Abstract
Purpose of Review
Established telehealth practices in pediatrics and pediatric cardiology are evolving rapidly. This review examines several concepts in contemporary telemedicine in our field: recent changes in direct-to-consumer (DTC) pediatric telehealth (TH) and practice based on lessons learned from the pandemic, scientific data from newer technological innovations in pediatric cardiology, and how TH is shaping global pediatric cardiology practice.
Recent Findings
In 2020, the global pandemic of COVID-19 led to significant changes in healthcare delivery. The lockdown and social distancing guidelines accelerated smart adaptations and pivots to ensure continued pediatric care albeit in a virtual manner. Remote cardiac monitoring technology is continuing to advance at a rapid pace secondary to advances in the areas of Internet access, portable hand-held devices, and artificial intelligence.
Summary
TH should be approached programmatically by pediatric cardiac healthcare providers with careful selection of patients, technology platforms, infrastructure setup, documentation, and compliance. Payment parity with in-person visits should be advocated and legislated. Newer remote cardiac monitoring technology should be expanded for objective assessment and optimal outcomes. TH continues to be working beyond geographical boundaries in pediatric cardiology and should continue to expand and develop.
Keywords: Telehealth, Digital, Pediatric cardiology, Post-pandemic telemedicine, Global telehealth
Introduction
Technological innovations and widespread Internet connectivity have catapulted progress in telehealth (TH). A thorough scientific statement from the American Heart Association was published in 2017, outlining telemedicine in pediatric cardiology (1). In the last 5 years since then, there has been robust growth in applications of TH, with a peak during the lockdown phase of the pandemic. The purpose of this review is to explore the general progress in TH in the last 5 years, which includes this unique time period of the COVID-19 pandemic.
Direct-to-consumer telehealth
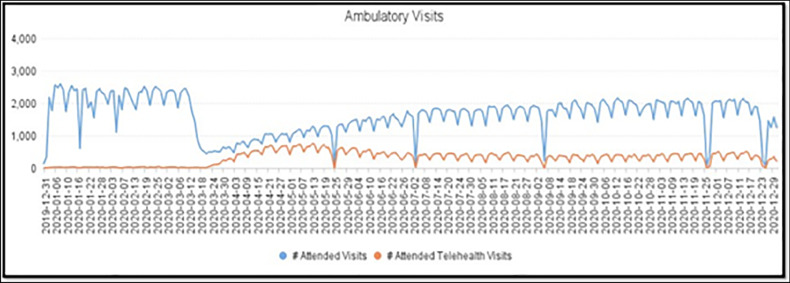
The field of pediatric cardiology has been a front runner in implementing telehealth to maximize access to care, efficiency, and patient satisfaction. Barriers to TH such as federal and state laws pertaining to privacy, licensing, insurance reimbursements, and organizational structures have received considerable attention but continue to be a challenge [2]. Recently, centralized departments for TH in hospitals as well as national and global initiatives have refocused efforts on improving access to healthcare in remote locations, management of chronic conditions, and shortening of patient wait periods. Social distancing guidelines during the COVID-19 pandemic provided the right impetus [3••, 4••] for the expansion of TH services. Direct-to-consumer TH is a broad term that encompasses various platforms and technologies that enable patients to obtain medical testing, consultation, and/or treatment via electronic media (e.g., computer, telephone, or smartphone) directly at their home. While tele-echocardiography, neonatal teleconsultations, and remote monitoring have been a routine practice for several years, DTC telecardiology visits have had the greatest scope for adoption during the pandemic. As an example, at Children’s Mercy Hospital, Kansas City, Missouri, USA, at its peak during the lockdown period, nearly 65% of ambulatory visits were performed through TH (Fig. 1). During this shift, patients continued to report very high levels of satisfaction. However, progress in TH and DTC visits has been nonuniform due to varying organizational support and resources, licensure requirements, and reimbursement. In this article, we share our experience and provide suggestions to conduct well-functioning telecardiology visits both domestically and internationally.
Fig. 1.
Number of ambulatory clinic visits at the Children’s Mercy Hospital in Kansas City, MO, vs weeks from December 31, 2019, to December 29, 2020. The blue line represents total visits, and the orange line represents the number of telehealth visits (mostly DTC). Note that the two lines are closest between mid-March 2020 to mid-May 2020 suggesting higher percentage of clinic visits (nearly 65%) being TH visits during that time. Image courtesy: Morgan Waller, Director of Telemedicine program at Children’s Mercy Hospital
Security and regulatory aspects of DTC telemedicine
Digital platforms that bring healthcare to the patient’s doorstep may pose a privacy threat. Choosing a secure platform is key. To date, the United States Department of Health and Human Services recognizes the following platforms as secure under the Health Insurance Portability and Accountability Act (HIPAA) of 1996: Skype for business/Microsoft Teams, Updox, VSee, Zoom for healthcare, Doxy.me, Google G Suite Hangouts Meet, Cisco Webex Meetings/Webex Teams, Amazon Chime, GoToMeeting, and Spruce Health Care Messenger. Physician licensing remains another barrier to telemedicine across state and national boundaries [5]. The provider needs a license to practice at the site of care, which is considered to be the patient’s location [2]. Interstate compacts are increasingly being used across the USA to aid physicians practicing in multiple states via TH [6].
Organizational preparation towards telehealth
Organizations need a formal business agreement with a chosen secure platform. Using multilinguistic platforms promotes equity and improves access to care. Workshops/training sessions should be provided regarding the new interface for all the team members, including trainees. System-wide education must address critical aspects of TH like virtual consent, documentation, and etiquette. The staff should be provided quiet zones where telemedicine visits can be conducted. Institutional websites should be designed to educate patients from all backgrounds regarding a TH visit and provide portals for document sharing and asynchronous communication.
Patient selection
TH visits are a great adjunct to in-person visits rather than simply an alternative [7••]. Careful selection of patients is needed to ensure high quality of care during TH visits. During the pandemic, TH has been particularly well received in preventive cardiology, cardiac neurodevelopmental (CND), and dysautonomia clinics [7••, 8, 9]. Patients have been assessed in their home environments during the visit, and factors influencing adherence further investigated in areas of the home, such as the kitchen, pantry, and home exercise areas. CND visits for children through hand-held devices put the patient at ease in their home environments, leading to accurate assessments [8]. TH visits have improved cost and time efficiency of CND clinics for screening, providing time-sensitive interventions and follow-up of established patients. DTC visits can provide novel counseling strategies using videos and infographics that appeal to adolescent patients. TH has been proven to enhance the patient–provider relationship by rendering the provider as more accessible and approachable, with options for asynchronous communication.
Pre-visit preparation
The TH visit requires preparation by the patient and provider to ensure efficiency and accurate assessments.
Patient’s responsibility
Digital consent must be obtained prior to TH visits. Pre-registration forms for demographics, review of systems, medication, and preferred pharmacy information are often provided in advance and requested to be completed prior to the visit. Additional questionnaires are often provided to the patient, particularly for CND assessments, and need to be filled out either prior to or during the visit [8]. A caregiver must accompany the patient for the entire visit. While TH saves patients the time and cost of travel and childcare for siblings, it is important to limit interruptions by surrounding family members during the visit. Patients should attempt to find a quiet place with good lighting during the visit.
Care team and provider’s responsibility
The provider has the responsibility to provide the best possible quality of care, uncompromised by the virtual interface. An in-depth chart review including a review of vital signs, growth data, and examination from any recent in-person encounters as well as pre-filled patient questionnaires can aid in accurate assessments. Information from home pulse oximeters or manometers should be considered. The provider should remain in a quiet area with proper lighting, wear professional attire, and silence non-emergency notifications. Ancillary asynchronous testing, if indicated, should be considered prior to the visit. In USA, the patient’s location needs to be confirmed, prior to the visit, to match the provider’s medical license.
Role of ancillary staff
Office personnel with knowledge of or interest in TH, documentation, and billing are best suited to participate in TH visits. The staff member must ensure that all the data provided by the patient are available for review before the visit. The staff member preferably meets the patient at the beginning of the visit to explain the proceedings including the layout of the digital platform at the consumer end. The need for a language interpreter should be assessed prior to the visit.
During the visit
Technology
Devices used must have front-facing cameras. Caregivers of young children are advised to use devices with both front/back facing cameras to help assess children as they move around the home. Any technical challenges should be clearly communicated. Information from consumer devices such as Apple watches/Fitbit may be considered for vital signs and heart rate variability [7••].
Clinical encounter/assessment
Detailed history taking is not compromised but enhanced by DTC visits due to the information often gained about the patient’s living conditions and home environment. In addition, good-quality video conferencing allows overall assessment, inspection of color and work of breathing of the patient, and a qualitative assessment of nutritional status. Limited neurological and behavioral assessments are possible with the advantage of having the patient at ease in their home. Patients with home monitoring devices that provide vital information are well suited for follow-up visits that don’t require further investigations. The use of technology in TH assessments is discussed further in a later section.
Telehealth complements in-person visits
While telemedicine cannot replace in-person provider–patient interactions, it may be preferred in some scenarios. Because patients’ perceptions and acceptance of digital counseling are variable and individual, providers must cater to a diverse patient population, including those who may be skeptical of virtual care visits. The opportunity for virtual multidisciplinary meetings with the patient and caregiver promotes patient-centered care models. Overall, DTC visits have proven highly effective in this regard as seen by high patient satisfaction rates and no reports of resultant adverse outcomes [10•, 11••, 12]. While the aim of DTC visits is to improve efficiency, it must not compromise quality of care. If an in-person evaluation or investigation is needed, it must be arranged without hesitancy. Chronic patients may need both periodic in-person and virtual visits; the combination may limit the patient’s lost hours of school or work and prove to be more efficient.
After the telehealth visit
Billing and coding
Documentation should reflect the complexity of care provided and time spent for the visit and should mention limitations in assessments. Designated billing and reimbursement payments for DTC virtual care that are on par with those for in-person visits should be universally established.
Reimbursement
DTC visits are attractive to self-paying patients and insurance companies due to the partially or completely waived facility fee. In the past, DTC visits were notorious for lower reimbursement rates despite their cost efficiency [13]. The pandemic has led to more appropriate reimbursement rates for DTC visits, which has been an excellent incentive for further progress. However, Medicaid has recently significantly reduced reimbursement rates for TH when compared to commercial insurance. We reviewed a total of 49 recent TH encounters between 12/01/2021 and 02/28/2022 for average reimbursements as a % of charges billed for CPT codes 99,212–99,205 with TH modifier code at our institution. While the reimbursement rate % was 23.8% for Blue Cross Blue Shield, it was 7.5% for Kansas Medicaid and 4.7% for Missouri Medicaid.
Malpractice
Despite the limited assessment and interaction in DTC visits, reports of malpractice suits in telemedicine have been extremely rare. On the contrary, DTC visits have resulted in high patient satisfaction rates. This phenomenon may be due to improved provider transparency and approachability in DTC visits, in addition to a high level of caution exercised by providers during TH assessments. In the USA, it must be noted that malpractice laws from both provider and patient location are applicable to the encounter, unlike licensing regulations.
Challenges
A key factor to the sustainability of TH is its appropriate use. DTC visits are not universally suitable for pediatric cardiology; e.g., referrals for new murmurs and for follow-up of known congenital heart disease often need in-person imaging and testing. Some devices used for teleauscultation have shown variable reliability among children [2]. While the rates of reimbursement have improved from the past, they remain nonuniform with little information on the future trend. Infrastructure needed for DTC visits continues to be unavailable among smaller organizations, thus limiting access to care in some locations [2]. Social and language barriers in addition to inadequate health literacy limit access to telemedicine among lower socio-economic groups, the population that needs it the most [14].
Technology in pediatric cardiology: the present and future in telehealth
Technology and innovation remain the key drivers of telecardiology [1••]. Explosive growth continues in technology, permeating nearly every aspect of the field, from areas such as advanced TH platforms, which are enhancing the way we currently experience virtual visits, to wearable technology and many other areas in-between. All these advances ultimately contribute to enabling patient and family empowerment and allowing optimal engagement in their own healthcare. Beyond mobile applications on smartphones and tablets, the widespread adoption of technology incorporated into the daily lives of patients has made it easier than ever to offer quick “virtual office” check-ins and closely monitor high-risk patients, rather than frequent in-person follow-up visits. This provides meaningful, relevant, and timely communication between the patient and their physician. Without question, and not surprisingly, the onset of the COVID-19 pandemic created a noticeable increase in the use and adoption of telecardiology.
While in-person care is undoubtedly necessary for certain situations, digital technology helps overcome key challenges and limitations for patient care provided via virtual visits. Evaluation of a patient or parts of their care that were previously thought to require in-person clinic visits is now easily accomplished through remote and mobile tools that are available to patients in the comfort of their own home.
Electrophysiology
The identification of clinically relevant cardiac arrhythmias has come a long way since the 1960s, when Holter’s vision of remote ambulatory ECG became a reality [15]. Today, continuous ambulatory monitors are ubiquitous, and the field of cardiac electrophysiology is at the forefront of advanced digital technologies. Patients routinely use medical devices and wearable technology, along with advanced healthcare mobile apps, in order to take advantage of “behind the scenes” computer algorithms that synthesize the data [16]. The available devices range from low-profile monitors such as Baxter’s BardyDx and iRhythm’s Ziopatch to truly innovative wearables like Healthwatch’s hWear 3–15 lead ECG T-shirt, Cardioskin’s 12-lead smart T-shirt, or Carré Techologies’ Hexoskin that reportedly provide continuous cardiac, pulmonary, and sleep data. For the curious consumer, devices such as the AliveCor’s KardiaMobile, Qardio’s QardioCore, and various ECG watchbands (like Apple Watch) do not require a physician’s prescription and are often patient-initiated purchases, marketed directly at the customer. Given the often-paroxysmal nature of arrhythmias in children, coupled with the difficulty in capturing these episodes at times, it is understandable that these wearable devices continue to gain popularity.
Ongoing research continues to show that compared with traditional measures, these innovative newer devices are similarly capable of passively or actively acquiring high-quality rhythm strips, transmitting them wirelessly, and enabling a remote electrophysiologist to promptly review them [17]. For example, a clinical trial assessing the Cardioskin smart T-shirt in 30 healthy subjects (age 18 and older) studied a 3-min period of rest in 3 positions (supine, seated, standing) as well as while walking. The authors demonstrated during this short duration that 12‐lead ECG acquisition was comparable to that of a Holter recording [18]. Similarly, a single-center study from Washington University in St. Louis assessed the utility of KardiaMobile (KM) — a single-channel or 6-lead ECG tracing with an algorithm approved by the Food and Drug Administration for detecting atrial fibrillation in adults[19] — in the pediatric population. The researchers obtained a standard 12-lead ECG followed by a 30-s KM tracing in 30 patients (67% had structural and/or conduction abnormalities) and found that a majority of the KM tracings (27/30, 90%) were of diagnostic quality on first attempt [20]. It seems clear that TH electrophysiology will continue to expand as part of mainstream practice, and it will be interesting to see if the advancement of AI and machine learning equates to even further advances in this area.
Echocardiography
Tele-echocardiography continues to be used across the ages and subspecialties in pediatric cardiology. Its benefit to fetal and neonatal cardiology has been previously described, as have the implications for training of sonographers in community-based settings [1••]. While the possibility of remote-controlled echocardiography probes with robotic arms in the absence of a trained sonographer remains on the horizon [21], members of the care team, specifically parents, continue to be trained to obtain high-quality diagnostic and surveillance images. In fact, a recent clinical trial by Chen et al. showed that parents were able to obtain adequate focused echocardiographic images via a hand-held device on their children with Marfan syndrome for more dynamic and complete TH visits [22]. Similarly, parents who received 1-h training with a hand-held device were able to acquire adequate parasternal short-axis and apical views for qualitative assessment of LV systolic function with no discrepancy compared with clinical echocardiograms in their children who were heart transplant recipients [23]. Although quantitative assessment of LV systolic function and retention of the skill set without additional training were reported to be suboptimal, capitalizing on opportunities afforded by advancements in portable echocardiography and telemedicine offers an incredible opportunity to enhance patient care and remote surveillance and to empower parents in the process.
Home monitoring programs
The success of interstage home monitoring programs for infants with single-ventricle physiology has led this practice to become a defining framework and model for close telemedical surveillance of the medically fragile and complex infant [24]. At present, with the widespread use of telemedicine and virtual visits, Foster et al. showed that incorporating the traditionally collected objective data (such as daily weight, oxygen saturation, and enteral intake) with the contextual narrative of a caregiver via video visits and video/photo sharing enhanced the overall impact of the program [25••]. Families that participated in this study felt reassured by the added oversight these virtual visits provided. They also identified logistical and clinical value to these virtual visits compared to simply transmitting the objective data.
Additionally, telemedicine-enabled technologies such as web-based exercise programs have also emerged as a novel way to engage the patient population outside inpatient and clinic-based settings. A large and growing body of literature continues to reiterate the positive impact of cardiac rehabilitation and exercise training programs in children with Congenital heart defects [CHD] both before and after cardiac surgery [26, 27, 28]. More recently, Meyer et al. studied a 24-week E-health exercise intervention program in 70 pediatric patients with moderate or complex CHD [29]. Although they found no improvement in health-related physical fitness or health-related quality of life scores, it is notable that 60 min of exercise per week was found to be safe without any adverse events in their patient population. Similarly, in those with Fontan circulations, routine exercise improves exertional capacity and self-reported quality of life [30]. While many of these training programs are hospital-based, home-based programs were found to be equally efficacious and safe [31]. Leveraging telemedicine and advances in technology, Khoury et al. were able to use a novel telemedicine ergometer, MedBIKE, to establish a high-intensity interval training protocol in children with Fontan physiology. The study found similar physiological responses as compared to a traditional cardiopulmonary exercise test ergometer [32]. It is notable that the MedBIKE is a custom telemedicine ergometer, incorporating a video game platform and live feed of patient video/audio, electrocardiography, pulse oximetry, and power output for remote medical supervision and modulation of work. Similar interventions have also been applied to the pediatric heart transplant population [33].
In the context of the COVID-19 pandemic, many pediatric solid transplant recipients, including those who received an orthotopic heart transplant and were deemed high risk due to their immunocompromised state, benefited greatly from receiving continued care through TH [34, 35]. Those exposed to the COVID-19 virus could additionally be monitored or triaged through virtual visits in an effort to further minimize their risk. The development, implementation, and success of programs such as these suggest that similar remote TH programs may develop for other pediatric cardiac patient populations for which we provide care.
Teleauscultation
Teleauscultation remains the subject of scrutiny across a variety of studies due to various perceived challenges. Nonetheless, researchers and cardiologists alike continue to highlight the improvements in technology that are enabling high-fidelity acquisition and transmission of a variety of heart sounds and murmurs for the purpose of physician interpretation. A recent study by Behere et al. demonstrated that sounds recorded by Eko’s Core stethoscope revealed a high percentage of agreement with in-person auscultation and echocardiographic findings. There was moderate inter-rater reliability, as well as an ability to discern major types of pathological murmurs [36]. Additionally, a large study conducted in rural China provides further evidence that screening for heart murmurs can be accomplished using a digital stethoscope (HeartLink teleauscultation system) and an Internet-based transmission in order to deliver phonocardiograms to an experienced observer. The authors reported overall test accuracy of 91%, with 78.5% sensitivity and 92.6% specificity [37]. Teleauscultation will likely remain an important part of TH despite various challenges and offers hope for expanding the quality of pediatric cardiac TH encounters provided in low-resource settings.
Global telehealth
Cardiovascular disease is one of the most common causes of global mortality [38] and reduced quality of life [39] and occurs in younger age groups in low-middle income countries (LMICs) [40]. Hypertensive disease, rheumatic heart disease (RHD), and dilated cardiomyopathies account for over 75% of cases of heart failure in Africa [41, 42]. CHD is also an important cause of morbidity and mortality in the young, especially in children under 5 years of age [43, 44]. Over 40 million people worldwide are living with RHD, and RHD is responsible for over 300,000 deaths and 10 million disability adjusted life years each year [38]. Between 15 and 40 of 1000 children in sub-Saharan Africa have evidence of early RHD when penicillin prophylaxis has the potential to prevent late complications [45, 46]. Unfortunately, many patients present late with heart failure as young adults, secondary to advanced heart valve disease [47, 48].
The management of cardiovascular disease in LMICs requires access to specialists for accurate diagnosis and timely initiation of therapy [49, 50]. While cardiologists trained in echocardiography provide care in capital cities throughout sub-Saharan Africa and other LMICs, 80% of the population lives in rural settings [51, 52]. Specialists in rural areas are rare, resulting in long waiting times, substantial transportation costs, and high out-of-pocket payments, limiting universal access to care [41, 53, 54]. Decentralization of care through task shifting has demonstrated promise for improving access in this setting [55]. Task shifting can improve care by increasing access, decreasing cost, and freeing higher-level providers to engage in more complex tasks [56]. The combination of task shifting and innovative telemedicine (that can overcome challenges from limited bandwidth), primarily tele-echocardiography, can provide remote populations in LMICs expanded access to medical services [55, 57]. A recently published randomized controlled trial showed that penicillin prevents progression of valvular disease in children with latent RHD (detected by echocardiography prior to clinical findings) [58], providing the most compelling evidence to date for scaling early detection programs. Task shifting of focused echocardiography to non-physician workers with limited training using highly portable hand-held ultrasound devices is feasible for the diagnosis of RHD in LMICs [59, 60, 61]. Using TH (most commonly asynchronous type of telecardiology) to complement task shifting holds the potential to allow cardiologists in tertiary care centers around the world to provide consultative services to patients with RHD and other cardiovascular diseases [62]. Sharing of images via cloud-based technology can advance research and clinical collaboration [57, 61]. More rapid, portable, and innovative uses of tele-echocardiography can provide remote populations in LMICs expanded access to medical services. These include enhanced data compression technology [63], novel training methods for international support [64], and the use smartphones for near-instantaneous image review [65]. Artificial intelligence focused on both image acquisition guidance [66•] and automatic diagnoses [67] can further increase the power of telemedicine for the detection of RHD and other CVD.
Many publications from high-income countries describe telemedicine use for echocardiography interpretation, education, and training [1••,68–79]. A recent publication from Uganda described the clinical characteristics, technical implementation, patient/parent satisfaction, and cost of a telecardiology program. Patients received an electrocardiogram and echocardiogram (Philips Lumify) performed by a local nurse in Gulu (northern Uganda) which were stored and transmitted using a cloud-storage/PAC solution (Imaging the World [80]) to the Uganda Heart Institute in the capital of Kampala (6-h drive from Gulu) for remote consultation by a cardiologist. Results were relayed to patients/families following cardiologist interpretation. A total of 1324 patients utilized telemedicine services during the 2-year study period: including 140 children between 0 and 5 years old, 424 patients between 6 and 21 years old, 360 patients between 22 and 50, and 400 patients over the age of 50. Most of the visits, 1285 (97%), were new patient visits. Valvular heart disease (predominantly rheumatic heart disease) was the most common diagnosis in the older three age groups. Medications were prescribed to 31%, 31%, 24%, and 48% of patients in the four age groups. A thematic analysis of focus group transcripts displayed an overall acceptance and appreciation for telemedicine, citing cost- and time-saving benefits. The cost of telemedicine was $29.48/visit. In summary, these data show that transmission and interpretation of echocardiograms from a remote clinic in northern Uganda are feasible, serve a population with a high burden of heart disease, have a significant impact on patient care, are favorably received by patients, and can be delivered at low cost.
Conclusion
Current and upcoming telehealth technologies greatly enhance the quality of care for children and adults with congenital heart disease. Telehealth continues to open new avenues of healthcare access for vulnerable populations globally in a cost-effective manner. There has been pivot to greater utilization of DTC telehealth during the pandemic lockdown with considerable success. With appropriate reimbursement regulations, the healthcare systems will be able to maintain and build on telehealth infrastructure for DTC visits and, ultimately, enhance optimal and inclusive access to care.
Acknowledgements
We thank the Medical Writing Center at Children's Mercy Kansas City for editing this manuscript.
Declarations
Conflict of interests
Sanket S. Shah declares no competing interests. Amulya Buddhavarapu declares no competing interests. Majid Husain declares no competing interests. Craig Sable declares no competing interests. Gary Satou declares no competing interests.
Footnotes
Key points
• Telehealth in pediatric cardiology continues to be robust and multi-dimensional.
• Increasing broadband access and novel cardiac technologies are optimizing the TH experience for patients and providers.
• Time-efficient and cost-saving aspects of TH are facilitating global pediatric cardiovascular disease care.
This article is part of the Topical Collection on Cardiology/CT Surgery.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.••.Satou GM, Rheuban K, Alverson D et al. Telemedicine in pediatric cardiology: a scientific statement from the American Heart Association. Circulation 2017. This is the most comprehensive and most cited manuscript for Pediatric cardiac telehealth. [DOI] [PubMed]
- 2.Olson CA, McSwain SD, Curfman AL, Chuo J. The current pediatric telehealth landscape. Pediatrics 2018;141. [DOI] [PubMed]
- 3.••.Cohen E, Cohen MI. COVID-19 will forever change the landscape of telemedicine. Curr Opin Cardiol. 2021;36:110. doi: 10.1097/HCO.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 4.••.Niaz T, Hope K, Fremed M, et al. Role of a pediatric cardiologist in the COVID-19 pandemic. Pediatr Cardiol. 2021;42:19–35. doi: 10.1007/s00246-020-02476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Hahsd. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. 2021.
- 6.US Hahsd. Telehealth licensing requirements and interstate compacts. 2021.
- 7.••.Chowdhury D, Hope KD, Arthur LC, et al. Telehealth for pediatric cardiology practitioners in the time of COVID-19. Pediatr Cardiol. 2020;41:1081–1091. doi: 10.1007/s00246-020-02411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox SM, Butcher JL, Sadhwani A, et al. Integrating telehealth into neurodevelopmental assessment: a model from the Cardiac Neurodevelopmental Outcome Collaborative. J Pediatr Psychol. 2022;47:707–713. doi: 10.1093/jpepsy/jsac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colglazier E, Brown A. PH professional network: the benefits and challenges in delivering telehealth in pediatric pulmonary hypertension. Adv Pulm Hypertens. 2021;20:22–25. doi: 10.21693/1933-088X-20.1.22. [DOI] [Google Scholar]
- 10.•.Rangachari P, Mushiana SS, Herbert K. A narrative review of factors historically influencing telehealth use across six medical specialties in the United States. Int J Environ Res Public Health. 2021;18:4995. doi: 10.3390/ijerph18094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.••.Mejia E, Zalewski J, Plummer ST. Adapting interstage home monitoring with the use of telemedicine during the COVID-19 pandemic. Pediatr Cardiol. 2022;43:1136–1140. doi: 10.1007/s00246-022-02835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curfman AL, Hackell JM, Herendeen NE et al. Telehealth: improving access to and quality of pediatric health care. Pediatrics 2021;148. [DOI] [PMC free article] [PubMed]
- 13.Phillips AA, Sable CA, Atabaki SM, et al. Ambulatory cardiology telemedicine: a large academic pediatric center experience. J Investig Med. 2021;69:1372–1376. doi: 10.1136/jim-2021-001800. [DOI] [PubMed] [Google Scholar]
- 14.Franciosi EB, Tan AJ, Kassamali B, et al. The impact of telehealth implementation on underserved populations and no-show rates by medical specialty during the COVID-19 pandemic. Telemedicine and e-Health. 2021;27:874–880. doi: 10.1089/tmj.2020.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corday E, Bazika V, Lang T-W, et al. Detection of phantom arrhythmias and evanescent electrocardiographic abnormalities: use of prolonged direct electrocardiocording. JAMA. 1965;193:417–421. doi: 10.1001/jama.1965.03090060007001. [DOI] [PubMed] [Google Scholar]
- 16.Tarakji KG, Silva J, Chen LY, et al. Digital health and the care of the patient with arrhythmia: what every electrophysiologist needs to know. Circ: Arrhythmia Electrophysiol. 2020;13:e007953. doi: 10.1161/CIRCEP.120.007953. [DOI] [PubMed] [Google Scholar]
- 17.Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol. 2018;41:487–494. doi: 10.1111/pace.13317. [DOI] [PubMed] [Google Scholar]
- 18.Fouassier D, Roy X, Blanchard A, Hulot JS. Assessment of signal quality measured with a smart 12-lead ECG acquisition T-shirt. Ann Noninvasive Electrocardiol. 2020;25:e12682. doi: 10.1111/anec.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galloway CD, Albert DE, Freedman SB. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165:193–194. doi: 10.1016/j.ijcard.2013.01.220. [DOI] [PubMed] [Google Scholar]
- 20.Gropler MR, Dalal AS, Van Hare GF, Silva JNA. Can smartphone wireless ECGs be used to accurately assess ECG intervals in pediatrics? A comparison of mobile health monitoring to standard 12-lead ECG. PLoS ONE. 2018;13:e0204403. doi: 10.1371/journal.pone.0204403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boman K, Olofsson M, Forsberg J, Boström S-Å. Remote-controlled robotic arm for real-time echocardiography: the diagnostic future for patients in rural areas? Telemed e-Health. 2009;15:142–147. doi: 10.1089/tmj.2008.0079. [DOI] [PubMed] [Google Scholar]
- 22.Chen A, Punn R, Collins RT, et al. Tele-clinic visits in pediatric patients with Marfan syndrome using parentally acquired echocardiography. J Pediatr. 2021;232:140–146. doi: 10.1016/j.jpeds.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Dykes JC, Kipps AK, Chen A, et al. Parental acquisition of echocardiographic images in pediatric heart transplant patients using a handheld device: a pilot telehealth study. J Am Soc Echocardiogr. 2019;32:404–411. doi: 10.1016/j.echo.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Rudd NA, Ghanayem NS, Hill GD, et al. Interstage home monitoring for infants with single ventricle heart disease: education and management: a scientific statement from the American Heart Association. J Am Heart Assoc. 2020;9:e014548. doi: 10.1161/JAHA.119.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.••.Foster CC, Steltzer M, Snyder A, et al. Integrated multimodality telemedicine to enhance in-home care of infants during the interstage period. Pediatr Cardiol. 2021;42:349–360. doi: 10.1007/s00246-020-02489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer M, Brudy L, García-Cuenllas L, et al. Current state of home-based exercise interventions in patients with congenital heart disease: a systematic review. Heart. 2020;106:333–341. doi: 10.1136/heartjnl-2019-315680. [DOI] [PubMed] [Google Scholar]
- 27.Singh T, Curran T, Rhodes J. Cardiac rehabilitation improves heart rate recovery following peak exercise in children with repaired congenital heart disease. Pediatr Cardiol. 2007;28:276–279. doi: 10.1007/s00246-006-0114-0. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes J, Curran TJ, Camil L, et al. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics. 2005;116:1339–1345. doi: 10.1542/peds.2004-2697. [DOI] [PubMed] [Google Scholar]
- 29.Meyer M, Brudy L, Fuertes-Moure A, et al. E-health exercise intervention for pediatric patients with congenital heart disease: a randomized controlled trial. J Pediatr. 2021;233:163–168. doi: 10.1016/j.jpeds.2021.01.058. [DOI] [PubMed] [Google Scholar]
- 30.Cordina RL, O'Meagher S, Karmali A, et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2013;168:780–788. doi: 10.1016/j.ijcard.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland N, Jones B, Aguero SW, et al. Home-and hospital-based exercise training programme after Fontan surgery. Cardiol Young. 2018;28:1299–1305. doi: 10.1017/S1047951118001166. [DOI] [PubMed] [Google Scholar]
- 32.Khoury M, Phillips DB, Wood PW, et al. Cardiac rehabilitation in the paediatric Fontan population: development of a home-based high-intensity interval training programme. Cardiol Young. 2020;30:1409–1416. doi: 10.1017/S1047951120002097. [DOI] [PubMed] [Google Scholar]
- 33.Chen AC, Rosenthal DN, Couch SC, et al. Healthy hearts in pediatric heart transplant patients with an exercise and diet intervention via live video conferencing—design and rationale. Pediatr Transplant. 2019;23:e13316. doi: 10.1111/petr.13316. [DOI] [PubMed] [Google Scholar]
- 34.Chen AC, Tierney ESS. Telehealth in pediatric heart transplant patients: exercise, nutrition, and parental imaging. Pediatr Clin. 2020;67:635–639. doi: 10.1016/j.pcl.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Doná D, Torres Canizales J, Benetti E, et al. Pediatric transplantation in Europe during the COVID-19 pandemic: early impact on activity and healthcare. Clin Transplant. 2020;34:e14063. doi: 10.1111/ctr.14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behere S, Baffa JM, Penfil S, Slamon N. Real-world evaluation of the Eko electronic teleauscultation system. Pediatr Cardiol. 2019;40:154–160. doi: 10.1007/s00246-018-1972-y. [DOI] [PubMed] [Google Scholar]
- 37.Pyles L, Hemmati P, Pan J, et al. Initial field test of a cloud-based cardiac auscultation system to determine murmur etiology in rural China. Pediatr Cardiol. 2017;38:656–662. doi: 10.1007/s00246-016-1563-8. [DOI] [PubMed] [Google Scholar]
- 38.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okello S, Abeya FC, Lumori BAE, et al. Validation of heart failure quality of life tool and usage to predict all-cause mortality in acute heart failure in Uganda: the Mbarara heart failure registry (MAHFER) BMC Cardiovasc Disord. 2018;18:1–10. doi: 10.1186/s12872-018-0959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aliku TO, Lubega S, Namuyonga J, et al. Pediatric cardiovascular care in Uganda: current status, challenges, and opportunities for the future. Ann Pediatr Cardiol. 2017;10:50. doi: 10.4103/0974-2069.197069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damasceno A, Cotter G, Dzudie A, et al. Heart failure in sub-Saharan Africa: time for action. J Am Coll Cardiol. 2007;50:1688–1693. doi: 10.1016/j.jacc.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Sani MU, Davison BA, Cotter G, et al. Echocardiographic predictors of outcome in acute heart failure patients in sub-Saharan Africa: insights from THESUS-HF. Cardiovasc J Afr. 2017;28:60–67. doi: 10.5830/CVJA-2016-070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman J. The global burden of congenital heart disease. Cardiovasc J Afr. 2013;24:141–145. doi: 10.5830/CVJA-2013-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/S0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 45.Beaton A, Lu JC, Aliku T, et al. The utility of handheld echocardiography for early rheumatic heart disease diagnosis: a field study. Eur Heart J Cardiovasc Imaging. 2015;16:475–482. doi: 10.1093/ehjci/jeu296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beaton A, Okello E, Lwabi P, et al. Echocardiography screening for rheumatic heart disease in Ugandan schoolchildren. Circulation. 2012;125:3127–3132. doi: 10.1161/CIRCULATIONAHA.112.092312. [DOI] [PubMed] [Google Scholar]
- 47.Okello E, Longenecker CT, Beaton A, et al. Rheumatic heart disease in Uganda: predictors of morbidity and mortality one year after presentation. BMC Cardiovasc Disord. 2017;17:20. doi: 10.1186/s12872-016-0451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okello E, Wanzhu Z, Musoke C, et al. Cardiovascular complications in newly diagnosed rheumatic heart disease patients at Mulago Hospital. Uganda Cardiovasc J Afr. 2013;24:80–85. doi: 10.5830/CVJA-2013-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwan GF, Bukhman AK, Miller AC, et al. A simplified echocardiographic strategy for heart failure diagnosis and management within an integrated noncommunicable disease clinic at district hospital level for sub-Saharan Africa. JACC Heart Fail. 2013;1:230–236. doi: 10.1016/j.jchf.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Carlson S, Duber HC, Achan J, et al. Capacity for diagnosis and treatment of heart failure in sub-Saharan Africa. Heart. 2017;103:1874–1879. doi: 10.1136/heartjnl-2016-310913. [DOI] [PubMed] [Google Scholar]
- 51.Frenk J, Chen L, Bhutta ZA, et al. Health professionals for a new century: transforming education to strengthen health systems in an interdependent world. Lancet. 2010;376:1923–1958. doi: 10.1016/S0140-6736(10)61854-5. [DOI] [PubMed] [Google Scholar]
- 52.Freers J, Mayanja-Kizza H, Ziegler JL, Rutakingirwa M. Echocardiographic diagnosis of heart disease in Uganda. Trop Doct. 1996;26:125–128. doi: 10.1177/004947559602600310. [DOI] [PubMed] [Google Scholar]
- 53.Maro EE, Kaushik R. The role of echocardiography in the management of patients with congestive heart failure. "Tanzanian experience". Cent Afr J Med. 2009;55:35–39. doi: 10.4314/cajm.v55i5-8.63638. [DOI] [PubMed] [Google Scholar]
- 54.Oyoo GO, Ogola EN. Clinical and socio demographic aspects of congestive heart failure patients at Kenyatta National Hospital. Nairobi East Afr Med J. 1999;76:23–27. [PubMed] [Google Scholar]
- 55.DeWyer A, Scheel A, Otim IO, et al. Improving the accuracy of heart failure diagnosis in low-resource settings through task sharing and decentralization. Glob Health Action. 2019;12:1684070. doi: 10.1080/16549716.2019.1684070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogedegbe G, Gyamfi J, Plange-Rhule J, et al. Task shifting interventions for cardiovascular risk reduction in low-income and middle-income countries: a systematic review of randomised controlled trials. BMJ Open. 2014;4:e005983. doi: 10.1136/bmjopen-2014-005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beaton A, Okello E, Scheel A, et al. Impact of heart disease on maternal, fetal and neonatal outcomes in a low-resource setting. Heart. 2018;105:755–760. doi: 10.1136/heartjnl-2018-313810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beaton A, Okello E, Rwebembera J, et al. Secondary antibiotic prophylaxis for latent rheumatic heart disease. N Engl J Med. 2022;386:230–240. doi: 10.1056/NEJMoa2102074. [DOI] [PubMed] [Google Scholar]
- 59.Beaton A, Nascimento BR, Diamantino AC, et al. Efficacy of a standardized computer-based training curriculum to teach echocardiographic identification of rheumatic heart disease to nonexpert users. Am J Cardiol. 2016;117:1783–1789. doi: 10.1016/j.amjcard.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Diamantino A, Beaton A, Aliku T et al. A focussed single-view hand-held echocardiography protocol for the detection of rheumatic heart disease. Cardiol Young 2017:1–10. [DOI] [PubMed]
- 61.Ploutz M, Lu JC, Scheel J, et al. Handheld echocardiographic screening for rheumatic heart disease by non-experts. Heart. 2016;102:35–39. doi: 10.1136/heartjnl-2015-308236. [DOI] [PubMed] [Google Scholar]
- 62.Lopes EL, Beaton AZ, Nascimento BR, et al. Telehealth solutions to enable global collaboration in rheumatic heart disease screening. J Telemed Telecare. 2016;24:101–109. doi: 10.1177/1357633X16677902. [DOI] [PubMed] [Google Scholar]
- 63.Cavero E, Alesanco A, Castro L, et al. SPIHT-based echocardiogram compression: clinical evaluation and recommendations of use. IEEE J Biomed Health Inform. 2013;17:103–112. doi: 10.1109/TITB.2012.2227336. [DOI] [PubMed] [Google Scholar]
- 64.LaGrone LN, Sadasivam V, Kushner AL, Groen RS. A review of training opportunities for ultrasonography in low and middle income countries. Trop Med Int Health. 2012;17:808–819. doi: 10.1111/j.1365-3156.2012.03014.x. [DOI] [PubMed] [Google Scholar]
- 65.Choi BG, Mukherjee M, Dala P, et al. Interpretation of remotely downloaded pocket-size cardiac ultrasound images on a web-enabled smartphone: validation against workstation evaluation. J Am Soc Echocardiogr. 2011;24:1325–1330. doi: 10.1016/j.echo.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 66.•.Narang A, Bae R, Hong H, et al. Utility of a deep-learning algorithm to guide novices to acquire echocardiograms for limited diagnostic use. JAMA Cardiol. 2021;6:624–632. doi: 10.1001/jamacardio.2021.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martins J, Nascimento ER, Nascimento BR, et al. Towards automatic diagnosis of rheumatic heart disease on echocardiographic exams through video-based deep learning. J Am Med Inform Assoc. 2021;28:1834–1842. doi: 10.1093/jamia/ocab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finley JP, Sharratt GP, Nanton MA, et al. Paediatric echocardiography by telemedicine–nine years' experience. J Telemed Telecare. 1997;3:200–204. doi: 10.1258/1357633971931165. [DOI] [PubMed] [Google Scholar]
- 69.Fisher JB, Alboliras ET, Berdusis K, Webb CL. Rapid identification of congenital heart disease by transmission of echocardiograms. Am Heart J. 1996;131:1225–1227. doi: 10.1016/S0002-8703(96)90103-9. [DOI] [PubMed] [Google Scholar]
- 70.Houston A, McLeod K, Richens T, et al. Assessment of the quality of neonatal echocardiographic images transmitted by ISDN telephone lines. Heart. 1999;82:222–225. doi: 10.1136/hrt.82.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewin M, Xu C, Jordan M, et al. Accuracy of paediatric echocardiographic transmission via telemedicine. J Telemed Telecare. 2006;12:416–421. doi: 10.1258/135763306779378636. [DOI] [PubMed] [Google Scholar]
- 72.Sable C, Roca T, Gold J, et al. Live transmission of neonatal echocardiograms from underserved areas: accuracy, patient care, and cost. Telemed J. 1999;5:339–347. doi: 10.1089/107830299311907. [DOI] [PubMed] [Google Scholar]
- 73.Sable CA, Cummings SD, Pearson GD, et al. Impact of telemedicine on the practice of pediatric cardiology in community hospitals. Pediatrics. 2002;109:E3. doi: 10.1542/peds.109.1.e3. [DOI] [PubMed] [Google Scholar]
- 74.Scholz TD, Kienzle MG. Optimizing utilization of pediatric echocardiography and implications for telemedicine. Am J Cardiol. 1999;83:1645–1648. doi: 10.1016/S0002-9149(99)00171-X. [DOI] [PubMed] [Google Scholar]
- 75.Sobczyk WL, Solinger RE, Rees AH, Elbl F. Transtelephonic echocardiography: successful use in a tertiary pediatric referral center. J Pediatr. 1993;122:S84–S88. doi: 10.1016/S0022-3476(09)90049-X. [DOI] [PubMed] [Google Scholar]
- 76.Webb CL, Waugh CL, Grigsby J, et al. Impact of telemedicine on hospital transport, length of stay, and medical outcomes in infants with suspected heart disease: a multicenter study. J Am Soc Echocardiogr. 2013;26:1090–1098. doi: 10.1016/j.echo.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 77.Awadallah S, Halaweish I, Kutayli F. Tele-echocardiography in neonates: utility and benefits in South Dakota primary care hospitals. S D Med. 2006;59:97–100. [PubMed] [Google Scholar]
- 78.Krishnan A, Fuska M, Dixon R, Sable CA. The evolution of pediatric tele-echocardiography: 15-year experience of over 10,000 transmissions. Telemed J E Health. 2014;20:681–686. doi: 10.1089/tmj.2013.0279. [DOI] [PubMed] [Google Scholar]
- 79.McCrossan BA, Grant B, Morgan GJ, et al. Diagnosis of congenital heart disease in neonates by videoconferencing: an eight-year experience. J Telemed Telecare. 2008;14:137–140. doi: 10.1258/jtt.2008.003011. [DOI] [PubMed] [Google Scholar]
- 80.Horn D, Edwards E, Ssembatya R, et al. Association between antenatal ultrasound findings and neonatal outcomes in rural Uganda: a secondary analysis. BMC Pregnancy Childbirth. 2021;21:756. doi: 10.1186/s12884-021-04204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]