23.0 | 88.0 |
| KD05 | 15.3 | 19.2 | 5.0 | 5.2 | 4.8 | 8.0 | 88.0 | 92.0 |
| JS100 | 9.6 | 11.7 | 0.6 | 0.5 | 4.8 | 7.5 | 44.0 | 35.0 |
| MT001 | 8.9 | 11.1 | 1.2 | 1.1 | 5.2 | 8.8 | 26.0 | 33.0 |
| Gadc2cy | 0.9 | 2.6 | 1.0 | 0.9 | 0.9 | 2.6 | 6.6 | 23.4 |
| Gadc2 | 7.7 | 7.9 | 1.2 | 1.3 | 2.7 | 3.4 | 22.7 | 33.6 |
| Gadcy | 2.0 | 3.6 | 1.4 | 1.3 | 2.3 | 3.7 | 25.4 | 22.2 |
| ME127 | 5.3 | 6.3 | 5.1 | 5.3 | 4.6 | 5.6 | 1.3 | 2.6 |
| BC17 | 3.8 | 3.8 | 3.7 | 3.7 | 3.8 | 3.8 | 39.0 | 41.8 |
In these assays, 0.2 mM NADH, 5 mM succinate, 2 mM ascorbate, 0.2 mM DCIP, 50 μM horse heart cyt c, 50 μM CN−, and 2 μM Myx were used as appropriate. Strain genotypes are given in Table 1.
Remarkably, the artificial electron donor DCIP reduced cyt cy better than cyt c2, as indicated by the lower oxidative rates observed in its presence in appropriate mutants (e.g., cyt cy− cbb3-Cox− [KD04] versus cyt c2− cbb3-Cox− [KD02] mutants [3.6 versus 19.3 μmol of O2 consumed/mg of protein/min], or cyt cy− aa3-Cox− [KD03] versus cyt c2− aa3-Cox− [KD05] mutants [23.0 versus 88.0 μmol of O2 consumed/mg of protein/min]) (Table 3). The small amounts of Cox activities detected in membranes of the cyt cy− cbb3-Cox− (KD04) and cyt c2− cyt cy− (Gadc2cy) mutants using reduced DCIP (3.6 and 6.6 μmol of O2 consumed/mg of protein/min, respectively) also differed from the increase of activity seen upon addition of cyt c (43.0 and 23.4 μmol of O2 consumed/mg of protein/min, respectively). Thus, DCIP did not act as a good electron donor when cyt cy and cbb3-Cox (KD04) or both cyt c2 and cyt cy (Gadc2cy) were absent, whereas horse heart cyt c acted as a good substitute for cyt c2.
Interestingly, no more than one-fifth of the total O2 consumption activity remained upon addition of either 2 μM Myx or 50 μM CN− to membrane fragments of the wild-type strain Ga (Table 3). This remnant activity was in line with NADH oxidation detected in membranes of the cbb3-Cox− aa3-Cox− (ME127), cyt c2− cyt cy− (Gadc2cy), or cyt bc1− (BC17) mutant. Virtually no Cox was detected in ME127 under all assay conditions, monitoring either NADH-, succinate-, or ascorbate-dependent O2 consumption, and both in the presence and in the absence of exogenous cyt c (Table 3). The limited O2 consumption activity detected was not inhibited by 0.1 mM CN− and possibly corresponded to other some kind of terminal oxidase, such as Qox (13). On the other hand, the cyt bc1− mutant BC17 contained Cox activities (39 or 42 μmol of 0.1 mM CN−-sensitive O2 consumed/mg of protein/min when assayed with ascorbate plus DCIP or exogenous cyt c, respectively) comparable to those seen with the wild-type strain Ga (56 or 57 μmol of O2/mg of protein/min using DCIP or cyt c, respectively). Lastly, the cbb3-Cox− (MT001) and aa3-Cox− (JS100) mutants contained about one-half of the total Cox activity found in the wild-type strain Ga (11.7 and 11.1 μmol of O2/mg of protein/min of NADH oxidase activity in the presence of exogenous cyt c in JS100 and MT001, respectively). Clearly, elimination of either the cbb3-Cox or the aa3-Cox decreased the total amount of the Cox activity but did not abolish it completely (37).
Most striking findings were obtained using the cyt c2− cbb3-Cox− (KD02), cyt cy− aa3-Cox− (KD03), cyt cy− cbb3-Cox− (KD04), and cyt c2− aa3-Cox− (KD05) mutants, which contained simpler electron transport pathways beyond the cyt bc1 complex (Fig. 1). The data indicated that higher O2 consumption activities were found in mutants containing the cbb3-Cox (KD03 and KD05), and among them that harboring only cyt cy (i.e., KD05) exhibited the highest Cox activity. Conversely, lower O2 consumption abilities were encountered in mutants lacking the cbb3-Cox (KD02 and KD04), and among them that lacking cyt cy (i.e., KD04) exhibited the lowest activity (Table 3). Therefore, both cyt c2 and cyt cy donated electrons to both the cbb3-Cox and the aa3-Cox in vitro, but to different extents. The data suggested that the respiratory electron transfer pathways via the cbb3-Cox may be more active than those via the aa3-Cox subbranches in cells grown under semiaerobic conditions. However, the amounts of various components varied between various mutants (Table 2), precluding firmer conclusions. Further dissection of the Cox activities into fractions corresponding to the cbb3-Cox and aa3-Cox activities exclusively linked to cyt c2 or cyt cy was impossible due to the assays used being unable to discriminate between the enzymes and their substrates.
NADH-induced cyt c oxidoreduction kinetics in various R. sphaeroides strains.
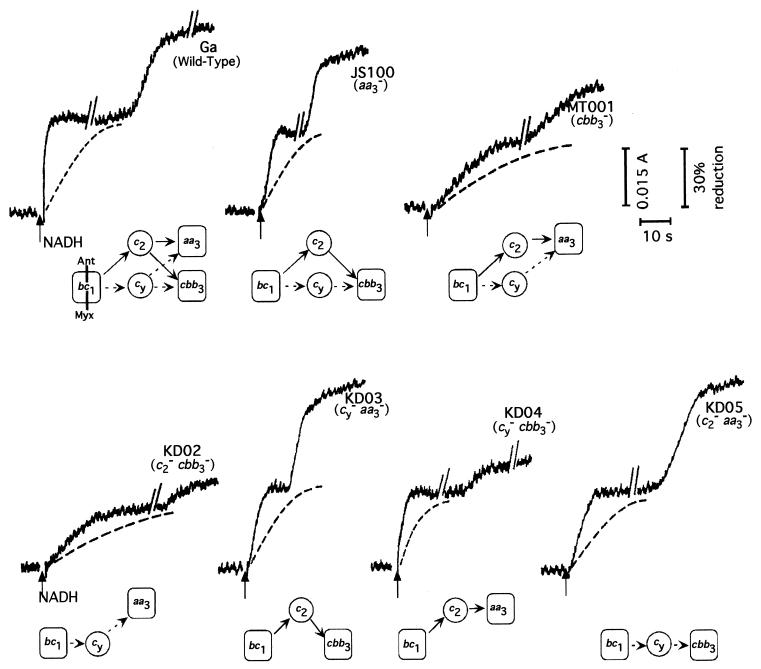
NADH-induced cyt c oxidoreduction kinetics in membranes from wild-type and various R. sphaeroides mutants were monitored in the presence or absence of oxygen in order to assess directly the electron transfer abilities of cyt cy and cyt c2 to the cbb3-Cox and aa3-Cox enzymes. In the wild-type strain Ga, approximately 50% of the total c-type cytochromes in membranes were rapidly reduced (half-life [t1/2] of <1 s) upon addition of NADH (Fig. 5). This respiration-dependent steady-state cyt c reduction (i.e., first reduction phase) lasted for several minutes, depending on the amount of membrane fragments used, and was sensitive to the cyt bc1 complex inhibitors Myx and Ant A (2 μM each) (Fig. 5). Thus, a large fraction of the c-type cytochrome complement of membranes from wild-type cells is in rapid equilibrium with the respiratory electron transport system. Similar but slower oxidoreduction kinetics (t1/2 = 1 to 2 s) were observed using membranes from the aa3-Cox− (JS100), cyt c2− aa3-Cox− (KD05), and cyt cy− aa3-Cox− (KD03) mutants. Even slower cyt c reduction kinetics (2 s, <t1/2 <10 s) were seen with membranes from the cbb3-Cox− (MT001), cyt c2− cbb3-Cox− (KD02), and cyt cy− cbb3-Cox− (KD04) mutants.
FIG. 5.
Cyt c reduction kinetics in R. sphaeroides wild-type strain Ga and various mutants. Cyt c reduction kinetics were monitored at 551 to 540 nm using membrane fragments of various mutants as described in Materials and Methods. In all experiments, the first reduction wave was initiated by addition of 0.2 mM NADH at the time indicated by the arrow, while the second reduction wave followed the onset of anaerobiosis the time of which was dependent on the rate of oxygen consumption. Dashed traces represent cyt c reduction patterns observed upon addition of Ant A or Myx (2 μM each) before the addition of NADH. Protein concentrations of membrane fragments used were 0.8 mg/ml and 2 to 3 mg/ml for the wild-type strain Ga and its mutant derivatives, respectively. Below the traces, schemes depicting the available electron transport pathways are shown.
Upon depletion of O2, and hence inhibition of the Cox activities, cyt c reduction further increased in the wild-type strain Ga and in various aa3-Cox− mutants to about 95% (second reduction phase). In the cbb3-Cox− mutants, the amounts of the c-type cytochromes reduced under steady-state respiration were between 30 and 45% of their total content, reaching a maximum of about 50 to 60% under anaerobic conditions (Fig. 5). This second phase of cyt c reduction revealed the amount of cyt c that had been oxidized during the first phase when cyt c oxidases were active and hence illustrated at least partly electron conduction to these enzymes by their electron carriers. Therefore, the data obtained with mutants containing linear electron transport pathways between the cyt bc1 complex and the cyt c oxidases demonstrated that both cyt c2 and cyt cy indeed donated electrons to either the cbb3-Cox or aa3-Cox enzyme. However, the cyt cy → aa3-Cox (KD02) and cyt c2 → aa3-Cox (KD04) pathways appeared to be less efficient (i.e., smaller second phases), suggesting that under our experimental conditions the aa3-Cox played in O2 consumption a less prominent role than the cbb3-Cox.
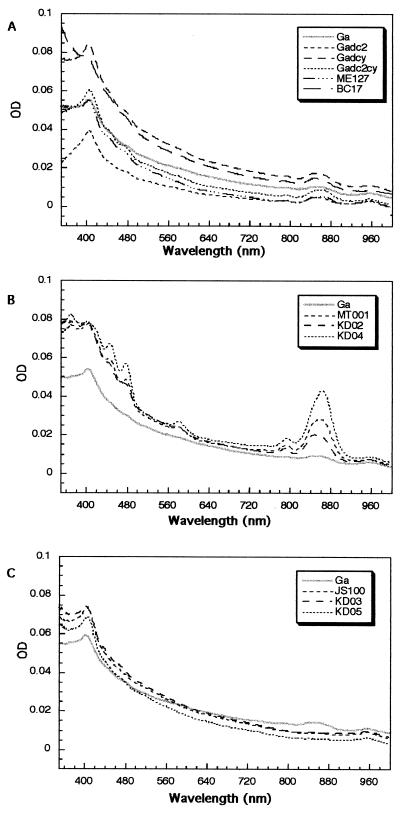
The light-harvesting antenna complexes are induced in the presence of oxygen in R. sphaeroides mutants lacking either both cyt c2 and cyt cy or the cbb3-Cox activity (28, 29). During our studies we also noted that R. sphaeroides strain MT001 lacking the cbb3-Cox and its derivatives lacking in addition either cyt c2 or cyt cy (KD02 or KD04) formed highly pigmented colonies under Res growth conditions. This pigmentation was especially pronounced in a mutant lacking both cyt cy and the cbb3-Cox (KD04). Indeed, spectral examination of chromatophore membranes of various mutants confirmed the presence of prominent absorption peaks in the 800- to 875-nm region (Fig. 6). Thus, the absence of the cbb3-Cox and its electron donors modulated the production of the light-harvesting complexes in the presence of oxygen. Remarkably, the pigment complexes were either absent or much less pronounced in the other R. sphaeroides Ga derivaties used in this work, including the cbb3-Cox− aa3-Cox− (ME127), cyt bc1− (BC17), and cyt c2− cyt cy− (Gadc2cy) mutants.
FIG. 6.
Absorption spectra of membrane fragments of various R. sphaeroides mutants. Membrane fragments were prepared from cells grown by respiration at an optical density (OD) at 630 nm of 0.4, and their absorption spectra were recorded as described in Materials and Methods. Spectra were obtained for the wild-type strain Ga and the cyt c2− (Gadc2), cyt cy− (Gacy), cyt c2− cyt cy− (Gadc2cy), cbb3-Cox− aa3-Cox− (ME127), and cyt bc1− (BC17) mutants (A), the wild-type strain Ga and the cbb3-Cox− (MT001), cyt c2− cbb3-Cox− (KD02), and cyt cy− cbb3-Cox− (KD04) mutants (B), and the wild-type strain Ga and the aa3-Cox− (JS100), cyt cy− aa3-Cox− (KD03), and cyt c2− aa3-Cox− (KD05) mutants (C). See the text for further details.
DISCUSSION
In this work we used a biochemical genetic approach, which consisted of disrupting simultaneously one of the two electron carrier c-type cytochromes and one of the two cyt c oxidases, to dissect the complicated respiratory electron transport pathways of R. sphaeroides (Fig. 1). The double mutants thus obtained simplified the crisscrossed pathways and enabled us to analyze the abilities of the different electron carriers to convey electrons from the cyt bc1 complex to the terminal cyt c oxidases. Prior to this work, it was well known that the soluble cyt c2 donates electrons to the aa3-Cox; indeed, it could be assumed correctly that it also donates electrons to the cbb3-Cox (15, 27). On the other hand, not much was known about the electron carrier properties of the more recently discovered membrane-anchored cyt cy in R. sphaeroides (27, 51). Studies reported here confirmed for cyt c2, and demonstrated for the first time for cyt cy, that both are efficient electron donors to both the cbb3-Cox and the aa3-Cox during the respiratory growth of R. sphaeroides. Neither of the two electron carriers is restricted to function solely with either of the cyt c oxidases, and clearly all four subbranches appear to be functional (Fig. 1). Whether or not all of these pathways are also proficient in vivo to support respiratory growth of R. sphaeroides in the absence of its Qox-dependent alternate branch(es) remains to be seen.
At least under the semiaerobic growth conditions used here, the cbb3-Cox subbranches appear to be more prominent in catalyzing electron transfer between the cyt bc1 complex and the cyt c oxidases in vitro. However, whether this prominence is due to the larger amounts of the components of these pathways or to their better catalytic abilities remains unknown until determination of their amounts in each case. Among the electron carriers, while cyt c2 can diffuse between the RC and the cyt bc1 complexes of the photosynthetic electron transfer chains (10), cyt cy is conceivably restricted to interact with a limited number of partners due to its membrane anchor (26, 27). Nonetheless, it is noteworthy that the alpha group proteobacterium Rickettsia prowazekii, thought to be closely related to mitochondria, lacks a soluble cyt c but contains a membrane-attached cyt cy homologue (1, 23).
Availability of otherwise isogenic mutants lacking different c-type cytochromes of R. sphaeroides allowed us to identify each of these proteins unambiguously and confirmed our previous assignments of the cytochromes c2, cy, c1, co, and cp in R. sphaeroides (18, 27). It is noteworthy that in this bacterium cyt cy runs ahead of the cyt co subunit of the cbb3-Cox (Fig. 3), which is different from what has been observed with R. capsulatus cyt cy (26). Whether the inability of R. sphaeroides cyt cy to donate electrons to the RC is linked to its smaller size remains to be seen (27).
The mutants described here, eliminating systematically various respiratory components, allowed us to estimate the amounts of various b- and c-type cytochromes present in membranes of R. sphaeroides cells grown under semiaerobic conditions. It appears that in a wild-type strain like Ga, elimination of the cbb3-Cox decreased roughly one-half of the total amount of the b-type cytochromes and two-thirds of the c-type cytochromes in membranes. Similarly, deletion of the cyt bc1 complex also decreased about one-half of the total amount of both b- and c-type cytochromes. These estimations are in agreement with the subunit composition of the cbb3-Cox and the cyt bc1 complex and suggest that these complexes may be present at similar amounts in R. sphaeroides membranes under the growth conditions used here. However, it should be noted that the amounts of various cytochromes change in different mutants as a function of the presence or absence of various components of the electron transport chain. For example, the absence of electron-carrying c-type cytochromes or the cyt bc1 complex also decreased the amounts of the cbb3-Cox or the aa3-Cox (Table 3). Thus, caution should be exercised in extrapolating the results obtained using membrane fragments to intact cells grown under various physiological conditions.
Estimation of the Cox-independent respiratory pathway in the wild-type strain Ga and in cbb3-Cox− aa3-Cox− (ME127), cyt bc1− (BC17), and cyt c2− cyt cy− (Gadc2cy) mutants indicated that in R. sphaeroides about one-fifth of the respiratory capacity is insensitive to 100 μM CN− and is possibly mediated via a Qox enzyme(s). This situation is unlike that observed in R. capsulatus (15), where the analogous CN-resistant NADH-dependent respiration represents about 75 to 85% of NADH oxidation rate. Moreover, in R. sphaeroides, restraining the mitochondrial-like respiratory electron flow by eliminating some of its components does not affect the levels of CN-resistant respiration. Membranes of an R. sphaeroides mutant lacking the cyt c2 and cyt cy (Gadc2cy) exhibit very low NADH oxidation activity (Table 3), unlike its R. capsulatus counterpart FJ2 (cyt c2− cyt cy−), which has a CN-resistant NADH oxidation activity roughly three times higher than that of its parental wild-type strain MT1131 (15). This phenomenon was suggested to reflect a regulatory response of one of the respiratory branches over the other (49), but the molecular basis of this effect is unknown. No similar response is obvious in R. sphaeroides, for the mutants analyzed here exhibited a CN-resistant respiratory activity similar to that present in the wild-type membranes. The marked inefficiency of the alternate respiratory pathway, in addition to the photosynthetic incompetence of cyt cy and the presence of an aa3-Cox, constitutes yet another striking difference between the growth abilities of the closely related species R. capsulatus and R. sphaeroides.
Increased synthesis of the photosynthetic apparatus in the presence of oxygen in mutants lacking the cbb3-Cox has been documented previously in R. sphaeroides strain 2.4.1 (28, 52). Recently, a similar situation has also been described in derivatives of the same strain lacking either the cyt bc1 complex or the cytochromes c2 and cy (29). In these mutants, expression of the photosynthetic apparatus is not repressed by O2, unlike in the wild-type strain, thus leading to the accumulation of increased amounts of light-harvesting antenna complexes and photosynthetic pigments. In R. sphaeroides strain Ga and its derivatives used here, the light-harvesting complexes were also highly induced in the cbb3-Cox− (MT001), cyt c2− cbb3-Cox− (KD02), and especially cyt cy− cbb3-Cox− (KD04) mutants, while this induction was less apparent in the cyt bc1− (BC17), cbb3-Cox− aa3-Cox− (ME127), and cyt c2−, cyt cy− (Gadc2cy) mutants. Why similar mutants derived from R. sphaeroides strains 2.4.1 and Ga behave differently in this respect is unclear. In any event, close examination of the electron transport pathways (Fig. 1) suggests that either restricting the electron flow via the cbb3-Cox subbranches, as proposed earlier (29, 52), or forcing this flow via the aa3-Cox subbranches, or even a combination of these possibilities, may lead to the accumulation of the pigment proteins. Clearly, the creation of an imbalance between the aa3-Cox and cbb3-Cox subbranches without taking into account the availability of O2 might explain at least partly the pigmentation phenotypes observed in various mutants used in this work.
In summary, the work presented here establishes for the first time that both of the electron carriers cyt c2 and cyt cy are efficient electron donors to both the cbb3-Cox and the aa3-Cox during the respiratory growth of R. sphaeroides. It remains to be seen whether or not the electron flow via each of the subbranches defined here is alone sufficient to support the Res growth of R. sphaeroides under physiological growth conditions.
ACKNOWLEDGMENTS
This work was supported by DOE grants 91ER20052 to F.D., CRP/TUR99-01 to S.M., and MURST-COFIN 99 to D.Z.
We thank E. Darrouzet for help with the figures and critical reading of the manuscript.
REFERENCES
- 1.Andersson S G, Zomorodipour A, Andersson J O, Sicheritz-Ponten T, Alsmark U C M, Podowski R M, Naslund A K, Ericksonn A-S, Winkler H H, Kurland C G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Caffrey M, Davidson E, Cusanovich M, Daldal F. Cytochrome c2 mutants of Rhodobacter capsulatus. Arch Biochem Biophys. 1992;292:419–426. doi: 10.1016/0003-9861(92)90011-k. [DOI] [PubMed] [Google Scholar]
- 4.Choudhary M, Mackenzie C, Mouncey N J, Kaplan S. RsGDB, the Rhodobacter sphaeroides genome database. Nucleic Acids Res. 1999;27:61–62. doi: 10.1093/nar/27.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton R K. Spectroscopic analyses of bacteriochlorophylls in vitro and in vivo. Photochem Photobiol. 1963;5:669–677. [Google Scholar]
- 6.Daldal F, Davidson E, Cheng S. Isolation of the structural genes for the Rieske Fe-S protein, cytochrome b and cytochrome c1, all components of the ubiquinol: cytochrome c oxidoreductase complex of Rhodopseudomonas capsulata. J Mol Biol. 1987;195:1–12. doi: 10.1016/0022-2836(87)90322-6. [DOI] [PubMed] [Google Scholar]
- 7.Daldal F, Cheng S, Applebaum J, Davidson E, Prince R C. Cytochrome c2 is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc Natl Acad Sci USA. 1986;83:2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donohue T J, McEwan A G, Van Doren S, Crofts A R, Kaplan S. Phenotypic and genetic characterization of cytochrome c2 deficient mutants of Rhodobacter sphaeroides. Biochemistry. 1988;27:1918–1925. doi: 10.1021/bi00406a018. [DOI] [PubMed] [Google Scholar]
- 9.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene. 1987;52:147–153. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Valesco J, Crofts A R. Complexes or supercomplexes: inhibitor titrations show that electron transfer in chromatophores from Rhodobacter sphaeroides involves a dimeric UQH2:cyt c2 oxidoreductase, and is delocalized. Biochem Soc Trans. 1991;19:588–593. doi: 10.1042/bst0190588. [DOI] [PubMed] [Google Scholar]
- 11.Fonstein M, Nikolskaya T, Kogan Y, Haselkorn R. Genome encyclopedias and their use for comparative analysis of Rhodobacter capsulatus strains. Electrophoresis. 1998;19:469–477. doi: 10.1002/elps.1150190403. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Horsman J A, Berry E, Shapleigh J P, Alben J O, Gennis R B. A novel cytochrome c oxidase from Rhodobacter sphaeroides that lacks CuA. Biochemistry. 1994;33:3113–3119. doi: 10.1021/bi00176a046. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Horsman J A, Barquera B, Rumbley J, Ma J, Gennis R B. The superfamily of heme-copper respiratory oxidases. J Bacteriol. 1994;176:5587–6000. doi: 10.1128/jb.176.18.5587-5600.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray K A, Grooms M, Myllykallio H, Moomaw C, Slaughter C, Daldal F. Rhodobacter capsulatus contains a novel cb-type cytochrome c oxidase without a CuA center. Biochemistry. 1994;33:3120–3127. doi: 10.1021/bi00176a047. [DOI] [PubMed] [Google Scholar]
- 15.Hochkoeppler A, Jenney F E, Jr, Lang S E, Zannoni D, Daldal F. Membrane-associated cytochrome cy of Rhodobacter capsulatus is an electron carrier from the cytochrome bc1 complex to the cytochrome c oxidase during respiration. J Bacteriol. 1995;177:608–613. doi: 10.1128/jb.177.3.608-613.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosler J P, Fetter J, Tecklenburg M M, Espe M, Lerma C, Ferguson-Miller S. Cytochrome aa3 of Rhodobacter sphaeroides as a model for mitochondrial cytochrome c oxidase. Purification, kinetics, proton pumping and spectral properties. J Biol Chem. 1992;267:24264–24272. [PubMed] [Google Scholar]
- 17.Jenney F E, Daldal F. A novel membrane-associated c-type cytochrome, cyt cy can mediate the photosynthetic growth of Rhodobacter capsulatus and Rhodobacter sphaeroides. EMBO J. 1993;12:1283–1292. doi: 10.1002/j.1460-2075.1993.tb05773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenney F E, Prince R C, Daldal F. The membrane-bound cytochrome cy of Rhodobacter capsulatus is an electron donor to the photosynthetic reaction of Rhodobacter sphaeroides. Biochim Biophys Acta. 1996;1273:159–164. doi: 10.1016/0005-2728(95)00137-9. [DOI] [PubMed] [Google Scholar]
- 19.Koch H-G, Hwang O, Daldal F. Isolation and characterization of Rhodobacter capsulatus mutants affected in cytochrome cbb3 oxidase activity. J Bacteriol. 1998;180:969–978. doi: 10.1128/jb.180.4.969-978.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch H-G, Winterstein C, Saribas A S, Alben J O, Daldal F. Roles of the ccoGHIS gene products in the biogenesis of the cbb3-type cytochrome c oxidase. J Mol Biol. 2000;297:49–65. doi: 10.1006/jmbi.2000.3555. [DOI] [PubMed] [Google Scholar]
- 21.La Monica R F, Marrs B L. The branched respiratory system of photosynthetically grown Rhodopseudomonas capsulata. Biochim Biophys Acta. 1976;423:431–439. doi: 10.1016/0005-2728(76)90198-5. [DOI] [PubMed] [Google Scholar]
- 22.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Margulis L. Symbiosis in cell evolution. W. H. New York, N.Y: Freeman; 1981. [Google Scholar]
- 24.Marrs B L, Gest H. Genetic mutations affecting the respiratory electron transport system of the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol. 1973;114:1045–1051. doi: 10.1128/jb.114.3.1045-1051.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouncey N J, Choudhary M, Kaplan S. Characterization of genes encoding dimethyl sulfoxide reductase of Rhodobacter sphaeroides 2.4.1T: an essential metabolic gene function encoded on chromosome II. J Bacteriol. 1997;179:7617–7624. doi: 10.1128/jb.179.24.7617-7624.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myllykallio H, Jenney F E, Jr, Slaughter C, Daldal F. Cytochrome cy of Rhodobacter capsulatus is attached to the cytoplasmic membrane by an uncleaved signal sequence-like anchor. J Bacteriol. 1997;179:2623–2631. doi: 10.1128/jb.179.8.2623-2631.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myllykallio H, Zannoni D, Daldal F. Rhodobacter sphaeroides cyt cy is a membrane-attached electron carrier that is deficient in photosynthesis but proficient in respiration. Proc Natl Acad Sci USA. 1999;96:4348–4353. doi: 10.1073/pnas.96.8.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh J-I, Kaplan S. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry. 1999;38:2688–2696. doi: 10.1021/bi9825100. [DOI] [PubMed] [Google Scholar]
- 29.Oh J-I, Kaplan S. Redox signaling: globalization of gene expression. EMBO J. 2000;19:4237–4247. doi: 10.1093/emboj/19.16.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paddock M L, Rongey S H, Feher G, Okamura M Y. Pathway of proton transfer in bacterial reaction centers: replacement of glutamic acid 212 in the L subunit by glutamine inhibits quinone (secondary acceptor) turnover. Proc Natl Acad Sci USA. 1989;86:6602–6606. doi: 10.1073/pnas.86.17.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 32.Prince R C, Daldal F. Physiological electron donors to the photochemical reaction center of Rhodobacter capsulatus. Biochim Biophys Acta. 1987;894:370–378. doi: 10.1016/0005-2728(87)90115-0. [DOI] [PubMed] [Google Scholar]
- 33.Rott M A, Witthuhn V C, Schilke B A, Soranno M, Ali A, Donohue T J. Genetic evidence for the role of isocytochrome c2 in photosynthetic growth of Rhodobacter sphaeroides spd mutants. J Bacteriol. 1993;175:358–366. doi: 10.1128/jb.175.2.358-366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki T, Notokawa Y, Kikuchi G. Occurrence of both a-type and o-type cytochromes as the functional terminal oxidases in Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1970;197:284–291. doi: 10.1016/0005-2728(70)90039-3. [DOI] [PubMed] [Google Scholar]
- 35.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 36.Shapleigh J P, Gennis R B. Cloning, sequencing and deletion from the chromosome of the gene encoding subunit I of the aa3-type cytochrome c oxidase of Rhodobacter sphaeroides. Mol Microbiol. 1992;6:635–642. doi: 10.1111/j.1365-2958.1992.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 37.Shapleigh J P, Hill J J, Alben J O, Gennis R B. Spectroscopic and genetic evidence for two heme-Cu-containing oxidases in Rhodobacter sphaeroides. J Bacteriol. 1992;174:2338–2343. doi: 10.1128/jb.174.7.2338-2343.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw A, Hochkoeppler A, Bonora P, Zannoni D, Hanson G, McEwan A. Characterization of DorC from Rhodobacter capsulatus, a c-type cytochrome involved in electron transfer to dimethyl sulfoxide reductase. J Biol Chem. 1999;274:9911–9914. doi: 10.1074/jbc.274.15.9911. [DOI] [PubMed] [Google Scholar]
- 39.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 40.Sistrom W. A requirement for sodium in the growth of Rhodopseudomonas sphaeroides. J Gen Microbiol. 1960;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- 41.Thomas P E, Ryan D, Levin D W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976;75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- 42.Toledo-Cuevas M, Barquera B, Gennis R B, Wikstrom M, Garcia-Horsman J A. The cbb3-type cytochrome c oxidase from Rhodobacter sphaeroides, a proton-pumping heme-copper oxidase. Biochim Biophys Acta. 1998;1365:421–434. doi: 10.1016/s0005-2728(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 43.Williams J, Steiner L, Feher G. Primary structure of the reaction center from Rhodopseudomonas sphaeroides. Proteins. 1986;11:312–325. doi: 10.1002/prot.340010405. [DOI] [PubMed] [Google Scholar]
- 44.Youvan D, Bylina E, Alberti M, Begusch H, Hearst J. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from Rhodopseudomonas capsulata. Cell. 1984;37:949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- 45.Yun C-H, Beci R, Crofts A R, Kaplan S, Gennis R B. Cloning and DNA sequencing of the fbc operon encoding the cytochrome bc1 complex from Rhodobacter sphaeroides. Eur J Biochem. 1990;194:399–411. doi: 10.1111/j.1432-1033.1990.tb15633.x. [DOI] [PubMed] [Google Scholar]
- 46.Zannoni D. Aerobic and anaerobic electron transport chains in anoxygenic phototrophic bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 949–971. [Google Scholar]
- 47.Zannoni D, Daldal F. The role of c-type cytochromes in catalyzing oxidative and photosynthetic electron transport in the dual functional plasmamembrane of facultative phototorophs. Arch Microbiol. 1993;160:413–423. doi: 10.1007/BF00245301. [DOI] [PubMed] [Google Scholar]
- 48.Zannoni D, Melandri B A, Baccarini-Melandri A. Composition and function of the branched oxidase system in wild type and respiratory mutants of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1976;423:413–430. doi: 10.1016/0005-2728(76)90197-3. [DOI] [PubMed] [Google Scholar]
- 49.Zannoni D, Moore A L. Measurement of the redox state of the ubiquinone pool in Rhodobacter capsulatus membrane fragments. FEBS Lett. 1990;271:123–127. doi: 10.1016/0014-5793(90)80387-x. [DOI] [PubMed] [Google Scholar]
- 50.Zannoni D, Venturoli G, Daldal F. The role of the membrane bound cytochromes of b- and c-type in the electron transport chain of Rhodobacter capsulatus. Arch Microbiol. 1992;157:367–374. [Google Scholar]
- 51.Zeilstra-Ryalls J, Kaplan S. Aerobic and anaerobic regulation in Rhodobacter sphaeroides 2.4.1: role of the fnrL gene. J Bacteriol. 1995;177:6422–6431. doi: 10.1128/jb.177.22.6422-6431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeilstra-Ryalls J, Gomelsky M, Eraso J M, Yeliseev A, O'Gara J, Kaplan S. Control of photosystem formation in Rhodobacter sphaeroides. J Bacteriol. 1998;180:2801–2809. doi: 10.1128/jb.180.11.2801-2809.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]