Abstract
Objectives
To describe the concept of toxic stress, present the basics of epigenetics and discuss their relationship with child development.
Data source
Narrative literature review through a search in the SciELO, Lilacs, Medline databases using the terms Adverse Childhood Experience OR Early Life Stress, Epigenomic OR Epigenetic, Child Development OR Infant Development.
Data synthesis
Continuing stress response, known as toxic stress, can occur when a child experiences intense, frequent, and/or prolonged adversity—such as physical or emotional abuse, chronic neglect, for example—without adequate adult support. This toxic stress can have harmful effects on learning, behavior, and health throughout life. Epigenetics, an emerging scientific research area, shows how environmental influences affect gene expressions and explains how early experiences can impact throughout life.
Conclusions
Toxic stress causes changes in the human body response systems that can be explained in part by epigenetic changes, which can be temporary or long-lasting. Pediatricians must be aware of these mechanisms and their consequences, seeking to prevent them and thus promote the health, well-being, and quality of life of children, contributing to their full development.
Keywords: Adverse experiences in childhood, Toxic stress, Epigenetics, Child development
Introduction
In recent years, the knowledge about the effects of stress in the first years of life has been researched and expanded, as well as its consequences for health during later years. Scientific societies and those that include professionals from different areas of health have emphasized this topic, especially in the current periods of the Covid-19 pandemic. Even in times prior to the pandemic, the Brazilian Society of Pediatrics has been concerned with this topic and produced informative material to expand the knowledge and guide pediatricians in preventive actions, aiming to reduce the effects of this important problem that affects humans beings globally.1 Aiming at contributing to these initiatives, the present article aims to review the concepts of stress that can affect children, especially toxic stress in the first years of life; present the basics of epigenetics, a relatively new area of investigation; and discuss the relationship between toxic stress and epigenetics, and its effects on child development.
Methods
Objectives
To describe the concept of toxic stress, present the basics of epigenetics and discuss their relationship with child development.
Study design
This is a narrative literature review carried out by searching the SciELO, Lilacs, and Medline databases using the terms Adverse Childhood Experience OR Early Life Stress, Epigenomic OR Epigenetic, Child Development OR Infant Development.
Data synthesis
Toxic stress
It is important to differentiate between three types of stress responses that occur in humans: the positive, the tolerable, and the toxic one. These three terms refer to the effects of stress response systems on the human body, not the stressful event or the experience itself. The “positive” response to stress is a normal and essential part of healthy development, characterized by brief increases in heart rate and mild elevations in hormone levels. Several situations can trigger a positive stress response, for instance, when a child is introduced to a new teacher or when they are taken to a health unit for an injectable vaccine.
The tolerable stress response activates the human body's alert systems to a greater degree than positive stress. It results from more serious and long-lasting difficulties, such as the loss of a loved one, a natural disaster, or a painful lesion such as a contusion. If this activation is limited in time and the child receives help from adults to adapt, sometimes referred to as “buffered activation”, the brain and other organs recover from what could otherwise be harmful effects.
The most severe and least desirable response to stress, the one considered toxic, being therefore called “toxic stress”, can occur when a child experiences intense, frequent and/or prolonged adversity – such as physical or emotional abuse, chronic neglect, drug abuse or mental illness in parents or guardians, exposure to violence and/or due to accumulated consequences of economic family difficulties – without receiving adequate support from adults.2 In short, toxic stress occurs when the child goes through unusual and threatening situations constantly and repeatedly, for prolonged periods and without the support of an adult caregiver. In addition to the abovementioned situations, one can also mention exposure to domestic violence, such as constant fights between parents, a history of drug addiction in the family, bullying, mental problems in the family, and cases of extreme poverty.3
Epigenetics
The term epigenetics was created by Conrad Waddington, a scholar of genetics and developmental biology, in the mid-20th century to define a branch of biology that studied the interactions between genes and their products that originate the phenotype. It was based on a highly deterministic view, in which tissue development outcome, although it might show some variation due to environmental exposure, was inexorably determined by the genes and not the environment. In the mid-1970s, a molecular model was proposed to explain the activation or inactivation of gene activity and its hereditary transmission, based on the idea that DNA modification in the gene promoter regions, through enzymatic methylation of cytosine, could have the effect of “turning on” and “turning off” the genes during the development. Interestingly, the term epigenetics was not used in any of the studies on DNA methylation and gene expression at that time, probably because it had been previously used in completely different contexts. In the late 1980s, an article published by Holliday (1987) in the journal Science under the title “The inheritance of epigenetic defects” represented a milestone in the history of epigenetics. In that article, the author reviews the term introduced by Waddington and uses it to characterize situations in which DNA methylation causes changes in genetic activity, discussing its role in cancer and aging, stimulating interest in the epigenetic phenomena, and investments in research aimed at their investigation.4
Although the recent sequencing of the human genome has contributed greatly to the understanding of several complex diseases, it was soon realized that epigenetics played an important role in gene expression. The change in focus from genetic studies, from DNA molecule structure to “beyond the DNA molecule” mechanisms using new epigenetic concepts provided the basis for the association of genetics and environmental factors to the origin of a vast number of diseases, ranging from molecular pathway disorders during embryonic and fetal development to immunologically mediated diseases such as allergies, autoimmune disorders and cancer.5
The current definition of epigenetics refers to DNA modifications (or associated factors that contain information) that do not modify the DNA nucleotide sequence (these are not gene mutations), are maintained during cell division, are influenced by the environment, and cause stable changes in gene expression. This definition results from the understanding of the deep effect of the environment on developmental plasticity, mainly related to aging and disease susceptibility. Thus, the epigenetic setting is now seen more dynamically than Waddington's deterministic view.6
In parallel with the term epigenetics, a vast vocabulary associated with this field of study has emerged. The term epigenome refers to the genomic distribution of epigenetic alterations and encompasses all the nuclear information that is hereditarily transmitted during cell division and that controls development, tissue differentiation, and cell responsiveness. Epigenetics explains why cells from different organs and tissues, containing essentially the same DNA, perform such different functions and retain their identity as their cells divide. This cell identity is only possible through information added to genes, which is epigenetic information. These are controlled by the genome sequence, environmental exposure, and random factors. Epigenetics, therefore, is at the interface of the genome, development and environmental exposure.6
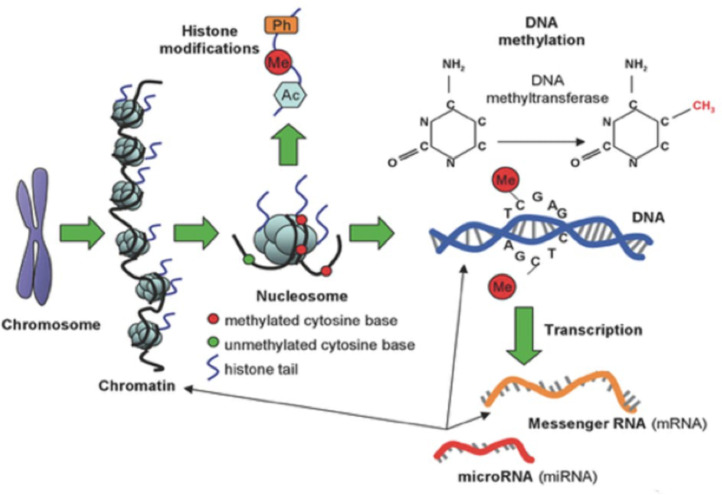
There are several different epigenetic mechanisms that interact to regulate gene expression, modifying the DNA access to transcription or through other regulatory factors. The three fundamental mechanisms include DNA methylation, histone modifications, and non-coding RNA (Fig. 1). Methylation is the most often studied mechanism.
Figure 1.
Types of epigenetic alterations: DNA methylation, histone modifications and microRNA (miRNA).
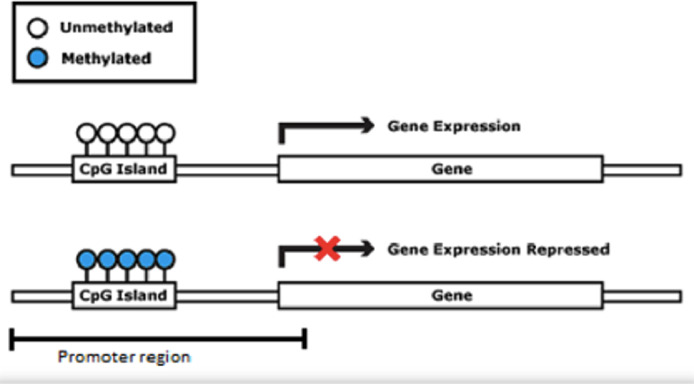
DNA methylation consists of the addition of a methyl group (CH3) to a cytosine base, most often in a CpG (Cytosine-phosphate-Guanine) dinucleotide, catalyzed by a DNA methyltransferase. This DNA modification occurs in a region rich in CpG residues (CpG islands), often found in regulatory (promoter) regions of the DNA molecule (Fig. 2). Inside the CpG islands, cytosine methylation can silence or activate gene expression (the gene transcription capacity), depending on preprogrammed tissue functions or environmental influences, acting as an “on-off switch”. Methylation patterns in CpG islands are conserved during cell-to-cell mitosis. DNA methylation can be reversible, meaning that genes can be turned on or off throughout life, but they can also be permanently methylated through cell differentiation.7 DNA demethylation is accepted as necessary for the embryonic development and cell differentiation process, during which embryonic stem cells gradually narrow their identities into different cell types, along with the loss of pluripotency.8
Figure 2.
Gene promoter region with methylated and unmethylated CpG islands.
Histones consist of eight proteins that support DNA, organizing themselves in the cell nucleus into packages called nucleosomes. Each nucleosome is formed by a histone surrounded by two DNA loops and several nucleosomes assembled in a linear array form the chromatin, which exists under two configurations: the euchromatin, present in embryonic stem cells, is an “uncoiled” and metabolically active form, in which exposed nucleosomes enable transcription; heterochromatin, present in differentiated cells, which is a metabolically inactive form, where compacted nucleosomes limit the access of transcription factors to the gene promoter regions.5 Histone tail amino acids can undergo epigenetic modifications (acetylation, methylation, phosphorylation, ubiquitination, or SUMOylation), which can result in changes in chromatin structure, leading to changes in the binding capacity of the gene promoter regions to transcription sites.9
In turn, non-coding RNAs are short, single-stranded RNAs, called MicroRNAs (miRNA), which act in a regulatory manner and are capable of suppressing protein production. DNA methylation can influence miRNA levels and, conversely, miRNA can target the translation of enzymes involved in histone modification and DNA methylation.9
The laboratory measurement of epigenetic alterations is mainly limited to the analysis of DNA methylation. There are currently several methods to do it, and describing them in detail is beyond the scope of this review. One of the DNA methylation quantification techniques is the treatment of these molecules with sodium bisulfite, which transforms the unmethylated cytosines into uracil and preserves the methylated cytosines. The subsequently performed genomic sequencing reveals the proportion of methylated CpG islands. This method offers wide genomic coverage, accurate quantification, and good reproducibility, but at a high cost.10 Alternative methods to bisulfite use have been developed, using specific antibodies to recognize DNA methylation, methyl-binding domain proteins, or restriction enzymes, followed by high-throughput sequencing.10 The new generation of sequencing platforms with microarray hybridization technology enabled the study of methylation in the total genome with a resolution of individual base pairs, allowing the preparation of DNA methylation genomic maps.8
Some barriers limit the application of these methods, such as some time-consuming and arduous steps, false results due to errors, high cost of high-performance technologies, and complexity to operate in standard laboratories. None of the DNA methylation analysis methods are adequate for all purposes, and the best approach depends on the goals of each study and the conditions under which the tests are performed.10
Relationships of toxic stress with epigenetics and its repercussions on child development.
Scientific research has accumulated, corroborating the epigenetic hypothesis that environmental influences can affect how genes are expressed and under what conditions it occurs. Early life experiences can determine how genes are turned on and off and even whether some are expressed or not. During development, the DNA that comprises our genes accumulate chemical marks that constitute the epigenome and determine the quantity of expressed genes. The different experiences that children have throughout their development reorganize these chemical marks. This explains why genetically identical twins can show different behaviors, abilities, health, and achievements.11
Epigenetic modifications can occur as isolated events or, more commonly, they can act together with genetic factors. That is, there is a relationship between epigenome and genome. Genetic polymorphisms, which are variations in DNA sequences between individuals, may be associated with DNA methylation at specific sites and certainly influence the way genes are expressed and how the proteins encoded by these genes will function.9 Genes inherited from parents, therefore, do not define a child's future development alone. However, the environment in which the child develops provides significant experiences that chemically modify certain genes, which, in turn, define how much and when they will be expressed. Therefore, while genetic factors exert strong influences, environmental factors have the ability to alter the function of inherited genes.11
The timing of epigenetic modifications throughout the life of a human being is also of great importance. There are some well-defined critical periods in epigenetic programming, which occur most notably before and shortly after birth and especially in early childhood. It is likely that the earlier the epigenetic modification, the more extensive its effects are.12 Epigenetic processes are, therefore, natural and essential for many body functions, but if they occur inappropriately, they can cause severe adverse effects on health and behavior.13
Adverse fetal and early childhood experiences can lead to physical and chemical alterations in the brain, which can last a lifetime. Harmful experiences, such as malnutrition, exposure to toxins or chemical drugs, and toxic stress before birth or in early childhood are incorporated into the architecture of the developing brain through the epigenome. The “biological memories” associated with these epigenetic alterations can affect multiple organ systems and increase the risk not only of poor outcomes related to physical and mental health but also of future behavior and learning capacity impairments.11
Research has shown that specific epigenetic alterations occur in brain cells as cognitive abilities (such as learning and memory) develop and that the repeated activation of brain circuits dedicated to learning and memory, through interaction with the environment, such as reciprocal “intense” interaction with adults facilitates these positive epigenetic changes. It is also known that good quality maternal and fetal nutrition, combined with positive socio-emotional support of children in their family and community environments, reduces the likelihood of negative epigenetic alterations that increase the risk of further damage to physical and mental health.11
In brief, the epigenome can be affected by positive experiences, such as supportive relationships and learning opportunities, or negative influences, such as environmental toxins or stressful life circumstances that leave a unique epigenetic “signature” on the genes. These signatures can be temporary or permanent, and both types affect how easily the genes are turned on or off. Recent research also demonstrates that there may be ways to reverse certain negative alterations and restore healthy functionality, but this takes a lot more effort and may not be successful in changing all aspects of the signatures, in addition to being costly. Therefore, the best strategy would be to support responsive relationships and reduce toxic stress early on, helping children to grow up to be healthy and productive members of society.11
Consequences of toxic stress on child development
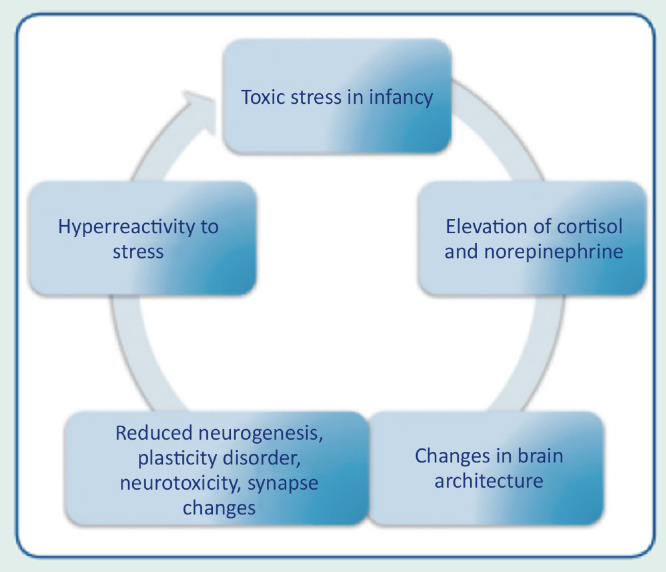
The prolonged activation of stress-response systems that occurs in toxic stress can disrupt the expected development of the child's brain and other organ systems and increase the risk of diseases and cognitive impairment in childhood and adulthood (Fig. 3). Therefore, it can cumulatively affect an individual's physical and mental health over one's lifetime. The more adverse the childhood experiences, the greater the likelihood of developmental delays and subsequent health problems.
Figure 3.
Relationship between toxic stress, epigenetic alterations and child development.
Early and constant exposure to stress in childhood stimulates the incessant release of cortisol, causing changes in synaptic connections, limiting the brain structural capacities. The more toxic the stress, the greater the risk of severe health consequences in the short, medium and long term. Toxic stress also promotes alterations in the brain architecture, causing reduced brain volume, dysfunction of the neuroendocrine and limbic systems, in addition to affecting structural and functional neuroplasticity.14, 15, 16 Individuals who were exposed to toxic stress in childhood are more vulnerable to chronic diseases in adulthood, such as systemic arterial hypertension, diabetes mellitus, pulmonary diseases, ischemic heart disease, stroke and autoimmune diseases. The risk also increases significantly for the incidence of neuropsychiatric and behavioral disorders, such as depression, generalized anxiety disorder, obsessive compulsive disorder, increased risk of substance abuse, autism spectrum disorder, and attention-deficit/hyperactivity disorder.17, 18, 19, 20 Research indicates that supportive and responsive relationships by caring adults as early as possible in life can prevent or reverse the harmful effects of the body's response to toxic stress.
Final considerations
The future of any society depends on its ability to promote the healthy development of the next generation. Extensive research into the biology of stress shows that healthy development can be altered by excessive or prolonged activation of stress response systems in the body and brain. This toxic stress can have harmful effects on learning, behavior and health throughout life.
It is the pediatrician's role to be aware of the mechanisms of toxicity and their aiming to improve the promotion of health, well-being, and quality of life of children during the short, medium and long-term stages of their growth and neuropsychomotor development.1
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Study conducted at Universidade Federal do Rio de Janeiro (UFRJ), Faculdade de Medicina, Rio de Janeiro, RJ, Brazil.
References
- 1.Sociedade Brasileira de Pediatria (SBP) Departamento Científico de Pediatria do Desenvolvimento e Comportamento; 2017. O Papel do Pediatra na Prevenção do Estresse Tóxico na Infância. Manual de Orientação; p. 3.https://www.sbp.com.br/fileadmin/user_upload/2017/06/Ped.-Desenv.-Comp.-MOrient-Papel-pediatra-prev-estresse.pdf [Internet]Available from. [cited 30 Aug 2021] [Google Scholar]
- 2.Harvard University. Center on the Developing Child. Key Concepts: Toxic Stress. [Internet]. Available from:https://developingchild.harvard.edu/science/key-concepts/toxic-stress/. [cited 30 Aug 2021].
- 3.Instituto Geração Amanhã. Estresse Tóxico na Infância: O Que é, Quais as Consequências e Como Evitar. [Internet]. Available from: https://geracaoamanha.org.br/estresse-toxico-na-infancia/ [cited 30 Aug 2021].
- 4.Holliday R. Epigenetics: a historical overview. Epigenetics. 2006;1:76–80. doi: 10.4161/epi.1.2.2762. [DOI] [PubMed] [Google Scholar]
- 5.Bellanti JA. Epigenetic studies and pediatric research. Pediatr Res. 2020;87:378–384. doi: 10.1038/s41390-019-0644-9. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg AP. The key role of epigenetics in human disease prevention and mitigation. N Engl J Med. 2018;378:1323–1334. doi: 10.1056/NEJMra1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Academic Press; San Diego, CA: 2017. Handbook of Epigenetics: The New Medical and Molecular Genetics. [Google Scholar]
- 8.Li S, Tollefsbol TO. DNA methylation methods: Global DNA methylation and methylomic analyses. Methods. 2021;187:28–43. doi: 10.1016/j.ymeth.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linnér A, Almgren M. Epigenetic programming-The important first 1000 days. Acta Paediatr. 2020;109:443–452. doi: 10.1111/apa.15050. [DOI] [PubMed] [Google Scholar]
- 10.Khodadadi E, Fahmideh L, Khodadadi E, Dao S, Yousefi M, Taghizadeh S, et al. Current advances in DNA methylation analysis methods. Biomed Res Int. 2021;2021 doi: 10.1155/2021/8827516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvard University . 2021. Center on the Developing Child. O Que é Epigenética.https://developingchild.harvard.edu/translation/o-que-e-epigenetica/ [Internet]. Available from: [cited 30 Aug] [Google Scholar]
- 12.Nafee TM, Farrell WE, Carroll WD, Fryer AA, Ismail KM. Epigenetic control of fetal gene expression. BJOG. 2008;115:158–168. doi: 10.1111/j.1471-0528.2007.01528.x. [DOI] [PubMed] [Google Scholar]
- 13.Weinhold B. Epigenetics: the science of change. Environ Health Perspect. 2006;114:A160–A167. doi: 10.1289/ehp.114-a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Scientific Council on the Developing Child (2005/2014). Excessive Stress Disrupts the Architecture of the Developing Brain: Working Paper No. 3. Updated Edition. Available from: www.developingchild.harvard.edu [cited 30 Aug 2021].
- 15.National Scientific Council on the Developing Child . Vol. 2. 2004. pp. 1–9. (Children's Emotional Development is Built into the Architecture of their Brains). Working Paper [Internet]. Available from: http://46y5eh11fhgw3ve3ytpwxt9r.wpengine. netdna-cdn.com/wp-content/uploads/2004/04/Childrens-Emotional-Development-Is-Builtinto-the-Architecture-of-Their-Brains.pdf [cited 30 Aug 2021] [Google Scholar]
- 16.Shonkoff JP, Garner AS. Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 17.Flaherty EG, Thompson R, Litrownik AJ, Zolotor AJ, Dubowitz H, Runyan DK, et al. Adverse childhood exposures and reported child health at age 12. Acad Pediatr. 2009;9:150–156. doi: 10.1016/j.acap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Quartilho M. “A Infância dura toda a vida” sobre a importância e o impacto das experiências de adversidade precoce. Rev Soc Portug Med Fís Reabil. 2012;22:49–52. [Google Scholar]
- 19.Sanders MR, Markie-Dadds C, Tully LA, Bor W. The triple P-positive parenting program: a comparison of enhanced, standard, and self-directed behavioral family intervention for parents of children with early onset conduct problems. J Consult Clin Psychol. 2000;68:624–640. [PubMed] [Google Scholar]
- 20.Turner KM, Sanders MR. Dissemination of evidence-based parenting and family support strategies: learning from the Triple P—Positive Parenting Program system approach. Aggr Viol Behavior. 2005;11:176–193. [Google Scholar]