Abstract
Shiga toxins (Stxs), encoded by the stxA and stxB genes, are important contributors to the virulence of Escherichia coli O157:H7 and other Stx-producing E. coli (STEC) strains. The stxA and stxB genes in STEC strains are located on the genomes of resident prophages of the λ family immediately downstream of the phage late promoters (pR′). The phage-encoded Q proteins modify RNA polymerase initiating transcription at the cognate pR′ promoter which creates transcription complexes that transcend a transcription terminator immediately downstream of pR′ as well as terminator kilobases distal to pR′. To test if this Q-directed processive transcription plays a role in stx2AB expression, we constructed a mutant prophage in an O157:H7 clinical isolate from which pR′ and part of Q were deleted but which has an intact pStx, the previously described stx2AB-associated promoter. We report that production of significant levels of Stx2 in this O157:H7 isolate depends on the pR′ promoter. Since transcription initiating at pR′ ultimately requires activation of the phage lytic cascade, expression of stx2AB in STEC depends primarily on prophage induction. By showing this central role for the prophage in stx2AB expression, our findings contradict the prevailing assumption that phages serve merely as agents for virulence gene transfer.
Escherichia coli O157:H7 and other Shiga toxin (Stx)-producing E. coli (STEC) strains are responsible for outbreaks and sporadic cases of diarrhea. In some patients, exposure to STEC leads to hemorrhagic colitis and hemolytic uremic syndrome that may lead to death (20). Two major classes of Stxs, Stx1 and Stx2, encoded respectively by stx1AB and stx2AB, have been identified in STEC (2). The severe clinical consequences of STEC infections are thought to be caused by the activities of Stxs, although Stx2 appears to be more closely associated with these sequelae than does Stx1 (7, 28, 36). Shiga toxins are of the A-B type, with the glycolipid-binding B subunits being involved in the transport of the enzymatic A subunits into the eukaryotic cell where the A subunit, acting as a glycosylase, catalyzes a cleavage at a unique site in the 28S rRNA (2). The resulting inactivation of the ribosome leads to an inhibition of protein synthesis. More than 60 serotypes of STEC have been associated with human disease (1). The stx genes of many, if not all, STEC strains are in the genomes of prophages of the lambdoid family (19, 23, 27). This fact probably accounts for the wide dissemination of these genes in diverse E. coli serotypes.
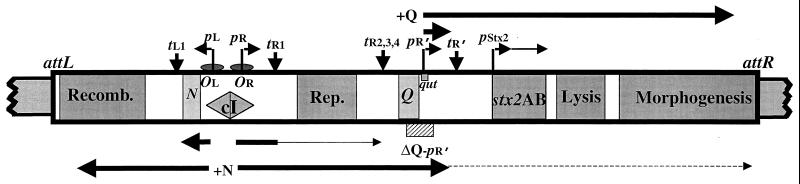
Comparison of lambdoid phage genomes (9) has revealed a common arrangement of functionally similar genes and a shared strategy governing gene expression (Fig. 1). In the lysogenic state, the repressor silences transcription of most phage genes (30). Removal of repression, which can occur when DNA damage activates the bacterial SOS response causing RecA-mediated cleavage of the repressor (21), leads to a cascade of regulatory events beginning with expression of the N transcription antitermination protein (30). Terminator read-through mediated by the N protein results in expression of delayed early genes that encode products involved in replication and prophage excision, as well as the Q antitermination protein (15). Q acts at a site, qut, within the phage late promoter, pR′, modifying RNA polymerase to a highly processive form that reads through downstream terminators (39), including the strong Rho-independent terminator, tR′, located directly downstream of pR′ (32, 33). Thus, late gene expression by lambdoid prophages is a consequence of prophage induction; i.e., it follows removal of repression of the early promoters, pR and pL.
FIG. 1.
Presumed genome arrangement and transcription patterns of Stx2-encoding phage Φ361. This diagram is based on the maps of characterized lambdoid phages (16, 26), including the Stx2-encoding phage 933W (29), and the information we have on Φ361 (not drawn to scale). Shown are relevant genes, promoters, terminators, operators, and the site of the ΔQ-pR′ deletion. The attL and attR sites are the junctions of the integrated prophage with the bacterial DNA. Below are shown the patterns of transcription initiating at the early promoters, pL and pR, in the absence and presence of N. Above are shown the patterns of transcription initiating at the late promoter pR′ in the absence and presence of Q. Induction inactivates the repressor (cI), resulting in expression of the N protein-encoded antiterminator. N modification of RNA polymerase allows read-through of the tL1 and tR1 terminators, resulting in production of proteins catalyzing excision and replication of the phage genome. The N-modified RNA polymerase also reads through the tR2, -3, and -4 terminators, leading to synthesis of the Q antiterminator. The Q-modified RNA polymerase transcends transcription termination at tR′ and subsequent downstream terminators, allowing expression of late genes, which include those encoding proteins involved in lysis and morphogenesis. Recomb., recombination; Rep., replication.
Although previous studies have identified functional promoters immediately upstream of stx genes (8, 34), recent evidence suggests that prophage induction and the resulting transcription from the phage pR′ late promoters are likely to be important in stx expression (26). First, stx genes are located directly downstream of the pR′ promoters and upstream of the phage lysis genes (25, 26, 29). Second, agents that induce Stx phages also increase Stx expression by their host STEC strains (24, 40). Third, the Q protein from Stx phage H-19B acting in trans directs high-level expression from the stx genes of repressed H-19B and 933W prophages, two phages that share nearly identical Q, qut, and pR′ sequences but different stx genes (25, 29). Thus, these observations suggest that the regulatory circuits of Stx-encoding phages play a direct role in STEC pathogenesis. Moreover, if phage-directed lysis is important in Stx release from STEC cells, Q-activated transcription of stx and downstream lysis genes may serve as the primary mechanism for coordinating production and release of toxin during a STEC infection (26, 38). We present evidence that transcription from the late phage promoter pR′ resulting from prophage induction plays a major role in the production of toxin from the Stx2-encoding O157:H7 enterohemorrhagic E. coli clinical isolate 1:361.
MATERIALS AND METHODS
Bacteria and plasmids.
Strain 1:361, a clinical E. coli isolate of serotype O157:H7, has previously been described (37). Strain 1:361ΔQ-pR′ was derived from 1:361 using the allele exchange vector pΔQ-pR′. This plasmid is a derivative of the sacB counterselectable vector pCVD442 (13), which contains an ∼900-bp DNA fragment from the pR′ region of Φ361 with a 189-bp deletion that includes pR′. The inserted fragment was synthesized using the procedure involving PCR-based splicing by overlap extension (18) with the 1:361 chromosomal DNA serving as the template. Four primers were employed in constructing this fragment: the upstream primer 5′CACCGTAAAAACCATTCCTGACATGCTCC, corresponding to sequences in the Q gene; the overlapping primers 5′CCTTTCTGTGTACTTTCCGCCAGCATCATCAGCATGCC and 5′GGCATGCTGATGATGCTGGCGGAAAGTACACAGAAAGG, corresponding to sequences upstream and downstream of the deleted region; and 5′GCCACCACATTAACTGAAAAGATAAC, corresponding to sequences downstream of pR′. pΔQ-pR′ was introduced into 1:361 by conjugation from E. coli strain SM10λpir. Exconjugates were selected as streptomycin- and ampicillin-resistant colonies. Haploid cells were then selected as sucrose-resistant colonies as described previously (13) and subsequently screened for ΔQ-pR′ mutations. DNA sequence analysis confirmed that 1:361ΔQ-pR′ contains the proper deletion.
RNA preparation.
RNA from mid-log-phase culture of 1:361 or 1:361ΔQ-pR′ was prepared using an RNeasy kit (Qiagen). Northern blotting was performed using standard procedures (3) with a 32P-labeled stx2A riboprobe. The stx2A probe was synthesized using T7 polymerase (Ambion), with plasmid pPW58 (which contains 258 bp of stx2A coding sequence subcloned into plasmid pCR2.1-TOPO [Invitrogen]) as the template.
Animal model.
A modified version of the streptomycin-treated-mouse model of enterohemorrhagic E. coli infection (35) was used to compare the levels of colonization and intestinal Stx2 production of 1:361 and 1:361ΔQ-pR′. Four-week-old CD-1 mice were given drinking water containing streptomycin (2 g/liter) throughout each experiment. Two days after streptomycin treatment was begun, mice were inoculated intragastrically with ∼1010 CFU of either 1:361 or 1:361ΔQ-pR′. Fecal samples were collected daily beginning 1 day after inoculation. Numbers of streptomycin-resistant CFU of either 1:361 or 1:361ΔQ-pR′ and fecal Stx2 concentration were determined as described previously (41).
Toxin assay.
Stx levels were measured using an enzyme-linked immunosorbent assay (ELISA) essentially as previously described (14).
RESULTS
Prophage construction.
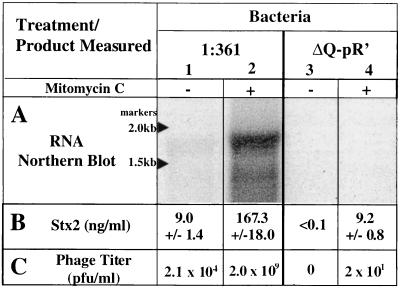
E. coli strain 1:361, isolated from a patient with bloody diarrhea, contains two Stx2-encoding lambdoid prophages (data not shown). One of these phages was found to be defective, and the other, designated Φ361, shares, at a minimum, the Q, pR′, and stx2 sequences found in phage 933W (37) (Fig. 1). We constructed a derivative of 1:361, called 1:361ΔQ-pR′, in which a 189-bp region including the 3′ end of Q and the pR′ late promoter of the Φ361 prophage was deleted (Fig. 1). This deletion left intact the stx2A and -B genes and the previously identified stx2 promoter, pStx2. As expected for a lysogen carrying a lambdoid prophage defective in transcription from pR′, 1:361ΔQ-pR′ did not produce phage detectable by plaque formation following induction with mitomycin C (Fig. 2C).
FIG. 2.
Production of stx2 RNA, Stx2, and phage from STEC strains 1:361 and 1:361ΔQ-pR′. (A) Measurement by Northern blotting of stx mRNA levels. Total RNA was prepared from 3-h cultures of 1:361 and 1:361ΔQ-pR′ grown with (+) or without (−) 0.5 μg of mitomycin C per ml. Six micrograms of RNA was run on a 1% agarose gel, transferred to a nylon membrane, and then probed with a 32P-labeled Stx2-encoding sequence riboprobe. (B) Total Stx2 was measured from the same cultures used in the Northern blot, using a previously described ELISA (14). Values with standard deviations from three independent measurements are shown. (C) Phage titers were determined using E. coli C600 as the indicator strain (3). This procedure leaves uncounted mutant phage producing very low bursts.
Transcription levels.
Northern blotting was performed to directly assess the role of transcription initiating at pR′ in stx expression (Fig. 2A). Strain 1:361 produced low levels of the stx transcript (lane 1), while strain 1:361ΔQ-pR′ (lane 3) failed to produce detectable levels of the stx transcript. Following induction with mitomycin C, strain 1:361 produced high levels of the stx transcript (lane 2), while strain 1:361ΔQ-pR′ again failed to produce observable levels of the stx transcript (lane 4). Based on studies with λ (33), Q-mediated antitermination of transcription initiating at pR′ is expected to result in a >25-kb message; however, the relatively small size of the stx transcript is not surprising for an mRNA initiating at pR′ since the λ pR′ message is known to be processed (11). Therefore, these experiments provide evidence that the Q-pR′ region in Φ361, which is necessary for transcription of late phage genes, is also important for stx2 transcription.
Toxin levels.
Stx levels in cultures of 1:361 and 1:361ΔQ-pR′, measured by an ELISA (14), provide further evidence that transcription from pR′ contributes significantly to stx expression (Fig. 2B). Uninduced cultures of strain 1:361 produced low levels of Stx2, and under identical conditions, cultures of 1:361ΔQ-pR′ failed to produce measurable levels of Stx2. Thus, the low level of Stx produced by the untreated culture most likely resulted from Q-stimulated transcription from pR′ in the small fraction of lysogens in which there was spontaneous induction of the prophage. Induced cultures of 1:361 (treated with mitomycin C) produced high levels of Stx2, while similarly treated cultures of 1:361ΔQ-pR′ produced toxin levels that were nearly 20-fold less (Fig. 2B). These results confirm the conclusion from the Northern analysis that the Q-pR′ region exerts a critical role in regulating Stx2 synthesis in vitro.
Stx production in vivo.
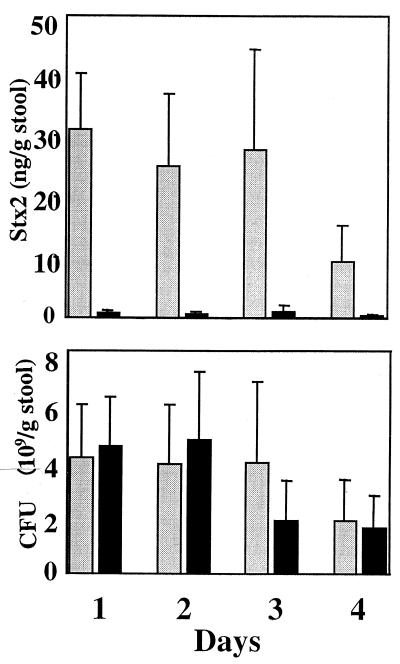
To assess the physiological relevance of these findings, Stx2 production by strains 1:361 and 1:361ΔQ-pR′ was examined in vivo, using a mouse model system (35). In these experiments, CD-1 mice were inoculated intragastrically with either 1:361 or 1:361ΔQ-pR′. Over the course of 4 days, stool samples were collected and assayed both for CFU of 1:361 or 1:361ΔQ-pR′ and for levels of Stx2. In striking contrast to the similar numbers of 1:361 and 1:361ΔQ-pR′ CFU recovered, the difference in the amounts of Stx in the stool specimens between the mice inoculated with the two strains was dramatic (Fig. 3). There was an approximately 30-fold lower concentration of Stx2 in stools from animals inoculated with 1:361ΔQ-pR′ than the concentration of Stx2 in stools from mice inoculated with 1:361. Thus, Stx production by strain 1:361 in the animal gut, as in vitro culture, largely derives from the small fraction of the bacteria in which there is prophage induction.
FIG. 3.
Fecal cell counts of 1:361 and 1:361ΔQ-pR′ and Stx2 concentration. Streptomycin-treated CD-1 mice were intragastrically inoculated with either 1:361 or 1:361ΔQ-pR′. Stool samples were collected daily, and the Stx2 concentration (top) and the number of CFU (bottom) of either 1:361 or 1:361ΔQ-pR′ were determined. Gray bars represent mice inoculated with 1:361, and black bars represent mice inoculated with 1:361ΔQ-pR′. There were eight mice in each group. Means and standard deviations are presented.
DISCUSSION
Previous studies identified a functional promoter, pStx, immediately upstream of stx genes in the Stx1-encoding phage H-19B (8) and the Stx2-encoding phage 933W (34). These findings appeared to confirm the idea that, although stx genes can be carried by phages, once the phage is established as a prophage, stx is expressed independently of the regulatory system governing phage gene expression. More recent studies led to the suggestion that phage-encoded regulatory circuits that result in Q modification of transcription initiating at the phage pR′ promoter and subsequent processive transcription of downstream genes, including stxA and stxB, may play a role in stxAB expression (26). We directly tested this hypothesis by deleting the Q-pR′ region while leaving intact the pStx promoter of a prophage that carries the stx2A and -B genes in an E. coli O157:H7 clinical isolate.
Our experiments comparing levels of Stx2 production by the parent clinical strain and an isogenic derivative with a deletion of the Q-pR′ region of the prophage provide compelling evidence supporting the idea that Q-activated pR′-promoted processive transcription plays a critical role in Stx2 production. Since Q expression depends on transcription from pR, a promoter that is under repressor control, stx expression, according to this idea, ultimately depends on induction of the prophage. Induction is expected to result in increased stx copy number and increased transcription of phage late genes initiating from the early promoter pR. However, copy number and increased transcription of phage late genes by N-antiterminated transcription from the early promoter pR are not expected to be affected by the ΔQ-pR′ deletion (17). Therefore, our results strongly suggest that high-level Q-modified transcription initiating at the Φ361 pR′ late promoter is responsible for the increase in Stx2 production observed upon induction of strain 1:361. Residual toxin production by 1:361ΔQ-pR′ may be due to low-level transcription from the early pR′ promoter (10) or pStx2 and possibly from the other defective Stx2-encoding phage present in 1:361. Our results are consistent with the hypothesis that Q-modified transcription (32) beginning at pR′ proceeds through the terminator tR′, into the stx genes, past termination signals, and through the downstream lysis genes, providing a unified mechanism for coupling Stx production and release. Previously, Stx phages were considered to be important in the dissemination of but not the regulation of expression of toxin genes (38). Our results favor the view that stx2 expression is regulated as one of the late phage genes and suggest that, far from being a mere vector for toxin gene transfer, Φ361 directs production of Stx2 as part of its lytic cycle. If our model is correct, it would mean that an induced subpopulation of the total infecting STEC population is responsible for significant levels of Stx production. This does not appear to be the exclusive mode for regulating virulence gene expression in lambdoid phages. The prototypical λ itself encodes bor and lom, genes encoding proteins having significant homology to known bacterial virulence factors (5) and, in the case of bor, shown to confer a virulence phenotype (6). The biologically relevant expression of these genes, unlike that of the stx genes, occurs from the repressed prophage.
Our study raises a question about the evolution of stx-carrying phages. Since the subpopulation of bacteria postulated to be the primary producers of Stx would not survive to be infectious, how can evolution select for stx if its production is coupled with phage-mediated lysis? The diarrhea induced by Stx likely contributes to the dissemination of the remaining population, an effect that may outweigh selection against toxin producers. There is precedence for the idea that the death of a minority of a bacterial population contributes to the survival advantage of the majority. Bacteriocins, molecules produced by some bacteria that are lethal to other bacteria of the same and closely related species, are produced by a subpopulation that is lysed in the process of releasing the bacteriocin (31). A possible role for the phage in expression of phage-borne virulence genes has been considered since the early demonstration that diphtheria toxin is carried by a phage (4). As far as we know, the results of our study provide the first evidence directly linking a phage regulatory cascade with expression of a phage-encoded virulence factor.
Expression of virulence factors, even when carried by mobile genetic elements, is generally considered in the context of overall coordination of the infectious strategy of the host bacterium (22). Here we present evidence that the bacteriophage life cycle is the dominant, if not the only, important factor involved in Stx2 expression. It will be interesting to learn whether phage control of virulence gene expression is unique to stx or can serve as a paradigm that explains regulation of expression of other phage-encoded virulence factors. In light of our findings, an understanding of the conditions in the intestine that trigger prophage induction and subsequent up-regulation of Stx expression takes on new significance. Notable pharmacologic agents which induce prophages include mitomycin C, used in chemotherapy, and numerous antibiotics, including the fluoroquinolones, often used in treatment of diarrhea (41). Interestingly, endogenous products of inflammatory cells, such as H2O2, are also known to induce lambdoid prophages (12). While it remains unknown to what extent such exogenous and endogenous factors contribute to STEC pathogenesis, our study suggests that clinical intervention to prevent prophage induction may reduce Stx production during STEC infection.
ACKNOWLEDGMENTS
P.L.W. and M.N.N. contributed equally to this work.
We thank Andrew Camilli, Victor DiRita, Michelle Swanson, and Bridgid Davis for critical reading of the manuscript. Eric Olson is thanked for helpful discussions and encouragement.
This work was supported by grants from the NIH (D.I.F., M.K.W., and D.W.K.A.), the Pew Foundation (M.K.W.), the FDA (D.W.K.A.), the HHMI (P.L.W. and M.K.W.), and an NIH biotechnology training grant (M.N.N.).
REFERENCES
- 1.Acheson D W K, Keusch G T K. Which Shiga toxin-producing types of Escherichia coli are important? ASM News. 1996;62:302–306. [Google Scholar]
- 2.Acheson W K, Donohue-Rolfe A, Keusch G T. The family of Shiga and Shiga-like toxins. In: Alouf J E, Freer J H, editors. Sourcebook of bacterial protein toxins. London, United Kingdom: Academic Press; 1991. pp. 415–433. [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 4.Barksdale L, Arden S B. Persisting bacteriophage infections, lysogeny, and phage conversion. Annu Rev Microbiol. 1974;28:265–297. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- 5.Barondess J J, Beckwith J. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature. 1990;346:871–874. doi: 10.1038/346871a0. [DOI] [PubMed] [Google Scholar]
- 6.Barondess J J, Beckwith J. bor gene of phage lambda, involved in serum resistance, encodes a widely conserved outer membrane lipoprotein. J Bacteriol. 1995;177:1247–1253. doi: 10.1128/jb.177.5.1247-1253.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boerlin P, McEwen S A, Boerlin-Petzold F, Wilson J B, Johnson R P, Gyles C L. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol. 1999;37:497–503. doi: 10.1128/jcm.37.3.497-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderwood S B, Auclair F, Donohue-Rolfe A, Keusch G T, Mekalanos J J. Nucleotide sequence of the Shiga-like toxin genes of Escherichia coli. Proc Natl Acad Sci USA. 1987;84:4364–4368. doi: 10.1073/pnas.84.13.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell A. Comparative molecular biology of lambdoid phages. Annu Rev Microbiol. 1994;48:193–222. doi: 10.1146/annurev.mi.48.100194.001205. [DOI] [PubMed] [Google Scholar]
- 10.Dambly C, Couturier M. A minor Q-independent pathway for expression of late genes in bacteriophage lambda. Mol Gen Genet. 1971;113:244–250. doi: 10.1007/BF00339545. [DOI] [PubMed] [Google Scholar]
- 11.Daniels D L, Subbarao M N, Blattner F R, Lozeron H A. Q-mediated late gene transcription of bacteriophage lambda: RNA start point and RNase III processing sites in vivo. Virology. 1988;167:568–577. [PubMed] [Google Scholar]
- 12.DeMarini D M, Brooks H G. Induction of prophage lambda by chlorinated organics: detection of some single-species/single-site carcinogens. Environ Mol Mutagen. 1992;19:98–111. doi: 10.1002/em.2850190204. [DOI] [PubMed] [Google Scholar]
- 13.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donohue-Rolfe A, Acheson D W, Kane A V, Keusch G T. Purification of Shiga toxin and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect Immun. 1989;57:3888–3893. doi: 10.1128/iai.57.12.3888-3893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman D I, Court D L. Transcription antitermination: the lambda paradigm updated. Mol Microbiol. 1995;18:191–200. doi: 10.1111/j.1365-2958.1995.mmi_18020191.x. [DOI] [PubMed] [Google Scholar]
- 16.Hendrix R W, Roberts J W, Stahl F W, Weisberg R A. Lambda II. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1983. [Google Scholar]
- 17.Herskowitz I, Signer Control of transcription from the r strand of bacteriophage lambda. Cold Spring Harbor Symp Quant Biol. 1970;35:355–368. [Google Scholar]
- 18.Horton R M, Cai Z L, Ho S N, Pease L R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- 19.Huang A, Friesen J, Brunton J L. Characterization of a bacteriophage that carries the genes for production of Shiga-like toxin I in Escherichia coli. J Bacteriol. 1987;169:4308–4312. doi: 10.1128/jb.169.9.4308-4312.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaper J B, O'Brien A D. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: American Society for Microbiology; 1998. [Google Scholar]
- 21.Little, J. W. The SOS regulatory system, p. 453–479. In E. C. C. Linn and A. S. Lynch (ed.), Regulation of gene expression in Escherichia coli. R. G. Landis Co., Georgetown, Tex.
- 22.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizutani S, Nakazono N, Sugino Y. The so-called chromosomal verotoxin genes are actually carried by defective prophages. DNA Res. 1999;6:141–143. doi: 10.1093/dnares/6.2.141. [DOI] [PubMed] [Google Scholar]
- 24.Mühldorfer I, Hacker J, Keusch G T, Acheson D W, Tschape H, Kane A V, Ritter A, Olschlager T, Donohue-Rolfe A. Regulation of the Shiga-like toxin II operon in Escherichia coli. Infect Immun. 1996;64:495–502. doi: 10.1128/iai.64.2.495-502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neely M N, Friedman D I. Arrangement and functional identification of genes in the regulatory region of lambdoid phage H-19B, a carrier of a Shiga-like toxin. Gene. 1998;223:105–113. doi: 10.1016/s0378-1119(98)00236-4. [DOI] [PubMed] [Google Scholar]
- 26.Neely M N, Friedman D I. Functional and genetic analysis of regulatory regions of coliphage H-19B: location of shiga-like toxin and lysis genes suggest a role for phage functions in toxin release. Mol Microbiol. 1998;28:1255–1267. doi: 10.1046/j.1365-2958.1998.00890.x. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien A D, Newland J W, Miller S F, Holmes R K, Smith H W, Formal S B. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 28.Ostroff S M, Tarr P I, Neill M A, Lewis J H, Hargrett-Bean N, Kobayashi J M. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J Infect Dis. 1989;160:994–998. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 29.Plunkett G, III, Rose D J, Durfee T J, Blattner F R. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J Bacteriol. 1999;181:1767–1778. doi: 10.1128/jb.181.6.1767-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ptashne M. A genetic switch. 2nd ed. Blackwell Scientific Publications, Ca: Cell Press; 1992. mbridge, Mass. [Google Scholar]
- 31.Riley M A. Molecular mechanisms of bacteriocin evolution. Annu Rev Genet. 1998;32:255–278. doi: 10.1146/annurev.genet.32.1.255. [DOI] [PubMed] [Google Scholar]
- 32.Roberts J. Antitermination and the control of transcription elongation. In: McKnight S L, Yamamoto K R, editors. Transcription regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1992. pp. 389–406. [Google Scholar]
- 33.Roberts J W, Yarnell W, Bartlett E, Guo J, Marr M, Ko D C, Sun H, Roberts C W. Antitermination by bacteriophage Lambda Q protein. Cold Spring Harbor Symp Quant Biol. 1998;63:319–325. doi: 10.1101/sqb.1998.63.319. [DOI] [PubMed] [Google Scholar]
- 34.Sung L M, Jackson M P, O'Brien A D, Holmes R K. Transcription of the Shiga-like toxin type II and Shiga-like toxin type II variant operons of Escherichia coli. J Bacteriol. 1990;172:6386–6395. doi: 10.1128/jb.172.11.6386-6395.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadolkowski E A, Burris J A, O'Brien A D. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1990;58:2438–2445. doi: 10.1128/iai.58.8.2438-2445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wadolkowski E A, Sung L M, Burris J A, Samuel J E, O'Brien A D. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun. 1990;58:3959–3965. doi: 10.1128/iai.58.12.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner P L, Acheson D W, Waldor M K. Isogenic lysogens of diverse Shiga toxin 2-encoding bacteriophages produce markedly different amounts of Shiga toxin. Infect Immun. 1999;67:6710–6714. doi: 10.1128/iai.67.12.6710-6714.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldor M K. Bacteriophage biology and bacterial virulence. Trends Microbiol. 1998;6:295–297. doi: 10.1016/s0966-842x(98)01320-1. [DOI] [PubMed] [Google Scholar]
- 39.Yarnell W S, Roberts J W. Mechanism of intrinsic transcription termination and antitermination. Science. 1999;284:611–615. doi: 10.1126/science.284.5414.611. [DOI] [PubMed] [Google Scholar]
- 40.Yee A J, De Grandis S, Gyles C L. Mitomycin-induced synthesis of a Shiga-like toxin from enteropathogenic Escherichia coli H.I.8. Infect Immun. 1993;61:4510–4513. doi: 10.1128/iai.61.10.4510-4513.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, McDaniel A D, Wolf L E, Keusch G T, Waldor M K, Acheson D W. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis. 2000;181:664–670. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]