Abstract
A β-1,4-xylan hydrolase (xylanase A) produced by Erwinia chrysanthemi D1 isolated from corn was analyzed with respect to its secondary structure and enzymatic function. The pH and temperature optima for the enzyme were found to be pH 6.0 and 35°C, with a secondary structure under those conditions that consists of approximately 10 to 15% α-helices. The enzyme was still active at temperatures higher than 40°C and at pHs of up to 9.0. The loss of enzymatic activity at temperatures above 45°C was accompanied by significant loss of secondary structure. The enzyme was most active on xylan substrates with low ratios of xylose to 4-O-methyl-d-glucuronic acid and appears to require two 4-O-methyl-d-glucuronic acid residues for substrate recognition and/or cleavage of a β-1,4-xylosidic bond. The enzyme hydrolyzed sweetgum xylan, generating products with a 4-O-methyl-glucuronic acid-substituted xylose residue one position from the nonreducing terminus of the oligoxyloside product. No internal cleavages of the xylan backbone between substituted xylose residues were observed, giving the enzyme a unique mode of action in the hydrolysis compared to all other xylanases that have been described. Given the size of the oligoxyloside products generated by the enzyme during depolymerization of xylan substrates, the function of the enzyme may be to render substrate available for other depolymerizing enzymes instead of producing oligoxylosides for cellular metabolism and may serve to produce elicitors during the initiation of the infectious process.
Next to cellulose, hemicellulose is the most prominent structural polysaccharide fraction of all higher plants. The common structural polymer found in hemicelluloses is β-1,4-xylan. Variations in the structure of the xylan occur in the extent of substitution by other carbohydrate residues, e.g., 4-O-methyl-d-glucuronopyranosyl and l-arabinofuranosyl residues (31), as well as in the extent of esterification by O-acetyl, p-coumaroyl, and feruloyl groups (25). The predominant structural polymer of the hemicellulose fraction of hardwoods has been well characterized and consists of a linear β-1,4-xylan that is somewhat regularly substituted with 4-O-methyl-glucuronopyranosyl (MeGA) residues linked α-1,2 to internal xylose (Xyl) residues. The ratio of Xyl to MeGA may vary from 5 to more than 20, depending on the source. Due to the prevalence of the MeGA substitutions, this polymer is generally referred to as glucuronoxylan. The glucuronoxylan from monocots (grasses and cereals) differs from that from hardwoods primarily in the higher degrees of substitution of l-arabinose, p-coumaroyl, and feruloyl groups.
As a significant and underutilized resource, the hemicellulose fraction of plant biomass has recently received attention as a carbohydrate substrate for fermentation to alternative fuels and other biobased products (27). The application of acid hydrolysis for the release of fermentable xylose has resulted in limited yields as well as the production of inhibitors of fermentation (36, 37, 38), and several groups are looking at enzymatic means for depolymerizing xylan. A number of endoxylanases have been purified from xylanolytic microorganisms (21). Based upon the primary sequence classification scheme of Henrissat and Bairoch (14), these have been assigned to families 10 and 11 of the glycosyl hydrolases. The two families differ significantly in molecular mass, isoelectric points, substrate preferences, and the oligoxylosides generated as products (2). For the enzymes of each family for which the tertiary structures have been solved, significant differences in structure between the two families have been observed. Members of family 10 have molecular masses of approximately 48 kDa and fold into an α/β barrel, with approximately 40% of the secondary structure of the enzyme being α-helices (9). Members of family 11 are generally much smaller, with molecular masses ranging from 19 to 31 kDa, and fold into structures composed primarily of β-strands (32). The evolution of these two families with their different structural domains implies significant functional differences that have yet to be fully defined. These differences may be due to the abundance and heterogeneity of hemicellulose, which has facilitated the evolution of many different enzymes by xylanolytic microorganisms in order to maximize the utilization of the polymer. One such organism is the phytopathogen Erwinia chrysanthemi.
E. chrysanthemi is a well-characterized phytopathogen which secretes a diverse array of enzymes to aid in infection and maceration of plant tissue, including pectate and pectin lyases, pectinesterase, exo-polygalacturonase, cellulases, proteases, and, in the case of strains isolated from monocots, an endoxylanase (6). The mature endoxylanase (XynA) was purified from cultures of E. chrysanthemi SR120A, isolated from corn (4). The active enzyme was found to have a molecular mass of 42 kDa and an isoelectric point of 8.8. The xynA gene was isolated from a cosmid library of another strain of E. chrysanthemi isolated from corn, strain D1 (20). The recombinant protein thus produced was identical to that obtained from E. chrysanthemi SR120A culture supernatants, as determined by N-terminal sequencing. Sequence analysis of XynA revealed that the enzyme does not have significant homology with xylanases from either family 10 or 11 of the glycosyl hydrolases, but rather is homologous to the endoglucanases (family 5) and human cerebrosidases (family 30). Previous work (20) on xylanase A has shown that removal of the xynA structural gene does not significantly alter the virulence of E. chrysanthemi D1, despite its production only in Erwinia strains infecting host plants with high xylan content. This observation leads to interesting questions regarding the role of XynA in pathogenesis and, in light of the novel structure of the enzyme predicted by its amino acid sequence, the process catalyzed by the enzyme during depolymerization of lignocellulosic substrates. In the current study, we have attempted to answer some of these questions by studying the structural and enzymatic properties of E. chrysanthemi xylanase A.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Escherichia coli BL21(DE3) (lab collection) was transformed with pNTK136, containing the structural gene (xynA) for the 42-kDa xylanase isolated from E. chrysanthemi D1 (20), according to standard procedures (28). Plasmid pNTK136 was generously provided by Noel Keen, Department of Plant Pathology, University of California, Riverside. E. coli BL21(DE3) cultures harboring the pNTK136 plasmid were grown aerobically in Luria-Bertani broth supplemented with 50 mg ampicillin per liter at 30°C with shaking (110 rpm). To induce xynA transcription, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM when previously inoculated cultures had reached an optical density at 600 nm of 0.5. The cultures were grown for 18 h after IPTG addition and harvested by centrifugation (1,000 × g for 20 min at 4°C).
Purification of XynA.
Spheroplasts of E. coli BL21(DE3)(pNTK136) cells harvested as previously described were prepared by the method of Witholt et al. (33). The final supernatants obtained were dialyzed against 5 mM Tris-HCl(pH 6.5) using a 7-kDa cutoff dialysis membrane (Pierce Inc.). The retained dialysate was loaded onto a carboxymethyl cellulose (CM)-Sephadex column (Sigma, Inc.), washed with 5 mM Tris-HCl (pH 6.5), and eluted with a gradient of 0 to 0.5 M NaCl in the same buffer. Peak fractions were identified by measuring the absorbance of the gradient eluate at 280 nm and screened for xylanase activity by determining the change in reducing sugar concentration (23) over time in a 0.1% (wt/vol) solution of beechwood xylan (Sigma, Inc.) in 50 mM sodium acetate (pH 5.5). Fractions containing xylanase activity were pooled and concentrated into 5 mM Tris-HCl (pH 6.5) by filtration using a 10-kDa cutoff membrane filter (PM10; Amicon, Inc.), and the protein concentration of the resulting concentrate was determined using bicinchoninic acid (29), with bovine serum albumin as a standard. The purity of the concentrate was verified by denaturing sodium-dodecyl sulfate-polyacrylamide gel electrophoresis (22), with a single prominent band of 40 kDa detected with Coomassie R250 following electrophoresis of 5.4 μg of protein. While a very faint band was detected with a mobility corresponding to a mass of less than 29 kDa, based upon the intensity of the 40-kDa component, the XynA was judged to be greater than 95% pure.
CD measurements of XynA.
For measurement of the circular dichroism (CD) spectra of XynA in response to different pHs and temperatures, 5.0 μM solutions of purified enzyme in 10 mM sodium phosphate buffer (pH 6.0) were used. For measurements at different pHs, aliquots (1 ml) of 5 μM XynA solutions were each dialyzed against 1 liter of 10 mM sodium phosphate buffer at pH 4.7, 5.2, 6.2, 7.0, 8.0, and 8.7 overnight at 4°C using 1,000-kDa cutoff Slidealyzer cassette units (Pierce, Inc.). Temperature was controlled using a circulating water bath (Neslab RTE-9). Sample measurement was performed in a 10-mm pathlength, water-jacketed quartz cell (170-μl volume). The instrument was calibrated with (+)-10-camphorsulfonic acid (12) prior to obtaining spectra. Baseline correction for each spectrum prior to accumulation averaging was made using the spectrum of 10 mM sodium phosphate buffer at a pH matching that of the sample. The CD spectra of each solution were measured and analyzed as previously described (16). Noise reduction of the averaged spectra was performed using the Jasco J-700 for Windows standard analysis software (version 1.50.01; Jasco, Inc.). Data were analyzed using a mean residue molarity of 0.0019 mol/liter, and deconvolutions of the CD spectra obtained were made using Dicropro version 2.5 (Gilbert Deleage, Institut de Biologie et Chimie des Proteines, CNRS-UPR412, 7, Passage du Vercors, 69 367 Lyon Cedex 07, France).
Substrates used.
Beechwood, birchwood, and a 4-O-methyl-d-glucurono-d-xylan (source unknown) were obtained from Sigma Chemical Co, St. Louis, Mo. Glucuronoxylan was prepared from 10-foot-tall sweetgum tree (Liquidamber styraciflua) stems (43 to 45 mm thick) by the method of Jones et al. (18). 4-Deoxy-hexenuronic acid-substituted xylan was prepared from the commercially prepared beechwood xylan following the method of Telemann et al. (30). 4-O-Methyl-glucoxylan was produced from sweetgum sawdust by methods modified from those of Anderson and Stone (1). 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC; Sigma Chemical Co.) was coupled to the 4-O-methyl-glucuronic acid substituents of the xylan chain in 10 mM sodium phosphate buffer (pH 4.7) for 12 h, followed by reduction for 2 h at room temperature in 200 mM sodium phosphate buffer (pH 8.0) with NaBH4. Both the EDC and the NaBH4 were added to 10-fold excess relative to the empirically determined amounts of glucuronic acid present in the polymer. Confirmation of glucuronic acid reduction was determined following complete acid hydrolysis by high-pressure liquid chromatography (HPLC) analysis (17). Percentages of xylose and glucuronic acid in all xylans used were determined by 13C nuclear magnetic resonance (NMR). Average degrees of polymerization of each xylan were calculated from the ratio of total carbohydrate present, as determined by the method of Dubois et al. (10), to the amount of total reducing sugars, determined by the method of Nelson (23). All samples were run in triplicate with glucose, xylose, and glucuronic acid standards used as controls.
Enzymatic activity as a function of pH and temperature.
Enzyme assays were performed in 0.6-ml volumes containing 2.7 μg of purified XynA. Reactions were started by the addition of enzyme, with the amounts of reducing sugars present determined every 20 min by the method of Nelson (23) and all analyses made in triplicate. One unit of enzyme is that which generates 1 μmol equivalent of reducing sugar in 1 h.
For the comparison of different substrates, solutions containing 1.0% (wt/vol) of the commercially prepared xylans and the sweetgum xylan extract in 50 mM sodium acetate were prepared at pH 5.0, 6.0, 7.0, and 8.0. Some samples were heated (50°C) for 15 min to facilitate solubilization of the polymers. Aliquots (500 μl) of each solution were dispensed into borosilicate tubes, and 100 μl of a 27-μg/ml solution of purified XynA in deionized H2O was added to each tube.
For determination of the effects of temperature, reactions containing XynA in 50 mM sodium acetate (pH 6.0) and 1.0% (wt/vol) 4-O-methyl-glucuronoxylan (Sigma Chemical Co.) in 50 mM sodium acetate (pH 6.0) were prepared. Substrate solutions and necessary volumes of enzyme were incubated for 5 min in a waterbath (Precision, Inc.) at each of the temperatures employed (25 to 60°C) prior to initiation of the reactions by the addition of enzyme.
Analysis of xylosaccharides generated by XynA digestion of sweetgum xylan.
A 50-ml solution of 10% (wt/vol) sweetgum xylan in 50 mM sodium acetate (pH 6.0) was incubated with 138 μg of XynA at 35°C for 24 h with constant shaking (150 rpm). Sweetgum xylan was chosen as the substrate because of both its abundance and the small size of the putative products generated by enzymatic hydrolysis, as predicted by the low ratio of xylose to MeGA calculated for the polymer. The solution was then concentrated by filtration (Amicon PM10 membrane; Millipore, Inc.) to 15 ml. The filtrate (filtrate 1, ca. 35 ml) was concentrated to 1 ml by flash evaporation at 45°C. The retained solution was taken back to 50 ml with the addition of 50 mM sodium acetate (pH 6.0) and had a 1.0-ml sample removed (retentate 1) after mixing thoroughly to disrupt the gel, followed by the addition of another 138 μg of XynA. The solution was incubated under the initial conditions for another 24 h, whereupon the concentration procedure was performed again, resulting in another set of filtrate (filtrate 2) and retentate (retentate 2) fractions. All four fractions were lyophilized and subjected to matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrographic analysis (26) and 13C-NMR analysis as previously described (19). To determine the position of the 4-O-methyl-glucuronic acid-substituted xylose residue in the xylosaccharide products, filtrates 1 and 2 were incubated in the presence of β-xylosidase isolated from Aspergillus niger (Sigma Chemical). β-Xylosidase (1 U) was added to solutions (1%, wt/vol) of both filtrate samples in 50 mM sodium acetate (pH 5.0), followed by incubation at 40°C for 24 h. Xylose released from the nonreducing termini of the xylosaccharide substrate molecules was quantified by HPLC (17).
RESULTS
Structural properties of XynA.
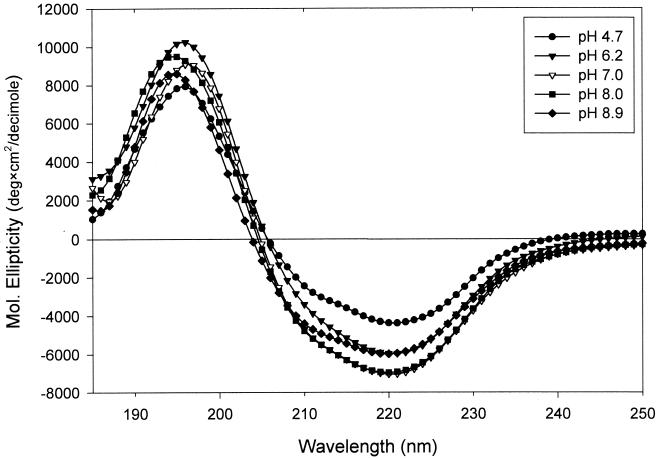
As shown in Fig. 1 and 2, E. chrysanthemi D1 XynA displays local maxima ellipticity values at 196 nm (positive ellipticity) and at 209 and 221 nm (negative ellipticities), indicative of the presence of α-helical secondary-structural elements. The intensity of the ellipticity bands at these wavelengths increases with pH (Fig. 1), reaching a maximum in samples incubated and measured at pH 7.0 and 8.0, then decreasing in intensity as the pH is raised to 8.9. In the positive-ellipticity band observed at 196 nm, a total change of +1,340 deg · cm2 · dmol−1 is observed, and ellipticity changes of −2,257 and −2,552 deg · cm2 · dmol−1 are observed at 209 and 221 nm, respectively, as the pH is decreased to 8.0 from 4.7. Estimation of the α-helical content of the proteins based upon the spectra found in Fig. 1 using the formula of Chen et al. (5) results in a range of values from 10.98% at pH 4.7 to 14.85% at pH 8.9, with a maximum of 17.66% estimated at pH 7.0.
FIG. 1.
Far-UV CD spectra of E. chrysanthemi D1 xylanase A in 10 mM sodium phosphate buffer at different pHs. Spectra shown are the averages of four accumulations obtained at 25°C on a Jasco-J500C spectropolarimeter according to the procedure given in Materials and Methods.
FIG. 2.
Far-UV CD spectra of E. chrysanthemi D1 xylanase A in 10 mM sodium phosphate buffer (pH 6.0) at different temperatures. Spectra shown are the averages of four accumulations obtained on a Jasco-J500C spectropolarimeter according to the procedure given in Materials and Methods.
Xylanase A maintains its secondary structure through the initial 20°C temperature range studied (25 to 40°C), as shown in Fig. 2. Only slight changes in the ellipticity values at 196, 209, and 221 nm are seen in this range, with changes of −282, +289, and +421 deg · cm2 · dmol−1 observed, respectively. As the temperature is increased from 45 to 60°C, the secondary structure of the enzyme is significantly disrupted, with total molecular ellipticity changes of −8,458, +3,118, and +5,032 deg · cm2 · dmol−1 observed at 196, 209, and 221 nm, respectively. Corresponding to the similarities observed in the molecular ellipticities observed in the samples from 25 to 40°C, the α-helical content of the enzyme in this temperature range is also constant at 10.9%, but then drops as the temperature is increased further, with no α-helical content estimated in the enzyme at temperatures above 50°C.
Enzymatic activity as a function of xylan substrate and pH.
The pH optimum of XynA was found to be 6.0, as noted in Table 1. Maximal xylanase activity is observed towards all four natural xylan substrates at pH 6.0, with the highest activities throughout the pH range tested observed in reactions containing the xylans with the highest degrees of 4-O-methyl-glucuronic acid substitution on the xylan backbone, i.e., sweetgum xylan and 4-O-methyl-d-glucuronoxylan. The degree of polymerization does not significantly influence the observed activity of the enzyme, as 4-O-methyl-glucuronoxylan was the shortest of the polymers examined and yet, due to the low ratio of xylose to 4-O-methyl-glucuronic acid, was the best substrate for XynA. Assuming an acid-catalyzed reaction mechanism for enzyme-catalyzed glycosyl bond hydrolysis, it is interesting that the enzyme is still active at pH 8.0 for all substrate types examined.
TABLE 1.
Substrate properties and xylanase activity as a function of pH for xylan substrates
| Xylan type | Substrate propertya
|
Xylanase activityb (U) at pH:
|
||||
|---|---|---|---|---|---|---|
| Est DP | Xyl:GlcUA | 5.0 | 6.0 | 7.0 | 8.0 | |
| Sweetgum | 208 | 6:1 | 0.44 ± 0.03 | 1.75 ± 0.06 | 0.55 ± 0.01 | 0.46 ± 0.03 |
| 4-O-Methyl-d-glucoxylan | 208 | — | ND | 0.33 ± 0.01 | ND | ND |
| 4-O-Methyl-d-glucuronoxylan | 83 | 5:1 | 1.58 ± 0.28 | 1.84 ± 0.32 | 0.77 ± 0.05 | 0.85 ± 0.12 |
| Beechwood | 125 | 16:1 | 0.58 ± 0.08 | 0.77 ± 0.05 | 0.25 ± 0.06 | 0.09 ± 0.05 |
| 4-Deoxy-hexenuronosylxylan | 125 | — | ND | 0.85 ± 0.05 | ND | ND |
| Birchwood | 100 | 26:1 | 0.37 ± 0.01 | 0.47 ± 0.02 | 0.32 ± 0.02 | 0.21 ± 0.01 |
Est DP, estimated degree of polymerization, as determined by the ratio of total anhydrous carbohydrate monomer to total reducing sugars; Xyl:GlcUA, ratio of xylose to glucuronic acid, as determined by commercial sources and verified by 13C-NMR as described in Materials and Methods. —, not determined.
Xylanase activity as reported here is taken from triplicate reactions sampled at multiple times to ensure a linear reaction rate. Activity observed is reported in units, where 1 U of xylanase activity is defined as the amount of enzyme necessary to produce 1 umol of xylose equivalent in 1 h at room temperature. ND, not determined.
The activity of XynA towards the chemically modified substrates at the empirically determined optimum pH is also shown in Table 1. Xylanase A displayed nearly equivalent activity towards 4-deoxy-hexenuronosyl beechwood xylan, a substrate in which the methyl esters on the glucuronic acid substituents have been removed, as was seen in reactions containing unmodified beechwood xylan. This behavior was not seen in reactions with 4-O-methyl-d-glucoxylan, a substrate in which the uronic acid has been reduced to glucose. Xylanase A displayed 81% lower activity towards this modified substrate relative to the activity observed using unmodified sweetgum glucuronoxylan as the substrate.
Enzymatic activity measurements as a function of temperature.
Xylanase A activity was determined at different temperatures at pH 6.0, using commercial 4-O-methylglucuronoxylan, as described in Materials and Methods. Mean activities measured at 25, 30, 35, 40, 45, 55, and 60°C were 1.95 ± 0.12, 2.40 ± 0.15, 2.76 ± 0.03, 2.94 ± 0.12, 2.85 ± 0.18, 2.88 ± 0.06, 2.37 ± 0.21, and 1.13 ± 0.06 U, respectively. Xylanase A is thus relatively thermotolerant, maintaining over 50% of its activity at 60°C relative to the activity observed at 25°C. As the temperature increased from 25 to 40°C, a steady increase (up to 35%) in xylanase activity was observed. This level of xylanase activity was retained in samples up to 50°C, when the observed activity was 1.5-fold the activity observed in samples incubated at 25°C.
Analysis of xylosaccharides generated by XynA digestion of sweetgum xylan.
The sweetgum xylan extract was hydrolyzed by XynA to a range of products detectable by MALDI-TOF mass spectrometry, all of which contained two glucuronic acid substitutions (Fig. 3). Based upon signal intensity, the most prevalent products formed were either 9 or 11 xylose residues in length. The glucuronic acid substitution ratios obtained by the mass spectrograms of the filtrate samples deviated slightly from those calculated by spectrophotometric techniques but were in good agreement with those obtained from total hydrolysis of the sweetgum glucoxylan (Table 1). The presence of two glucuronic acid residues in each reaction product and the wide range of xylose-to-MeGA ratios observed suggest that the positioning of the substitutions is varied. These observations also suggest that a critical distance between substitution points on the xylan backbone exists, and at distances smaller than this threshold, XynA cannot hydrolyze the xylan within that particular region of the polymer. This is supported by the lack of any activity by XynA in 1% (wt/vol) solutions of each of the filtrate samples (data not shown). Preliminary analyses indicate that both filtrate samples serve as substrates for a family 11 xylanase isolated from Trichoderma viride (Sigma Chemical Co.) (data not shown). This observation confirms the mass spectral data, as the oligoxylosides generated by XynA should serve as substrates for the T. viride enzyme, with a xylosidic β-1,4 bond between the glucuronic acid substitution points on the oligoxyloside products hydrolyzed by the fungal enzyme.
FIG. 3.
MALDI-TOF mass spectrum of the products generated by xylanase A cleavage of sweetgum xylan. Putative oligoxylosides and their calculated masses are given above each detected mass signal. Spectrum was obtained on a Voyager Benchtop MALDI-TOF mass spectrometer (PE Biosystems) externally calibrated with low-molecular-weight standard peptides according to the manufacturer's protocols.
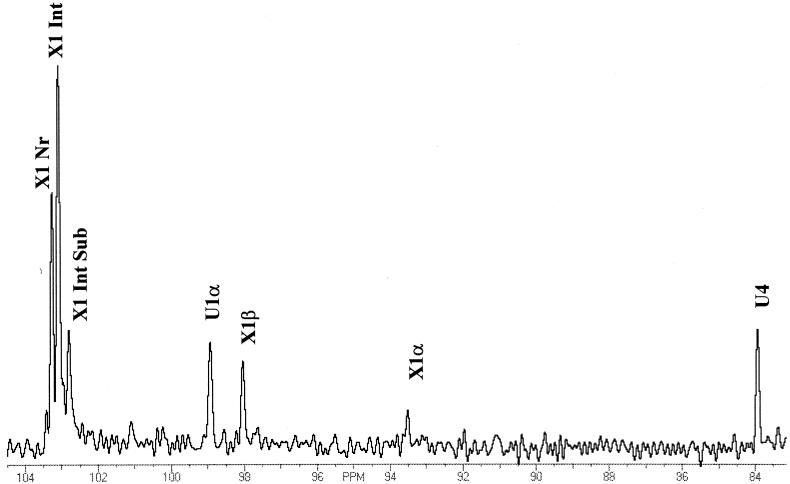
Based upon 13C-NMR, the products generated by XynA-mediated hydrolysis of sweetgum xylan were not significantly different from those generated by a xylanase from family 11 (2) with respect to the lack of MeGA substitution on the nonreducing terminal xylose residues (Fig. 4). The resonance at approximately 102.7 ppm is attributed to carbon 1 of a glucuronic acid-substituted xylose residue not on the nonreducing terminus of the oligoxylosidic products. This resonance is clearly differentiable from the carbon 1 resonances of internal xylose residues (103 ppm) and at the nonreducing termini of the oligoxylosides (103.3 ppm). The internal position of the glucuronic acid-substituted xylose residue was confirmed by incubation of 1.0% (wt/vol) solutions of either filtrate with β-xylosidase, which resulted in stoichiometric release of xylose, as determined by HPLC (data not shown). In 1-ml reaction solutions containing 10 mg of filtrate 1, 0.9 mg of xylose was detected in solution, and in reactions with equivalent amounts of filtrate 2, 0.82 mg of xylose was detected. This result indicates that a single xylose residue lies on the nonreducing terminal side of the 4-O-methyl-d-glucuronic acid-substituted xylose in the oligoxyloside products generated by XynA-catalyzed hydrolysis of sweetgum xylan. The combined data from the product characterization experiments lead to the proposed mode of action of XynA on xylan substrates shown in Fig. 5.
FIG. 4.
Anomeric region of the 13C-NMR spectrum of the products generated by xylanase A cleavage of sweetgum xylan. A total of 2,000 data acquisitions were obtained on a Nicolet FT 300 NMR spectrometer at a frequency of 75.47 MHz, spectral width of 0.02 MHz, at 22°C. Data were processed using FELIX (Molecular Simulations Inc.). Resonance assignments are based on published data (19).
FIG. 5.
Proposed sites of hydrolysis of E. chrysanthemi D1 xylanase A on xylan substrates. The enzyme recognizes the carboxylate group of an α-1,2-linked 4-O-methyl-d-glucuronic acid substituent and hydrolyzes the β-1,4 bond between xylose residues in the xylan backbone at the positions marked by the arrows. The number of xylose residues represented by n varies with xylan source.
DISCUSSION
At its optimum pH and temperature (pH 6.0 and 40°C, respectively), E. chrysanthemi D1 XynA appears to fold into a conformation that is approximately 10 to 15% α-helices. Deconvolution of the CD spectra of XynA under these conditions by least-squares fitting using several reference sets, including model polypeptides (11) and protein sets (3, 5, 34), yields similar mean percentages of α-helical content. It is interesting that despite significant secondary-structure changes at temperatures higher than 40°C and pHs higher than 6.2, the enzyme is still active. This suggests that the chemical environment surrounding the active site of the enzyme is not severely perturbed despite the pronounced conformational changes undergone by the enzyme in response to changing solution conditions. The α-helical content derived for XynA lies between those found in the two families of glycosyl hydrolases (13, 14) containing other endoxylanases. In comparison, members of glycosyl hydrolase family 10 are approximately 30 to 40% α-helical in nature (9), and those proteins in family 11 are approximately 3 to 5% α-helical in nature (33). The lack of structural identity to either glycosyl hydrolase family 10 or 11 was predicted by amino acid sequence comparison (20), with the same comparison identifying homology between E. chrysanthemi D1 XynA and members of glycosyl hydrolase families 5 and 30. The three-dimensional structures of proteins in family 5 are 39 to 43% α-helical (7, 8, 15), much higher than the 10 to 15% observed for XynA. This suggests that XynA may not have a significant degree of structural homology with members of glycosyl hydrolase family 5, despite having many amino acids in common with those proteins, and may in fact represent a new class of glycosyl hydrolase.
Given the lack of homology between E. chrysanthemi D1 XynA and other endoxylanases, the novel approach to depolymerization of xylan exhibited by the enzyme is not unexpected. As identified in this work, XynA requires 4-O-methyl-d-glucuronic acid substitutions on the xylan backbone for enzymatic activity. Based upon the 4-deoxy-hexenuronosylxylan activity experiments, the importance of the methyl ester on carbon 4 of the uronate substituent is apparently minimal. The presence of the C-6 carboxylic acid on the sugar substituent is, however, not negotiable. Reduction of this carbon to the corresponding alcohol results in significant reduction in enzymatic activity, as seen in the activity of XynA towards 4-O-methyl-d-glucoxylan. This group may serve to properly align the substrate molecule for hydrolysis of the xylosidic bond between carbon 4 of the substituted xylose residue and carbon 1 of the adjacent xylose in the polymer. The presence of a single xylose on the nonreducing terminal side of the 4-O-methyl-d-glucuronic acid-substituted xylose in the oligoxyloside products places XynA with members of family 11 of the glycosyl hydrolases (2) with respect to function despite the significant differences between the enzymes. However, the oligoxylosides generated by E. chrysanthemi D1 XynA are suitable substrates for a family 11 endoxylanase from T. viride, indicating a unique feature of the Erwinia enzyme, namely, the lack of any cleavage of β-1,4-xylosidic bonds between 4-O-methyl-d-glucuronic acid substitution points on the xylan backbone. To date, only one other xylanase with a requirement for glucuronosyl substitutions has been identified (24). The Bacillus subtilis enzyme demonstrating this requirement for glucuronosyl moieties cleaved the xylosidic bond between the first and second xylose residues on the reducing terminal side of the xylose bearing the glucuronosyl substitution.
The MALDI-TOF mass spectrograms of the reaction products are quite interesting and raise several questions regarding the role of the glucuronic acid substitutions on the xylan backbone. As shown in Fig. 3, a majority of the products produced by XynA have odd numbers of xylose residues, and the ratio of xylose to glucuronic acid in the detected products ranges from 2 to 7.5. This variability in glucuronosyl substitution, with a propensity for odd-numbered xylose-to-glucuronic acid ratios, has not been documented before and is very intriguing. Due to its mode of action, the Erwinia xylanase may be ideally suited to probe other xylan types to gain a better understanding of the composition and structure of the polymer and the role of MeGA substitutions in the development of higher plants.
The combined structural data regarding XynA and its reaction products obtained in this work serve to illustrate its novelty. Xylanase A has a high molecular mass, like most family 10 endoxylanases, and yet possesses a basic isoelectric point, like members of family 11. The functional form of the enzyme is structurally similar to neither family, and the enzyme is active in a wider pH range than those displayed by members of either family 10 or 11. Xylanase A generates products from xylan that show some similarity with respect to the nonreducing terminal structure of those generated by endoxylanases from family 11, yet those same products serve as substrates for a family 11 endoxylanase. The requirement of XynA for MeGA substitutions on the β-1,4-xylan backbone for enzymatic activity narrows the acceptable substrate range of the enzyme to one much smaller than that of either family 10 or 11.
The unique MeGA substitution requirement and mode of action of E. chrysanthemi D1 XynA raise a few very interesting questions regarding the role of the enzyme in the life cycle of the organism. Xylanase A may be one of several enzymes of the E. chrysanthemi D1 xylanolytic system. However, to date, only one other enzyme that would be part of the same system, a β-glucosidase/xylosidase (BgxA), has been discovered in this organism (32). In most xylanolytic enzyme systems, several exogenous enzymes participate in the breakdown of xylans to components suitable for cellular uptake and metabolism (21), with xylanases from glycosyl hydrolase families 10 and 11 acting to depolymerize xylans to suitable substrates for β-xylosidases. Xylanase A only cuts out the glucuronosyl repeating units of xylans, generating oligoxylosides that are not suitable substrates for the known β-glucosidase/xylosidase. Barring the discovery of another endoxylanase in E. chrysanthemi D1, this suggests that the role of XynA may be to loosen the cell wall structure of the host, thereby allowing other enzymes secreted by the pathogen to gain better access to the host tissue, rather than to participate in a pathway for the catabolism of the hemicellulose. XynA may also participate directly or indirectly in the formation of elicitor molecules from host tissue. Both possibilities are supported by observations that xylanase A is produced constitutively and acts synergistically with known virulence factors secreted by phytopathogenic Erwinia species, e.g., the pectate lyases (4), and that XynA alone is not absolutely required for pathogenesis in monocots (20). Future experiments with xylanase A may better identify the role of the enzyme in the infection process, as well as serving as a probe to analyze the differences in glucuronoxylan structure and function.
ACKNOWLEDGMENTS
This work was supported by U.S. Department of Energy grant DE FC36-99GO10476, the Consortium for Plant Biotechnology Research Project No. OR22072-94, and the Institute of Food and Agricultural Sciences, University of Florida Experiment Station, as CRIS Project MCS 03763.
We especially thank M. Buszko of the IFAS NMR Facility for his assistance, as well as K. T. Shanmuggam, L. O. Ingram, J. D. Gander, and J. D. Rice for the comments and suggestions provided during the preparation of the manuscript.
Footnotes
Florida Agricultural Experiment Station Journal Series no. R-07770.
REFERENCES
- 1.Anderson M A, Stone B A. A radiochemical approach to the determination of carboxylic acid groups in polysaccharides. Carbohydr Polymers. 1985;5:115–129. [Google Scholar]
- 2.Biely P, Vrsanska M, Tenkanen M, Kluepfel D. Endo-beta-1,4,-xylanase families: differences in catalytic properties. J Biotechnol. 1997;57:151–166. doi: 10.1016/s0168-1656(97)00096-5. [DOI] [PubMed] [Google Scholar]
- 3.Bolotina I A, Chekhov V O, Lugauskas V, Ptitsyn O B. Determination of the secondary structure of proteins from their cicular dichroism spectra. II. Estimation of the contribution of β-pleated sheets. Mol Biol (USSR) 1980;14:902–908. [PubMed] [Google Scholar]
- 4.Braun E J, Rodrigues C A. Purification and properties of an endoxylanase from a corn stalk rot strain of Erwinia chrysanthemi. Phytopathology. 1993;83:332–338. [Google Scholar]
- 5.Chen Y H, Yang J T, Chau K H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974;13:3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- 6.Collmer A, Keen N T. The role of pectic enzymes in plant pathogenesis. Annu Rev Phytopathol. 1986;24:383–409. [Google Scholar]
- 7.Cutfield S M, Davies G J, Murshudov G, Anderson B F, Moody P C E, Sullivan P A, Cutfield J F. The structure of the exo-β-(1,3)-glucanase from Candida albicans in native and bound forms: relationship between a pocket and groove in family 5 glycosyl hydrolase. J Mol Biol. 1999;294:771–783. doi: 10.1006/jmbi.1999.3287. [DOI] [PubMed] [Google Scholar]
- 8.Davies G J, Dauter M, Brzozowski A M, Bjornvad M E, Andersen K V, Schulein M. Structure of the Bacillus agaradherans family 5 endoglucanase at 1.6 Å and its cellobiose complex at 2.0 Å resolution. Biochemistry. 1998;37:1926–1932. doi: 10.1021/bi972162m. [DOI] [PubMed] [Google Scholar]
- 9.Derewenda U, Swenson L, Green R, Wei Y, Morosoli R, Shareck F, Kluepfel D, Derewenda Z S. Crystal structure, at 2.6 Angstrom resolution, of the Streptomyces lividans xylanase A, a member of the F family of beta-1,4-d-glycanases. J Biol Chem. 1994;269:20811–20814. [PubMed] [Google Scholar]
- 10.Dubois M, Gilles K, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 11.Greenfield N, Fasman G E. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969;8:4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- 12.Hennessey J P, Johnson W C. Experimental errors and their effect on analyzing circular dichroism spectra of proteins. Anal Biochem. 1982;125:177–188. doi: 10.1016/0003-2697(82)90400-6. [DOI] [PubMed] [Google Scholar]
- 13.Henrissat B. A classification of glycosyl hydrolases based upon amino acid sequence similarities. Biochem J. 1991;280:309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henrissat B, Bairoch A. New families in the classification of glycosyl hydrolases based upon amino acid sequence similarities. Biochem J. 1993;293:781–788. doi: 10.1042/bj2930781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilge M, Gloor S M, Rypniewski W, Sauer O, Heightman T D, Zimmermann W, Winterhalter K, Piontek K. High-resolution native and complex structures of thermostable β-mannanase from Thermomonospora fusca—substrate specificity in glycosyl hydrolase family 5. Structure. 1998;6:1433–1444. doi: 10.1016/s0969-2126(98)00142-7. [DOI] [PubMed] [Google Scholar]
- 16.Hurlbert J C, Preston J F. Functional implications of the beta-helical protein fold: differences in chemical and thermal stabilities of Erwinia chrysanthemi EC16 pectate lyases B, C, and E. Arch Biochem Biophys. 2000;381:264–272. doi: 10.1006/abbi.2000.1982. [DOI] [PubMed] [Google Scholar]
- 17.Ingram L O, Conway T, Clark D P, Sewell G W, Preston J F. Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol. 1987;53:2420–2425. doi: 10.1128/aem.53.10.2420-2425.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones J K N, Purves C B, Timmel T E. Constitution of a 4-O-methylglucuronoxylan from the wood of trembling aspen (Populus tremuloides MICHX) Can J Chem. 1961;39:1059–1066. [Google Scholar]
- 19.Kardosova A, Matulova M, Malovikova A. (4-O-Methyl-α-d-glucurono)-d-xylan from Rudbeckia fulgida var. sullivantii (Boynton et Beadle) Carbohydr Res. 1998;308:99–105. doi: 10.1016/s0008-6215(98)00072-x. [DOI] [PubMed] [Google Scholar]
- 20.Keen N T, Boyd C, Henrissat B. Cloning and characterization of a xylanase gene from corn strains of Erwinia chrysanthemi. Mol Plant-Microbe Interact. 1996;9:651–657. doi: 10.1094/mpmi-9-0651. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni N, Shendye A, Rao M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev. 1999;23:411–456. doi: 10.1111/j.1574-6976.1999.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- 24.Nishitani K, Nevins D. Glucuronan xylanohydrolase: a unique xylanase with the requirement for appendant glucuronosyl units. J Biol Chem. 1991;266:6539–6543. [PubMed] [Google Scholar]
- 25.Puls J P, Schusell J. Chemistry of hemicelluloses: relationship between hemicellulose structure and enzymes required for hydrolysis. In: Coughlan M P, Hazelwood G P, editors. Hemicelluloses and hemicellulases. London, U.K: Portland Press; 1992. pp. 1–26. [Google Scholar]
- 26.Rydlund A, Dahlman O. Oligosaccharides obtained by enzymatic hydrolysis of birch kraft pulp xylan: analysis by capillary zone electrophoresis and mass spectrometry. Carbohydr Res. 1997;300:95–102. doi: 10.1016/s0008-6215(97)00038-4. [DOI] [PubMed] [Google Scholar]
- 27.Samain E, Debeire P, Touzel J P. High level production of a cellulase-free xylanase in glucose limited fed batch cultures of a thermophilic Bacillus strain. J Biotechnol. 1997;58:71–78. doi: 10.1016/s0168-1656(97)00140-5. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Smith P K, Krohn R I, Hermanson G T, Mallia F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenck D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 30.Telemann A, Hausalo T, Tenkanen M, Vuorinen T. Identification of the acidic degradation products of hexenuronic acid and characterization of hexenuronic acid-substituted xylooligosacchardies by NMR spectroscopy. Carbohydr Res. 1996;280:197–208. doi: 10.1016/0008-6215(95)00309-6. [DOI] [PubMed] [Google Scholar]
- 31.Timmel T E. Recent progress in the chemistry of wood hemicelluloses. Wood Sci Technol. 1967;1:45–70. [Google Scholar]
- 32.Vroemen S, Heldens J, Boyd C, Henrissat B, Keen N T. Cloning and characterization of the bgxA gene from Erwinia chrysanthemi D1 which encodes a β-glucosidase/xylosidase enzyme. Mol Gen Genet. 1995;246:465–477. doi: 10.1007/BF00290450. [DOI] [PubMed] [Google Scholar]
- 33.Wakarchuk W W, Campbell R L, Sung W L, Davoodi J, Yaguchi M. Mutational and crystallographic analysis of the active site residues of the Bacillus circulans xylanase. Protein Sci. 1994;3:467. doi: 10.1002/pro.5560030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witholt B, Boekhout M, Brock M, Kingma J, Van Heerikhuizen H, DeLeij H. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976;74:160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- 35.Yang J T, Wu C S, Martinez H M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]
- 36.Zaldivar J, Martinez A, Ingram L. Effect of selected aldehydes on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol Bioeng. 1999;65:24–33. doi: 10.1002/(sici)1097-0290(19991005)65:1<24::aid-bit4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Zaldivar J, Martinez A, Ingram L. Effect of organic acids on the growth and fermentation of ethanologenic Escherichia coli LY01. Biotechnol Bioeng. 1999;66:203–210. doi: 10.1002/(sici)1097-0290(1999)66:4<203::aid-bit1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Zaldivar J, Martinez A, Ingram L. Effect of alcohol compounds found in hemicellulose hydrolysate on the growth and fermentation of ethanologenic Escherichia coli LY01. Biotechnol Bioeng. 1999;68:524–530. doi: 10.1002/(sici)1097-0290(20000605)68:5<524::aid-bit6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]