Abstract
This narrative review aims at providing an update on the management of inherited cerebellar ataxias (ICAs), describing main clinical entities, genetic analysis strategies and recent therapeutic developments. Initial approach facing a patient with cerebellar ataxia requires family medical history, physical examination, exclusions of acquired causes and genetic analysis, including Next-Generation Sequencing (NGS). To guide diagnosis, several algorithms and a new genetic nomenclature for recessive cerebellar ataxias have been proposed. The challenge of NGS analysis is the identification of causative variant, trio analysis being usually the most appropriate option. Public genomic databases as well as pathogenicity prediction software facilitate the interpretation of NGS results. We also report on key clinical points for the diagnosis of the main ICAs, including Friedreich ataxia, CANVAS, polyglutamine spinocerebellar ataxias, Fragile X-associated tremor/ataxia syndrome. Rarer forms should not be neglected because of diagnostic biomarkers availability, disease-modifying treatments, or associated susceptibility to malignancy. Diagnostic difficulties arise from allelic and phenotypic heterogeneity as well as from the possibility for one gene to be associated with both dominant and recessive inheritance. To complicate the phenotype, cerebellar cognitive affective syndrome can be associated with some subtypes of cerebellar ataxia. Lastly, we describe new therapeutic leads: antisense oligonucleotides approach in polyglutamine SCAs and viral gene therapy in Friedreich ataxia. This review provides support for diagnosis, genetic counseling and therapeutic management of ICAs in clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-022-11383-6.
Keywords: Cerebellar ataxia, Genetics, Next generation sequencing, Phenotype
Introduction
Inherited cerebellar ataxias (ICAs) belong to a large group of rare and complex neurodegenerative diseases affecting the cerebellum, but also frequently the spinal cord and the peripheral nerves. ICAs are clinically dominated by progressive cerebellar syndrome, that may lead to significant disability [1]. Most patterns of inheritance have been described in ICAs. Over the past 25 years, and especially since the advent of the next-generation sequencing (NGS), there has been a tremendous improvement in this field with the description of more than 100 new entities, most of them being recessively inherited [2, 3]. In addition, up to 500 genes have been related to neurological disease whose clinical picture may include cerebellar ataxia [2, 3]. Taken together, the prevalence of ICA is close to 1:10 000, making them a common motive for outpatient referral to neurogenetics center [4]. Because of the recent and rapid progression in cerebellar ataxia knowledge, periodic reviews are required to provide up-to-date guidance for the diagnosis and management of this disorder. We consequently aimed at providing such an update on ICA literature by presenting the main clinical entities, proposing genetic analysis strategies and describing the present and future therapeutic perspectives.
Initial approach to a patient with cerebellar ataxia
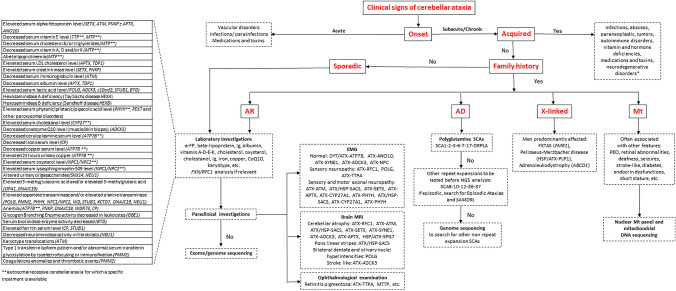
Cerebellar ataxia (CA) is usually diagnosed in a patient complaining gait and balance difficulties. Beside gait ataxia, neurological examination may reveal cerebellar dysarthria, dysmetria during nose–finger and heel–shin tests and hypotonia. Cerebellar eye signs are very frequent and can occur as the earliest clinical features suggesting the cerebellar motor syndrome [5, 6] (Video 1–7). Facing such patients, many acquired causes of CA should be ruled out before considering any genetic analysis (Table 1). The family history should then be investigated in three generations at least (Fig. 1). It should be kept in mind that the lack of family history of CA is frequent in autosomal-recessive cerebellar ataxia (ARCA). The more reliable the clinical status of each member of the family and the higher number of subjects whose DNA may be collected, the more likely to establish the molecular diagnosis.
Table 1.
Main causes of acquired cerebellar ataxia
| Acute ataxias | Vascular disorders |
Cerebellar ischemia Cerebellar hemorrhage |
| Medications and toxins |
Antiepileptic drugs (phenytoin, carbamazepine, oxcarbazepine, lacosamide, lamotrigine, zonisamide, rufinamide. With benzodiazepines, felbamate, phenobarbital and valproic acid in the setting of hyperammonemia) Chemotherapy (cytarabine, fluorouracil, capecitabine, hexamethylmelamine, procarbazine, vincristine, cisplatin and oxaliplatin) Antiarrhythmic (amiodarone, procainamide) Antibiotics (metronidazole, polymyxins) Toxins and poisons (alcohol, carbon tetrachloride, heavy metals, phencyclidine, toluene, pesticides) Lithium |
|
| Infections |
Acute cerebellitis (Epstein-Barr virus, varicella-zoster, herpes simplex virus 1, human herpesvirus 6, influenza A and B, mumps, coxsackie virus, rotavirus, echovirus, SARS-CoV2, enterovirus, hepatitis A, measles, parvovirus B19, typhoid fever, malaria) Bacterial infection (Mycoplasma pneumoniae, Listeria monocytogenes, Streptococcus pneumoniae, Neisseria meningitidis, tuberculosis) |
|
| Subacute ataxias | Autoimmune disorders |
Multiple sclerosis, ADEM, celiac disease/gluten ataxia, GAD antibody-associated ataxia, anti-NMDA receptor antibodies, anti-P/Q voltage-gated calcium channel antibodies, Homer-3 autoantibodies, contactin-associated protein-like 2 antibodies, anti-M-phase phosphoprotein-1 antibodies, Hashimoto thyroiditis/encephalopathy, histiocytosis X, anti-GQ1b antibody syndromes, Bickerstaff encephalitis, neurosarcoidosis, postinfectious cerebellitis, Behçet syndrome, polyarteritis nodosa, systemic lupus erythematosus, Sjögren syndrome Paraneoplastic cerebellar degeneration |
| Infections | Lyme disease, Whipple disease, JC virus, HIV, syphilis, tuberculosis and Prion disease | |
| Structural causes |
Primary or metastatic tumors Abscess Liver failure (hepatocerebral degeneration) |
|
| Chronic ataxias | Vitamin and hormone deficiencies |
Deficiency in vitamin B1 (Wernicke encephalopathy), B12, E Hypothyroidism, Hypoparathyroidism |
| Toxins | Alcohol, heavy metals, phencyclidine, toluene, solvents, pesticides | |
| Medications | Antiepileptic drugs, chemotherapy | |
| Infections | HIV, tuberculosis, syphilis, Lyme disease, Creutzfeldt–Jakob disease | |
| Autoimmune disorders | Progressive multiple sclerosis | |
| Neurodegenerative disorders | Multiple system atrophy, progressive supranuclear palsy | |
| Other | Arnold–Chiari malformation, normal pressure hydrocephalus, superficial siderosis, psychogenic ataxia |
ADEM acute disseminated encephalomyelitis, GAD glutamic acid decarboxylase, HIV human immunodeficiency virus
Fig. 1.
Flowchart of the diagnostic process in cerebellar ataxias. This flowchart shows the steps in evaluating a patient with cerebellar ataxia based on clinical and family history, physical evaluation and paraclinical tests. First, the onset should be differentiated between acute and subacute/chronic; second, the acquired causes have to be ruled out by paraclinical investigations as neuroimaging, blood and cerebrospinal fluid exams; third, if family history is positive for inherited cerebellar ataxia, the clinician should determine the transmission pattern by pedigree in three generations at least. Sporadic forms should be explored as recessive ones especially in the case of early-onset (before the age of 40). The main laboratory investigations for the diagnosis of autosomal-recessive cerebellar ataxias are reported in the table on the left. *For sporadic patients with unknown or censored family history, autosomal forms should be also considered. AD autosomal dominant, AR autosomal recessive, α-FP alpha-fetoprotein, CoQ10 Coenzyme Q10, Ig Immunoglobulin, Mt mitochondrial
Most patients with ICA are referred to neurologist, pediatrician or geneticist due to insidious symptoms occurrence associated with slowly progressive worsening of gait imbalance on several months or years. Rarely, ICA is characterized by acute onset, especially when related to inborn error of metabolism, such as nonketotic hyperglycinemia, pyruvate dehydrogenase deficiency, or mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS).
An algorithm for investigations into a patient with suspected cerebellar ataxia
Several algorithms [7, 8] have been proposed to guide the diagnosis workup and the genetic analysis in ICA. To ask for the age at onset and to assess the speed of disease progression according to the current functional disability is the first major step (Fig. 1). Among the most frequent ARCA, an early AO (< 10 years) is usual suggestive of ATX–ATM (ataxia telangiectasia), ATX/HSP–SACS (ARSACS) and ATX–APTX (AOA1) for instance while ATX–SETX (AOA2), ATX–SYNE1, ATX–ANO10 and POLG-related disease have an onset usually after 10 years of age. For ATX–FXN (Friedreich’s), the most frequent ARCA, age of onset generally falls in the first two decades. Regarding disease progression, patients affected with ATX–FXN, ATX–ATM, ATX–APTX are confined to wheelchair faster (in less than 15 years) than those with ATX–SYNE1, ATX–ANO10 or ATX/HSP–SACS. In autosomal dominant CA (ADCA), age at onset is usually earlier and disease progression slower in case of missense mutations (SCA5/STPBN2, SCA13/KCNC3, SCA14/PRKCG, SCA21/TMEM240, SCA28/AFG3L2) compared to SCAs due to CAG triplet repeat expansions (SCA3/ATXN3, SCA2/ATXN2, SCA1/ATXN1, SCA6/CACNA1A, SCA7/ATXN7, SCA17/TBP) whose age at onset is usually around 30 to 35 years but disease worsening faster. It should be pointed out that for SCA3 and SCA6, age of onset can be later as the sixth or seventh decade and for the latter the disease progression is the slowest among polyglutamine SCAs [9].
The collection of the clinical signs associated to CA is the second crucial step in the assessment (Table 2). When the formers are lacking, the following pure forms should be considered: SCA6/CACNA1A, SCA5/STPBN2, SCA11/TTBK2, SCA15–16/ITPR1, SCA22/KCND3, SCA 31/BEAN, SCA 41/TRCP3, ATX–SYNE1, ATX–ANO10.
Table 2.
List of diseases for which cerebellar ataxia is associated with other main clinical features
| Clinical feature | Diseases |
|---|---|
| Chorea |
ATX–ATM, ATX–APTX, ATX–SETX, ATX–MRE11A, ATX–OPA3 NBIA/DYT/PARK-CP XFE/ERCC4 SCA2/ATXN2 (large CAG expansion) SCA17/TBP, DRPLA/ATN1 (in the course of the disease) SCA48/STUB1 |
| Myoclonus |
ATX–ADCK3 MYC/ATX–GOSR2, MYC–SCARB2, MYC/ATX–KCTD7, MYC/ATX–NEU1 PRICKLE1 SCA2/ATXN2, DRPLA/ATN1 SCA13/KCNC3, SCA14/PRKCG, SCA19/KCND3, SCA21/TMEM240 |
| Dystonia |
ATX–ATM, ATX–APTX, ATX–SETX, ATX–NPC ATX/HSP–HEXA, ATX/HSP–HEXB, NBIA/DYT/PARK–PLA2G6 PNKP, MARS2 HSP/ATX/NBIA–FA2H DYT/ATX–ATP7B SCA2/ATXN2, SCA3/ATXN3, SCA17/TBP DYT/PARK/NBIA–PLA2G6 ATX–POLR3A/ATX–POLR3B |
| Parkinsonism |
ATX–ATM, ATX–CYP27A1 POLG DYT/ATX–ATP7B NBIA/DYT/PARK–PLA2G6 Fragile X-associated tremor ataxia syndrome (FXTAS) SCA2/ATXN2, SCA3/ATXN3, SCA17/TBP DYT/PARK/NBIA–PLA2G6 |
| Spastic paraplegia |
ATX–CYP27A1, ATX/HSP–SACS, ATX/HSP–SPG7, ATX/HSP–POLR3A, ATX/HSP–CLCN2, HSP/ATX/NBIA–FA2H MARS2, GBE1, MTPAP, ATX/HSP–DARS2, HSD17B4 DYT/PARK/NBIA–PLA2G6 |
| Pyramidal signs |
ATX–ANO10 SCA1/ATXN1, SCA3 /ATXN3, SCA7/ATXN7, SCA17/TBP SCA8/ATXN8, SCA10/ATXN10, SCA14/PRKCG, SCA15/ITPR1 SCA35/TGM6, SCA40/CCDC88C, SCA43/MME ATX–POLR3A/ATX–POLR3B |
| Oculomotor apraxia |
ATX–ATM, ATX–APTX, ATX–SETX, ATX–MRE11 AOA4/PNKP |
| Strabismus or diplopia |
ATX–SETX SCA3/ATXN3 |
| Square wave jerks |
ATX–FXN, POLG FXTAS SCA3/ATXN3 |
| Hypometric, slow saccades | SCA2/ATXN2 |
| Vertical supranuclear saccades palsy | ATX–NPC, ATX/HSP–SACS, ATX–STUB1 |
| Intellectual deficiency |
ATX–GRID2, ATX–L2HGDH, ATX–POLR3A, ATX–APTX ATX–KIAA0226, SCAR12/WWOX, ATX–SNX14, SCAR22/VWA3B, SCAR23/TDP2, MYC/ATX–KCTD7, ATX–PEX10, ATX/MYC–TPP1, ATX–SPTBN2, MARS2, ACO2, ATX–WDR73, MGR1, ATX–PMPCA, ATX–POLR3B, ATX–KCNJ10, SPAX4/MTPAP, OPA3, ATX–VLDLR, SCAR5/WDR73, ATX–CA8, ATX–BTD, NBIA/DYT/PARK–PLA2G6, SCA13/KCNC3, SCA19–22/KCND3, SCA21/TMEM240 |
| Cognitive decline |
ATX–NPC, ATX–CYP27A1, ATX–ANO10, ATX/HSP–PNPLA6, ATX–ADCK3 NBIA/DYT/PARK–CP, MYC/ATX–KCTD7, GRM1, NBIA/DYT/PARK–PLA2G6 SCA2/ATXN2, SCA17/TBP, DRPLA/ATN1, SCA48/STUB1 FXTAS |
Laboratory investigations and brain imaging
Numerous biological markers presented in Fig. 1 have been associated with CA, mainly with ARCA. Of note, some of these diseases, which are summarized in Table 3, are accessible to specific disease-modifying treatments, and should be consequently systematically searched for.
Table 3.
Treatments available for inherited cerebellar ataxias
| Disease | Treatment |
|---|---|
|
ATX–TTP (Ataxia with vitamin E deficiency) |
α-tocopherol (vitamin E) |
|
ATX–CYP27A1 (Cerebrotendinous Xanthomatosis) |
Chenodeoxycholic acid, ursodeoxycholic acid, cholic acid and taurocholic acid |
|
ATX–PHYH (Refsum’s disease) |
Diet with phytanic acid restriction, plasmapheresis for acute presentation |
|
ATX–NPC1 (Niemann–Pick disease type C1) |
Miglustat |
|
ATX–ADCK3 (ARCA2/SCAR9) |
Oral supplementation of coenzyme Q10 |
|
ATX–ATM (Ataxia–telangiectasia) ERCC4 (XFE progeroid syndrome) |
Avoid exposure to sun and radiations |
| DYT/ATX–ATP7B (Wilson’s disease) | D-penicillamine, trientine, zinc acetate/sulfate and liver transplantation in acute forms |
|
MTTP (Abetalipoproteinemia) |
Fat-soluble vitamins (vitamin A, E, D, K) low-fat diet |
|
PxMD–SLC2A1 (GLUT1 deficiency) |
Ketogenic diet and triheptanoin |
|
PxMD–KCNA1 (Episodic ataxia type 1) |
Carbamazepine |
|
PxMD–CACNA1A (Episodic ataxia type 2) |
Acetazolamide 4-aminopyridine or baclofen (useful for downbeat nystagmus treatment) |
Ophthalmological evaluation, including fundus and optical tomography coherence, might be helpful to identify peculiar ocular characteristics, such as optic atrophy in ATX–SPG7 [10] and cone rod dystrophy in SCA7 [11].
Electroneuromyography findings are of paramount importance for categorizing ARCAs [7], which can be divided into 3 groups (listed according to frequency of the genetic from among each group): (i) ARCA with sensory neuronopathy, such as ATX–FXN and CANVAS/RFC1, POLG-related diseases and ataxia with vitamin E deficiency (ATX–TTPA); (ii) ARCA with both sensory and motor axonal neuropathy, such as ATX–ATM, ATX/HSP–SACS, ATX–SETX, ATX–APTX, ATX–CYP27A1, ATX–PHYH; (iii) ARCA with a supplemental demyelinating component, such as ATX/HSP–SACS, ATX–CYP27A1 and ATX–PHYH. Patients displaying peripheral neuropathy are usually more severely disabled and more frequently experience falls. In ADCA, peripheral neuropathy is also frequently found in SCA3/ATXN3 (with frequent disabling pain) or SCA2/ATXN2 for instance.
Brain MRI allows to identify the presence or the absence of a marked cerebellar atrophy. Obvious cerebellar atrophy is reminiscent of ATX–ATM, ATX/HSP–SACS, ATX–SETX, ATX–SYNE1, ATX–ADCK3, ATX–APTX (which are ARCA), or SCA14/PKRG, SCA5/STBN2, SCA13/KCNC3 (which are ADCA due to missense mutations). Conversely, the lack of cerebellar atrophy is suggestive of ATX–FXN or ATX–TTPA. Facing a prominent pons atrophy combined with a more subtle cerebellar atrophy, CAs due to CAG triplet repeat expansions should be considered, such as (in order of relative frequency), SCA3/ATXN3, SCA2/ATXN2, SCA1/ATXN1, SCA7/ATXN7, SCA6/CACNA1A, SCA17/TBP and DRPLA/ATN1. Other imaging clues suggestive of a specific entity may also be found on brain MRI such as linear stripes of the pons in T2-weighted or FLAIR axial slides in ATX/HSP-SACS, white matter hyperintensities in corpus callosum, middle cerebellar peduncle and cerebral white matter in FXTAS/FMR1, bilateral dentate nuclei and olivary nuclei hyperintensities in POLG-related CA or ATX–PRDX3 as well as cortical hyperintensities during epileptic fits or stroke-like episodes in ATX–ADCK3.
Genetic analysis strategy
Expansion detection
ICA is the group of inherited diseases that includes the largest number of distinct short tandem repeat mutations, including dominant coding together with dominant and recessive noncoding expansions. Initial screening for the most frequent expansions, which are listed in Table 4, is an efficient initial approach for the diagnosis of ICA, even in the absence of a family history if the phenotype is thought compatible with these genes. Molecular diagnosis can be easily achieved by simple PCR fragment size analyses or by repeat-primed PCR amplifications, while this would be missed with classical short read exome sequencing. More sophisticated techniques including Southern blot analyses, long range PCR or repeat sequencing, might be required to detect some of these mutations, such as biallelic expansions in RFC1 responsible for CANVAS. Future developments of long-read genome sequencing are awaited to hopefully detect both expansions and point mutations.
Table 4.
Genetics and presentation of hereditary ataxias due to repeat expansions to be tested before NGS analysis
| Disease | Gene | Pathological expansion | Protein | Transmission | Age at onset | Clinical phenotype | MRI |
|---|---|---|---|---|---|---|---|
|
FA ATX–FXN |
FXN |
> 38 GAA FA: > 700 GAA LOFA: < 500 GAA |
Frataxin |
Recessive Mostly isolated case, or ≥ 2 sibs in same generation, unaffected parents |
FA: 7–25 y LOFA: 25–40 y VLOFA: > 40 y |
FA: sensory neuropathy, cerebellar ataxia, absent tendon reflexes, Babinski sign, scoliosis, pes cavus, impairment of position and vibratory senses, hearing loss, optic neuropathy, diabetes, cardiomyopathy LOFA: normal tendon reflexes, Babinski sign, spastic ataxia VLOFA: normal tendon reflexes, Babinski sign, spastic ataxia |
Spine atrophy |
|
CANVAS ATX–RFC1 |
RFC1 |
400–2000 AAGGG |
Replication factor C |
Recessive Mostly isolated case, or ≥ 2 sibs in same generation, unaffected parents |
54 y (35–73) | Cerebellar ataxia, sensory neuropathy, vestibular areflexia, chronic cough | Cerebellar vermis atrophy |
|
SCA3 ATX–ATXN3 |
ATXN3 | > 51 CAG | ATXN3 |
Dominant Founder mutation: Portugal (Azores Islands), Germany, Japan |
0–20 y: 11% 21–40 y: 43% > 40 y: 46% |
Small repeat: axonal neuropathy, dopa-responsive parkinsonism Medium repeat: cerebellar ataxia, pyramidal signs, diplopia Large repeat: dystonia, pyramidal signs Gaze-evoked nystagmus, hypometric saccades |
Cerebellar, brainstem and spine atrophy |
|
SCA2 ATX–ATXN2 |
ATXN2 | > 32 CAG | ATXN2 |
Dominant with several cases in successive generations. Juvenile case can be apparently isolated Founder mutation: Cuba, West Indies |
0–20 y: 17% 21–40 y: 45% > 40 y: 38% |
Small repeat: postural tremor Medium repeat: cerebellar ataxia, decreased reflexes Large repeat: cerebellar ataxia, chorea, dementia, myoclonus, dystonia Very large repeat: cardiac failure, retinal degeneration Slow saccades |
Cerebellar (vermis) and brainstem atrophy |
|
SCA6 ATX–CACNA1A |
CACNA1A | > 19 CAG | α1A-Subunit of voltage-dependent calcium channel of P/Q type |
Dominant, but can be censured due to late onset |
45y (19–73) |
Small repeat: Episodic ataxia Downbeat nystagmus |
Cerebellar atrophy |
|
SCA1 ATX–ATXN1 |
ATXN1 | > 38 CAG (without CAT interruption) | ATXN1 | Dominant with several cases in successive generations |
0–20 y: 15% 21–40 y: 42% > 40 y: 43% |
Medium repeat: cerebellar ataxia, pyramidal syndrome Large repeat: amyotrophic lateral sclerosis-like disorder Very large repeat: developmental delay Hypermetric saccades |
Cerebellar (vermis) and brainstem atrophy |
|
SCA7 ATX–ATXN7 |
ATXN7 | > 36 CAG | ATXN7 |
Dominant with several cases in successive generations. Juvenile case can be apparently isolated Founder mutation: Scandinavian countries, South Africa and Mexico |
0–20 y: 25% 21–40 y: 48% > 40 y: 27% |
Small repeat: cerebellar ataxia without visual loss Medium repeat: cerebellar ataxia, cone rod dystrophy Large repeat: visual loss (cone rod dystrophy) before cerebellar syndrome Very large repeat: cardiac and renal failure |
Cerebellar and brainstem atrophy |
|
SCA8 ATX–ATXN8 |
ATXN8 | CTA/CTG repeat in 3′ untranslated region | ATXN8 | Dominant | Cerebellar ataxia, pyramidal syndrome, sensory neuropathy, cognitive impairment, depression, | Cerebellar atrophy | |
|
SCA36 ATX–NOP56 |
NOP56 | > 650 GGCCTG (normal 3–14) | Nucleolar protein 56 | Dominant |
0–20 y: 21% 21–40 y: 10% > 40 y: 69% |
Cerebellar ataxia, amyotrophy, hearing loss | Cerebellar atrophy |
|
DRPLA ATX–ATN1 |
ATN1 | > 47 CAG | DRPLA | Dominant | 31y (1–67) |
Small repeat: chorea, ataxia, psychiatric manifestations Large repeat: progressive myoclonus, epilepsy, developmental delay, mild ataxia Very large repeat: myoclonic epilepsy, chorea, cognitive impairment |
Cerebellar and brainstem atrophy, white matter lesions in cerebrum, thalamus, globus pallidus |
|
SCA12 ATX–PPP2R2B |
PPP2R2B | CAG repeat in 5’ untranslated region | Protein phosphatase 2, regulatory subunit B | Dominant | 10–55 y | Cerebellar ataxia, tremor, dystonia, dementia, polyneuropathy | Cerebellar atrophy |
|
SCA31 ATX–TK2 |
BEAN–TK2 | Intronic TGGAA repeat insertion |
Brain-expressed protein associating with NEDD4 homologue |
Dominant | 56 y (45–72) | Pure cerebellar ataxia | Cerebellar atrophy |
|
SCA17 ATX–TBP |
TBP | > 48 CAG | TATA-box-1-binding protein | Dominant, incomplete penetrance | 34 y (3–75) |
Small repeat: Huntington’s disease-like phenotype, parkinsonism Medium repeat: ataxia, dementia, chorea and dystonia, pyramidal signs Large repeat: ataxia, dementia, spasticity, epilepsy Very large repeat: growth retardation |
Diffuse cerebral atrophy |
|
SCA10 ATX–ATXN10 |
ATXN10 | Intronic ATTCT repeat insertion | ATXN10 | Dominant | 10–40 y | Cerebellar ataxia, epilepsy | Cerebellar atrophy |
|
SCA37 ATX–DAB1 |
DAB1 | ATTTC insertion in 5’ untranslated region ranging from 31 to 75 repeats | Disabled homologue 1′ | Dominant | 48 y (18–64) | Cerebellar ataxia, saccadic pursuit (vertical > horizontal) | Cerebellar atrophy |
|
FXTAS ATX–FMR1 |
FMR1 | 55–200 CGG | Fragile X mental retardation protein | X-linked | > 50 y | Intention tremor, cerebellar ataxia, parkinsonism, axonal neuropathy, cognitive impairment |
White matter lesions in middle cerebellar peduncle, splenium of the corpus callosum and cerebrum Cerebral atrophy |
The autosomal-dominant ataxias are listed according to available data regarding worldwide prevalence
CANVAS Cerebellar ataxia, neuropathy and vestibular are flexia syndrome, DRPLA Dentatorubral–pallidoluysian atrophy, FA Friedreich ataxia, FXTAS Fragile X-associated tremor/ataxia syndrome, SCA Spinocerebellar ataxia, y years
Sanger sequencing
Dideoxynucleotide chain termination sequencing, also named Sanger sequencing, is a method of genetic sequencing that only allows detection of a few genetic variants per testing. Its use is now restricted to cases with prior knowledge of the defective gene (usually based on the biochemical screen, such as for ATX–TTPA or other metabolic diseases), confirmation of familial segregation for a mutation already identified in an affected relative, genetic counseling and confirmation of a mutation previously identified by Next-Generation Sequencing (NGS).
Next-generation sequencing
Next-Generation Sequencing (NGS) is defined by the parallel sequencing of multiple genes in a single test. Given the large number of genes involved in ICA and the important clinical overlap between the different entities, NGS has become the method of choice for the genetic diagnosis of ICA. Panel sequencing, which consists in the testing of a predefined list of genes, is a suboptimal option because of low positive yield and the absence of the most recently identified genes [12]. Thus, exome (all protein-coding genes), “Mendelian”-exome (all known disease genes), and whole genome sequencing are increasingly being used as first tier diagnostic tools [13]. The major challenge for positive diagnosis is variant interpretation and sorting among the wealth of generated data. Thus, the most effective approach is usually trio analysis, which requires the parallel sequencing of the patient and both parents. Trio analyses solve the mode of inheritance issue: recessive mutations must be biallelic, i.e. each parent has to be heterozygous for the mutation while the patient has to be compound heterozygous or homozygous, dominant mutation must be inherited from the affected parent (or from the affected branch in case of reduced penetrance) or be de novo, meaning absent from both parents if they are both unaffected.
Another difficulty related to NGS is pathogenicity prediction for missense variants, which are defined by the replacement of a single amino acid by another one in a protein sequence, especially when facing atypical clinical presentations. An average of 9000 missense variants are identified in each exome or genome sequencing, while only a single of them accounts for the phenotype in Mendelian disorders. Pathogenicity prediction based on conservation and structure (such as SIFT, PolyPhen2 and CADD algorithms) have a reasonable sensitivity but low specificity. In contrast, truncating variants (occurrence of a stop codon or short frameshift indel) and copy number variations (deletions and duplications) usually have a higher pathogenicity score but are less common. When a trio analysis is combined with pathogenicity assessment and matching with disease variants and common polymorphisms reported in public databases, the outcome usually yields a short list of variants that can be confronted with clinical data and subsequent targeted paraclinical investigations. Despite the recent advances in the genetics of ICA, the incapacity to identify the causative variant still occurs in many families, indicating that challenges lie ahead to unravel the missing genetic causes of this phenotype.
The genetic nomenclature of ARCAs
A new transparent, adaptable nomenclature of ARCA has been proposed according to the International Parkinson and Movement Disorder Society [2]. Sixty-two entities were indicated with ATX prefix followed by gene name presenting ataxia as the main feature, and 30 have a double prefix when ataxia is combined with another prominent movement disorder (e.g. ATX/HSP, combination of ataxia and spastic paraparesis). Another interesting clinical and pathophysiological classification of ARCA was proposed excluding forms for which ataxia is minor or rare feature and identifying 59 main disorders [14].
Clue clinical features for the diagnosis of inherited cerebellar ataxia
Friedreich–ataxia (ATX–FXN)
ATX-FXN is a multisystem disorder with an age at onset mostly before 25, and a progressive evolution with CA, sensory neuronopathy, pyramidal signs, leading to wheelchair after 10 years of evolution. Cardiomyopathy, diabetes, scoliosis, deafness and optic neuropathy may occur. Brain MRI usually demonstrates no cerebellar atrophy, but cervical spinal cord atrophy is common. Despite a less severe phenotype, late (> 25 years) and very late (> 40 years) onset Friedreich ataxia should not be missed. These forms are characterized by a spastic ataxia with preserved or even brisk deep tendon reflexes. Dysarthria, muscle wasting, ganglionopathy, scoliosis and cardiomyopathy occur less frequently than in typical early onset Friedreich ataxia [15]. ATX–FXN is due in the majority of cases to biallelic GAA repeat expansions in the first intron of the FXN gene, and in 2% of cases, to combination of one GAA expansion and one FXN point mutation or deletion in the other allele [16]. Frataxin, encoded by FXN, is involved in mitochondrial iron homeostasis and the assembly and transfer of iron–sulfur clusters to various mitochondrial enzymes and components. FXN mutations should be searched for firstly in case of suspected ARCA when the phenotype is compatible.
Cerebellar ataxia, neuropathy and vestibular areflexia syndrome (CANVAS)
Cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS) was first described by Migliaccio et al. [17]. The classic phenotype-associated sensory neuronopathy, cerebellar and sensory ataxia, vestibular areflexia, dysautonomia and chronic cough. Additional clinical features, including parkinsonism or motor neuron disorders have recently been described [18]. Brain MRI showed frequent cerebellar atrophy and optional cervical posterior columns changes. Biallelic intronic AAGGG repeat expansions in the RFC1 gene encoding a subunit of a DNA polymerase accessory protein have been recently identified as the cause of CANVAS [19]. In CANVAS, the number of the pentanucleotide repeat expansion of RFC1 is increased from 11 up to more than 400 repeats (400–2000) and there is a modification of the AAAAG or AAAGG reference sequence. Although further works are warranted to determine the mechanism underlying RFC1-related disorders, the absence of RFC1 expression alteration in mutation carriers argue against a loss of function mechanism [19]. Owing to the frequency of the heterozygous AAGGG repeat expansion in the normal population (0.7%), CANVAS/RFC1 appear to be a common cause of late-onset ARCA [19].
Ataxia telangiectasia (ATX/ATM)
ATX–ATM, the second most frequent ARCA after ATX–FXN, is characterized by CA, telangiectasias, oculomotor apraxia, dystonia, sensorimotor axonal neuropathy as well as elevated AFP serum level (markedly increased in 90% of patients, often above 100 µg/L (normal < 5 µg/L) [20]. ATX–ATM mostly starts around 2 years of age then progressively worsens, leading to the loss of independent walking by 10 years and to death before 18 years [21], although some patients experience later onset, milder phenotype or longer life span. ATX–ATM patients are prone to recurrent infections because of immunodeficiency and to increased cancer risk (especially hematologic malignancies) and sensitivity to ionizing radiations. ATM gene encodes a phosphatidylinositol-3-kinase involved in cell cycle progression, cellular response to DNA alterations and maintenance of genome stability [22].
ATX/HSP-SPG7
Disease onset is generally in the fourth decade [23] and the CA can be the main feature and precede spastic paraparesis. Optic neuropathy, progressive external ophthalmoplegia, parkinsonism and cognitive impairment can enrich the phenotype [10]. In the last few years, SPG7 appeared as a frequent cause of slowly progressive CA [13] beyond spastic paraplegia. Brain MRI revealed cerebellar atrophy [23, 24], and in some cases dentate nucleus hyperintensity on T2 sequences [24]. SPG7 is due to mutation in the gene encoding paraplegin, a mitochondrial protein [25]. Genotype–phenotype correlations have been described for this gene since p.Ala510Val variants was associated with a later onset cerebellar phenotype as compared to loss of function variants with spasticity-predominant phenotype [23].
SCA3/ATXN3
SCA3, or Machado–Joseph disease, is the most frequent SCA worldwide especially in Portugal, Brazil, China, the Netherlands, Japan and Germany [26]. Founder mutations are responsible for the higher prevalence in these countries, in particular for families of Portuguese–Azorean ancestry and other founder haplotypes are reported in Japan and Germany [27]. Disease occurs in adulthood, and the phenotype also includes pyramidal signs, parkinsonism, dystonia, hypometric saccades, diplopia, painful axonal neuropathy and depression [28]. Facial myokymia are also highly prevalent in SCA3 but not particularly specific. Polyglutamine (polyQ) expansion in ATNX3 gene is responsible for SCA3 and provokes protein aggregates that form intranuclear inclusions with subsequent neuronal loss. Recent publications have suggested specific retinal architecture alteration in SCA3 [29].
SCA36/NOP56
CA is associated with tongue atrophy and fasciculations then skeletal muscle atrophy in limbs and trunk, lower motor neuron disease being confirmed by electroneuromyography. Other clinical signs are ptosis, postural tremor, mild cerebellar cognitive affective syndrome (CCAS) [30] and deafness. For the latter, its association with cerebellar ataxia may guide the clinician toward a specific diagnosis as SCA36 [31]. Large hexanucleotide GGCCTG repeat expansions in the first intron of the NOP56 gene are responsible for SCA36 [32]. Nop56 protein is highly expressed in Purkinje cells, motor neurons of hypoglossal nucleus and the spinal cord anterior horn explaining the phenotype [32].
SCA37/DAB1
SCA37 is a late-onset pure, slowly progressive cerebellar ataxia with a severe lower limbs dysmetria, dysphagia and oculomotor abnormalities [33]. Indeed, a remarkable clinical feature is the early vertical eye movement disorders as saccadic pursuit and dysmetria, which may anticipate CA. SCA37 is the last reported SCA associated with repeat expansion and due to insertion of ATTTC pentanucleotide in the noncoding region of DAB1 [34]. This insertion modifies the normal allele (ATTTT)7–400 in the pathogenic variant (ATTTT)60–79(ATTTC)31–75(ATTTT)58–90, with a negative correlation between age at disease onset and repeat size of ATTTC.
SCA48/STUB1
SCA48 is due to STUB1 (STIP1 Homology And U-Box Containing Protein 1) gene mutation [35] encoding the CHIP protein, a ubiquitin ligase/co-chaperone. STUB1 biallelic mutations were first described as responsible for ARCA with hypogonadism and short stature [36] then in a Spanish family for dominantly inherited CA and cognitive impairment [35]. CCAS or Schmahmann’s syndrome, based on the concept of “dysmetria of thought” created in’90 s [37], which includes executive deficits, attentional dysfunctions, language difficulties, visual–spatial memory impairment, impaired socio-emotional processes with social cognition deficits, apathy and disinhibited behavior is commonly found in SCA48 [35]. The median age at disease onset is 42 years and in several cases cognitive impairment can preceed cerebellar ataxia [35]. Novel STUB1 mutations were reported in six Italian families and in a larger French cohort with dominant ataxia, confirming this phenotype and its high frequency among dominant ataxias [38].
Ataxia–pancytopenia syndrome/SCA49/SAMD9L
Ataxia–pancytopenia syndrome is characterized by CA, cytopenia (that must be searched for) and myeloid malignancies. Missense mutations in SAMD9L cause the autosomal-dominant Ataxia–Pancytopenia syndrome [39]. SAMD9L acts as a tumor suppressor with antiproliferative function. Given the hematopoietic mosaicism, the germline variant should be searched for in skin fibroblasts rather than in blood. It is important to test family members because of the risk for the carriers to develop hematological malignancies and the possibilities to be healthy hematopoietic stem cells donors [40].
FXTAS/FMR1
Fragile X-associated tremor/ataxia syndrome (FXTAS) includes action tremor, CA, cognitive decline, peripheral neuropathy, parkinsonism, depression and anxiety [41]. Typical MRI findings are brain atrophy including cerebellar atrophy and typical but not specific middle cerebellar peduncles, periventricular area and corpus callosum splenium hyperintensities. FXTAS is due to fragile X premutation (55 up to 200 CGG triplet repeat) that is a 5′ untranslated region of the FMR1 gene (whose complete mutation is responsible for fragile X syndrome (FXS) mainly characterized by intellectual deficiency) [42]. FXTAS is due to sequestration of proteins by abnormal FMR1 mRNA, production of toxic FMRPolyG protein as well as consequences of DNA damage repair process activation [42]. FXTAS affects 40–75% of ageing male premutation carriers and 15–20% of women premutation carriers. The diagnosis of FXTAS is crucial to provide appropriate genetic counseling especially to patients’ daughters who are at risk of developing FXTAS, primary ovarian insufficiency and of having sons affected with Fragile X syndrome.
What is controversial or remains to be elucidated?
Conditions with both dominant and recessive presentations
Both dominant and recessive inheritance patterns are described for several genes involved in cerebellar ataxia. Few cases with dominant inheritance have been reported for SPG7. In the largest cohort of 241 SPG7 patients, carrying two variants, 6% of patients presented with a dominant pattern of transmission [23]. Heterozygous relatives may present impaired balance and mild cerebellar atrophy, occurring later than homozygous carriers do [10]. Some variants seem to be associated with dominant cases, as p.Arg485_Glu487del [43]. However, for these cases, the presence of pathogenic variant in another gene cannot be excluded.
Pathogenic STUB1 variants were initially described in autosomal-recessive ATX–STUB1 [36] then in autosomal-dominant SCA48 [35].
SPTBN2 can be inherited in both autosomal recessive and dominant fashion, responsible for ATX–SPTBN2 [44] and SCA5 [45], respectively. SCA5 is an adult-onset, slowly progressive pure CA whereas ATX–SPTBN2 is characterized by early-childhood onset and complicated phenotype.
GRID2 may also be either recessively or dominantly inherited, the latter being reported with the variant p.Leu656Val [46]. Before that, only null mutations in homozygous state were described as responsible for SCAR18 [47] with a more severe phenotype.
Ataxia or spastic paraplegia?
It is frequent that clinicians encounter patients presenting with both spastic paraparesis and cerebellar features. Prominent spastic phenotype might lead to difficulties in assessing cerebellar ataxia. This is the case in polyQ SCAs, with clear genotype–phenotype correlation for large expansions in SCA1/ATXN1, SCA3/ATXN3 and SCA7/ATXN7 presenting as spastic paraplegia. In addition, ATX/HSP–SACS (ARSACS) in childhood and ATX/HSP–SPG7 in adulthood, present as spastic ataxia, both with autosomal-recessive transmission [48]. A slow progression and a clinical triad characterize the first: cerebellar ataxia, lower-limb spasticity and axonal and demyelinating sensorimotor neuropathy. Some investigations can help in diagnosis as fundoscopy allowing the identification of myelinated retinal nerve fibers and brain MRI showing linear pontine hypointensities and cerebellar atrophy. Adulthood onset, though rare, has been reported [49]. Other important spastic ataxias are cerebrotendinous xanthomatosis (ATX–CYP27A1), late-onset Friedreich ataxia and adult-onset Alexander disease. Among new genes, CAPN1 was identified initially as a HSP-related gene (SPG76), but has been recently linked to spastic ataxia [50].
Unique genotyping strategies
With the emergence of NGS, which is increasingly available, cheap and efficient, and permit the identification of many variants (including those with unknown significance) one may be tempted to perform NGS early on in the diagnosis work for a given patient, sometimes even before any laboratory investigation. The strategy to first genotype, that could be less expensive and more pragmatic, requires a close collaboration between geneticists and clinicians to interpret the genetic results based on phenotype. However, suitable clinical examination, exclusion of acquired causes, a precise delineation of the phenotype and follow-up of the patients are thoroughly recommended.
Treatments and future therapeutic development perspectives
Only a limited number of ICA patients can benefit from a specific disease-modifying treatment (in Table 3). A Guideline Development Group composed by ataxia experts from United Kingdom provided recommendations with levels of evidence for medical interventions [51]. Supportive treatments proposed to the patients are physical therapy, speech therapy and occupational therapy. With regular practice of physiotherapy, which is recommended, improvement of CA was demonstrated [52]. Speech therapy is suggested for management of dysarthria and swallowing difficulties as well as psychological support. For more information, there are dedicated websites: http://ataxia-global-initiative.net/, a platform for clinical research. https://spatax.wordpress.com, international research network; https://ataxia.org and https://www.ataxia.org.uk/ for research support and education, as well as the website of the European Rare Disease network for webinars and fellowship applications http://www.ern-rnd.eu. Transcranial direct current stimulation (tDCS), aiming to modulate the excitability of the cerebellum, reported encouraging, but not replicated, results [53].
Curative therapies are in development for several CA. As for Huntington Disease, they derive from antisense oligonucleotides (ASOs) for SCA1/ATXN1 [54], SCA2/ATXN 2[55], SCA3/ATXN3 [56] and SCA7/ATXN7 [57] mice model. For SCA36/NOP56, ASOs showed RNA-foci reduction in patient iPSCs [58]. The perspective of ASOs clinical trials in humans in the next few years is realistic and the first clinical trial for SCA3 patients began in early 2022 (NCT05160558). Accumulated ataxin 1, 2, 3 and 7 proteins are promising target engagement biomarkers. Specific bioassays to quantify mutant ataxin-3 in blood, CSF, peripheral blood mononuclear cells and fibroblasts are used [59]. Allele-specific approaches are advisable since consequences due to wild type protein decrease could be harmful. Other therapies targeting RNA inhibiting gene expression are RNA interference (RNAi), micro RNA (miRNA), short hairpin RNA (shRNA) [60], gene editing strategies (CRISPR/Cas9) [61]. For now, in ATX-FXN, the only treatment that has shown positive results is omaveloxolone. In a phase 2 trial, omaveloxolone administration (150 mg/day) for 48 weeks showed significant neurological improvement as decreased of mFARS scores as compared to placebo [62, 63]. In ATX–FXN, delivery of frataxin-expressing AAV showed sensory neuropathy rescue in a conditional mouse model with complete frataxin deletion [64]. Another strategy to restore the frataxin expression was tested on mouse model YG8R by CRISPR/Cas9-mediated genome editing [65].
Although, gene therapies have been successful in mice models, major difficulties as technological challenges, lack of biomarkers and economic efforts arise in using these therapies in patients [61].
Genetic counseling
Genetic counseling covers transmission pattern in the family, information about possibilities of presymptomatic testing especially in dominant diseases and prenatal diagnosis. For entities that present both, autosomal dominant and recessive inheritance patterns, genetic counseling is challenging. In diseases where no preventive measures exist, but the severity leads to the wish to find out about the genetic status, presymptomatic testing is a choice that is not only medical in the absence of preventive treatment. The major reason for taking the test for SCAs is to plan a family, but a minority of expansion carriers choose to perform prenatal testing or preimplantation genetic diagnosis [66]. A specific care setting should be provided to support parents and families through these life choices.
Conclusion
In this review, we provide to clinicians the key points for clinical, laboratory and genetic investigations when inherited cerebellar ataxia is suspected. The most frequent entities for each transmission pattern are discussed more in detail in order not to miss the diagnosis. New genes are constantly identified thanks to the increased availability of exome/genome and the development of new techniques as long-read sequencing. This lead to broaden phenotypic spectrum for some genes and to describe either recessive or dominant inheritance patterns, complicating the diagnostic process. As for therapy, curative treatments are not available, except for some subtypes, which is why gene therapies are eagerly awaited.
Supplementary Information
Below is the link to the electronic supplementary material.
Video 1. SPG7 patient carrying pathogenic variants c.618+3G>C (p.?) and c.2240 T>C (p.Ile747Thr), age at onset 32, age at examination 49, with spastic−ataxic gait with permanent support, distal lower limbs muscular deficit, left big toe dystonia. At second 18, oculomotor examination showed saccadic pursuit, horizontal gaze-evoked nystagmus and horizontal and vertical ophthalmoplegia (MP4 41470 KB)
Video 2. SCA3 patient carrying pathogenic expanded ATNX3 allele (69 CAG), age at onset 33, age at examination 62. The gait (from the beginning to second 6) was ataxic with postural instability requiring intermittent support of the wall, small steps and loss of arm swing. Cerebellar dysmetria at nose−finger test (at second 7), SARA score 25/40. At second 13, oculomotor examination showing saccadic pursuit, horizontal gaze-evoked nystagmus, vertical ophthalmoplegia. Facial myokimia were also present (MP4 33653 KB)
Video 3. SCA3 patient carrying pathogenic expanded ATNX3 allele (71 CAG), age at onset 40, age at examination 50. Ataxic gait requiring one stick, SARA score 18/40. Bilateral big toe and left arm dystonia (MP4 4807 KB)
Video 4. SCA1 patient carrying pathogenic expanded ATNX1 allele (50 CAG), age at onset 35, age at examination 45. Ataxic gait with half-turn difficulties, tandem walking (at second 7−18) not possible without aid. Cerebellar dysmetria at nose−finger test (at second 19−26). At second 27, bulging eyes, mydriasis and slow saccades (MP4 35081 KB)
Video 5. Patient with ataxia with vitamin E deficiency carrying homozygous pathogenic variant c.744delA, age at onset 34, age at examination 44, showing ataxic and dystonic gait, trunk dystonia and distal lower limbs muscular deficit (MP4 4640 KB)
Video 6. Patient with ataxia with vitamin E deficiency carrying homozygous pathogenic variant c.744delA, age at onset 9, age at examination 27, showing upper limbs dysmetria (from the beginning to second 9), severe postural head tremor (strongly improved after botulinum toxin injections in bilateral splenius, levator scapulae and longissimus muscles) and oculomotor examination without horizontal or vertical limitation (second 10−32), mild lower limbs dysmetria (second 33) (AVI 23351 KB)
Video 7. Patient with ataxia−oculomotor apraxia type 1 carrying homozygous pathogenic variant p.Trp279*, age at onset 3, age at examination 19. From the beginning to second 7, the video shows an ataxic and dystonic gait requiring intermittent support, trunk and upper and lower limbs dystonia, distal lower limbs muscular deficit, chorea at hands (second 6-10), bilateral cerebellar dysmetria at nose−finger test and heel-to-shin test (second 11−23). At second 24, oculomotor examination showed saccadic pursuit, dysmetric and slow saccades, horizontal gaze-evoked nystagmus and oculomotor apraxia (MP4 43335 KB)
Author Contributions
GC: research project: A. conception, B. organization, C. execution; manuscript preparation: A. writing of the first draft, B. review and critique. TW: research project: A. conception, B. organization, C. execution; manuscript preparation: A. writing of the first draft, B. review and critique. CT: research project: A. conception, B. organization, C. execution; manuscript preparation: A. writing of the first draft, B. review and critique. MK: research project: A. conception, B. organization, C. execution; manuscript preparation: A. writing of the first draft, B. review and critique. AD: research project: A. conception, B. organization, C. execution; manuscript preparation: A. writing of the first draft, B. review and critique. MA: research project: A. conception, B. organization, C. execution; manuscript preparation: A. writing of the first draft, B. review and critique.
Declarations
Conflicts of interest
The authors report no competing interests.
References
- 1.Joyce MR, Nadkarni PA, Kronemer SI, et al. Quality of life changes following the onset of cerebellar ataxia: symptoms and concerns self-reported by ataxia patients and informants. Cerebellum Lond Engl. 2022;21:592–605. doi: 10.1007/s12311-022-01393-5. [DOI] [PubMed] [Google Scholar]
- 2.Rossi M, Anheim M, Durr A, et al. The genetic nomenclature of recessive cerebellar ataxias: genetic nomenclature of recessive ataxias. Mov Disord. 2018;33:1056–1076. doi: 10.1002/mds.27415. [DOI] [PubMed] [Google Scholar]
- 3.Marras C, Lang A, van de Warrenburg BP, et al. Nomenclature of genetic movement disorders: recommendations of the international Parkinson and movement disorder society task force. Mov Disord. 2016;31:436–457. doi: 10.1002/mds.26527. [DOI] [PubMed] [Google Scholar]
- 4.Salman MS. Epidemiology of cerebellar diseases and therapeutic approaches. Cerebellum Lond Engl. 2018;17:4–11. doi: 10.1007/s12311-017-0885-2. [DOI] [PubMed] [Google Scholar]
- 5.Moscovich M, Okun MS, Favilla C, et al. Clinical evaluation of eye movements in spinocerebellar ataxias: a prospective multicenter study. J Neuro-Ophthalmol Off J N Am Neuro-Ophthalmol Soc. 2015;35:16–21. doi: 10.1097/WNO.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephen CD, Schmahmann JD. Eye movement abnormalities are ubiquitous in the spinocerebellar ataxias. Cerebellum Lond Engl. 2019;18:1130–1136. doi: 10.1007/s12311-019-01044-2. [DOI] [PubMed] [Google Scholar]
- 7.Anheim M, Tranchant C, Koenig M. The autosomal recessive cerebellar ataxias. N Engl J Med. 2012;366:636–646. doi: 10.1056/NEJMra1006610. [DOI] [PubMed] [Google Scholar]
- 8.Renaud M, Tranchant C, Martin JVT, et al. A recessive ataxia diagnosis algorithm for the next generation sequencing era. Ann Neurol. 2017;82:892–899. doi: 10.1002/ana.25084. [DOI] [PubMed] [Google Scholar]
- 9.Jacobi H, du Montcel ST, Romanzetti S, et al. Conversion of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 to manifest ataxia (RISCA): a longitudinal cohort study. Lancet Neurol. 2020;19:738–747. doi: 10.1016/S1474-4422(20)30235-0. [DOI] [PubMed] [Google Scholar]
- 10.Klebe S, Depienne C, Gerber S, et al. Spastic paraplegia gene 7 in patients with spasticity and/or optic neuropathy. Brain. 2012;135:2980–2993. doi: 10.1093/brain/aws240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marianelli BF, Filho FMR, Salles MV, et al. A proposal for classification of retinal degeneration in spinocerebellar ataxia type 7. Cerebellum Lond Engl. 2021;20:384–391. doi: 10.1007/s12311-020-01215-6. [DOI] [PubMed] [Google Scholar]
- 12.Montaut S, Tranchant C, Drouot N, et al. Assessment of a targeted gene panel for identification of genes associated with movement disorders. JAMA Neurol. 2018;75:1234. doi: 10.1001/jamaneurol.2018.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutelier M, Hammer MB, Stevanin G, et al. Efficacy of exome-targeted capture sequencing to detect mutations in known cerebellar ataxia genes. JAMA Neurol. 2018;75:591. doi: 10.1001/jamaneurol.2017.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaudin M, Matilla-Dueñas A, Soong B-W, et al. The classification of autosomal recessive cerebellar ataxias: a consensus statement from the society for research on the cerebellum and ataxias task force. Cerebellum Lond Engl. 2019;18:1098–1125. doi: 10.1007/s12311-019-01052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecocq C, Charles P, Azulay J-P, et al. Delayed-onset Friedreich’s ataxia revisited. Mov Disord Off J Mov Disord Soc. 2016;31:62–69. doi: 10.1002/mds.26382. [DOI] [PubMed] [Google Scholar]
- 16.Cossée M, Campuzano V, Koutnikova H, et al. Frataxin fracas. Nat Genet. 1997;15:337–338. doi: 10.1038/ng0497-337. [DOI] [PubMed] [Google Scholar]
- 17.Migliaccio AA, Halmagyi GM, McGarvie LA, Cremer PD. Cerebellar ataxia with bilateral vestibulopathy: description of a syndrome and its characteristic clinical sign. Brain J Neurol. 2004;127:280–293. doi: 10.1093/brain/awh030. [DOI] [PubMed] [Google Scholar]
- 18.Huin V, Coarelli G, Guemy C, et al. Motor neuron pathology in CANVAS due to RFC1 expansions. Brain. 2021 doi: 10.1093/brain/awab449. [DOI] [PubMed] [Google Scholar]
- 19.Cortese A, Simone R, Sullivan R, et al. Biallelic expansion of an intronic repeat in RFC1 is a common cause of late-onset ataxia. Nat Genet. 2019;51:649–658. doi: 10.1038/s41588-019-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothblum-Oviatt C, Wright J, Lefton-Greif MA, et al. Ataxia telangiectasia: a review. Orphanet J Rare Dis. 2016;11:159. doi: 10.1186/s13023-016-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micol R, Ben Slama L, Suarez F, et al. Morbidity and mortality from ataxia-telangiectasia are associated with ATM genotype. J Allergy Clin Immunol. 2011;128:382–389.e1. doi: 10.1016/j.jaci.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 22.Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 23.Coarelli G, Schule R, van de Warrenburg BPC, et al. Loss of paraplegin drives spasticity rather than ataxia in a cohort of 241 patients with SPG7. Neurology. 2019;92:e2679–e2690. doi: 10.1212/WNL.0000000000007606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewamadduma CA, Hoggard N, O’Malley R, et al. Novel genotype-phenotype and MRI correlations in a large cohort of patients with SPG7 mutations. Neurol Genet. 2018;4:e279. doi: 10.1212/NXG.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casari G, De Fusco M, Ciarmatori S, et al. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell. 1998;93:973–983. doi: 10.1016/S0092-8674(00)81203-9. [DOI] [PubMed] [Google Scholar]
- 26.Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Primer. 2019;5:24. doi: 10.1038/s41572-019-0074-3. [DOI] [PubMed] [Google Scholar]
- 27.Martins S, Calafell F, Gaspar C, et al. Asian origin for the worldwide-spread mutational event in Machado-Joseph disease. Arch Neurol. 2007;64:1502–1508. doi: 10.1001/archneur.64.10.1502. [DOI] [PubMed] [Google Scholar]
- 28.Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–894. doi: 10.1016/S1474-4422(10)70183-6. [DOI] [PubMed] [Google Scholar]
- 29.Rezende Filho FM, Jurkute N, de Andrade JBC, et al. Characterization of retinal architecture in spinocerebellar ataxia type 3 and correlation with disease severity. Mov Disord. 2021 doi: 10.1002/mds.28893. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Regueiro R, Arias M, Cruz R, et al. Cerebellar cognitive affective syndrome in Costa da Morte ataxia (SCA36) The Cerebellum. 2020;19:501–509. doi: 10.1007/s12311-020-01110-0. [DOI] [PubMed] [Google Scholar]
- 31.Barsottini OG, Pedroso JL, Martins CR, et al. Deafness and vestibulopathy in cerebellar diseases: a practical approach. Cerebellum Lond Engl. 2019;18:1011–1016. doi: 10.1007/s12311-019-01042-4. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi H, Abe K, Matsuura T, et al. Expansion of intronic GGCCTG hexanucleotide repeat in NOP56 causes SCA36, a type of spinocerebellar ataxia accompanied by motor neuron involvement. Am J Hum Genet. 2011;89:121–130. doi: 10.1016/j.ajhg.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matilla-Dueñas A, Volpini V (1993) Spinocerebellar Ataxia Type 37. In: Adam MP, Mirzaa GM, Pagon RA, et al (eds) GeneReviews®. University of Washington, Seattle, Seattle (WA) [PubMed]
- 34.Seixas AI, Loureiro JR, Costa C, et al. A Pentanucleotide ATTTC repeat insertion in the non-coding region of DAB1, mapping to SCA37, causes spinocerebellar ataxia. Am J Hum Genet. 2017;101:87–103. doi: 10.1016/j.ajhg.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genis D, Ortega-Cubero S, San Nicolás H, et al. Heterozygous STUB1 mutation causes familial ataxia with cognitive affective syndrome (SCA48) Neurology. 2018;91:e1988–e1998. doi: 10.1212/WNL.0000000000006550. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Wang J, Li J-D, et al. Identification of CHIP as a novel causative gene for autosomal recessive cerebellar ataxia. PLoS ONE. 2013;8:e81884. doi: 10.1371/journal.pone.0081884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmahmann J. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 38.SPATAX network. Roux T, Barbier M, et al. Clinical, neuropathological, and genetic characterization of STUB1 variants in cerebellar ataxias: a frequent cause of predominant cognitive impairment. Genet Med. 2020 doi: 10.1038/s41436-020-0899-x. [DOI] [PubMed] [Google Scholar]
- 39.Chen D-H, Below JE, Shimamura A, et al. Ataxia-pancytopenia syndrome is caused by missense mutations in SAMD9L. Am J Hum Genet. 2016;98:1146–1158. doi: 10.1016/j.ajhg.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed IA, Farooqi MS, Vander Lugt MT, et al. Outcomes of hematopoietic cell transplantation in patients with germline SAMD9/SAMD9L mutations. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2019;25:2186–2196. doi: 10.1016/j.bbmt.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salcedo-Arellano MJ, Dufour B, McLennan Y, et al. Fragile X syndrome and associated disorders: clinical aspects and pathology. Neurobiol Dis. 2020;136:104740. doi: 10.1016/j.nbd.2020.104740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagerman RJ, Hagerman P. Fragile X-associated tremor/ataxia syndrome—features, mechanisms and management. Nat Rev Neurol. 2016;12:403–412. doi: 10.1038/nrneurol.2016.82. [DOI] [PubMed] [Google Scholar]
- 43.van Gassen KLI, van der Heijden CDCC, de Bot ST, et al. Genotype-phenotype correlations in spastic paraplegia type 7: a study in a large Dutch cohort. Brain J Neurol. 2012;135:2994–3004. doi: 10.1093/brain/aws224. [DOI] [PubMed] [Google Scholar]
- 44.Lise S, Clarkson Y, Perkins E, et al. Recessive mutations in SPTBN2 implicate β-III spectrin in both cognitive and motor development. PLoS Genet. 2012;8:e1003074. doi: 10.1371/journal.pgen.1003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elsayed SM, Heller R, Thoenes M, et al. Autosomal dominant SCA5 and autosomal recessive infantile SCA are allelic conditions resulting from SPTBN2 mutations. Eur J Hum Genet EJHG. 2014;22:286–288. doi: 10.1038/ejhg.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coutelier M, Burglen L, Mundwiller E, et al. GRID2 mutations span from congenital to mild adult-onset cerebellar ataxia. Neurology. 2015;84:1751–1759. doi: 10.1212/WNL.0000000000001524. [DOI] [PubMed] [Google Scholar]
- 47.Utine GE, Haliloğlu G, Salanci B, et al. A homozygous deletion in GRID2 causes a human phenotype with cerebellar ataxia and atrophy. J Child Neurol. 2013;28:926–932. doi: 10.1177/0883073813484967. [DOI] [PubMed] [Google Scholar]
- 48.Pedroso JL, Vale TC, França Junior MC, et al. A diagnostic approach to spastic ataxia syndromes. Cerebellum Lond Engl. 2021 doi: 10.1007/s12311-021-01345-5. [DOI] [PubMed] [Google Scholar]
- 49.Briand M-M, Rodrigue X, Lessard I, et al. Expanding the clinical description of autosomal recessive spastic ataxia of Charlevoix-Saguenay. J Neurol Sci. 2019;400:39–41. doi: 10.1016/j.jns.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Shetty A, Gan-Or Z, Ashtiani S, et al. CAPN1 mutations: Expanding the CAPN1-related phenotype: from hereditary spastic paraparesis to spastic ataxia. Eur J Med Genet. 2019;62:103605. doi: 10.1016/j.ejmg.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 51.de Silva R, Greenfield J, Cook A, et al. Guidelines on the diagnosis and management of the progressive ataxias. Orphanet J Rare Dis. 2019;14:51. doi: 10.1186/s13023-019-1013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milne SC, Corben LA, Georgiou-Karistianis N, et al. Rehabilitation for individuals with genetic degenerative ataxia: a systematic review. Neurorehabil Neural Repair. 2017;31:609–622. doi: 10.1177/1545968317712469. [DOI] [PubMed] [Google Scholar]
- 53.Benussi A, Dell’Era V, Cantoni V, et al. Cerebello-spinal tDCS in ataxia: a randomized, double-blind, sham-controlled, crossover trial. Neurology. 2018;91:e1090–e1101. doi: 10.1212/WNL.0000000000006210. [DOI] [PubMed] [Google Scholar]
- 54.Friedrich J, Kordasiewicz HB, O’Callaghan B, et al. Antisense oligonucleotide-mediated ataxin-1 reduction prolongs survival in SCA1 mice and reveals disease-associated transcriptome profiles. JCI Insight. 2018 doi: 10.1172/jci.insight.123193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scoles DR, Meera P, Schneider MD, et al. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature. 2017;544:362–366. doi: 10.1038/nature22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLoughlin HS, Moore LR, Chopra R, et al. Oligonucleotide therapy mitigates disease in spinocerebellar ataxia type 3 mice. Ann Neurol. 2018;84:64–77. doi: 10.1002/ana.25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niu C, Prakash TP, Kim A, et al. Antisense oligonucleotides targeting mutant Ataxin-7 restore visual function in a mouse model of spinocerebellar ataxia type 7. Sci Transl Med. 2018 doi: 10.1126/scitranslmed.aap8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuzono K, Imamura K, Murakami N, et al. Antisense oligonucleotides reduce RNA foci in spinocerebellar ataxia 36 patient iPSCs. Mol Ther Nucleic Acids. 2017;8:211–219. doi: 10.1016/j.omtn.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prudencio M, Garcia-Moreno H, Jansen-West KR, et al. Toward allele-specific targeting therapy and pharmacodynamic marker for spinocerebellar ataxia type 3. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.abb7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitoma H, Manto M, Gandini J. Recent advances in the treatment of cerebellar disorders. Brain Sci. 2019 doi: 10.3390/brainsci10010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vázquez-Mojena Y, León-Arcia K, González-Zaldivar Y, et al. Gene therapy for polyglutamine spinocerebellar ataxias: advances, challenges, and perspectives. Mov Disord Off J Mov Disord Soc. 2021;36:2731–2744. doi: 10.1002/mds.28819. [DOI] [PubMed] [Google Scholar]
- 62.Lynch DR, Johnson J. Omaveloxolone: potential new agent for Friedreich ataxia. Neurodegener Dis Manag. 2021;11:91–98. doi: 10.2217/nmt-2020-0057. [DOI] [PubMed] [Google Scholar]
- 63.Ghanekar SD, Kuo S-H, Staffetti JS, Zesiewicz TA. Current and emerging treatment modalities for spinocerebellar ataxias. Expert Rev Neurother. 2022;22:101–114. doi: 10.1080/14737175.2022.2029703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piguet F, de Montigny C, Vaucamps N, et al. Rapid and complete reversal of sensory ataxia by gene therapy in a novel model of Friedreich ataxia. Mol Ther. 2018;26:1940–1952. doi: 10.1016/j.ymthe.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rocca CJ, Rainaldi JN, Sharma J, et al. CRISPR-Cas9 gene editing of hematopoietic stem cells from patients with Friedreich’s ataxia. Mol Ther Methods Clin Dev. 2020;17:1026–1036. doi: 10.1016/j.omtm.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goizet C, Lesca G, Dürr A. Presymptomatic testing in Huntington’s disease and autosomal dominant cerebellar ataxias. Neurology. 2002;59:1330–1336. doi: 10.1212/01.wnl.0000032255.75650.c2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. SPG7 patient carrying pathogenic variants c.618+3G>C (p.?) and c.2240 T>C (p.Ile747Thr), age at onset 32, age at examination 49, with spastic−ataxic gait with permanent support, distal lower limbs muscular deficit, left big toe dystonia. At second 18, oculomotor examination showed saccadic pursuit, horizontal gaze-evoked nystagmus and horizontal and vertical ophthalmoplegia (MP4 41470 KB)
Video 2. SCA3 patient carrying pathogenic expanded ATNX3 allele (69 CAG), age at onset 33, age at examination 62. The gait (from the beginning to second 6) was ataxic with postural instability requiring intermittent support of the wall, small steps and loss of arm swing. Cerebellar dysmetria at nose−finger test (at second 7), SARA score 25/40. At second 13, oculomotor examination showing saccadic pursuit, horizontal gaze-evoked nystagmus, vertical ophthalmoplegia. Facial myokimia were also present (MP4 33653 KB)
Video 3. SCA3 patient carrying pathogenic expanded ATNX3 allele (71 CAG), age at onset 40, age at examination 50. Ataxic gait requiring one stick, SARA score 18/40. Bilateral big toe and left arm dystonia (MP4 4807 KB)
Video 4. SCA1 patient carrying pathogenic expanded ATNX1 allele (50 CAG), age at onset 35, age at examination 45. Ataxic gait with half-turn difficulties, tandem walking (at second 7−18) not possible without aid. Cerebellar dysmetria at nose−finger test (at second 19−26). At second 27, bulging eyes, mydriasis and slow saccades (MP4 35081 KB)
Video 5. Patient with ataxia with vitamin E deficiency carrying homozygous pathogenic variant c.744delA, age at onset 34, age at examination 44, showing ataxic and dystonic gait, trunk dystonia and distal lower limbs muscular deficit (MP4 4640 KB)
Video 6. Patient with ataxia with vitamin E deficiency carrying homozygous pathogenic variant c.744delA, age at onset 9, age at examination 27, showing upper limbs dysmetria (from the beginning to second 9), severe postural head tremor (strongly improved after botulinum toxin injections in bilateral splenius, levator scapulae and longissimus muscles) and oculomotor examination without horizontal or vertical limitation (second 10−32), mild lower limbs dysmetria (second 33) (AVI 23351 KB)
Video 7. Patient with ataxia−oculomotor apraxia type 1 carrying homozygous pathogenic variant p.Trp279*, age at onset 3, age at examination 19. From the beginning to second 7, the video shows an ataxic and dystonic gait requiring intermittent support, trunk and upper and lower limbs dystonia, distal lower limbs muscular deficit, chorea at hands (second 6-10), bilateral cerebellar dysmetria at nose−finger test and heel-to-shin test (second 11−23). At second 24, oculomotor examination showed saccadic pursuit, dysmetric and slow saccades, horizontal gaze-evoked nystagmus and oculomotor apraxia (MP4 43335 KB)