Abstract
Objective
To describe the participation of the environment in the childhood obesity epidemic, since childhood obesity currently represents a great challenge, with high prevalence worldwide, including in Brazil.
Data source
Survey of articles published in the last 10 years in PubMed, evaluating the interface between the environment and childhood obesity.
Data synthesis
Recent studies show that the environment is very important in the etiopathogenesis of obesity and its comorbidities. Therefore, factors such as air pollution, exposure to chemical substances that interfere with the metabolism, excessive consumption of ultra-processed foods, changes in the intestinal microbiota, and sedentary lifestyle are associated with increased obesity, insulin resistance, type 2 diabetes, and changes in lipid metabolism. These factors have a greater impact on some stages of life, such as the first thousand days, as they affect the expression of genes that control the adipogenesis, energy expenditure, and the mechanisms for hunger/satiety control.
Conclusions
Environmental aspects must be taken into account in the prevention and treatment of childhood obesity, both from the individual and the population point of view, with adequate and comprehensive public health policies.
Keywords: Childhood obesity, Ultra-processed food, Epigenetics, Sedentary lifestyle, Endocrine disruptors, Microbiota
Introduction
In 2019, approximately 38.2 million children under the age of 5 were overweight or obese in the world. Once considered a problem of high-income countries, overweight and obesity are increasing in low- and middle-income countries, especially in the urban areas. In Africa, the number of children under five years of age with excess weight has increased by almost 24% since 2000. In Brazil, obesity in the age group from 5 to 9 years old has reached 17.6% and 12.4% of girls and boys, respectively. In this age group, about a third of children have excess weight, a warning sign for the risk of obesity in childhood. Obesity threatens to undo the gains that have been obtained in life expectancy over the past two centuries and has become one of the most important global health challenges of the 21st century.1
This whole scenario seems to have been aggravated during the COVID-19 Pandemic. A recent study analyzed the electronic health records of 191,509 American individuals between 5 and 17 years old, collected from March 2019 to January 2021. The researchers found an increase in overweight and obesity, especially in children aged between 5 and 11 years, whose percentage increased by 8.7% during the Pandemic. Currently, 45.7% of children in this age group are overweight. Among adolescents aged 12 to 15 years, overweight increased by 5.2% compared to the period before the Pandemic, and among individuals aged 16 to 17 years, the increase was 3.1%.2
The increase in the prevalence of excess weight in children has contributed to the increase in the global burden of chronic diseases, such as obesity in adulthood, mental health problems, diabetes, cardiovascular disease, and some types of cancer. The obesogenic environment, one of the factors responsible for the increase in obesity rates in children and adolescents, is defined as "the sum of influences that the surroundings, opportunities, or conditions of life have on promoting obesity in individuals or populations". Modifiable environmental factors manifest themselves with an indirect effect on the individual's eating behavior and physical activity.3
Although previous publications generally addressed associated individual factors, such as knowledge, motivation, and also genetic factors, more recent epidemiological studies place obesity in a broader and more complex socioecological context, where the environment also plays a key role in the formation of individual behaviors, emphasizing the importance of intersectoral actions.
The mechanisms that explain the associations between multiple urban exposures and the development of childhood obesity are still poorly understood. Air pollution can interfere with molecular mechanisms that participate in the pathogenesis of obesity. Noise has been associated with sleep deprivation and the increased production of stress-related hormones. Green spaces built environments, and the circulation of vehicles can partially determine the levels of air and noise pollution and, in turn, influence the prevalence of excess weight. Additionally, the urban environment can modulate a variety of weight-related behaviors, including well-established obesity risk factors such as diet, physical activity, sedentary behavior, sleep duration, and well-being. The interrelationship between all the aforementioned factors highlights the complexity of the involved mechanisms and the challenge of intervention proposals.
A systematic review and meta-analysis showed a strong association between air pollution and obesity in the pediatric age group, through biological and behavioral mechanisms,4 while greater availability and exposure to green spaces has been associated with increased levels of physical activity and hours of sleep.5 A better understanding of the associations between urban exposures and behaviors related to excess weight is essential for the development of effective health promotion strategies in the community, but there are few studies on the topic in children, especially in country.6
In Brazil, Ordinance GM/MS N. 1862 of August 10th, 2021, instituted the National Strategy for the Prevention and Care of Childhood Obesity, aiming at preventing the advance of obesity and contributing to the improvement of children's health and nutrition. Among the actions, the Ministry of Health launched a campaign to prevent childhood obesity, of which the motto is: "Let's prevent childhood obesity: 1,2,3, now!". The action reinforces the need to encourage children to adopt a healthy diet, combined with the practice of physical activities. This year, the Physical Activity Guide for the Brazilian Population was also launched, aiming to support managers and health professionals in encouraging this habit.7
A recent systematic review and meta-analysis were carried out aiming to identify the interventions being carried out in municipalities to reduce the prevalence of obesity, and thus provide recommendations that may be useful for researchers, public policymakers, political and health system leaders in the creation of strategies to prevent and fight obesity. The publications were predominantly from the USA and showed that interventions at different levels and components, at the individual, community, and regional levels, carried out simultaneously and altogether, are necessary to deal with obesity. The authors emphasized that such strategies will also be beneficial to the environment and will make the health of the individual and that of the planet be considered as one.8
Finally, it is worth reflecting on something when thinking about the causes of obesity –an inadequate diet associated with the lack of physical activity soon comes to our minds. Thus, it seems logical for many to treat individuals with obesity as unmotivated to adopt healthy lifestyle habits. In this individualized, simplified, and at times stigmatizing look, its complex and multifactorial etiology is not taken into account, which involves a deep relationship with the way the socioecological environment is structured.
Epigenetics, environment and obesity (focus on the first one thousand days of life)
In early life, represented by the intrauterine period and the first years of life, cells, tissues, organs, and systems are immature and have greater plasticity, therefore being more susceptible to environmental influences and nutritional factors in their development and maturation.9
The exposome can be defined as the cumulative exposure that an organism can be exposed to from birth to death, with an impact on health or the development of diseases. This exposure can be either endogenous (e.g., infections and psychological stress) or exogenous (e.g., pollution and smoking). Some of these exposures can result in responses, such as epigenetic changes secondary to environmental and nutritional factors; therefore, the exposome may be related to the risk of developing chronic non-communicable diseases, such as obesity.
The environment and nutritional factors can modify the exposome through permanent structural changes, cell aging acceleration and gene expression modification (epigenetic factors).
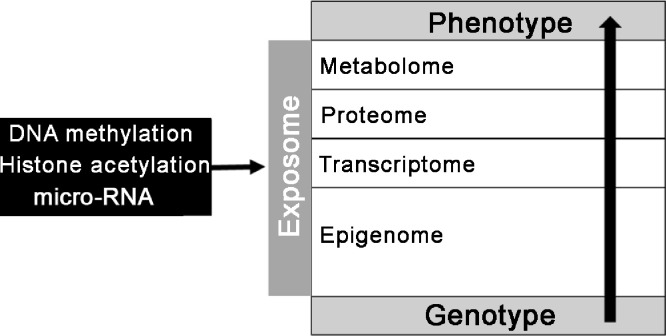
The epigenetic factors involve DNA methylation, histone acetylation, and the presence of micro-RNAs. They interfere with DNA transcription and translation in a complex and dynamic way, increasing or reducing gene expression. The main target of epigenetic factors are the CpG islands, which are regions located close to the gene promoter regions.10
DNA methylation occurs when a methyl group is incorporated into the structure of one or more genes. Methylated and hypomethylated genes have their expression silenced and increased, respectively. Histones are structures in which the DNA is coiled and compacted; when they are in the deacetylated (grouped) form, there is greater difficulty in DNA transcription, and in the acetylated (relaxed) form, the DNA strand is more exposed and, therefore, can be more easily transcripted. Micro-RNAs are small RNAs (21 to 24 nucleotides) that influence the translation of messenger RNA and, therefore, protein synthesis10 (Fig. 1).
Figure 1.
Schematic representation of the exosome.
During pregnancy, the maternal nutritional status (malnutrition and obesity), diseases (diabetes and hypertension), infections, exposure to chemical factors (tobacco, dioxins, pesticides, bisphenol A and arsenic), and stress influence the risk of obesity in the offspring, through of epigenetic factors.10 Paternal smoking and obesity are also related to epigenetic markers in germ cells that increase the risk of obesity in the offspring throughout life.11
Publications related to the Dutch Hunger Winter Cohort describe the long-term consequences of acute malnutrition during pregnancy on the health of the offspring (children and grandchildren). The children of malnourished women in early pregnancy were at greater risk for developing cardiovascular disease, diabetes, obesity, and some types of cancer; in turn, the male grandchildren of women who went hungry at the end of the pregnancy are at greater risk of obesity, especially the perivisceral type.12
Some of these findings by the Dutch Hunger Winter Cohort can be attributed to epigenetic factors. Individuals who were exposed to hunger in the intrauterine period had, almost six decades later, reduced DNA methylation of the insulin-like growth factor 2 (IGF-2) gene – an important mediator of cell proliferation, growth and differentiation – when compared to unexposed siblings of the same sex.13
The UK Pregnancies Better Eating and Activity Trial (UPBEAT-Trial) is a clinical trial of over 1500 obese pregnant women in the UK who were randomly assigned to two groups. The control group received the traditional prenatal guidelines proposed by the NHS (National Health System) of the United Kingdom, and the intervention group was submitted to eight in-person meetings that involved systematized strategies for nutritional guidance, encouraging the practice of physical activities and lifestyle improvement. The authors followed the women from the beginning of pregnancy until the delivery and continued to follow their children.14 In this study, the women from the intervention group adopted more adequate eating habits and lifestyles and gained 0.55 kg less weight during the pregnancy when compared to the control group.14 The children born to the women from the intervention group also had lower adiposity at six months of life, and this protective effect was greater in the group of breastfed infants.15
In the UPBEAT trial, maternal glycemic fluctuations during pregnancy were associated with significant changes in the epigenome of newborns in a different way in the intervention and control groups, suggesting that changes in lifestyle, diet, and physical activity influenced the epigenetic markers of the children. These findings open a positive perspective on the possibility of interventions based on education and lifestyle change that lead to the modification of the course of chronic non-communicable diseases through epigenetic factors in children of women from risk groups, such as obese pregnant women.16
Diet and postnatal growth also interfere with the risk of obesity throughout life17 and it is known that this association also occurs, at least in part, through epigenetic factors that have been previously described in human studies. In the ALSPAC cohort study (UK Avon Longitudinal Study of Parents and Children), the authors identified that excessive weight gain in the first year of life (weight-for-age z-score variation > 0.67) was associated with higher methylation (1%) in two CpG islands,cg01379158 (NT5M) and cg11531579 (CHFR), up to the age of 7 years. Methylation of the cg11531579 island (CHFR) was also associated with the persistence of overweight/obesity in adolescence.18
The practice of breastfeeding is one of the factors associated with protection against obesity throughout life. A systematic review described that breastfeeding is associated with a reduction of about 25% in the risk of overweight in adulthood.19 Some of the proposed mechanisms to explain this relationship are the composition of breast milk, which adapts to the infant's needs, including aspects related to the number of nutrients; the presence of adipokines, such as leptin, which influence the hypothalamic hunger/satiety center; the way the infant empties the breast (more slowly compared to the supply of milk from the bottle), appetite self-regulation and, more recently, epigenetic factors in this process have also been described.
Breastfeeding influences the expression of several genes through DNA methylation and the presence of numerous micro-RNAs in breast milk; these micro-RNAs are species-specific and act on energy expenditure, immune system, inflammatory response, and the maturation of the gastrointestinal tract.20
A cohort study compared the influence of breastfeeding on DNA methylation in infants who were exclusively, partially breastfed, or those fed exclusively formula. The authors showed that exclusive breastfeeding up to 4 to 6 months of life was associated with a higher percentage and diversity of DNA methylation between birth and ten years of age.21
In another publication, the authors assessed the impact of maternal obesity on the amount of six micro-RNAs in human milk, which are known to be involved in adipogenesis and glucose metabolism (miR-148a, miR-30b, miR-29a, miR-29b, miR-let-7a, and miR-32). The amounts of miR-148a and miR-30b were, respectively, 30% and 42% lower in the breast milk of overweight/obese women when compared to those with normal BMI in the first month of lactation. The amount of miR-148a and miR-30b was positively and negatively associated, respectively, with the weight, percentage of fat mass, and lean mass of the one-month-old infant. This proves that the maternal nutritional condition influences the infant's weight and body composition through epigenetic factors.22
In Brazil, approximately 15%, 30%, and 60% of infants, children/adolescents, and the adult population are overweight/obese. Moreover, less than 50% of children are exclusively breastfed until they are six months of age. From this perspective and in light of current knowledge, health promotion and integrated care for women and children, involving healthy eating, physical activity, monitoring of nutritional status, and the encouragement to promote breastfeeding, become central points to reduce the risk of excess weight for this and future generations.
Food and health risks: fresh and ultra-processed foods
The World Health Organization (WHO) and the Food and Agriculture Organization (FAO) of the United Nations have recommended for more than 20 years that governments draw up food guides. After all, people consume foods and not specific nutrients. These guides should ultimately advise and encourage people to adopt healthier lifestyles and make better food choices. Thus, food guides are an essential health technology to improve food and nutrition standards and promote the health of populations.
The Food Guide for the Brazilian Population, one of the most advanced ones, uses the NOVA classification as a parameter, which organizes foods according to the purpose and extent of processing – and not according to the type of nutrient predominant in the food.23 In the NOVA classification, foods are categorized into four groups: fresh and minimally processed, culinary ingredients, processed and ultra-processed. Ultra-processed foods (UPFs) should be avoided; they are industrial formulations comprising mostly substances extracted from foods (oils, fats, sugar, starch, proteins), derived from food constituents (hydrogenated fats, modified starch), or synthesized in the laboratory (dyes, flavors and flavor enhancers). They have more than five ingredients on their labeling; they are hyper-palatable, easy to prepare, and have a longer shelf life. Moreover, they harm the environment due to a large amount of packaging that contains this type of food. These products are aggressively marketed by the food industry to promote their purchase and shape food preferences, and children are the main consumers of UPFs. Currently, UPFs represent 65.4% and 66.2% of the energy consumption of schoolchildren in the United Kingdom and the United States, respectively.24,25 In the context of the importance of the first one thousand days of life in the individual's health trajectory, in 2019, the Ministry of Health launched the Food Guide for children under two years old, which emphasizes the importance of exclusive breastfeeding up to two years old and complemented by a healthy, opportune and responsive diet for up to two years of age or older.26
The rising consumption of UPFs, including in low- and middle-income countries, has resulted in a parallel increase in the prevalence of obesity worldwide, suggesting that excessive consumption may be a key driver of the obesity epidemic and other diet-related chronic non-communicable diseases.
A cohort study involving 9025 British children described that the trajectories of body mass index, fat mass index, weight, and abdominal circumference from 7 to 24 years of age showed a greater increase in children who consumed the highest quintile of UPFs when compared to those who consumed the lowest consumption quintile. The findings emphasize the importance of robust public health measures that promote the consumption of fresh and minimally processed foods and discourage the consumption of UPFs among children.27 Faster and more effective public health actions aimed to reduce UPF consumption and exposure to UPF advertising are urgently needed to address childhood obesity. Unfortunately, UPF advertising goes in the opposite direction to the Guides. The authors need to progress in the regulation of UPFs and food education to amplify the effective implementation of the 'Guides' proposals. Healthy eating involves much larger issues, as it is also related to sustainability, the food production chain, regional foods, and economic issues, among others.
Environmental contaminants and obesity
Recently, the association between endocrine disruptors (ED) and the global obesity epidemic has gained prominence. Some experimental studies indicate that these substances, also called obesogenic substances, can influence the development and progression of obesity.28
The ED is defined as chemical substances exogenous to the body and that can interfere with hormonal axes and cause adverse health events to an individual, their offspring and the environment.29
More than 800 chemicals are listed in the World Health Organization/United Nations Environment Program book, and that list continues to grow, including industrial products, pesticides, plastic-derived products, phytoestrogens, and several heavy metals.
Obeso gens can act directly on adipose cells, causing adipocyte hyperplasia or hypertrophy, but they can also act indirectly by controlling hunger/satiety, reducing the basal metabolic rate, and interfering with the intestinal microbiota.30 Obeso gens can cause changes in hormonal function through the activation or inhibition of nuclear receptors (estrogen receptor, androgen receptor, thyroid hormone receptor, Retinoic acid X alpha receptor/peroxisome proliferator-activated receptor gamma (PPARG), glucocorticoid receptor). Activation of the heterodimer Retinoic acid X receptor/PPARG favors the differentiation of pre-adipocyte into adipocyte and regulates lipid biosynthesis and storage.31
Several substances have been associated with adipogenesis, glucose intolerance, and diabetes, such as bisphenol A, phthalates, and some metals, such as arsenic.30
A recent systematic review included several articles from different countries, with studies on the association of exposure to different ED and obesity, in children and adults. The authors found a positive association between exposure to bisphenol A and obesity (both generalized and centripetal) in adults and between exposure to phthalates (more specifically to some types of phthalate) and obesity in children and adolescents.32
Epidemiological studies suggest that prolonged and persistent exposure to low concentrations of organic pollutants, e.g., pesticides, may be associated with the risk of obesity and complications such as high blood pressure, diabetes, and dyslipidemia. The main pollutants associated with obesity in adults are dichlorodiphenyltrichloroethane (DDT), dichlorodiphenyldichloroethylene (DDE), hexachlorobenzene (HCB), and beta-hexachlorocyclohexane (beta-HCH).33 These data were also observed in experimental studies that describe the activation of PPARG as a possible mechanism of action of these substances. In addition, exposure to these pollutants during intrauterine life is a matter of great concern, as it can lead to future harmful consequences for health.29
Food processing and packaging are common sources of ED; therefore, it can be assumed that food and beverage packaging may also be associated with a higher risk of obesity, as they contain plastic compounds, such as bisphenol A, in their composition. Some authors suggest that policies to fight obesity should take into account obese substances present in the environment.34
A recent review of recommendations to reduce the risk of obesity by avoiding contact with obesogenic substances emphasized the role of individual actions, such as reducing the consumption of ultra-processed and canned foods, the use of cosmetics with parabens, phthalates, triclosan, the use of plastic containers to store food and, finally, not to heat the food in the microwave oven (especially during the fetal/neonatal stage); actions by the scientific communities, such as the development of research for faster elimination of ED by the body, prevention/treatment of metabolic diseases secondary to exposure to ED and government actions (regulation of the use of these substances).34
Therefore, it is important to emphasize that we are exposed to several disrupting substances in our daily lives, with different chemical characteristics and action mechanisms, and that, according to experimental studies, they can alter the metabolism, leading to a greater risk of obesity and its comorbidities. However, it is important to remember that clinical studies have greater difficulty in demonstrating this association since there is still much controversy regarding the way these substances are quantified. Moreover, little is known about the summation effect of exposure to different substances. Anyway, the importance of these studies is suggesting that disruptors may also show action on metabolism and, thus, individuals can assume the precautionary principle and choose to avoid exposure to these substances.
Sedentary lifestyle, lifestyle and urbanization
The increasing concentration of families in cities with less mobility increased urban violence, and the scarcity of spaces available for play, recreation, and sports practices play an important role in the development of obesity.35
A sedentary lifestyle in the pediatric age group, represented by low physical activity and high screen time, is a serious public health problem. Only 23% and 19% of children aged 11 and 13, respectively, reach the recommended level of physical activity, which is 60 min of moderate-to-vigorous activity per day, according to a survey involving 38 countries.36 In parallel, evidence suggests that today's children and adolescents spend less time in outdoor activities than their parents at the same age.37 Unfortunately, this situation got much worse after the start of the COVID-19 epidemic.38
The change in this scenario, according to recent research, involves increased exposure to adequate environments for outdoor leisure activities. Places with less vehicle traffic, homes with access to backyards, and the presence of green areas in the surroundings are associated with more time for playing and recreational activities in general,39 and these changes in habits are associated with a reduction in the sedentary lifestyle and improvement in cardiorespiratory fitness in children and adolescents.40
The school is also a promoter of the practice of lifestyle changes and increase in a physical activity aiming at the prevention41,42 and reduction of overweight/obesity43,44 in the pediatric age group in developed and developing countries.
The greater availability of spaces and equipment that can be used by the general population, safely and free of charge, for outdoor activities, is essential to reduce sedentary lifestyles in the general population. A scientific document published in 2019 by the Brazilian Society of Pediatrics in partnership with Instituto Alana's Child and Nature Project provides practical guidelines for the promotion of outdoor leisure activities for the pediatric age group.45
In 2021, the Brazilian Ministry of Health launched the Physical Activity Guide for the Brazilian Population, which provides recommendations for children and adolescents in the pediatric age group, in line with those of the World Health Organization (Table 1), aiming to promote a healthy lifestyle for the family as a whole.
Table 1.
Guidelines for the practice of physical activities for children and adolescents.
| Age | Time | Examples |
|---|---|---|
| Less than one year | At least 30 min a day on their stomach, which can be distributed throughout the day | Plays and games involving activities that place the child on their stomach or sitting, moving arms and legs and stimulating the child to reach, hold, pull, push, crawl, crawl, roll, balance with or without support, sit and stand, among others. |
| 1 to 2 years | At least 3 h a day of physical activities of any intensity, which can be distributed throughout the day. | Plays and games involving activities such as the balance on two feet, balance on one foot, turning, crawling, walking, running, hopping, climbing, jumping, throwing, tossing, bouncing, and holding, among others; |
| 3 to 5 years | At least 3 h a day of physical activities of any intensity, with at least 1 h of moderate-to-vigorous intensity activity that can be accumulated throughout the day. | Play and games involving activities such as walking, running, spinning, kicking, throwing, jumping, and crossing or climbing objects, among others. At this age, physical activity can also be performed during the school physical education class, swimming, gymnastics, wrestling, dancing, and sports. Also, through active commuting, such as on foot or by bicycle, always accompanied by parents or guardians. |
| 6 to 17 years | At least 60 min or more of moderate-to-intense physical activity during the day (child or adolescent can speak with some difficulty while moving and cannot sing). This time can be divided into small blocks or all at once. As part of these 60 min or more a day, including at least 3 days a week of muscle and bone-strengthening activities (jumping, jumping rope, pulling, and pushing). | Choose and alternate activities that the child or adolescent likes the most (walking, running, flying a kite, dancing, swimming, cycling, surfing, playing soccer, volleyball, basketball, bocce ball, tennis, shuttlecock or frescobol, doing gymnastics or martial arts, or participating in games and plays such as hide-and-seek, tag, jump rope, elastic jump, dodgeball, play tacobol, among others. Include activities of daily living such as commuting to school, recess, physical education, and household chores shared with the family. |
Source: Ministry of Health. Guia de Atividade Física para a População Brasileira, 2021.7
Although the document does not have specific recommendations for overweight and obese children and adolescents, all strategies to reduce the sedentary lifestyle should be encouraged in this group, aiming at better weight control.46
Microbiome, environment and obesity
The intestinal microbiota is made up of trillions of microorganisms, with more than 100 times the amount of genes compared to the human genome. These microorganisms that reside in the gastrointestinal tract, mainly in the distal colon, are of great importance in maintaining the ' 'host's metabolism homeostasis.47
The human microbiota develops from birth to around two years of age and is relatively stable after 31 months of life.48 During birth, the newborn is exposed to several types of bacteria, depending on factors such as type and duration of delivery and oxygenation. The magnitude of the influence of these factors on the microbiota is still controversial in the literature. After birth, the growth of the intestinal microbiota continues, and after approximately 31 months of age, the microbiota is similar to that of the adult and is unique for each individual. The microbiota depends on factors such as type of diet, presence, and duration of maternal breastfeeding, use of antibiotics, physical activity, hygiene, sleep quality, and stress.49 The ' 'host's genome is essential for controlling the composition of the intestinal microbiota, but external factors can also contribute to altering the populations of intestinal microorganisms.50
The Human Microbiome Project Consortium revealed that the most frequently found microorganisms in the human microbiota are Bacteroidetes, Firmicutes, and Proteobacteria.51,52 The microbiota of thin individuals and individuals with obesity show mostly the presence of the phyla Bacteroidetes (23%) and Firmicutes (64%).53 Despite the importance of Firmicutes and Bacteroidetes, studies have not been consistent regarding the ratio between the amounts of the two phyla and metabolic risk. Obesity has been more associated with a reduction in the diversity of microorganisms (lower variety of gene load) than the amount of each species.49 Studies have demonstrated that individuals with lower gene counts of microorganisms also have a higher risk of insulin resistance, high concentrations of leptin, free fatty acids, and triglycerides, in addition to a greater pro-inflammatory profile.54
One of the action mechanisms of the microbiota in metabolic control is through the fermentation of non-digestible dietary fibers, producing short-chain fatty acids (SCFA), which are responsible for up to 10% of daily energy needs. The SCFA mainly include propionate, butyrate and acetate, and supply energy for the colonic epithelium, liver, and peripheral tissues.49 The SCFA act in the ' 'host's metabolism through some G protein-coupled receptors (GPR 41, GPR43, GPR119, GPR109A), which are abundant in adipocytes, intestinal epithelial, and immune cells. Each receptor can be activated by a different SCFA and has different actions. For instance, the activation of GPR41 and GPR43 induces the secretion of peptide YY (PYY) by intestinal cells. PYY acts directly on hypothalamic centers, reducing food intake. Moreover, the activation of GPR 41 also increases leptin expression by adipocytes. The activation of these receptors reduces lipolysis, decreasing the serum concentration of free fatty acids and promoting an anti-inflammatory state.53
The SCFA stimulates the secretion of glucagon-like peptide-1 (GLP-1), and the total concentration of SCFA, propionate and acetate is inversely proportional to insulin resistance.49
Another action mechanism is mediated by the reduced activity of fasting-induced adipose factor (FIAF). This factor inhibits lipoprotein lipase (LPL) and is produced by the intestine, liver, and adipose tissue. The intestinal microbiota suppresses FIAF in the ileum and increases LPL activity, and therefore, increases the cellular uptake of free fatty acid and triglyceride storage in the adipocyte.53
The microbiota is also important in bile acid metabolism. The change in the population of intestinal microorganisms prevents the conversion of primary bile acids, resulting in their accumulation and the reduction of secondary bile acids. Primary bile acids act more specifically on G protein-coupled receptors (TGR5), while secondary bile acids act mainly on the Farnesoid X receptor (FXR). The activation of these receptors results in changes in lipid and carbohydrate metabolism, energy expenditure, and inflammation.55
Another important role of the microbiota is strengthening the intestinal mucosal barrier. Injury to this barrier is associated with an increased risk of infection, low-grade inflammation and increased oxidative stress.53
Therefore, the intestinal microbiota has an important influence on the host's metabolism. However, in the case of obesity and its comorbidities, it is still difficult to determine what is the cause or the consequence due to controversial studies, differences in responses in animal and human studies, and the complex interaction of trillions of microorganisms and the host, with the activation of several molecular mediators. It remains a large field of research, not only to determine the molecular mechanisms involved but also in the search for an intervention that can lead to a healthier microbiota and, thus, promote a better metabolic balance.
Conclusion
The increasing prevalence of obesity in the pediatric age group has contributed to the increase in the global burden of chronic diseases such as adult obesity, mental health problems, diabetes, cardiovascular disease, and cancer. The disease etiology is complex and multifactorial and has been largely explained by the interaction of genetic factors, lifestyle behaviors, and environmental exposures. It is also known that the formation of eating habits in childhood has a prolonged effect and, therefore, the preference for an exaggerated consumption of ultra-processed foods is a matter of concern, especially in this age group. In addition to their high caloric content at the expense of unhealthy carbohydrates and fats, they contain salt and/or numerous chemical additives, which make them attractive and hyper-palatable, encouraging excessive and careless consumption. In this sense, the dissemination of information contained in the Food Guides that refer to the priority consumption of fresh or minimally processed foods and emphasize the importance of avoiding ultra-processed foods, together with the proposal by the National Health Surveillance Agency (ANVISA, Agência Nacional de Vigilância Sanitária) which approved in October 2020, a label template for processed and ultra-processed foods containing a warning seal informing of the excess salt, sugar and saturated fat contents are essential to support measures for success in facing the current scenario.
There is also growing evidence regarding the epigenetic aspects in early life, such as intrauterine life and the first two years of life, which can define the trajectory of health or diseases such as obesity. Therefore, currently, considering the new knowledge, the exclusive role of the health team in the prevention/treatment of childhood obesity focusing on nutritional advice, fighting sedentary lifestyles, and encouraging the regular practice of physical activity is not enough. Changes in eating patterns and physical activity practice are often the results of environmental and social changes associated with the development and the lack of supportive policies in sectors such as health, agriculture, transport, urban planning, environment, food processing, distribution, marketing, and education. Intersectoral measures, combined and public health measures, are urgent and must take into account the aspects mentioned in the prevention and fight against obesity.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.World Obesity Federation. Atlas of childhood obesity. [cited 2021 Jul 31]. Available from:ttps://data.worldobesity.org/publications/11996-Childhood-Obesity-Atlas-Report-ART-V2.pdf
- 2.Woolford S.J., Sidell M., Li X., Else V., Young DR., Resnicow K., et al. Changes in body mass index among children and adolescents during the COVID-19 pandemic. JAMA. 2021;326:1434–1436. doi: 10.1001/jama.2021.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia P. Obesogenic environment and childhood obesity. Obes Rev. 2021;22(S1):e13158. doi: 10.1111/obr.13158. [DOI] [PubMed] [Google Scholar]
- 4.Parasin N., Amnuaylojaroen T., Saokaew S. Effect of air pollution on obesity in children: a systematic review and meta-analysis. Children. 2021;8:327. doi: 10.3390/children8050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo Y.N., Huang W.Z., Liu X.X., Markevych I., Bloom M.S., Zhao T., et al. Greenspace with overweight and obesity: a systematic review and meta-analysis of epidemiological studies up to 2020. Obes Rev. 2020;21:e13078. doi: 10.1111/obr.13078. [DOI] [PubMed] [Google Scholar]
- 6.de Bont J., Márquez S., Fernández-Barrés S., Warembourg C., Koch S., Persavento C., et al. Urban environment and obesity and weight-related behaviours in primary school children. Environ Int. 2021;155 doi: 10.1016/j.envint.2021.106700. [DOI] [PubMed] [Google Scholar]
- 7.Ministério da Saúde do Brasil. Guia de Atividade Física para a População Brasileira. Brasília – Distrito federal, 2021: 50p. [Cited 2021 Sep 5]. Available from:http://bvsms.saude.gov.br/bvs/publicacoes/guia_atividade_fisica_populacao_brasileira_material_suplementar.pdf
- 8.Danielli S., Coffey T., Ashrafian H., Darzi A. Systematic review into city interventions to address obesity. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2020.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson M.A., Cooper C., Aihie Sayer A., Eendebak R.J., Clough G.F., Beard J.R. Developmental aspects of a life course approach to healthy ageing. J Physiol. 2016;594:2147–2160. doi: 10.1113/JP270579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Twinn D.S., Hjort L., Novakovic B., Ozanne S.E., Saffery R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia. 2019;62:1789–1801. doi: 10.1007/s00125-019-4951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huypens P., Sass S., Wu M., Dyckhoff D., Tschöp M., Theis F., et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet. 2016;48:497–499. doi: 10.1038/ng.3527. [DOI] [PubMed] [Google Scholar]
- 12.Bleker L.S., de Rooij S.R., Painter R.C., Ravelli A.C., Roseboom TJ. Cohort profile: the Dutch famine birth cohort (DFBC)- a prospective birth cohort study in the Netherlands. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-042078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heijmans B.T., Tobi E.W., Stein A.D., Putter H., Blauw G.J., Susser E.S., et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poston L., Bell R., Briley A.L., Godfrey K.M., Nelson S.M., Oteng-Ntim E., et al. NIHR Journals Library; Southampton (UK): 2017. Improving pregnancy outcome in obese women: the UK pregnancies better eating and activity randomised controlled Trial. PMID: 28671801. [PubMed] [Google Scholar]
- 15.Patel N., Godfrey K.M., Pasupathy D., Levin J., Flynn A.C., Hayes L., et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int J Obes. 2017;41:1018–1026. doi: 10.1038/ijo.2017.44. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antoun E., Kitaba N.T., Titcombe P., Dalrymple K.V., Garratt E.S., Barton S.J., et al. Maternal dysglycaemia, changes in the infant's epigenome modified with a diet and physical activity intervention in pregnancy: secondary analysis of a randomised control trial. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilario-Christensen S., Grenov B., Larnkjær A., Mølgaard C., Michaelsen K.F. Early nutrition and its effect on growth, body composition and later obesity. World Rev Nutr Diet. 2021;123:122–135. doi: 10.1159/000516440. [DOI] [PubMed] [Google Scholar]
- 18.Robinson N., Brown H., Antoun E., Godfrey K.M., Hanson M.A., Lillycrop K.A., et al. Childhood DNA methylation as a marker of early life rapid weight gain and subsequent overweight. Clin Epigenetics. 2021;13:8. doi: 10.1186/s13148-020-00952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Victora C.G., Bahl R., Barros A.J., França G.V., Horton S., Krasevec J., et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 20.Melnik B.C., Stremmel W., Weiskirchen R., John S.M., Schmitz G. Exosome-derived microRNAs of human milk and their effects on infant health and development. Biomolecules. 2021;11:851. doi: 10.3390/biom11060851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smyczynska U., Bartlomiejczyk M.A., Stanczak M.M., Sztromwasser P., Wesolowska A., Barbarska O., et al. Impact of processing method on donated human breast milk microRNA content. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah K.B., Chernausek S.D., Garman L.D., Pezant N.P., Plows J.F., Kharoud H.K., et al. Human milk exosomal microRNA: associations with maternal overweight/obesity and infant body composition at 1 month of life. Nutrients. 2021;13:1091. doi: 10.3390/nu13041091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica . 2. ed. Departamento de Atenção Básica; 2014. Guia alimentar para a população brasileira /Ministério da Saúde, Secretaria de Atenção à Saúde. –1. reimpr. – Brasília: Ministério da Saúde. [Google Scholar]
- 24.Onita B.M., Azeredo C.M., Jaime P.C., Levy R.B., Rauber F. Eating context and its association with ultra-processed food consumption by British children. Appetite. 2021;157 doi: 10.1016/j.appet.2020.105007. [DOI] [PubMed] [Google Scholar]
- 25.Neri D., Martinez-Steele E., Monteiro C.A., Levy R.B. Consumption of ultra-processed foods and its association with added sugar content in the diets of US children, NHANES 2009-2014. Pediatr Obes. 2019;14:e12563. doi: 10.1111/ijpo.12563. [DOI] [PubMed] [Google Scholar]
- 26.Brasil. Ministério da Saúde . Secretaria de Atenção Primaria à Saúde, Departamento de Promoção da Saúde. – Brasília: Ministério da Saúde; 2019. Secretaria de Atenção Primaria à Saúde. Departamento de Promoção da Saúde. Guia alimentar para crianças brasileiras menores de 2 anos /Ministério da Saúde. [Google Scholar]
- 27.Chang K., Khandpur N., Neri D., Touvier M., Huybrechts I., Millett C., et al. Association between childhood consumption of ultraprocessed food and adiposity trajectories in the avon longitudinal study of parents and children birth cohort. JAMA Pediatr. 2021;175 doi: 10.1001/jamapediatrics.2021.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., Prins G.S., et al. EDC-2: the endocrine society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:E1–150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González-Casanova J.E., Pertuz-Cruz S.L., Caicedo-Ortega N.H., Rojas-Gomez D.M. Adipogenesis regulation and endocrine disruptors: emerging insights in obesity. Biomed Res Int. 2020;2020 doi: 10.1155/2020/7453786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heindel J.J., Blumberg B. Environmental obesogens: mechanisms and controversies. Annu Rev Pharmacol Toxicol. 2019;59:89–106. doi: 10.1146/annurev-pharmtox-010818-021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J.T., Lee H.K. Childhood obesity and endocrine disrupting chemicals. Ann Pediatr Endocrinol Metab. 2017;22:219–225. doi: 10.6065/apem.2017.22.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro C.M., Beserra B.T., Silva N.G., Lima C.L., Rocha P.R., Coelho M.S., et al. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-033509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren X.M., Kuo Y., Blumberg B. Agrochemicals and obesity. Mol Cell Endocrinol. 2020;515 doi: 10.1016/j.mce.2020.110926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobstein T., Brownell KD. Endocrine-disrupting chemicals and obesity risk: a review of recommendations for obesity prevention policies. Obes Rev. 2021:1–10. doi: 10.1111/obr.13332. [DOI] [PubMed] [Google Scholar]
- 35.Lam T.M., Vaartjes I., Grobbee D.E., Karssenberg D., Lakerveld J. Associations between the built environment and obesity: an umbrella review. Int J Health Geogr. 2021;20:7. doi: 10.1186/s12942-021-00260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallal P.C., Andersen L.B., Bull F.C., Guthold R., Haskell W., Ekelund U., et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 37.Bassett D.R., John D., Conger S.A., Fitzhugh E.C., Coe D.P. Trends in physical activity and sedentary behaviors of United States youth. J Phys Act Health. 2015;12:1102–1111. doi: 10.1123/jpah.2014-0050. [DOI] [PubMed] [Google Scholar]
- 38.Riazi N.A., Wunderlich K., Gierc M., Brussoni M., Moore S.A., Tremblay M.S., et al. "You cannot go to the park, you cannot go here, you cannot go there": exploring parental experiences of COVID-19 and its impact on their children's movement behaviours. Children (Basel) 2021;8:219. doi: 10.3390/children8030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambert A., Vlaar J., Herrington S., Brussoni M. What is the relationship between the neighbourhood built environment and time spent in outdoor play? A systematic review. Int J Environ Res Public Health. 2019;16:3840. doi: 10.3390/ijerph16203840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray C., Gibbons R., Larouche R., Sandseter E.B., Bienenstock A., Brussoni M., et al. What is the relationship between outdoor time and physical activity, sedentary behaviour, and physical fitness in children? A systematic review. Int J Environ Res Public Health. 2015;12:6455–6474. doi: 10.3390/ijerph120606455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Podnar H., Jurić P., Karuc J., Saez M., Barceló M.A., Radman I., et al. Comparative effectiveness of school-based interventions targeting physical activity, physical fitness or sedentary behaviour on obesity prevention in 6- to 12-year-old children: a systematic review and meta-analysis. Obes Rev. 2021;22:e13160. doi: 10.1111/obr.13160. [DOI] [PubMed] [Google Scholar]
- 42.Jacob C.M., Hardy-Johnson P.L., Inskip H.M., Morris T., Parsons C.M., Barrett M., et al. A systematic review and meta-analysis of school-based interventions with health education to reduce body mass index in adolescents aged 10 to 19 years. Int J Behav Nutr Phys Act. 2021;18:1. doi: 10.1186/s12966-020-01065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oosterhoff M., Joore M., Ferreira I. The effects of school-based lifestyle interventions on body mass index and blood pressure: a multivariate multilevel meta-analysis of randomized controlled trials. Obes Rev. 2016;17:1131–1153. doi: 10.1111/obr.12446. [DOI] [PubMed] [Google Scholar]
- 44.Martin A., Booth J.N., Laird Y., Sproule J., Reilly J.J., Saunders DH. Physical activity, diet and other behavioural interventions for improving cognition and school achievement in children and adolescents with obesity or overweight. Cochrane Database Syst Rev. 2018;1 doi: 10.1002/14651858.CD009728.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sociedade Brasileira de Pediatria. Grupo de Trabalho em Saúde e Natureza . Publicação eletrônica; 2019. Benefícios da Natureza no Desenvolvimento de Crianças e Adolescentes; p. 28.https://www.sbp.com.br/fileadmin/user_upload/manual_orientacao_sbp_cen1.pdf [Cited 2021 Sep 5]. Available from: Available from: [Google Scholar]
- 46.Wijndaele K., White T., Andersen L.B., Bugge A., Kolle E., Northstone K., et al. Substituting prolonged sedentary time and cardiovascular risk in children and youth: a meta-analysis within the international children's accelerometry database (ICAD) Int J Behav Nutr Phys Act. 2019;16:96. doi: 10.1186/s12966-019-0858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Son J., Koekkoek L.L., La Fleur S.E., Serlie M.J., Nieuwdorp M. The role of the gut microbiota in the gut–brain axis in obesity: mechanisms and future implications. Int J Mol Sci. 2021;22:2993. doi: 10.3390/ijms22062993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart C.J., Ajami N.J., 'O'Brien J.L., Hutchinson D.S., Smith D.P., Wong M.C., et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–588. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee C.J., Sears C.L., Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann N Y Acad Sci. 2020;1461:37–52. doi: 10.1111/nyas.14107. [DOI] [PubMed] [Google Scholar]
- 50.Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 51.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Human Microbiome Jumpstart Reference Strains Consortium. Nelson K.E., Weinstock G.M., Highlander S.K., Worley K.C., Creasy H.H., et al. A catalog of reference genomes from the human microbiome. Science. 2010;328:994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cunningham A.L., Stephens J.W., Harris D.A. A review on gut microbiota: a central factor in the pathophysiology of obesity. Lipids Health Dis. 2021;20:65. doi: 10.1186/s12944-021-01491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 55.Grüner N., Mattner J. Bile acids and microbiota: multifaceted and versatile regulators of the liver-gut axis. Int J Mol Sci. 2021;22:1397. doi: 10.3390/ijms22031397. [DOI] [PMC free article] [PubMed] [Google Scholar]