Abstract
Juglans regia L. commonly known as walnut is used as the most extensive and economical tree in the world. This review aims to study the ethnomedicinal, phytochemical and pharmacological potential of walnut. The literature has been collected from different online sources like science Direct, Scopus, ResearchGate, Google Scholar, PubMed, etc. based on inclusion and exclusion criteria. An ethnomedicinal survey has also been conducted to document the traditional knowledge and uses of walnut among the local peoples of the Union Territory of Jammu and Kashmir. On surveying the local peoples in the different major walnut-producing areas, it has been followed that the walnut is locally used as a medicinal, nutritional, and commercial plant to treat common diseases and disorders in the locality. The survey has been conducted first time in the area and no study has been reported till now in the Jammu Division while some work has been reported in the Kashmir Division. Among the many bioactive compounds present in various plant parts, Juglone has been reported a significant anti-cancer compound in treating deadly cancer. This systematic review describes the significant knowledge and traditional information collected on ethnomedicinal uses, phytochemistry, habitat, macro-morphology, area of distribution, and pharmacological importance.
Keywords: Ethnomedicine, Morphology, Phytochemistry, Pharmacology, Walnut
Introduction
Plants are a valuable source of a wide range of secondary metabolites, which are used as pharmaceuticals, agrochemicals, flavors, fragrances, colors, and biopesticides. Since ancient times, walnut is used to treat various diseases and disorders. The walnut tree possesses a wide range of medicinal properties in almost every plant part like bark, kernel, fruit, leaves, green husk, and flower. There are present phytochemicals such as alkaloids, flavonoids, and polyphenolic compounds in these plant parts which exhibit medicinal values. Oil extracted from the fruits is used widely in Ayurveda and another medical system. Herbal medicine is getting more popularized in exponential growth in every part of the world because of its natural origin, high nutrient values, and almost negligible side effects as compared to modern medicines. Hence, herbal medicine is popular all over the world (Girzu et al. 1998). There are roughly 20 species of Juglans in the genus Juglans, all of which produce edible nuts. The English or Persian walnut is the most commonly utilized species among these species (McGranahan and Hand Leslie 1990). The Western Himalayan region of India provides suitable agroclimatic conditions for producing high-quality walnuts. The Union Territory of Jammu and Kashmir (JKUT) in India produces a major share of an export quality walnut and is the hub of the production of walnut in India (Hassan et al. 2013). Walnut is produced in two divisions of JKUT. It is cultivated in all districts of Kashmir divisions, and Ramban, Kisthwar, Doda, Rajouri, and Poonch districts of the Jammu division. Walnut trade is maximum Jammu & Kashmir and is considered as backbone pillar of the economy of the UT as the fruit is exported to different countries because it has a high quality of fruit and this is the major earning source for the local peoples living in the remote areas of J&K. The peoples living in the Union Territory has a diversity of culture like Brahmins, Rajput’s, Gujjars, Tribes living in the remote areas have present immense traditional information that was gained orally from their grand forefathers. This vital Ethnomedicinal Knowledge about the uses of different medicinal plant species growing in the wild is a boon in the treatment of different diseases and disorders. It contains noteworthy ethnomedicinal properties and huge economic potential, nutritional and medicinal values (Khan et al. 2013). The plant species has therapeutic, economic, and nutritional qualities. Walnuts are on the FAO's priority plant list because of their nutritional content (Hassan et al. 2013). Almost everywhere in the world, walnuts are commercially cultivated. It contains a high amount of protein and essential fatty acids, making it known as a high-density nutrient nut. Walnut production has increased rapidly in recent years, especially in Asian countries, which value these products for their nutritional benefits. As far as nutritional aspects are concerned, due to its valuable kernels, it is highly caloric with 654 kcal per 100 g and with high number of proteins content (15.2 g), lipids (65.2 g), carbohydrates (13.7 g), and micronutrients (FAOSTAT 2020). Walnuts are well known as one of the most vital nuts globally. A prominent component of the Mediterranean diet due to its highly nutritious nature, it is highly beneficial in nature. On the basis of the recent studies, walnut has gained huge attention for their increasing health-promoting properties that is maximum contributed by abundance of vital bioactive compounds such as plant sterols, dietary fiber and polyphenols. Many pharmacological functions can be attributed to walnuts including antioxidation, antibacterial, antiviral, anticancer, anti-inflammatory, improvement of cognitive function, and reduction of immunotoxic effects (Zhang et al. 2021). In traditional medicine, walnuts were used to treat cancer, inflammation, diabetes, antiradical, antidiarrheic, prostate and cardiovascular disorders. There has been much research that suggests that the entire plant parts are effective against a wide range of health disorders. The extracts from nuts inhibited oxidative damages, inflammation, tumour growth, antiwrinkle, and photoaging. Kernels as a dietary food, against diabetes, hypoxia, some skin diseases, and inflammation, leaves as antidiarrheals, anthelmintic, depurative and also mixed with long term stored-grains as an effective insecticide and fungicide. Some studies also reported that stem bark as an astringent, anthelmintic, depurative, bactericide, diuretic, digestive, laxative, stimulant, detergent, and insecticidal. Walnut dried shells are reported for polishing gun-casings, jewellery, and metal material and is also used as media to separate water and crude oil (Muzaffer and Paul 2018; Vieria et al. 2019a, b). Walnut is a good source of flavonoids, Polyphenols, flavanols, carbohydrates, fatty acids, cardiac glycosides, steroids, minerals, tannins, protein, dietary fiber, melatonin, plant sterols, α-tocopherol, folate, tannins, vitamin A, vitamin C, and vitamin E family compound. Several studies proved the antimicrobial activity of phenolic extracts making them as best substitute to antibiotics and food preservatives. Food industry professionals view this natural product as of high economic importance. The product is popular and widely consumed as royal food worldwide, and it is valued for its nutritional, health, and sensory qualities. There is an extended interest in using natural antimicrobial compounds, due to the increasing resistance to large spectrum of antibiotics (Rusu et al. 2020; Wang et al. 2020). The dried bark is tough and has been used as brush for mechanical tooth cleaning purposes due to its tough fibrous texture. It contains Juglone as its main and most important constituent and it works as an anti-viral, anti-parasitic, anti-fungal, anti-bacterial, anti-inflammatory and anti-cancerous agent. In dentistry, it is considered as an effective anti-plaque, anti-fungal, anti-bacterial, anti-cariogenic and tooth whitening material. In recent years, scientists and researchers have shown an increasing interest in studying the chemicals and compounds in bark, and using it in dental products to improve dental care (Khattak et al. 2022). Walnut fruit green husk extract obtained was reported as suitable natural hair dyeing agent and showed maximum antimicrobial activity compared with modern semi-synthetic and commercial hair dyes. It can be used as an economical, valuable, eco-friendly and safe source of dyeing and antimicrobial agents for modern cosmetic products (Bieki et al. 2018). Therefore, this review had first time reported the traditional uses of walnut and its different plant part like kernel, shell, husk, leaves, bark and root found in the different regions of J&K UT. Functional foods, their expansion in the pharmaceutical industry, are essential for the beneficial effects of walnuts on the human body. This will expand the knowledge regarding the phytochemical profile and to discover some new pharmacological importance in order to make the best use of it as a source of valuable bioactive compounds for food and pharmaceutical industries (Ni et al. 2021). Therefore, the present review reports an updated knowledge on the ethnobotanical importance, phytochemical composition and their pharmacological uses that has a great significance for the welfare of mankind.
Methodology
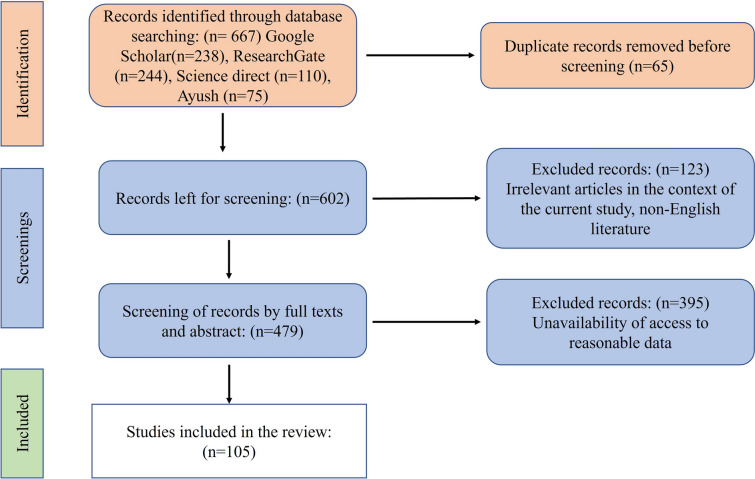
The purpose of this study was to describe the ethnomedicinal use, pharmacology, nutritional value, phytochemistry, macro-morphology, habitat, and area of distribution, and other uses of walnut produced in JKUT. Comprehensive literature was searched using relevant keywords in internet portals such as Google Scholar, PubMed, Science Direct, and the AYUSH site to obtain precise information. The PRISMA methodology used for systematic review has been illustrated in Fig. 1. Some relevant published articles have been included that were related to the keywords and subject. Not- relevant articles that have not complete information, not full text available, and not related to walnut were excluded out. An ethnomedicinal survey had been conducted in different walnut-producing areas which are major production areas of walnut. Some open-end discussions and semi-structured interviews among the local peoples and medicine men were carried out to document the traditional knowledge and various uses of walnut.
Fig. 1.
Systematic methodology adopted for literature screening
Results and discussion
First, 667 search results were identified using electronic databases and assessed based on the title and abstract, using the above-mentioned search strategy and selection criteria. We simply deleted unsuitable and identical articles after screening relevant data by title and abstract. 583 articles did not fulfill the inclusion requirements; thus, they were eliminated, leaving only 90 articles that met all of the criteria and contained the most significant information. The complete information on the various parameters like morphology, ethnomedicinal uses, phytochemistry, and pharmacology are studied. To obtain secret traditional knowledge from villagers and herbalists in accessible portions of the regions. Field surveys and semi-structured interviews were conducted. During the current study, an appropriate methodology was used. Typically, the survey in each locality began with interviews of aged and experienced members of the community, herbalists, and medicine men. In addition, residents of the examined areas who had used this plant species were interviewed. Local residents were also consulted for information on the economic significance of the plant species. All of the information acquired, including the part(s) utilized, method of preparation, manner of use, and dosage, was meticulously recorded (Table 1). The plant species have been documented as being used to treat different ailments. The information acquired from one locality was cross-checked with that of others to ensure accuracy. As a result, there is evidence that local people often share traditional information.
Table 1.
Nutritional content value of various constituents in walnut
| Principle compounds | Values per 100 g |
|---|---|
| Vitamins | |
| Vitamin A | 20 mg |
| Vitamin C | 1.3 mg |
| Vitamin E | 20.83 mg |
| Vitamin K | 207 mg |
| Folates | 98 mg |
| Niacin | 1.125 mg |
| Pantothenic acid | 0.570 mg |
| Pyridoxine | 0.537 mg |
| Riboflavin | 0.150 mg |
| Thiamin | 0.541 mg |
| Minerals | |
| Aluminum | 0.58 mg |
| Phosphorus | 346 mg |
| Calcium | 98 mg |
| Potassium | 441 mg |
| Magnesium | 158 mg |
| Sodium | 2 mg |
| Iron | 2.9 mg |
| Copper | 1.5 mg |
| Manganese | 3.8 mg |
| Zinc | 3.09 mg |
| Fatty acids | |
| Unsaturated fatty acid | |
| Palmitoleic acid C16:1 | 0.77 |
| Oleic acid C18:1 | 25.26 |
| Gadoleic acid C20:1 | 0.05 |
| Total MUFA | 22.37 |
| Linoleic acid C18:2 | 57.10 |
| Linolenic acid C18:3 | 10.34 |
| Total PUFA | 4.29 |
| Saturated fatty acid | |
| Myristic acid C14:0 | 0.24 |
| Palmitic acid C16:0 | 4.28 |
| Stearic acid C18:0 | 1.85 |
| Arachidic acid C20:0 | 0.19 |
| Total SFA | 7.21 |
| PUFA/SFA | 9.91 |
Vernacular names
“The Plant List includes 110 scientific plant names of species rank for the genus Juglans. Of these 21 are accepted species names (http://www.theplantlist.org/1.1/browse/A/Juglandaceae/Juglans/)”. It is recognized by diverse names all around the world and it is called Persian walnut in English, Akhrot in Hindi language, Doon in local Kashmiri, and Gardhghani in Unani language (Verma and Sharma 2020).
Area and production
Walnut is one of India's most important dried fruits, with exports to more than 40 countries and annual revenues of more than INR 300 crores in capital inflows. Jammu and Kashmir, Himachal Pradesh, Uttarakhand, and Arunachal Pradesh are the primary producers. Jammu & Kashmir, on the other hand, is the country's top walnut producer, accounting for more than 88 percent of total production. Pulwama, Anantnag, Kupwara, Budgam, Baramulla, and Srinagar are the major walnut-growing areas in Kashmir. Ramban and Doda districts in Jammu have the most walnut farming, followed by Poonch and Udhampur, with lesser amounts cultivated in Rajouri and Kathua districts. Kullu, Mandi, Shimla, Kinnaur, Sirmour, and Chamba are major walnut-growing districts in Himachal Pradesh, whereas Nanital, Dehradun, Pauri, Tehri, Chamoli, and Pithoragarh are major walnut-growing districts in Uttarakhand. Superior quality walnuts are those that grow at altitudes of 1500 m or have a light-colored kernel and a thin shell. Because of the high temperatures at the time of ripening at lower elevations, the kernel frequently becomes brown (Ahmed et al. 2018).
Taxonomy and systematics
Walnuts belong to the Juglandaceae family, with the genus Juglans L. including roughly 60 species, 21 of which are classified as Juglans. All nuts are edible, but none are as huge and easy to crack as the Persian or English walnut, Juglans regia L. Juglans species have a somatic chromosomal number (2n) of 32. Based mostly on morphology, the genus is divided into four divisions (Manning 1978; Ahmed et al. 2018).
Taxonomical Classification of Juglans regia (Faramarz Zakavi et al. 2013).
| Domain | Eukaryota |
| Kingdom | Plantae |
| Division | Magnoliophyta |
| Subdivision | Angiospermae |
| Class | Magnoliopsida |
| Family | Juglandaceae |
| Genus | Juglans |
| Species | regia |
Section a: Juglans
Juglans regia is the only species. There are two sub species Turcominica and fallax commonly known as English or Persian walnut and range from south-eastern Europe, Iran to the Himalayas, and China.
Section b: Rhysocaryon (Black walnut)
It has sixteen species viz. Juglans australis, Juglans boliviana, Juglans californica, Juglans hindsii, Juglans hirsute, Juglans jamaicensis, Juglans microcarpa, Juglans major, Juglans mollis, Juglans neotrapa, Juglans nigra, Juglans pyriformis, Juglans olanchana, Juglanssortensis, Juglans steyermarkii, Juglans venezuelensis. Black walnut is well-known for its good quality wood and is the most important walnut (Ahmed 2018). It is a wild tree found mainly in south-central and south- eastern United States. Nuts are mostly hard and difficult to crack. Shells of this variety has been mostly utilized in sand slats cleaners and metal polishers. It has indehiscent, thick, adherent husks, four-celled nuts, and thick ridged shells. Besides this other significant black walnut species are Juglans California (southern California black walnut, Juglans hindsii (northern California black walnut) Juglans major (Arizona black walnut) Juglans microcarpa (Texas black walnut) but among these Juglans hindsii is significant (root for Persian walnut).
Section c: Cardiocaryon
Juglans ailantifoliavarcardiformis, Heartnut is the most popular species in the section found in Japan. They are easily cracked nuts. J. ailantifolia (Japanese walnut) J. Cathayensis (Chinese walnut) and J. mandsinuria (Manchurian walnut) are other important species. In this particular section, nuts are twin celled with almost four to eight ridges, indehiscent, and are present on long clusters of nuts.
Section d: Trachycaryon
Juglans cineria L. Butternut is the only species in the section. In New England, these are used to make maple butternut candies, they have very thick shells with almost eight ridges, indehiscent husks with two-celled nuts, and four ribs along with small fennels that tend to easily shatter when cracked (Shah et al. 2014; Ahmed et al. 2018).
Ecological aspects
Altitude
In temperate climates, it grows between 850 and 3600 msl. However, commercial walnut production beyond 2500 m is not recommended because it has resulted in unpredictability and even entire crop loss in some years when there is late spring frost (Ahmed et al. 2018).
Temperature
It requires a chilly autumn time to induce leaf fall as well as the physiological processes of plant hardening and dormancy induction. During deep hibernation, the plant can withstand temperatures as low as − 110 °C without suffering major harm, but late spring frosts can be fatal. As soon as growth commences after dormancy, the temperature even two or three degrees below freezing point totally damages aerial parts leaves, shoots, and flowers and thus leading to crop failure. The tree thrives in cool locations with little or no frost in the spring, but it does not thrive in hotter temperatures. It is not suggested in places where late spring or early fall frosts are widespread, because cold temperatures damage the growth point of walnut trees, reducing yield. Sunburning of hulls and withering of kernels caused by high temperatures of 380 °C resulted in empty nuts in some cases. If the humidity is low and the temperature approaches 400 °C, severe damage is exacerbated (Sharma et al. 2020). As a result, walnut production is limited by a cool or short growing season. In order to generate optimal vegetative development and flowering, walnut requires a particular amount of chilling time in the winter to break dormancy. The bud opening and flowering are irregular and delayed in the lack of adequate cooling, leading to poor and dieback of shoots (Ahmed et al. 2018; Sharma et al. 2020).
Rainfall
It necessitates 760–800 mm of rainfall or equivalent irrigation. Avoid planting walnut in drought-prone locations if you want a good yield. Spring rains are linked to an upsurge in walnut blight concerns (Ahmed et al. 2018).
Wind tolerance
If the plant is exposed to stronger breezes, it will grow and crop considerably more slowly. It's also prone to breaking its limbs. As a result, shelter is critical for healthy growth throughout the establishment years.
Soil nature
Walnut trees prefer somewhat acidic soil with a pH of 5.4–6.8. It is vulnerable to zinc and boron deficiency; thus, enough zinc levels and a slightly acidic pH are necessary for the soil. The walnut thrives on deep, friable loamy soil, silt loam, or clay loam that has been treated with lime and is high in humus, with roots that can reach depths of 3–5 m. In addition to having adequate topsoil, the subsoil should be devoid of impermeable layers such as clay and rock gravel, as well as anaerobic conditions (clayey soil coupled with high rainfall). Walnuts cannot survive on moist soils for long periods of time. Water logging can cause serious harm in just a few hours. Slopes that are gentler are easier to manage. Because walnut plantations in Kashmir are likely to be on steep slopes, there are two key considerations:
Select a location that has southerly and southwest-facing slopes. In the spring, it warms up more quickly and cools down more slowly in the autumn, thus extending the growing season to a higher altitude. North and east aspects tend to be colder and more prone to frost damage at higher altitudes but may allow walnut production at lower altitudes.
Select a location with sufficient frost drainage. The free flow of cool air down the slope is essential and important, particularly in spring where a build-up of cold air in the pocket behind a line of trees or in a fold in the ground can cause crop loss due to late frosts (Ahmed et al. 2018; Sharma et al. 2020).
Macro-morphology
Walnut is a huge, deciduous tree that grows to a height of 27–40 m and has a trunk diameter of up to 2.5 m. It normally has a short base trunk and a wide upper crown, but in dense forest competition, it grows higher and narrower. As a light-demanding or sun-loving species, walnut trees thrive in direct sunlight. When the tree is young, the bark is smooth and olive-brown, but as it becomes older, the bark becomes silvery-grey with a coarser texture and some dispersed large fissures. The leaves are 20–40 cm long, odd-pinnate with 5–9 leaflets, and alternately paired with a single terminal leaflet. Male flowers are present as drooping catkins 4–10 cm long, while female flowers are found at the terminal, often in groups of 2 to 5, which form a green fruit with a fleshy outer husk and a brown corrugated nut in the autumn season. In the autumn, the entire fruit, including the husk, falls; the seed is enormous, with a thin shell, and delicious, with a rich flavor (AbuTaha and Wadaan 2011; Sharma et al. 2011). The morphological illustrations are shown in (Fig. 2). Almost all parts of the walnut are well illustrated and clearly shown.
Fig. 2.
a Mature harvested fruits, b mature unharvested fruits, c, d immature fruits, e–g drooping of male catkins, h–i, k macro-morphology of walnut tree during different seasons. j root and stem of walnut seedling
Walnut composition and nutritional value
Since ancient times walnut has been used in human nutrition. It is being included in the priority list of plants in the world due to the rich phytochemistry that makes it a source of rich nutrients. The kernels have high protein and oil content which make this fruit essential for human nutrition. Walnut is rich in nutrients due to the presence of a high number of proteins, vitamins, fats, and minerals (Table 1). Besides these, they are also an efficient source of polyphenols, phenolic acids, and flavonoids (Gandev 2007; AbuTaha and Wadaan 2011). The nutrient values and content of phytochemicals are influenced by genotypes, cultivar, ecology, and nature of the soil. Fats are found to be present in higher amounts about 68% and proteins are also present at about 16% in the kernels of walnut fruits (Martinez et al. 2010; Muradoglu et al. 2010; Gupta et al. 2019). Walnuts have long played a significant part in the market for functional foods because of their well-known nutritional and therapeutic advantages. According to Ni et al. (2021) walnuts' lipid profile has attracted a lot of scientific attention since it combines a wide range of biological advantages and functions for achieving health. Over the years, researchers have emphasized the physiological importance of the various nutrients (polyphenols and vitamins) found in the walnut's flower, pellicle, and kernel. As a result, various studies have been conducted on the potential protective role of walnut consumption against a variety of diseases, including cancer, gut dysbiosis, cardiovascular, and neurological diseases. The ameliorative effects of a walnut-enriched diet in chronic illnesses can be linked to the synergistic or individual actions of walnut components, notably through anti-oxidative and anti-inflammatory activities.
Ethnomedicinal uses
An extensive survey was carried out in the major walnut-producing areas of JKUT. The traditional ethnomedicinal knowledge was documented by taking interviews and open-end discussions about the uses of walnut as a medicinal plant. The local peoples include inhabitants, herbalists, medicinal healers, tribes, Gujjars, and Bakerwals and were from 18 to 80 years of age range. The interview revealed that Tribes, Gujjars, and Bakerwals use the plant more frequently than local inhabitants. This means that there is improper sharing of ethnomedicinal knowledge. Among all, they cited the use of walnut maximum, and hence, walnut possesses high Use-Value (UV-index) and most preferred medicinal plant. After collecting all the information, it has been revealed that walnut fruit is used maximum and treats almost 10 different types of diseases followed by leaves that are used to treat 5 types of diseases. Almost all plant parts are utilized in one way or the other way but the fruits (nuts) have been put to maximum use each plant part is used raw or in combinations to treat many diseases. The various traditional uses of walnut are documented (Mouhajir et al. 2001; Vaidyaratnam 2005; Ibrar and Sultan 2007). Walnut plants are used for scrofula, eczema, dermal inflammation, and excessive hand and feet perspiration. The leaves are used for sunburns, itching and to treat scalp dandruff. Leaves are also used on the body or forehead to reduce fever and also applied on swollen joints to cure rheumatic pain. The branches and bark are used for toothache and also used as a brush on daily basis for cleaning teeth. Fruit peel is used to treat ringworm infestations in the feet. However, results had been also compiled from published literature and revealed immense ethnomedicinal uses of walnut (Ahmed et al. 2018). The medicinal uses of walnut plant parts and mode of applications are tabulated in Table 2.
Table 2.
Ethnomedicinal uses, mode of preparation, and prescription of walnut
| S. no. | Part used | Ethnomedicinal uses | Methods of preparation | Prescription |
|---|---|---|---|---|
| 1 | Leaves | Mosquito repellant | Leaves are directly used | The leaves are kept fresh within the homes |
| Itching and acne | 600 g fresh and tender leaves in 5 L of water are boiled for 1 h | This water washes the damaged body parts | ||
| Lice killer | Fresh leaves are boiled in 5 L of water for 1 h | The hairs are washed with water | ||
| 10 g of fresh leaves are boiled in 1 L of water for 1and half hour | 4 teaspoons full of mixture is given twice a day | |||
| Frost bite | 200 g of fresh leaves are boiled in 2 L of water and then completely Shaked | The combination was used to wash the damaged regions of the body | ||
| Against rheumatic pain | Thick paste of freshly crushed leaves is used | Paste is applied on swollen joints | ||
| 2 | Kernel | Brain tonic | 5 g of fruit kernels are boiled in half liters of milk for 10 min and little honey or sugar is also added | Early in the morning, the mixture is given orally |
| Aphrodisiac | Kernels of fruit 30 g and poppy seeds 5 g are boiled with salt tea 500 mL for 10–20 min | The tea is taken twice a day | ||
| Constipation | 5 g of fruit kernel are boiled in 300 mL milk for 10 min and some sugar is added to the mixture | The tea is consumed twice daily | ||
| Dandruffs | Oil is extracted from kernels | The oil can be applied twice a day to the hairs | ||
|
Rheumatism Muscular pain |
Oil of kernel is little warmed Oil extracted from kernels |
The warm oil is applied twice a day The oil is rubbed into sore limbs in particular |
||
| Improve eye sight | 5–10 g of fruit kernels are boiled in 300 mL of fresh milk | At bedtime, the combination is administered orally | ||
| Against cold | 5 g of fruit kernels is added mixed to cinnamon and liquorice 5 g each. The mixture is completely boiled in water and prepared in the form of tea | Tea is consumed orally two to three times each day | ||
| Memory booster | The fruit kernel 5 g is boiled in mixture of milk, cream and sugar | The combination is consumed first thing in the morning | ||
| Anti-diabetic | Fruits kernels (40 g) and few poppy seeds (5 g) are boiled with salt and tea (half liters) for 10–20 min | The tea is consumed twice daily | ||
| 3 | Roots | Hair fall | The tender roots are kept in bottles containing sufficient mustard oil under soil for 2–3 months | Once a day, the oil is massaged into the hair |
| 4 | Root bark, leaves and twigs | Tooth ache and tooth decay | Fresh roots, bark twigs are cut into tiny pieces and dried | Early in the morning, the little bits are chewed and used as a brush |
| 5 | Root bark, leaves | Antiseptic | The root, bark and leaves are grounded rigorously and transform into thick paste | Skin wounds are treated with a thick paste that acts as an antibacterial |
| 6 | Fruit cover (raw) | To heal the wounds | The fruit epicarp is completely crushed and some quantity mustard oil has been added in it | The paste combination that was placed to the wounds on the skin |
Kernel
Walnut kernels, the edible component, make up around half of the overall weight of the fruit. Walnut kernels are heavy in protein, lipids, and minerals, as well as being an excellent source of energy. It is the richest source of vitamin B-6 and has a considerable amount of B group vitamins. These are used as raw as a good tonic for the brain. It is good for enhancing memory and longevity. It is cardioprotective and prevents bone loss.
Leaves
The walnut green leaves are used for skin disorders, eye irritations, eye pain, and conjunctivitis, and are also used in enhancing low appetite. An infusion is made from leaves that are used in eye washing to get rid of irritations and to treat conjunctivitis. Also, the same infusion made from leaves is used on wounds, acne, and skin allergy to heal wounds and get rid of skin diseases.
Shell
Its powder is one of the main ingredients used in beauty products to treat suntan and sunburn on the skin.
Inner bark
Both a decoction and a tincture can be made from the walnut's inner bark. The decoction can be used to treat constipation, and poor digestion, as a liver stimulant, and even to treat skin conditions.
Phytoconstituents and their pharmacological activities
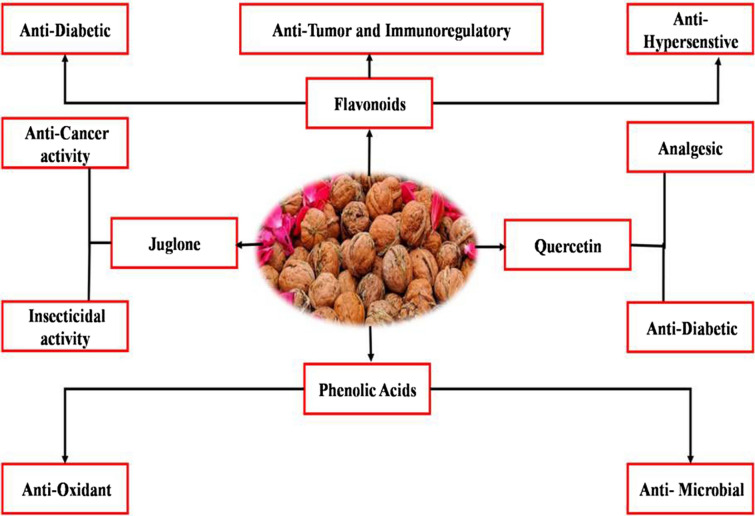
The chemical composition of phytoconstituents differs depending on geographical location, meteorological conditions, and soil type. Because of the presence of several bioactive chemicals, walnut leaves, bark, and fruits have been found to have medicinal activity in studies conducted all over the world (Kong et al. 2008; Shimoda et al. 2009; Parivash et al. 2011; Omwenga et al. 2015; Panth et al. 2016). Walnut contains numerous potential neuroprotective components such as gamma-tocopherol (vitamin E) and long-chain omega-3 fatty acids and is a strong supply of vital fatty acids and tocopherols. Furthermore, the previous study had proven its involvement in the treatment of dementia and Alzheimer's disease (Muthaiyah et al. 2014; Subhan and Bagchi 2017). Walnut is a rich source of phenolic acids and tannins. The phenolic composition of walnut leaves has already been researched by several experts, and its pharmacological effect has already been determined (Martinez et al. 2010). A complete summarized information of the active constituents of walnut is represented and illustrated in (Table 3 and Fig. 3). The oil obtained from walnut contains majorly triacylglycerols which comprise polyunsaturated fatty acids (PUFAs) and monounsaturated fatty acids (MUFAs). The oil of walnut also contains linoleic acid and oleic acids. Saturated fatty acids present are Stearic acid and palmitic acid. Some other compounds are Magnesium, Calcium, Potassium, Phosphorous, Manganese, and Sodium are present in kernels (Thakur et al. 2011; Muthaiyah et al. 2014; Raisi et al. 2015; Subhan and Bagchi 2017; Ragab and Zhongli 2019). The various phytopharmacological uses are reported from time to time by different researchers (Mori 2014; Bamberger et al. 2017; Zehr and Walker 2018; Vieira et al. 2019a, b). It has been reported that the due to the presence of bioactive molecules in different plant parts is responsible for the medicinal and pharmacological properties of walnut (Fig. 3 and Table 3). By using the important plant part of Juglans regia like walnut flower and walnut pellicle 50 substances were extracted and identified. These 50 phenols compounds were characterized as phenolic acids, flavonoids, tannins, and some coumarin derivatives. There were clear variances between the phenolic compounds found in different walnut parts. Among these 29 compounds were found in walnut flower, while 32 compounds were found in walnut pellicle. Ellagic acid was found to be the phenol in walnuts with the highest concentration. The greater phenol concentration in walnut pellicle extractions may have contributed to their superior antioxidant efficacy (Zhang et al. 2020).
Table 3.
Phytoconstituents of Walnut with its pharmacological activity
| Plant parts | Phytochemical compound | Pharmacological activity | References |
|---|---|---|---|
| Leaves |
Phenolic acids, tannins, essential fatty acids, ascorbic acid, flavonoids, caffeic acid, para-coumaric acid, juglone; Alkaloid; Saponin Flavonoids-Quercetin, galactoside, arabinoside, xyloside and rhamnoside; Naphthoquinones |
Anti-oxidant activity, lipid-lowering effect, anti-hypertensive effect, anti-microbial effects, gastro-protective activity, hypercholesteremic activity Anti-diabetic effect, anti-cancer effect, hepato-protective activity, Anti-ageing activity |
Rahimipanah et al. (2010), Zhang et al. (2009), Fukuda et al. (2004), Verma and Sharma (2020), Emira et al. (2011), Mohammed et al. (2018), Fang et al. (2015) |
| Green husk of fruits | Tannins, Glucose, citric acid, malic acid, phosphate, calcium oxalate, Juglone, polyphenols | Liver and kidney protective | Hiroshi et al. (2008), Parivash et al. (2011) |
| Fruits | Fatty acids, tocopherols, phytosterols, total phenolic-tannins | Anti-microbial effects, anti-oxidant activity, thyroid hormone enhancing activity | Deshpande et al. (2011), Poyrazolu and Biyik (2010) |
| Seeds | Glutelin, globulins, albumin and prolamins | Wound healing, Anticancer | Ruijun (2015), Rosaria et al. (2019), Akram et al. (2013) |
| Walnut oil | Poly unsaturated fatty acids, monoacylglycerol, diacylglycerol, triacylglycerol, oleic and linoleic acid | Antinociceptive and anti-inflammatory activity | Ksenija et al. (2019), Verma et al. (2013), Verma and Sharma (2020) |
| Bark | Polyphenols | Antimicrobial activity, antimycobacterial activity, antioxidant activity, antifungal activity, platelet aggregation, bleeding time, plasma coagulation | Jamshid et al. (2011), Hubert and Grzegorg (2015), Tuqa et al. (2017), Verma and Sharma (2020) |
| Stem | Juglone, sitosterol, ascorbic acid, quercetin-3-larabinoside, phenols, flavonoids, naphthoquinones | Antifungal activity | Emira et al. (2011), Mohammed et al. (2018), Verma and Sharma (2020) |
| Flower | Gallic acid, coumarin, quercetin, polyphenols, flavonoids, sterols, fat, protein, vitamin, minerals | Antidepressant, antihypoxic, anti-inflammatory | Raheleh et al. (2016), Verma and Sharma (2020) |
Fig. 3.
Dominant phytoconstituents and their pharmacological properties of walnut
Antibacterial activity
Many researchers reported the antibacterial activity of walnut extracts by using agar streak and disc diffusion methods. The aqueous extract of leaves, bark, fruits, and fruit green husk obtained in hot and cold solvent resulted in antibacterial activity when tested against the gram+ and gram− strains of bacterial cultures (Poyrazolu and Biyik 2010; Deshpande et al. 2011). The aqueous extract obtained from the bark, green husk, and leaves shows negligible antimycobacterial activity. This antimycobacterial on the other hand exhibited by extract obtained from hexane and methanol using a Soxhlet extractor (Cruz-Vega et al. 2008). The chloroform and aqueous extract of walnut show microbicidal activity on air microorganisms also the leaves extract is very much beneficial in treating acne on the skin against Propioni bacterium acne which causes acne on the skin and other acne-causing bacteria (Qadan et al. 2005). The use of natural antimicrobial compounds in food preservatives is preferred over chemical preservatives to avoid their side effects in food and hence increase antibiotic resistance. So, various walnut plant parts extract and juglone is used against microbial infections and these extracts are used in pharmaceutics (Khattak et al. 2022).
Antifungal activity
On performing column chromatography various portions are separated from leaves extract and obtained bioactive compounds juglone and eugenol that are attributed to the antifungal properties of various fungal pathogens. Walnut leaves were estimated for antifungal activity by making four different extracts namely methanolic extract, alkaloid extract, ethyl acetate extract, and hydrolyzed methanolic extract against Candida albicans pathogenic isolates. From all the extracts, it was found that methanolic extract were showing maximum antifungal activity, alkaloid shows a slightly little low and ethyl acetate and hydrolyzable methanolic extract showed the minimum lowest antifungal activity (Oliveira et al. 2008). Methanolic extract, chloroform extract, acetone extract, and ethyl acetate extract from walnut bark showed antifungal properties against all Candida species, fusarium solani, Alternaria alternata, and Aspergillius niger fungal infections (Fang et al. 2015; Mohammed et al. 2018).
Antiviral activity
Antiviral activity is estimated by using 95% ethanolic and ethyl acetate extract of leaves that showed inhibition against tobacco mosaic virus while Sindbis virus is found to be inhibited by methanolic extract (Vardhini et al. 2007). It is found that antiviral compounds are present in leaf extract and also Juglone also showed antiviral properties because of proteins-ligand binding affinity against viruses in virtual docker In Silico method (Akram et al. 2013; Rosaria et al. 2019).
Antioxidant activity
All the extracts ethyl acetate, butanol, methanol, ether, and alcohol extract made from walnut different plant parts like kernels, leaves and husks showed antioxidant capacity by reducing power, lipid oxidation, and scavenging on free radical’s methods (Carvalho et al. 2010; Abbasi et al. 2010; Qamar and Sultana 2011). It shows potent antioxidant activity against DPPH, H2O2, and superoxide anion radicles (Zhang et al. 2009; Rahimipanah et al. 2010; Vu et al. 2018). Due to its abundance of phytochemicals, fat-soluble bioactive, and nutritional and non-nutrient antioxidants, walnuts are recognized as heart-healthy snack foods and dietary supplements. Walnuts are a fantastic option for meeting basic dietary requirements and also benefit human hosts physiologically. Their skin, known as the pellicles, has a high phytochemical content and can function as an antioxidant. The constituents of walnuts have additive or synergistic effects on lowering oxidative stress and inflammation, two significant factors in the development of many diseases. Additionally, studies have shown that walnuts' anti-oxidative qualities are attained via reducing free radical levels and boosting antioxidant defense. Consuming walnuts over time may enhance cognitive function and reduce the risk of contracting other diseases like dementia, depression, and cardiovascular disease and type 2 diabetes. Various studies have already shown that consuming walnuts can be a nutritious supplement to a dietary that is helpful in preventing metabolic issues. The information acquired about the chemical composition of walnuts leads to the industrial usage of a number of chemical compounds that are found there and can be exploited as a source of distinctive natural antioxidants for developing novel anticancer drugs (Ni et al. 2021). The phenolics present in walnut contributed to outstanding antioxidant effect, with the highest effect observed in walnut pellicle. The potential to bind Fe2+ shields the tissues and cells from oxidative stress and the easy passage of hydroxyl radicals through cell membranes. Therefore, the ability to chelate ferrous ions and quench hydroxyl radicals is thought to be a key determinant of antioxidant activity (Zhang et al. 2020).
Antidiabetic activity
Walnut possesses a rich content of polyphenols that showed a strong inhibition against different enzymes like amylase, maltase, sucrose, and glycosidase. The polyphenolic compounds Tellimagradin I, Tellimagradin II, and Casuarictin showed antidiabetic activity and have a lowering effect on triglycerides and urine peroxidase in Type II genetically inherited diabetes mellitus (Fukuda et al. 2004; Jelodar et al. 2007; Jafari et al. 2013; Anwar et al. 2020).
Antihelmenthic activity
Acetone extract from walnut bark possesses significant activity against Eicinia feotida. Also, ethanol, methanol, and benzene extract of bark showed significant antihelminthic activity against Pheretima posthumana (Upadhyay et al. 2010a) and concluded that stem bark has a maximum antihelminthic activity.
Hepatoprotective activity
Some researchers reported that walnut polyphenols from kernel pellicle orally given to liver damage in mice model induced by CCL4. This result showed that polyphenols are a higher hepatoprotective agent than commonly used famous curcumin. It was found that polyphenolic constituents were the main principal phytoconstituents present in different parts of the walnut and are responsible for oxidative damage to hepatoprotective activity. Tellimagrandins I one of the polyphenolics was found as the vital constituents of hepatoprotective agent (Hiroshi et al. 2008; Christopoulos and Tsantili 2011; Choudhary et al. 2020).
Anticancer activity
Anticancer properties had been reported Juglone as a promising chemopreventive agent against many cancerous cells. Researchers had done an invitro study revealed juglone as a potent cytotoxic agent against human tumor cell lines. Juglone induced apoptosis and inhibited the growth of sarcoma cells (Avanzato 2010; Ji et al. 2011; Bennacer and Cherif 2016; Al-Snafi 2018). Walnut leaf, bark, green husk, fine powder as petroleum, and methanolic extract shows significant anticancer properties by growth inhibition, antiproliferative efficiency, activation of mitochondrial death pathway, and generation of reactive oxygen species (ROS) mechanisms (Arya et al. 2020). The molecular basis of juglone-induced cell cycle arrest and apoptosis in human endometrial cancer cells was investigated. Juglone was extracted and characterized after the green husk had been purified. Juglone significantly decreased the proliferation of Ishikawa cells as shown by a S phase arrest brought on by the inactivation of the cyclin A protein. Following juglone exposure, the levels of ROS significantly increase, p21 mRNA and protein expression levels rise, and CDK2, CDC 25A, CHK1, and cyclin A expression levels decrease. The expression of Bcl-2 and Bcl-xL was significantly downregulated, whilst Bax, Bad, and cyto-c were elevated (Zhang et al. 2019). This later supported the idea that the mitochondrial pathway is involved in juglone-induced apoptosis. Juglone can be investigated further as a powerful natural anticancer drug by the in vitro studies. Ishikawa cells from endometrial cancer undergo ferroptosis when exposed to the novel ferroptosis activator juglone. The function of juglone, a compound derived from green peel, in promoting autophagy and preventing the migration of endometrial cancer cells. The accumulation of Fe2+, lipid peroxidation, the depletion of GSH, the upregulation of HMOX1, and the conversion of heme to Fe2+ were verified the effectiveness. The new hallmarks of cancer treatment, including the induction of autophagy, inhibition of cell migration, and endoplasmic reticulum stress, were attained by juglone. By inducing programmed cell death in endometrial cancer cells through the activation of oxidative stress, juglone, a functional food ingredient, suggests a unique therapeutic strategy for the treatment and prevention of endometrial cancer. Juglone, a naturally occurring quinone derived from the green peel, may exhibit its anti-cancer effect by triggering iron-dependent autophagy and preventing the migration of endometrial cancer cells (Zhang et al. 2021).
Memory booster activity
Walnut enhances memory and learning skills and acts as a memory booster was reported and concluded that it increases the serotonin level in the brain which results in the advancement in memory and learning capacity (Haider 2011; Asadi-Shekaari et al. 2013). One more study conducted showed the effect of polyphenol extracts from green husk and kernels magnificently increases the learning and memory function of the brain (Eidi 2013; Shi 2014).
Immunity booster activity
It has been reported that fruits of walnut increase and boost the immunity of the immune system by increasing phagocytosis against various macrophages and enhancing proliferation of lymphocytes (Ruijun 2015; David Hayes et al. 2015; Delaviz et al. 2017; Danh et al. 2020). Hence, due to its immune-boosting activity walnut can be one of the key medicinal plants used in Covid-19 viral infections also.
Other uses
According to findings reported by Ali et al (2020) huge quantities of shells are obtained as crop leftovers and wasted or burned as fuel in the walnut fruit processing industries. The fruit is made up of the kernel, skin, shell, and husk, which are its four basic components. The precious kernels of walnuts are primarily responsible for their significance. However, because of the advantages of the shells, they are currently receiving just as much interest as their kernels. Walnut shell has received a lot of attention in recent years as a naturally inert plant-based biosorbent. The discarded by-products of walnut fruit make up around 40 to 60% of the fruit's weight and are easily accessible in walnut production sites as a source of beneficial substances like phenolics. These left-over products, which are produced in large quantities when the walnut fruit is processed to remove its kernel, are typically discarded or burned as fuel. Due to their numerous beneficial features and valuable chemicals, recent studies on various walnut fruit portions have demonstrated that their waste products could be utilized efficiently. Another consideration is the strong potential for applications in the removal of various hazardous contaminants such as heavy metal ions, dyes, oils, and other compounds from aqueous solution by using low cost raw or chemically activated plant products, such as walnut shell powder (Ali et al. 2020).
A major portion of walnuts are produced in the North-Western states of the Himalayan region including Jammu and Kashmir, Himachal Pradesh, and Uttarakhand, among these Jammu and Kashmir producing the highest in terms of quantity and quality of nuts (Shah et al. 2021). Therefore, the demand for healthy and nutrition products is significantly increased at present time. Many studies reported walnuts from different production areas differ significantly in appearance, value, and nutritional composition. This may be due to genetic differences or variations in the growing environment (Bieki et al. 2018). People are increasingly interested in various health benefits related to nuts, which are becoming vital components of a healthy diet. It is a plant-based food material that is naturally packed with vital nutrients (Qamar et al. 2020). The consumption of common walnuts is a healthy choice due to the presence of high vitamin E content, flavonoids that are considered the major phenolic compounds and good source of proteins present in walnut kernels. Also, Linoleic acid, oleic acid, linolenic acid, stearic acid, and palmitic acid are the major fatty acids found in walnuts (Geng et al. 2021). Although all plant parts are valuable and has been investigated for different biological properties, till now no study regarding the phytochemical analysis, antioxidant and antimicrobial activity has been reported yet for the male inflorescence of walnut from Himalayan region (Muzaffer and Paul 2018). The intake of walnuts improved nutrient and food intake and partially replaced total energy, total dietary fiber, total protein, total carbohydrate, total fat, and total carbohydrate. Adding walnuts to a diet can change nutrient intake profiles in a way that may prevent chronic diseases (Iordanescu et al. 2021). Thus, nutrient and food displacement may be a mechanism to explain the favorable association between walnut intake and improved diet (Natto et al. 2022). Epidemiological and clinical reports have clearly shown that daily consumption of nuts can be beneficial for the betterment of several health disorders such as cardiovascular, and chronic diseases (de Souza et al. 2017), cancer (Berkey et al. 2020), diabetes (Anwar et al. 2020), obesity (Crovesy et al. 2021).
Conclusion and future prospective
This systematic review mainly focuses on documentations of the ethnomedicinal uses known by local peoples of hilly remote areas and also collected many research studies conducted in the past on the pharmacological properties of walnut and its various bioactive constituents. Walnut is one of the important medicinal plants having great therapeutic potential. The available data recommended extensive research conducted on the ethnopharmacological effects of walnut. The wide range of ethnopharmacological activities makes the walnut a promising medicinal plant with high effectiveness and safety. On conducting detailed study, it has been concluded that walnut has many medicinal properties that make it worth more valuable. In addition to offering basic nutritional functions, walnuts serve as an excellent choice for their physiological benefits on human hosts due to their plethora of phytochemicals, fat-soluble bioactive, nutrient and non-nutrient antioxidants, which make them a good choice as food additives and heart-healthy snacks. Taking into consideration the experimental confirmations contained in this review, we summarized the information about walnut polyphenols and the obvious role they play in disease prevention and treatment. In vitro studies were mainly used to describe walnut polyphenols' molecular functions until a few years ago. The growing amount of research on their bioavailability and in vivo efficacy has provided a way to correlate the exact mechanism of bioactive compounds with their biological responses. We have reviewed the reports which highlight the importance of including walnuts into a daily healthy diet. More methods are needed in future to improve walnut extraction in order to fully utilize each part of the walnut. However, further research is needed to evaluate the long-term efficacy of walnut consumption and resulting health improvements. Juglone is considered as good anti-cancer compound that in future research may be effective in most commonly occurring cancers. In spite of this, existing evidence supports the bioactive properties of walnut polyphenols, which should be explored more thoroughly in the future.
Acknowledgements
The authors thank Prof. Pardeep Kumar, Head, Department of Plant sciences, Central University of Himachal Pradesh for providing the necessary facilities. MS1 is thankful to Central University of Himachal Pradesh for Financial support in the form of fellowship.
Author contributions
MS1 suggested the idea and designed the study. MS2 collected the data and involved in draft preparation for the review. The final editing was done by MS3* and approved the final version.
Declarations
Conflict of interest
The author declares no conflict of interest. The manuscript has not been submitted for publication in other journal.
Ethical approval
Not applicable.
References
- Abbasi MA, Raza A, Riaz T, Shahzadi T, Rehman A, Jahangir M, Shahwar D, Siddiqui SZ, Chaudhary AR, Ahmad N. Investigation on the volatile constituents of Juglans regia and their in vitro antioxidant potential. Pakistan Acad. Sci. 2010;47:137–141. [Google Scholar]
- AbuTaha N, Wadaan M. Utility and importance of walnut, Juglans regia Linn: a review. Afr. J. Microbiol. Res. 2011;5(32):5796–5805. [Google Scholar]
- Ahmad, N., Singh, S., Bakshi, M., Mir, H.: Walnut. Fruit Production of India. 660–672 (2018)
- Akram E, Jalal ZM, PejmanM SR, Somayeh O. Hepatoprotective effects of Juglans regia extract against CCl4-induced oxidative damage in rats. Pharm. Biol. 2013;51(5):558–565. doi: 10.3109/13880209.2012.749920. [DOI] [PubMed] [Google Scholar]
- Al-Snafi AE Chemical constituents, nutritional, pharmacological and therapeutic importance of Juglans regia- a review. IOSR J. Pharm. 2018;8(11):1–21. [Google Scholar]
- Ali JE, Rana JE, Mahnaz T, Leila R, Ryszard A. Recent advances in the use of walnut (Juglans regia L.) shell as a valuable plant-based bio-sorbent for the removal of hazardous materials. RSC Adv. 2020 doi: 10.1039/C9RA10084A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar M, Birch EJ, Ding Y, Bekhit ED. Water-soluble non-starch polysaccharides of root and tuber crops: extraction, characteristics, properties, bioactivities, and applications. Crit. Rev. Food Sci. Nutr. 2020 doi: 10.1080/10408398.2020. [DOI] [PubMed] [Google Scholar]
- Arya AK, Arora M, Singh FMA. A review on pharmacological activity of Juglans regia. Int. J. Pharm. 2020;7(1):1–11. doi: 10.13040/IJPSR.0975-8232.IJP.7(1).1-11. [DOI] [Google Scholar]
- Asadi-Shekaari M, Karimi A, Shabani M, Sheibani V, Esmaeilpour K. Maternal feeding with walnuts (Juglans regia) improves learning and memory in their adult pups. Avicenna J. Phytomed. 2013;3(4):341–346. [PMC free article] [PubMed] [Google Scholar]
- Avanzato D. Traditional and modern uses of walnut. Acta Hortic. 2010;861:89–96. doi: 10.17660/ActaHortic.2010.861.11. [DOI] [Google Scholar]
- Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Stark RG, Altenhofer J, Henze K, Parhofer KGA. Walnut-enriched diet reduces lipids in healthy Caucasian subjects, independent of recommended macronutrient replacement and time point of consumption: a prospective, randomized, controlled trial. Nutr. J. 2017;9(10):1097. doi: 10.3390/nu9101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiki T, Najafpour GD, Hosseini M. Evaluation of antimicrobial and dyeing properties of walnut (Juglans regia L.) green husk extract for cosmetics. Color Technol. 2018;134:71–81. [Google Scholar]
- Bennacer A, Cherif HS. Contribution to the ethnobotanical, phytochemical, antimicrobial and antioxidant study of the leaves aqueous extract of the common walnut Juglans regia L. Int. J. Pharmacol. Phytochem. Ethnomed. 2016;7:41–52. [Google Scholar]
- Berkey CS, Tamimi RM, Willett WC, Rosner B, Hickey M, Toriola AT, Frazier AL, Colditz GA. Adolescent alcohol, nuts, and fiber: combined effects on benign breast disease risk in young women. NPJ Breast Cancer. 2020;6(1):61. doi: 10.1038/s41523-020-00206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jenimo C, Silva BM. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010;48:441–447. doi: 10.1016/j.fct.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Christopoulos MV, Tsantili E. Effects of temperature and packaging atmosphere on total antioxidants and colour of walnut (Juglans regia L.) kernels during storage. Sci. Hortic. 2011;131:49–57. [Google Scholar]
- Chudhary, Z., Khera, R.A., Hanif, M.A., Ayub, M.A., Hamrouni, L.: Walnut. In: Medicinal Plants of South Asia. Elesiver, pp. 671–684 (2020). 10.1016/B978-0-08-102659-5.00049-5.
- Clark AM, Jurgens TM, Hufford CD. Antimicrobial activity of juglone. Phytother. Res. 1990;4:11–14. [Google Scholar]
- Crews C, Hough P, Godward J, Brereton P, Lees M, Guiet S. Study of the main constituents of some authentic walnut oils. J. Agric. Food Chem. 2005;53:4853–4860. doi: 10.1021/jf0478354. [DOI] [PubMed] [Google Scholar]
- Crovesy L, Kaipperf VC, Santos L, Marcelly CO, Magno FCCM, Fialho E, Rosado EL. Profile of polyphenols intake by women with different class of obesity: Consumption of these compounds does not reflect healthy eating. Nutrition. 2021;82:111045. doi: 10.1016/j.nut.2020.111045. [DOI] [PubMed] [Google Scholar]
- Cruz-Vega DE, Verde-Star MJ, Salinas-Gonzalez N, Rosales-Hernandez B, Estrada-Garcia I, Mendez-Aragon P, Carranza-Rosales P, Gonzalez-Garza MT, Castro-Garza J. Antimycobacterial activity of Juglans regia, Juglans mollis, Caryaillinoensi, and Bocconiafrutescens. Phytother. Res. 2008;22:557–559. doi: 10.1002/ptr.2343. [DOI] [PubMed] [Google Scholar]
- Danh CV, Trang HD, Nguyenb TL. An overview of phytochemicals and potential health-promoting properties of black walnut. RSC Adv. 2020;10:33378. doi: 10.1039/d0ra05714b. [DOI] [Google Scholar]
- David H, Michael JA, Joe T, Christina D. Walnuts (Juglans regia) chemical composition and research in human health. Crit. Rev. Food Sci. Nutr. 2015 doi: 10.1080/10408398.2012.760516. [DOI] [PubMed] [Google Scholar]
- de Souza RGM, Schincaglia RM, Pimentel GD, Mota JF. Nuts and human health outcomes: a systematic review. Nutrition. 2017;9(12):1311. doi: 10.3390/nu9121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaviz H, Mohammadi J, Ghalamfarsa G, Mohammadi B, Farhadi N. A review study on phytochemistry and pharmacology applications of Juglans regia plant. Phcog. Rev. 2017;11:145–152. doi: 10.4103/phrev.phrev_10_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande RR, Kale AR, Ruikar AD, Panvalkar PS, Kulkarni AA, Deshpande NR, Salvekar JP. Antimicrobial activity of different extracts of Juglans Regia L. against oral microflora. Int. J. Pharm. Sci. 2011;3:200–201. [Google Scholar]
- Eidi A. Hepatoprotective effects of Juglans regia extract against CCl4-induced oxidative damage in rats. Pharm. Biol. 2013;51(5):558–565. doi: 10.3109/13880209.2012.749920. [DOI] [PubMed] [Google Scholar]
- Emira N, Mejdi S, Najla T, Hafedh H, Riadh K, Eulogio V, Amina B. Antibacterial, anticandidal and antioxidant activities of Salvadorar ersica and Juglans regia L. extracts. J. Med. Plant Res. 2011;5(17):4138–4146. [Google Scholar]
- Fang F, Yingxin Q, Ling Q, Qing F, Liangzhong Z, Shuang C, Qiang L, Duo Z, Liguo W. Juglone exerts antitumor effect in ovarian cancer cells. Iran J. Basic Med. Sci. 2015;18:544–548. [PMC free article] [PubMed] [Google Scholar]
- Faramarz Z, Leila GH, Arash D, Ahmad FS, Arsham D, Zahra LS. Antibacterial effect of Juglans regia bark against oral pathologic bacteria. Int. J. Dent. 2013;10:1–5. doi: 10.1155/2013/854765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Ito H, Yoshida T. Effect of the walnut polyphenol fraction on oxidative stress in type 2 diabetes mice. BioFactors. 2004;2:251–253. doi: 10.1002/biof.552210148. [DOI] [PubMed] [Google Scholar]
- Gajendra M, Sharique A. A recent update on the medicinal properties and use of aloe vera in the treatment of various ailments. Biosci. Biotech. Res. Commun. 2016;9(2):273–288. [Google Scholar]
- Gandev S. Budding and grafting of the walnut (Juglans regia L.) and their effectiveness in Bulgaria (review) Bulgar. J. Agri. Sci. 2007;13:683–689. [Google Scholar]
- Geng S, Ning D, Ma T, Chen H, Zhang Y, Sun X. Comprehensive analysis of the components of walnut kernel (Juglans regia L.) in China. J. Food Qual. 2021 doi: 10.1155/2021/9302181. [DOI] [Google Scholar]
- Girzu M, Carnat A, Privat AM, Fialip J, Carnat AP, Lamaison JL. Sedative effect of walnut leaf extract and Juglone, an isolated constituent. Pharm. Biol. 1998 doi: 10.1076/phbi.36.4.280.4580. [DOI] [Google Scholar]
- Gupta A, Behl T, Panichayupakaranan P. A review of phytochemistry and pharmacology profile of Juglans regia. Obes. Med. 2019;16:1–7. doi: 10.1016/j.obmed.2019.100142. [DOI] [Google Scholar]
- Haider S. Effects of walnuts (Juglans regia) on learning and memory functions. Plant Foods Hum. Nutr. 2011;66(4):335–340. doi: 10.1007/s11130-011-0260-2. [DOI] [PubMed] [Google Scholar]
- Hassan GA, Bilal AT, Ahmad TA, Wani S, Irshad N. Economic and ethno-medicinal uses of Juglans regia L. in Kashmir Himalaya. UJAHM. 2013;1(3):64–67. [Google Scholar]
- Hiroshi S, Tanaka J, Kikuchi M, Fukuda T, Ito H, Hatano T, Yoshida T. Walnut polyphenols prevent liver damage induced by carbon tetrachloride and d-galactosamine hepatoprotective hydrolyzable tannins in the kernel pellicles of walnut. J. Agric. Food Chem. 2008;56:4444–4449. doi: 10.1021/jf8002174. [DOI] [PubMed] [Google Scholar]
- Hubert S, Grzegorz C. Anti-fungal activity of Juglans regia leaf extract against Candida albicans isolates. Pol. J. Environ. Stud. 2015;24(3):1339–1348. [Google Scholar]
- Ibrar MFH, Sultan A. Ethnobotanical studies on plant resources of Ranyal Hill, District Shangla Pakistan. Pak J Bot. 2007;39:329–337. [Google Scholar]
- Iordănescu OA, Radulov I, et al. Physical, nutritional and functional properties of walnuts genotypes (Juglans regia L.) from Romania. Agronomy. 2021;11(6):1092. doi: 10.3390/agronomy11061092. [DOI] [Google Scholar]
- Jafari T, Fallah AA, Azadbakht L. Role of dietary n-3 polyunsaturated fatty acids in type 2 diabetes: a review of epidemiological and clinical studies. Maturitas. 2013;74:303–308. doi: 10.1016/j.maturitas.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Jamshid M, Khalil S, Hamdollah D, Bahram M. Anti-diabetic effects of an alcoholic extract of Juglans regia in an animal model. Turk. J. Med. Sci. 2011;41(4):685–691. [Google Scholar]
- Jelodar G, Mohsen M, Shahram S. Effect of walnut leaf, coriander and pomegranate on blood glucose and histopathology of pancreas of alloxan induced diabetic rats. Afr. J. Trad. CAM. 2007;43:299–305. doi: 10.4314/ajtcam.v4i3.31223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, HongYuan QZ, Xiang Z. Juglone induced apoptosis in human gastric cancer SGC-7901 cells via the mitochondrial pathway. Exp. Toxicol. Pathol. 2011;63:69–78. doi: 10.1016/j.etp.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Khan SA, Bhatia S, Tripathi N. Entomological studies of chaetoproctaodata, an important pest on walnut trees (Juglans regia L.) in Kashmir valley. JAIR. 2013;2(6):378–381. [Google Scholar]
- Khattak P, Khalil TF, Bibi S, Jabeen H, Muhammad N, Khan MA, Liaqat S. Juglans regia (walnut tree) bark in dentistry. PBMJ. 2022 doi: 10.54393/pbmj.v5i2.201. [DOI] [Google Scholar]
- KongY ZL, Yang Z, Han C, Hu L, Jiang H, Shen X. Natural product juglone targets three key enzymes from Helicobacter pylori: inhibition assay with crystal structure characterization. Acta Pharmacol. Sin. 2008;29:870–876. doi: 10.1111/j.1745-7254.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksenija K, Vanja T, Miroslav S, Snezana C. Antioxidant activity of Juglans regia L. juglandaceae pericarp originated from Sumadija region. Pons. Med. J. 2019;16(1):3–8. [Google Scholar]
- Liu L, LiW KoikeK, Zhang S, Nikaido T. Newalpha-tetralonylglucosides from the fruit of Juglans mandshurica. Chem. Pharm. Bull. 2004;52:566–569. doi: 10.1248/cpb.52.566. [DOI] [PubMed] [Google Scholar]
- Manning WE. The classification within the Juglandandaceae. Ann. Mo. Bot. Gard. 1978;59:1058–1087. [Google Scholar]
- Martinez ML, Labuckas DO, LamarqueAL MDM. Walnut (Juglans regia L.) genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010;90:1959–1967. doi: 10.1002/jsfa.4059. [DOI] [PubMed] [Google Scholar]
- McGranahan G, Hand Leslie C. Walnuts (Juglans) Acta Hortic. 1990;290:905–951. [Google Scholar]
- Mohammed N, Kasim H, Hussein AS, Emad AM, Hassan AM, Mohammed AJ. Antifungal activity of alcoholic extract of Juglans regia against Phytopathogenic Rhizoctonia solani. Chem. Res. J. 2018;3(4):105–109. [Google Scholar]
- Mori T. Dietary n-3 PUFA and CVD: a review of the evidence. Proc. Nutr. Soc. 2014;73(1):57–64. doi: 10.1017/S0029665113003583T. [DOI] [PubMed] [Google Scholar]
- Mouhajir F, Hudson JB, Rejdali M, Towers GHN. Multiple antiviral activities of endemic medicinal plants used by Berber people of Morocco. Pharm. Biol. 2001;39:364–374. [Google Scholar]
- Muradoglu FH, Oguz I, Yildiz K, Yilmaz H. Some chemical composition of walnut (Juglans regia L.) selections from Eastern Turkey. Afr. J. Agric. Res. 2010;5:2379–2385. [Google Scholar]
- Muthaiyah B, Musthafa E, Lee M, Chauhan M, Kaur V, Chauhan K. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer's disease. J. Alzheimer's Dis. 2014 doi: 10.3233/JAD-140675. [DOI] [PubMed] [Google Scholar]
- Muzaffer U, Paul VI. Phytochemical analysis, invitro antioxidant and antimicrobial activities of male flower of Juglans regia L. Int. J. Food Prop. 2018;21(1):345–356. doi: 10.1080/10942912.2017.1409762. [DOI] [Google Scholar]
- Natto ZS, Siapco G, Jaceldo-Siegl K, Haddad EH, Sabate J. Food and nutrient displacement by walnut supplementation in a randomized crossover study. Nutrients. 2022;14:1017. doi: 10.3390/nu14051017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Zhang YG, Chen SX, Thakur K, Wang S, Zhang JG, Shang YF, Wei ZJ. Exploration of walnut components and their association with health effects. Crit. Rev. Food Sci. Nutr. 2021 doi: 10.1080/10408398.2021.1881439. [DOI] [PubMed] [Google Scholar]
- Oliveira I, Sousa A, Ferreira ICFR, Bento A, Stevinhol LE, Pereira JA. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008;46:2326–2331. doi: 10.1016/j.fct.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Omwenga E, Hensel A, Shitandi A, Goycoolea F. Ethnobotanical survey of traditionally used medicinal plants for infections of skin, gastrointestinal tract, urinary tract and the oral cavity in Borabu sub-county Nyamira county Kenya. J. Ethnopharmacol. 2015 doi: 10.1016/j.jep.2015.11.032. [DOI] [PubMed] [Google Scholar]
- Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J. Integr. Med. 2016;14(5):359–373. doi: 10.1016/S2095-4964(16)60274-1. [DOI] [PubMed] [Google Scholar]
- Parivash R, Najmeh K, Sedigheh A, Mahbubeh S. Anti-diabetic effects of walnut oil on alloxan-induced diabetic rats. Afr. J. Pharmacy Pharmacol. 2011;5(24):2655–2661. [Google Scholar]
- Pereira JA, Oliveira I, Sousa A, Ferreira ICFR, Bento A, Estevinho L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008;46:2103–2111. doi: 10.1016/j.fct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Poyrazolu EC, Biyik H. Antimicrobial activity of the ethanol extracts of some plants natural growing in Aydin Turkey. Afr. J. Microbiol. Res. 2010;4:2318–2323. [Google Scholar]
- Qadan F, Al-Adham IS, Nahrstedt A. Characterization of antimicrobial polymeric procyanidins from Juglans regia leaf extract. Eur. J. Sci. Res. 2005;11:438–443. [Google Scholar]
- Qamar W, Sultana S. Polyphenols from Juglans regia L. (Walnut) kernel modulate cigarette smoke extract induced acute inflammation, oxidative stress and lung injury in Wistar rats. Hum. Exp. Toxicol. 2011;30:499–506. doi: 10.1177/0960327110374204. [DOI] [PubMed] [Google Scholar]
- Qamar S, Manrique YJ, Parekh H, Falconer JR. Nuts, cereals, seeds and legumes proteins derived emulsifiers as a source of plant protein beverages: a review. Crit. Rev. Food Sci. Nutr. 2020;60(16):2742–2762. doi: 10.1080/10408398.2019.1657062. [DOI] [PubMed] [Google Scholar]
- Ragab, K., Zhongli, P.: Walnuts. In: Integrated Processing Technologies for Food and Agricultural By-Products (2019). 10.1016/B978-0-12-814138-0.00016-2
- Raheleh J, Mahmood S, Ghaffari M, Salami K, Houri V, et al. Antioxidant and anticancer activities of walnut (Juglans regia L.) protein hydrolysates using different proteases. Plant Foods Hum. Nutr. 2016;71:402–409. doi: 10.1007/s11130-016-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimipanah M, Hamedi M, Mirzapour M. Antioxidant activity and phenolic contents of Persian walnut (Juglans regia L.) green husk extract. Afr. J. Food Sci. Technol. 2010;1:105–111. [Google Scholar]
- Raisi M, Mohammad G, et al. Effect of storage atmosphere and temperature on the oxidative stability of almond kernels during long term storage. J. Stored Prod. Res. 2015 doi: 10.1016/j.jspr.2015.03.004. [DOI] [Google Scholar]
- Raja G, Shaker IA, Sailaja I, Swaminathan R, Kondaveeti SB, Saleem BS. Nutritional analysis of nuts extract of Juglans regia L. Int. J. Bioassays. 2012;1:68–73. [Google Scholar]
- Rosaria A, Floriana D, Giuseppe AM, Simone R, et al. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2019;35:1–5. doi: 10.1080/14786419.2019.1650352. [DOI] [PubMed] [Google Scholar]
- Ruijun W. Antitumor effects and immune regulation activities of a purified polysaccharide extracted from Juglan regia. Int. J. Biol. Macromol. 2015;72:771–775. doi: 10.1016/j.ijbiomac.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Rusu ME, Fizesan I, Pop A, Mocan A, Gheldiu AM, et al. Walnut (Juglans regia L.) septum: assessment of bioactive molecules and in vitro biological effects. Molecules. 2020;25(9):2187. doi: 10.3390/molecules25092187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah TA, Sharma E, Ahmad G. Juglans regia Linn: a phytopharmacological review. World J. Pharm. Res. 2014;2(4):364–373. [Google Scholar]
- Shah RA, Bakshi P, Sharma N, Jasrotia A, Itoo H, Gupta R, Singh A. Diversity assessment and selection of superior Persian walnut (Juglans regia L.) trees of seedling origin from North-Western Himalayan region. Resour. Environ. Sustain. 2021;3:100015. doi: 10.1016/j.resenv.2021.100015. [DOI] [Google Scholar]
- Sharma P, Tomar L, Bachwani M, Bansal V. Review on neem: thousand problems one solution. IRJP. 2011;2(12):97–102. [Google Scholar]
- Sharma M, Sharma AK, Sharma M. Ethno-botanical study of medicinal plants from unexplored area of District Ramban (J&K) India. Indian J. Agric. Res. 2020;54:1–7. doi: 10.18805/IJARe.A-5561. [DOI] [Google Scholar]
- Shi D. Effects of walnut polyphenol on learning and memory functions in hypercholesterolemia mice. J. Food Nutr. Res. 2014;2:450–456. [Google Scholar]
- Shimoda H, Tanaka J, Kikuchi M, Fukuda T, Ito H, Hatano T, Yoshida T. Effect of polyphenol-rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver. J. Agric. Food Chem. 2009;57:1786–1792. doi: 10.1021/jf803441c. [DOI] [PubMed] [Google Scholar]
- Subhan S, Bagchi M. Phytopharmaceuticals for brain health. Boca Raton: CRC Press; 2017. [Google Scholar]
- Thakur M, Singh K. Walnut (Juglan regia L.): a complete health and brain food. Asian J. Biol. Sci. 2013;8(2):276–288. [Google Scholar]
- Thakur ND, Apraj V, Bhagwat A, Mallya R, Sawant L, Pandita N. Pharmacognostic and phytochemical investigation of Juglans regia Linn. bark. Pharmacogn. J. 2011;3:39–43. doi: 10.5530/pj.2011.25.7. [DOI] [Google Scholar]
- Tuqa A, Alaa A, Shima A, Nora A, et al. Antibacterial effect of Juglans regia L. bark extract at different concentrations against human salivary microflora. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017;3(4):214–217. [Google Scholar]
- UN Food and Agriculture Organization, Corporate Statistical Database (FAOSTAT). (2020) Accessed 25 Aug 2022
- Upadhyay V, Kambhoja S, Harshaleena K, Veeresh DK. Anthelmintic activity of the stem bark of Juglans regia Linn. Res. J. Pharm. Phytochem. 2010;2:465–467. [Google Scholar]
- Upadhyay V, Kambhoja S, Harshaleena K. Antifungal activity and preliminary phytochemical analysis of stem bark extracts of Juglans regia linn. IJPBA. 2010;1:442–447. [Google Scholar]
- Vaidyaratnam PSV. Indian medicinal plants a compendium of 500 species. Orient Longman Private Limited, Chennai. 2005;3:264–265. [Google Scholar]
- Vardhini SR. Exploring the antiviral activity of juglone by computational method. J. Recept Signal Transduct. Res. 2007;34(6):456–457. doi: 10.3109/10799893.2014.917325. [DOI] [PubMed] [Google Scholar]
- Verma G, Sharma VA. A scientific update on Juglans regia Linn. Asian J. Pharm. Res. Dev. 2020;8(3):166–175. doi: 10.22270/ajprd.v8i3.741. [DOI] [Google Scholar]
- Verma R, Padalia R, Chauhan A, Thul S. Phytochemical analysis of the leaf volatile oil of walnut tree (Juglans regia L.) from western Himalaya. Ind. Crops Prod. 2013;42:195–201. doi: 10.1016/j.indcrop.2012.05.032. [DOI] [Google Scholar]
- Vieira V, Pereira C, et al. Phenolic profile, antioxidant and antibacterial properties of Juglans regia L. (walnut) leaves from the Northeast of Portugal. Ind. Crop Prod. 2019;134:347–355. doi: 10.1016/j.indcrop.2019.04.020. [DOI] [Google Scholar]
- Vieira V, Pereira C, Pires T, Calhelha R, Alves M, Ferreira O, Barros L, Ferreira I. Phenolic profile, antioxidant and antibacterial properties of Juglans regia L. (walnut) leaves from the Northeast of Portugal. Ind. Crops Prod. 2019;134:347–355. doi: 10.1016/j.indcrop.2019.04.020. [DOI] [Google Scholar]
- Vu D, Vo P, Coggeshall M, Lin CH. Identification and characterization of phenolic compounds in black walnut kernels. J. Agric. Food Chem. 2018 doi: 10.1021/acs.jafc.8b01181. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhong D, Wu S, Han Y, Zheng Y, et al. The phytochemical profiles for walnuts (J. regia and J. sigillata) from China with protected geographical indications. Food Sci. Technol. 2020;41:695–701. doi: 10.1590/fst.30320. [DOI] [Google Scholar]
- Zehr KR, Walker MK. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: a review. Prostaglandins Other Lipid Mediat. 2018;134:131–140. doi: 10.1016/j.prostaglandins.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liao L, Moore J, Wua T, Wang Z. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.) Food Chem. 2009;113:160–165. [Google Scholar]
- Zhang YY, Zhang F, Zhang YS, Thakur K, Zhang JG, Liu Y, Kan H, Wei ZJ. Mechanism of Juglone-induced cell cycle arrest and apoptosis in Ishikawa human endometrial cancer cells. J. Agric. Food Chem. 2019;67(26):7378–7389. doi: 10.1021/acs.jafc.9b02759. [DOI] [PubMed] [Google Scholar]
- Zhang YG, Kan H, Chen SX, Thakur K, Wang S, Zhang JG, Shang YF, Wei ZJ. Comparison of phenolic compounds extracted from diaphragma juglandis fructus, walnut pellicle, and flowers of Juglans regia using methanol, ultrasonic wave, and enzyme assisted-extraction. Food Chem. 2020 doi: 10.1016/j.foodchem.2020.126672. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Ni Z, Elam E, Fan Z, Thakur K, Wang S, Zhang JG, Wei ZJ. Juglone, a novel activator of ferroptosis induces cell death in endometrial carcinoma Ishikawa cells. Food Funct. 2021 doi: 10.1039/D1FO00790D. [DOI] [PubMed] [Google Scholar]