Abstract
Objectives
Tricuspid valve (TV) surgery remains understudied and little data exist describing the surgical indications, outcomes, and prognostic factors for pediatric patients with non-Ebstein 2-ventricle congenital TV lesions. This study aims to describe early and late outcomes of pediatric patients with non-Ebstein congenital TV lesions undergoing isolated TV procedures at a single institution.
Methods
All patients who underwent TV surgery for non-Ebstein congenital TV disease between 2006 and 2018 were included. Patients who had missing preoperative data, patients with single-ventricle physiology, congenitally corrected transposition of the great arteries, and patients undergoing TV intervention as part of repair of an atrioventricular canal defect were excluded. The primary end point was the occurrence of TV reintervention or TV regurgitation (TR) ≥ moderate.
Results
A total of 85 patients were included. The tricuspid lesion was isolated TR in 80 (94.1%), isolated tricuspid stenosis in 3 (3.5%) and mixed disease in 2 (2.4%) patients. Median age at surgery was 33 years (interquartile range, 12-53 years). TV repair and TV replacement were performed in 66 (77.6%) and 19 (22.4%) patients, respectively. One (1.2%) patient underwent TV reoperation during the same admission. There was no in-hospital mortality. Median follow-up was 3.3 years (interquartile range, 0.1-4.7 years). The overall cumulative incidence of TV reintervention or TR deemed moderate or greater at 1, 3, and 5 years was 3% ± 2%, 11% ± 4%, and 20% ± 8%. In multivariable analysis, age younger than 12 years (P = .04) and mitral valve regurgitation deemed moderate or greater (P = .01) were independent risk factors for TV reintervention or recurrent TR deemed to be moderate or greater at last follow-up.
Conclusions
TV surgery in patients with non-Ebstein congenital TV disease can be performed with good outcomes. TV reintervention or TR deemed moderate or greater occurred in 20% of patients on midterm follow-up. Patients younger than age 12 years are at higher risk for recurrent TR or TV reintervention, whereas preoperative MR deemed moderate or greater increases this risk, especially in patients older than age 12 years. There was no difference in outcomes between TV replacement and repair.
Key Words: congenital, tricuspid valve
Abbreviations and Acronyms: ccTGA, congenitally corrected transposition of the great arteries; ECMO, extracorporeal membrane oxygenation; MR, mitral regurgitation; RVOTO, right ventricular outflow tract obstruction; SV, single ventricle; TR, tricuspid valve regurgitation; TS, tricuspid stenosis; TV, tricuspid valve
Graphical abstract
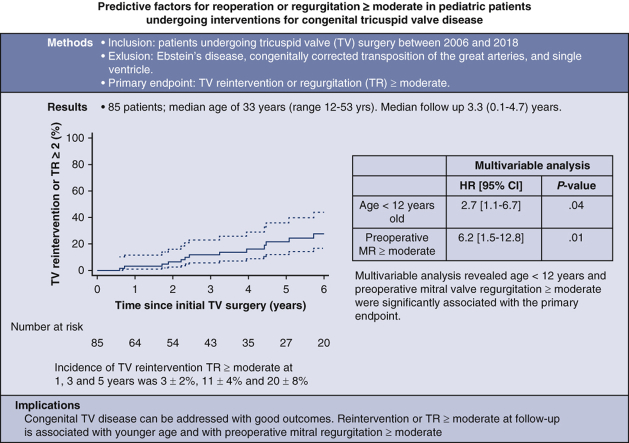
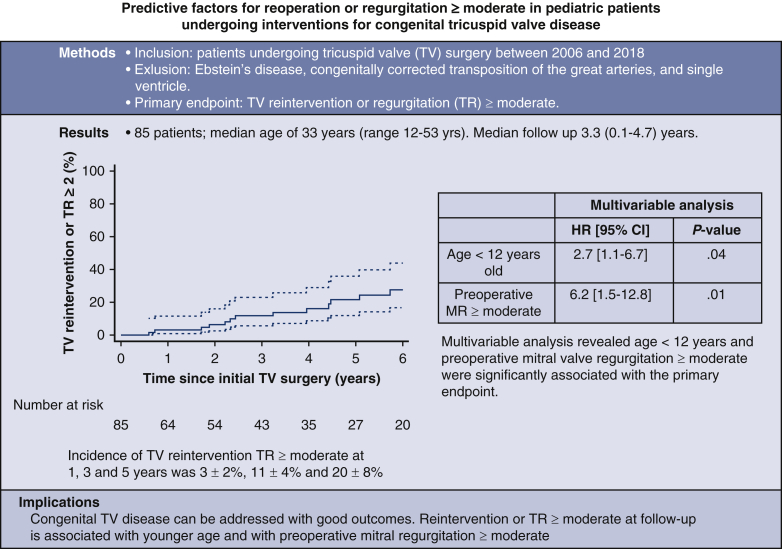
Predictive factors for reoperation or regurgitation deemed moderate or greater in pediatric patients undergoing interventions for congenital tricuspid valve disease.
Kaplan-Meier cumulative incidence of TV reintervention or TR deemed moderate or greater.
Central Message.
TV surgery in patients with non-Ebstein 2-ventricle congenital anomaly can be performed with good outcomes. In our experience, younger patients as well as those with moderate mitral regurgitation are more prone to adverse outcome.
Perspective.
TV surgery remains understudied and little data exist describing the surgical indications, outcomes, and prognostic factors for pediatric patients with non-Ebstein congenital TV lesions. The aim of this study was to describe early and late outcomes of pediatric patients with non-Ebstein congenital TV lesions undergoing isolated TV procedures at a single institution.
There has been increasing recognition of the clinical impact of tricuspid valve (TV) disease and observational studies of its natural history have demonstrated a decreased survival in the adult population, both with congenital and acquired disease.1, 2, 3 Operative intervention is not without risk. It has been shown that operative mortality is markedly higher for patients undergoing isolated TV surgery compared with other single-valve procedures in adult populations.4,5 In the population with congenital disease, TV interventions and outcomes in the setting of Ebstein anomaly has been an area of focus for both pediatric and adult populations.6,7 However, there are little data in regard to TV interventions and outcomes for patients with congenital TV disease overall, with even less looking exclusively at non-Ebstein disease.8
Patients with congenital disease involving the TV represent a diverse population. Some of the underlying etiologies include TV disease in patients with single-ventricle anatomy, congenitally corrected transposition of the great arteries (ccTGA), and atrioventricular canal defects. Each of these is a unique population and has been relatively well studied.8 Other etiologies, such as TV diseases related to right heart volume or pressure overload from intracardiac shunts, right ventricular outflow tract obstruction (RVOTO), iatrogenic TV injury, and isolated congenital TV disease have been described but remain relatively understudied.8, 9, 10, 11, 12, 13, 14, 15 Comprehensive studies on TV surgery remain rare and thus little data exist describing the surgical indications, outcomes, and prognostic factors for patients without Ebstein, single-ventricle (SV), or ccTGA lesions. We describe these elements for patients with non-Ebstein TV lesions in the setting of biventricular physiology and a right-sided TV.
Methods
Patients and Study Design
This study was approved by the Columbia University Institutional Review Board (Protocol IRB-AAAR3476; approval date May 22, 2020) and the need for informed consent was waived. All patients undergoing TV repair or replacement at New York Presbyterian Morgan Stanley Children's Hospital/Columbia University and Komansky Children's Hospital/Weill Cornell from 2006 to 2018 were included. Exclusion criteria were an underlying diagnosis of Ebstein anomaly (n = 56), or a systemic TV as in the case of SV physiology (n = 15) or congenitally ccTGA (n = 9). Patients undergoing right atrioventricular valve repair as part of repair of an atrioventricular canal defect were also excluded (n = 11). Additionally, patients who underwent prior repair at an outside institution for which preoperative and operative characteristics at that time were not available were excluded (n = 7).
Patients with anatomic TV pathology (n = 17 [20%]) included those with an isolated congenital dysplastic TV and those with iatrogenic injury. Patients who developed functional TV pathology (n = 68 [80%]) were categorized in 2 groups: those with right heart volume overload and evidence of left-to-right shunting (defined as a Qp:Qs > 1.5:1) and those with RVOTO or pulmonary stenosis (defined as a peak gradient >30 mm Hg). The severity of TV disease was characterized by preoperative echocardiography.
A retrospective review of medical records was performed for all patients meeting the inclusion criteria. Patient demographic and preoperative characteristics included comorbid disease, clinical features, and preoperative echocardiographic findings. The severity of the TV lesion was graded through independent assessment by pediatric cardiologists in accordance with national guidelines.4 Moderate tricuspid valve regurgitation (TR) was defined as a moderate central, parabolic, or triangular jet on qualitative Doppler, vena contracta width between 0.3 and 0.69 cm, proximal isovelocity surface area radius between 0.6 and 0.9 cm, and systolic blunting of hepatic veins flow. Severe TR was defined as a severe central or eccentric dense and triangular jet on qualitative Doppler, color flow jet >10 cm2, vena contracta width >0.7 cm, proximal isovelocity surface area radius >0.9 cm, and systolic flow reversal in the hepatic veins. A mean TV gradient of 5 to 10 mm Hg was considered moderate stenosis and a gradient >10 mm Hg was considered severe stenosis. TV intervention was considered in the presence of symptoms, progressive moderate-to-severe or severe TV disease, or if an operative intervention was planned for other indications in the setting of tricuspid stenosis (TS) or TR deemed moderate or greater. Intraoperative data collected included cardiopulmonary bypass parameters and procedural details. Perioperative outcomes included duration of intubation, intensive care and hospital length of stay, perioperative complications and morbidity, operative mortality (ie, death within 30 days or during same hospitalization), and predischarge echocardiographic data. Perioperative complications included were requirement for extracorporeal membrane oxygenation, reoperation for any reason, and any reoperation on the TV. Follow-up data included echocardiographic assessment of TV function (presence and degree of TV regurgitation or stenosis) and the need for repeat intervention on the TV. All follow-up echocardiography was performed at our institution. For analysis of TR and TS categories, the higher grade was recorded for those patients with severity assessed between grades (eg, mild-moderate disease was categorized as moderate). The primary end point was a composite of TV reintervention or TR moderate or greater. Mean follow-up was 3.3 years (range, 0.1-4.7 years).
Statistical Analysis
Continuous data are presented as mean ± SD for normally distributed data and median (interquartile range) for nonnormally distributed data. Categorical variables are presented as total number and frequency. Data comparisons were performed using χ2 testing for categorical data and either Student t test, Fisher exact test, or the Mann Whitney U test for continuous data where appropriate. Predictors of TV reintervention or TR deemed moderate or greater were assessed using a time-related semiparametric model (ie, Cox model). Variables screened as potential confounders were the preoperative baseline characteristics. All variables with a P value < .20 upon univariate analysis were considered as having a potential confounding effect. Variables with a P value < .05 were retained in the final multivariable model. The model was then internally validated by generating 1000 bootstrap samples derived from the original dataset. As a sensitivity analysis, 2 separate univariate analyses were performed for patients younger than age 12 years and aged 12 years or older with the primary end point as the dependent variable. Linearized occurrence rates of midterm outcomes (ie, death, TR deemed moderate or greater, and TV reintervention) were calculated ([number of events/number of patient-years] × 100). Kaplan-Meier curves for TV reintervention or TR was performed for the whole cohort, repair versus replacement and by age groups. Statistical analyses were performed using STATA version 14 (Stata Corp).
Results
Patient Characteristics and Preoperative Risk Factors
A total of 85 patients met inclusion criteria. Median age at the time of TV intervention was 33 years (range, 12-53 years); median body surface area was 1.6 m2 (range, 1.3-1.9 m2). Preoperative characteristics are further described in Table 1. The primary TV lesion was isolated TR in 94.1% (n = 80), isolated TS in 3.5% (n = 3), and mixed disease in 2.4% (n = 2). A history of a previous cardiac surgery was present in 61.2% (n = 52) of patients. The most common previous interventions were pulmonary valve/RVOTO procedures (n = 19 [22.4%]), tetralogy of Fallot repair (n = 19 [22.4%]), and Blalock-Taussig shunt (n = 11 [12.9%]). Etiologies for TV disease included TV disease secondary to RVOTO (n = 39 [45.9%]), TV disease secondary to right heart volume overload (n = 29 [34.1%]), congenital dysplastic TV lesions (n = 12 [14.1%]), and iatrogenic TV disease (n = 5 [5.9%]). Preoperative mitral regurgitation (MR) deemed moderate or greater was present in 6 patients (7%; n = 2 severe) (Table E1). Preoperative right ventricle dysfunction greater than moderate was present in 18.8% (n = 16) of patients.
Table 1.
Preoperative characteristics
| Variable | Result |
|---|---|
| Male | 40 (47.1) |
| Age at surgery (y) | 33 (12-53) |
| BSA (m2) | 1.6 (1.3-1.9) |
| Genetic syndromes present | 4 (4.7) |
| Cardiac malformations present | 72 (84.7) |
| Prior cardiac interventions | 52 (61.2) |
| Pulmonary valve/RVOT procedure | 19 (22.4) |
| TOF repair | 19 (22.4) |
| Blalock-Taussig shunt | 11 (12.9) |
| RV-PA connection revision | 9 (10.6) |
| Isolated ASD | 6 (7.1) |
| Isolated VSD | 2 (2.4) |
| Glenn | 1 (1.2) |
| Cardiac transplantation | 5 (5.9) |
| PA band | 1 (1.2) |
| Aortic valve intervention | 2 (2.4) |
| Other | 6 (7.1) |
| Fontan | 1 (1.2) |
| Mitral valve replacement | 1 (1.2) |
| Arterial switch | 1 (1.2) |
| Rastelli | 1 (1.2) |
| Hybrid PCI | 1 (1.2) |
| Coronary unroofing | 1 (1.2%) |
| Primary TV lesion | |
| TR | 80 (94.1) |
| TS | 3 (3.5) |
| Mixed TR + TS | 2 (2.4) |
| Etiology of TV lesion | |
| RV/RVOT/PA obstruction | 39 (45.9%) |
| Right heart volume overload | 29 (34.1) |
| Isolated congenital dysplastic TV lesion | 12 (14.1) |
| Iatrogenic lesion | 5 (5.9) |
| Lesion post-VSD repair | 2 (2.4) |
| Iatrogenic injury | 2 (2.4) |
| Lesion from transvenous lead | 1 (1.2) |
| RV dysfunction | |
| Mild | 23 (27.1) |
| Moderate | 15 (17.6) |
| Severe | 1 (1.2) |
| LV dysfunction∗ | |
| Mild | 10 (11.8) |
| Moderate | 5 (5.8) |
| Mitral regurgitation | |
| Moderate | 2 (2.4) |
| Severe | 4 (4.7) |
Values are presented as median (interquartile range) or frequency (%). BSA, Body surface area; RVOT, right ventricular outflow tract; TOF, tetralogy of Fallot; RV, right ventricle; PA, pulmonary artery; ASD, atrial septal defect; VSD, ventricular septal defect; PCI, percutaneous coronary intervention; TV, tricuspid valve; TR, tricuspid regurgitation; TS, tricuspid stenosis; LV, left ventricle.
LV dysfunction was defined as mild (ejection fraction 40%-49%) or moderate (ejection fraction 30%-39%).
Intraoperative Data
Operative characteristics are described in Table E2. Median cardiopulmonary bypass time was 101 minutes (interquartile range, 77-126 minutes) and median crossclamp time was 54 minutes (range, 41-78 minutes). TV repair was performed in 77.6% (n = 66) of patients. These procedures included annuloplasty in 50.6% (n = 43), commissuroplasty in 12.9% (n = 11), subvalvular intervention in 10.6% (n = 9), and leaflet intervention in 5.9% (n = 5). TV replacement was performed in 22.4% (n = 19). In all instances of TV replacement, a bioprosthetic valve was implanted. The valves used included Carpentier-Edwards (Edwards Lifesciences) bovine pericardial valves (n = 7 [36.8%]), Medtronic porcine valves (n = 5 [26.3%]), and St Jude porcine valves (n = 7 [36.8%]). Concomitant procedures were performed at the time of TV intervention in 98.9% (n = 84) of cases. The most common procedures were atrial septal defect repair (n = 35 [41.2%]), pulmonary valve replacement (n = 23 [27.1%]), pulmonary artery plasty (n = 12 [14.1%]), and ventricular septal defect repair (n = 8 [9.4%]). The 2 Glenn procedures were performed in patients with pulmonary atresia and intact ventricular septum. Of the patients with preoperative MR deemed moderate or greater, 4 underwent concomitant mitral valve intervention with 1 undergoing mitral valve replacement and the remainder undergoing mitral valve repair. Two patients did not undergo concomitant mitral valve intervention, both of whom had undergone prior heart transplantation and presented with severe TR and moderate MR and both underwent a TV replacement only.
Immediate Postoperative Course
Median length of ICU stay was 2 days (range, 0-1 day) and median length of hospital stay was 6 days (range, 5-310 days). Common postoperative complications included arrhythmias in 25.9% (n = 22), with 3.5% (n = 3) experiencing complete atrioventricular block and requiring a permanent pacemaker. One patient (1.1%) underwent reoperation during the same admission. The reason for reoperation was repair of detached papillary muscle. There were no patients who required extracorporeal membrane oxygenation postoperatively. There was no instance of in-hospital mortality. There was 1 patient with MR deemed moderate or greater at discharge echocardiography.
Midterm Outcomes
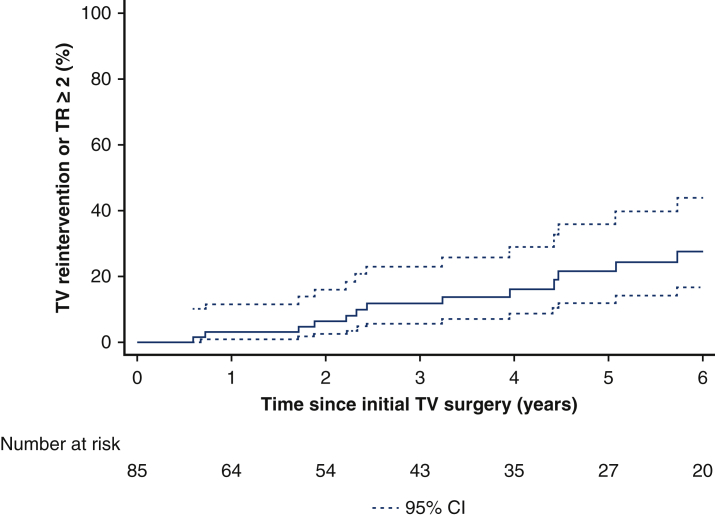
Midterm outcomes are described in Table 2. Mean follow-up was 3.3 years (range, 0.1-4.7 years). The overall cumulative incidence of TV reintervention or TR deemed moderate or greater at 1, 3, and 5 years was 3% ± 2%, 11% ± 4%, and 20% ± 8%. Kaplan-Meier long-term freedom from reintervention or TR deemed moderate or greater at 5 years is provided in Figure 1. Table 2 presents the outcomes at last follow-up for the overall cohort and for patients undergoing TV repair versus replacement, age younger than 12 years versus age 12 year or older, and preoperative MR deemed less than moderate versus MR deemed moderate or greater. There was a significant difference in mortality between patients with preoperative MR moderate or greater (n = 1 out of 6; 4.0% per patient-year) versus less than moderate (n = 1 out of 79; 0.4% per patient-year) (P = .001) and in TR moderate or greater at follow-up for patients younger than age 12 years (n = 8 out of 20; 12.2% patient-years) and aged 12 years and older (n = 11 out of 65; 4.4% patient-years) (P = .002). Significant differences were also noted in the composite end point for patients younger than age 12 years (n = 8 out of 20; 12.2% patient-years) versus age 12 years or older (n = 12 out of 65; 4.8% patient-years) (P = .005) and also for patients with preoperative MR deemed moderate or greater (n = 3 out of 6; 58.1% patient-years) and MR deemed less than moderate (n = 17 out of 79; 6.7% patient-years) (P = .005). Multivariate analysis for TV reintervention or TR deemed moderate or greater (Table 3) showed that age younger than 12 years (hazard ratio, 2.7; 95% CI, 1.1-6.7; P = .04) and MR deemed moderate or greater (hazard ratio, 6.2; 95% CI, 1.5-12.8; P = .01) are independent risk factors for TV reintervention or TR deemed moderate or greater.
Table 2.
Late outcomes∗
| Variable | Overall |
Type of intervention |
Patient age |
Preoperative MR |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 85) | Repair (n = 66) | Replacement (n = 19) | P value | <12 y (n = 20) | ≥12 y (n = 65) | P value | Moderate or greater (n = 6) | Less than moderate (n = 79) | P value | |
| Death | 2/0.6 | 1/0.4 | 1/1.6 | .07 | 0/0 | 2/0.8 | .62 | 1/4.0 | 1/0.4 | .001 |
| TR moderate or greater | 19/6.1 | 16/6.3 | 3/4.7 | .96 | 8/12.2 | 11/4.4 | .002 | 3/58.1 | 16/6.3 | .005 |
| TV reintervention | 9/2.8 | 8/3.2 | 1/1.6 | .82 | 4/6.1 | 5/2.0 | .006 | 0/0 | 9/3.1 | .52 |
| TR moderate or greater or TV reintervention | 20/6.3 | 16/6.3 | 4/6.3 | .58 | 8/12.2 | 12/4.8 | .005 | 3/58.1 | 17/6.7 | .005 |
Values are presented as events/% patient-years. Bold values highlight statistically significant findings. TR, Tricuspid regurgitation; TV, tricuspid valve.
α = 0.004 after Bonferroni correction of multiple comparison.
Figure 1.
Kaplan-Meier long-term freedom from reintervention or tricuspid valve regurgitation (TR) deemed moderate or greater. TV, Tricuspid valve; CI, confidence interval.
Table 3.
Univariate and multivariate analysis: Tricuspid valve (TV) reintervention or tricuspid valve regurgitation (TR) deemed moderate or greater
| Variable | Univariate analysis |
Multivariable analysis∗ |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.0 (0.9-1.0) | .07 | ||
| Age <12 y | 3.1 (1.3-7.4) | .01 | 2.7 (1.1-6.7) | .04 |
| Male | 0.9 (0.4-2.3) | .87 | ||
| Previous cardiac surgery | 1.0 (0.4-2.4) | .95 | ||
| Age at first cardiac intervention (y) | 0.9 (0.8-1.1) | .23 | ||
| Genetic syndromes present | 0.6 (0.1-5.0) | .67 | ||
| Other cardiac malformations | 0.7 (0.2-2.2) | .57 | ||
| No. of previous cardiac interventions = 0 | 0.82 (0.32-2.5) | .82 | ||
| No. of previous cardiac interventions = 1 | 1 (0.8-1.8) | |||
| No. of previous cardiac interventions ≥2 | 1.2 (0.7-3.5) | |||
| BSA | 0.6 (0.3-1.1) | .09 | ||
| Weight | 1.0 (0.9-1.0) | .20 | ||
| Diameter of TV annulus (mm) | 1.0 (0.9-1.0) | .82 | ||
| AS moderate or greater | 0.5 (0.1-3.8) | .51 | ||
| AI moderate or greater | 1.8 (0.4-8.5) | .44 | ||
| PR moderate or greater | 1.0 (0.4-2.6) | .96 | ||
| MS moderate or greater | 0.7 (0.1-4.9) | .68 | ||
| MR moderate or greater | 8.7 (2.1-12.6) | .002 | 6.2 (1.5-12.8) | .01 |
| Preoperative degree of TR† | ||||
| Mild | 2.3 (0.1-17) | .55 | ||
| Moderate | 1.7 (0.2-14) | .62 | ||
| Severe | 2.1 (0.3-15) | .47 | ||
| RV dysfunction | 0.9 (0.3-2.6) | .82 | ||
| LV dysfunction‡ | 2.9 (0.7-8.5) | .16 | ||
| Etiology of TV lesion | ||||
| RV/RVOT/PA obstruction | 0.9 (0.4-2.1) | .81 | ||
| Right heart volume overload | 1.8 (0.7-4.1) | .20 | ||
| Iatrogenic lesion | 0.5 (0.1-3.5) | .46 | ||
| Congenital/isolated TV lesion | 1.6 (0.4-7.1) | .52 | ||
| Crossclamp time (per min) | 1.0 (1.0-1.1) | .16 | ||
| CPB time (per min) | 1.0 (1.0-1.1) | .77 | ||
| Repair§ | 0.8 (0.3-2.4) | .65 | ||
Bold values highlight statistically significant findings. HR, Hazard ratio; CI, confidence interval; BSA, body surface area; AS, aortic stenosis; AI, aortic insufficiency; PR, pulmonary regurgitation; MS, mitral stenosis; MR, mitral regurgitation; RV, right ventricle; LV, left ventricle; RVOT, right ventricular outflow tract; PA, pulmonary artery; CPB, cardiopulmonary bypass.
Corrected C index = 0.62 after bootstrapping × 1000.
Reference category is no TR.
Defined as EF <50%.
Reference category is replacement.
Repair Versus Replacement
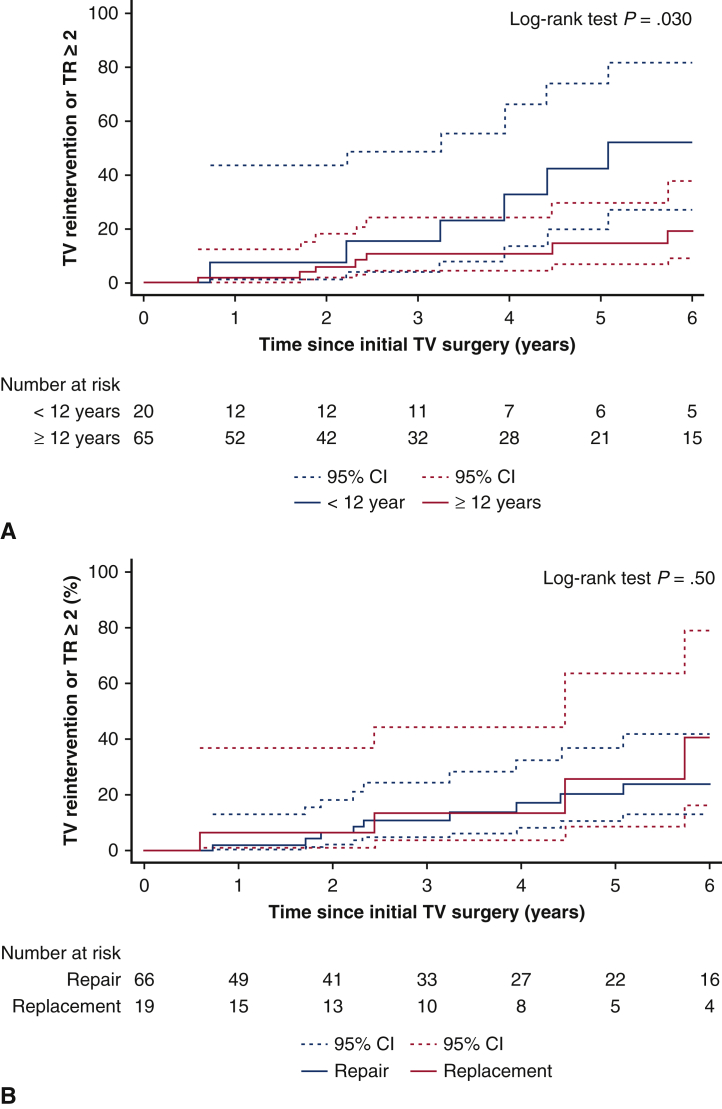
A comparison between patients undergoing TV repair and replacement is summarized in Table 4. Patients undergoing TV repair were significantly younger (age 29 vs 53 years; P < .001) and smaller by body surface area (1.6 vs 1.7 m2; P = .02). Etiologies for TV disease were not different between groups. There was no difference between groups in terms of TV reintervention or in-hospital mortality. There was no difference in cumulative incidence of TV reintervention or TR deemed moderate or greater between repair and replacement (18% ± 6% vs 26% ± 14%, respectively, at 5 years; P = .49) (Figure 2).
Table 4.
Patient demographic characteristics in tricuspid valve (TV) repair and TV replacement groups and for patients younger than age 12 years or 12 years and older∗
| Variable | TV repair group (n = 66) | TV replacement group (n = 19) | P value | Patient age <12 y (n = 20) | Patient age ≥12 y (n = 65) | P value |
|---|---|---|---|---|---|---|
| Patient characteristic | ||||||
| Age (y) | 29 (6-45) | 53 (31-62) | .001 | 0.8 (0.4-4.3) | 40 (29-56) | .001 |
| Male sex | 37 (52.9) | 6 (31.6) | .10 | 11 (52.4) | 32 (47.1) | .67 |
| BSA (m2) | 1.6 (0.9-1.9) | 1.7 (1.5-2.0) | .02 | 0.6 (0.3-1.4) | 1.8 (1.5-2.0) | .001 |
| Genetic syndromes present | 4 (6.1) | 0 (0) | .29 | 2 (10.0) | 2 (3.1) | .20 |
| TV demographic characteristic | ||||||
| Lesion | .54 | .004 | ||||
| TV regurgitation | 61 (92.4) | 19 (100) | 16 (80.0) | 64 (98.5) | ||
| TV stenosis | 3 (4.5) | 0 (0) | 3 (15.0) | 0 (0) | ||
| Mixed TR + TS | 3 (3.1) | 0 (0) | 1 (5.0) | 1 (1.5) | ||
| Tricuspid regurgitation | ||||||
| Mild | 5 (7.6) | 0 (0) | .03 | 4 (20) | 1 (1.5) | < .001 |
| Moderate | 23 (34.8) | 1 (5.3) | 9 (45) | 15 (23.1) | ||
| Severe | 38 (57.6) | 18 (94.7) | 7 (35) | 49 (75.4) | ||
| Etiology | .33 | .06 | ||||
| RV/RVOT/PA obstruction | 30 (45.5) | 9 (47.4) | 10 (47.6) | 29 (42.7) | ||
| Right heart volume overload | 20 (30.4) | 5 (26.3) | 5 (23.8) | 21 (35.9) | ||
| Iatrogenic lesion | 8 (12.1) | 1 (5.2) | 5 (23.8) | 4 (5.9) | ||
| Congenital/isolated TV lesion | 5 (7.6) | 4 (21.1) | 0 (0.0) | 9 (13.2) | ||
| Other | 3 (4.5) | 0 (0) | 1 (4.8) | 2 (2.9) | ||
| Replacement | 0 (0) | 19 (29.9) | .006 | |||
| Repair | 20 (100) | 46 (70.1) | ||||
Values are presented as median (range) or n (%). BSA, Body surface area; TR, tricuspid regurgitation; TS, tricuspid stenosis; RV, right ventricle; RVOT, right ventricular outflow tract; PA, pulmonary artery.
α = 0.004 after Bonferroni correction of multiple comparison.
Figure 2.
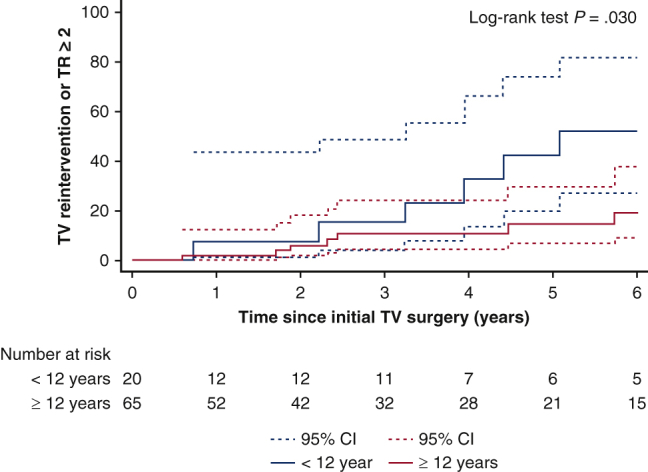
A, Kaplan-Meier cumulative incidence of tricuspid valve (TV) reintervention or tricuspid valve regurgitation (TR) deemed moderate or greater for patients aged <12 years or ≥12 years. B, Kaplan-Meier cumulative incidence of TV reintervention or TR deemed moderate or greater for TV repair versus replacement. CI, Confidence interval.
Age Younger Than 12 Years Versus 12 Years and Older
Table 4 describes demographic differences between patients based on age younger than 12 years or 12 years and older. There was no difference in the etiology of TV disease based on patient age. Older patients had significantly more isolated TR and less TS (P = .004), more severe TR (P < .001), and were more likely to undergo TV replacement (29.9% vs 0.0% for those younger than age 12 years; P = .006). There was no difference between age groups in terms of TV reintervention or in-hospital mortality (Table E3). There was a significant difference in Kaplan-Meier cumulative incidence of TV reintervention or TR deemed moderate or greater based on age younger than 12 years or 12 years and older (38% ± 14% vs 10% ± 4%, respectively, at 5 years; P = .01) (Figure 3).
Figure 3.
Predictive factors for reoperation or regurgitation deemed moderate or greater in pediatric patients undergoing interventions for congenital tricuspid valve (TV) disease. TR, Tricuspid valve regurgitation; HR, hazard ratio; CI, confidence interval; MR, mitral valve regurgitation.
Discussion
The present study underscores important findings in this population of patients with congenital heart disease undergoing surgical intervention for TV disease. First, a significant proportion of these patients required TV reintervention or developed TR deemed moderate or greater during the follow-up period. With early to midterm follow-up, patients younger than age 12 years are at higher risk for reintervention or recurrent TR, and preoperative MR deemed moderate or greater increases this risk, especially in patients older than age 12 years. Moreover, whereas TV repair was performed more commonly in younger patients, no difference in outcomes was found between patients undergoing TV replacement versus repair.
The present study included a diverse patient population in regard to TV pathophysiology. Patients with SV physiology were excluded because this population represents a distinct and complex cohort that has already been studied in detail.16, 17, 18 For similar reasons, patients with a systemic TV in the setting of ccTGA were excluded.19, 20, 21 Reports of midterm results for patients with biventricular physiology undergoing surgical intervention on the TV across the spectrum of right-sided congenital TV lesions are sparse throughout the literature.
Based on our results, 1 patient out of 5 will experience a recurrence of TR moderate or greater or require TV reintervention on midterm follow-up. Similar studies looking specifically at patients with adult congenital heart disease undergoing TV intervention reported high rates of TR recurrence following TV intervention. Indeed, Lo Rito and colleagues22 analyzed 128 patients with adult congenital heart disease undergoing TV surgery. Of these, 74% had functional TR, 18% dysplastic valve and 8% systemic TV. The incidence of TR deemed moderate or greater after 5 years of follow-up was 34%. Similarly, Martin-Garcia and colleagues23 reported that the incidence of moderate to severe TR was 11% at 1 year of follow-up for patients undergoing concomitant TV intervention at the time of atrial septal defect repair or pulmonary valve replacement. Similarly, The The Surgical Correction Of Tricuspid Insufficiency in Adult congenital patients requiring Pulmonary Valve Replacement (SCOTIA-PVR) trial sought to evaluate the influence of TV intervention on 542 adults with congenital heart disease undergoing pulmonary valve replacement.24 Although TV annuloplasty was effective in reducing TR severity when compared with nonintervention, 16% of the patients who underwent a concomitant TV intervention had TR deemed moderate or greater on discharge. Such early failure of TV repair mirrors previous reports on TV annuloplasty in adult patients with left-sided lesions, where significant TR was seen in 14% of patients at discharge.25 In our cohort, the incidence of TV reintervention or TR deemed moderate or greater at 1 year was only 3%. Therefore, the recurrence of TR seems to be more closely related to a gradual progression of the underlying disease process rather than early repair failure.
In our series, younger age at the time of the operation was found to be a significant independent risk factor for TR recurrence or reintervention. Indeed, there was a 2-fold increase in the risk of TV disease recurrence in patients younger than age 12 years versus those aged 12 years and older. Previous studies reported even worse clinical outcomes in infants and children undergoing TV intervention, mainly in the setting of a systemic TV and single ventricles.16, 17, 18,26 This finding can be explained by the use of complex TV repair techniques to avoid prosthetic valve replacement in these patients. Indeed, in our cohort, all patients younger than age 12 years underwent a TV repair. This strategy reflects the current philosophy in the field, which aims to minimize the risk of prosthetic valve degeneration or the need for long-term anticoagulation with a mechanical valve in younger patients, at the expense of an increased risk for recurrent TR or TV reoperation.27, 28, 29
Our results establish an association between significant MR in the preoperative setting and TR recurrence or reintervention after TV surgery. In the sensitivity analysis of age subgroups, this association was only seen for patients older than age 12 years. Similarly, recent large cohort studies on adults with acquired heart disease have reported such an association when MR was greater than moderate.30,31 The progression of TV annular dilatation and subsequent functional TR are caused by further worsening of pulmonary hypertension, atrial arrhythmias, and right and left ventricle interdependence.32 Although the mechanism of TR recurrence was not systematically available in our study, we hypothesize that the mechanism by which preoperative MR contributes to TR recurrence in our cohort of patients with congenital heart disease is similar. Therefore, the clinical significance of left-sided lesions and the need for lower thresholds for concomitant intervention at the time of TV surgery should be assessed further in properly designed studies.
This study contains a diverse patient population in regard to TV pathophysiology. In our center, all patients with TR deemed moderate or greater in the setting of another surgical indication underwent TV intervention. Concomitant TV intervention associated with either RV pressure or volume overload in congenital heart disease has led to controversial conclusions in previous reports.13,33,34 Most recent studies have shown TV repair offers significant reduction of TR with little added morbidity,13,21,24 therefore supporting more proactive surgical management of significant concomitant TR. Indeed, RV volume or pressure overload as the underlying etiology for TV disease was not associated with TV reintervention, TR deemed moderate or greater at a follow-up, or mortality, and these patients overall did well with repair in our study. Among patients in our series, primary TV disease was present in 20%, with these being divided in 2 main subgroups: iatrogenic lesions and congenital TV disease. This patient population represents a complex subset of TV disease, where leaflet restriction is the main underlying mechanism of TV dysfunction.11,35 In the present study, these patients did well with repair and did not experience added complications on follow-up.
There are several limitations to the current study. This is a retrospective study with a heterogeneous population within an experienced single center. This limits the broader generalizability of any conclusions formed from this work. Seven patients (8%) were excluded for missing preoperative, operative, and follow-up data. A sensitivity analysis could not be undertaken for those missing data. Analysis is also limited by the small numbers in each etiologic category of TV dysfunction and by the limited number of events throughout the follow-up period. Additionally, these small numbers limited the ability to account for individual surgeon volumes or variations in outcomes by era. With a mean follow-up of 3.3 years, this study is also unable to make conclusions regarding long-term outcomes. The multivariable model was not externally validated and therefore calibration and discrimination cannot be provided. Finally, follow-up echocardiograph data were collected retrospectively and therefore interobserver variability was not available.
Conclusions
TV surgery in patients with non-Ebstein congenital TV disease yields good outcomes. TR recurrence after surgery is not infrequent. Patients younger than age 12 year are at higher risk for recurrent TR or TV reintervention at early to midterm follow-up. Preoperative MR deemed moderate or greater increases the risk of recurrent TR or TV reintervention, especially in patients older than age 12 years at early to midterm follow-up. TV replacement itself is not a risk factor for recurrent TR or reoperation with medium-term follow-up.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Supported in part by National Institutes of Health grant R01-HL-155381 to Dr Kalfa.
Accepted for the 100th Annual Meeting of The American Association for Thoracic Surgery.
Drs Blitzer and Bouhout contributed equally to this article.
Appendix E1
Table E1.
Preoperative, operative, and postoperative characteristics for patients with mitral regurgitation (MR) deemed moderate or greater on preoperative echocardiography
| Patient No. | Preoperative TR | Preoperative MR | LV dysfunction | TV surgery∗ | MV surgery† | TR at follow-up | MR at follow-up | Last follow-up (mo) | Composite end point‡ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Severe | Moderate | None | Replacement | No | Severe | Moderate | 7 | Yes |
| 2 | Severe | Moderate | None | Replacement | No | None | Moderate | 72.6 | No |
| 3 | Severe | Severe | None | Repair | Yes | Mild | None | 83.6 | No |
| 4 | Moderate | Moderate | None | Repair | Yes | Moderate | Severe | 60.8 | Yes |
| 5 | Severe | Moderate | Moderate | Replacement | Yes | Moderate | None | 53.5 | Yes |
| 6 | Moderate | Severe | None | Repair | Yes | None | None | 22.7 | No |
TR, Tricuspid valve regurgitation; LV, left ventricle; TV, tricuspid valve; MV, mitral valve.
Repair or replacement.
Yes or no.
TV reintervention or TR moderate or greater.
Table E2.
Intraoperative characteristics
| Variable | Result |
|---|---|
| CPB/perfusion time (min) | 101 (77-126) |
| Crossclamp time (min) | 54 (41-78) |
| Need for ECMO | 0 (0) |
| TV procedure | |
| TV replacement | 19 (22.4) |
| TV repair | 66 (77.6) |
| Commissuroplasty | 11 (12.9) |
| Annuloplasty∗ | 43 (50.6) |
| Leaflet intervention | 5 (5.9) |
| Subvalvular intervention | 9 (10.6) |
| Concomitant surgery/procedure | 84 (98.8) |
| Pulmonary procedure† | 37 (43.5) |
| ASD closure | 35 (41.2) |
| VSD closure | 8 (9.4) |
| PA plasty | 9 (10.6) |
| Maze | 9 (10.6) |
| Mitral valve procedure | 6 (7.1) |
| Glenn | 2 (2.4) |
| Take down of RV-PA shunt | 3 (3.6) |
| Pulmonary venous baffle | 3 (3.6) |
| RV overhaul | 3 (3.6) |
| Aortic valve repair | 2 (2.4) |
| PDA ligation | 3 (3.6) |
| Pulmonary valvotomy | 2 (2.4) |
| TOF repair | 2 (2.4) |
| RA reduction | 1 (1.2) |
| CABG | 2 (2.4) |
| Other | 6 (7.1) |
| PTE | 1 (1.2) |
| Central shunt | 1 (1.2) |
| Ascending aortic aneurysm repair | 1 (1.2) |
| Coronary unroofing | 1 (1.2) |
| Hybrid coronary stent | 1 (1.2) |
Values are presented as median (range) or frequency (%). CPB, Cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; TV, tricuspid valve; ASD, atrial septal defect; VSD, ventricular septal defect; PA, pulmonary artery; RV-PA, right ventricle to pulmonary artery; PDA, patent ductus arteriosus; TOF, tetralogy of Fallot; RA, right atrium; PTE, pulmonary thromboendarterectomy.
Includes suture/DeVega/ring.
Includes PA plasty, arterioplasty, TOF repair, PA plication, PV implantation, and PV replacement.
Table E3.
Univariate analysis: Tricsupid valve (TV) reintervention or TV regurgitation deemed moderate or greater in patients older than age 12 years
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| Age | 1.0 (0.9-1.0) | .11 |
| Male sex | 1.1 (0.3-3.1) | .88 |
| Previous cardiac surgery | 1.0 (0.3-2.9) | .87 |
| Age at first cardiac intervention (y) | 0.9 (0.8-1.5) | .53 |
| Genetic syndromes present | 0.8 (0.7-4.2) | .47 |
| Other cardiac malformations | 0.7 (0.5-1.8) | .59 |
| BSA | 0.9 (1.0-1.1) | .15 |
| Weight | 1.0 (0.9-1.0) | .37 |
| MR moderate or greater | 2.2 (3.1-14.6) | .01 |
| RV dysfunction | 0.7 (0.9-1.6) | .10 |
| LV dysfunction | 3.1 (0.9-8.5) | .23 |
| Etiology of TV lesion | ||
| RV/RVOT/PA obstruction | 0.9 (0.4-2.1) | .81 |
| Right heart volume overload | 1.8 (0.7-4.1) | .20 |
| Iatrogenic lesion | 0.7 (0.4-3.1) | .52 |
| Congenital/isolated TV lesion | 1.7 (0.8-4.3) | .72 |
| Crossclamp time (per min) | 1.0 (1.0-1.1) | .23 |
| CPB time (per min) | 1.0 (1.0-1.1) | .83 |
| Repair∗ | 0.7 (0.5-2.4) | .48 |
Bold values highlight statistically significant findings. CI, Confidence interval; BSA, body surface area; MR, mitral valve regurgitation; RV, right ventricle; LV, left ventricle; RVOT, right ventricular outflow tract; PA, pulmonary artery; CPB, cardiopulmonary bypass.
Reference category is replacement.
References
- 1.Lewis M.J., Ginns J.N., Ye S., Chai P., Quaegebeur J.M., Bacha E., et al. Postoperative tricuspid regurgitation after adult congenital heart surgery is associated with adverse clinical outcomes. J Thorac Cardiovasc Surg. 2016;151:460–465. doi: 10.1016/j.jtcvs.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Nath J., Foster E., Heidenreich P.A. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 3.Aaron B.L., Mills M., Lower R.R. Congenital tricuspid insufficiency. Definition and review. Chest. 1976;69:637–641. doi: 10.1378/chest.69.5.637. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., III, Guyton R.A., et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien S.M., Feng L., He X., Xian Y., Jacobs J.P., Badhwar V., et al. The Society of Thoracic Surgeons 2018 adult cardiac surgery risk models: part 2-statistical methods and results. Ann Thorac Surg. 2018;105:1419–1428. doi: 10.1016/j.athoracsur.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Holst K.A., Dearani J.A., Said S.M., Davies R.R., Pizarro C., Knott-Craig C., et al. Surgical management and outcomes of Ebstein anomaly in neonates and infants: a Society of Thoracic Surgeons Congenital Heart Surgery database analysis. Ann Thorac Surg. 2018;106:785–791. doi: 10.1016/j.athoracsur.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 7.Attenhofer Jost C.H., Connolly H.M., Scott C.G., Burkhart H.M., Warnes C.A., Dearani J.A. Outcome of cardiac surgery in patients 50 years of age or older with Ebstein anomaly: survival and functional improvement. J Am Coll Cardiol. 2012;59:2101–2106. doi: 10.1016/j.jacc.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Said S.M., Burkhart H.M., Dearani J.A. Surgical management of congenital (non-Ebstein) tricuspid valve regurgitation. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2012;15:46–60. doi: 10.1053/j.pcsu.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Petit C.J., Glatz A.C., Qureshi A.M., Sachdeva R., Maskatia S.A., Justino H., et al. Outcomes after decompression of the right ventricle in infants with pulmonary atresia with intact ventricular septum are associated with degree of tricuspid regurgitation: results from the Congenital Catheterization Research Collaborative. Circ Cardiovasc Interv. 2017;10:e004428. doi: 10.1161/CIRCINTERVENTIONS.116.004428. [DOI] [PubMed] [Google Scholar]
- 10.Li V.W., Wong J.Y., Wang C., Chow P.C., Cheung Y.F. Tricuspid regurgitation in adults after repair of right ventricular outflow obstructive lesions. Pediatr Cardiol. 2020;41:1153–1159. doi: 10.1007/s00246-020-02366-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang G., Ma K., Pang K., Zhang S., Qi L., Yang Y., et al. Tricuspid valvuloplasty for isolated tricuspid regurgitation in children. Cardiol Young. 2020;30:1076–1080. doi: 10.1017/S104795112000150X. [DOI] [PubMed] [Google Scholar]
- 12.Katogi T., Aeba R., Ito T., Goto T., Cho Y., Ueda T., et al. Surgical management of isolated congenital tricuspid regurgitation. Ann Thorac Surg. 1998;66:1571–1574. doi: 10.1016/s0003-4975(98)00753-x. [DOI] [PubMed] [Google Scholar]
- 13.Guler S., Reyhancan A., Kubat E., Onan I.S., Kadirogullari E., Onan B. Impact of additional annuloplasty on tricuspid valve and cardiac functions after atrial septal defect closure in adults. J Card Surg. 2020;35:2895–2901. doi: 10.1111/jocs.14905. [DOI] [PubMed] [Google Scholar]
- 14.Gaynor J.W., O'Brien J.E., Jr., Rychik J., Sanchez G.R., DeCampli W.M., Spray T.L. Outcome following tricuspid valve detachment for ventricular septal defects closure. Eur J Cardiothorac Surg. 2001;19:279–282. doi: 10.1016/s1010-7940(01)00577-2. [DOI] [PubMed] [Google Scholar]
- 15.Uehara K., Minakata K., Watanabe K., Sakaguchi H., Yamazaki K., Ikeda T., et al. Tricuspid valve repair for severe tricuspid regurgitation due to pacemaker leads. Asian Cardiovasc Thorac Ann. 2016;24:541–545. doi: 10.1177/0218492316654775. [DOI] [PubMed] [Google Scholar]
- 16.King G., Ayer J., Celermajer D., Zentner D., Justo R., Disney P., et al. Atrioventricular valve failure in Fontan palliation. J Am Coll Cardiol. 2019;73:810–822. doi: 10.1016/j.jacc.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Sinha R., Altin H.F., McCracken C., Well A., Rosenblum J., Kanter K., et al. Effect of atrioventricular valve repair on multistage palliation results of single-ventricle defects. Ann Thorac Surg. 2021;111:662–670. doi: 10.1016/j.athoracsur.2020.03.126. [DOI] [PubMed] [Google Scholar]
- 18.Muntaner C.D., King G., Zannino D., Alphonso N., Finucance K., Winlaw D., et al. Poor late outcomes after tricuspid valve repair in a single ventricle: experience of 103 patients. Ann Thorac Surg. 2021;111:987–994. doi: 10.1016/j.athoracsur.2020.05.070. [DOI] [PubMed] [Google Scholar]
- 19.Filippov A.A., Del Nido P.J., Vasilyev N.V. Management of systemic right ventricular failure in patients with congenitally corrected transposition of the great arteries. Circulation. 2016;134:1293–1302. doi: 10.1161/CIRCULATIONAHA.116.022106. [DOI] [PubMed] [Google Scholar]
- 20.Koolbergen D.R., Ahmed Y., Bouma B.J., Scherptong R.W., Bruggemans E.F., Vliegen H.W., et al. Follow-up after tricuspid valve surgery in adult patients with systemic right ventricles. Eur J Cardiothorac Surg. 2016;50:456–463. doi: 10.1093/ejcts/ezw059. [DOI] [PubMed] [Google Scholar]
- 21.Mongeon F.P., Connolly H.M., Dearani J.A., Li Z., Warnes C.A. Congenitally corrected transposition of the great arteries ventricular function at the time of systemic atrioventricular valve replacement predicts long-term ventricular function. J Am Coll Cardiol. 2011;57:2008–2017. doi: 10.1016/j.jacc.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Lo Rito M., Grandinetti M., Muzio G., Varrica A., Frigiola A., Micheletti A., et al. Results for tricuspid valve surgery in adults with congenital heart disease other than Ebstein's anomaly. Eur J Cardiothorac Surg. 2019;56:706–713. doi: 10.1093/ejcts/ezz093. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Garcia A.C., Dimopoulos K., Boutsikou M., Martin-Garcia A., Kempny A., Alonso-Gonzalez R., et al. Tricuspid regurgitation severity after atrial septal defect closure or pulmonic valve replacement. Heart. 2020;106:455–461. doi: 10.1136/heartjnl-2019-315287. [DOI] [PubMed] [Google Scholar]
- 24.Deshaies C., Trottier H., Khairy P., Al-Aklabi M., Beauchesne L., Bernier P.L., et al. Tricuspid intervention following pulmonary valve replacement in adults with congenital heart disease. J. Am Coll Cardiol. 2020;75:1033–1043. doi: 10.1016/j.jacc.2019.12.053. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy P.M., Bhudia S.K., Rajeswaran J., Hoercher K.J., Lytle B.W., Cosgrove D.M., et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004;127:674–685. doi: 10.1016/j.jtcvs.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Bartlett H.L., Atkins D.L., Burns T.L., Engelkes K.J., Powell S.J., Hills C.B., et al. Early outcomes of tricuspid valve replacement in young children. Circulation. 2007;115:319–325. doi: 10.1161/CIRCULATIONAHA.106.618652. [DOI] [PubMed] [Google Scholar]
- 27.Hwang H.Y., Kim K.H., Kim K.B., Ahn H. Treatment for severe functional tricuspid regurgitation: annuloplasty versus valve replacement. Eur J Cardiothorac Surg. 2014;46:e21–e27. doi: 10.1093/ejcts/ezu224. [DOI] [PubMed] [Google Scholar]
- 28.Moraca R.J., Moon M.R., Lawton J.S., Guthrie T.J., Aubuchon K.A., Moazami N., et al. Outcomes of tricuspid valve repair and replacement: a propensity analysis. Ann Thorac Surg. 2009;87:83–88. doi: 10.1016/j.athoracsur.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Vassileva C.M., Shabosky J., Boley T., Markwell S., Hazelrigg S. Tricuspid valve surgery: the past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg. 2012;143:1043–1049. doi: 10.1016/j.jtcvs.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Bertrand P.B., Overbey J.R., Zeng X., Levine R.A., Ailawadi G., Acker M.A., et al. Cardiothoracic Surgical Trials Network (CTSN) Progression of tricuspid regurgitation after surgery for ischemic mitral regurgitation. J Am Coll Cardiol. 2021;77:713–724. doi: 10.1016/j.jacc.2020.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kavsur R., Iliadis C., Spieker M., Brachtendorf B.M., Tiyerili V., Metze C., et al. Predictors and prognostic relevance of tricuspid alterations in patients undergoing transcatheter edge-to-edge mitral valve repair. EuroIntervention. 2021;17:827–834. doi: 10.4244/EIJ-D-20-01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiran A., Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol. 2009;53:401–408. doi: 10.1016/j.jacc.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 33.Kurkluoglu M., John A.S., Cross R., Chung D., Yerebakan C., Zurakowski D., et al. Should tricuspid annuloplasty be performed with pulmonary valve replacement for pulmonary regurgitation in repaired tetralogy of Fallot? Semin Thorac Cardiovasc Surg. 2015;27:159–165. doi: 10.1053/j.semtcvs.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Lueck S., Bormann E., Rellensmann K., Martens S., Rukosujew A. Impact of additional tricuspid valve annuloplasty in TOF patients undergoing pulmonary valve replacement. J Cardiovasc Surg (Torino) 2019;60:268–273. doi: 10.23736/S0021-9509.18.10385-5. [DOI] [PubMed] [Google Scholar]
- 35.Bol-Raap G., Weerheim J., Kappetein A.P., Witsenburg M., Bogers A.J. Follow-up after surgical closure of congenital ventricular septal defect. Eur J Cardiothorac Surg. 2003;24:511–515. doi: 10.1016/s1010-7940(03)00430-5. [DOI] [PubMed] [Google Scholar]