Abstract
Objective
To describe the relationship of oxidative stress and antioxidant biomarkers in cord blood of premature newborns and the prognosis of diseases in the neonatal period.
Sources
This study consists of an integrative review. Searches were conducted in electronic databases Scopus, PubMed, Web of Science, and Medline/Lilacs through the Virtual Library on Health Issues, using the descriptors: “premature infants”, “preterm infants”, “preterm birth”, “preterm”, “oxidative stress”, “antioxidants”, “infant, premature, diseases” and “cord blood”. Original articles published between 2016 and 2021 in Portuguese, English, or Spanish, which analyzed oxidative stress and/or antioxidant levels through cord blood of premature newborns and evaluated clinical outcomes, were included.
Summary of the findings
Of the 1,003 studies reviewed, after exclusion of duplicate articles, analysis of titles, abstracts, and full texts, 18 articles were included. 72.2% (n = 13) of analyzed studies reported a positive association between oxidative stress and the development of prematurity-related diseases; 27.7% (n = 5) showed no significant relation. Outcomes that showed a positive association were: intrauterine growth restriction, necrotizing enterocolitis, bronchopulmonary dysplasia, intraventricular hemorrhage, fetal inflammatory response syndrome, early-onset neonatal sepsis, retinopathy of prematurity, morbidity, and mortality.
Conclusion
The analysis of oxidative stress and antioxidants in cord blood of premature newborns may be useful in the prognosis of some pathologies. The consequences of oxidative damage are known to be associated with increased morbidity in the short and long term. Further investigation is needed in this population in order to define normality parameters of biomarkers, clinical manifestations, diagnosis and treatment of these conditions.
Keywords: Infant, premature; Oxidative stress; Antioxidants; Diseases; Cord blood
Introduction
Prematurity is a major public health problem. Complications from premature birth are the leading cause of death in children under five years of age.1 In 2017, of the 2.5 million newborns who died from preventable causes, nearly two-thirds were premature.2
Oxidative stress has been implicated as a possible pathophysiological condition to contribute to this unfavorable situation. Premature neonates lack well-developed antioxidant and immune defense mechanisms, making them more susceptible to oxidative stress injury.3, 4, 5 The main reasons for this susceptibility are a hypoxic-hyperoxic challenge, presence of infections, deficiency in antioxidant defense, and high levels of free iron.6,7
The transition from intrauterine to the extrauterine environment greatly increases free radical production, which is normally downregulated by the antioxidant defense system.8 Oxidative stress is caused by excessive reactive oxygen species (ROS), which are generated when there is an imbalance in this regulation. In oxidative stress, there is an inability of the antioxidant defense system to repair the ROS damage8,9 due to either an excessive formation or impaired inactivation of ROS, or a combination of both.9
ROS include free radicals and oxygenated molecules of non-free radicals. Both of them can generate oxidative stress and redox reaction imbalance.6 Oxygen-free radicals are extremely reactive chemical species; they react with various cellular molecules – such as phospholipids, amino acids, and nucleic acids – and lead to lipid peroxidation, DNA strand breaks, and other damaging processes which culminate in cellular injury.6,8,10, 11, 12
Oxidative stress-induced damage has an important role in several pathological pathways involved in neonatal diseases.6,13,14 For example, some studies point out that most complications of prematurity, such as bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), and white matter lesions seem to be related to oxidative injury.6,15
According to Ozsurekci et al.,6 there are still gaps in knowledge about the potential role of oxidative injury in the pathogenesis of neonatal diseases. New studies should be conducted to investigate more extensively diagnostic and prognostic values of various oxidative stress and antioxidant biomarkers in order to reduce oxidative tissue injury in neonates.
Cord blood has been the most widely used matrix when evaluating mechanisms related to prematurity16 as are allows monitoring of biomarkers without causing any distress to the babies and providing relevant information.17
From this perspective, the objective of this study is to describe the relationship between oxidative stress and antioxidant biomarkers in cord blood of premature newborns and the development of diseases in the neonatal period of preterm newborns.
Method
This study consists of an integrative literature review, which is a method that enables the analysis and synthesis of results in a systematized manner, providing support for decision making and improvement of clinical practice. The preparation of this review respected all the pre-established phases for its realization, covering the following steps: formulation of guiding questions; selection and retrieval of articles according to inclusion and exclusion criteria; data collection; evaluation of selected studies; discussion and interpretation of results, and presentation of the review.18
Studies that evaluated biomarkers of oxidative stress and/or antioxidant levels in cord blood of preterm newborns and evaluated clinical outcomes were included. Searches were conducted from January to March 2021, and studies published between 2016 and 2021 in Portuguese, English, or Spanish were included. Original peer-reviewed articles and indexed in the electronic databases Scopus (Elsevier), PubMed (via National Library of Medicine), Web of Science (main collection), and Medline/Lilacs through the Virtual Health Library were chosen. Specific descriptors and their synonyms were used, according to the Medical Subject Headings (MeSH) terms and their equivalents in Portuguese, established by the Health Sciences Descriptors (DeCS). The terms were combined using the Boolean operators “AND” and “OR” to compose the search strategy. The following terms were used in searches: “premature infants” OR “preterm infants” OR “preterm birth” OR “preterm” AND “oxidative stress” OR “antioxidants” AND “infant, premature, diseases.” The search equation used was: (“premature infants” OR “preterm infants” OR “preterm birth” OR preterm) AND (“oxidative stress” OR antioxidants) AND diseases.
To be included in this review, studies should evaluate oxidative stress and/or antioxidant biomarkers in cord blood of preterm infants (i.e. born before 37 weeks of gestational age (GA)) and associate them with a clinical outcome (a clinical condition, disease or epidemiological indicators, such as morbidity or mortality). Quantitative or qualitative data were extracted from included articles. Studies were excluded if they were duplicate publications; review studies; if they evaluated oxidative stress and/or antioxidants in premature newborns but did not associate it with a clinical outcome; studies that did not evaluate oxidative stress and/or antioxidants using cord blood; experimental studies with animals; or studies with objectives out of the scope of this review.
Two authors independently screened the titles and identified by the searches, and those which met the eligibility criteria were selected for the full-text review. The selected full-text articles were further evaluated by two independent authors, and the studies were definitively included in the review when they met all the inclusion criteria. Any differences between the two reviewers were resolved through a third independent author. The analysis of the studies was conducted in a systematized way, using a structured instrument containing the following information: title of the article; journal; database; Qualis evaluation; authors; country; language; year; objectives; sample characteristics; analyzed variables; data analysis; results; and conclusions. The studies were critically analyzed regarding their authenticity, methodological quality, the relevance of the information, and representations to ensure the scientific integrity of the review.
Results
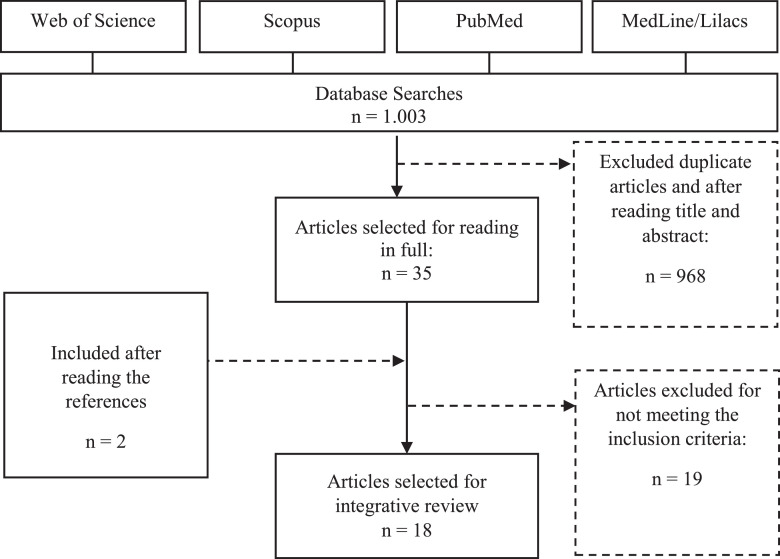
One thousand and three articles were identified by the search strategy. Were excluded 968 articles after title/abstract analysis and after excluding duplicates, so 35 articles were fully assessed for eligibility. Two additional studies were included by assessing article references. After full-text analysis, 19 articles were excluded because they did not meet the inclusion criteria. Thus, a total of 18 studies were included in the review. Figure 1 shows the flowchart of the article selection process. Included studies were presented as follows concerning the year of publication: 5 in 2016, 3 in 2017, 2 in 2018, 3 in 2019, 3 in 2020, and 2 publications in 2021. Synthesis of the articles, year of publication, type of study, sample, source of specimen, biomarkers, diseases evaluated, and the main outcomes are described in Table 1.3, 4, 5,8, 9, 10,12,13,19, 20, 21, 22, 23, 24, 25, 26, 27, 28
Figure 1.
Flowchart of the study selection process for the integrative review on oxidative stress in premature newborns.
Table 1.
Studies reviewed by oxidative stress biomarker category.
| Article / Country | Type of study | Sample | Source of specimen | Oxidative stress and antioxidants biomarkers | Diseases evaluated | Main outcomes |
|---|---|---|---|---|---|---|
| Moore et al., 201619 United States |
Prospective | 31 PTN | Cord blood and urine | 8-OHdG | CLD IVH BPD ROP |
CLD was associated with lower levels of 8-OHdG. There were no significant differences regarding IVH, BPD, and ROP incidences. |
| Perrone et al., 201613 Italy | Cohort | 120 PTN | Cord blood | IsoPs NPBI AOPP |
IUGR | Placental injuries (inflammation or impaired perfusion) were associated with elevated OS levels. PTN with vascular perfusion lesions had higher levels of AOPP, low GA, and the IUGR. |
| Dietze et al., 201620 United States | Prospective | 31 PTN | Cord blood and saliva | Cortisol | NEC IVH BPD ROP |
The higher the GA at the beginning of prenatal care, the lower cord blood cortisol and tended to have a higher risk of NEC. In salivary cortisol, NB whose mothers smoked had a higher risk of IVH. |
| Ghany et al., 201610 Egypt | Case-control study | 200 NB 100 PTN 100 FT NB |
Cord blood and venous blood | MDA; TAC Catalase Vitamin A Vitamin E |
NEC BPD IVH |
Positive relationship between reduced antioxidant levels at birth and the risk of neonatal morbidities, including BPD, IVH, NEC. |
| Bandyopadhyay et al., 20178 India | Transversal | 109 NB 27 PTN 82 FT NB |
Cord blood | MDA 8-OHdG |
IUGR | Term NB and late PTN with IUGR had higher levels of MDA and 8-OHdG compared to infants suitable for GA through meconium-stained amniotic fluid. |
| Norishadkam et al., 20174 Iran | Case-control study | 50 NB 25 PTN 25 FT NB |
Cord blood | MDA Catalase SOD TAC |
DNA damage |
There was no significant association between MDA, SOD, TAC, catalase, and early DNA damage in cord blood plasma for PTN. |
| Ozalkaya et al., 201721 Turkey | Prospective | 51 PTN | Cord blood |
TAC PON-1 TOS OSI |
FIRS RDS IVH BPD ROP Sepsis |
Higher levels of TOS were associated with NB with PPROM; Higher PON-1 was associated with higher risk of PPROM, FIRS or both. NB with PPROM and FIRS had higher incidence of RDS. NB with FIRS had higher mortality. |
| Bharadwaj et al., 20173 India | Cohort | 143 NB 71 w/ GA >35 e 72 w/ GA >39 |
Maternal blood and cord blood | Protein carbonyls MDA TAC |
Neurologic deseases IUGR Sepsis |
OS increases in NB and mothers with PE. Decreased maternal TAC is associated with negative neuromotor results. Maternal TAC during PE is useful to predict poor motor development at the corrected age of one year. |
| Coutinho et al., 201822 Brazil | Transversal | 21 PTN | Cord blood, saliva, and urine | MDA SOD GPx Catalase |
Sepsis | GPx in cord blood can be a diagnostic tool for suspicion of early-onset neonatal sepsis in PTNs of mothers with risk factors for sepsis. |
| Armann et al., 201823 Turkey | Case-control study | 80 PTN | Cord blood | TAC TOS OSI |
Cardiac functions |
TAC, TOS, and OSI were significantly higher for NB of mothers with PE. Echocardiographic parameters are not affected by the oxidant state. |
| Elkabany et al., 201912 Egypt | Prospective Case-control study |
40 PTN | Cord blood | MDA; AOPP 8-OHdG TAC Copper; Zinc |
RDS | AOPPs and 8-OHdG can be used as serum biomarkers for OS among NBs with RDS to monitor disease progression. |
| Dekker et al., 2019 Netherlands24 | Clinical trial | 52 PTN | Cord blood and venous blood | 8iPGF2α | Grade III IVH | 8iPGF2α did not differ among groups. There were also no differences regarding intubation, the incidence of grade III IVH, or death before hospital discharge. |
| Silva et al., 2019 Brazil25 |
Transversal | 140 NB 54 PTN and 76 FT NB |
Cord blood | Vitamin E | IUGR | IUGR was more frequent in PTB; most of the infants had low vitamin E levels. |
| Stefanov et al., 20209 United States | Prospective Pilot study |
63 NB 50 PTN and 13 FT NB |
Cord blood and venous blood | MDA Glutathione |
Endothelial dysfunction | MDA was higher in cord blood than at 24 hours of life, regardless of GA. PTN had higher ET-1 levels in cord blood than 24 hours of life, but overall, ET-1 had no significant association with OS. |
| Liu et al., 2020 China26 |
Case-control study | 816 NB 182 PTN 634 FT NB |
Cord blood | MDA SOD ROSs |
Morbidity and mortality | Higher levels of MDA and ROSs are associated with lower Apgar scores; NICU admission and ventilation, i.e., are significantly associated with higher morbidity and mortality. |
| Pajai e Bezalwar 20205 India | Observational Analytic | 45 PTN | Cord blood | MDA Nitrates Vitamin C Vitamin E |
Morbidity and mortality | PTN shows higher MDA and nitrates levels and reduced levels of vitamins C and E, especially in males, indicating increased morbidity and mortality. |
| Agrawal et al., 2021 Índia27 |
Nested case-control | 189 PTN | Cord blood and venous blood (both from NB and mother) | MDA Copper Zinc Vitamin A |
ROP | MDA and vitamin A in umbilical cord plasma were independent predictive variables of ROP. |
| Coviello et al., 2021 Netherlands28 |
Prospective observational study | 44 PTN | Cord blood and plasma | F2-isoprostanes | White matter injury | Early verification of plasma IsoPs can help discriminate abnormal WMI scores at term-equivalent age; and represents an early biomarker for identifying PTN at risk of brain injury. |
AOPP, advanced oxidative protein products; BPD, bronchopulmonary dysplasia; CLD, chronic lung disease; DNA, deoxyribonucleic acid; ET-1, endothelin-1; FIRS, fetal inflammatory response syndrome; FT, full-term; GA, gestational age; GPx, glutathione peroxidase; IVH, intraventricular hemorrhage; IsoPs, isoprostanes; IUGR, intrauterine growth restriction; MDA, malondialdehyde; NB, newborns; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; NPBI, non-protein bound iron; OS, oxidative stress; OSI, oxidative stress index; PE= preeclampsia; PON-1, paraoxonase-1; PPROM, preterm premature rupture of membrane; PTN, preterm newborn; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity; ROS, reactive oxygen species; SOD= superoxide dismutase; TAC, total antioxidant capacity; TOS, total oxidant status; WMI, white matter injury; 8-OHdG, 8- hydroxydeoxyguanosine; 8iPGF2α, 8-iso-prostaglandin F2.
Most of the studies were published in the English language (94.4%, n = 17), and one was in Portuguese. Regarding study methodology, six articles were case-control studies, five were prospective studies, three were cross-sectional studies, two were cohort studies, one was a clinical trial, and one was an observational analytic study.
Most studies had their sample composed only of premature newborns (61.1%, n = 11). In the remaining (38.8%, n = 7), term newborns were also included. Sample specimens differed in some articles. 50% (n = 9) evaluated only cord blood, 16.6% (n = 3) analyzed cord blood and venous blood a few hours after birth, and 33.3% (n = 6) evaluated cord blood together with urine, saliva, maternal blood, and venous blood.
The following biomarkers of oxidative stress were assessed in the studies: 8-hydroxydeoxyguanosine (8-OHdG) (n = 3); malondialdehyde (MDA) (n = 10), isoprostanes (n = 2) and 8-iso-prostaglandin (n = 1); advanced oxidative protein products (AOPP) / carbonyls protein (n = 3); total antioxidant capacity (TAC) (n = 6); total oxidant status (TOS) and oxidative stress index (OSI) (n = 1); cortisol (n = 1); non-protein bound iron (NPBI) (n = 1). ROS and nitrate markers were also analyzed (n = 1). Antioxidants evaluated in the studies were superoxide dismutase (SOD), catalase, vitamin E (n = 3); copper, zinc, vitamin A (n = 2); glutathione, paraoxonase-1 (PON-1), glutathione peroxidase (GPx) and vitamin C (n = 1).
Evaluated pathologies included: HIV (5 articles); IUGR, BPD, ROP (4 articles); sepsis (3 articles); NEC, RDS and morbidity/mortality (2 articles); and chronic lung disease (CLD), white matter injury, DNA damage, neurological disease, fetal inflammatory response syndrome (FIRS), cardiac functions and endothelial dysfunction (one article).
The studies that reported a positive association between oxidative stress and/or development of diseases related to prematurity were 72.2% (n = 13); 27.7% (n = 5) showed no significant association. Positive correlations were found in IUGR;8,13,25 NEC;10,20 morbidity and mortality;5,26 BPD, IVH;10 FIRS;21 early-onset neonatal sepsis;22 RDS12 and ROP.27 CLD,19 DNA damage,4 IVH,24 cardiac functions,23 and endothelial dysfunction9 were not associated with oxidative stress levels in included studies.
Table 25,8,10,12,13,20, 21, 22,25, 26, 27 shows levels of oxidative stress and/or antioxidants correlated with clinical outcomes. It was observed the following associations: 1) MDA and vitamin A were associated with ROP;27 2) AOPP,13 MDA, 8-OHdG8 and vitamin E25 were associated with intrauterine growth restriction; 3) Cortisol,20 vitamin A and vitamin E10 were associated with NEC; 4) Vitamins A and E were associated with BPD and IVH;10 5) PON-1 was associated with FIRS;21 6) GPx was associated with sepsis;22 7) AOPP and 8-OHdG were associated with RDS12 and 8) MDA;5,26 and ROSs,26 nitrates, vitamin C, and vitamin E5 were associated with morbidity and mortality.
Table 2.
Oxidative stress biomarkers and antioxidants correlated with evaluated conditions.
| Condition | Biomarker |
|---|---|
| ROP | ↑ MDA27 ↓ Vitamin A27 |
| IUGR | ↑ AOPP13 ↑ MDA8 ↑ 8-OHdG8 ↓ Vitamin E25 |
| NEC | ↑ Cortisol20 ↓ Vitamin A10 ↓ Vitamin E10 |
| BPD | ↓ Vitamin A10 ↓ Vitamin E10 |
| IVH | ↓ Vitamin A10 ↓ Vitamin E10 |
| FIRS | ↓ PON-121 |
| Sepsis | ↓ GPx22 |
| RDS | ↑ AOPP12 ↑ 8-OHdG12 |
| Morbidity and mortality | ↑ MDA5,26 ↑ ROS26 ↑ Nitrates5 ↓ Vitamin C5 ↓ Vitamin E5 |
AOPP, advanced oxidative protein products; BPD, bronchopulmonary dysplasia; FIRS, fetal inflammatory response syndrome; GPx, glutathione peroxidase; IVH, intraventricular hemorrhage; IUGR, intrauterine growth restriction; MDA, malondialdehyde; NEC, necrotizing enterocolitis; PON-1, paraoxonase-1; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity; ROS, reactive oxygen species; 8-OHdG, 8- hydroxydeoxyguanosine.
Three of the 13 studies which found an association between oxidative biomarkers and disease development used different samples to analyze data: venous blood drawn a few hours after birth,28 maternal blood,3 and salivary cortisol.20
In general, studies have shown that premature newborns have a higher level of oxidative stress biomarkers compared to term newborns due to several factors. Moreover, worse prognosis (low Apgar score, admission to the neonatal intensive care unit, assisted ventilation and hospital stay time) and increased morbidity and mortality have been related to higher levels of oxidative stress and lower levels of antioxidants with the development of some pathologies.
Discussion
In premature newborns, oxidative stress is a physiological event during the normal transition from the intrauterine to the extrauterine environment. Outside the uterus, free radical production increases significantly and must be counterbalanced by the antioxidant defense system. Healthy full-term newborns can tolerate these drastic changes; however, when intrauterine development is incomplete or abnormal, this tolerance might be affected.8 The identification of reliable biomarkers to analyze the oxidant-antioxidant system dysregulation is essential to improve neonatal care. Furthermore, evaluation of oxidative stress through cord blood may be useful in determining the prognosis of some pathologies.29
Most of the studies included in this review showed a relationship between increased levels of oxidative stress biomarkers and/or decreased levels of antioxidants in cord blood and higher risk of clinical outcomes, such as neonatal diseases and morbimortality. Among the conditions that showed a greater association with increased oxidative stress and/or reduced antioxidant levels were ROP,27 IUGR,8,13,25 RDS,12 sepsis22 and morbidity and mortality.5,26 Falsaperla et al.11 also observed that the imbalance between the newborn's oxidant and antioxidant factors seems to play an important role in the onset of the main pathologies of the preterm infant, such as BPD, ROP, NEC, IVH, periventricular leukomalacia, and white matter lesions.
One of the selected studies evaluated 189 premature newborns (38 with ROP and 151 without ROP) in a case-control study and showed that MDA and vitamin A concentration in cord blood are independent predictor variables of ROP.27 ROP is an eye disease that affects 7 to 15% of premature infants and is caused by abnormal vascular growth in the retina and may cause significant visual impairment or even blindness.20
To monitor ROP progression among neonates, AOPPs and 8-OHdG might be relevant biomarkers of oxidative stress. In a study conducted by Elkabany et al.,12 these markers were measured in the cord blood of 80 premature newborns at <34 weeks GA (40 newborns with RDS and 40 newborns without RDS), with a positive association. Another marker that might be considered as a diagnostic tool is GPx. One study evaluated 21 preterm infants (30 and 36 weeks) and showed that early-onset neonatal sepsis had a significant correlation with GPx levels in preterm infants from mothers with risk factors for this disease.22
A review by Casavant et al.30 showed that, compared to term infants, premature newborns had lower levels of antioxidants, vitamin A, vitamin E and catalase (an enzyme that neutralizes ROS and is linked to increased rates of NEC and BPD). In a case-control study conducted by Ghany et al.10 with 100 preterm and 100 full-term newborns, levels of vitamin A, vitamin E, catalase, TAC, and MDA were analyzed. The study described a significant relationship between decreased antioxidant levels at birth and the risk of neonatal morbidities, including BPD, NEC, and IVH. In addition, cortisol was another marker that showed a significant association with NEC. NEC is the main cause of morbidity and mortality in premature babies, with a mortality rate of up to 30% and an increased risk of delayed neurological development, especially in pregnancies with inadequate prenatal care.20
Among the reports which evaluated IUGR, Silva et al.25 analyzed the concentration of vitamin E in umbilical cord serum in 140 newborns (64 premature and 76 terms) to test a correlation between the biomarker and intrauterine growth. Results showed that IUGR was more frequent in premature newborns, and most of them had low vitamin E levels. IUGR is a complication of pregnancy, often described when the fetus is estimated to be small for the GA and has an incidence ranging between 3 and 7% of births. In the study by Perrone et al.13 with 129 premature newborns, it was observed that newborns with vascular perfusion lesions had higher levels of AOPP, low GA, and IUGR. Bandyopadhyay et al.8 evaluated 109 newborns (27 premature and 82 term ones) and related IUGR to higher levels of AOPP, MDA and 8-OHdG.
The study by Pajai and Bezalar5 associated higher morbidity and mortality rates with increased levels of MDA and nitrates and decreased levels of vitamins C and E, especially in premature male newborns. Liu et al.26 stated an association between MDA and ROS levels and a higher risk of a low Apgar score, NICU admission and mechanical ventilation. Ozalkaya et al.21 determined TAC, PON-1, TOS, and OSI levels in 51 preterm infants (<34 weeks). They showed a significant association between PON-1 levels and PPROM and FIRS, alongside a higher incidence of RDS and higher mortality of infants with FIRS.
Alternative markers used in the studies are the antioxidant activity levels. Antioxidant activity measurement may be generally more useful than oxidative stress levels because the results allow a greater understanding of potential mechanisms and therapeutic interventions.14 In this review, antioxidants that showed a significant association between their levels and the development of morbidities were PON-1,21 GPx,22 and vitamins A, C, and E.5,10 It is essential to measure multiple antioxidants and include measures that identify specific ROS or RNS that may be associated with imbalanced antioxidant levels or activity. The inclusion of these additional measures provides a more comprehensive comprehension of the biological processes involving antioxidants.14
Also, data obtained from some studies reveal a positive association between oxidative stress and neurological diseases. However, these analyses were not obtained from cord blood. One of them stated that analysis of plasma IsoPs (24 and 48 h after birth) might represent an early biomarker to identify the risk of brain damage in premature patients.28 Bharadwaj et al.3 evaluated the neurodevelopment of 71 children and measured oxidative/antioxidant stress levels. They concluded that maternal TAC in PE is useful for predicting impaired motor development at one year of corrected age. Dietze et al.20 observed through salivary cortisol analysis that newborns whose mothers smoked more than ten cigarettes per day were at higher risk for IVH. IVH affects 30 to 60% of preterm infants and is characterized as white matter lesion due to microvascular events that occur in the germ matrix, putting the infant at increased risk for neurodevelopmental delays and additional brain damage.20
On the other hand, several studies did not confirm the association between oxidative stress biomarkers and the development of pathologies, morbimortality or prognosis. Moore et al.19 examined 31 premature newborns to evaluate the association between CLD and 8-OHdG levels. They observed that CLD was associated with lower levels of oxidative stress, which contradicts previous studies. Authors justify these conflicting results by different methodologies used in sample collection and analysis, alongside other additional factors that might have affected 8-OHdG levels. Dekker et al.24 evaluated 52 preterm infants for 8iPGF2α levels in order to find a higher risk of developing IVH, but no significant association was found. Norishadkam et al.4 compared 25 premature and 25 term newborns to verify the relationship between oxidative stress and DNA damage and also found no significant association. Stefanov et al.9 evaluated the relationship between glutathione, MDA, and endothelin-1 levels to analyze endothelial dysfunction in 63 infants born between 24 to 42 weeks. Still, there was no significant relationship between the two factors analyzed. Finally, Arman et al.23 evaluated the global oxidant and antioxidant status in newborns of mothers with and without PE; there was no statistical difference between groups. The absence of significant associations in these studies may be justified by clinical factors affecting biomarkers levels, the small sample size in most studies, and the complexity of the neonatal transition period, in which a not fully comprehended mixed-redox state might occur.9
The present study has some limitations. Although the utility of cord blood samples for the assessment of oxidative stress biomarkers and their possible clinical implications is evident, they cannot be used in follow-up analysis after birth. Alternative non-invasive monitoring options that have shown some positive results are newborn saliva and urine samples, which may be used for longitudinal follow-ups.29 Also, different methodologies, biomarkers, and the limited number of participants in each study make results unreliable when extrapolating to the general population of preterm babies.
An accurate assessment of oxidative damage and the possibility of targeted treatments might improve neonatal care.31 Currently, none of these biomarkers are used in clinical practice. However, further researches on the field might help to overcome the technical and economic barriers and enable their routine use. As a future perspective, in the next few years, diagnostic strategies directed to the identification of the risk of oxidative stress-related pathologies might be developed, and guidelines for their prevention and treatment might be updated, reducing the morbidity and mortality of premature newborns.
The authors of the present study conclude that the analysis of oxidative stress and antioxidant levels in cord blood of premature newborns might be useful in assessing the diagnosis and prognosis of some clinically relevant pathologies. Oxidative stress and antioxidant activity are involved in the pathophysiology of the development of several neonatal diseases, and their consequences are associated with increased short- and long-term morbidity, impaired neurodevelopment, and increased mortality. More information and research in the area are needed to impact these clinical outcomes.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Study conducted at the Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre, RS, Brazil.
References
- 1.World Health Organization. Preterm birth –19 February 2018. [Internet]. World Health Organization; c2018 [Cited 2021 Mar 5]. Available from: http://www.who.int/mediacentre/factsheets/fs363/en/
- 2.Organização Pan-Americana da Saúde. Organização Mundial da Saúde. OPAS Brasil Quase 30 Milhões de Recém-Nascidos Prematuros e Doentes Necessitam de Tratamento Para Sobreviver Todos os anos. [Internet]. Organização Pan-Americana da Saúde; c2018 [Cited 2021 Mar 5]. Available from: https://www.paho.org/bra/index.php?option=com_content&view=article&id=5821:quase-30-milhoes-de-recem-nascidos-prematuros-e-doentes-necessitam-de-tratamento-para-sobreviver-todos-os-anos&Itemid=820#:∼:text=De%20acordo%20com%20o%20relat%C3%B3rio,beb%C3%AAs%20que%20morrem%20nasceram%20prematuros
- 3.Bharadwaj SK, Bhat BV, Vickneswaran V, Adhisivam B, Bobby Z, Habeebullah S. Oxidative stress, antioxidant status and neurodevelopmental outcome in neonates born to pre-eclamptic mothers. Indian J Pediatr. 2018;85:351–357. doi: 10.1007/s12098-017-2560-5. [DOI] [PubMed] [Google Scholar]
- 4.Norishadkam M, Andishmand S, Zavar reza J, Sakhvidi MJ, Hachesoo VR. Oxidative stress and dna damage in the cord blood of preterm infants. Mutat Res. 2017;824:20–24. doi: 10.1016/j.mrgentox.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Pajai SS, Bezalwar AP. Oxidative stress in preterm neonates: an analysis of oxidative stress biomarkers and antioxidant profiles. J Clin Diagn Res. 2020;14 CC01-3. [Google Scholar]
- 6.Ozsurekci Y, Kubra A. Oxidative stress related diseases in newborns. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/2768365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrone S, Santacroce A, Longini M, Proietti F, Bazzini F, Buonocore G. The free radical diseases of prematurity: from cellular mechanisms to bedside. oxid med cell longev. 2018;2018 doi: 10.1155/2018/7483062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandyopadhyay T, Bhatia BD, Khanna HD. A study of oxidative stress in neonates delivered through meconium-stained amniotic fluid. Eur J Pediatr. 2017;176:317–325. doi: 10.1007/s00431-016-2845-0. [DOI] [PubMed] [Google Scholar]
- 9.Stefanov G, Briyal S, Pais G, Puppala B, Gulati A. Relationship between oxidative stress markers and endothelin-1 levels in newborns of different gestational ages. Front Pediatr. 2020;8:279. doi: 10.3389/fped.2020.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghany EA, Elhamed WA, Ali AA, Youness ER, Hussein JS. Antioxidant profiles and markers of oxidative stress in preterm neonates. Paediatr Int Child Health. 2016;36:134–140. doi: 10.1179/2046905515Y.0000000017. [DOI] [PubMed] [Google Scholar]
- 11.Falsaperla R, Lombardo F, Filosco F, Romano C, Saporito MA, Puglisi F, et al. Oxidative stress in preterm infants: overview of current evidence and future prospects. Pharmaceuticals. 2020;13:145. doi: 10.3390/ph13070145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkabany ZA, El-Farrash RA, Shinkar DM, Ismail EA, Nada AS, Farag AS, et al. Oxidative stress markers in neonatal respiratory distress syndrome: advanced oxidation protein products and 8-hydroxy-2-deoxyguanosine in relation to disease severity. Pediatr Res. [DOI] [PMC free article] [PubMed]
- 13.Perrone S, Tataranno ML, Negro S, Longini M, Toti MS, Alagna MG, et al. Placental histological examination and the relationship with oxidative stress in preterm infants. Placenta. 2016;46:72–78. doi: 10.1016/j.placenta.2016.08.084. [DOI] [PubMed] [Google Scholar]
- 14.Moore TA, Ahmad IM, Zimmerman MC. Oxidative stress and preterm birth: an integrative review. Biol Res Nurs. 2018;20:497–512. doi: 10.1177/1099800418791028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuffrè M, Rizzo M, Scaturro G, Pitruzzella A, Gammazza AM, Cappello F, et al. Oxidative stress markers at birth: Analyses of a neonatal population. Acta Histochem. 2015;117:486–491. doi: 10.1016/j.acthis.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Caldas JP, Vilela MM, Braghini CA, Mazzola TN, Marba ST. Antenatal maternal corticosteroid administration and markers of oxidative stress and inflammation in umbilical cord blood from very low birth weight preterm newborn infants. J Pediatr (Rio J) 2012;88:61–66. doi: 10.2223/JPED.2158. [DOI] [PubMed] [Google Scholar]
- 17.Slater L, Asmerom Y, Boskovic DS, Bahjri Plank MS, Angeles KR, et al. Procedural pain and oxidative stress in premature neonates. J Pain. 2012;13:590–597. doi: 10.1016/j.jpain.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendes KD, Silveira RC, Galvão CM. Integrative literature review: a research method to incorporate evidence in health care and nursing. Texto Contexto Enferm. 2008;17:758–764. [Google Scholar]
- 19.Moore TA, Schmid KK, Anderson-Berry A, Berger AM. Lung disease, oxidative stress, and oxygen requirements in preterm infants. Biol Res Nurs. 2016;18:322–330. doi: 10.1177/1099800415611746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietze TR, Rosea FF, Moore TA. Maternal variables associated with physiologic stress and perinatal complications in preterm infants. J Neonatal Perinatal Med. 2016;9:271–277. doi: 10.3233/NPM-16915134. [DOI] [PubMed] [Google Scholar]
- 21.Ozalkaya E, Karatekin G, Topçuoglu S, Karatepe HO, Taner Hafızoglu T, Baran P, et al. Neonatology oxidative status in preterm infants with premature preterm rupture of membranes and fetal inflammatuar response syndrome. Pediatr Neonatol. 2017;58:437–441. doi: 10.1016/j.pedneo.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Coutinho FG, Diniz EM, Ingrid K, Cianciarullo MA, Santos NR. Assessment of oxidative damage and enzymatic antioxidant system activity on the umbilical cord blood and saliva from preterm newborns with risk factors for early-onset neonatal sepsis. Rev Assoc Med Bras (1992) 2018;64:888–895. doi: 10.1590/1806-9282.64.10.888. [DOI] [PubMed] [Google Scholar]
- 23.Arman D, Ercin S, Topcuoğlu S, Kaya A, Yavuz T, Karatekin G, et al. The association between oxidative stress and cardiac functions in infants born to preeclamptic mothers. Am J Perinatol. 2019;36:1205–1210. doi: 10.1055/s-0038-1676481. [DOI] [PubMed] [Google Scholar]
- 24.Dekker J, Martherus T, Lopriore E, Giera M, McGillick EV, Hutten J, et al. The effect of initial high vs. low fio2 on breathing effort in preterm infants at birth: a randomized controlled trial. Front Pediatr. 2019;7:504. doi: 10.3389/fped.2019.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva AB, Medeiros JF, Lima MS, Mata AM, Andrade ED, Bezerra DS, et al. Intrauterine growth and the vitamin E status of full-term and preterm newborns. Rev Paul Pediatr. 2019;37:291–296. doi: 10.1590/1984-0462/;2019;37;3;00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Zheng B, Jiang Z, Wu S, Jin Q, Lin P, et al. Elevated cord blood oxidative stress biomarkers negatively affect neonatal outcomes of mothers with severe preeclampsia: a case-control study. BMC Pregnancy Childbirth. 2020;1:1–19. [Google Scholar]
- 27.Agrawal G, Dutta S, Prasad R, Dogra MR. Fetal oxidative stress, micronutrient deficiency and risk of retinopathy of prematurity: a nested case-control study. Eur J Pediatr. 2021;180:1487–1496. doi: 10.1007/s00431-020-03896-x. [DOI] [PubMed] [Google Scholar]
- 28.Coviello C, Perrone S, Buonocore G, Negro S, Longini M, Dani C, et al. Isoprostanes as biomarker for white matter injury in extremely preterm infants. Front Pediatr. 2021;8 doi: 10.3389/fped.2020.618622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peña-Bautista C, Durand T, Vigor C, Oger C, Galano JM, Cháfer-Pericás C. Non-invasive assessment of oxidative stress in preterm infants. Free Radic Biol Med. 2019;142:73–81. doi: 10.1016/j.freeradbiomed.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Casavant SG, Cong X, Fitch RH, Moore J, Rosenkrantz T, Starkweather A. Allostatic load and biomarkers of stress in the preterm infant: an integrative review. Review. Biol Res Nurs. 2019;21:210–223. doi: 10.1177/1099800418824415. [DOI] [PubMed] [Google Scholar]
- 31.Perrone S, Laschi E, Buonocore G. Oxidative stress biomarkers in the perinatal period: diagnostic and prognostic value. Semin Fetal Neonatal Med. 2020;25 doi: 10.1016/j.siny.2020.101087. [DOI] [PubMed] [Google Scholar]