Abstract
The d-alanylation of membrane-associated lipoteichoic acid (LTA) in gram-positive organisms requires the d-alanine–d-alanyl carrier protein ligase (AMP) (Dcl) and the d-alanyl carrier protein (Dcp). The dlt operon encoding these proteins (dltA and dltC) also includes dltB and dltD. dltB encodes a putative transport system, while dltD encodes a protein which facilitates the binding of Dcp and Dcl for ligation with d-alanine and has thioesterase activity for mischarged d-alanyl-acyl carrier proteins (ACPs). In previous results it was shown that d-alanyl-Dcp donates its ester residue to membrane-associated LTA (M. P. Heaton and F. C. Neuhaus, J. Bacteriol. 176: 681–690, 1994). However, all efforts to identify an enzyme which catalyzes this d-alanylation process were unsuccessful. It was discovered that incubation of d-alanyl-Dcp in the presence of LTA resulted in the time-dependent hydrolysis of this d-alanyl thioester. d-Alanyl-ACP in the presence of LTA was not hydrolyzed. When Dcp was incubated with membrane-associated d-alanyl LTA, a time and concentration-dependent formation of d-alanyl-Dcp was found. The addition of NaCl to this reaction inhibited the formation of d-alanyl-Dcp and stimulated the hydrolysis of d-alanyl-Dcp. Since these reactions are specific for the carrier protein (Dcp), it is suggested that Dcp has a unique binding site which interacts with the poly(Gro-P) moiety of LTA. It is this specific interaction that provides the functional specificity for the d-alanylation process. The reversibility of this process provides a mechanism for the transacylation of the d-alanyl ester residues between LTA and wall teichoic acid.
The biosynthesis of d-alanyl-lipoteichoic acid (LTA) requires the 56-kDa d-alanine–d-alanyl carrier protein ligase (AMP) (Dcl) and the 8.8-kDa d-alanyl carrier protein (Dcp) (11, 12). Heaton and Neuhaus (12) showed that d-alanyl-Dcp donates its d-alanyl residue to the poly(Gro-P) moiety of membrane-associated LTA. Neither the mechanism nor the topology of this d-alanylation reaction is known. To address this key reaction, several groups have identified the genes in a number of organisms containing the dlt operon (11, 21, 23). In addition to the genes encoding Dcl (dltA) and Dcp (dltC), dltB and dltD encode a putative transport protein (20) and a protein which facilitates the binding of Dcl and Dcp for ligation with d-alanine and has thioesterase activity for mischarged d-alanyl acyl carrier proteins (ACPs) (5), respectively. A gene encoding an enzyme which catalyzes the transfer of the d-alanine residue from d-alanyl-Dcp to membrane-associated LTA has not been identified.
Dcp provides the essential link between the ligase (Dcl) and the incorporation of d-alanine into LTA. This carrier protein is a homologue of those ACPs which function in fatty acid biosynthesis and metabolism (4, 26). However, it was unexpected to find that Dcl will ligate d-alanine to ACPs from Escherichia coli, Vibrio harveyi, Saccharopolyspora erythraea, and Bacillus subtilis (12). Nevertheless, only Dcp participates in the d-alanylation of LTA. These observations suggested that there are at least two determinants for interaction of Dcp with its cognate partners, one of which is recognized by Dcl and one of which is recognized by the membrane acceptor LTA. It is this second determinant which is the focus of structural studies on Dcp (B. F. Volkman, Q. Zhang, D. Debabov, E. Rivera, G. Kresheck, and F. C. Neuhaus, unpublished results), and the biochemical studies to be reported here.
For Staphylococcus aureus, it was found that growth in the presence of NaCl resulted in a lower d-alanine ester content in LTA (8). The mechanism by which the ester content was reduced during growth in NaCl is unknown and was not correlated with the reaction catalyzed by DltD (5). Instead, an NaCl-activated, thioesterase-like activity specific for d-alanyl-Dcp which is distinct from that catalyzed by DltD was discovered (5, 20).
Our goal here was to establish the mechanism of d-alanine transfer from d-alanyl-Dcp to LTA. To accomplish this goal, the thioesterase-like activity of LTA specific for d-alanyl-Dcp was examined in incubations containing LTA in different microenvironments: (i) purified, (ii) membrane associated, and (iii) membrane associated (salt treated). The results suggested that complex formation between d-alanyl-Dcp and LTA is one of the features resulting either in the transfer of d-alanine from d-alanyl-Dcp to LTA when this amphiphile is membrane associated or in the hydrolysis of d-alanyl-Dcp when the LTA is not membrane associated.
MATERIALS AND METHODS
Strains and growth of bacteria.
Lactobacillus casei 102S was generously provided by Bruce Chassy (University of Illinois). L. casei 102S dltD::cat was from Debabov et al. (5). These strains were grown in Lactobacilli MRS broth (Difco Laboratories).
Chemicals and reagents.
Purified samples of LTA prepared by the procedure of Fischer et al. (9) were generously provided by Werner Fischer (Universitat Erlangen-Nürnberg): These samples were isolated from S. aureus DSM20231 (Ala/P 0.43), B. subtilis JH542 (Ala/P 0.32), Enterococccus faecium MT9 (not purified by hydrophobic chromatography) (Ala/P 0.47; Glc/P 0.38; Glc2/P 0.05), Streptococcus sanguis DSM 20567 (Ala/P 0.36), Enterococcus hirae (ca. 60% of Gro glycosylated Glc, Glc2, Glc3, and Glc4), and Lactobacillus garvieae [glycosylated by Gal(α1-2)].
The concentrations of LTA are presented as micromolar concentrations in phosphorus, and thus in the results the concentrations are designated as micromolar concentrations of LTA-phosphorus. Since the poly(Gro-P) chains of LTA are heterogeneous in length, it is not possible to extrapolate LTA-phosphorus to micromolar concentrations of LTA. In the case of S. aureus, the average number of Gro-P residues is 25, with a variation between 4 and 30 (7).
E. coli fatty acid synthase ACP and holo-ACP synthase were generously provided by Ralph H. Lambalot, Roger S. Flugel, and Christopher T. Walsh (Harvard University) (16). d-[14C]alanine (43 mCi/mmol) was the product of ICN Biochemicals, Inc. Metricel filter membranes (GN-6) and Econo-Safe scintillation cocktail were purchased from Gelman Sciences and RPI Corp., respectively. Nanosep centrifugal concentrators (10K and 30K) were the products of Pall Filtron Corp.
Expression and purification of Dcp and Dcl.
Dcp was expressed, purified, and converted to holo-Dcp using recombinant holo-ACP synthase (4). The expression of Dcl and its purification from inclusion bodies were as previously described (5, 11).
Preparation of d-[14C]alanyl-Dcp and d-[14C]alanyl-ACP.
For the preparation of either d-[14C]alanyl-Dcp or d-[14C]alanyl-ACP, reaction mixtures contained either 15 μM recombinant holo-Dcp or holo-ACP, 0.23 mM d-[14C]alanine (43 mCi/mmol), 15 U of recombinant Dcl, 30 mM bis-Tris (pH 6.5), 10 mM ATP, 10 mM MgCl2, and 1 mM dithiothreitol (DTT). The mixture was incubated at 37°C for 90 min, and proteins with a mass of >30 kDa were separated from d-[14C]alanyl-Dcp or d-[14C]alanyl-ACP with the Nanosep (30K) concentrator. d-[14C]alanyl-Dcp or d-[14C]alanyl-ACP in the filtrate was desalted and separated from ATP, DTT, MgCl2, and d-[14C]alanine using four cycles of filtration with the Nanosep (10K) concentrator. During the course of this process the buffer was changed to 5 mM sodium acetate (pH 4.5).
Preparation of membrane-associated d-[14C]alanyl-LTA.
Membranes from L. casei 102S and L. casei 102S dltD::cat were prepared according to a previous procedure (12) using a French pressure cell for the disruption of bacteria. The incorporation of d-[14C]alanine from d-[14C]alanyl-Dcp into membrane-associated d-[14C]alanyl-LTA was carried out as previously described (4, 12) in 50 μl with 100 μg of membranes prepared from Lactobacillus rhamnosus 102S and 5 nmol of recombinant d-[14C]alanyl-Dcp (43 mCi/mmol). After incubation for 90 min, the labeled membranes were separated from d-[14C]alanyl-Dcp using Nanosep (30K) centrifugal concentrators and used without further washing before storage at −80°C.
Assay of d-[14C]alanyl-Dcp and d-[14C]alanyl-ACP.
d-[14C]alanyl-Dcp was determined by precipitation with 10% trichloroacetic acid (TCA) and filtration through 0.45-μm-pore-size Metricel filters, which retained 97% of the radiolabeled carrier protein. The filters were dissolved in 3.5 ml of ethyl acetate and assayed for radiolabel. In the hydrolytic reactions of d-[14C]alanyl-Dcp in the presence of LTA, the amount of d-alanine was expressed as the difference between the added d-alanyl-Dcp and the remaining d-alanyl-Dcp at the termination of the reaction.
RESULTS
Hydrolysis of d-alanyl-Dcp in the presence of LTA.
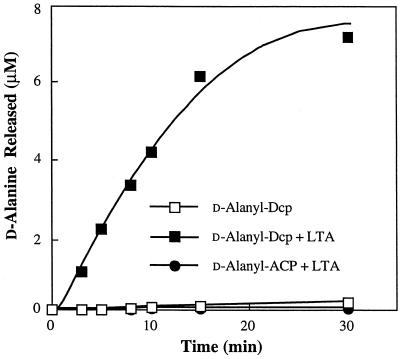
Heaton and Neuhaus (12) showed that d-alanyl-Dcp donates its d-alanyl substituent to membrane-associated LTA in the absence of Dcl. To simplify this system, attempts were made to transfer d-alanine from d-alanyl-Dcp to isolated, purified LTA in the absence of membranes. However, instead of transacylation to LTA, incubation of d-alanyl-Dcp with pure LTA resulted in the time-dependent hydrolysis of d-alanyl-Dcp (Fig. 1). In contrast, d-alanyl-ACP was not hydrolyzed in the presence of LTA. d-Alanyl-ACP, a homologue of d-alanyl-Dcp, apparently does not provide the necessary determinant for interaction with LTA for the cleavage of the d-alanyl substituent. On the basis of these observations we hypothesized that d-alanyl-Dcp forms a complex with the poly(Gro-P) moiety of LTA and that within this complex a thioesterase-like activity occurs.
FIG. 1.
Hydrolysis of d-[14C]alanyl-Dcp in the presence of LTA. The reaction mixture contained 4.4 μM LTA-phosphorus from S. aureus, 8 μM d-[14C]alanyl-Dcp (6,700 cpm/nmol), and 30 mM bis-Tris (pH 6.5) in a total of 350 μl. Samples (50 μl) were removed and added to 950 μl of 10% TCA. The amount of radiolabeled d-alanyl-Dcp was determined as described in Materials and Methods, and the amount of d-alanine released was determined by subtraction.
A variety of LTAs was examined in the hydrolysis reaction for d-alanyl-Dcp (Table 1). Using a fixed-time assay (5 min), we found that the most effective samples of LTA are those which are nonglycosylated. Prior removal of the d-alanyl ester residues from the LTA resulted in a 25% increase of d-alanyl-Dcp hydrolysis activity (Table 1, S. aureus and S. sanguis). The addition of 5 mM MgCl2 to these reaction mixtures had no effect on the velocity of d-alanine formation. For the studies presented here, LTA with its d-alanine ester content of 0.43 from S. aureus prepared by the method of Fischer et al. (9) was used. Because of the polydispersity of LTA, micromolar concentrations of LTA-phosphorus were used for comparisons.
TABLE 1.
Specificity of d-alanyl-Dcp hydrolysis in the presence of LTA
| LTA source | d-[14C]alanine releaseda (μM/min) |
|---|---|
| None, control | 0.001 |
| None, 5 mM Gro-P(α, 1) | 0.001 |
| S. aureus | 2.31 (2.94)b |
| S. sanguis | 2.35 (3.01)b |
| B. subtilis | 2.09 |
| E. faeciumc | 1.57 |
| E. hiraec | 0.79 |
| L. garvieaec | 0.74 |
The reaction mixture (50 μl) contained 30 mM bis-Tris (pH 6.5), 5 μM d-[14C]alanyl-Dcp (6,700 cpm/nmol), and 4.4 μM concentrations of the indicated LTA-phosphorus. The reaction time was 5 min at 37°C. The amount of d-alanyl-Dcp remaining was assayed as described in the legend to Fig. 1, and the amount of d-alanine released was calculated by subtraction.
Values in parenthesis refer to LTA sample that was treated with 0.5 M H2NOH for 30 min at 37°C, dialyzed, and adjusted to the same concentration using Nanosep centrifugal concentrators.
Samples which contained glycosylated LTA (see Materials and Methods).
The putative formation of a complex between LTA and d-alanyl-Dcp implied that saturation kinetics should be observed between d-alanyl-Dcp and LTA (Fig. 2). Increasing the concentrations of d-alanyl-Dcp gave saturation kinetics from which an apparent Km of 7.5 μM for d-alanyl-Dcp was established. The calculation of this value assumes that the LTA has a fixed number of binding sites for d-alanyl-Dcp. Since the velocity of d-alanyl-Dcp hydrolysis is high relative to the estimated concentration of LTA, it would appear that LTA turnover occurs. Thus, while it is not clear how this catalysis is effected, it is apparent that the reaction might be catalyzed by LTA and that d-alanyl-Dcp is the substrate.
FIG. 2.
Effect of increasing d-alanyl-Dcp on the velocity of hydrolytic cleavage. The reaction mixture contained increasing concentrations of d-[14C]alanyl-Dcp in the presence of 4.4 μM LTA-phosphorus in the reaction mixture described in the legend to Fig. 1. The mixtures were incubated for 5 min prior to termination with 10% TCA, and the amounts of d-alanyl-Dcp remaining were determined for calculating the initial velocities of d-alanine released. In the inset a double-reciprocal plot is presented from which Vmax (5.0 μM/min) and Km (7.5 × 10−6 M) were calculated.
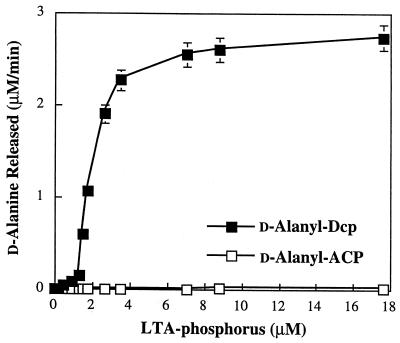
Increasing concentrations of LTA in the presence of a fixed concentration of d-alanyl-Dcp gave a sigmoidal response with a defined saturation curve (Fig. 3). The Km for LTA is roughly 2 μM LTA-phosphorus. This response implies a fixed number of sites in LTA for binding d-alanyl-Dcp. Samples of glycosylated LTA gave lower velocities of hydrolysis (Table 1) and, thus, it was concluded that the glycosyl substituents have an effect on the interaction of d-alanyl-Dcp and LTA. Since d-alanyl-ACP was not hydrolyzed in this reaction (Fig. 3), it is concluded that ACP and LTA do not form a complex.
FIG. 3.
Effect of increasing LTA-phosphorus on the hydrolysis of d-[14C]alanyl-Dcp. The reaction mixtures (50 μl) contained the indicated concentrations of LTA-phosphorus from S. aureus, 8 μM d-[14C]alanyl-Dcp (6,700 cpm/nmol), and 30 mM bis-Tris (pH 6.5). The amounts of d-alanine released were determined as described in the legend to Fig. 1.
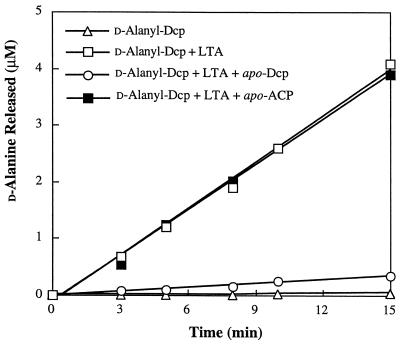
If Dcp has a specific site for binding LTA, apo-Dcp will inhibit the hydrolysis reaction while apo-ACP will not. As shown in Fig. 4, the addition of apo-Dcp to the reaction mixture inhibited the hydrolytic cleavage of d-alanyl-Dcp, while the addition of apo-ACP had no effect on the cleavage. For comparison, it is observed that d-alanyl-Dcp in the absence of LTA is stable in the reaction mixture. Thus, apo-Dcp binds to LTA in an interaction that is not dependent on the phosphopantetheine prosthetic group.
FIG. 4.
Inhibition of d-alanyl-Dcp hydrolysis. The reaction mixture contained 4.4 μM LTA-phosphorus and 8 μM d-[14C]alanyl-Dcp (6,700 cpm/nmol) in the presence of either 85 μM apo-Dcp or 85 μM apo-ACP. Samples were removed at the indicated times, and the amounts of d-alanyl-Dcp or d-alanyl-ACP were determined as described in Materials and Methods. The amounts of d-alanine released were determined as described in the legend to Fig. 1.
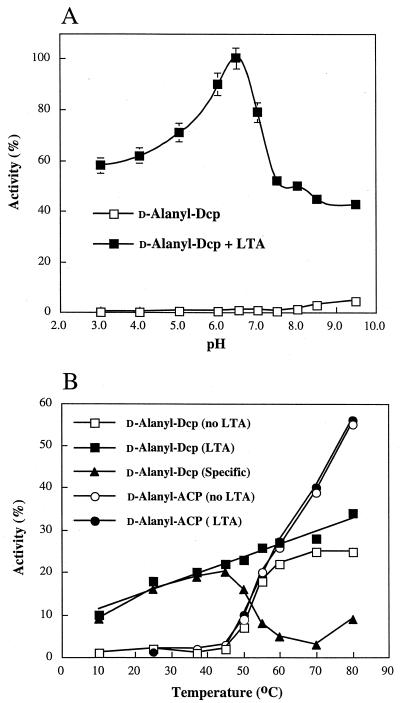
Variation of pH on the hydrolysis of d-alanyl-Dcp in the presence of LTA gave a pH optimum of 6.5 (Fig. 5A). However, both the acidic and the basic limbs of the pH response are nontraditional, i.e., the hydrolytic rate at the extremes of the pH curve do not approach the control velocity. It would appear that both an ionizable group(s) and a binding cleft are functional in Dcp. Additional pH studies may provide clues to those interactions which are essential for this cleavage reaction. The effect of temperature (Fig. 5B) on the hydrolytic rate was complex and would appear to reflect more than one process. The velocity of d-alanyl-Dcp hydrolysis was dependent on LTA and specific for Dcp from 10 to 45°C.
FIG. 5.
(A) Effect of pH on the hydrolysis of d-alanyl-Dcp in the presence of LTA. (B) Effect of temperature on the specific and nonspecific hydrolyses of d-alanyl-Dcp and d-alanyl-ACP in the presence of LTA. The reaction mixtures for panel A contained either 30 mM sodium acetate (pH 3.0 to 5.0), bis-Tris (pH 6.0 to 7.0), or Tris-HCl (pH 7.5 to 9.5) in the presence of 4.4 μM LTA-phosphorus and 8 μM d-[14C]alanyl-Dcp. The reaction mixtures for panel B contained 30 mM bis-Tris (pH 6.5), either 8 μM d-[14C]alanyl-Dcp or d-[14C]alanyl-ACP, and 4.4 μM LTA-phosphorus where indicated. The reaction mixtures for panel A were incubated for 5 min at 37°C and at the indicated temperature for panel B. The amounts of d-alanyl-Dcp remaining were determined as described in Materials and Methods. In panel A, 100% activity is given by the velocity at pH 6.5 in the presence of LTA. In panel B, 100% activity is given by the amount of d-[14C]alanyl-Dcp added to the reaction mixture. The amount of specific hydrolysis is the difference between the hydrolyses observed for d-alanyl-Dcp (LTA) and d-alanyl-Dcp (no LTA).
Transfer of d-[14C]alanine from d-[14C]alanyl-LTA to Dcp in membranes.
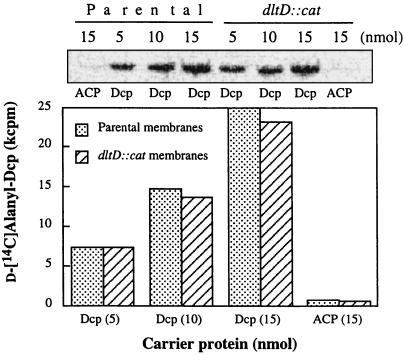
A second approach for studying the putative thioesterase-like activity of the Dcp-LTA complex was to test whether Dcp will stimulate the hydrolytic cleavage of the d-alanyl esters of membrane-associated d-[14C]alanyl-LTA. However, incubation of Dcp with membrane-associated d-[14C]alanyl-LTA revealed a new facet of the d-alanine incorporation system. Instead of hydrolysis, d-[14C]alanyl-Dcp was formed (Fig. 6). When increasing concentrations of Dcp were incubated with membrane-associated d-[14C]alanyl-LTA, increasing amounts of d-[14C]alanyl-Dcp are formed according to reaction 1. In contrast, these radiolabeled membranes do not transfer the d-alanyl residues to ACP membrane as follows (reaction 1):
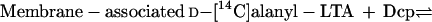 |
 |
1 |
Debabov et al. (5) proposed that DltD facilitates the binding of Dcp and Dcl for ligation of Dcp with d-alanine. Thus, it was essential to establish whether DltD is responsible for the reverse reaction illustrated in reaction 1. As shown in Fig. 6, membranes prepared from L. casei 102S dltD::cat also catalyze the synthesis of d-[14C]alanyl-Dcp from membrane-associated d-[14C]alanyl-LTA and Dcp to approximately the same extent as the parental membranes. Thus, DltD plays no role in catalyzing the transfer of d-alanine from membrane-associated d-alanyl-LTA to Dcp.
FIG. 6.
Effect of Dcp concentration on the formation of d-alanyl-Dcp from membrane-associated d-alanyl-LTA. The reaction mixture contained 20 μg of membrane-associated d-[14C]alanyl-LTA (6,700 cpm/nmol), the indicated concentration of Dcp or ACP, and 30 mM bis-Tris buffer (pH 6.5) in a total volume of 15 μl. It was incubated for 30 min at 37°C. The amounts of d-[14C]alanyl-Dcp formed were quantified by nondenaturing polyacrylamide gel electrophoresis by the method of Heaton and Neuhaus (12).
NaCl-stimulated thioesterase-like activity of membranes for d-alanyl-Dcp.
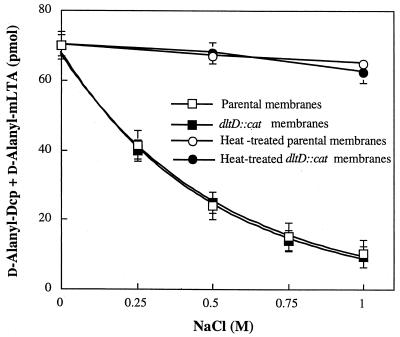
Growth of S. aureus in the presence of high concentrations of NaCl resulted in a lower d-alanine ester content in LTA (8). While the precise mechanism of the NaCl effect is unknown, we have used this salt effect to modulate the thioesterase-like activity for d-alanyl-Dcp in membranes. The addition of increasing concentrations of NaCl to membranes stimulated the thioesterase-like activity for d-alanyl-Dcp (Fig. 7). It is hypothesized that NaCl increases the accessibility of the endogenous membrane-associated LTA, making it available for participation in the hydrolytic cleavage specific for d-alanyl Dcp. To establish whether DltD participates in this NaCl-stimulated hydrolysis of d-alanyl-Dcp, membranes from the L. casei 102S dltD::cat were also examined in the salt-induced thioesterase-like reaction. As shown in Fig. 7, these membranes were not different from parental membranes in their ability to cleave d-alanyl-Dcp in the presence of increasing concentrations of NaCl. The fact that DltD is not responsible for the NaCl-stimulated thioesterase-like activity for d-alanyl-Dcp suggests that a different mechanism is responsible for the cleavage of d-alanyl-Dcp than that for the DltD-catalyzed hydrolysis of d-alanyl-ACP.
FIG. 7.
Hydrolysis of d-[14C]alanyl-Dcp by membranes in the presence of increasing concentrations of NaCl. Because d-alanyl-Dcp can transfer its activated d-alanine to membrane-associated LTA, the ordinate represents the aggregate of d-alanyl-Dcp and d-alanyl-LTA. The reaction mixtures contained 30 mM bis-Tris (pH 6.5) and 0.25 mg of membranes from either L. casei 102S or the L. casei 102S dltD::cat mutant (5) per ml in the presence of increasing concentrations of NaCl in a volume of 50 μl. The reaction time was 30 min at 37°C. The amount of hydrolysis was quantified by the procedure described in Materials and Methods.
NaCl sensitivity of d-alanyl-Dcp formation from membrane-associated d-alanyl-LTA.
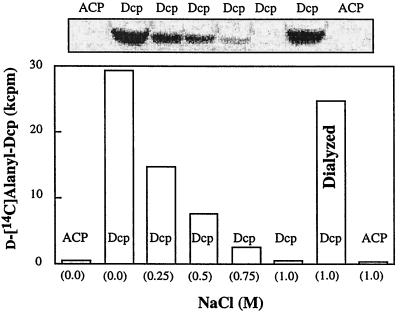
Because the salt sensitivity of d-alanyl-Dcp hydrolysis (Fig. 7) and the salt sensitivity of the d-[14C]alanyl-Dcp formation utilizing d-[14C]alanyl-LTA and Dcp are similar, a relationship may exist between these activities. For example, the addition of increasing concentrations of NaCl to a series of reaction mixtures containing membrane-associated d-[14C]alanyl-LTA and Dcp results in the formation of lower amounts of d-alanyl-Dcp in the reverse reaction (Fig. 8). The concentration of salt which gave 50% inhibition is 0.30 M. This is essentially the same concentration of NaCl as that required to observe the salt-activated thioesterase-like activity for d-alanyl-Dcp (Fig. 7; 0.25 M for 50% stimulation). The correlation of these activities to salt sensitivity provided support for the suggestion that NaCl enhances the accessibility of membrane-associated LTA and hence makes the LTA available for catalyzing the hydrolytic cleavage of d-alanyl-Dcp. This cleavage is similar to that described with purified LTA and d-alanyl-Dcp (Fig. 1 and Table 1).
FIG. 8.
Effect of increasing concentrations of NaCl on the formation of d-[14C]alanyl-Dcp from membrane-associated d-[14C]alanyl-LTA and Dcp. The reaction mixture contained 30 mM bis-Tris (pH 6.5) and 20 μg of d-[14C]alanyl-LTA (6,700 cpm/nmol) in a volume of 15 μl. The reaction time was 20 min at 37°C. The reaction mixtures were desalted using Nanosep microconcentrators. In the reaction mixture designated “Dialyzed” the d-[14C]alanyl-LTA was treated with 1.0 M NaCl for 20 min and then dialyzed before incubation with Dcp by using the Nanosep concentrator (10K). It was incubated in the reaction mixture described above for the indicated time. The amount of d-alanyl-Dcp formed was quantified by nondenaturing polyacrylamide gel electrophoresis (12).
DISCUSSION
The results of these experiments, together with previous observations (12, 20), describe three reactions. These are as follows: (i) the hydrolysis of d-alanyl-Dcp in the presence of isolated LTA, (ii) the formation of d-alanyl-Dcp from membrane-associated d-alanyl-LTA and Dcp, and (iii) the transacylation of the activated d-alanyl residue from d-alanyl Dcp to membrane-associated LTA. We propose that each of these activities is, in fact, the result of a previously unrecognized feature of Dcp and LTA, i.e., the binding of this carrier protein to LTA. Binding of d-alanyl-Dcp with LTA can mimic an enzyme reaction and, for the purposes of the present study, LTA will be designated an “enzyme mimic” and d-alanyl-Dcp will be designated the “substrate.” The specificity for the substrate suggests the presence of a specific binding site on Dcp for its acceptor target LTA.
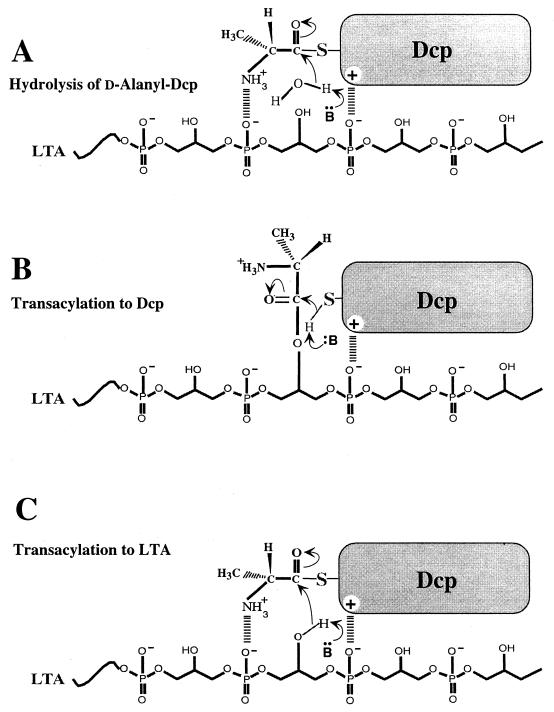
To consider the mechanisms underlying these reactions, three illustrations are presented in Fig. 9. In each case the nucleophilic acceptor generated with a common proton acceptor is shown: HO:, R-S:, and R-O: (Fig. 9A, B, and C, respectively). In panel A, the binding of d-alanyl-Dcp to LTA followed by nucleophilic attack of HO: from water on the electrophilic carbonyl results in hydrolysis. In panel B, nucleophilic attack of the R-S: of the phosphopantetheine prosthetic group of Dcp on the electrophilic carbonyl of the d-alanyl residue of the membrane-associated LTA results in the transacylation of the d-alanyl residue to Dcp. In panel C, nucleophilic attack of the R-O: of the Gro-P residue on the electrophilic carbonyl of the d-alanyl residue results in the transacylation of d-alanine to the membrane-associated LTA. Depending on the nucleophilic acceptor, each of these activities is correlated with the specific interaction of the carrier protein, Dcp or d-alanyl-Dcp, with LTA.
FIG. 9.
Proposed mechanisms for the hydrolysis of d-alanyl-Dcp in the presence of purified LTA (A), the formation of d-alanyl-Dcp from membrane-associated d-alanyl-LTA (B), and the formation of membrane-associated d-alanyl-LTA from d-alanyl-Dcp (C). B·· and ∶B indicate an unknown proton acceptor for generating the nucleophile. The electrostatic interaction between Dcp phosphodiester anion may be the result of Arg-64 (see Discussion). Events up to the tetrahedral intermediate stage are shown.
The addition of NaCl to the reaction mixture apparently changes the accessibility of the membrane-associated LTA. In the native state, the associated LTA is thought to be sequestered from the bulk solvent. Membranes in the presence of NaCl resulted (i) in the inhibition of transacylation of the activated d-alanine from d-alanyl-Dcp to the R-OH of LTA and (ii) in the stimulation of the hydrolytic cleavage of the d-alanyl thioester. Alternatively in the reverse reaction, the addition of NaCl inhibited the formation of d-alanyl-Dcp from membrane-associated d-alanyl-LTA and Dcp and stimulated hydrolysis. It is proposed that membrane-associated LTA in the presence of NaCl provides access to the bulk solvent, resulting in the hydrolysis of d-alanyl-Dcp. As shown in Fig. 8, removal of the NaCl by dialysis allowed the LTA to assume its original organization and sequestration from the aqueous solvent.
The functional specificity of acyl carrier proteins is a hallmark of this protein family. For example, in the case of the rhizobial carrier proteins, four ACPs each have specific nodulation functions (17). Thus, each of these carrier proteins is targeted to their respective site of action. In the case of Dcp from L. rhamnosus, a specific binding cleft for LTA would appear to exist on the protein surface, which may allow for this targeting (B. F. Volkman et al., unpublished). While the cleft is not completely defined at this time, there are candidate residues which may play a role in this binding. For example, in the case of the Dcp, one of the candidates for the electrostatic attraction of Dcp with LTA is Arg-64. It is found in the highly conserved Dcp motif R64KEW67D. The conserved Trp-67 plays an important role in positioning helix III and hence Arg-64 in a unique orientation for its putative interaction with LTA. This Arg residue is part of a crescent of positive charges not found in E. coli ACP. All attempts to use ACPs in the d-alanine incorporation system failed, even though all could be ligated with d-alanine by Dcl in the absence of DltD. These included ones from V. harveyi, S. erythraea, and B. subtilis (12, 20). Thus, the experiments described here further address the unique functional specificity of Dcp.
There are at least two examples where an ACP catalyzes a reaction (13, 27). In the first the type II polyketide (PKS) ACP catalyzes self-malonylation with malonyl-coenzyme A, and in the second PKS ACP catalyzes the malonylation of ACP involved in type II fatty acid biosynthesis. While these findings were unexpected, it demonstrated that the PKS ACP has the ability to catalyze a reaction in the presence of its cognate donor and acceptor. Thus, the hydrolysis-transacylation reactions observed in the present work with d-alanyl-Dcp may have some similarity to those in the PKS system.
The reversibility of the transacylation reaction, i.e., the formation of d-alanyl-Dcp from d-alanyl-LTA and Dcp, implies that Dcp and LTA can effect the transacylation of the d-alanyl residues between LTA molecules, as well as between wall teichoic acid and LTA. Haas et al. (10) showed that under in vivo conditions the d-alanine esters of LTA are the precursor of the d-alanine esters of wall teichoic acid. In addition, the d-alanyl esters of LTA from either S. aureus (15) or L. casei (2) are randomly distributed along the poly(Gro-P) moiety. The results presented here also provide a possible mechanism for the migration and redistribution of these residues. Thus, it is proposed that d-alanyl-Dcp is translocated to the extramembranal site of LTA acylation, and it is further suggested that Dcp can be ligated with d-alanine from preexisting d-alanyl-LTA for subsequent transacylation to adjacent LTA as well as WTA.
The enhanced reactivity of the d-alanyl ester residues of LTA was recognized by Shabarova et al. (24) and correlated with the presence of the vicinal phosphodiester links. The ability of Dcp to effect the transacylation of these ester residues in the cell wall matrix could provide a mechanism for modulating surface charge and hence ligand binding and autolysin activity. While transacylation of d-alanyl esters was reported between short-chain LTA and long-chain LTA (2, 19), the rate of this reaction was relatively slow. Thus, it is hypothesized that transacylation in the presence of Dcp is accelerated and hence Dcp plays a major role in distributing d-alanine esters from one location of the wall matrix to another.
One of the caveats to this suggestion concerns the topology of these processes. While a putative channel (DltB) for the translocation of d-alanyl-Dcp has been proposed (20), proof for its role in the secretion of d-alanyl-Dcp is lacking. Thus, it is not known whether the d-alanylation of LTA takes place during the course of LTA assembly or whether d-alanylation takes place in the wall matrix in concert with d-alanyl-Dcp. The fact that d-alanylation of WTA takes place at the expense of d-alanyl-LTA (10, 15) argues that these events may take place in the extramembranal cell wall matrix.
The importance of the dlt operon in the physiology of the gram-positive organism is illustrated by the many phenotypes of mutants which have been observed. For example, inactivation of dltC in Streptococcus mutans resulted in a loss of acid tolerance (1). In Streptococcus gordonii DL1 (Challis), inactivation of dltA resulted in a loss of intrageneric coaggregation and in the formation of a variety of pleomorphs (3). In B. subtilis, deletion of either dltA, -B, -C, or -D resulted in mutants with enhanced autolytic activity (26). Additional results with this organism revealed that these deletions in the dlt operon partially suppressed the secretion deficiency resulting from a defective PrsA protein (14). In Lactococcus lactis (6), mutants defective for DltD have enhanced UV sensitivity. Insertional inactivation of this gene in Lactobacillus casei 102S resulted in increased cellular length and enhanced antimicrobial activity to cetyltrimethylammonium bromide and chlorhexidine (5). In the case of S. aureus, inactivation of the dlt operon confers sensitivity to defensins, protegrins, and other cationic peptides (23). This deficiency in d-alanine esters also increased its sensitivities to vancomycin and lysostaphin (22). In S. mutans, a knockout mutation in the promoter of the dlt operon resulted in the defective synthesis of intracellular polysaccharides (25). It is apparent from these different phenotypes that the d-alanyl esters of LTA play an important role in the physiology of the gram-positive organism and, thus, an understanding of the d-alanylation mechanism is essential.
ACKNOWLEDGMENTS
This research was supported by Public Health Service grant RO1 GM51623 to F.C.N.
We are especially grateful to Werner Fischer for the characterized samples of LTA from six organisms and for discussions of these results. We also thank Michael P. Heaton for a critical reading of the manuscript and discussions and Richard B. Silverman for comments on the mechanisms.
Footnotes
This paper is dedicated to the memory of Werner Fischer of the Universität Erlangen-Nürnberg.
REFERENCES
- 1.Boyd D A, Cvitkovitch D G, Bleiweis A S, Kiriukhin M Y, Debabov D V, Neuhaus F C, Hamilton I R. Defects in d-alanyl-lipoteichoic acid synthesis in Streptococcus mutans results in acid sensitivity. J Bacteriol. 2000;182:6055–6065. doi: 10.1128/jb.182.21.6055-6065.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Childs W C, III, Taron D J, Neuhaus F C. Biosynthesis of d-alanyl-lipoteichoic acid by Lactobacillus casei: interchain transacylation of d-alanyl ester residues. J Bacteriol. 1985;162:1191–1195. doi: 10.1128/jb.162.3.1191-1195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemens D L, Kolenbrander P E, Debabov D V, Zhang Q, Lunsford R D, Sakone H, Whittaker C J, Heaton M P, Neuhaus F C. Insertional inactivation of genes responsible for the d-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregations. Infect Immun. 1999;67:2464–2474. doi: 10.1128/iai.67.5.2464-2474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debabov D V, Heaton M P, Zhang Q, Stewart K D, Lambalot R H, Neuhaus F C. The d-alanyl carrier protein in Lactobacillus casei: cloning, sequencing, and expression of dltC. J Bacteriol. 1996;178:3869–3876. doi: 10.1128/jb.178.13.3869-3876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debabov D V, Kiriukhin M Y, Neuhaus F C. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in d-alanylation. J Bacteriol. 2000;182:2855–2864. doi: 10.1128/jb.182.10.2855-2864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duwat P, Cochu A, Ehrlich S D, Gruss A. Characterization of Lactococcus lactis UV-sensitive mutants obtained by IS-1 transposon. J Bacteriol. 1997;179:4473–4479. doi: 10.1128/jb.179.14.4473-4479.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer W. Molecular analysis of lipid macroamphiphiles by hydrophobic interaction chromatography, exemplified with lipoteichoic acids. Anal Biochem. 1993;208:49–56. doi: 10.1006/abio.1993.1007. [DOI] [PubMed] [Google Scholar]
- 8.Fischer W, Rosel P. The alanine ester substitution of lipoteichoic acid (LTA) in Staphylococcus aureus. FEBS Lett. 1980;119:224–226. doi: 10.1016/0014-5793(80)80257-2. [DOI] [PubMed] [Google Scholar]
- 9.Fischer W, Koch H U, Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983;133:523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 10.Haas R, Koch H U, Fischer W. Alanyl turnover from lipoteichoic acid to teichoic acid in Staphylococcus aureus. FEMS Microbiol Lett. 1984;21:27–31. [Google Scholar]
- 11.Heaton M P, Neuhaus F C. Biosynthesis of d-alanyl-lipoteichoic acid: cloning, nucleotide sequence, and expression of the Lactobacillus casei gene for the d-alanine-activating enzyme. J Bacteriol. 1992;174:4707–4717. doi: 10.1128/jb.174.14.4707-4717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heaton M P, Neuhaus F C. Role of the d-alanyl carrier protein in the biosynthesis of d-alanyl-lipoteichoic acid. J Bacteriol. 1994;176:681–690. doi: 10.1128/jb.176.3.681-690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hitchman T S, Crosby J, Byrom K J, Cox R J, Simpson T J. Catalytic self-acylation of type II polyketide synthase acyl carrier proteins. Chem Biol. 1998;5:35–47. doi: 10.1016/s1074-5521(98)90085-0. [DOI] [PubMed] [Google Scholar]
- 14.Hyyrylainmen H-L, Vitikainen M, Thwaite J, Wu H, Sarvas M, Harwood C R, Kontinett V P, Stephenson K. d-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J Biol Chem. 2000;275:26696–26703. doi: 10.1074/jbc.M003804200. [DOI] [PubMed] [Google Scholar]
- 15.Koch H U, Doker R, Fischer W. Maintenance of d-alanine ester substitution of lipoteichoic acid by reesterification in Staphylococcus aureus. J Bacteriol. 1985;164:1211–1217. doi: 10.1128/jb.164.3.1211-1217.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambalot R H, Walsh C T. Cloning, overproduction, and characterization of the Escherichia coli holo-acyl carrier protein synthase. J Biol Chem. 1995;270:24658–24661. doi: 10.1074/jbc.270.42.24658. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Lara I M, Geiger O. Expression and purification of four different rhizobial acyl carrier proteins. Microbiology. 2000;146:839–849. doi: 10.1099/00221287-146-4-839. [DOI] [PubMed] [Google Scholar]
- 18.Mitharu A-L, Cox R J, Crosby J, Brom K J, Simpson T J. MCAT is not required for in vitro polyketide synthesis in a minimal actinorhodin polyketide synthase from Streptomyces coelicolor. Chem Biol. 1998;5:699–711. doi: 10.1016/s1074-5521(98)90663-9. [DOI] [PubMed] [Google Scholar]
- 19.Neuhaus F C. Inter-chain transacylation of d-alanine esters of lipoteichoic acid: a unique mechanism of membrane communication. Biochem Soc Trans. 1985;13:987–990. doi: 10.1042/bst0130987. [DOI] [PubMed] [Google Scholar]
- 20.Neuhaus F C, Heaton M P, Debabov D V, Zhang Q. The dlt operon in the biosynthesis of d-alanyl-lipoteichoic acid in Lactobacillus casei. Microb Drug Resist. 1996;2:77–84. doi: 10.1089/mdr.1996.2.77. [DOI] [PubMed] [Google Scholar]
- 21.Perego M, Glaser P, Minutello A, Strauch M A, Leopold K, Fischer W. Incorporation of d-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis: identification of genes and regulation. J Biol Chem. 1995;270:15595–15606. doi: 10.1074/jbc.270.26.15598. [DOI] [PubMed] [Google Scholar]
- 22.Peschel A, Vuong C, Otto M, Gotz F. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob Agents Chemother. 2000;44:2845–2847. doi: 10.1128/aac.44.10.2845-2847.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peschel A, Otto M, Jack R W, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 24.Shabarova Z A, Hughes N A, Baddiley J. The influence of adjacent phosphate and hydroxyl groups on amino acid esters. Biochem J. 1962;83:216–219. doi: 10.1042/bj0830216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spatafora G, Sheets A M, June R, Luyimbazi D, Howard K, Holbert R, Barnard D, El Janne M, Hudson M C. Regulated expression of the Streptococcus mutans dlt genes correlates with intracellular polysaccharide accumulation. J Bacteriol. 1999;181:2363–2372. doi: 10.1128/jb.181.8.2363-2372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wecke J, Perego M, Fischer W. d-Alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects autolytic activity. Microb Drug Resist. 1997;2:2953–2960. doi: 10.1089/mdr.1996.2.123. [DOI] [PubMed] [Google Scholar]
- 27.Zhou P, Florova G, Reynolds K A. Polyketide synthase acyl carrier protein (ACP) as a substrate and a catalyst for malonyl ACP biosynthesis. Chem Biol. 1999;6:577–584. doi: 10.1016/S1074-5521(99)80090-8. [DOI] [PubMed] [Google Scholar]