Abstract
Background
To prevent further spread of the disease and secondary deformity, musculoskeletal tuberculosis (TB) remains a challenge in terms of early diagnosis and treatment. This study gives an overview on TB trends in Austria (pulmonary and extrapulmonary TB) (A) and analyses a retrospective series of musculoskeletal TB cases diagnosed and treated at an Austrian tertiary centre (B).
Methods
(A) We analysed data obtained from the Austrian national TB registry to provide information on TB patients´ demographics and manifestation sites between 1995 and 2019. (B) Furthermore, we performed an observational study of all patients with a confirmed diagnosis of musculoskeletal TB who were admitted to the Department of Orthopaedics and Trauma, Medical University of Graz (2005–2019). Demographic, diagnostic, clinical and follow-up data were retrieved from the medical records.
Results
(A) From 1995 to 2019, a significant linear reduction in overall Austrian tuberculosis incidence rates occurred (p < 0.001). In the period investigated, Austria recorded a total of 307 patients with musculoskeletal TB. (B) Our retrospective case-series included 17 individuals (9 males, 8 females; average follow-up 48.4 months; range 0–116). There was a biphasic age distribution with a peak in elderly native Austrians (median 69, range 63–92), and a second peak in younger patients with a migration background (median 29, range 18–39). Sites of manifestation were the spine (n = 10), peripheral joints (n = 5), and the soft tissues (n = 2). Diagnosis was based on histology (n = 13), PCR (n = 14), and culture (n = 12). Eleven patients underwent surgery (64.7%). Secondary deformities were frequent (n = 9), and more often observed in patients with spinal TB (n = 6).
Conclusion
Musculoskeletal TB should be considered if untypical joint infections or nonspecific bone lesions occur in younger patients with a migration background or in patients with specific risk factors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00402-022-04615-x.
Keywords: Tuberculosis, Bone, Joint, Mycobacterium tuberculosis, Extrapulmonary tuberculosis
Introduction
Tuberculosis (TB) is one of the oldest diseases and has plagued humankind since prehistoric ages [5]. It is thought that the “white death” has caused more deaths than any other infectious disease [5, 12]. Characteristic signs of TB-induced deformities, particularly in the spine, have been detected in skeletal remains and mummies of various ages all over the world [5, 7, 8, 22]. It was not until the nineteenth century that the infectious pathogenesis of TB was elucidated [5]. In 1882, Dr Robert Koch finally identified the tubercle bacillus (Mycobacterium tuberculosis) as an underlying pathogen [5, 12]. TB usually affects the lungs (pulmonary TB) and occurs upon inhalation of bacteria [10]. Less frequently, also other sites are affected (these findings are known as extrapulmonary TB) [10].
Even though TB has never been extinct in developed countries, the USA and Europe have seen an increase in extrapulmonary TB infections over the last few decades [10, 11, 13, 17, 18, 22]. This increase is largely due to migration, as well as to human immunodeficiency virus (HIV) infections [10, 11, 13, 22]. Spinal tuberculosis (“Pott’s disease”) is the most common form of skeletal TB and mainly occurs in thoracic vertebrae [8, 17, 22]. It is caused by haematogenous dissemination of tubercle bacteria from a primary (usually pulmonary) focus and typically presents with back pain or neurological complications [8, 22]. Apart from the spine, TB can affect almost any peripheral bone or joint (“peripheral osteoarticular TB”), though it preferably occurs in long bones and weight-bearing joints (e.g., the hip and the knee joint) [22]. In addition, an involvement of the sacroiliac joints, the ribs, or the sternoclavicular joints has been reported [21, 22]. Cardinal symptoms are cold abscess formations, pain, and swellings, often suggestive of a tumour [22, 23]. Systemic antimicrobials are the treatment of choice; as in Pott´s disease, surgery is only performed in selected cases [22].
In all forms of bone and joint TB, early diagnosis is warranted to prevent the further spread of the disease and secondary deformity [22]. However, as symptoms of musculoskeletal TB are often nonspecific and misleading, and the physicians in developed countries might not have been frequently exposed to these TB presentations, diagnosis is often delayed [3, 22, 23].
This study consists of two parts: first, it aims to give a brief overview of general TB trends in Austria (pulmonary and extrapulmonary TB) between 1995 and 2019. Second, it provides a descriptive analysis of a retrospective series of bone and joint TB cases diagnosed and treated at an Austrian university hospital between 2005 and 2019, summarising the radiological, histological, and molecular findings to guide doctors in their differential diagnoses and help avoid unnecessary delays.
Patients and methods
Tuberculosis trends in Austria
First, we submitted an inquiry to the Austrian national TB reference centre to obtain information about the newly reported TB rates in Austria and their development within the last 25 years (1995–2019) [23]. This centre is hosted by the Austrian Agency for Health and Food Safety (AGES; www.ages.at), which documents all reportable diseases within the Austrian population. By law, every case of newly diagnosed TB needs to be reported to the health authorities within three days from diagnosis. Registry data are released in annual reports. Apart from disease numbers, the AGES also documents the organs primarily affected and co-affected by TB at first diagnosis. According to the WHO classification, they distinguish between pulmonary and extrapulmonary TB manifestations. Amongst extrapulmonary manifestations, various affected organ sites are listed. We analysed the organ affections throughout the period investigated herein (1995–2019) (Table S1) [23].
Retrospective case series
Second, we retrospectively analysed all patients diagnosed or treated with musculoskeletal TB since the initiation of the electronic recordings in 2005 at the Medical University of Graz, Graz, Austria [23]. We searched our hospital´s electronic register for the term “tuberculosis” in any context (i.e., diagnosis, treatment, and others more) for patients treated at the Department of Orthopaedics and Trauma. Data regarding patients´ demographics, clinical presentations, diagnostic modalities, time to diagnosis, surgical and medical treatments, and follow-up data were retrieved from the electronic files. Furthermore, we conducted a search to assess documented risk factors for TB retrospectively [23].
Patients’ selection
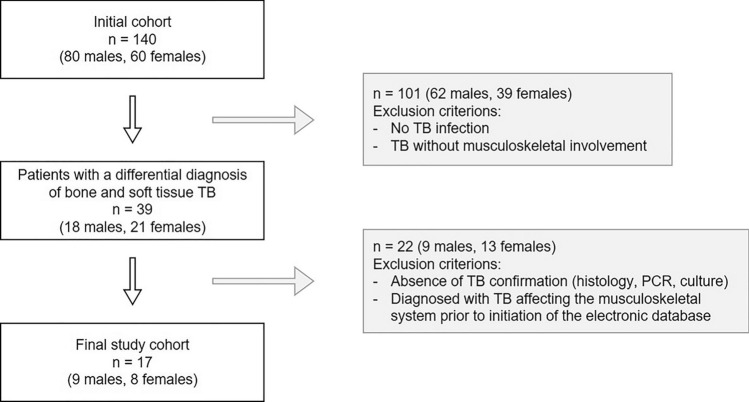
As illustrated in Fig. 1, our search yielded a total of 140 patients (80 males and 60 females) who had been admitted to our institution with a differential diagnosis of TB [23]. After reviewing these patients’ medical histories, we identified 39 patients (18 males and 21 females) who presented with a differential diagnosis of TB affecting the musculoskeletal system. Subsequently, we excluded 20 patients (7 males and 13 females), as their TB was not confirmed by standard methodology (histology, PCR, or culture) [23]. In addition, two more male patients were excluded as their disease diagnosis and treatment dated back before the initiation of electronic recordings at our hospital (2005) [23].
Fig. 1.
Patients’ selection. Flow chart illustrating the selection criteria for patients diagnosed with TB of the musculoskeletal system as extracted from our hospital´s electronic database [23]
Finally, a total of 17 patients (9 males and 8 females) were included in our study suffering from confirmed tuberculosis affecting their bones or soft tissues. Data of these patients were anonymised and recorded in a password-protected file using Microsoft Excel 2019 (Microsoft, Redmond, WA, USA). This analysis included clinical data (age, gender, symptoms, risk factors, surgical and medical treatments, and follow-up) as well as radiological, histological, serological, and/or molecular findings [23].
Radiological findings
A retrospective image evaluation, including plain radiography (RTG), computed tomography (CT), and magnetic resonance imaging (MRI), was performed by the musculoskeletal radiologist (JI). The analysis included: site, number of lesions, size, margin, signal intensity, contrast enhancement pattern, and changes of the surrounding tissue. Depending on the clinical presentation and bones or joints involved, radiographs in at least two projections were analysed. Lateral radiographs were used for evaluating spinal lesions. Axial CT images in soft tissue and "high resolution" bone windows with multiplanar reconstructions were used to detect early disease and analyse the spinal lesions. MRI analysis included delineating the extent of infection, depicting additional foci, demonstrating spinal disease for the extent of abscess formation, possible cord compression, and joint involvement. The study's minimal MRI protocol included T1-weighted and fluid-sensitive, fat-saturated (FS) and non-FS sequences in three imaging planes. If available, the post contrast sequences after intravenous application of gadolinium (Gd) were included in the analysis.
Histological analysis and polymerase chain reaction amplification of Mycobacterium tuberculosis genes
Formalin-fixed, paraffin-embedded (FFPE) paraffin tissue blocks were cut in 4 µm-thick whole tissue sections, stained with haematoxylin & eosin (HE) and evaluated by a bone and soft tissue pathologist (IB). In addition, FFPE tissue blocks were cut with a microtome using a new sterile blade. For each case 20–30 3 μm thick whole tissue sections were cut. According to the manufacturer's recommendations, genetic DNA was extracted with the Maxwell 16 FFPE Plus LEV DNA purification kit (Promega, Mannheim, Germany). The concentration and quality of DNA were determined fluorometrically with a Quantus™ Fluorometer and the Invitrogen™ Quant iT™ PicoGreen™ dsDNA assay (Thermo Fisher Scientific, Waltham, MA, USA). For PCR amplification, the LCD-Assay MYCO Direkt 1.9 (BioProducts, Stockerau, Austria) was used. The Mycobacterium genus-specific real-time PCR amplification was performed on a LightCycler 2.0 instrument using the LightCycler® TaqMan® Master (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's recommendations with 200–750 ng input DNA. The M. kansasii DNA control (Vircell, Granada, Spain) served as a positive control.
Statistical analysis
Data were analysed using Microsoft Excel 2019 (Microsoft, Redmond, Washington, USA), and Stata/BE 17.0 (StataCorp LLC, TX, USA). First, we conducted a descriptive analysis of the different variables. Second, we used a regression model to describe a trend in the reduction of incidence rates. A two-sample Wilcoxon rank-sum (Mann–Whitney) test was conducted to describe differences between the groups (spinal vs extraspinal). p values < 0.05 were considered statistically significant.
Ethic’s permission
This retrospective study was approved by the Institutional Review Board of the Medical University of Graz, Graz, Austria (reference number 32–148 ex 19/20).
Results
Overall TB trends in Austria, 1995–2019
According to the AGES registry, a total number of 23.120 patients were newly diagnosed with TB of any site between 1995 and 2019 in Austria. Except for three years (2004, 2013, and 2016), a continuous downward trend has been recorded (Fig. 2): In 1995, a total number of 1.633 newly reported cases were documented [23]. Since then, the rate of newly reported cases constantly declined to 474 in 2019 (71% reduction) [23]. Bivariate regression analysis describes a significant linear reduction in tuberculosis incidence rates from 1995 to 2019, on average by b = − 0.61 cases per 100.000 people/year (p < 0.001) (Fig. 2). Most patients were diagnosed with pulmonary TB (n = 18.436; 79.7%; Fig. S1 and Table S1) [23]. A total of 4.213 patients (18.2%) presented with extrapulmonary TB (Fig. S1 and Table S1) [23]. A minority of patients (n = 471, 2%) had no site of involvement specified (Fig. S1). Detailed organ involvements are listed in Table S1. The most frequently affected extrapulmonary sites were lymph nodes and the pleura with 1.919 and 858 reported cases, respectively. In contrast, the rarest single organ involvement was the central nervous system including the meninges (n = 98) (Table S1). Only 88 patients were diagnosed with disseminated TB [23].
Fig. 2.
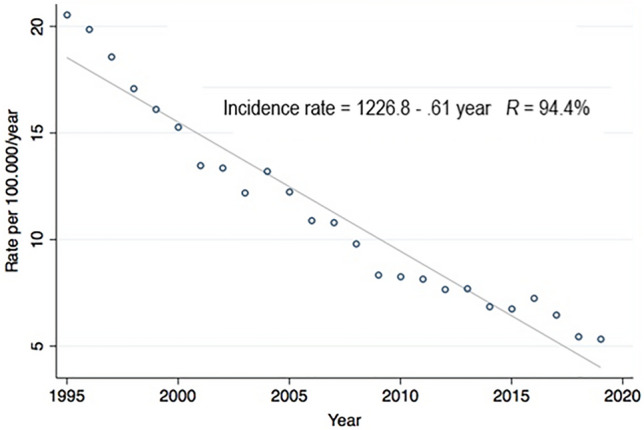
Incidence rates of diagnosed TB in Austria between 1995 and 2019. Regression analysis indicates a significant linear reduction in tuberculosis incidence rates from 1995 to 2019, on average by b = − 0.61 cases per 100.000 people/year (p < 0.001)
Bone and joint TB in Austria, 1995–2019
Between 1995 and 2019, musculoskeletal TB was first diagnosed in 307 patients in Austria [23]. Out of those 140 presented with spinal TB (“Pott’s disease”), and 167 with extraspinal musculoskeletal TB. Patients with musculoskeletal TB accounted for 1.3% of all TB cases recorded in Austria in this period and for 7.3% of extrapulmonary TB cases. Thus, musculoskeletal TB is fourth-ranked amongst extrapulmonary sites, after affections of lymph nodes, the pleura, and the urogenital tract [23]. A detailed summary of patient numbers and sites for each year is given in Tab. S1. In addition to patients who presented with symptomatic musculoskeletal TB, the bones or joints were co-affected in 149 patients with symptomatic TB of any other site. Amongst those, 77 presented with spinal and 72 with extraspinal manifestations (Table S1). On average, 6 new cases of spinal and 7 cases of extraspinal bone and joint TB were recorded in Austria between 1995 and 2019 every year [23].
Case series of patients with musculoskeletal TB treated at an Austrian tertiary centre, 2005–2019
The search of our hospital´s electronic database retrieved 17 patients who were diagnosed and/or treated with musculoskeletal TB at our institution between 2005 and 2019 (Fig. 1) [23]. The cohort consisted of eight female (47.1%) and nine male (52.9%) patients. Nine patients were native Austrians (52.9%) (Table 1). The remaining patients were of different origins, most of them from the Asian continent (India, Pakistan, Afghanistan, Nepal, New-Guinea). Two of the non-Austrian born patients were refugees from Gambia and Jemen (Table 2) [23]. The patients´ median age at diagnosis was 63 years (range 18–92). Native Austrian patients were diagnosed at a median age of 69 years (range 63–92), whereas for patients with a recent migration background, the median age at diagnosis was 29 years (range 18–39) [23]. None of the patients had a known concomitant HIV infection.
Table 1.
Demographics and affected sites
| Origin | Total number of patients (n) | Spinal affection (n) | Joint affection (n) | Soft tissue abscess (n) | Age at diagnosis |
|---|---|---|---|---|---|
| EU (Austria) | 9 | 5 | 3 | 1 | 69 (median) (range 63–92) |
| Non-EU | 8 | 5 | 2 | 1 | 29 (median) (range 18–39) |
| Total (n) | 17 | 10 | 5 | 2 | 63 (median) 52.1 (mean) (range 18–92) |
Summary of demographics and sites of affection in our cohort of n = 17 patients suffering from TB of the musculoskeletal system [23]
Table 2.
Radiological findings, clinical presentation, surgery and secondary deformities in patients presenting with musculoskeletal TB
| Sex | Age | CO | Location | Imaging | Lung | BME | Bone Destr | Path. Fx | Soft Tissue | Abscess | Spinal Canal | TBC Arthritis | No of Bones | Major Symptoms | Biopsy | Surgery | Sec. Deform | FUP months (status) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 69 | A | Tibia | MR | N | Y | Y | N | Y | Y | NA | Y | 2 | S (P) | N | Y | Y | 2 (DOC) |
| F | 74 | A | MCP1 | MR, RTG | N | Y | Y | N | Y | N | NA | Y | 2 | S (P) | N | Y | Y | 47 (NED) |
| M | 39 | I | SC joint | CT, SONO, RTG, MR | Lymph- adenopathy | Y | Y | N | Y | Y | NA | Y | 2 | T (S) | Ya | N | Y | 61 (NED) |
| F | 81 | A | Femur | RTG | N | NA | NA | NA | NA | NA | NA | Y | 1 | F (S) | N | Y | N | 69 (DOC) |
| M | 19 | NG | Elbow | RTG | Post specific changes | Y | Y | N | Y | Y | NA | Y | 2 | S (P) | N | Y | N | 15 (NED) |
| M | 29 | I | Thoracic wall | RTG, SONO, CT | Pleuritis | NA | Y | Y | Y | Y | N | N | 1 | T (S) | Yb | Y | N | 32 (NED) |
| M | 92 | A | Ischium | RTG, CT | N | NA | Y | N | Y | Y | N | N | 1 | S (P) | N | Y | N | 50 (NED) |
| M | 37 | G | Spine thoracic | RTG, CT, MR | N | Y | Y | N | Y | Y | Y | N | 2 | N (S) | N | Y | Y | 116 (NED) |
| M | 22 | AG | Spine thoracic | RTG, CT, MR | N | Y | Y | Y | Y | Y | Y | N | 4 | T (P) | Yc | N | Y | 71 (NED) |
| M | 63 | A | Spine cervical, thoracic | CT | Pneumonia, miliary | NA | Y | Y | Y | Y | Y | N | 3 | N (S) | N | N | NA | 0 (DOD) |
| M | 29 | N | Spine lumbar, sacral | RTG, CT | Tree-in-bud infiltration | NA | Y | Y | Y | Y | Y | N | 5 | S (P) | Yd | N | Y | 74 (NED) |
| M | 30 | P | Spine lumbar | RTG, CT, MR | N | Y | Y | Y | Y | Y | Y | N | 1 | N (P) | Yc | Y | N | 70 (NED) |
| F | 18 | J | Spine sacral | RTG, CT, MR | N | Y | Y | N | Y | Y | Y | Y | 4 | T (N) | Yd | N | Y | 33 (NED) |
| F | 65 | A | Spine thoracic | RTG, CT | N | NA | Y | N | Y | N | Y | N | 1 | T | Yc | N | N | 29 (NED) |
| F | 69 | A | Spine thoracic | CT, MR | Post specific changes | Y | Y | N | Y | Y | Y | N | 2 | N (P) | Yc | Y | Y | 105 (NED) |
| F | 69 | A | Spine lumbar | RTG, CT, MR | Miliary | Y | Y | N | N | N | Y | N | 1 | N (P) | Ya | Y | Y | 44 (NED) |
| F | 80 | A | Spine lumbar | RTG, CT | N | NA | Y | N | Y | N | Y | N | 2 | S | N | Y | N | 5 (DOC) |
Sex: female (F); male (M). Country of origin (CO): Austria (A); India (I); New-Guinea (NG); Gambia (G); Afghanistan (AG); N (Nepal); Pakistan (P); Jemen (J). Location: MCP1: first metacarpophalangeal joint; SC joint: sternoclavicular joint. Imaging: RTG: conventional two-planar radiographs; SONO: sonography; CT: computed tomography; MR: magnetic resonance imaging; Imaging analyses: Y: yes; N: no; NA: not applicable. BME: bone marrow edema; Bone Destr.: bone destruction; Path. Fx: pathological fracture; Soft Tissue: soft tissue component; Abscess: abscess formation; Spinal Canal: involvement of spinal canal. Major symptoms and differential diagnoses: pain (P)/neurology (N)/fracture (F)/septic process, i.e., abscess formation or septic arthritis (S)/osseous destruction, suspicious of tumourous process (T). Biopsy: open biopsy (a); core needle biopsy (b); CT-guided biopsy (c), with drainage (d) of an abscess formation. Sec. Deform.: secondary deformity. Status at last follow-up (FUP): death of other cause (DOC), no evidence of disease (NED), death of disease (DOD) [23]
Manifestation sites and radiological evaluation
Manifestation sites of extrapulmonary TB in our cohort were the spine (n = 10) the joints (n = 5), and the soft tissues (n = 2) (Tables 1 and 2) [23]. In patients with spinal TB, the thoracic (n = 5) and the lumbar (n = 4) spine were particularly affected, followed by the sacral region (n = 1) (Table 2) [23]. Furthermore, the cervical spine and the sacral region were co-affected in one case, each. Joint involvements included the hip, the knee (Fig. 3c–d), and the elbow joint (Fig. 3e–g), the thumb saddle, and the sternoclavicular joint (n = 1, each). Two cases of soft-tissue manifestations presented in the posterior chest wall, and the gluteal region. However, in all cases of joint and soft tissue manifestations, radiology saw an affection of the adjacent bones, so that an osseous origin of all these cases can be assumed, as indicated in Table 2. Multifocal disease affecting more than one anatomic location was present in five patients at the time of diagnosis (Table 2): Three patients with spinal involvement had concomitant pulmonary TB. One of these patients presented with miliary TB; this patient had a severe affliction to the lungs, spleen, liver, and kidney and died shortly after diagnosis. A fourth patient suffered from extraspinal TB of the sternoclavicular joint and showed a simultaneous affection of the supraclavicular lymph nodes. Another patient with soft-tissue TB of the lateral thoracic wall showed a concomitant pleural affection (Table 2) [23].
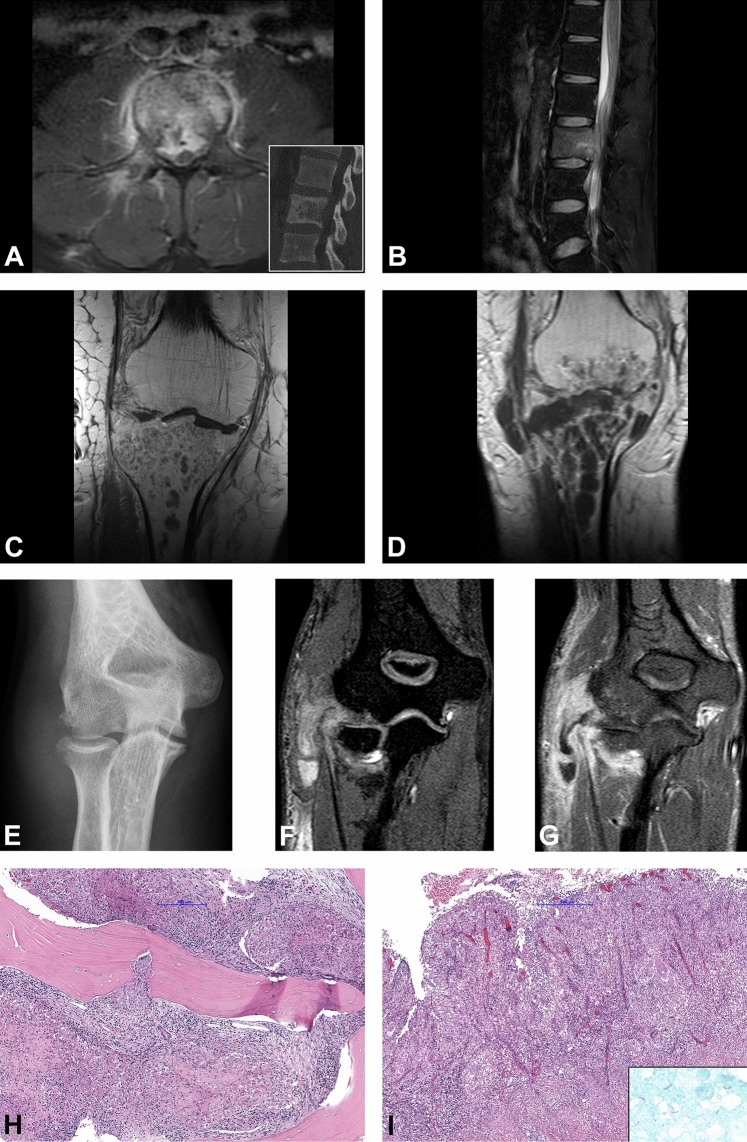
Fig. 3.
Radiological and histological findings. a, b 31-year-old male patient with a diffuse pathological contrast enhancement of the L2 and paravertebral soft tissue component on axial T2w fat-saturated MRI image (a). Osteolytic destruction of the vertebral body on sagittal CT image (inset). Sagittal T2w fat-saturated image shows bone marrow edema of the vertebral body with epidural abscess and spinal cord compression (b). Timely evolution of the intraosseous abscess formations in the proximal tibia in the 69-year-old-male patient on coronal post contrast T1w sequences baseline MRI (c) and after 1 month (d). Radiological signs of pyogenic arthritis with the osteo destruction of the tibial plateau and newly developed involvement of the distal femur and para-articular soft tissues medially. Antero-posterior plain radiograph of the right elbow in a 20-year-old female patient shows osteo destruction of the humeral capitellum with soft tissue edema (e). 3-dimensional T1w WATS (Water Selective Excitation) coronal image (f) shows a widening of the radio-humeral joint with osteo destruction of the periarticular bones, synovial proliferation, and pathological contrast enhancement of the periarticular soft tissue with abscess formation along the flexor muscles of the forearm (g). Granulomatous inflammation composed of granulomas with central necrotic areas surrounded by the palisading histiocytes involving the bone (h) and the soft tissue (i). Ziehl–Neelsen staining shows scattered acid-fast organisms (i inset)
Clinical presentation
Overall, most patients presented with septic or inflammatory conditions (n = 7), neurological deficits (n = 5), or a suspected neoplastic process (n = 5) [23].
Lead symptoms in patients with spinal TB were neurological deficits and pain (n = 5), osseous destructions suspicious of a tumourous process (n = 3), and/or inflammatory conditions (e.g., abscess formations in the psoas muscle) (n = 2). Patients with extraspinal TB usually presented with inflammatory conditions, e.g., septic arthritis or abscess formations (n = 4), soft-tissue swellings suspicious of a tumourous process (n = 2), and a non-recent bony fracture due to osteomyelitis (n = 1) (Table 2) [23].
The period from the initial presentation of the patients at our department to confirmed diagnosis ranged from 7 to 405 days (median 14 days, IQR 7–30 days). Two out of 17 patients already presented with a confirmed diagnosis of TB. They had been diagnosed with TB at external hospitals seven and 42 days before their initial presentation at our department, respectively [23].
Spinal TB was diagnosed on the 31st day after initial presentation at our department (median, range 7–405). In comparison, extraspinal musculoskeletal TB, which frequently presented as a joint infection or suspected neoplastic process, was diagnosed on the 22nd day (median, range 10–74 d) [23].
Diagnosis
A biopsy was performed in nine cases; it consisted of open biopsy (n = 2), core-needle biopsy (n = 1), or CT-guided biopsy (n = 6) (Tab. 2) [23]. In most cases (n = 5), a biopsy was conducted to rule out a neoplastic lesion. Biopsies were also performed in cases of neurological deficits (n = 3) and inflammatory conditions (n = 1). In two cases, an abscess formation was drained in the course of a CT-guided biopsy. Of those eight patients that did not undergo biopsy, seven were diagnosed with TB based on their surgical specimens. The eighth patient died from severe multi-organ affections. In this case, TB was diagnosed on tissue specimens obtained during autopsy (Table 2) [23].
The diagnosis was based on histologic examination of biopsy/surgical specimens, PCR, and/or culture verification of TB. In most patients (n = 15, 88.2%), the diagnosis was confirmed by at least two of these methods. In two cases, only histology was available; one patient had a history of recurrent TB and presented with a typical histomorphology including a positive Ziehl–Neelsen staining (Fig. 3i), and the second patient was diagnosed with miliary TB at autopsy [23].
Histology showed typical granulomatous inflammation composed of granulomas with central caseous necrosis surrounded by the palisading histiocytes (n = 13; 76.5%) (Fig. 3h–i). In ten cases, the granulomatous inflammation was found in the affected bone and the surrounding soft tissues, and in three patients the changes were found in soft tissue (in these cases no bone tissue was received for the examination). In one patient, only necrosis was found.
An additional Ziehl–Neelsen staining was performed in four cases and in one case, the acid-fast organisms were identified (Fig. 3i).
PCR was conducted in most of the cases (n = 14, 82.4%), and was positive in n = 11/14 (78.6%). As mentioned above, in one case without PCR validation, Ziehl–Neelsen staining was positive, and the patient had recurrent TB; the second patient was diagnosed with miliary TB at autopsy; in the third case a positive TB culture was available. The other three PCR-negative cases had a positive histology and TB culture [23].
TB culture was performed in n = 12 cases (70.6%). It was positive in n = 11/12 (91.7%), and negative in one case. In the latter, TB was confirmed by histology and PCR [23].
Risk factors
One patient received systemic corticosteroid therapy due to systemic sarcoidosis which was diagnosed prior to TB. Another patient had a long-term therapy with corticosteroids and TNF-alpha inhibitors due to an underlying autoimmune disease (systemic lupus erythematosus). One patient with rheumatoid arthritis was on TNF-alpha blocker treatment without another immunosuppressant. Another patient had received systemic chemotherapy due to a follicular lymphoma. One patient had diabetes (type 2) [23].
Surgical treatment
Eleven out of 17 patients underwent surgery (64.7%) (Table 2). All patients had surgery performed due to septic conditions (n = 7) and/or neurologic deficits (n = 4) [23].
Patients who were operated due to peripheral joint infections all showed osteomyelitis and necrosis of the joint forming bones (n = 4) (Table 2 and Fig. 3c–g). These patients underwent joint lavage, synovectomy, necrectomy, and debridement of periarticular abscess formations. One patient underwent prosthetic hip joint replacement due to a non-recent fracture of the femoral neck caused by chronic osteomyelitis. Another patient had an additional removal of her trapezium bone. Patients with soft-tissue manifestations of TB had their abscess formations drained and/or resected (n = 2); in one patient with an abscess formation of the thoracic wall, surgery included a partial resection of the affected rib [23].
Amongst the patients with spinal TB, 50% underwent surgery: Four patients had surgery due to neurological deficits (Table 2 and Fig. 3a, b), another one had a severe manifestation of spondylodiscitis with osseous destruction of the bones involved. Surgery consisted of a decompression of the spinal cord via (hemi-) laminectomy and stabilization in four of these patients. The fifth patient was initially diagnosed with spinal TB via CT-guided biopsy. He then developed a progressive kyphotic spinal deformity, which caused neurological symptoms and incipient paraplegia. Therefore, this patient was treated with corpectomy and the implantation of an obelisk cage. Even though more than half of the patients of our series developed secondary deformities (n = 9; 52.9%), most commonly in patients with spinal TB (n = 6) (Table 2), this was the only patient who required secondary surgical treatment [23].
Antibiotic treatment
Eleven out of 17 patients (64.7%) received the initial standard four-drug treatment as suggested by the WHO, consisting of isoniazid (INH), rifampicin (RMP), pyrazinamide (PZA), and ethambutol (EMB) (Table 3) [23].
Table 3.
Medical treatment as received by 16 patients with musculoskeletal TB
| Medical treatment | Spinal (n) | Extraspinal (n) | Total (n) |
|---|---|---|---|
| Four-drug therapy (INH, RMP, PZA, EMB) | 6 | 5 | 11 |
| Alternative four-drug regimen | 2 | 0 | 2 |
| Three-drug therapy (INH, RMP, EMB) | 1 | 2 | 3 |
| No drug therapy | 1 | 0 | 1 |
Isoniazid (INH), rifampicin (RMP), pyrazinamide (PZA), and ethambutol (EMB) [23]
Two patients with spinal involvement received a different four-drug regimen due to drug-related intolerances (Table 3). In those cases, the quadruple-therapy was modified by administering streptomycin instead of isoniazid, and moxifloxacin instead of rifampin, respectively. Three patients received a three-drug regimen. In one of these cases, the standard four-drug therapy was initiated. However, due to the subsequent onset of severe urticaria, PZA was terminated, and the therapy was continued as a three-drug combination regimen. Unfortunately, one patient died prior to treatment initiation (Table 3) [23].
After an average follow-up of 48.4 months (range 0–116), 13 patients showed no evidence of disease (NED) (76.5%); three patients had died of other causes (DOC) (17.6%). One patient died from severe miliary TB (death of disease, DOD) (5.9%) (Table 2) [23].
Discussion
The aims of this study were twofold: first, to provide a brief overview of general TB trends in the Austrian population between 1995 and 2019 including pulmonary, as well as extrapulmonary TB. Second, it describes a retrospective case series of bone and joint TB diagnosed and treated at an Austrian tertiary orthopaedic referral centre between 2005 and 2019 [23].
As stated previously, TB is one of the oldest diseases and has plagued humankind since prehistoric ages [5]. Eventually, TB rates declined in developed parts of the world due to public health measures and the BCG (Bacille Calmette–Guérin) vaccine in the second half of the twentieth century. Furthermore, the development of drug treatments, such as streptomycin and isoniazid, and improved living conditions and healthcare lead to lower TB rates [5, 23].
However, according to the World Health Organization (WHO), TB is still endemic in large parts of the world. Globally, 10 million TB infections were reported in 2018 [10, 23]. Epidemiologically, there is a much greater prevalence in low-income countries worldwide: so is the reported TB incidence 220 per 100,000 in African and Southeast Asian countries, and less than 30 in the United States and Europe [1, 10]. TB was the leading cause of death amongst single infectious diseases with 1.4 million TB-related deaths in 2019 [10]. Despite successful drug treatment regimens that can cure up to 85% of patients with active TB [10] and comprehensive prevention strategies [10], the global fight against TB faces hurdles. These include prevailing poverty, undernutrition, limited access to healthcare in large parts of the world, and the impact of the acquired immunodeficiency syndrome (AIDS) pandemic, particularly in these areas [10].
In recent years, some of the high-income countries are experiencing an increase in the incidence of TB as well, mainly because of increased migration from disease-endemic regions [1]. However, as reported by the AGES [14] and illustrated by our data (Fig. 2) [23], this trend was not seen in Austria, as the annual overall incidence of TB has constantly been decreasing within the last decades and reached 5.5 per 100,000 in 2018.
Extrapulmonary TB infections of the bone and joints have been reported in 10–15%, accounting for 2–4% of all TB cases [17, 19, 22]. In general, the spine is the most frequently affected site amongst all cases of musculoskeletal TB (Pott’s disease, 1–2%) [6, 9, 19]. According to the data provided by the Austrian Reference Centre for Tuberculosis, musculoskeletal TB was the fourth most common manifestation of extrapulmonary TB (7.3%) in Austria after pleural, lymphatic, and urogenital disease between 1995 and 2019 (Table S1) [23]. In total, 45.6% of TB patients registered in Austria within this period presented with a spinal TB manifestation (Table S1) [23]. Reports from other countries, such as England (65%) or Denmark (54.2%), confirm a high percentage of spinal affections in musculoskeletal TB [3, 15]. In line with the published literature, we observed a slight male predominance in our cohort (53%) [8, 23].
The collective we investigated herein showed a varying age distribution. In fact, we could clearly distinguish between two affected age groups: approximately half of the patients were diagnosed between the age of 20 and 40 (n = 8), the other half were diagnosed at an age over 60 (n = 9) [23]. All patients affected in the younger age group had a migration background from countries outside the EU, whereas all patients in the older group were native Austrians (Table 1) [23]. The fact that extrapulmonary TB was particularly common in EU migrants was also stated in a recent cross-sectional analysis of database entries collected over 23 years (1995–2017) from 32 countries of the EU and the European Free Trade Association [13]. In addition, a bimodal age distribution due to a migration and/or HIV background of younger patients has previously been reported by other authors and single-country studies, for instance from the UK or the Netherlands [4, 18, 22, 26].
Patients with skeletal tuberculosis usually present with pain, deformity, a characteristic cold abscess formation, and/or a fistulation [1, 8, 22]. These conditions are often accompanied by general symptoms, such as low-grade evening fever, anorexia, and weight loss [8]. An active concomitant pulmonary infection is rarely seen [21, 22]. Particularly in cases of spinal TB, the onset is perfidious, typically progressing over months [21]. Clinically, a gibbus might be seen, corresponding to the radiological finding of kyphosis due to a collapse of the anterior vertebral column [8, 16, 22, 24]. Furthermore, paraspinal abscesses may be present [4, 6, 8, 16]. Neurological deficit is common and reported between 23 and 76% of cases, with a higher prevalence in cases of cervical and thoracic involvement [6, 22]. We also observed rather unspecific symptoms in our collective: In line with the literature, most patients reported a dump pain in the affected area, which had developed and persisted over months [23]. Thirty percent of patients already presented at our institution with a neurologic deficit. Remarkably, 6 out of 10 patients (60%) already presented with or consecutively developed a visible spinal deformity, such as levoscoliosis or a kyphotic deformation of the affected region of the spine [23]. Approximately 30% showed a concomitant pulmonary co-affection or a miliary form of TB. In three out of ten patients (30%) an underlying malignant disease was suspected due to an advanced osseous destruction of the affected region and/or lytic bone lesions [23].
Long-lasting unspecific signs and symptoms potentially result in a delayed presentation at a specialised center, and a consecutively delayed diagnosis [3, 4]. For instance, Colmenero et al. reported diagnostic delays in tuberculous vertebral osteomyelitis of more than 6 months [4]. These delays may explain the high percentage of neurologically impaired patients, as well as the considerably high proportion of patients with clinically apparent secondary deformities at the time of presentation [22]. In line with these findings, also patients treated at our institution faced delays in diagnosis, even though these were not as extensive as reported in the literature [3, 4, 23]. This statement is limited, however, by the fact that we are lacking detailed information on the patients´ symptom durations prior to their admission to our facility. So was the median time between the initial presentations at our department to diagnosis 31 days for patients with spinal TB [23]. This is largely because, apart from patients who had previously been diagnosed with TB, Pott’s disease was not amongst the differential diagnoses considered by the admitting, nor by the treating physicians. In comparison, extraspinal musculoskeletal TB, which is frequently presented as a suspected joint infection or neoplastic process, was diagnosed after a median time of 22 days after presentation [23]. Due to the frequently present infectious/septic condition of the affected joints and soft-tissues, patients underwent surgical treatment faster and more often than patients with spinal TB. Therefore, six out of seven patients with extraspinal TB underwent surgery (85.7%), compared to five out of ten (50%) patients with spinal TB [23]. Nevertheless, secondary deformities were observed frequently in those patients. Particularly functional impairments resulting from bony ankylosis or surgically performed resection arthroplasties were seen in a high percentage (42.9%) of patients with extraspinal manifestations [23].
Even though there is no final consensus regarding the role of surgery in TB in the existing literature [16], surgical treatment was required in the majority of patients, which is in line with other reports [4, 8, 22–24, 28]. As illustrated by several well-described series, surgery is usually conducted in cases of progressive neurological impairment and spinal instability [8, 24, 25, 27, 28]. In our series, the main indications for surgery were neurological deficits, large abscess formations, septic arthritis of an affected joint, or an established or predicted deformity of the spine. Frequently, we observed a combination of more than one of these factors (e.g., a neurological deficit and an abscess formation or a spinal deformity) [23]. In line with previous reports, surgery resulted in the postoperative improvement of neurological deficits, pain, and the restoration of spinal alignment in these patients [8, 24, 25, 27, 28].
Regarding the drug treatment of musculoskeletal TB, the WHO recommends treatment in two phases: an intensive phase and a continuation phase [2, 19, 20]. In our series, the majority of patients (n = 11, 65%) received a standard drug treatment regimen. In a total of five cases, the treatment regimen had to be amended due to intolerance (n = 3) or resistance issues (n = 2) [23]. We were not aware of any case of concomitant HIV infection. One of the patients included in this retrospective series suffered from recurrent TB, and one patient (5.9%) died from a severe, generalised course of the disease [23]. Again, this is in concordance with other published series reporting comparatively low relapse rates and mortality for spinal TB [22].
Our study is limited by the fact that the retrospective case series presented herein was derived from a single centre analysis. However, this study was conducted at an Austrian tertiary reference centre recognized as a specialised institution for orthopaedics, infectious diseases, and musculoskeletal tumours. Therefore, it can be assumed that patients with bone and joint TB in the southern parts of Austria are largely covered by this retrospective cohort in the period investigated. In addition, we included nationwide data from the national TB registry, so that Austrian trends in bone and joint TB are provided. A second limitation is that most patients had been symptomatic for an unknown period prior to their referral to our department. Therefore, and due to the retrospective study-design, we were unable to define the exact time from the onset of signs and symptoms to diagnosis. However, irrespective of the retrospective study design, we were able to gain thorough information on the treatment and follow-up of the patients included in our case series by collaborating with a resident lung specialist who treated and followed-up these patients, and who co-authors the study presented herein.
Conclusion
In summary, this study illustrates that TB is by far not extinct in developed countries, even though Austrian TB incidence rates are continuously declining. In an era of increased mobility and migration, doctors should consider a differential diagnosis of musculoskeletal TB in cases of untypical joint infections or nonspecific bone lesions in younger patients with a migration background or in patients with specific risk factors.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1 Tuberculosis in Austria. Distribution of pulmonary, extrapulmonary, and musculoskeletal TB amongst newly diagnosed cases of TB in Austria 1995 – 2019 [23] (TIF 399 KB)
Table S1 Cases of newly diagnosed TB in Austria, 1995 – 2019. Detailed organ involvements of sites which are mainly affected (a) and co-affected (b) by TB are listed. A summary of organs mainly affected by TB is provided (c). Musculoskeletal cases are sub-classified in spinal (Pott´s disease) and extraspinal TB (c) [23] (XLSX 16 KB)
Table S2 TB incidence rates in Austria, 1995–2019 [23] (XLSX 12 KB)
Acknowledgements
The authors would like to thank the Austrian Reference Centre for Tuberculosis, particularly Dr Indra, for their support in providing the data of the Austrian tuberculosis registry. Thanks to Ms Andrea Schlemmer for retrieving the data from the electronic files of our hospital. We would also like to thank Dr Tanja Kraus, and Professor Dr Mathias Glehr, who were involved in the treatment of patients presented in our series and helped with the images provided. Finally, the authors thank all other medical practitioners who were involved in the treatment of these patients, and all physicians who supported us in obtaining the data presented herein.
Author contributions
IV investigation, formal analysis, methodology, visualisation, supervision, writing-original draft preparation. LP data curation, formal analysis, investigation, visualisation, writing-original draft preparation. IT data curation, methodology, investigation, writing-review and editing. JI data curation, visualisation, investigation, writing-original draft preparation. IB data curation, visualisation, investigation, writing-original draft preparation. TV data curation, investigation, writing-original draft preparation. UW data curation, visualisation, writing-review and editing. RZ formal analysis, supervision, writing-review and editing. PS methodology, supervision, writing-review and editing. AL supervision, project administration, writing-review and editing. SF formal analysis, visualisation, supervision, writing-original draft preparation. SS conceptualisation, methodology, project administration, supervision, validation, writing-original draft preparation. All authors critically reviewed the manuscript and approved its final version.
Funding
This study did not receive any external funding.
Data availability
A detailed summary of the Austrian tuberculosis incidence rates 1995–2019 as presented in this manuscript is provided in Supplementary Tables 1 and 2 (Tables S1 and S2).
Declarations
Conflict of interest
IV and PS received research funding from Johnson & Johnson Austria. SS partnered a research project funded by Roche Austria. PS received funding from Alphamed, and Medacta. AL reports institutional educational grants by Johnson & Johnson, Alphamed, Implantec, and Medacta. The other authors declare that they have no conflicts of interest. This manuscript is based on a diploma thesis submitted by LP [23].
Ethical approval
This study has been approved by the Institutional Review Board (IRB) of the Medical University of Graz, Graz, Austria (32-148 ex 19/20). The study was performed in accordance with the Declaration of Helsinki.
Informed consent
Not applicable, as retrospective observational case series.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agashe VM, Johari AN, Shah M, et al. Diagnosis of osteoarticular tuberculosis: perceptions, protocols, practices, and priorities in the endemic and non-endemic areas of the world—a WAIOT view. Microorganisms. 2020;8(9):1312. doi: 10.3390/microorganisms8091312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 3.Broderick C, Hopkins S, Mack DJF, et al. Delays in the diagnosis and treatment of bone and joint tuberculosis in the United Kingdom. Bone Jt J. 2018;100B(1):119–124. doi: 10.1302/0301-620X.100B1.BJJ-2017-0357.R1. [DOI] [PubMed] [Google Scholar]
- 4.Colmenero JD, Jimenez-Mejias ME, Reguera JM, et al. Tuberculous vertebral osteomyelitis in the new millennium: still a diagnostic and therapeutic challenge. Eur J Clin Microbiol Infect Dis. 2004;23(6):477–483. doi: 10.1007/s10096-004-1148-y. [DOI] [PubMed] [Google Scholar]
- 5.Daniel TM. The history of tuberculosis. Respir Med. 2006;100(11):1862–1870. doi: 10.1016/j.rmed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Dunn RN, Ben Husien M. Spinal tuberculosis: review of current management. Bone Jt J. 2018;100B(4):425–431. doi: 10.1302/0301-620X.100B4.BJJ-2017-1040.R1. [DOI] [PubMed] [Google Scholar]
- 7.Evinger S, Bernert Z, Fothi E, et al. New skeletal tuberculosis cases in past populations from Western Hungary (Transdanubia) Homo. 2011;62(3):165–183. doi: 10.1016/j.jchb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Fuentes Ferrer M, Gutierrez Torres L, Ayala Ramirez O, Rumayor Zarzuelo M, del Prado GN. Tuberculosis of the spine. A systematic review of case series. Int Orthop. 2012;36(2):221–231. doi: 10.1007/s00264-011-1414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautam MP, Karki P, Rijal S, Singh R. Pott's spine and paraplegia. JNMA J Nepal Med Assoc. 2005;44(159):106–115. [PubMed] [Google Scholar]
- 10.Global tuberculosis report 2020. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO
- 11.Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72(9):1761–1768. [PubMed] [Google Scholar]
- 12.Gradmann C. Robert Koch and the white death: from tuberculosis to tuberculin. Microbes Infect. 2006;8(1):294–301. doi: 10.1016/j.micinf.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Hayward SE, Rustage K, Nellums LB, et al. Extrapulmonary tuberculosis among migrants in Europe, 1995–2017. Clin Microbiol Infect. 2020;27:1347. doi: 10.1016/j.cmi.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Indra A, Schmid D (2018) Nationale Referenzzentrale für Tuberkulose. Jahresbericht 2018 [National Reference Centre for Tuberculosis. Annual Report 2018](ed)^(eds). Austrian Agency for Health and Food Safety GmbH; Federal Ministry Republic of Austria Social Affairs, Health, Care and Consumer Protection, Vienna
- 15.Johansen IS, Nielsen SL, Hove M, et al. Characteristics and clinical outcome of bone and joint tuberculosis from 1994 to 2011: a retrospective register-based study in Denmark. Clin Infect Dis. 2015;61(4):554–562. doi: 10.1093/cid/civ326. [DOI] [PubMed] [Google Scholar]
- 16.Jutte PC, van Loenhout-Rooyackers JH (2006) Routine surgery in addition to chemotherapy for treating spinal tuberculosis. Cochrane Database Syst Rev (5):CD004532 [DOI] [PubMed]
- 17.Jutte PC, van Loenhout-Rooyackers JH, Borgdorff MW, van Horn JR. Increase of bone and joint tuberculosis in The Netherlands. J Bone Jt Surg Br. 2004;86(6):901–904. doi: 10.1302/0301-620X.86B6.14844. [DOI] [PubMed] [Google Scholar]
- 18.Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999–2006. Thorax. 2009;64(12):1090–1095. doi: 10.1136/thx.2009.118133. [DOI] [PubMed] [Google Scholar]
- 19.Leonard MK, Blumberg HM (2017) Musculoskeletal tuberculosis. Microbiol Spectr 5(2) [DOI] [PubMed]
- 20.Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papagelopoulos PJ, Papadopoulos E, Mavrogenis AF, Themistocleous GS, Korres DS, Soucacos PN. Tuberculous sacroiliitis. A case report and review of the literature. Eur Spine J. 2005;14(7):683–688. doi: 10.1007/s00586-004-0831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pigrau-Serrallach C, Rodriguez-Pardo D. Bone and joint tuberculosis. Eur Spine J. 2013;22(Suppl 4):556–566. doi: 10.1007/s00586-012-2331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putzl L (2022) Differential diagnosis TB: analysis and evaluation of patients with tuberculosis affecting the bones and soft tissues, a retrospective study. Diploma Thesis, Medical University of Graz
- 24.Rajasekaran S. Kyphotic deformity in spinal tuberculosis and its management. Int Orthop. 2012;36(2):359–365. doi: 10.1007/s00264-011-1469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi S, Ying X, Fei J, Hu S (2021) One-stage surgical treatment of upper thoracic spinal tuberculosis by posterolateral costotransversectomy using an extrapleural approach. Arch Orthop Trauma Surg [DOI] [PubMed]
- 26.te Beek LA, van der Werf MJ, Richter C, Borgdorff MW. Extrapulmonary tuberculosis by nationality, The Netherlands, 1993–2001. Emerg Infect Dis. 2006;12(9):1375–1382. doi: 10.3201/eid1209.050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Cui Y, Gong L et al (2021) Comparison between single anterior and single posterior approaches of debridement interbody fusion and fixation for the treatment of mono-segment lumbar spine tuberculosis. Arch Orthop Trauma Surg [DOI] [PMC free article] [PubMed]
- 28.Yuan B, Zhao Y, Zhou S, Wang Z, Chen X, Jia L. Treatment for tuberculosis of the subaxial cervical spine: a systematic review. Arch Orthop Trauma Surg. 2021;141(11):1863–1876. doi: 10.1007/s00402-020-03572-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Tuberculosis in Austria. Distribution of pulmonary, extrapulmonary, and musculoskeletal TB amongst newly diagnosed cases of TB in Austria 1995 – 2019 [23] (TIF 399 KB)
Table S1 Cases of newly diagnosed TB in Austria, 1995 – 2019. Detailed organ involvements of sites which are mainly affected (a) and co-affected (b) by TB are listed. A summary of organs mainly affected by TB is provided (c). Musculoskeletal cases are sub-classified in spinal (Pott´s disease) and extraspinal TB (c) [23] (XLSX 16 KB)
Table S2 TB incidence rates in Austria, 1995–2019 [23] (XLSX 12 KB)
Data Availability Statement
A detailed summary of the Austrian tuberculosis incidence rates 1995–2019 as presented in this manuscript is provided in Supplementary Tables 1 and 2 (Tables S1 and S2).