Abstract
Objective
Emerging literature has described using venoarterial extracorporeal membranous oxygenation (ECMO) as a bridge to transplant or left ventricular assist device (LVAD) placement. We sought to identify the incremental cost-effectiveness ratio (ICER) of ECMO used as a bridge to cardiac transplant or LVAD.
Methods
Patients with refractory cardiogenic shock who received venoarterial ECMO and were bridged to either cardiac transplant (n = 7) or a HeartMate 3 LVAD (n = 6) placement were included. Markov modeling was used, comparing ECMO bridging with non–ECMO-bridged patients. Cohorts entered the model alive and at every 1-year cycle, were exposed to risk of death, and ran forward for 20 years after transplant or LVAD.
Results
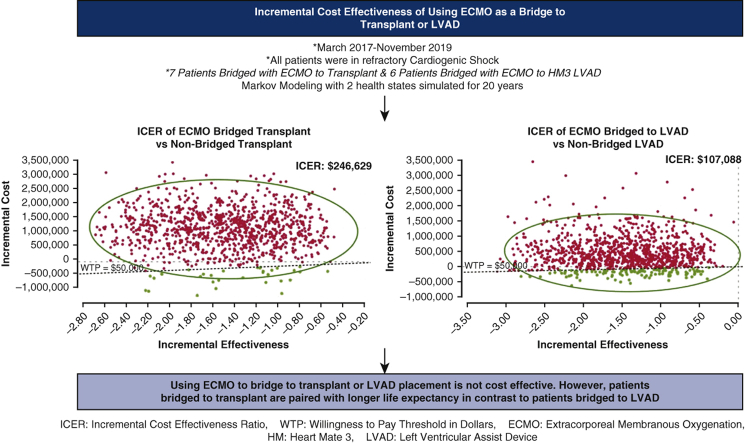
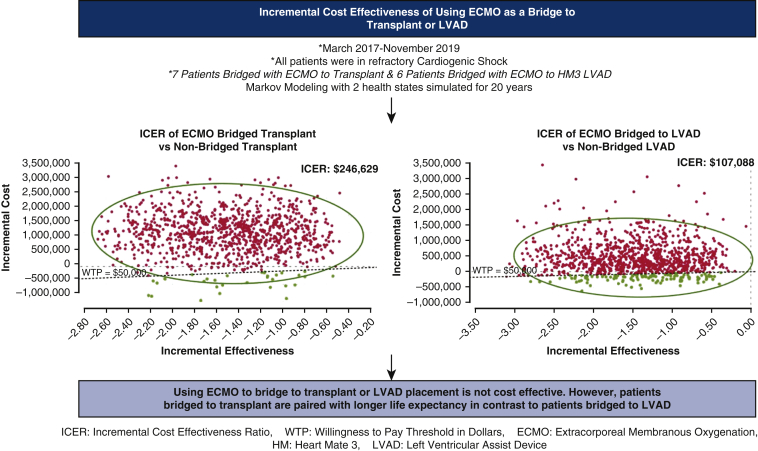
Patients bridged with ECMO to cardiac transplant were stratified as group 1 whereas those bridged with ECMO to LVAD were stratified as group 2. The average ECMO run was 3 days in group 1 versus 11 days in group 2. Among group 1 patients, the ICER was $246,629 but was paired with a longer life expectancy. The ICER of group 2 patients was –$107,088 and was not paired with a longer life expectancy. The average inpatient cost for group 1 was found to be $636,023 versus $769,471 for group 2 patients. The average inpatient costs for patients not bridged to ECMO who received cardiac transplant or LVAD was $538,928 and $325,242, respectively.
Conclusions
Using ECMO to bridge to transplant or LVAD placement is not cost effective. However, patients bridged to transplant are paired with longer life expectancy in contrast to patients bridged to LVAD.
Key Words: ECMO, transplant, LVAD, MCS
Abbreviations and Acronyms: ECMO, extracorporeal membranous oxygenation; HM3, HeartMate 3; ICER, incremental cost-effectiveness ratio; ICU, intensive care unit; LVAD, left ventricular assist device; MCS, mechanical circulatory support; NYHA, New York Heart Association; QALY, quality-adjusted life year; VA, venoarterial
Graphical abstract
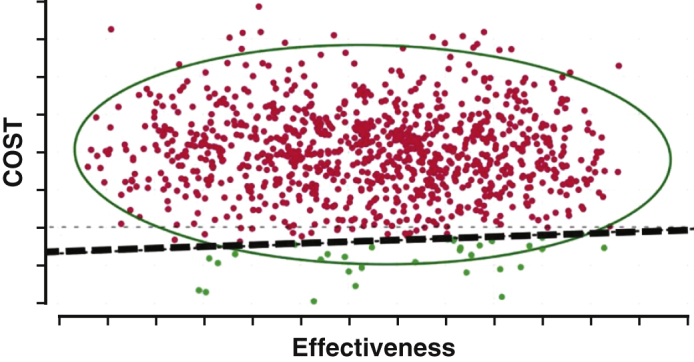
Bridging patients with ECMO to transplant or LVAD is above the cost-effective threshold.
Central Message.
Bridging patients in refractory cardiogenic shock with VA-ECMO to transplant or LVAD is not cost effective, and improved life expectancy among transplanted patients is not shared by LVAD patients.
Perspective.
Complex clinical questions are encountered when managing patients with refractory cardiogenic shock on VA-ECMO, with cost-effectiveness being an important consideration. Bridging patients to transplant or LVAD is not cost effective. In contrast to ECMO bridged transplant, a shorter life expectancy after bridging to LVAD compounds the negative impact on cost-effectiveness.
The incidence of heart failure continues to increase in the United States, leading to a significant financial burden for the health care system.1,2 Despite improvements in medical therapy, hospitalization resulting from decompensated heart failure continues to carry significant mortality and can exceed 70% in those with refractory cardiogenic shock.3 Advances in biomedical engineering have led to the development of technology enabling the rescue of patients with refractory cardiac failure, including percutaneous devices, implantable continuous-flow ventricular assist devices, and extracorporeal membranous oxygenation (ECMO). While cardiac transplant remains the gold standard for selected patients, the scarcity of this resource remains a paramount factor in providing a durable intervention to prolong life in these patients.4
In the dramatic setting of rapid cardiac decompensation, venoarterial (VA) ECMO has been increasingly employed as a therapeutic intervention to treat acute cardiac failure, potentially as a bridge to recovery or continuous-flow ventricular assist device placement.5, 6, 7, 8 Conversely, the application of VA-ECMO used as a bridge to orthotopic heart transplant has not demonstrated consistent benefit, with recent retrospective data demonstrating an increased risk of early mortality.4
Therapeutic advancements such as ECMO are expensive and require specialized care teams in specialized intensive care units (ICUs) as well as specialized equipment. Over the last several years, cost-utility evaluations of VA-ECMO have begun to populate the literature, describing various clinical scenarios and patient populations in which ECMO has been applied. However, fiscal evaluation has been challenged by multiple factors, including variability in economic modeling, heterogeneity of both the study population, and device implanted.9 In the pediatric population, which comprises the largest proportion of ECMO volume, more robust financial analyses are available, and some of these reports have described the cost-effectiveness of ECMO as a bridge to heart transplant.10,11 However, economic analysis in which VA-ECMO has been used as a bridge to either cardiac transplant or LVAD has not been thoroughly explored in the adult population. The purpose of our study is to report on the incremental cost-effectiveness ratio (ICER) of ECMO used as a bridge to transplant or LVAD with a single device among adult patients with acute heart failure.
Methods
We retrospectively analyzed a prospectively maintained database of patients who were treated at AdventHealth Orlando between March 2017 and November 2019 (Table E1). Patients with refractory cardiogenic shock who required VA-ECMO and were bridged to either HeartMate 3 (HM3) LVAD (Abbott) or cardiac transplant were evaluated. Patients in postcardiotomy shock were excluded. Patients not bridged to ECMO who underwent transplant during the same month as a patient bridged to ECMO were used as a comparison group for the model. A similar strategy was used to select the non–ECMO-bridged HM3 LVAD comparison group. Patients who received intra-aortic balloon pump support were considered medically managed for our model. Patients undergo multidisciplinary review before being offered LVAD or transplant. Data were collected from review of the medical record and from the financial analysists at the institution. After review, informed written consent was deemed not required. The institutional review board at AdventHealth Orlando approved this study protocol and publication of the study data (institutional review board #1517126-1, approved November 14, 2019).
Model Structure
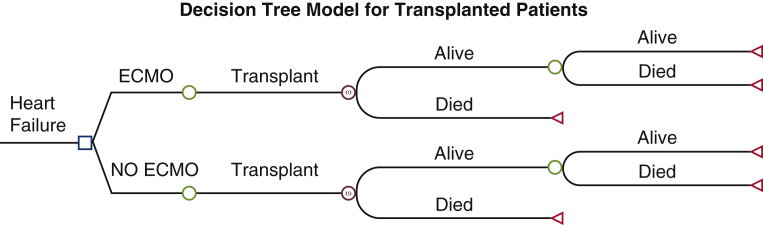
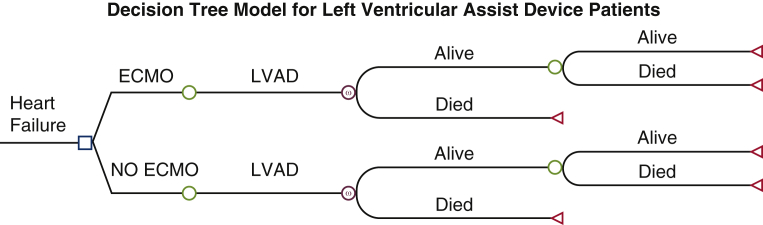
We employed a Markov model to estimate health outcomes and costs for patients who underwent heart transplantation or LVAD placement. Among the transplant patients, some were bridged with VA-ECMO before transplantation. Similarly, some patients were bridged with VA-ECMO to LVAD placement. For each patient population (transplant or LVAD), we compared patients bridged with ECMO with non-ECMO bridged patients. These non-bridged patients were not in cardiogenic shock and underwent transplant or LVAD during the study period. The structure used 2 health states: “alive” and “dead” for the model (Figures E1 and E2). Cohorts entered the model alive and at every 1-year cycle were exposed to risk of death. The Markov model applied variable mortality rates every cycle and ran forward for 20 years after transplantation or LVAD placement, using a half-cycle correction to increase the accuracy of the estimates. The model does not directly account for death after cannulation. Costs and quality-adjusted life year (QALY) weights were modeled in a time varying manner where appropriate. Discounting of health outcomes and costs was applied at 3%. All cost estimates used in analysis, including those from previous studies, were adjusted to reflect 2020 prices using the consumer price index for medical care. The model was performed in TreeAge Pro Healthcare 2021 Software.
Figure E1.
Decision tree modeling clinical course of transplanted patients. The square represents the decision point where extracorporeal membranous oxygenation (ECMO) is used or not. The circle represents the downstream consequences of the decision. The triangle represents the cost and effects end of the pathway.
Figure E2.
Decision tree modeling clinical course of patients receiving left ventricular assist device placement (LVAD). The square represents the decision point where extracorporeal membranous oxygenation (ECMO) is used or not. The circle represents the downstream consequences of the decision. The triangle represents the cost and effects end of the pathway.
Model Inputs
Costs
Costs occurred before discharge of transplant or LVAD placement were obtained from a review of patients treated between 2017 and 2019. These inpatient costs included ICU cost, ward cost, cost for transplant or LVAD, etc.
Costs occurred after discharge following transplantation or LVAD placement were obtained from previous studies.12 The estimated annual costs after cardiac transplant were $155,512 for the first year and $34,905 afterword. The estimated annual costs after LVAD placement were $164,831 for the first year and $46,835 afterword. The estimated cost of death was $62,324 due to end-of-life care (Table 1).
Table 1.
Model parameters
| Variable | Value | Reference |
|---|---|---|
| Cost∗ | ||
| Transplant | ||
| Inpatient costs for ECMO-bridged transplant | $636,023 | Patient-level data |
| Inpatient costs for non–ECMO-bridged transplant | $538,928 | Patient-level data |
| LVAD | ||
| Inpatient costs for ECMO-bridged LVAD | $769,471 | Patient-level data |
| Inpatient costs for non–ECMO-bridged LVAD | $325,242 | Patient-level data |
| Annual cost after transplant | ||
| Year 1 | $155,512 | Long and colleagues, 201412 |
| Year 2 and beyond | $34,905 | Long and colleagues, 201412 |
| Annual cost after LVAD | ||
| Year 1 | $164,831 | Long and colleagues, 201412 |
| Year 2 and beyond | $46,835 | Long and colleagues, 201412 |
| End-of-life care costs | $62,324 | Long and colleagues, 201412 |
| QALY weights | ||
| Postheart transplant | ||
| NYHA I | 0.90 | Göhler and colleagues, 200913 |
| NYHA II | 0.83 | Göhler and colleagues, 200913 |
| NYHA III | 0.74 | Göhler and colleagues, 200913 |
| NYHA IV | 0.60 | Göhler and colleagues, 200913 |
| Post-LVAD | ||
| ECMO-bridged QALY | 0.82 | Unai and colleagues, 201714 |
| Non–ECMO-bridged QALY | 0.79 | Unai and colleagues, 201714 |
| Survival | ||
| Years after transplant | ||
| ECMO-bridged survival | ||
| 1 Year | 0.707 | DeFilippis and colleagues, 202115 |
| 2 Year | 0.666 | DeFilippis and colleagues, 202115 |
| 5 Year | 0.618 | DeFilippis and colleagues, 202115 |
| Non–ECMO-bridged survival | ||
| 1 Year | 0.92 | Mishra and colleagues, 201716 |
| 3 Year | 0.87 | Mishra and colleagues, 201716 |
| 5 Year | 0.81 | Mishra and colleagues, 201716 |
| 7 Year | 0.77 | Mishra and colleagues, 201716 |
| Years after LVAD placement | ||
| ECMO-bridged survival | ||
| 1 Year | 0.692 | DeFilippis and colleagues, 202115 |
| 2 Year | 0.626 | DeFilippis and colleagues, 202115 |
| 5 Year | 0.565 | DeFilippis and colleagues, 202115 |
| Non–ECMO-bridged survival | ||
| 1 Year | 0.88 | Han and colleagues, 201817 |
ECMO, Extracorporeal membranous oxygenation; LVAD, left ventricular assist device; QALY, quality-adjusted life years; NYHA, New York Heart Association.
Cost in 2020 US dollars.
Healthcare Utilities
Health outcomes were measured in QALYs, which jointly determined by survival and health care utilities represented by QALY weights. We determined the QALY weights of patients who had heart transplant by using the relationship between the EuroQol 5-dimensions and New York Heart Association (NYHA) information as reported by Göhler and colleagues.13 NYHA class was recorded at the first follow-up after transplant. For patients who had LVAD, the QALY weights were based on the measurements from the clinical trial in Unai and colleagues.14 The utility weights were generated on a scale of zero to one, where zero indicated extremely poor life quality and one indicated perfect health. The post-LVAD QALY weighs of ECMO bridged patients and non-ECMO bridged patients were 0.82 and 0.79, respectively.
Survival Probabilities
We employed published 5-year survival data following cardiac transplant patients in patients who were bridged with ECMO and 7-year survival data for transplant patients who were not bridged. Similarly, we employed 5-year survival estimates for LVAD patients who were not bridged with ECMO, and 1-year survival estimates for LVAD patients who were also not bridged with ECMO. Survival probabilities for transplant patients and LVAD patients who were bridged with ECMO were obtained from the most recent findings published by DeFilippis and colleagues.15 Survival probabilities for transplant patients and LVAD patients who were not bridged with ECMO were obtained from Mishra and colleagues. and Han and colleagues, respectively.16,17 This approach allowed us to estimate the impact of ECMO as a bridge of transplant or LVAD more accurately. We assumed that the mortality rates after the data available periods were consistent with the US Actuarial Life Table published by Social Security Administration. The posttransplant survival probabilities of ECMO patients for years 1 through 5 were 70.7%, 66.6%, and 61.8%, respectively. The posttransplant survival probabilities of non-ECMO bridged patients for years 1, 3, 5, and 7 were 92%, 87%, 81%, and 77%, respectively. The post-LVAD survival probabilities of ECMO bridged patients for years 1, 2, and 5 were 69.2%, 62.6%, and 56.5%, respectively. The post-LVAD survival probability of non-ECMO bridged patients for 1 year was 88%.
Analysis Results
Our cohort came from real-world patients treated at our institution. Patients with cardiac failure who were bridged with ECMO and then underwent cardiac transplant were considered group 1 (n = 7). Patients who were treated with ECMO and then received placement of an LVAD comprised group 2 (n = 6). These groups informed the inputs form cost of care in our model. The average age in these groups was 47.6 and 49.3 years, respectively.
As shown in Table 2, the ICER of using ECMO as a bridge to transplant was $246,629. To be specific, the cost per QALY gained among the ECMO patients was $153,266 (measured by $1,909,702/12.46 QALY), whereas it was $131,913 (measured by $1,338,917/10.15 QALY) for the non-ECMO bridged group. Cost-effective analysis results for the LVAD patient groups are reported in Table 2. The cost per QALY gained among the ECMO bridged patients was $299,198 (measured by $2,028,565/6.78 QALY), whereas it was $129,157 (measured by $1,505,979/11.66 QALY) for the non-ECMO group. Therefore, the ICER of using ECMO as a bridge to LVAD was –$107,088.
Table 2.
Cost-effectiveness of bridge to transplant or LVAD
| Strategy | Cost | QALY | IC | IE | ICER (IC/IE) |
|---|---|---|---|---|---|
| ECMO bridge to transplant | $1,909,702 | 12.46 | $570,785 | 2.31 | $246,629 |
| Non-bridge to transplant | $1,338,917 | 10.15 | – | – | – |
| ECMO bridge to LVAD | $2,028,565 | 6.78 | $522,585 | –4.88 | –$107,088 |
| Non-bridge to LVAD | $1,505,979 | 11.66 | – | – | – |
QALY, Quality-adjusted life years; IC, incremental cost; IE, incremental effectiveness; ICER, incremental cost-effectiveness ratio; ECMO, extracorporeal membranous oxygenation; LVAD, left ventricular assist device.
Sensitivity Analysis
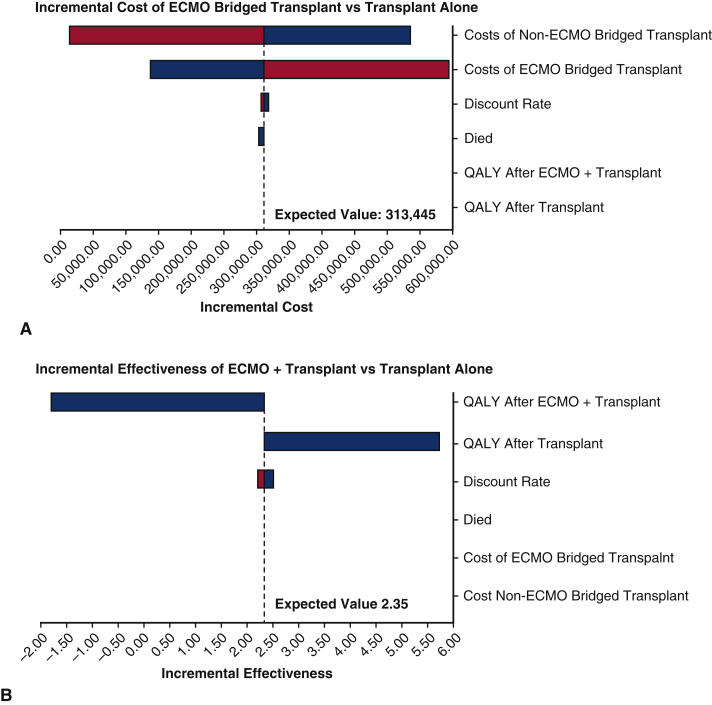
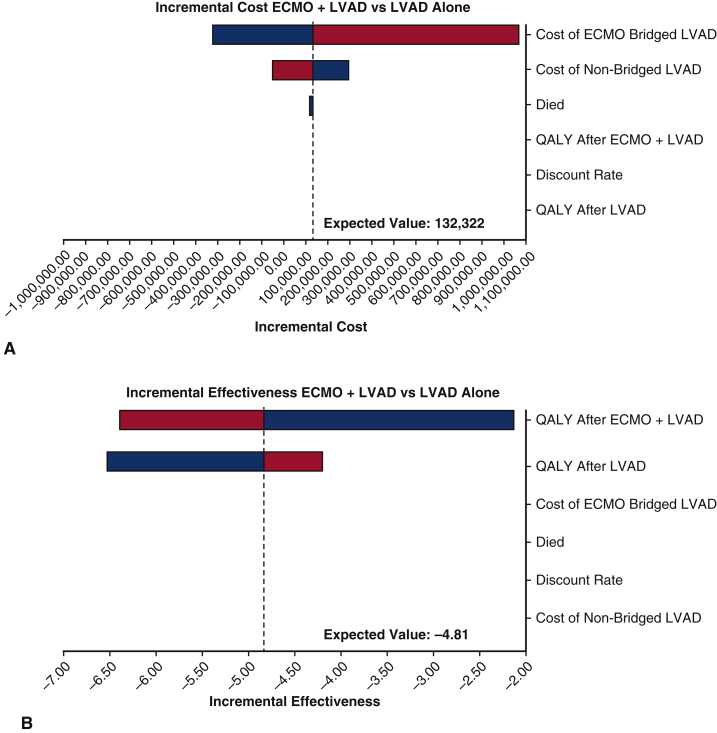
We performed a series of sensitivity analyses to check the robustness of the results. We ran the first-order simulation trials in a sensitivity analysis for 1000 samples, with stabilization of random walks across strategies. Variables included in the sensitivity analysis were discount rate, end-of-life care cost, inpatient costs for patients who had ECMO and subsequent transplant, inpatient costs for patients who did not receive ECMO but were transplanted, inpatient costs for patients who were bridged with ECMO to LVAD, inpatient costs for patients who were not ECMO bridged to LVAD, posttransplant QALY weights, and post-LVAD QALY weights. We assumed that the low and high values for discount rate were 2% and 4%, respectively. The range of the end-of-life care cost was between $0 and $62,324. Details for other parameters used in the sensitivity analysis are shown in Table 3. One-way sensitivity analyses were conducted for transplant patients and LVAD patients, respectively, as plotted in the tornado diagrams (Figures 1, A and B and 2, A and B). The variables were ordered by their influences on the incremental cost and the incremental effectiveness. That is, variables that caused the most variation in the incremental value are shown on the top, followed by the second largest change, and so on. Bars are colored by variable range. The expected value lines represent the incremental value between the ECMO and non-ECMO strategies using the base-case value for each variable. According to Figure 1, A, the inpatient costs of the patients who had transplant without using ECMO had the greatest impact on the incremental cost. Similarly, the incremental effectiveness of using ECMO as a bridge to transplant was largely affected by the posttransplant QALY weight, as shown in Figure 1, B. Figure 2, A and B, show similar results for comparison between using ECMO as a bridge of LVAD and using LVAD only. The inpatient costs of the patients who were not ECMO bridged to LVAD had the greatest impact on the incremental cost, whereas the incremental effectiveness of using ECMO as a bridge to LVAD was largely affected by the post-LVAD QALY weight.
Table 3.
Parameters for sensitivity analysis
| Variable | Value | Reference |
|---|---|---|
| Cost∗ | ||
| Transplant | ||
| Inpatient costs for ECMO-bridged transplant | Mean: $636,023 SD: $166,369 Min: $461,201 Max: $917,322 |
Patient-level data |
| Inpatient Costs for non–ECMO-bridged transplant | Mean: $538,928 SD: $157,331 Min: $317,404 Max: $836,799 |
Patient-level data |
| LVAD | ||
| Inpatient costs for ECMO-bridged LVAD | Mean: $769,471 SD: $500,431 Min: $317,201 Max: $1708,318 |
Patient-level data |
| Inpatient Costs for non–ECMO-Bridged LVAD | Mean: $325,242 SD: $114,812 Min: $160,363 Max: $504,643 |
Patient-level data |
| Survival after transplant | ||
| Years after transplant | ||
| ECMO-bridged survival | ||
| 1 Year | 0.70 | Mishra and colleagues, 201716 |
| 2 Year | 0.70 | Mishra and colleagues, 201716 |
| 5 Year | 0.70 | Mishra and colleagues, 201716 |
| 1 Year | 0.70 | Mishra and colleagues, 201716 |
| Non–ECMO-bridged survival | 0.86 | Long and colleagues, 201412 |
| 1 Year | 0.82 | Long and colleagues, 201412 |
| 3 Year | 0.79 | Long and colleagues, 201412 |
| 5 Year | 0.66 | Long and colleagues, 201412 |
| 7 Year | 0.86 | Long and colleagues, 201412 |
| Years after LVAD placement | ||
| ECMO-bridged survival | ||
| 1 Year | 0.77 | Han and colleagues, 201817 |
| Non–ECMO-bridged survival | ||
| 1 Year | 0.77 | Long and colleagues, 201412 |
| 2 Year | 0.62 | Long and colleagues, 201412 |
ECMO, Extracorporeal membranous oxygenation; SD, standard deviation; LVAD, left ventricular assist device.
Cost in 2020 Dollars.
Figure 1.
A, Tornado diagram analysis of influential parameters affecting incremental cost among transplanted patients. The tornado diagram is a one-way sensitivity analysis that demonstrates the range of incremental cost-effectiveness. The variables depicted were ordered by their influence on the incremental cost with the most influential listed at the top. The range is a colored bar where blue represents the parameter range from the low uncertainty value to the base value, and red represents the parameter range from the base value to the high uncertainty value. The expected value (EV) lines represent the incremental value between the extracorporeal membranous oxygenation (ECMO) and non-ECMO strategies among transplanted patients using the base case value for each variable. Among non–ECMO-bridged transplant patients, the incremental value decreases as the parameter increases (from blue to red), whereas for ECMO-bridged transplant patients, the incremental value increases as the parameter value increases (from blue to red). B, Tornado diagram analysis of influential parameters affecting incremental effectiveness among transplanted patients. The tornado diagram is a one-way sensitivity analysis that evaluates the potential impact of incremental cost-effectiveness of one variable while the others are held constant, determining which variable has the greatest potential impact on cost-effectiveness. The variables listed were ordered by their influence on the incremental cost with the most influential listed at the top. The expected value (EV) lines represent the incremental value between the extracorporeal membranous oxygenation (ECMO) and non-ECMO strategies among transplanted patients using the base case value for each variable. In this figure, quality adjusted life-years (QALY) after ECMO bridged transplant was the most influential and the incremental value increases as the parameter value increases toward the base value.
Figure 2.
A, Tornado diagram analysis of influential parameters affecting incremental cost among patients who received a left ventricular assist device (LVAD). The tornado diagram is a one-way sensitivity analysis that evaluates the potential impact of incremental cost-effectiveness of one variable while the others are held constant, determining which variable has the greatest potential impact on cost-effectiveness. The variables listed were ordered by their influence on the incremental cost with the most influential listed at the top. The expected value (EV) lines represent the incremental value between the extracorporeal membranous oxygenation (ECMO) and non-ECMO strategies among patients who received an LVAD using the base case value for each variable. In this figure, inpatient costs among ECMO-bridged patients were the most influential where the incremental value increases as the parameter increases (from blue to red); while for the cost of non-bridged LVAD, the incremental value decreases as the parameter increases (from blue to red). This finding is the opposite of the effect observed in ECMO bridged transplant patients. B, Tornado diagram analysis of influential parameters affecting incremental effectiveness among patients who received LVAD. The tornado diagram is a one-way sensitivity analysis that evaluates the potential impact of incremental cost-effectiveness of one variable while the others are held constant, determining which variable has the greatest potential impact on cost-effectiveness. The variables listed were ordered by their influence on the incremental effectiveness with the most influential listed at the top. The EV lines represent the incremental value between the ECMO and non-ECMO strategies among patients who received an LVAD. In this figure, quality adjusted life-years (QALY) after ECMO bridged LVAD placement was the most influential where the incremental value decreases as the parameter increases (from blue to red).
Discussion
In the United States, the most frequent reason for hospital admission is heart failure and, in parallel, there has been an increasing number of patients who require mechanical circulatory support (MCS).8,18 Costs associated with providing MCS support can exceed $200,000, with fewer than 25% of patients ultimately being discharged from the inpatient setting.8,19 In this analysis, we used patient-level data to estimate the cost-effectiveness of using ECMO as a bridge to cardiac transplant or LVAD placement.
ECMO Bridging to Cardiac Transplant
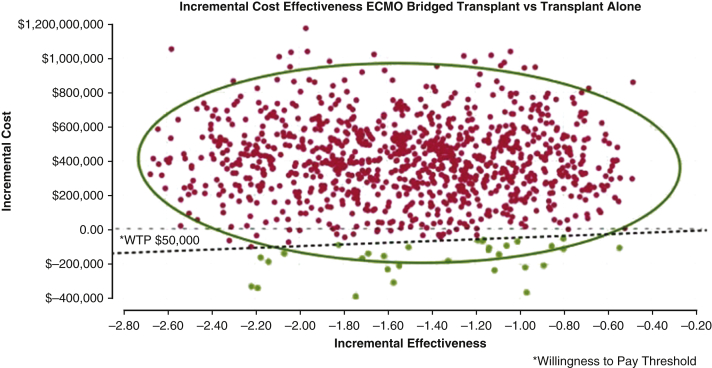
There is a paucity of data evaluating the clinical long-term and economic outcomes in this population, as employing ECMO as a bridge to transplant or LVAD placement is not widely practiced in the United States.4,20 We identified the ICER of ECMO as a bridge to transplant to be $246,629, which is more than twice the ICER of $94,000-$97,000 reported for non-ECMO bridged cardiac transplant patients.1,12 Historically, the willingness-to-pay threshold has been estimated to be $50,000.1,21 Using this value as base reference, we found bridging patients to durable intervention (transplant or LVAD) was not cost effective (Figures E3 and E4). This is demonstrated in Figure 3. Accounting for changes in the gross domestic product, updated cost-effectiveness has been suggested to range between $100,000 and $120,000/QALY.22 Although there is no previously published data against which to compare this value, bridging patients with ECMO to transplant is not cost effective even when measured with more contemporary cost-effectiveness estimates. However, this finding is important, as it estimates economic parameters that can add to the complex discussion about rescuing patients with refractory cardiogenic shock who are reliant on ECMO. The largest contributors to costs associated with providing ECMO support are related to specialized care teams and ICU stay rather than ECMO itself.21,23,24 Therefore, one strategy to improve costs may be to reduce time on ECMO. Data from a European series reported patients received ECMO a median of 9 days before transplant, where we observed a median of 3 days, which likely demonstrates the impact of the recent organ allocation changes enacted in October 2018 as we analyzed patients transplanted after this transition in policy.16,20 In our data, the mean length of stay after cardiac transplant in ECMO bridged and non-bridged patients was 21 days versus 24 days, respectively. From a policy perspective, it appears that the recent changes in transplant allocation have had a positive effect on reducing the duration of ECMO prior to transplant while maintaining similar posttransplant length of stay.
Figure E3.
Monte Carlo scatter plot: incremental cost-effectiveness of ECMO bridged transplant versus transplant alone. Monte Carlo plots show repeated random samples from the model are depicted by one dot, which represents the average quality adjusted life year (QALY) gained and the incremental cost of that sampling based on the model. The 0-value horizontal line demarks the point above which, the intervention is not cost effective. The inclining line represents the willingness to pay (WTP) threshold. Above this line, the intervention is efficacious but not cost effective. The red dots demonstrate the simulated incremental cost estimate above the willingness to pay threshold of $50,000 whereas the green dots demonstrate estimates below the WTP threshold. The scale represented by dollar amount along the Y axis among the transplanted patients is narrower than for left ventricular assist device patients. Fewer simulations identified incremental cost estimates below the willingness to pay threshold in contrast to those that exceed it. ECMO, Extracorporeal membranous oxygenation.
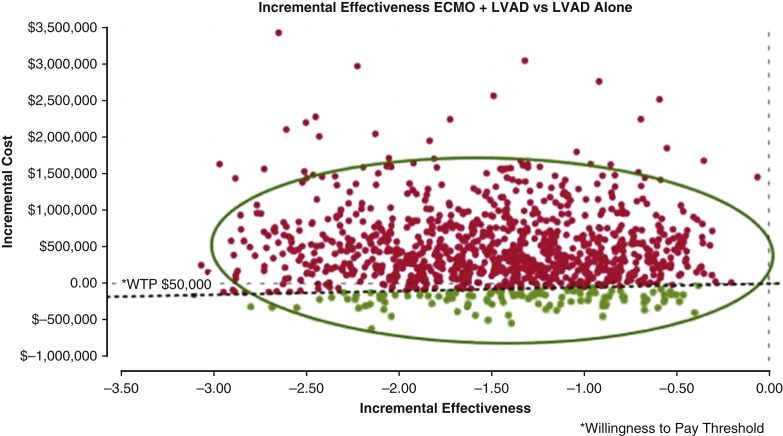
Figure E4.
Monte Carlo scatter plot: incremental cost-effectiveness of ECMO bridged left ventricular assist device (LVAD) versus LVAD alone. Monte Carlo plots show repeated random samples from the model are depicted by one dot, which represents the average quality-adjusted life year (QALY) gained and the incremental cost of that sampling based on the model. The 0-value horizontal line demarks the point above which, the intervention is not cost effective. The inclining line represents the willingness to pay (WTP) threshold. Above this line, the intervention is efficacious but not cost effective. The red dots demonstrate the simulated incremental cost estimate above the WTP threshold of $50,000, whereas the green dots demonstrate estimates below the WTP threshold. Fewer simulations identified incremental cost estimates below the WTP threshold in contrast to those that exceed it. The scale represented by dollar amount along the Y axis among the patients who received and LVAD is broader than for the transplant patients. ECMO, Extracorporeal membranous oxygenation.
Figure 3.
Between March 2017 and November 2019, 7 patients with refractory cardiogenic shock cannulated on venoarterial extracorporeal membranous oxygenation (VA-ECMO) were bridged to cardiac transplant. Six patients with refractory cardiogenic shock were cannulated on VA-ECMO and bridged to left ventricular assist device placement (LVAD) with a HeartMate 3 (HM3) device. Markov modeling was used to estimate the incremental cost-effectiveness of bridging patients with cardiogenic shock to transplant or LVAD placement. In both scenarios, bridging patients with VA-ECMO who are in refractory cardiogenic shock to transplant or LVAD placement was not cost effective, with cost effective estimates above the willingness to pay willingness-to-pay (WTP) threshold of $50,000. ICER, Incremental cost-effectiveness ratio.
The main determinant of outcome estimates in this analysis is survival. Identifying a population of similar patients in published literature is challenging, given the limited number of patients bridged to transplant with ECMO. Fukuhara in 2018 reported on 107 patients selected from the United Network of Organ Sharing Thoracic Registry, finding increased early and midterm survival whereas data from Europe analyzing survival after ECMO bridging to transplant found 1-year posttransplant survival of 70%.4,16,20 The more recent paper by DeFilippis and colleagues15 evaluating ECMO patients bridged to transplant or LVAD has also demonstrated 1-year and 5-year survival estimates of 70% and 61.8%. To improve the accuracy of our analysis, we used these more contemporary estimates of posttransplant survival after ECMO bridging to generate the survival estimates for our model. In addition, while bridging to transplant is not cost effective, this strategy appears to be paired with improved life expectancy.
Finally, our model employed NYHA classification to estimate quality of life among patients with heart failure. We excluded other causes for cardiogenic shock such as postcardiotomy or extracorporeal life support, creating a more homogenous population for analysis. Other authors evaluating cost-effectiveness have employed the EuroQol 5-dimensions questionnaire to estimate quality of life after ECMO.25,26 This questionnaire evaluates 5 dimensions from which QOL estimates are generated. However, accuracy of the estimated utility and subsequent cost-effectiveness estimates may be negatively impacted by recall bias associated with data obtained from questionnaires, compromising the accuracy of the estimated ICER. To reduce the possibility of this bias in our analysis, we used NYHA class to estimate heart failure, which has been linked to utilities when used as health states in Markov modeling.13 The NYHA class was designated by the treating posttransplant heart failure specialist and obtained from the medical record.
ECMO Bridging to LVAD
The rate of LVAD implantation has increased significantly overtime.15 Evolution in technology, patient selection, and clinical experience has resulted in improved survival after device implantation, which has translated into improved cost-effectiveness over time.2,27 The previous estimates by Long and colleagues12 reported $206,300/QALY for patients bridged to transplant with an LVAD versus $198,184 to $802,700/QALY in destination therapy patients. Baras Shreibati and colleagues27 recently reported an ICER of $209,400 in ambulatory patients with inotrope dependent heart failure who underwent LVAD placement. However, comparing these cost-effectiveness estimates with our results is limited, as these reports estimate cost-effectiveness in non-ECMO bridged patients. As with estimating the cost-effectiveness of ECMO as a bridge to cardiac transplant, there are no data available to compare our estimate of the cost-effectiveness of ECMO as a bridge to HM3 LVAD placement.
Disease states used in this analysis were alive and dead, and thus survival after LVAD placement was the main determinant in estimating cost-effectiveness. In contrast to the clinical outcomes reported after bridging with ECMO to transplant, the outcomes after ECMO bridging to LVAD have been less favorable.15,16,28 Han and colleagues17 in 2018 compared 18 Interagency Registry for Mechanically Assisted Circulatory Support-1 patients who were bridged to LVAD with VA-ECMO to 17 Interagency Registry for Mechanically Assisted Circulatory Support-1 patients who received LVAD placement who did not require ECMO. One-year survival was 88% but was bolstered by the fact that approximately 50% of the remaining patients in each group underwent transplant during the follow-up period. Similar findings were reported by Unai and colleagues.14 In both series, multiple devices were implanted, challenging interpretation of their data. Goldstein and colleagues29 recently reported on 317 destination therapy patients in the Multi-center Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 (MOMENTUM 3), observing a 2-year survival of just greater than 60% after HM3 implantation. The possibility of cardiac transplantation in this population has reduced the number of patients available for long-term survival analysis. Recently, DeFilippis and colleagues15 addressed this issue. This group evaluated 587 patients who were bridged to LVAD with VA-ECMO between 2006 and 2019, reporting 1-, 2-, and 5-year survival to be 69%, 62.6%, and 56.5%, respectively. When adjusting for those who received cardiac transplant, they found no significant difference in mortality after LVAD placement in ECMO-bridged patients. In our model, we identified that patients bridged to LVAD had greater costs but did not have a longer life expectancy in contrast to what we observed in the ECMO bridged to transplant patients, which is reflected by the negative value of the ICER in the ECMO bridged to LVAD patients.
In the context of refractory cardiogenic shock, cannulation onto VA-ECMO in patients with refractory cardiogenic shock as a bridge to cardiac transplant or LVAD placement may have acceptable long-term outcomes.15 Improving the short-term survival of patients bridged to LVAD increases the opportunity for orthotopic heart transplant in the following months, bolstering long-term survival.30,31
We found that ECMO bridging to transplant was not cost effective and did not impact posttransplant length of stay when compared with the posttransplant length of stay of non–ECMO -bridged transplant patients. This suggests that pretransplant care may have a significant influence on overall cost. The reduction in time on ECMO before transplant may be reflections of the recent changes in organ allocation when compared to data from Europe. Similarly, we also found bridging patients with VA-ECMO to LVAD was not cost effective. In contrast to ECMO-bridged transplant patients, the additional costs incurred with ECMO bridging to LVAD were not paired with longer life expectancy, negating the ICER. This is not surprising in that even among patients who undergo LVAD placement in the absence of ECMO bridging, published data suggests LVAD placement is not considered cost-effective.27
Health care costs for the management of heart failure will continue to increase and are expected to approach 70 billion dollars annually, by 2030.32 Estimating cost-effectiveness in treating patients with advanced heart failure including medical and procedural/surgical management is challenged by the impact of confounding variables on outcomes. For example, Urbich and colleagues32 demonstrated that comorbidities are a stronger predictor of costs than reduced ejection fraction. However, when hospitalization is factored in, elevated costs are driven primarily by recurrent hospitalization. The competing variables of comorbidity, degree of heart failure, readmission, age, etc, challenge the ability to estimate cost-effectiveness of medical versus surgical treatment of patients with heart failure.
This study has several limitations. While the cost estimates reflect real-world values, the number of patients studied may underestimate the variability of hospital charges associated with the care of this subpopulation of patients. Our model endeavored to simulate cost-effectiveness among a specific group of patients with cardiogenic shock. The generalizability of our results may be reduced due the limitations incurred from developing a model from a small number of patients. In addition, there is limited data describing functional outcomes such as ability to live independently after HM3 placement and such data would be helpful to build a more accurate model. However, we built a model that reflected outcomes specific to the HM3 LVAD as different MCS devices have different complication profiles, which may impact quality of life. We also included patients with a narrowed clinical picture to reduce the heterogeneity which has been a source of criticism of other reports on this topic. We observed the posttransplant and LVAD hospitalization length of stay were similar to patients who were admitted from home for both these interventions, which suggests the increases in cost when comparing bridged and non-bridged patients may occur in the preintervention phase of hospitalization, although we were unable to specifically characterize this impact. However, despite these challenges, we have identified the ICER of ECMO employed as a bridge to cardiac transplant or LVAD, for which limited data exist.
In conclusion, we observed that employing ECMO as a bridge to direct cardiac transplant or LVAD placement is not cost-effective. There appears to be a reduction in the time to transplant after ECMO cannulation, although the costs associated with ECMO itself may contribute to less than 10% of overall costs incurred during hospitalization.21 While either approach is not cost-effective, our results are consistent with other non–cost-effective extraordinary life-saving interventions that employ ECMO, as observed in patients undergoing lung transplant.33 While ECMO has been shown to be cost-effective in some scenarios (Table E2), parity has not been demonstrated among patients bridged to transplant or LVAD.34
While cost-effectiveness models ascribe value to intervention, further analysis of the factors that drive costs may be more important than the estimated ICER itself. Identifying the areas of care in which excessive cost is incurred can further innovation to reduce cost and improve quality of life.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix E1
Table E1.
Demographics
| ECMO indication | Age, y | Days on ECMO | INR | CRRT | INTERMACS | LOS after surgery, d |
|---|---|---|---|---|---|---|
| Transplant | ||||||
| Cardiogenic shock | 18 | 5 | 1.3 | No | 1 | 25 |
| Cardiogenic shock | 56 | 1 | 1.01 | No | 1 | 27 |
| Cardiogenic shock | 58 | 3 | 1.22 | Yes | 1 | 32 |
| Cardiogenic shock | 54 | 4 | 1.43 | No | 1 | 17 |
| Cardiogenic shock | 59 | 3 | 1.5 | No | 1 | 26 |
| Cardiogenic shock | 40 | 3 | 1.24 | No | 1 | 21 |
| Cardiogenic shock | 48 | 5 | 1.1 | No | 1 | 23 |
| LVAD | ||||||
| Cardiogenic shock | 58 | 5 | 2.2 | No | 1 | 34 |
| Cardiogenic shock | 33 | 19 | 1.6 | No | 1 | 32 |
| Cardiogenic shock | 46 | 18 | 3.7 | No | 1 | 20 |
| Cardiogenic shock | 47 | 8 | 1.49 | No | 1 | 40 |
| Cardiogenic shock | 57 | 12 | 1.36 | No | 1 | 28 |
| Cardiogenic shock | 55 | 4 | 1.32 | Yes | 1 | 20 |
ECMO, Extracorporeal membranous oxygenation; INR, international normalized ratio; CRRT, continuous renal replacement therapy; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LOS, length of stay; LVAD, left ventricular assist device.
Table E2.
Cost-effectiveness of other surgical procedures
| Study | Year | Cost-effectiveness ratio (US dollars) |
|---|---|---|
| Cost-Effectiveness of Implantable Cardioverter-DefibrillatorsE1 | 2005 | $34,000-$70,200/QALY |
| Cost-Effectiveness of Remote Cardiac Monitoring with the CardioMEMS Heart Failure SystemE2 | 2017 | $44,832/QALY |
| Cost-Effectiveness analysis of mitral valve repair with the MitraClip delivery system for patients with mitral regurgitation: a systematic reviewE3 | 2021 | $55,600/QALY |
| Cost-Effectiveness of Coronary Artery Bypass Grafting and Percutaneous Coronary Intervention in Patients with Chronic Kidney Disease and Acute Coronary Syndromes in the US Medicare ProgramE4 | 2021 | $101,565/QALY CABG |
| Cost-Effectiveness of Transcatheter vs Surgical Aortic Valve Replacement in Patients with Severe Aortic Stenosis at Intermediate RiskE5 | 2017 | $44,062/QALY TAVR vs 46,968/QALY SAVR |
QALY, Quality-adjusted life years; CABG, coronary artery bypass grafting; TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement.
References
- 1.Shih T., Dimick J.B. Reducing the cost of left ventricular assist devices: why it matters and can it be done? J Thorac Cardiovasc Surg. 2018;155:2466–2468. doi: 10.1016/j.jtcvs.2017.12.156. [DOI] [PubMed] [Google Scholar]
- 2.Silvestry S.C., Mahr C., Slaughter M.S., Levy W.C., Cheng R.K., May D.M., et al. Cost-effectiveness of a small intrapericardial centrifugal left ventricular assist device. ASAIO J. 2020;66:862–870. doi: 10.1097/MAT.0000000000001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrell A.J., Pellegrino V.A., Wolfe R., Wong W.K., Cooper D.J., Kaye D.M., et al. Long-term survival of adults with cardiogenic shock after venoarterial extracorporeal membrane oxygenation. J Crit Care. 2015;30:949–956. doi: 10.1016/j.jcrc.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Fukuhara S., Takeda K., Kurlansky P.A., Naka Y., Takayama H. Extracorporeal membrane oxygenation as a direct bridge to heart transplantation in adults. J Thorac Cardiovasc Surg. 2018;155:1607–1618.e1606. doi: 10.1016/j.jtcvs.2017.10.152. [DOI] [PubMed] [Google Scholar]
- 5.Garan A.R., Eckhardt C., Takeda K., Topkara V.K., Clerkin K., Fried J., et al. Predictors of survival and ability to wean from short-term mechanical circulatory support device following acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2018;7:755–765. doi: 10.1177/2048872617740834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravesteijn B.Y., Schluep M., Voormolen D.C., van der Burgh A.C., Miranda D.D.R., Hoeks S.E., et al. Cost-effectiveness of extracorporeal cardiopulmonary resuscitation after in-hospital cardiac arrest: a Markov decision model. Resuscitation. 2019;143:150–157. doi: 10.1016/j.resuscitation.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Sun T., Guy A., Sidhu A., Finlayson G., Grunau B., Ding L., et al. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for emergency cardiac support. J Crit Care. 2018;44:31–38. doi: 10.1016/j.jcrc.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Shah M., Patnaik S., Patel B., Ram P., Garg L., Agarwal M., et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107:287–303. doi: 10.1007/s00392-017-1182-2. [DOI] [PubMed] [Google Scholar]
- 9.Harvey M.J., Gaies M.G., Prosser L.A. U.S. and international in-hospital costs of extracorporeal membrane oxygenation: a systematic review. Appl Health Econ Health Policy. 2015;13:341–357. doi: 10.1007/s40258-015-0170-9. [DOI] [PubMed] [Google Scholar]
- 10.Brown K.L., Wray J., Wood T.L., Mc Mahon A.M., Burch M., Cairns J. Cost utility evaluation of extracorporeal membrane oxygenation as a bridge to transplant for children with end-stage heart failure due to dilated cardiomyopathy. J Heart Lung Transplant. 2009;28:32–38. doi: 10.1016/j.healun.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell B.G., Powers A.J., Sheikh A.Y., Lee P.H., Lobato R.L., Wong J.K. Resource use trends in extracorporeal membrane oxygenation in adults: an analysis of the Nationwide Inpatient Sample 1998-2009. J Thorac Cardiovasc Surg. 2014;148:416–421.e411. doi: 10.1016/j.jtcvs.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Long E.F., Swain G.W., Mangi A.A. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail. 2014;7:470–478. doi: 10.1161/CIRCHEARTFAILURE.113.000807. [DOI] [PubMed] [Google Scholar]
- 13.Göhler A., Geisler B.P., Manne J.M., Kosiborod M., Zhang Z., Weintraub W., et al. Utility estimates for decision-analytic modeling in chronic heart failure--health states based on New York Heart Association classes and number of rehospitalizations. Value Health. 2009;12:185–187. doi: 10.1111/j.1524-4733.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 14.Unai S., Yamane K., Tanaka D., Cook G., Hirose H., Cavarocchi N., et al. Quality of life and mid-term survival of patients bridged with extracorporeal membrane oxygenation to left ventricular assist Device. ASAIO J. 2017;63:273–278. doi: 10.1097/MAT.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 15.DeFilippis E.M., Clerkin K., Truby L.K., Francke M., Fried J., Masoumi A., et al. ECMO as a bridge to left ventricular assist device or heart transplantation. JACC Heart Fail. 2021;9:281–289. doi: 10.1016/j.jchf.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Mishra V., Fiane A.E., Winsnes B.A., Geiran O., Sorensen G., Hagen T.P., et al. Cardiac replacement therapies: outcomes and costs for heart transplantation versus circulatory assist. Scand Cardiovasc J. 2017;51:1–7. doi: 10.1080/14017431.2016.1196826. [DOI] [PubMed] [Google Scholar]
- 17.Han J.J., Chung J., Chen C.W., Gaffey A.C., Sotolongo A., Justice C., et al. Different clinical course and complications in Interagency Registry for Mechanically Assisted Circulatory Support 1 (INTERMACS) patients managed with or without extracorporeal membrane oxygenation. ASAIO J. 2018;64:318–322. doi: 10.1097/MAT.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 18.Lee D.S., Lee J.S., Schull M.J., Borgundvaag B., Edmonds M., Ivankovic M., et al. Prospective validation of the emergency heart failure mortality risk grade for acute heart failure. Circulation. 2019;139:1146–1156. doi: 10.1161/CIRCULATIONAHA.118.035509. [DOI] [PubMed] [Google Scholar]
- 19.Enezate T., Eniezat M., Thomas J. Utilization and outcomes of temporary mechanical circulatory support devices in cardiogenic shock. Am J Cardiol. 2019;124:505–510. doi: 10.1016/j.amjcard.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Jasseron C., Lebreton G., Cantrelle C., Legeai C., Leprince P., Flecher E., et al. Impact of heart transplantation on survival in patients on venoarterial extracorporeal membrane oxygenation at listing in France. Transplantation. 2016;100:1979–1987. doi: 10.1097/TP.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 21.Chiu R., Pillado E., Sareh S., De La Cruz K., Shemin R.J., Benharash P. Financial and clinical outcomes of extracorporeal mechanical support. J Card Surg. 2017;32:215–221. doi: 10.1111/jocs.13106. [DOI] [PubMed] [Google Scholar]
- 22.Neumann P.J., Cohen J.T., Weinstein M.C. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 23.Godown J., Smith A.H., Thurm C., Hall M., Dodd D.A., Soslow J.H., et al. Mechanical circulatory support costs in children bridged to heart transplantation—analysis of a linked database. Am Heart J. 2018;201:77–85. doi: 10.1016/j.ahj.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oude Lansink-Hartgring A., van den Hengel B., van der Bij W., Erasmus M.E., Mariani M.A., Rienstra M., et al. Hospital costs of extracorporeal life support therapy. Crit Care Med. 2016;44:717–723. doi: 10.1097/CCM.0000000000001477. [DOI] [PubMed] [Google Scholar]
- 25.Camboni D., Philipp A., Rottenkolber V., Zerdzitzki M., Holzamer A., Floerchinger B., et al. Long-term survival and quality of life after extracorporeal life support: a 10-year report. Eur J Cardiothorac Surg. 2017;52:241–247. doi: 10.1093/ejcts/ezx100. [DOI] [PubMed] [Google Scholar]
- 26.Jäämaa-Holmberg S., Salmela B., Suojaranta R., Lemström K.B., Lommi J. Cost-utility of venoarterial extracorporeal membrane oxygenation in cardiogenic shock and cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2020;9:333–341. doi: 10.1177/2048872619900090. [DOI] [PubMed] [Google Scholar]
- 27.Baras Shreibati J., Goldhaber-Fiebert J.D., Banerjee D., Owens D.K., Hlatky M.A. Cost-effectiveness of left ventricular assist devices in ambulatory patients with advanced heart failure. JACC Heart Fail. 2017;5:110–119. doi: 10.1016/j.jchf.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Toda K., Fujita T., Seguchi O., Yanase M., Nakatani T. Role of percutaneous veno-arterial extracorporeal membrane oxygenation as bridge to left ventricular assist device. J Artif Organs. 2018;21:39–45. doi: 10.1007/s10047-017-0984-3. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein D.J., Naka Y., Horstmanshof D., Ravichandran A., Schroder J., Ransom J., et al. Association of clinical outcomes with left ventricular assist device use by bridge to transplant or destination therapy intent: the multicenter study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 (MOMENTUM 3) randomized clinical trial. JAMA Cardiol. 2020;5:411–419. doi: 10.1001/jamacardio.2019.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamdar F., John R., Eckman P., Colvin-Adams M., Shumway S.J., Liao K. Postcardiac transplant survival in the current era in patients receiving continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2013;145:575–581. doi: 10.1016/j.jtcvs.2012.09.095. [DOI] [PubMed] [Google Scholar]
- 31.Suarez-Pierre A., Zhou X., Fraser C.D., III, Grimm J.C., Crawford T.C., Lui C., et al. Survival and functional status after bridge-to-transplant with a left ventricular assist device. ASAIO J. 2019;65:661–667. doi: 10.1097/MAT.0000000000000874. [DOI] [PubMed] [Google Scholar]
- 32.Urbich M., Globe G., Pantiri K., Heisen M., Bennison C., Wirtz H.S., et al. A systematic review of medical costs associated with heart failure in the USA (2014-2020) Pharmacoeconomics. 2020;38:1219–1236. doi: 10.1007/s40273-020-00952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayanga J.W.A., Shigemura N., Aboagye J.K., Ensor C., Dew M.A., Hayanga H.K., et al. ECMO support in lung transplantation: a contemporary analysis of hospital charges in the United States. Ann Thorac Surg. 2017;104:1033–1039. doi: 10.1016/j.athoracsur.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Barrett K.A., Hawkins N., Fan E. Economic evaluation of venovenous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Crit Care Med. 2019;47:186–193. doi: 10.1097/CCM.0000000000003465. [DOI] [PubMed] [Google Scholar]
E-References
- Sanders G.D., Hlatky M.A., Owens D.K. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–1480. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- Schmier J.K., Ong K.L., Fonarow G.C. Cost-effectiveness of remote cardiac monitoring with the CardioMEMS heart failure system. Clin Cardiol. 2017;40:430–436. doi: 10.1002/clc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezapour A., Azari S., Arabloo J., Pourasghari H., Behzadifar M., Alipour V., et al. Cost-effectiveness analysis of mitral valve repair with the MitraClip delivery system for patients with mitral regurgitation: a systematic review. Heart Fail Rev. 2021;26:587–601. doi: 10.1007/s10741-020-10055-9. [DOI] [PubMed] [Google Scholar]
- Reynolds M.R., Gong T., Li S., Herzog C.A., Charytan D.M. Cost-effectiveness of coronary artery bypass grafting and percutaneous coronary intervention in patients with chronic kidney disease and acute coronary syndromes in the US Medicare Program. J Am Heart Assoc. 2021;10:e019391. doi: 10.1161/JAHA.120.019391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S.J., Wang K., House J.A., Magnuson E.A., Reynolds M.R., Makkar R., et al. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at intermediate risk. Circulation. 2019;139:877–888. doi: 10.1161/CIRCULATIONAHA.118.035236. [DOI] [PubMed] [Google Scholar]