Abstract
“Addiction modules” consist of two genes; the product of the second is long lived and toxic, while the product of the first is short lived and antagonizes the lethal action of the toxin. The extrachromosomal addiction module phd-doc, located on the P1 prophage, is responsible for the postsegregational killing effect (death of plasmid-free cells). The Escherichia coli chromosomal addiction module analogue, mazEF, is responsible for the induction of programmed cell death. Here we show that the postsegregational killing mediated by the P1 phd-doc module depends on the presence of the E. coli mazEF system. In addition, we demonstrate that under conditions of postsegregational killing, mediated by phd-doc, protein synthesis of E. coli is inhibited. Based on our findings, we suggest the existence of a coupling between the phd-doc and mazEF systems.
In Escherichia coli cultures, programmed cell death is mediated through “addiction modules” consisting of two genes; the product of the second gene is long lived and toxic, whereas the product of the first is short lived and antagonizes the lethal action of the toxin. Until recently, such genetic systems of bacterial programmed cell death have been found mainly in a number of E. coli extrachromosomal elements (for reviews, see references 5, 7, 8, and 15), where they are responsible for what is called the postsegregational killing effect; they are responsible for the death of plasmid-free cells. When bacteria lose the extrachromosomal elements, the cured cells are selectively killed because the unstable antitoxin is degraded faster than is the more stable toxin. One of the best-studied systems belonging to this category is the phd-doc module of plasmid prophage P1 (9, 10). This module consists of an operon the organization of which is similar to that of the operons of other addiction modules: the antitoxic gene, phd (prevents host death), precedes the toxic gene, doc (death on curing). Doc acts as a cell toxin to which the short-lived Phd protein is an antidote. Phd is degraded by the serine protease ClpPX (10). Like that of other previously described addiction modules, the expression of phd-doc is also subjected to an autoregulatory circuit (11, 12). The cellular target of Doc is not yet known. However, based on recent studies it is assumed to be a step in protein synthesis (7; M. Yarmolinsky, personal communication).
Members of our group have reported on the E. coli mazEF system, the first known regulatable prokaryotic chromosomal addiction module (1). It consists of two genes, mazE and mazF, located downstream from the relA gene in the rel operon (13). Through our work, we have found that mazEF has all the properties required for an addiction module. MazF is toxic and long lived, while MazE is antitoxic and short lived and is degraded by the ClpPA serine protease. MazE and MazF are coexpressed, and they interact. In addition, the mazEF system has a unique property: its expression is regulated by guanosine-3′5′-bispyrophosphate (ppGpp), which is synthesized by the RelA protein under conditions of amino acid starvation (3). Moreover, overproduction of ppGpp induces mazEF-mediated cell death (1, 6). These properties suggest that the mazEF addiction module may be responsible for programmed cell death in starving cultures of E. coli (1). In an accompanying report (14a), we are showing that cell death mediated by the E. coli mazEF addiction module can also be triggered by several antibiotics that are general inhibitors of transcription and/or translation. These antibiotics inhibit the continuous expression of the labile antitoxin MazE, and as a result, the stable toxin MazF causes cell death. This finding, together with the observation that the toxic protein Doc of the P1 phd-doc system inhibits protein synthesis (7; M. B. Yarmolinsky, personal communication), prompted us to ask whether the P1 phd-doc system exerts its postsegregational killing effect through the chromosomal E. coli mazEF system.
Here we show that the postsegregational killing effect mediated by the P1 phd-doc addiction module does depend on the presence of the E. coli chromosomal addiction module mazEF. In addition, we found that under conditions of P1 phd-doc postsegregational killing, protein synthesis of E. coli is inhibited. Therefore we suggest that the toxic protein Doc triggers cell death through the mazEF system by the inhibition of E. coli protein synthesis.
MATERIALS AND METHODS
E. coli strains and plasmids.
The E. coli strains used in this study included MC4100 (genotype, araD139 Δ(argF-lac)205 flb-5301 pstF25 rpsL150 deoC1 relA1) (2) and its derivatives, MC4100 mazEF::kan (ΔmazEF) (1). The plasmid pGB2ts::phd-doc (9) is a pGB2 derivative, thermosensitive for replication, carrying phd-doc genes and spectinomycin resistance genes (4). Plasmid pKK223mazEF was constructed by inserting the open reading frame of mazEF into the BamHI and HindIII sites of the overexpression plasmid pKK233-3 (Amersham Pharmacia Biotech).
Materials and media.
[35S]methionine (>800 Ci/mmol [1 Ci = 37 GBq]) was obtained from Amersham (Little Chalfont, England). Bacteria were grown in M9 medium (14) with a mixture of amino acids (20 μg/ml each) or in Luria-Bertani medium (LB) (14).
Conditions for the loss of plasmids pGB2ts and pGB2ts::phd-doc from the cells.
We used plasmid pGB2ts or pGB2ts::phd-doc to transform E. coli strain MC4100 and its ΔmazEF derivative. Transformants were selected on LB agar plates supplemented with spectinomycin (100 μg/ml) at 30°C. The postsegregational killing effect of the phd-doc addiction module was studied by growing the bacteria on LB agar plates, at 30 or 42°C or in liquid media. In liquid media, the cells were grown in LB medium or M9 medium at 30°C overnight. Plasmid loss was achieved by growing the cultures for at least eight generations at 42°C. For this purpose we grew the cultures from an optical density at 600 nm (OD600) of 0.07 to 0.7 at 42°C and diluted them 1:10; this procedure was repeated at least twice. Plasmid loss was confirmed by plating the cultures on spectinomycin plates.
Assay for protein synthesis under conditions of the loss of plasmids pGB2ts and pGB2ts::phd-doc.
We measured the incorporation of [35S]methionine into a trichloroacetic acid (TCA)-insoluble fraction. In these experiments we used E. coli strain MC4100 and its ΔmazEF derivative carrying plasmid pGB2ts::phd-doc or pGB2t as a control. We grew the tested E. coli cultures in LB liquid medium under conditions of plasmid loss as described in the previous section. The cells were washed, resuspended in M9 medium without methionine, and grown at 42°C for 1 h. The culture was labeled with 0.2 μCi/ml in [35S]methionine in a final concentration of 2 μg of unlabeled methionine/ml. At various time intervals, the reactions were stopped by the addition of TCA to a final concentration of 10%, after which the reaction tubes were put in ice. The samples were centrifuged at 14,000 rpm for 5 min in Eppendorf centrifuge 5417C. The pellets were washed twice with 5% TCA and then twice with acetone. The TCA-insoluble counts were determined by using a scintillation counter (BETAmatic I/II; KONTRON).
RESULTS
P1 phd-doc postsegregational killing is triggered by the loss of pGB2ts::phd-doc.
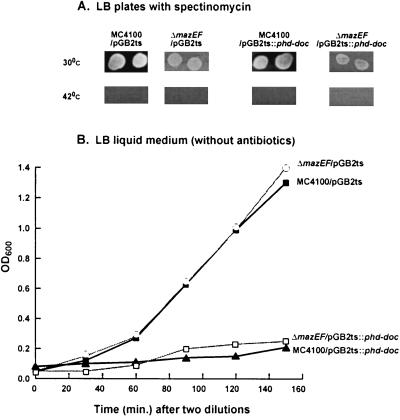
We used plasmid pGB2ts::phd-doc bearing the P1 phd-doc addiction module and pGB2ts as a control. Both plasmids are temperature sensitive for replication and carry a gene for spectinomycin resistance (9). As previously reported for E. coli MC4100 cells (10, 6) and confirmed here also for the ΔmazEF derivative, pGB2ts and pGB2ts::phd-doc are retained at a low temperature (30°C) but are lost when the cells are grown at a high temperature (42°C) (Fig. 1A). The presence of either plasmid in the cells was tested by plating the bacteria on LB plates supplemented with spectinomycin (Fig. 1A). Without spectinomycin the pattern of bacterial growth in LB liquid medium at 42°C (conditions of plasmid loss) depends upon the presence of phd-doc on the plasmid. This is shown in Fig. 1B, where the growth of E. coli MC4100 and its ΔmazEF derivative harboring the plasmid pGB2ts::phd-doc is inhibited relative to that of cells harboring pGB2ts. The growth of the latter at 42°C is similar in MC4100 and its ΔmazEF derivative (data not shown). It should be emphasized that according to our experiments, in order to get conditions for plasmid loss the cells should grow at 42°C for at least eight generations. This was achieved by growing the cultures from an OD600 of 0.07 to 0.7 followed by a 1:10 dilution; this procedure was repeated twice. Figure 1B shows the growth curves obtained after the second dilution.
FIG. 1.
The effect of the loss of the plasmids pGB2ts and pGB2ts::phd-doc on the growth of MC4100 and its ΔmazEF derivative. (A) Growth on LB plates with spectinomycin. E. coli MC4100 (wild type) and its ΔmazE derivative, both harboring either plasmid pGB2ts or pGB2ts::phd-doc, were plated on LB plates with 100 μg of spectinomycin/ml. The plates were incubated at 30 or at 42°C overnight. (B) Bacterial growth curves at 42°C in liquid LB medium without any added antibiotic. The bacteria were grown to an OD600 of 0.7 and then diluted to an OD600 of 0.07. This step was repeated twice in order to cure the cells from the plasmids. The curves represent the values after the second dilution. Results for MC4100 are illustrated by black lines, and those for its ΔmazEF derivative are illustrated by gray lines. Results with MC4100/pGB2ts (■), MC4100/pGB2ts::phd-doc (▴), ΔmazEF/pGB2ts ( ), and ΔmazEF/pGB2ts::phd-doc (□) are shown.
P1 phd-doc postsegregational killing is dependent on E. coli mazEF.
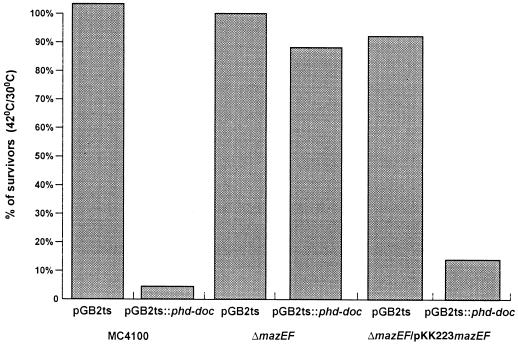
We determined quantitatively the postsegregational killing mediated by the loss of pGB2ts::phd-doc from cells. This was done by the comparison of the number of the surviving cells grown at 42°C to those grown at 30°C in LB medium without spectinomycin. In the case of the wild-type strain E. coli MC4100, only 5% of the population escaped the postsegregational killing effect (Fig. 2). As a control we used plasmid pGB2ts, which was also lost at 42°C (Fig. 1A), but cell growth was not inhibited (Fig. 1B) and the cells did not die (Fig. 2). We also studied the postsegregational killing effect mediated by the loss of plasmid pGB2ts::phd-doc on the ΔmazEF strain. As in the case of the wild-type strain, incubation at 42°C caused the loss of plasmids pGB2ts and pGB2ts::phd-doc from ΔmazEF cells (Fig. 1A), and the growth of ΔmazEF/pGB2ts::phd-doc cells was inhibited (Fig. 1B). However, in contrast to results with the wild-type strain MC4100, most of the ΔmazEF derivative cells (90%) survived the loss of the plasmid (Fig. 2). We also tested pGB2ts::phd-doc postsegregational killing in ΔmazEF cells in which the mazEF gene module was not present on the chromosome but was borne on the plasmid pKK223mazEF. In this case, we observed that postsegregational killing did take place and only 8% of the cells survived plasmid loss (Fig. 2). Thus, we found that the postsegregational killing effect of the phd-doc addiction module required the presence of the mazEF system.
FIG. 2.
The effect of the E. coli mazEF system on the postsegregational killing mediated by the P1 phd-doc addiction module. Plasmids pGB2ts and pGB2ts::phd-doc (Sptr) were used to transform E. coli MC4100 (wild type) and its ΔmazEF derivative. The transformant MC4100ΔmazEF/pGB2ts::phd-doc was further transformed by plasmid pKK223mazEF (Ampr). Transformants were grown in LB liquid medium at 30°C to mid-logarithmic phase and were plated on LB plates without antibiotics at 30 and 42°C. The percentage of cell survivors was calculated by comparing the numbers of CFU at 42°C versus 30°C.
E. coli protein synthesis is inhibited under conditions of pGB2ts::phd-doc plasmid loss.
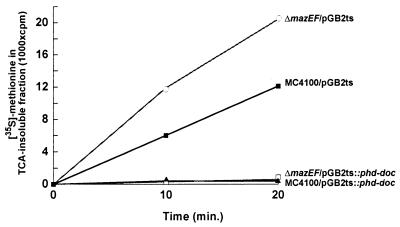
Note that although the ΔmazEF derivative cells survive the loss of plasmid pGB2ts::phd-doc (Fig. 2), their growth is inhibited in liquid LB medium as is that of the wild-type cells (Fig. 1B). Thus, the loss of plasmid pGB2ts::phd-doc is bactericidal for wild-type MC4100 cells but only bacteriostatic for the ΔmazEF derivative. This finding is similar to the effect of the translational inhibitor chloramphenicol which we show in the accompanying report (14a). There we found that in M9 minimal medium, chloramphenicol is bactericidal for the wild-type strain MC4100 but only bacteriostatic for the ΔmazEF derivative. Having considered our results described above, along with the observation that Doc inhibits protein synthesis (Yarmolinsky, personal communication) (reviewed in reference 7), we decided to study the effects of the loss of plasmid pGB2ts::phd-doc on the level of protein synthesis. We compared the level of protein synthesis in MC4100 and in its ΔmazEF derivative carrying pGB2ts::phd-doc or to the same cells carrying pGB2ts as a control. We determined the level of protein synthesis by measuring the incorporation of [35S]methionine into a TCA-insoluble fraction. At 42°C, protein synthesis was drastically inhibited in both MC4100 and its ΔmazEF derivative carrying pGB2ts::phd-doc relative to that in cells carrying pGB2ts (Fig. 3). Thus, under conditions of plasmid loss (Fig. 1A), protein synthesis (Fig. 3) and bacterial growth (Fig. 1B) are inhibited.
FIG. 3.
The effect of the P1 phd-doc system on protein synthesis. Plasmid pGB2ts::phd-doc or pGB2ts (as a control) were used to transform E. coli MC4100 (wild type) and the MC4100 ΔmazEF derivative. Plasmid curing was carried out in LB liquid medium as described in the legend to Fig. 1. The rate of protein synthesis was determined as described in Materials and Methods. Results for MC4100 are illustrated by black lines, and those for its ΔmazE derivative are shown by gray lines. Results with MC4100/pGB2ts (■), MC4100/pGB2ts::phd-doc (▴), ΔmazEF/pGB2ts ( ), and ΔmazEF/pGB2ts::phd-doc (□) are shown.
DISCUSSION
Addiction modules of extrachromosomal element (including P1 phd-doc) and their postsegregational killing effect have been thoroughly studied (reviewed in references 5, 7, 8, and 15). Here we found that P1 postsegregational killing is mediated by the E. coli chromosomal programmed cell death system mazEF (Fig. 2). Moreover, we show that while in E. coli MC4100 the loss of the pGB2ts::phd-doc plasmid is bactericidal, in its ΔmazEF derivative it is only bacteriostatic (Fig. 2). More specifically, under conditions of plasmid loss, protein synthesis (Fig. 3) and cell growth (Fig. 1B) were inhibited both in wild-type MC4100 cells and in ΔmazEF derivative cells; however, while the wild-type cells died, the ΔmazEF cells survived (Fig. 2). Furthermore, when we reintroduced the mazEF system on a plasmid to the ΔmazEF cells, the loss of the pGB2ts::phd-doc plasmid was again bactericidal (Fig. 2). In addition, we demonstrate that E. coli protein synthesis is inhibited under the conditions of postsegregational killing mediated by P1 phd-doc (Fig. 3).
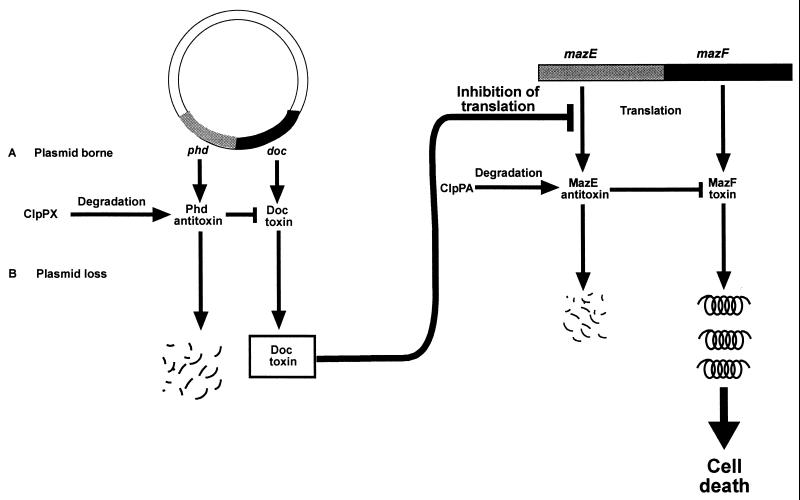
Based on our results, we suggest a model in which mazEF is required for the postsegregational killing process mediated by P1 phd-doc (Fig. 4). The extrachromosomal module phd-doc and the chromosomal module mazEF function analogously: in both systems, the second gene specifies for a toxic stable protein and the first gene specifies for an antitoxic labile protein. Each of these systems is triggered when the continuous expression of its labile antitoxic component is inhibited. In the case of the extrachromosomal phd-doc system, such a triggering occurs by the loss of the extrachromosomal element (9, 10). On the other hand, in the case of the chromosomal mazEF system, we have previously shown that the inhibition of the antitoxin expression can be triggered at least in two ways: (i) inhibition of transcription by ppGpp (1, 6) or by rifampin (14a) and (ii) inhibition of translation by chloramphenicol or spectinomycin (14a). Here we suggest an additional possible trigger for mazEF-mediated programmed cell death: through its action as a translational inhibitor, the toxic protein Doc can trigger the mazEF system. In this respect, Doc can be considered an analogue to the antibiotics chlorampenicol and spectinomycin. Moreover, Doc seems to trigger the mazEF system even more efficiently than do the drugs chloramphenicol and spectinomycin. In an accompanying report (14a), we reported that these antibiotics trigger the mazEF system only in a minimal medium, and they were not able to do it in LB medium. In contrast, the bactericidal effect of the postsegregational killing mediated by Doc is manifested both in LB (10) (Fig. 2) and in M9 (data not shown), and in both cases it requires the E. coli mazEF system.
FIG. 4.
A schematic representation for the coupling of the extrachromosomal P1 phd-doc system and the chromosomal mazEF addiction module. (A) In the presence of plasmid-borne phd-doc. When the plasmid-borne phd-doc system is expressed, there is a balance between the expression of the antitoxic Phd and its degradation by ClpX. This balance permits the neutralization of the toxic protein Doc by Phd. (B) When the plasmid is lost. Under conditions of plasmid loss, the P1 phd-doc system mediates postsegregational killing. Under these conditions, the level of the labile antitoxin Phd decreases below the threshold required for neutralizing Doc. Doc inhibits the translational machinery and thereby triggers programmed cell death mediated by the E. coli mazEF system. When protein translation is inhibited, the continuous expression of the labile antitoxic MazE protein is prevented. As a result, the stable toxin MazF causes cell death (for further details, see the text).
In summary, our results described here suggest that at least in the case of phd-doc, postsegregational killing is not a one-step process manifested by Doc. It seems that instead a “death cascade” is involved. Thus, our results suggest that by itself Doc is not a toxin; rather, it triggers the E. coli mazEF system in this cascade. The question of whether the toxic MazF is responsible by itself for the death or is also an intermediate in the death cascade is under investigation.
ACKNOWLEDGMENTS
We thank G. Glaser for kindly supplying us with plasmid pKK223mazEF. We thank Sudarsan Narasimhan for his help. We are deeply grateful to F. R. Warshaw-Dadon (Jerusalem, Israel) for her critical reading of the manuscript.
This research was supported by a grant of the Israel Science Foundation administrated by the Israel Academy of Science and Humanities.
REFERENCES
- 1.Aizenman E, Engelberg-Kulka H, Glaser G. An Escherichia colichromosomal “addiction module” regulated by guanosine-3′5′-bispyrophosphate: a model for programmed bacterial cell death. Proc Natl Acad Sci USA. 1996;93:6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadaban M J, Cohen S N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivoprobe for transcriptional control sequences. Proc Natl Acad Sci USA. 1979;76:4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cashel M, Gentry D R, Hernandez V Z, Vinella D. The stringent response. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B M, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1458–1496. [Google Scholar]
- 4.Clerget M. Site-specific recombination promoted by a short DNA segment of plasmid R1 and by a homologous segment in the terminus region of the Escherichia colichromosome. New Biol. 1995;3:780–788. [PubMed] [Google Scholar]
- 5.Couturier M, Bahassi E M, Van Melderen L. Bacterial death by DNA gyrase poisoning. Trends Microbiol. 1998;6:269–275. doi: 10.1016/s0966-842x(98)01311-0. [DOI] [PubMed] [Google Scholar]
- 6.Engelberg-Kulka H, Reches M, Narasimhan S, Schoulaker-Schwarz R, Klemes Y, Aizenman E, Glaser G. rexBof bacteriophage λ is an anti-cell death gene. Proc Natl Acad Sci USA. 1998;95:15481–15486. doi: 10.1073/pnas.95.26.15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelberg-Kulka H, Glaser G. Addiction modules and programmed cell death and antideath in bacterial cultures. Annu Rev Microbiol. 1999;53:43–70. doi: 10.1146/annurev.micro.53.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Jensen R B, Gerdes K. Programmed cell death in bacteria: proteic plasmid stabilization systems. Mol Microbiol. 1995;17:205–210. doi: 10.1111/j.1365-2958.1995.mmi_17020205.x. [DOI] [PubMed] [Google Scholar]
- 9.Lehnherr H, Magnuson R, Jafri S, Yarmolinsky M B. Plasmid addiction genes of bacteriophage P1: doc, which causes cell death on curing of prophage, and phd, which prevents host death when prophage is retained. J Mol Biol. 1993;233:414–428. doi: 10.1006/jmbi.1993.1521. [DOI] [PubMed] [Google Scholar]
- 10.Lehnherr H, Yarmolinsky M B. Addiction protein Phd of plasmid prophage P1 is a substrate of the ClpXP serine protease of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:3274–3277. doi: 10.1073/pnas.92.8.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnuson R, Lehnherr H, Mukhopadhyay G, Yarmolinsky M B. Autoregulation of the plasmid addiction operon of bacteriophage P1. J Biol Chem. 1996;271:18705–18710. doi: 10.1074/jbc.271.31.18705. [DOI] [PubMed] [Google Scholar]
- 12.Magnuson R, Yarmolinsky M B. Corepression of the P1 addiction operon by Phd and Doc. J Bacteriol. 1999;180:6342–6351. doi: 10.1128/jb.180.23.6342-6351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzger S, Dror I B, Aizenman E, Schreiber G, Toone M, Friesen J D, Cashel M, Glaser G. The nucleotide sequence and characterization of the relA gene of Escherichia coli. J Biol Chem. 1988;263:15699–15704. [PubMed] [Google Scholar]
- 14.Miller J H. Experiments in molecular genetics. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 205–210. [Google Scholar]
- 14a.Sat B, Hazan R, Fisher T, Khaner H, Glaser G, Engelberg-Kulka H. Programmed cell death in Escherichia coli: some antibiotics can trigger mazEFlethality. J Bacteriol. 2001;183:2041–2045. doi: 10.1128/JB.183.6.2041-2045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarmolinsky M B. Programmed cell death in bacterial population. Science. 1995;267:836–837. doi: 10.1126/science.7846528. [DOI] [PubMed] [Google Scholar]