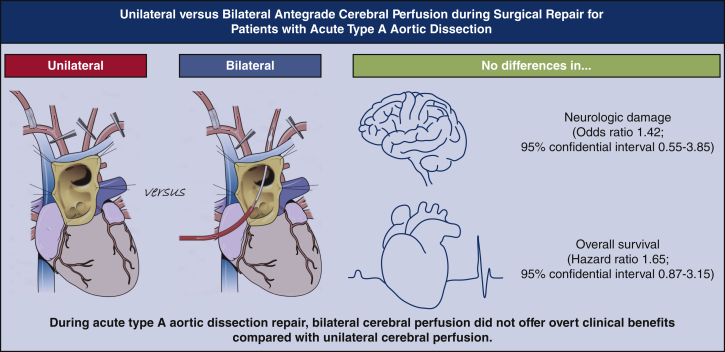
Abstract
Objectives
To compare unilateral versus bilateral antegrade cerebral perfusion (ACP) techniques on cerebral protection during acute type A aortic dissection repair.
Methods
Using an institutional database, we retrospectively reviewed patients who underwent acute type A aortic dissection repair with selective ACP techniques from October 2008 to December 2019. Primary end point was the detection of neurologic dysfunctions. The secondary end point was mortality. For baseline adjustment, the propensity score matching method was used. Multivariable logistic regression analysis was performed to determine the predictor of neurologic events.
Results
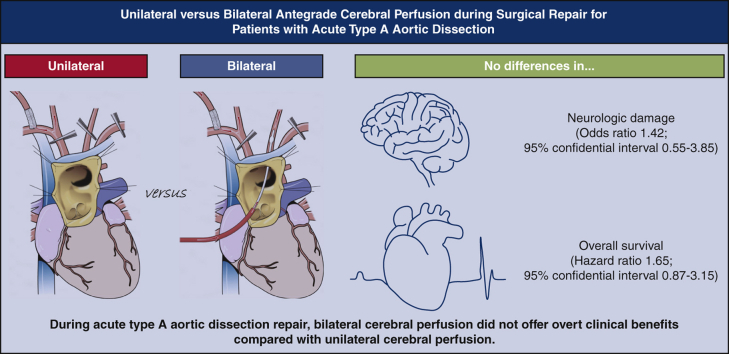
Among 522 patients (aged 62.0 ± 14.9 years; 45.7% women), unilateral and bilateral ACP techniques were used in 357 (64.7%) and 165 (35.3%) patients, respectively. Transient (19.6% vs 21.2%; P = .65) and permanent (7.0% vs 10.3%; P = .70) neurologic dysfunction rates were not significantly different in patients with unilateral versus bilateral ACP, respectively. Observed mortality rate was higher in the patients with bilateral ACP (hazard ratio, 2.05; 95% CI, 1.33-3.14; P = .001). Propensity-score matching yielded 94 pairs of patients. In matched analysis, bilateral ACP did not significantly lower the risks for transient (odds ratio, 0.87; 95% CI, 0.42-1.81; P = .71) and permanent (odds ratio, 1.42; 95% CI, 0.55-3.85; P = .47) neurologic dysfunction or death (hazard ratio, 1.65; 95% CI, 0.87-3.15; P = .13). In the multivariable analysis, the ACP technique was not significantly associated with perioperative neurologic deficit.
Conclusions
Despite additional supply, the patients undergoing bilateral ACP during acute type A aortic dissection repair did not have superior outcomes in neurologic and death events compared with the patients undergoing unilateral ACP.
Key Words: aortic dissection, selective cerebral perfusion, cerebral protection
Abbreviations and Acronyms: ATAAD, acute type A aortic dissection; ACP, antegrade cerebral perfusion; CPB, cardiopulmonary bypass; CT, computed tomography; PND, permanent neurologic deficit; TCA, total circulatory arrest; TND, transient neurologic deficit
Video Abstract
Graphical abstract
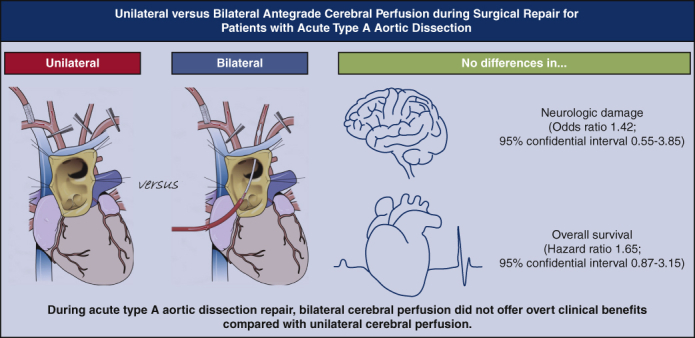
Bilateral cerebral perfusion was not related to better clinical outcomes.
Central Message.
Bilateral cerebral perfusion technique during acute type A aortic dissection repair seems not to offer significant clinical benefits over unilateral perfusion.
Perspective.
ATAAD is a highly devastating condition that requires swift surgical repair. Optimal brain protection during ATAAD repair is among the major concerns, and a better choice between unilateral and bilateral cerebral perfusion has long been debated.
Acute type A aortic dissection (ATAAD) is a highly devastating condition that requires swift surgical repair. Although lifesaving is a primary goal of ATAAD repair, recent arguments favor the extended arch repair considering potential rupture risk of the remaining arch after the limited AD repair.1, 2, 3 Reported neurologic damage rates after ATAAD repair have been diverse: From 5.5% to 33.3%. This is believed to be heavily dependent on the center's experience and surgical dogma.4,5 Because patients experiencing stroke are prone to mortality even after successful discharge, there have been substantial efforts to improve neurologic outcomes of arch surgery.6 As a result, lowering temperature and applying selective antegrade cerebral perfusion (ACP) techniques have been introduced for the purpose of brain protection.
Recent studies have shown that a mild degree of hypothermia (28-30 °C) may provide acceptable brain protection during 90 minutes of ACP.7 It seems that a consensus has been made on the hypothermia issue because the deeper temperatures portend more risks of prolonged cardiopulmonary bypass (CPB) time and bleeding events. Any arguments for adequate ACP technique are still inconclusive. Although several previous studies have shown noninferiority of the use of unilateral perfusion over bilateral ACP, these comparisons may be influenced by multiple factors, such as patients' various conditions and cannulation stratagem, as well as experience of the surgeon.8, 9, 10, 11 In light of this, we aimed to evaluate the influence of unilateral and bilateral ACP on neurologic and clinical outcomes in patients who underwent ATAAD repair.
Materials and Methods
Study Subjects and Outcome Measures
A total of 534 patients underwent ATAAD repair from October 2008 to December 2019 at our institution. After exclusion of ATAAD repair with triple ACP or without circulatory arrest, a total of 522 patients formed the study cohort. Among these, 357 (64.7%) and 165 (35.3%) received unilateral and bilateral ACP techniques, respectively (Figure 1).
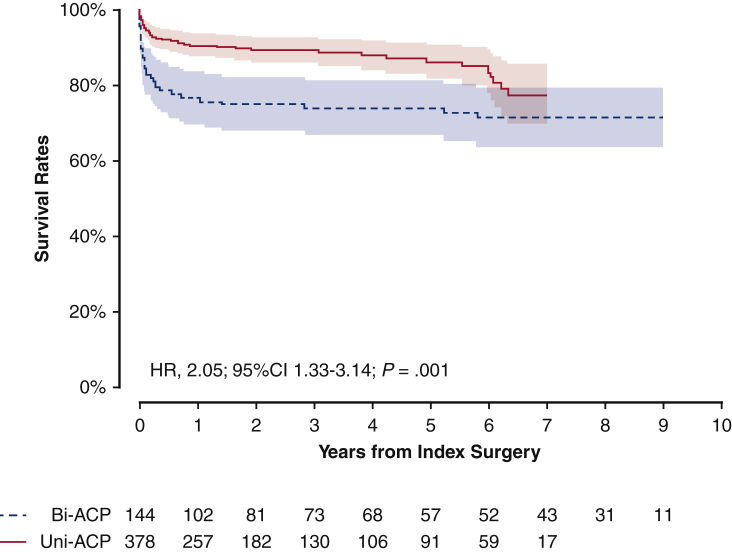
Figure 1.
Unadjusted Kaplan-Meier plot for survival rates depending on the types of anterior cerebral perfusion techniques. Solid red line indicates unilateral group; dashed blue line indicates bilateral group. Shaded bands indicate areas within 95% CIs. Bi-ACP, Bilateral antegrade cerebral perfusion; Uni-ACP, unilateral antegrade cerebral perfusion; HR, hazard ratio; CI, confidence interval.
The primary end point was set for the detection of neurologic deficit. The neurologic deficits were separately recorded as permanent and transient dysfunctions. The former was diagnosed when a newly found irreversible neurologic change was found with abnormal findings of a brain image. The latter was defined when a patient's neurologic symptoms disappeared before discharge, including postoperative confusion, transient delirium, agitation, and motor discomfort.12,13 Early adverse clinical events such as 30-day mortality, use of extracorporeal membrane oxygenation, re-exploration for bleeding, reintubation, tracheostomy, and renal failure depending on the type of ACP were additionally compared. The secondary end point was mortality during the follow-up.
The requirement for informed consent from individual patients was waived due to the retrospective nature of the study design. The Institutional Review Board of Gangnam Severance Hospital approved the present study (No. 3-2021-0167 [September 14, 2021]).
Operative Procedure and Follow-up
All operations were performed as emergency surgery, and the surgical incision was made no longer than 2 hours after entrance to the emergency department. Surgical details were described previously, but briefly, procedures were as follows.14 Under general anesthesia, right radial and left femoral artery pressure, central vein pressure, nasopharyngeal and rectal temperatures, and cerebral oximeter were monitored. Transesophageal echocardiography was used for overall patients. After median sternotomy, CPB was established. Arterial cannulation was performed via side-graft (8 mm polyethylene terephthalate graft) cannulation of both the right axillary and femoral arteries in all cases. Body temperature was lowered after CPB establishment. Rectal temperature was regarded as a reference of measurement, and the target was 28 °C. However, in most cases, the body temperature was fully dropped for 15 minutes after CPB initiation, and thereafter, the arch was opened regardless of the core temperature. During arch surgery, selective ACPs were maintained with clamping of the arch branch vessels (Figure 2). Our default setting was unilateral ACP through the right axillary artery. Even if unilateral ACP was primarily planned, an additional balloon catheter was directly introduced at the left carotid artery in the case that cerebral oximeter readings fell more than 15% of baseline. The additional balloon catheter was secured with the silk tape snaring technique. These patients were included in the unilateral ACP group. Direct cerebral perfusion flow was 10 mL/kg/min and managed under the monitoring of right radial artery pressure (40-50 mm Hg) during selective ACP. Surgical extent was principally dependent on the location of the primary tear site. If the arch tear existed, partial arch or total arch replacement was performed. After distal anastomosis completion, rewarming and lower body perfusion were started via the side branch of the artificial graft. Remaining proximal procedures and arch vessel anastomoses followed.
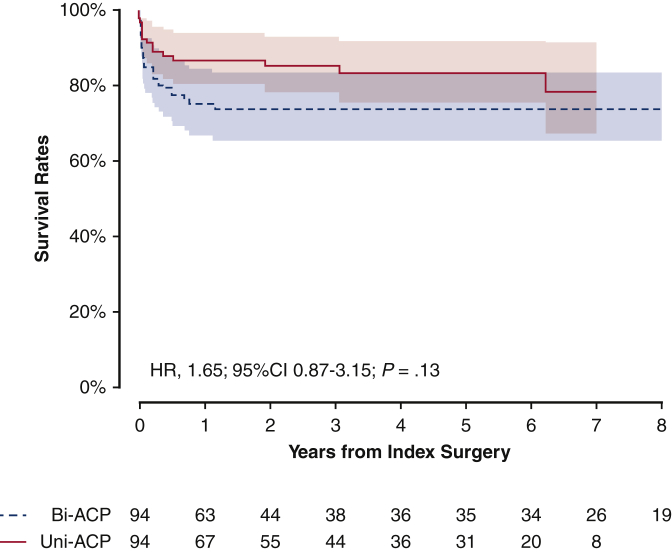
Figure 2.
Propensity score-matched Kaplan-Meier plot for survival rates depending on the types of anterior cerebral perfusion techniques. Solid red line indicates unilateral group; dashed blue line indicates bilateral group. Shaded bands indicate areas within 95% CI. Bi-ACP, Bilateral antegrade cerebral perfusion; Uni-ACP, unilateral antegrade cerebral perfusion; HR, hazard ratio; CI, confidence interval.
During the follow-up period of the present study, there was consistency in the surgical procedures because most of operations were performed by a single surgeon. We list details of patients and surgery by years of index operation in Table E1. The first postoperative computed tomography (CT) scan follow-up was performed before discharge. After discharge, CT follow-ups were done at postoperative 3 months, 12 months, and every 2 years thereafter. With any evidence of AD progression or aorta dilatation, the follow-up CT scan was performed in <3 months.
Statistical Analysis
The unilateral and bilateral ACP groups were categorized depending on the intention to treat. For further validation, we additionally performed same analyses in an as-treated manner. Categorical variables (listed as percentages or frequencies) and continuous variables (listed as mean ± SD or median with interquartile range) were estimated with the χ2 test, Fisher exact test, and Student t test, respectively. The permanent and transient neurologic events were regarded as early outcomes because those events occurred within 30 days of the operation. The comparisons of early outcomes were performed by logistic regression analyses. Survival was compared with Cox regression analyses. Kaplan-Meier analyses were used to assess the conditional probability of survival, and log-rank tests or Cox regression analyses were used to compare intergroup differences.
To reduce selection bias, we adjusted the differences in a patient's baseline variables with a propensity score matching method in both intention-to-treat and as-treated analyses. In the propensity score generation, long-term follow-up of the present study was regarded as a potential confounder by dividing surgical period in an interquartile manner as well as surgeon experience (numbers of aortic surgery) in addition to patients' baseline profiles and surgical details. (Tables 1, E2, and E3). Calibration of the propensity scores was tested by C statistics (area under the curve, 0.753; 95% CI, 0.708-0.798). Propensity score matching was processed based on the logit of the propensity score using calipers of width ≤0.10 of the SD. Propensity score generation in the as-treated analyses were the same as those of the intention-to-treat analyses. The propensity score calibration was tested by C statistics (area under the curve, 0.764; 95% CI, 0.720-0.809). Paired t test and McNemar test were used for continuous and categorical variables, respectively, in the propensity score-matched analyses.
Table 1.
Baseline characteristics depending on the type of cerebral perfusion technique (intention-to-treat groups)
| Characteristic | Overall cohort |
Propensity score-matched cohort |
||||||
|---|---|---|---|---|---|---|---|---|
| Unilateral (n = 378) | Bilateral (n = 144) | P value | STD | Unilateral (n = 94) | Bilateral (n = 94) | P value | STD | |
| Age (y) | 60.6 ± 15.3 | 61.8 ± 13.8 | .42 | 0.66 | 61.8 ± 14.2 | 62.2 ± 14.0 | .86 | 0.03 |
| Male gender | 177 (46.8) | 75 (52.1) | .33 | 0.11 | 42 (44.7) | 46 (48.9) | .66 | 0.09 |
| Hypertension | 231 (61.1) | 92 (63.9) | .63 | 0.18 | 55 (58.5) | 61 (64.9) | .45 | 0.13 |
| Diabetes mellitus | 40 (10.6) | 7 (4.9) | .06 | 0.22 | 6 (6.4) | 6 (6.4) | >.99 | 0 |
| Cerebrovascular disease | 23 (6.1) | 16 (11.1) | .08 | 0.18 | 11 (11.7) | 7 (7.4) | .46 | 0.14 |
| Chronic renal failure | 14 (3.7) | 4 (2.8) | .80 | 0.05 | 3 (3.2) | 2 (2.1) | >.99 | 0.07 |
| COPD | 6 (1.6) | 3 (2.1) | .99 | 0.04 | 1 (1.1) | 2 (2.1) | >.99 | 0.09 |
| Coronary arterial disease | 15 (4.0) | 8 (5.6) | .58 | 0.07 | 6 (6.4) | 5 (5.3) | >.99 | 0.05 |
| Marfan | 24 (6.3) | 9 (6.2) | >.99 | 0.004 | 6 (6.4) | 5 (5.3) | >.99 | 0.05 |
| DeBakey type 1 | 301 (79.6) | 111 (77.1) | .61 | 0.06 | 81 (86.2) | 73 (77.7) | .19 | 0.22 |
| DeBakey type 2 | 77 (20.4) | 33 (22.9) | .61 | 0.06 | 13 (13.8) | 21 (22.3) | .19 | 0.22 |
| Intramural hematoma | 80 (21.2) | 26 (18.1) | .51 | 0.08 | 18 (19.1) | 17 (18.1) | >.99 | 0.03 |
| Hemopericardium | 132 (34.9) | 53 (36.8) | .76 | 0.04 | 31 (33.0) | 31 (33.0) | >.99 | 0 |
| Details of surgery | ||||||||
| Arch surgery | <.001 | 0.55 | .36 | 0.02 | ||||
| 1-partial arch replacement | 65 (17.2) | 15 (10.4) | 17 (18.1) | 9 (9.6) | ||||
| 2-partial arch replacement | 110 (29.1) | 27 (18.8) | 22 (23.4) | 25 (26.6) | ||||
| Total arch replacement | 166 (43.9) | 46 (31.9) | 36 (38.3) | 36 (38.3) | ||||
| Root surgery | >.99 | 0.005 | .80 | 0.07 | ||||
| None | 338 (89.4) | 129 (89.6) | 84 (89.4) | 86 (91.5) | ||||
| Bentall's operation | 29 (7.7) | 12 (8.3) | 8 (8.5) | 5 (5.3) | ||||
| Valve sparing techniques | 11 (2.9) | 3 (2.1) | 2 (2.1) | 3 (3.2) | ||||
| Graft insertion in DTA | 41 (10.8) | 10 (7.0) | .38 | 0.19 | 13 (13.8) | 6 (6.4) | .23 | 0.25 |
Values are presented as mean ± SD or n (%). STD, Standardized mean difference; COPD, chronic obstructive pulmonary disease; DTA, descending thoracic aorta.
To find the risk factors of primary and secondary outcomes, stepwise backward multivariable logistic and Cox regression analyses were performed, respectively. Baseline variables within 0.20 of the P value in univariable regression models were included in the multivariable models, and, thereafter, stepwise backward elimination methods were conducted to leave variables within 0.10 of the P value in the final multivariable model. Regarding the surgeon factor, surgical experience was graded depending on surgical volume (ie, a more experienced surgeon scored a higher number). To test multicollinearity, we performed variance inflation factor tests using “VIF” in R version 3.6.0 (R Foundation for Statistical Computing). R was used for statistical analyses. All P values were 2-tailed.
Results
Preoperative Profiles and Surgical Details
The left column of Table 1 lists patient demographic characteristics, preoperative conditions, and surgical details depending on the type of ACP. Mean age of overall patients was 61.9 ± 14.9 years, and 270 (51.7%) were men. There was no significant difference in baseline profiles between the groups. Arch surgeries were more frequently performed in the unilateral than in the bilateral ACP group, but root surgeries were similar in the 2 groups. Mean lowest body temperature during ACP at the esophagus and rectum were lower in the unilateral and bilateral ACP groups, respectively. In 21 (5.6%) unilateral ACP patients, ACP type was converted because an additional catheter was introduced at the left common carotid artery. Total operation time was significantly shorter in the unilateral group (Table 1). Interquartile surgical period 1, 2, 3, and 4 included 130 (24.9%), 130 (24.9%), 131 (25.1%), and 131 (25.1%) patients, respectively. The operations were performed by 3 surgeons, as listed in the Table E2. Achieved body temperature and operation times, including total bypass, cardiac, and lower body ischemic times were not significantly different depending on the ACP technique.
Crude Clinical Outcomes
Among 522 patients, 42 (8.0%) permanent neurologic deficits (PNDs) were observed. During the total follow-up of 1572.07 patient-years, 86 deaths occurred.
The detection of transient neurologic deficit (TND) was comparable between the 2 groups (Table 2). The risk of permanent neurologic damage trended to be higher in patients who received bilateral ACP technique (odds ratio [OR], 1.89; 95% CI, 0.97-3.59; P = .054) compared with patients who underwent unilateral ACP technique. The risks for 30-day mortality (P < .001), use of extracorporeal membrane oxygenation (P < .001), and reintubation (P = .003) were significantly higher in the bilateral ACP group than in the unilateral ACP group (Table 2). Mortality rate was significantly higher in the patients undergoing bilateral ACP (hazard ratio [HR], 2.05; 95% CI, 1.33-3.14; P = .001) compared with patients undergoing unilateral ACP (Figure 1).
Table 2.
Crude early clinical outcomes depending on the type of cerebral perfusion technique (intention-to-treat groups)
| Overall cohort (N = 522) | Unilateral (n = 378) | Bilateral (n = 144) | Odds ratio∗ (95% CI) | P value |
|---|---|---|---|---|
| Hospital stay (d) | 16 (12-24) | 16 (11-24) | .90 | |
| Primary outcome | ||||
| TNDs | 70 (19.6) | 35 (21.2) | 0.89 (0.54-1.43) | .63 |
| PNDs | 25 (7.0) | 17 (10.3) | 1.89 (0.97-3.59) | .054 |
| Other early clinical outcomes | ||||
| 30-d mortality | 16 (4.5) | 24 (14.5) | 4.04 (2.10-7.92) | <.001 |
| Extracorporeal membrane oxygenation | 3 (0.8) | 10 (6.1) | 9.33 (2.81-42.1) | <.001 |
| Re-exploration for bleeding | 25 (7.0) | 14 (8.5) | 1.03 (0.48-2.08) | .93 |
| Reintubation | 9 (2.5) | 14 (8.5) | 3.65 (1.57-8.75) | .003 |
| Tracheostomy | 4 (1.1) | 3 (1.8) | 1.05 (0.15-4.94) | .95 |
| Renal failure | 24 (6.7) | 14 (8.5) | 1.40 (0.68-2.78) | .35 |
Values are presented as median (interquartile range) or n (%). CI, Confidence interval; TNDs, transient neurologic deficits; PNDs, permanent neurologic deficits.
Results from the patients with unilateral anterior cerebral perfusion group are references.
Adjusted Clinical Outcomes
The right columns of Tables 1, E2 and E3 list baseline variables of the propensity score-matched cohort. After propensity score matching, baseline demographic characteristics, preoperative conditions, and surgical details were well balanced (overall standardized mean differences were within 0.2). The bilateral ACP did not significantly lower the risks of transient (P = .71) and permanent (.47) neurologic deficits (Table 3). There were no significant differences in other early clinical outcomes, depending on the type of ACP technique (all P values > .1). Mortality rate of patients who received bilateral ACP was not significantly lower than the patients who received unilateral ACP (HR, 1.65; 95% CI, 0.87-3.15; P = .13) (Figure 2).
Table 3.
Adjusted early clinical outcomes depending on the types of cerebral perfusion techniques (intention-to-treat groups)
| Overall cohort (N = 188) | Unilateral (n = 94) | Bilateral (n = 94) | Odds ratio∗ (95% CI) | P value |
|---|---|---|---|---|
| Hospital stay (d) | 16 (12-22) | 15 (12-20) | .50 | |
| Primary outcome | ||||
| TNDs | 19 (20.2) | 17 (18.1) | 0.87 (0.42-1.81) | .71 |
| PNDs | 8 (8.5) | 11 (11.7) | 1.42 (0.55-3.85) | .47 |
| Other early clinical outcome | ||||
| 30-d mortality | 7 (7.4) | 14 (14.9) | 2.18 (0.86-5.99) | .11 |
| Extracorporeal membrane oxygenation | 2 (2.1) | 6 (6.4) | 3.14 (0.70-21.8) | .17 |
| Re-exploration for bleeding | 10 (10.6) | 6 (6.4) | 0.57 (0.19-1.61) | .30 |
| Reintubation | 2 (2.1) | 7 (7.4) | 3.70 (0.87-25.3) | .11 |
| Tracheostomy | 0 | 1 (1.1) | NA | |
| Renal failure | 7 (7.4) | 11 (11.7) | 1.65 (0.62-4.67) | .33 |
Values are presented as median (interquartile range) or n (%). CI, Confidence interval; TNDs, transient neurologic deficits; PNDs, permanent neurologic deficits.
Results from the patients with unilateral anterior cerebral perfusion group are references.
As-treated Analyses
For further validation of the study findings, we additionally performed same analyses depending on the as-treated groups of ACP techniques. Twenty-one patients who received additional balloon catheter insertion at the left carotid artery during ACP due to the decrement of cerebral oximeter reading were included in the bilateral ACP group. Baseline profiles depending on the as-treated categorization are listed in Table E4. The overall baseline variables were well balanced after baseline adjustment with the propensity score-matching method.
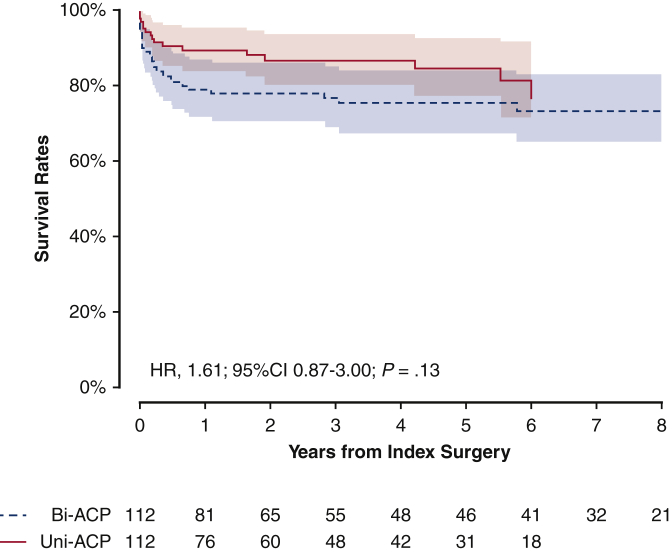
The primary outcome was not significantly different depending on the as-treated ACP techniques (OR, 1.96; 95% CI, 0.98-4.01; P = .06) (Table E5). In addition, the risk for secondary outcome was not significantly lower in the patients with bilateral ACP (HR, 1.61; 95% CI, 0.87-3.00; P = .13) compared with the patients undergoing unilateral ACP after baseline adjustment (Figure E1).
Figure E1.
Propensity score matched Kaplan-Meier plot for survival rates for the as-treated types of cerebral perfusion techniques. Solid red line indicates unilateral group; dashed blue line indicates bilateral group. Shaded bands indicate areas within 95% CIs. Bi-ACP, Bilateral antegrade cerebral perfusion; Uni-ACP, unilateral antegrade cerebral perfusion; HR, hazard ratio; CI, confidence interval.
Risk Factors for Permanent Neurologic Deficit and Mortality
Table 4 shows outcomes of multivariable analyses. In the final multivariable logistic regression model, intramural hematoma remained as a negative predictor of PND (OR, 0.34; 95% CI, 0.09-0.88), whereas the use of bilateral ACP was eliminated in the final model (OR, 1.83; 95% CI, 0.94-3.50).
Table 4.
Multivariable risk analyses for PND or 10-year mortality
| Variable | Univariable |
Multivariable |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| PND | ||||
| Bilateral antegrade cerebral perfusion | 1.89 (0.97-3.59) | .054 | ||
| Intramural hematoma | 0.39 (0.11-1.00) | .08 | 0.34 (0.09-0.88) | .046 |
| Hemopericardium | 1.56 (0.82-2.95) | .17 | ||
| Age | 1.02 (1.00-1.04) | .12 | ||
| Hazard ratio | Hazard ratio | |||
|---|---|---|---|---|
| Death | ||||
| Bilateral antegrade cerebral perfusion | 2.05 (1.33-3.14) | .001 | ||
| Hemopericardium | 1.47 (0.85-2.25) | .08 | ||
| Age | 1.04 (1.02-1.05) | <.001 | 1.04 (1.03-1.06) | <.001 |
| Chronic obstructive pulmonary disease | 4.59 (1.86-11.4) | <.001 | 4.26 (1.70-10.7) | .002 |
| Cardiopulmonary bypass time | 1.01 (1.00-1.01) | <.001 | 1.01 (1.00-1.01) | <.001 |
| Surgeon experience∗ | 0.43 (0.30-0.62) | <.001 | 0.49 (0.34-0.71) | <.001 |
Bold indicates variables mentioned in the “Risk Factors for Permanent Neurologic Deficit and Mortality” section. PND, Permanent neurologic deficit.
The surgeon experience was graded depending on the surgical volumes (more experienced surgeon was scored as the higher number).
On multivariable Cox regression analysis, old age (HR, 1.04; 95% CI, 1.03-1.06), the presence of chronic obstructive pulmonary disease (HR, 4.26; 95% CI, 1.70-10.7), longer CPB time (HR, 1.01; 95% CI, 1.00-1.01), and surgeon experience (HR, 0.49; 95% CI, 0.34-0.71) were positively correlated with mortality (Table 4). The use of bilateral ACP was eliminated at the second multivariable model (HR, 1.34; 95% CI, 0.83-2.15).
Discussion
ATAAD is a highly lethal disease and often has serious neurologic complications. Because consensus is lacking, many aortic surgery centers have developed different cerebral protection strategies in attempts to improve surgical outcomes and reduce adverse neurologic complications.15,16 There is no disagreement over the need for cerebral perfusion if an arch procedure takes more than 30 minutes because there is a significant difference in mortality between groups with and without adequate cerebral perfusion in these cases.6 However, the method of cerebral perfusion remains controversial, with each institution having its own strategies. Cerebral perfusion strategies can be categorized as ACP and retrograde cerebral perfusion. Retrograde cerebral perfusion has become less popular and recently ACP has been mainly used. ACP has the advantage of allowing higher-pressure perfusion in the natural flow direction. ACP has the risk of air embolism and dislodgment of debris, but these problems can be avoided through right axillary artery cannulation.6 In a study by Ghoreishi and colleagues17 involving 7353 patients in 772 centers, it was demonstrated that axillary cannulation lowered the risk of stroke compared with femoral cannulation (OR, 0.60; P < .001). The axillary artery is less likely to have dissection, and is easy to cannulate.18 In theory, bilateral ACP can provide sufficient cerebral perfusion, but because of the circle of Willis, unilateral ACP through the right axillary artery also can perfuse into the vertebral artery, the internal carotid artery, and the basilar artery, allowing entire cerebral perfusion.19 Previous studies comparing unilateral ACP to bilateral ACP demonstrated no relevant differences regarding outcomes, including operative mortality and PND.6,13,16 El-Sayed Ahmed and colleagues20 demonstrated that selective ACP with mild-to-moderate systemic hypothermic circulatory arrest (≥28 °C) can be applied safely for more than 1 hour.
Our strategy was selective ACP (unilateral ACP or bilateral ACP) with moderate hypothermia (around 28 °C). In our institution, bilateral ACP was mainly used until 2010, with unilateral ACP mainly used thereafter. Preoperative evaluation of the cerebral circulation is recommended because 6% to 17% of patients present with an incomplete circle of Willis.21,22 Because there were many cases of emergency surgery in our cohort, we did not routinely perform cerebral circulation imaging, adapting a strategy to convert to bilateral ACP only when deemed necessary.
Physiologically, bilateral ACP better secures cerebral perfusion. A meta-analysis by Malvindi and colleagues10 reviewing 3548 patients, 2949 of whom underwent bilateral ACP and 599 of whom underwent unilateral ACP, concluded that both techniques were acceptable but that if ACP time was expected to exceed 40 to 50 minutes, bilateral ACP was safer. However, bilateral ACP during arch surgery has several disadvantages. Concerns regarding cerebral embolism due to debris or air are always present when implementing bilateral ACP.6 Using unilateral ACP, these concerns can be avoided23 and cerebral malperfusion is clinically rare because cerebral perfusion is reinforced through the right vertebral artery and extracranial collaterals.24 Zierer and colleagues7 demonstrated that unilateral ACP offers as much cerebral and visceral organ protection as bilateral ACP and might have an added advantage of avoiding arch vessel manipulation. This study was not limited to aortic dissection but also included patients who underwent partial or total arch surgery.7 Lu and colleagues19 demonstrated that unilateral ACP offered cerebral and visceral protection not inferior to bilateral ACP in patients with ATAAD. Tong and colleagues13 compared 121 bilateral ACP and 203 unilateral ACP patients who underwent total arch replacement with ATAAD, showing no significant differences in PND or 30-day mortality rate between groups.
Bilateral ACP requires a more complicated CPB setup and an additional perfusion line. These prolong the total operation and cerebral perfusion times compared with those needed for unilateral ACP.6 In our study, after propensity score matching, the CPB and aortic crossclamp times were not significantly different between groups, but the total circulatory arrest (TCA) time was significantly longer in the bilateral ACP group (Table 3). In a study by Preventza and colleagues,16 TCA time was significantly higher with bilateral ACP than with unilateral ACP. TCA time >30 minutes was an independent risk factor for stroke despite an ACP ≤30 minutes.
After propensity score matching, our mortality rate of 18.9% and PND rate of 11.4% were consistent with previous studies.6,25,26 Our TND rate was 19.8% and 19.7% in matched patients after propensity score matching. This rate was somewhat higher than that in previous literature.13,19 To compare even minor outcomes, we investigated episodes of agitation, delirium, and confusion that were not severe or had very short durations as TND. Thus, the TND rate seemed higher. Neither TND nor PND rates were different between groups even after propensity score matching (17.5% vs 21.9% [P = .51] and 10.5% vs 12.3% [P = .84]) (Table 4). In the logistic regression analysis to determine risk factors for PND, ACP type was not associated with PND, both crude and after propensity score matching. After propensity score matching, the TCA time was significantly longer in the bilateral ACP group (45.5 ± 16.8 vs 50.0 ± 16.8 minutes; P = .04), but the TCA time was not a significant risk factor for PND. Tong and colleagues13 demonstrated no significant difference regarding PND in unilateral ACP versus bilateral ACP. They also stated that bilateral ACP did not significantly lower the incidence of TND. Preventza and colleagues16 found no differences in stroke and TNDs between unilateral ACP and bilateral ACP groups but found that, in multivariable regression analysis, prior coronary intervention and preoperative limb ischemia were independent predictors of stroke.
In 2006, Olsson and colleagues27 demonstrated that unilateral ACP was independently related to a PND, with an OR of 6.6, and that stroke was significantly more common among unilateral ACP patients. They performed this analysis after propensity score matching, but the patient size of each group was as small at 17. Ghoreishi and colleagues17 demonstrated that longer CPB and TCA times increased risks of postoperative stroke. Tong and colleagues13 noted that CPB and TCA times independently predicted PND. Our multivariable logistic regression analysis for PND identified preoperative intramural hematoma as independently predicting PND, whereas age, hemopericardium, and bilateral ACP did not. After propensity score matching, we found no significantly associated variables, including age, CPB time, TCA time, or bilateral ACP.
In this study, after PSM, similar to previous studies, there was no significant difference in the 30-day mortality rate between the unilateral ACP and bilateral ACP groups.6,13,19 Norton and colleagues11 demonstrated that operative mortality was not significantly different between the unilateral ACP and bilateral ACP groups (5.7% vs 9.6%; P = .21). But midterm survival was better in the unilateral ACP group compared with the bilateral ACP group (5-year survival: 84% vs 76%; P = .03). In a study by Preventza and colleagues,16 the survival rates for the unilateral ACP and bilateral ACP groups were similar in both groups (65.6% vs 73.0%; P = .96; median follow-up period, 3.57 years). In our study, after propensity score matching, survival rate between unilateral ACP and bilateral ACP group was not significantly different, but survival after unilateral ACP tended to be higher (P = .05) (Figure 3, B).
Figure 3.
Of 522 surgical repairs for acute type A aortic dissection, 357 unilateral and 165 bilateral antegrade cerebral perfusion techniques were used in the purpose of cerebral protection. In adjusted analysis using propensity score, 94 pairs of each group were matched. There was no significant difference in the early clinical outcomes and mortality rates.
Study Limitations
There were some limitations to this study because of its single-center retrospective observational nature with inherent biases in data collection. More bilateral ACP was performed during 2008 to 2010 and more unilateral ACP was performed during 2011 to 2019. We used the propensity score matching method to minimize these biases. In patients who underwent CT at another hospital and were transferred to ours for surgery, the initial CT results, and patient condition immediately before surgery, might have been different due to the disease characteristics of ATAAD. Brain imaging was not routinely performed in those patients who did not show any neurologic symptoms; thus, some cases of small, subclinical strokes may have been missed. In addition, the record of malperfusion was unavailable to review because the present study has a retrospective nature and more than 10 years of follow-up.
Conclusions
Despite additional complexity during ATAAD repair, bilateral ACP did not offer superiority regarding cerebral protection and survival (Figure 3 and Video Abstract).
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank Medical Illustration & Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work. Minyoung Baek, Department of Cardiovascular Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea, made the video clip.
Footnotes
Drs S. J. Song and Kim contributed equally to this article.
Appendix E1
Table E1.
Details of patients and surgery depending on the interquartile surgical periods
| Detail | Interquartile range |
P value | |||
|---|---|---|---|---|---|
| First | Second | Third | Fourth | ||
| Years of index surgery | 2008-2012 | 2012-2015 | 2015-2018 | 2018-2019 | |
| No. of patients | 130 (24.9) | 130 (24.9) | 131 (25.1) | 131 (25.1) | |
| Baseline characteristic | |||||
| Age (y) | 61.7 ± 14.9 | 59.4 ± 13.7 | 59.1 ± 14.5 | 63.4 ± 16.1 | .42 |
| Male gender | 66 (50.8) | 61 (46.9) | 59 (45.0) | 66 (50.4) | .75 |
| Types of aortic dissection | .12 | ||||
| DeBakey type 1 | 104 (80.0) | 108 (83.1) | 106 (80.9) | 94 (71.8) | |
| DeBakey type 2 | 26 (20.0) | 22 (16.9) | 25 (19.1) | 37 (28.2) | |
| Cerebral perfusion technique | <.001 | ||||
| Unilateral | 74 (56.9) | 92 (70.8) | 96 (73.3) | 116 (88.5) | |
| Bilateral | 56 (43.1) | 38 (29.2) | 35 (26.7) | 15 (11.5) | |
| Details of surgery | |||||
| Arch surgery | <.001 | ||||
| 1-partial arch replacement | 21 (16.2) | 20 (15.4) | 33 (25.2) | 6 (4.6) | |
| 2-partial arch replacement | 28 (21.5) | 21 (16.2) | 39 (29.8) | 49 (37.4) | |
| Total arch replacement | 38 (29.2) | 53 (40.8) | 48 (36.6) | 73 (55.7) | |
| Root surgery | .34 | ||||
| None | 119 (91.5) | 120 (92.3) | 114 (87.0) | 114 (87.0) | |
| Bentall operation | 10 (7.7) | 10 (7.7) | 12 (9.2) | 9 (6.9) | |
| Valve-sparing techniques | 1 (0.8) | 0 | 5 (3.8) | 8 (6.1) | |
| Graft insertion into DTA | 7 (5.4) | 23 (17.8) | 5 (3.8) | 16 (12.2) | <.001 |
Values are presented as range, n (%), or mean ± SD. DTA, Descending thoracic aorta.
Table E2.
Surgical periods and attending surgeons of the patients who received unilateral or bilateral antegrade cerebral perfusion techniques (intention-to-treat groups)
| Variable | Overall cohort |
Propensity score-matched cohort |
||||||
|---|---|---|---|---|---|---|---|---|
| Unilateral (n = 378) | Bilateral (n = 144) | P value | STD | Unilateral (n = 94) | Bilateral (n = 94) | P value | STD | |
| Years at operation | <.001 | 0.66 | .65 | 0.04 | ||||
| Quartile range 1 | 74 (19.6) | 56 (38.9) | 26 (27.7) | 31 (33.0) | ||||
| Quartile range 2 | 92 (24.3) | 38 (26.4) | 28 (29.8) | 21 (22.3) | ||||
| Quartile range 3 | 96 (25.4) | 35 (24.3) | 24 (25.5) | 27 (28.7) | ||||
| Quartile range 4 | 116 (30.7) | 15 (10.4) | 16 (17.0) | 15 (16.0) | ||||
| Surgeon | <.001 | 0.57 | .85 | 0.08 | ||||
| A | 367 (97.1) | 104 (72.2) | 86 (91.5) | 88 (93.6) | ||||
| B | 7 (1.9) | 33 (22.9) | 5 (5.3) | 4 (4.3) | ||||
| C | 4 (1.1) | 7 (4.9) | 3 (3.2) | 2 (2.1) | ||||
Values are presened as n (%). STD, Standardized mean difference.
Table E3.
Achieved body temperature and operation-related times for patients who received unilateral or bilateral antegrade cerebral perfusion (ACP) techniques (intention-to-treat groups)
| Variable | Overall cohort |
Propensity score-matched cohort |
||||||
|---|---|---|---|---|---|---|---|---|
| Unilateral (n = 378) | Bilateral (n = 144) | P value | STD | Unilateral (n = 94) | Bilateral (n = 94) | P value | STD | |
| Body temperatures during ACP | ||||||||
| Esophagus | 22.7 (20.3-24.3) | 23.6 (21.4-25.3) | .004 | 0.28 | 23.9 (22.0-25.2) | 23.9 (22.1-25.2) | .32 | 0.15 |
| Rectum | 27.8 (26.7-28.4) | 27.4 (26.6-28.0) | .002 | 0.27 | 27.5 (26.7-28.0) | 27.7 (27.0-28.1) | .09 | 0.25 |
| Additional ACP (conversion) | 21 (5.6) | 0 | 7 (7.4) | 0 | ||||
| Operation time (min) | ||||||||
| Total bypass time | 154 (133-186) | 182 (154-221) | <.001 | 0.53 | 172 (136-214) | 155 (131-189) | <.001 | 0.58 |
| Cardiac ischemia | 63 (48-82) | 70 (52-100) | .03 | 0.22 | 59 (43-80) | 56 (44-76) | .03 | 0.32 |
| Lower body ischemia | 44 (35-53) | 51 (42-62) | <.001 | 0.36 | 41 (32-56) | 43 (35-56) | <.001 | 0.59 |
Values are presented as mean (interquartile range) or n (%). STD, Standardized mean difference.
Table E4.
Baseline characteristics depending on type of cerebral perfusion technique (as-treated groups)
| Variable | Overall cohort |
Propensity-score matched cohort |
||||||
|---|---|---|---|---|---|---|---|---|
| Unilateral (n = 357) | Bilateral (n = 165) | P value | STD | Unilateral (n = 112) | Bilateral (n = 112) | P value | STD | |
| Characteristic | ||||||||
| Age (y) | 60.6 ± 15.6 | 61.6 ± 13.4 | .47 | 0.07 | 61.5 ± 15.5 | 61.4 ± 13.6 | .96 | 0.007 |
| Male gender | 193 (54.1) | 77 (46.7) | .14 | 0.15 | 57 (50.9) | 58 (51.8) | >.99 | 0.018 |
| Hypertension | 215 (60.2) | 108 (65.5) | .30 | 0.11 | 73 (65.2) | 76 (67.9) | .78 | 0.155 |
| Diabetes mellitus | 36 (10.1) | 11 (6.7) | .27 | 0.12 | 9 (8.0) | 9 (8.0) | >.99 | 0 |
| Cerebrovascular disease | 22 (6.2) | 17 (10.3) | .14 | 0.15 | 10 (8.9) | 10 (8.9) | >.99 | 0 |
| Chronic renal failure | 14 (3.9) | 4 (2.4) | .54 | 0.09 | 3 (2.8) | 3 (2.8) | >.99 | 0 |
| Coronary artery disease | 15 (4.2) | 8 (4.8) | .92 | 0.03 | 4 (3.6) | 5 (4.5) | >.99 | 0.045 |
| Marfan syndrome | 24 (6.7) | 9 (5.5) | .72 | 0.05 | 7 (6.2) | 6 (5.4) | >.99 | 0.04 |
| DeBakey type 1 | 281 (78.7) | 131 (79.4) | .95 | 0.02 | 87 (77.7) | 90 (80.4) | .74 | 0.07 |
| DeBakey type 2 | 76 (21.3) | 34 (20.6) | .95 | 0.02 | 25 (22.3) | 22 (19.6) | .74 | 0.07 |
| Intramural hematoma | 77 (21.6) | 29 (17.6) | .35 | 0.10 | 23 (21.7) | 17 (16.0) | .38 | 0.05 |
| Hemopericardium | 130 (36.4) | 55 (33.3) | .56 | 0.07 | 23 (20.5) | 21 (18.8) | .87 | 0.12 |
| Details of surgery | ||||||||
| Arch surgery, n (%) | <.001 | 0.54 | .26 | 0.008 | ||||
| 1-partial arch replacement | 59 (16.5) | 21 (12.7) | 26 (23.2) | 15 (13.4) | ||||
| 2-partial arch replacement | 105 (29.4) | 32 (19.4) | 26 (23.2) | 29 (25.9) | ||||
| Total arch replacement | 159 (44.5) | 53 (32.1) | 43 (38.4) | 45 (40.2) | ||||
| Root surgery | .79 | 0.04 | .82 | 0.06 | ||||
| None | 318 (89.1) | 149 (90.3) | 12 (10.7) | 10 (8.9) | ||||
| Bentall's operation | 28 (7.8) | 13 (7.9) | 10 (8.9) | 7 (6.2) | ||||
| Valve sparing techniques | 11 (3.1) | 3 (1.8) | 2 (1.8) | 3 (2.7) | ||||
| Graft insertion in DTA | 19 (5.3) | 13 (7.9) | .16 | 0.23 | 12 (10.7) | 11 (9.8) | .69 | 0.11 |
| Years at operation | <.001 | 0.63 | .93 | 0.05 | ||||
| Quartile range 1 | 67 (18.8) | 63 (38.2) | 34 (30.4) | 36 (32.1) | ||||
| Quartile range 2 | 84 (23.5) | 46 (27.9) | 34 (30.4) | 33 (29.5) | ||||
| Quartile range 3 | 90 (25.2) | 41 (24.8) | 27 (24.1) | 29 (25.9) | ||||
| Quartile range 4 | 116 (32.5) | 15 (9.1) | 17 (15.2) | 14 (12.5) | ||||
| Surgeon | <.001 | 0.61 | .36 | |||||
| A | 347 (97.2) | 124 (75.2) | 107 (95.5) | 109 (97.3) | ||||
| B | 7 (2.0) | 33 (20.0) | 3 (2.7) | 3 (2.7) | ||||
| C | 3 (0.8) | 8 (4.8) | 2 (1.8) | 0 | ||||
Values are presented as mean ± SD or n (%). STD, Standardized mean difference; DTA, descending thoracic aorta.
Table E5.
Adjusted early clinical outcomes depending on the type of cerebral perfusion technique (as-treated groups)
| Overall cohort (N = 224) | Unilateral (n = 112) | Bilateral (n = 112) | Odds ratio∗ (95% CI) | P value |
|---|---|---|---|---|
| Hospital stay (d) | 23.9 ± 27.3 | 23.7 ± 23.7 | .94 | |
| Primary outcome | ||||
| TNDs | 15 (13.4) | 26 (23.2) | 2.10 (0.72-6.93) | .19 |
| PNDs | 5 (4.5) | 10 (8.9) | 1.96 (0.98-4.01) | .06 |
| Other early clinical outcome | ||||
| 30-d mortality | 3 (2.7) | 11 (9.8) | 3.96 (1.20-17.9) | .04 |
| Extracorporeal membrane oxygenation | 0 | 5 (4.5) | NA | |
| Re-exploration for bleeding | 11 (9.8) | 10 (8.9) | 0.90 (0.36-2.23) | .82 |
| Reintubation | 3 (2.7) | 8 (7.1) | 2.79 (0.78-13.0) | .14 |
| Tracheostomy | 2 (1.8) | 2 (1.8) | 1.0 (0.12-8.45) | >.99 |
| Renal failure | 10 (8.9) | 11 (9.8) | 1.11 (0.45-2.78) | .82 |
Values are presented as mean ± SD or n (%). CI, Confidence interval; TNDs, transient neurologic deficits; PNDs, permanent neurologic deficits; NA, not available.
Results of unilateral cerebral perfusion group are references.
Supplementary Data
A total of 522 patients who received acute type A aortic dissection repair were reviewed. We found no significant differences on the occurrences of neurologic dysfunction or 10-year mortality depending on the type of cerebral perfusion technique. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00227-3/fulltext.
References
- 1.Shrestha M., Haverich A., Martens A. Total aortic arch replacement with the frozen elephant trunk procedure in acute DeBakey type I aortic dissections. Eur J Cardiothorac Surg. 2017;51(Suppl 1):i29–i34. doi: 10.1093/ejcts/ezw341. [DOI] [PubMed] [Google Scholar]
- 2.Matalanis G., Ip S. A new paradigm in the management of acute type a aortic dissection: total aortic repair. J Thorac Cardiovasc Surg. 2019;157:3–11. doi: 10.1016/j.jtcvs.2018.08.118. [DOI] [PubMed] [Google Scholar]
- 3.Poon S.S., Theologou T., Harrington D., Kuduvalli M., Oo A., Field M. Hemiarch versus total aortic arch replacement in acute type a dissection: a systematic review and meta-analysis. Ann Cardiothorac Surg. 2016;5:156–173. doi: 10.21037/acs.2016.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apostolakis E., Koletsis E.N., Dedeilias P., John N.K., George S., Argini P., et al. Antegrade versus retrograde cerebral perfusion in relation to postoperative complications following aortic arch surgery for acute aortic dissection type A. J Card Surg. 2008;23:480–487. doi: 10.1111/j.1540-8191.2008.00587.x. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka A., Ornekian V., Estrera A.L. Limited repair with tear-oriented approach for type A aortic dissection. J Cardiovasc Surg (Torino) 2020;61:278–284. doi: 10.23736/S0021-9509.20.11259-X. [DOI] [PubMed] [Google Scholar]
- 6.Kruger T., Weigang E., Hoffmann I., Blettner M., Aebert H., GERAADA Investigators Cerebral protection during surgery for acute aortic dissection type a: results of the German Registry for acute aortic dissection type A (GERAADA) Circulation. 2011;124:434–443. doi: 10.1161/CIRCULATIONAHA.110.009282. [DOI] [PubMed] [Google Scholar]
- 7.Zierer A., El-Sayed Ahmad A., Papadopoulos N., Moritz A., Diegeler A., Urbanski P.P. Selective antegrade cerebral perfusion and mild (28-30 °C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg. 2012;144:1042–1044. doi: 10.1016/j.jtcvs.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 8.Angeloni E., Benedetto U., Takkenberg J.J., Stigliano I., Roscitano A., Melina G., et al. Unilateral versus bilateral antegrade cerebral protection during circulatory arrest in aortic surgery: a meta-analysis of 5100 patients. J Thorac Cardiovasc Surg. 2014;147:60–67. doi: 10.1016/j.jtcvs.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Pacini D., Murana G., Di Marco L., Berardi M., Mariani C., Coppola G., et al. Cerebral perfusion issues in type a aortic dissection. J Vis Surg. 2018;4:77. doi: 10.21037/jovs.2018.03.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malvindi P.G., Scrascia G., Vitale N. Is unilateral antegrade cerebral perfusion equivalent to bilateral cerebral perfusion for patients undergoing aortic arch surgery? Interact Cardiovasc Thorac Surg. 2008;7:891–897. doi: 10.1510/icvts.2008.184184. [DOI] [PubMed] [Google Scholar]
- 11.Norton E.L., Wu X., Kim K.M., Patel H.J., Deeb G.M., Yang B. Unilateral is comparable to bilateral antegrade cerebral perfusion in acute type a aortic dissection repair. J Thorac Cardiovasc Surg. 2020;160:617–625.e5. doi: 10.1016/j.jtcvs.2019.07.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ergin M.A., Galla J.D., Lansman S.L., Quintana C., Bodian C., Griepp R.B. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J Thorac Cardiovasc Surg. 1994;107:788–797. [PubMed] [Google Scholar]
- 13.Tong G., Zhang B., Zhou X., Tao Y., Yan T., Wang X., et al. Bilateral versus unilateral antegrade cerebral perfusion in total arch replacement for type a aortic dissection. J Thorac Cardiovasc Surg. 2017;154:767–775. doi: 10.1016/j.jtcvs.2017.02.053. [DOI] [PubMed] [Google Scholar]
- 14.Song S.W., Yoo K.J., Shin Y.R., Lim S.H., Cho B.K. Effects of intermittent lower body perfusion on end-organ function during repair of acute DeBakey type I aortic dissection under moderate hypothermic circulatory arrest. Eur J Cardiothorac Surg. 2013;44:1070–1074. doi: 10.1093/ejcts/ezt145. [DOI] [PubMed] [Google Scholar]
- 15.Milewski R.K., Pacini D., Moser G.W., Moeller P., Cowie D., Szeto W.Y., et al. Retrograde and antegrade cerebral perfusion: results in short elective arch reconstructive times. Ann Thorac Surg. 2010;89:1448–1457. doi: 10.1016/j.athoracsur.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 16.Preventza O., Simpson K.H., Cooley D.A., Cornwell L., Bakaeen F.G., Omer S., et al. Unilateral versus bilateral cerebral perfusion for acute type a aortic dissection. Ann Thorac Surg. 2015;99:80–87. doi: 10.1016/j.athoracsur.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Ghoreishi M., Sundt T.M., Cameron D.E., Cornwell L., Bakaeen F.G., Omer S., et al. Factors associated with acute stroke after type A aortic dissection repair: an analysis of the society of thoracic surgeons national adult cardiac surgery database. J Thorac Cardiovasc Surg. 2020;159:2143–2154.e3. doi: 10.1016/j.jtcvs.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Ji B., Sun L., Liu J., Liu M., Sun G., Wang G., et al. The application of a modified technique of SCP under DHCA during total aortic arch replacement combined with stented elephant trunk implantation. Perfusion. 2006;21:255–258. doi: 10.1177/0267659106074766. [DOI] [PubMed] [Google Scholar]
- 19.Lu S., Sun X., Hong T., Yang S., Song K., Lai H., et al. Bilateral versus unilateral antegrade cerebral perfusion in arch reconstruction for aortic dissection. Ann Thorac Surg. 2012;93:1917–1920. doi: 10.1016/j.athoracsur.2012.02.090. [DOI] [PubMed] [Google Scholar]
- 20.El-Sayed Ahmad A., Papadopoulos N., Risteski P., Hack T., Ay M., Moritz A., et al. Is more than one hour of selective antegrade cerebral perfusion in moderate-to-mild systemic hypothermic circulatory arrest for surgery of acute type a aortic dissection safe? Thorac Cardiovasc Surg. 2018;66:215–221. doi: 10.1055/s-0037-1604451. [DOI] [PubMed] [Google Scholar]
- 21.Iqbal S. A comprehensive study of the anatomical variations of the circle of Willis in adult human brains. J Clin Diagn Res. 2013;7:2423–2427. doi: 10.7860/JCDR/2013/6580.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaladj N., Shrestha M., Meck S., Peterss S., Kamiya H., Kallenbach K., et al. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg. 2008;135:908–914. doi: 10.1016/j.jtcvs.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 23.Aebert H., Reber D., Kobuch R., Philipp A., Birnbaum D.E. Aortic arch surgery using moderate systemic hypothermia and antegrade cerebral perfusion via the right subclavian artery. Thorac Cardiovasc Surg. 2001;49:283–286. doi: 10.1055/s-2001-17806. [DOI] [PubMed] [Google Scholar]
- 24.Immer F.F., Moser B., Krahenbuhl E.S., Englberger L., Stalder M., Eckstein F.S., et al. Arterial access through the right subclavian artery in surgery of the aortic arch improves neurologic outcome and mid-term quality of life. Ann Thorac Surg. 2008;85:1614–1618. doi: 10.1016/j.athoracsur.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Cabasa A., Pochettino A. Surgical management and outcomes of type a dissection-the mayo clinic experience. Ann Cardiothorac Surg. 2016;5:296–309. doi: 10.21037/acs.2016.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Hara D., McLarty A., Sun E., Itagaki S., Tannous H., Chu D., et al. Type-a aortic dissection and cerebral perfusion: the Society of Thoracic Surgeons Database analysis. Ann Thorac Surg. 2020;110:1461–1467. doi: 10.1016/j.athoracsur.2020.04.144. [DOI] [PubMed] [Google Scholar]
- 27.Olsson C., Thelin S. Antegrade cerebral perfusion with a simplified technique: unilateral versus bilateral perfusion. Ann Thorac Surg. 2006;81:868–874. doi: 10.1016/j.athoracsur.2005.08.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A total of 522 patients who received acute type A aortic dissection repair were reviewed. We found no significant differences on the occurrences of neurologic dysfunction or 10-year mortality depending on the type of cerebral perfusion technique. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00227-3/fulltext.