Abstract
Background
Carbohydrate–lectin interactions are extremely specific as the lectin is capable of recognising monomeric and oligomeric sugars in a reversible manner. It has been known for a long time that lectins have antibacterial, antifungal, and insecticidal activities. Recently, it has been reported that many lectins can prevent the virus growth by interacting with the viral envelop surface glycoprotein. Spike protein, which is found on the surface of some enveloped viruses, is heavily mannosylated and will have strong affinity for mannose specific lectins. According to the findings, lectins have a high binding affinity for the glycans of the SARS-CoV-2 spike glycoprotein, which contains N-glycosylation sites. As a result, various lectins are being researched and developed as anti-viral agents.
Results
According to our in silico studies, the amino acid residues Asn487, Tyr489, Gln493, Lys417, and Tyr505 of the receptor binding domain (RBD) of SARS-CoV-2 formed an interaction with the model lectin Lablab purpureus lectin. Similar interaction for SARS-CoV-2 spike protein was observed with Griffithsin lectin (algal source) as well. These observations demonstrate that lectins could be one of the potential molecules for neutralising coronavirus infection.
Conclusion
This review focuses on anti-viral lectins isolated and characterized from plants and algae (last 5 years) and showed anti-viral properties against HIV, Influenza, and coronaviruses.
Keywords: COVID-19, Lectin, Molecular modeling, MOE, Pymol
Introduction
Viral infections are one of the most serious public health problems that exist today. [1–3]. The major concern is the lack of anti-viral therapy against most viruses. On several occasion, it can be noted that RNA viruses have been implicated during the outbreak of pandemics [2, 4, 5]. In 2002, an outbreak of coronavirus, an RNA virus in Guangdong province of China, has disseminated to several parts of the world including middle East countries, Taiwan, Hong Kong, Singapore, and United States, resulting in the deaths of thousands of individuals [6–8]. In December 2019, a new severe viral acute respiratory syndrome (SARS) virus, named as COVID-19, outbreak has occurred in Wuhan, China [9]. This virus has created mayhem worldwide and spread over more than 120 countries. According to the statistics in July 2022, more than five hundred seventy nine million COVID-19 cases were confirmed world-wide and over 6,414,976 deaths globally (as of 29 July 2022. https://www.worldometers.info/coronavirus/) [9–11]. Human Immunodeficiency Virus (HIV) infection is caused by an RNA virus that has spread throughout the world, with the majority of cases occurring in Africa, Asia, and Latin America. Around the world, the HIV epidemic has an impact on the lives of more than 40 million individuals [12]. There are also single-strand RNA influenza viruses, which are members of the orthomyxoviridae family of viruses. At this moment, two subtypes of influenza A viruses are found in the human population: influenza A (H1N1) and influenza A (H3N2). In 2009, the World Health Organization (WHO) estimated that more than 199 nations had officially reported 482,300 H1N1 cases, with 6071 deaths [13]. These RNA viruses employ a spike protein or surface glycoprotein that has been highly glycosylated to enter the human body in order to replicate. Glycoproteins are found in the HIV viral envelope and are composed of two subunits: the surface glycoprotein gp120 and the transmembrane glycoprotein gp41 [14]. The gp120 is responsible for binding to receptor molecules to hold 20–30 N-linked high mannose sugar structures. In the case of SARS/influenza, viruses enter the body via spike proteins that are likewise heavily glycosylated.
Lectins are glycoproteins that has the ability to bind reversibly to monomeric or oligomeric carbohydrates, can bind to the viral surface, crosslink with glycan, and stop interaction with co-receptor [15]. The presence of glycoproteins in the viral envelope opens up a plethora of possible applications for carbohydrate-binding proteins such as lectins, which can operate as antiviral agents. Lectins are found in a variety of organisms, including plants, mammals, algae, and bacteria, and are capable of distinguishing between different types of carbohydrates. Carbohydrates recognition domain (CRD) of lectin determine the specificity and avidity of sugar-binding properties. Primarily, lectins interact with sugars through hydrogen bonds and phenylalanine, aspartic acid, glutamic acid, and asparagine are important amino acids involved in these interactions.
In recent years, the role of lectin’s as anti-viral characteristics has been extensively researched [16–18]. Banana lectins (PDB ID-3MIT), jacalin (PDB ID-1UGW), and concanavalin A (PDB ID-6AHG) are a few examples of plant lectins that are known to exist and bind to high mannose sugar concentrations, and have been proven to have anti-viral activity against a range of viruses. In contrast, many lectins isolated from marine organisms and algae demonstrated anti-viral activity against various viruses [19]. This review has incorporated the last 5 years report on anti-viral lectin properties from plants and algae.
Plant lectins with antiviral properties
Plant lectins against HIV
Many lectins from various plants have showed anti-HIV activity, particularly mannose -binding lectin showed significant anti-viral properties not only against HIV as well as a number of other viruses as well (Fig. 1). HIV surface glycoprotein gp120 is highly glycosylated and contains mannose-rich glycans that interacts with CD4 that is mainly responsible for viral fusion and infection. Anti-viral lectin recognizes or binds to these glycans and breaks the interaction between viral surface protein and co-receptor like CD4 [20]. Mannose-binding lectin has formed interaction with glycan moieties of HIV surface glycoprotein, inhibiting the growth of virus and loss of attachment of virus on the host cells. Monocot lectin from Dioscorea bulbifera bulbils can bind with different mannose-rich sugars, including mannose asialofetuin, fetuin, mucin asialomucin and transferrin. This lectin inhibits HIV-1 reverse transcriptase activity effectively with IC50 of 1.3 µg and displayed anti-reverse transcriptase activity against HIV [21]. The lectin isolated from the tuber of Sauromatum guttatum is a β-sheet rich protein structurally. This lectin showed highest binding affinity towards [Man1-6(Man1-3) Manß1-4GlcNAcß1-4GlcNAcß] motif. Glycan array analysis of this lectin found a unique specificity towards various glycans that are part of gp120 of HIV-I and acts as a putative anti-HIV agent [22]. The binding of mannose-specific banana lectin with HIV surface protein was studied in detail and found as a promising anti-HIV lectin [19]. Horcolin, a lectin from Hordeum vulgare, showed sugar-binding specificity towards high mannose sugars (Man5/7/9) and confirmed that it can bind with recombinant gp120 and gp140 with high affinity and inhibition of HIV infection at 30–35 nM concentrations without mitogenicity [23] (Table 1).
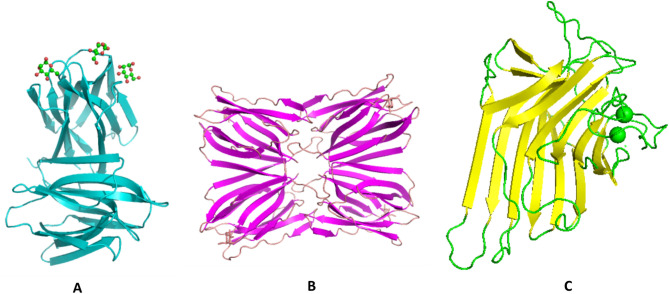
Fig. 1.
Three dimensional structure of various mannose specific lectins. A Banana (PDB ID-3MIT), B Jacalin (PDB ID-1UGW), C Concavalin (PDB ID-6AHG)
Table 1.
Anti-viral plant lectins
| S no. | Year | Source | Species | class | Sugar binding | Molecular weight (kDa) | Virus | Function |
|---|---|---|---|---|---|---|---|---|
| 1 | 2017 | Plant | Dioscorea bulbifera bulbils | Monocot | simple sugars or oligosaccharides | 24.49 | HIV | Anti antireverse transcriptase activity against HIV |
| 2 | 2017 | Plant | Sauromatum guttatum | Monocot | High mannose motif | Subunits of 11.4 and 11.9 | HIV | A putative anti-HIV agent |
| 3 | 2017 | Plant | Banana | Monocot | high-mannose N-linked glycan | 60 | HIV | Anti-HIV microbi-cides of great potential utility |
| 4 | 2020 | Plant | Hordeum vulgare | Monocot | High mannose sugars | 15 | HIV | Inhibition of HIV infection at nanomolar concentrations |
| 5 | 2018 | Plant | Tamarindus indica | Dicot | N-acetylglucosamine (NAG) | 33 | Alphaviruses | Anti-viral activity against alphaviruses |
| 6 | 2020 | Plant | Banana | Monocot | Mannose glycans | 10–11 | BoHV-1 | High levels of inhibition against BVDV-1 and BoHV-1 |
| 7 | 2020 | Plant | Lablab purpureus | Dicot | Mannose/ glucose | 112.1 tetramer in solution | Influenza and SARS-CoV-2 | Anti-influenza and anti-SARS-CoV-2 activity |
| 8 | 2020 | Plant | Galanthus nivalis | Monocot | Mannnose | Not specified | Influenza | Inhibit receptor binding and broadly neutralize recent human H3N2 viruses |
Plant lectins against influenza
The homotetrameric lectin from edible Lablab purpureus bean is a mannose/glucose-specific lectin. In the case of influenza, the surface envelop glycoprotein is rich in mannose and hybrid type N-glycans. Lectin from L. purpureus impounds influenza virions in cytoplasmic endosomes that prevent their nuclear entry [15]. Snowdrop lectin has showed inhibition of receptor binding and broadly neutralized human influenza viruses H3N2.
Plant lectins against coronaviruses
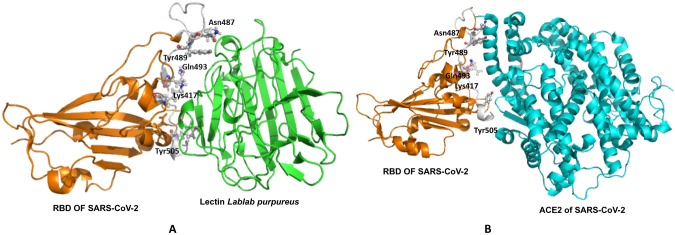
The highly glycosylated spike protein from SARS-CoV-2 interacts with hACE2, which aids coronavirus entry into humans. Spike protein was composed of 2 functional units, S1 and S2, with 22 and 3 potential sites for N-glycosylation and O-glycosylation, respectively. S1 protein participates in cell adhesion by binding to the human angiotensin receptor (hACE2) [18]. Lectin from the edible L. purpureus bean was shown to have mannose/glucose binding characteristics, which allowed it to bind to N-glycans and neutralise viruses on their envelopes.This lectin significantly neutralises SARS-CoV-2 by preventing viral protein production and cytopathic effect in the host cells [15]. Lectin L. purpureus sequence was retrieved from UniProt (P38662-1) and further model was developed using MOE software (https://www.chemcomp.com/Products.htm) template used PDB ID-2FMD.The model structure was validated with the help of http://molprobity.manchester.ac.uk/ and the prosa webserver. Further, modeled Lablab lectin was dock on receptor-binding (RBD) protein of SARS-CoV-2 retrieved from https://www.rcsb.org/ PDB ID-6M0J using MOE software setting. Best top ten scores based on binding energy were retrieved and analyzed using visualization software PYMOL and MOE. The tenth complex, which had a binding energy of − 62.21, occupied a location comparable to that of SARS-CoV-2 RBD to Angiotensin-converting enzyme 2 (ACE2). Our in silico investigations indicate that the amino acid residues Asn487, Tyr489, Gln493, Lys417, and Tyr505 of the RBD protein formed a contact with the model lectin L. purpureus lectin (Fig. 2). These residues are essential in the formation of the RBD-ACE2 interaction (PDB ID-6M0J). Lectin can be utilised to prevent the interaction between RBD and ACE2 from occurring.
Fig. 2.
Molecular interaction of RBD of SARS-CoV-2 and lectin lablab purpureus (modelled structure) (A) and RBD-ACE complex of SARS-CoV-2 (PDB ID-6M0J) (B)
Plant lectins against other viruses
Lectin isolated from Tamarind showed chitinase activity and hence named as chitinase like lectin. This lectin has sugar specificity towards N-acetylglucosamine (NAG). Decrease in virus RNA levels in the presence of Tamarind chitinase like lectin has validated the anti-viral activity against alphaviruses (chikungunya). At the same time, Tamarind lectin did not show any toxicity up to 250 μM against BHK-21 cells [24]. Lectin from Musa acuminata has a high affinity towards mannose glycans which are well known for their presence in several viral envelopes. 25 μg/mL of banana lectin has showed high levels of inhibition against bovine viral diarrhea virus-1 (BVDV-1) (99.98%) and bovine alpha herpes virus type 1 (BoHV-1) (99.68%) without affecting viability of Madin-Darby bovine kidney (MDBK) epithelial cells [25].
Algal lectins against antivirus properties
Algal lectins against HIV
Scytovirin is an algal lectin expressed in the Human vaginal Lactobacillus plantarum strain and characterized as lectin from Scytonema varium species. The recombinant scytovirin has formed interaction with HIV-1 gp160 and reduces the HIV-induced cytopathic effect to nearly 56.67% in R5 infected TZM-bl cells and 86.47% in X4 HIV-1 infected TZM-bl cells [14]. The cyanovirin-N, a cyanobacterial origin lectin was in Escherichia coli SHuffle® T7 Express lysY strain and was engineered to enhance correctly the disulfide-bonded proteins, which showed significant anti-HIV activity with an IC50 of 0.5–5 nM. In contrast, this lectin had negligible cytotoxicity up to 5 µM [26]. Griffithsin well-known anti-viral lectin, was expressed in Lactobacillus rhamnosus GR-1 probiotic strains for vaginal mucosal delivery of lectin to inhibit HIV transmission and subsequent replication [27]. Another research group has used Nicotiana benthamiana as the expression to over express Griffithsin lectin (as an HIV-1 entry inhibitor) by using three gene silencing suppressors, with a yield of 400 μg/g fresh weight after expression which was reduced to 287 μg/g after purification with a total recovery of 71.75% [28]. Later on, the monomeric Griffithsin lectin was linked in tandem repeats of two (2MG and 2MG3), three (3MG) and four (4MG) units and found that 2MG and 2MG3 tandemers had similar activity to Griffithsin against cell-free and cell-associated HIV-viruses, while 3MG and 4MG were significantly more potent than monomeric Griffithsin against HIV-viruses [29]. Another research with multi-layered nanoparticle (NPs) and electro spun fiber (NP-EF) composite was used in addition of Griffithsin lectin to provide sustained-release of Griffithsin lectin. Both NPs and NP-EF composites had inhibited HIV-1 infection (in vitro studies) [30].
Algal lectins against coronaviruses
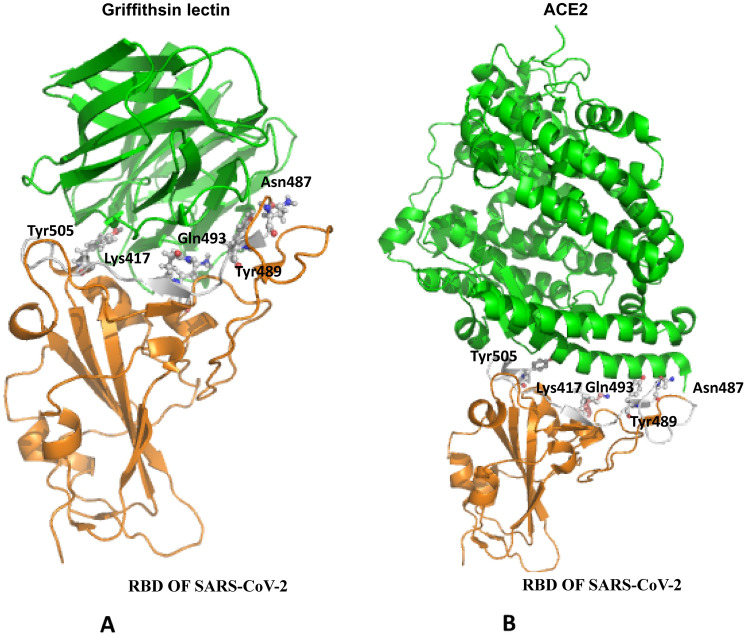
Griffithsin lectin was isolated from red marine alga and can bind to the glycoprotein of many viruses. Recombinant SARS-CoV spike protein was used in ELISA studies indicating the binding of Griffithsin lectin through spike protein of coronaviruses. To confirm this, the three-dimensional structure of Griffithsin lectin (PDB ID-2GTY) was downloaded and prepared for docking experiment (such as removing water and all bond length, missing atoms were rectified) and docked on RBD of SARS-CoV. Protein–protein docking was done using MOE software. The pattern of binding of Griffithsin lectin towards ACE2 was similar to ACE2-RBD with binding energy of − 61.7 (Fig. 3).
Fig. 3.
Molecular interaction of RBD of SARS-CoV-2 and Griffithsin lectin (PDB ID-2GTY) (A) and RBD of SARS-CoV-2 and ACE2 (PDB ID-6M0J) (B)
Algal lectins against other viruses
Griffithsin which acts as an anti-viral lectin against different viruses (Table 2) can also inhibits the growth of the Japanese encephalitis virus by binding on the mannose envelope proteins of the virus. However, binding ability was decreased by incubating Griffithsin lectin with mannose. This inhibition was found to abolish the anti-Japanese encephalitis virus effect of the lectin by blocking mannose-binding sites of the Griffithsin [31]. Another research with multi-layered NPs and NP-EF composite was used in addition to Griffithsin lectin to provide sustained-release of Griffithsin lectin. Both NPs and NP-EF composites showed protection against a lethal dose of HSV-2 infection in a murine model [22]. Lectin from cyanobacterium Lyngabya confervoides MK012409 has sugar specificity with Sugar alcohol (mannitol and sorbitol) and glycan polymer with α glycosidic bond (amylose, yeast mannan, and pectin) has showed virucidal activity against herpes simplex virus type-1 (HSV-1) with EC50 of 167 ± 0.52 ng/mL [32].
Table 2.
Anti-viral algal lectins
| S no. | Year | Source | Species | Sugar binding | Molecular weight (kDa) | Virus | Function |
|---|---|---|---|---|---|---|---|
| 1 | 2018 | Algae | Scytonema varium | Not specified | 14 | HIV | Reduces the HIV-induced cytopathic effect |
| 2 | 2018 | Algae | Griffithsia sp. | Not specified | 12 | HIV | Higher inhibition against both T-tropic and M-tropic HIV-1 strains |
| 3 | 2018 | Algae | Griffithsia sp. | Not specified | 12 | HIV | High-level production of the HIV-1 entry inhibitor griffithsin with a non-viral expression |
| 4 | 2020 | Algae | Griffithsia sp. | Not specified | 12 | HIV | Monomeric griffithsin (mGRFT) in tandem repeats of two (2MG and 2MG3), three (3MG) and four (4MG) units, 2MG and 2MG3 tandemers had similar activity to GRFT against cell-free and cell-associated viruses, while 3MG and 4MG were significantly more potent |
| 5 | 2020 | Algae | Griffithsia sp. | Not specified | 12 | HIV and HSV-2 | Dual-protection against HIV-1 and HSV-2 infections. Composites demonstrated high loading of GRFT NPs and achieved sustained-release of GRFT |
| 6 | 2020 | Algae | Nostoc ellipsosporum | Not specified | 11.96 | HIV | Anti-HIV |
| 7 | 2020 | Algae | Lyngabya confervoides MK012409 | Sugar alcohol (Mannitol and sorbitol) and glycan polymerwith α glycosidic bond (amylose, yeast mannan, and pectin) | Two subunits of 70 (140) | Herpes simplex virus type-1 (HSV-1) | Virucidal activity against HSV-1 |
| 8 | 2016 | Algae | Griffithsia sp. | Not specified | 12 | Japanese encephalitis virus | Inhibited by increasing concentrations of mannose; in turns abolished anti-JEV activity |
| 9 | 2016 | Algae | Griffithsia sp. | Interacts with mannoses of MERS-CoV | 12 | Human coronaviruses Middle East respiratory syndrome coronavirus (MERS-CoV) | Inhibitor of MERS-CoV infection and inhibits entry into host cells of particles pseudo typed with the MERS-CoV spike protein |
Conclusion
Lectins are found in abundance in nature, and anti-viral effects of lectins have been discovered in various plant species. Lectins are proteins that are unique to certain sugars such as mannose, lactose, and varied glycans, and they have a wide range of applications. Mannose-specific lectins, for example, has demonstrated anti-viral activity against various viruses and in future could be used in anti-viral therapy. Although there have been many studies on Coronaviruses in the last 18 months, HIV was the most studied virus in virus diseases. Due to the high variability within the strains and the high mutation rate of COVID-19 coronavirus, it is possible to constantly modify neutralising antibodies or vaccine candidates. It was discovered through the use of lectins from diverse sources that these molecules bind to the viral envelop glycoproteins. Anti-viral drugs target a variety of proteins and enzymes, such as proteases, that are needed for the life cycle of viruses, whereas monoclonal antibody therapy tries to neutralise virus envelope proteins, such as spike protein, that are important for the life cycle of viruses. Lectins from a variety of sources can be utilised to combat these viruses since many of the lectins formed complexes with or interacted with the virus’s envelope protein.
Acknowledgements
S.Naik thanks the Indian council of Medical Research (ICMR) for senior research fellowship (SRF). S.K thanks SERB for financial assistance under the Fast Track Scheme (Grant No. SB/FT/LS-190/2012). S.K thanks ICMR New-Delhi for financial assistance under adhoc project No. ISRM/12(38)/2019 ID NO-2019-14004. We thank Dr. Kali Kishore Reddy Tetala for his valuable and critical comments on the work.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Colomb F, Giron LB, Kuri-Cervantes L, et al. Sialyl-Lewisx glycoantigen is enriched on cells with persistent HIV transcription during therapy. Cell Rep. 2020;32:107991. doi: 10.1016/j.celrep.2020.107991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billon-Denis E, Tournier JN. COVID-19 and vaccination: a global disruption. Med Sci (Paris) 2020;36:1034–1037. doi: 10.1051/medsci/2020203. [DOI] [PubMed] [Google Scholar]
- 3.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson AJ, Cao L, Ma Y, et al. Human influenza virus hemagglutinins contain conserved oligomannose N-linked glycans allowing potent neutralization by lectins. Cell Host Microbe. 2020;27:725–735.e725. doi: 10.1016/j.chom.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roychoudhury S, Das A, Sengupta P, et al. Viral pandemics of the last four decades: pathophysiology, health impacts and perspectives. Int J Environ Res Public Health. 2020;17:9411. doi: 10.3390/ijerph17249411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar M, Roy A, Rawat RS, et al. Identification and structural studies of natural inhibitors against SARS-CoV-2 viral RNA methyltransferase (NSP16) J Biomol Struct Dyn. 2021 doi: 10.1080/07391102.2021.1997821. [DOI] [PubMed] [Google Scholar]
- 7.Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins V, Sohaei D, Diamandis EP, Prassas I. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2021;58:297–310. doi: 10.1080/10408363.2020.1860895. [DOI] [PubMed] [Google Scholar]
- 9.Millet JK, Séron K, Labitt RN, et al. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antiviral Res. 2016;133:1–8. doi: 10.1016/j.antiviral.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sifuentes-Rodríguez E, Palacios-Reyes D. COVID-19: the outbreak caused by a new coronavirus. Bol Med Hosp Infant Mex. 2020;77:47–53. doi: 10.24875/BMHIM.20000039. [DOI] [PubMed] [Google Scholar]
- 11.Seyed Hosseini E, Riahi Kashani N, Nikzad H, Azadbakht J, Hassani Bafrani H, Haddad Kashani H. The novel coronavirus disease-2019 (COVID-19): mechanism of action, detection and recent therapeutic strategies. Virology. 2020;551:1–9. doi: 10.1016/j.virol.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamoto M, Hidaka A, Toyama M, Baba M. Galectin-3 is involved in HIV-1 expression through NF-κB activation and associated with tat in latently infected cells. Virus Res. 2019;260:86–93. doi: 10.1016/j.virusres.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Al-Qahtani AA, Murugaiah V, Bashir HA, et al. Full-length human surfactant protein A inhibits influenza A virus infection of A549 lung epithelial cells: a recombinant form containing neck and lectin domains promotes infectivity. Immunobiology. 2019;224:408–418. doi: 10.1016/j.imbio.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Janahi EMA, Haque S, Akhter N, et al. Bioengineered intravaginal isolate of Lactobacillus plantarum expresses algal lectin scytovirin demonstrating anti-HIV-1 activity. Microb Pathog. 2018;122:1–6. doi: 10.1016/j.micpath.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Liu YM, Shahed-Al-Mahmud M, Chen X, et al. A carbohydrate-binding protein from the edible lablab beans effectively blocks the infections of influenza viruses and SARS-CoV-2. Cell Rep. 2020;32:108016. doi: 10.1016/j.celrep.2020.108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barre A, Van Damme EJM. Man-specific lectins from plants, fungi, algae and cyanobacteria, as potential blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) coronaviruses: biomedical perspectives. Cells. 2021 doi: 10.3390/cells10071619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohrab SS, Suhail M, Kamal MA, Ahmad F, Azhar EI. The emergence of human pathogenic coronaviruses: lectins as antivirals for SARS-CoV-2. Curr Pharm Des. 2020;26:5286–5292. doi: 10.2174/1381612826666200821120409. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento da Silva LC, Mendonça JSP, de Oliveira WF, et al. Exploring lectin-glycan interactions to combat COVID-19: lessons acquired from other enveloped viruses. Glycobiology. 2021;31:358–371. doi: 10.1093/glycob/cwaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopper JTS, Ambrose S, Grant OC, et al. The tetrameric plant lectin banlec neutralizes HIV through bidentate binding to specific viral glycans. Structure. 2017 doi: 10.1016/j.str.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell CA, Ramessar K, O’Keefe BR. Antiviral lectins: selective inhibitors of viral entry. Antiviral Res. 2017;142:37–54. doi: 10.1016/j.antiviral.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma M, Hotpet V, Sindhura BR, Kamalanathan AS, Swamy BM. Purification, characterization and biological significance of mannose binding lectin from dioscorea bulbifera bulbils. Int J Biol Macromol. 2017;102:1146–1155. doi: 10.1016/j.ijbiomac.2017.04.085. [DOI] [PubMed] [Google Scholar]
- 22.Thakur K, Kaur T, Singh J, et al. Sauromatum guttatum lectin: spectral studies, lectin-carbohydrate interaction, molecular cloning and in silico analysis. Int J Biol Macromol. 2017;104:1267–1279. doi: 10.1016/j.ijbiomac.2017.06.123. [DOI] [PubMed] [Google Scholar]
- 23.Jayaprakash NG, Singh A, Vivek R, et al. The barley lectin, horcolin, binds high-mannose glycans in a multivalent fashion, enabling high-affinity, specific inhibition of cellular HIV infection. J Biol Chem. 2020;295:12111–12129. doi: 10.1074/jbc.RA120.013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur R, Neetu MR, Jose J, Kumar P, Tomar S. Glycan-dependent chikungunya viral infection divulged by antiviral activity of NAG specific chi-like lectin. Virology. 2019;526:91–98. doi: 10.1016/j.virol.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 25.de Camargo LJ, Picoli T, Fischer G, de Freitas ACO, de Almeida RB, da Silva PL. Antiviral activity of native banana lectin against bovine viral diarrhea virus and bovine alphaherpesvirus type 1. Int J Biol Macromol. 2020;157:569–576. doi: 10.1016/j.ijbiomac.2020.04.125. [DOI] [PubMed] [Google Scholar]
- 26.Petrova MI, van den Broek MFL, Spacova I, et al. Engineering Lactobacillus rhamnosus GG and GR-1 to express HIV-inhibiting griffithsin. Int J Antimicrob Agents. 2018;52:599–607. doi: 10.1016/j.ijantimicag.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Habibi P, Soccol CR, O'Keefe BR, et al. Gene-silencing suppressors for high-level production of the HIV-1 entry inhibitor griffithsin in Nicotiana benthamiana. Process Biochem. 2018;70:45–54. doi: 10.1016/j.procbio.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexandre K, Malatji K, Mulaudzi T. Comparison of the antiviral activity of the microbicide candidate griffithsin and its tandemers derivatives against different modes of HIV-1 transmission. Virology. 2020;544:12–20. doi: 10.1016/j.virol.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Tyo KM, Lasnik AB, Zhang L, et al. Sustained-release griffithsin nanoparticle-fiber composites against HIV-1 and HSV-2 infections. J Control Release. 2020;321:84–99. doi: 10.1016/j.jconrel.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal R, Trivedi J, Mitra D. High yield production of recombinant cyanovirin-N (antiviral lectin) exhibiting significant anti-HIV activity, from a rationally selected Escherichia coli strain. Process Biochem. 2020;93:1–11. doi: 10.1016/j.procbio.2020.03.011. [DOI] [Google Scholar]
- 31.El-Fakharany EM, Saad MH, Salem MS, Sidkey NM. Biochemical characterization and application of a novel lectin from the cyanobacterium Lyngabya confervoides MK012409 as an antiviral and anticancer agent. Int J Biol Macromol. 2020;161:417–430. doi: 10.1016/j.ijbiomac.2020.06.046. [DOI] [PubMed] [Google Scholar]
- 32.Ishag HZ, Li C, Wang F, Mao X. Griffithsin binds to the glycosylated proteins (E and prM) of Japanese encephalitis virus and inhibit its infection. Virus Res. 2016;215:50–54. doi: 10.1016/j.virusres.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]