Abstract
Objectives
This review aimed to verify indoor and outdoor pollution, host and environmental microbiome, and the impact on the health of the pediatric population.
Sources
A review of the literature, non-systematic, with the search for articles since 2001 in PubMed with the terms “pollution” AND “microbiome” AND “children's health” AND “COVID-19”.
Summary of the findings
Prevention of allergic diseases includes the following aspects: avoid cesarean delivery, the unnecessary overuse of antibiotics, air pollution, smoking in pregnancy and second-hand tobacco smoke, stimulate breastfeeding, soil connection, consume fresh fruits and vegetables, exercise and outdoor activities and animal contact. The children's microbiota richness and diversity decrease the risk of immune disbalance and allergic disease development.
Conclusions
Lifestyle and exposure to pollutants, both biological and non-biological, modify the host and the environment microbiome provoking an immune disbalance with inflammatory consequences and development of allergic diseases.
Keywords: Children, COVID-19, Environment, Health, Microbiome
Introduction
There are live microbial colonies in the gastrointestinal, respiratory, and skin tracts promoting health or developing the disease. The microbiome is composed of trillions of microbes (bacteria, archaea and microbial eukaryotes) and viruses, genetic material, and communication with each ecologic site, while microbiota is the totality of microbes found in this niche. Dysbiosis is the imbalance in any microbial ecosystem. Current sequencing technologies detect and analyze the human microbiome with the greatest number of details ever described.1 Variation in the composition or function of microbial ecological systems was evaluated through the 16S, a small subunit of rRNA gene, and sequencing techniques.2
The hypothesis of the origins of health promotion and disease development (DOHaD) suggests that an environment where children live in the first 1000 days is associated with a higher risk of developing diseases.3 Environmental factors such as ultra-processed food, antibiotics, and infections interrupt adequate microbial succession, contributing to intergenerational and lifelong deficits in growth and development. The first 1000 days, starting in conception to age 2 are represented as a critical window for child growth and neurologic development. In this period of life, there is a maturation of immunological pathways, which maintain child health and normal growth.4,5
The function of the respiratory tract is the exchange of respiratory gases. The respiratory system microbiota can act like a guardian that offers resistance against colonization by pathogenic microorganisms. This microbiota may be associated with the maintenance of respiratory and immunological homeostasis. Factors influencing the microbial population in the respiratory system have been evaluated to determine how they affect respiratory health.6
Lower microbial exposure early in life decreases microbial diversity in urban lifestyles leading to changes in microbial stimulation and is being associated with increased predisposition to allergic diseases.7
The skin area is one of the widest in the human body, and its microbiome plays a major role in developing immunity and creates a barrier to protect from pathogenic microorganisms. This barrier shows a direct contact between the individual and the environment, and an important site for interactions of the microbiota with the immunologic system. The human skin is diverse in its microorganism community and its composition varies according to age, location, and period of analysis. The distribution of these bacteria on the skin is influenced by the degree of hydration, type of delivery, pollution, exposure to ultraviolet radiation, sex hormones, diet, among others.8
The analysis and interpretation of the human microbiome in the context of human host biology and the environment reveals the importance of microbiota for the immune response and shows the potential involvement of the microbiome in developing allergic diseases.9
This review aimed to verify indoor and outdoor pollution, host and environmental microbiome, and the impact on children's health.
Early-life microbiome and environmental influences
The microbiome has had a heavy influence on human health and development since it is established in early life. Potential variations in the composition and function of the microbiome in early life result from lifestyle, type of delivery, breastfeeding, dietetic habits, and use of antibiotics.10 The origins of the development hypothesis are based on variations in child programming that occur by environmental exposures in a critical period in the initial months of life.11
Non-pathogenic microorganisms were detected in the amniotic fluid or placenta of normal concepts, indicating an exchange of microbes from mother to fetus. The maternal microbiome in the prenatal period could modulate the baby's immunologic system. Colonization in pregnancy with Escherichia coli HA107 has been related to alter the innate immunologic response in the intestinal mucosa and affecting the offspring transcriptome.12
Babies born from cesarean section have microbiota similar to the human skin, and those born by non-cesarean section have similar to the maternal birth canal and intestinal microbiota. In the first 24 hours after birth, the microbiota of various parts of the body of children born by cesarean section is colonized with Staphylococcus spp. (like the residents on the mother's skin), and those born by natural delivery are colonized by Prevotella and Atopobium spp. (vaginal bacteria).13 Infants born by non-natural delivery had a significantly higher risk of asthma.14
Influenced by breastfeeding and dietetic habits, the gut microbiome became mature when its composition stabilizes. Breastfed babies are colonized by Bifidobacteria and Lactobacillus spp. and formula-fed children have higher proportions of clostridialis and proteobacteria.15 Breastfeeding can protect against wheezing in the initial months in high-risk babies of asthmatic mothers.16
Prenatal maternal exposure to antibiotics changed the diversity of both infant and mother microbiota. Antibiotics in children in the early months of life could alter gut colonization (Ruminococcus and clostridialis). Another study suggested that dysbiosis caused by antibiotics in childhood stimulates the development of childhood asthma.17
The composition and metabolic activity of the enteric microbiota in early life makes the gastrointestinal system a target for immunologic modulation and the balance of the immune response. There has been an interest to clarify the agents or nutrients involved in this process, especially with probiotics or prebiotics.18 These provoked several interventions with different probiotic strains in pregnancy, the postnatal period, or both for the prevention of allergies. Most of these studies focused primarily on outcomes such as atopic dermatitis and food allergy mediated by IgE, and most showed a significant reduction in the development of AD (25% to 50%), but no consistent effects on any other result in allergic diseases.19 Microbial metabolites (e.g. Butyrate) could protect against the development of allergic diseases by T-regulatory cells. Higher consumption of fresh foods and a lower intake of saturated fat is associated to a lower risk of childhood asthma or wheezing, particularly higher consumption of vegetables, fruits, and fish during pregnancy.19
Indoor environment
Household pollution involves biological agents, such as house dust mite allergens, insects, pollen, animal dander, fungi, in addition to bacterial endotoxins.20
Few studies have observed the relationship between microbiomes and the consequences observed by fungi and moisture. Fungal and moisture damage have been provoked changes in the composition of microbiomes in dwellings.21
Estimated risk suggests that high-level exposure to microbial was associated with a higher risk of respiratory symptoms (RR = 1.24). There was a strong association with some fungal species (Aspergillus, Penicillium, Cladosporium and Alternaria) exposure (RR = 1.73). There was a higher risk of wheezing (RR = 1.20) or allergic rhinitis (RR = 1.18) from any microbial exposure.22 Children exposed to higher intra-household mold concentrations are at higher risk for lower respiratory tract infections [Odds ratio (OR) = 1.20]. There was a greater risk the greater the exposure to the total concentration of fungi (OR = 1.27) than to visible molds (OR = 1.20).23
Living room dust samples were collected to verify if some individual bacterial genera in the indoor microbiota may predict asthma development in children. They were followed up to 10.5 years old. There were a higher household diversity of microorganisms in non-asthmatics children. The microbiota in the homes of asthmatics was phylogenetic different from that in homes of non-asthmatic subjects. The presence of Lactococcus increased the risk for asthma (adjusted OR = 1.36). The bacterial abundance of genera (especially order Actinomycetales) decreased the risk of asthma, but not independently.24 There was an association between indoor microbes exposure and severity of asthma in children. The severity of non-atopic asthma was related to the fungal levels (OR = 2.40). Volutella genus was related to the severity of asthma, especially in atopic patients. Kondoa yeast can be protective and Cryptococcus affects asthma severity.25
Pets can affect the internal microbial diversity and consequently the household exposure to microorganisms. The presence of animals, loss of water in the hydraulic system, abuse of air conditioning (AC), comparing homes in the suburban area with homes in the urban area, and measurements of dust composition were associated with microbial richness. Differences in microbiota were observed in the use of AC and household occupation characteristics (people and animals). Occupancy rates were related to good bacterial rates, such as Lactobacillus johnsonii.26
Children aged 6 to 17 years were assessed whether molds, house dust mites, and endotoxin in indoor settings increase the risk of allergic diseases. Mold/mildew odor increased the risk of developing asthma (OR = 1.60). Levels of IgE≥170 KU/L related to asthma (OR = 1.81). The greater the exposure to biological agents presents in the home (> 8 agents), the risk of eczema (OR = 0.17) and asthma (OR = 0.49), respectively, was reduced.27
Differences in microbial composition were noted between homes and schools. Eighty-six species of bacteria were found with different abundance between schools and homes. Some species have been in homes, such as Enterobacter cloacae, Escherichia coli, and Klebsiella compared with Serratia marcescens in the schools. In the classroom, a higher microbial diversity was related to asthma in children (OR = 1.07), while there was no association with domestic microbial diversity (OR = 1.00).28 Schools with greater fungal diversity had a low prevalence of allergic sensitization and a high prevalence of asthma. Increased exposure to endotoxin in the schools increased the allergic sensitization prevalence and higher levels of Penicillium spp also increased the number of children with atopic sensitization.29
Household non-biological pollutants are gases, particles, formaldehyde, and volatile organic compounds (VOCs). Domestic air pollution (PAH) resulting from the burning of polluting fuels such as coal, kerosene and biomass is a global environmental health problem.20 Secondhand smoke (SHS) has been extensively studied and contributes to the development of non-communicable diseases. Tobacco maternal exposure pre-and postnatal was related to the development of asthma in children.30
Outdoor environment
Clinical manifestations of asthma or allergic rhinitis are inversely related to exposure to environmental microorganisms. The vast diversity of microbial exposure of children living on farms protects against asthma risk. A wide range of microbes in farms largely explains the protective action of the agricultural environment on the risk of asthma in children.31
The potential contribution of microbiota to increasing the numbers of allergic patients has become the focus of many studies. These findings, related to the increased risk of allergic diseases, suggest that the movement from rural to urban life in Western society is one of the main factors for this change.32
Similar settings were used to study the relationship of environmental microbial exposures in the epidemiology of asthma and allergies in childhood. Children of the same genetic origin, living in different environmental conditions on farms were compared with children from urban areas. There were significant differences in asthma epidemiology. A large number of microbes in the farm environment could be protective against asthma and allergies onset.32
Lung function and nasopharyngeal swabs were collected in a prospective cohort in Ghana in a rural area. The lower diverse phenotype of nasopharyngeal microbiota showed greater resistance to small airways (R5-R20 = 17.9%) compared to the more diverse phenotype.33 Atopy was more common among school-age children in Finland Karelia compared to the same region from Russia. These neighboring regions have contrasting socioeconomic differences, although both have the same climatic and geographic characteristics. The preserved, developed and built natural environment is scarce in Russian Karelia. However, in Finnish Karelia, the environment and lifestyle of people are remarkably more Western and modern. The skin and nasal microbiota in both populations were evaluated to verify how lifestyle and environment could affect colonization. The difference in the microbiome of the skin and nasal microbiota could explain the possible mechanism that observation. There were an abundance and diversity of Acinetobacter that contribute to the low prevalence of allergic diseases in Russia Karelia. Proteobacteria were more common (33.3%) in the Finnish sample comparing to the Russian (19.7%). Firmicutes were less common in Finland (13.9%) comparing to Russia (47%).34
The impact of particulate pollutants on human health is not only due to direct effects, may also involve the effect on bacterial behavior in the host. The normal diversity of the microbiome and the number of species are fundamental to maintaining health. Carbon, the main component of particulate matter (PM), is implicated in the predisposition to infectious respiratory diseases, inducing changes in bacterial biofilms of Streptococcus pneumoniae and Staphylococcus aureus.35
Atmospheric PAHs concentrations were related to those of pollutants, such as PM10, NO2 and SO2. In the atmosphere a high level of the genus Micrococcus (Actinobacteria) and PAHs with high molecular weight, and Bacillus and PAHs with low molecular weight. Elevated urinary 1-OHPyrene levels have been associated with childhood asthma and correlated with elevated levels of PM2.5 and PM10. In addition, an abundance of oral Prevotella-7 was also found. PAHs can disrupt signaling pathways by microbiota imbalance, like a purine and lipid metabolism, a folate carbon reservoir, and pyrimidine metabolites which contribute to public health problems.36
Pre-exposure for one week to diesel exhaust decreased the clearance of Pseudomonas aeruginosa from murine bronchial epithelial cells, however, this did not occur at a pre-exposure of six months.37 Microbial characteristics, such as the growth and virulence of opportunistic bacteria (Pseudomonas aeruginosa, Enterococcus faecalis and Escherichia coli) were modified by internal and external dust.38
The high traffic-related air pollution (TRAP) promoted high levels of diversity for bacteria in the high level of exposure group compared to a low level of exposure group. There were no differences in the mother's asthma status, gender, or education. This study concluded that there was an alteration related to exposure to TRAP in the lower respiratory microbiota independently of asthma.39
Air pollution, mainly from rising CO2 levels, is the driving force behind global warming as a consequence of the greenhouse effect. The higher intensity and increased frequency of rainfall, storms, sandstorms, or extreme weather events such as heatwaves, droughts, blizzards, floods, and hurricanes are related to climate change.40 The growth of plants and microorganisms modifies the external exposome and results in disease, which is directly impacted by environmental changes.41
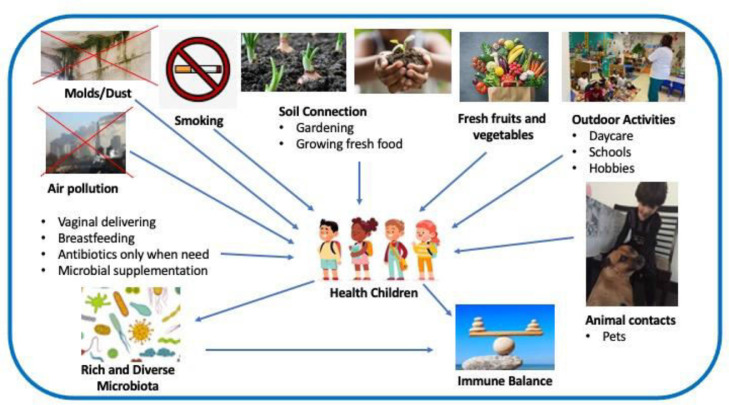
The genome to which the people are exposed is the result of the exposome both the external and internal environment. The exposome includes three wide domains: specific external, non-specific external, and internal domain. The host microbiome associated with external microbial exposure are essential to increase the risk of allergic diseases in the first years of life. The increasing prevalence of autoimmune or allergic diseases was blamed for the loss of biodiversity and climate change resulting from human action. The possible adverse consequences for humanity caused by the loss of biodiversity have been a global concern. Industrialization, pollution, and widespread use of chemical products that impact the environment and microorganisms are among the reasons for this loss42 (Fig. 143).
Figure 1.
Interaction between indoor and outdoor environment, microbiome and health. Biodiverse environment and lifestyle. (Modified from Haahtela et al.43).
Environment and COVID-19
Recent studies have shown an increase in mortality from COVID-19 in places where there has been long-term exposure to air pollution.44 In Korea, there was an association between the levels of NO2, CO, and SO2 and SARS-CoV-2 in confirmed cases.45 Air pollution has been associated to respiratory viral infection. An increase in mortality in the order of 3% (95% CI = 6%-13%) due to non-malignant respiratory disease is estimated when there is an increase of 10 μg/m3 in the concentration of PM2,5.46,47
Airway permeability is modified by gases such as nitrogen dioxide (NO2) or ozone (O3) and breathable particles (PM). Damage to epithelial cilia, the first line of defense against coronavirus, and the ability of macrophages to phagocytize the microorganism and prevent an effective immunologic response against the infectious agent occurs after exposure to fine particles or ultra-fine. Lifelong endemic exposure to air pollutants causes chronic systemic inflammation that leads to cardiovascular and respiratory diseases, metabolic diseases, etc., considered comorbidities to COVID-19, increasing the risk of serious illness or death in patients with SARS-CoV-2 infection. In addition, the angiotensin-converting enzyme receptor 2 (ACE-2), the receptor for SARS-CoV-2 on respiratory cells, is overexpressed in long-term exposure to air pollution. In fact, the lungs are damaged by pollution and increase the activity of the ACE-2 enzyme, consequently taking up the virus.48 This pattern was demonstrated in Italy.49
Airborne pollen constitutes an important fraction of bioaerosols (solid and liquid biological airborne particles) and functions as carriers of bacteria and viruses.50 Several bacterial and fungal pathogens transmitted through bioaerosols cause respiratory abnormalities, hypersensitivity reactions, and systemic infection.
There is a strong influence of temperature, relative humidity, precipitation, and wind, etc.51 and, together, they help to explain the effect on COVID-19.52,53 The ability of pandemic coronavirus to persist in the environment can cause eventual exposure to pollen bioaerosols, further altering seasonality and transmission rate.54
Conclusion
Lifestyle and early exposure to pollutants, biologics, and non-biologics change the host and the environment's microbiome, provoking an immune disbalance with inflammatory repercussions developing allergic diseases. Assessment of exposure to indoor and outdoor pollutants in the environment at pregnancy and post-natally should be a concern to pediatricians to implement for early detection and intervention on the environment.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Schwierzeck V, Hülpüsch C, Reiger M. Germs! Handb Exp Pharmacol. 2021. Microbiome of barrier organs in allergy: who runs the world? Epub ahead of print. PMID: 34228203. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock GM. Genomic approaches to studying the human microbiota. Nature. 2012;489:250–256. doi: 10.1038/nature11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott SL, Logan AC. Transforming life: a broad view of the developmental origins of health and disease concept from an ecological justice perspective. Int J Environ Res Public Health. 2016;13:1075. doi: 10.3390/ijerph13111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The human microbiome and child growth - first 1000 days and beyond. Trends Microbiol. 2019;27:131–147. doi: 10.1016/j.tim.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Chong-Neto HJ, Pastorino AC, Melo AC, Medeiros D, Kuschnir FC, Alonso ML, et al. Gut microbiota and its interface with the imune system. Arq Asma Alerg Imunol. 2019;3:406–420. [Google Scholar]
- 6.Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki Y, Nakamura Y, Núñez G. Role of the microbiota in skin immunity and atopic dermatitis. Allergol Int. 2017;66:539–544. doi: 10.1016/j.alit.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Huang YJ, Marsland BJ, Bunyavanich S, O'Mahony L, Leung DY, Muraro A, et al. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol. 2017;139:1099–1110. doi: 10.1016/j.jaci.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141 doi: 10.1542/peds.2017-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–388. doi: 10.1146/annurev.nutr.27.061406.093705. [DOI] [PubMed] [Google Scholar]
- 12.Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sevelsted A, Stokholm J, Bønnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135:e92–e98. doi: 10.1542/peds.2014-0596. [DOI] [PubMed] [Google Scholar]
- 15.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH) Anaerobe. 2011;17:478–482. doi: 10.1016/j.anaerobe.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Azad MB, Vehling L, Lu Z, Dai D, Subbarao P, Becker AB, et al. Breastfeeding, maternal asthma and wheezing in the first year of life: a longitudinal birth cohort study. Eur Respir J. 2017;49 doi: 10.1183/13993003.02019-2016. [DOI] [PubMed] [Google Scholar]
- 17.Hoskin-Parr L, Teyhan A, Blocker A, Henderson AJ. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose-dependent relationship. Pediatr Allergy Immunol. 2013;24:762–771. doi: 10.1111/pai.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Moura PN, Rosário Filho NA. The use of prebiotics during the first year of life for atopy prevention and treatment. Immun Inflamm Dis. 2013;1:63–69. doi: 10.1002/iid3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brough HA, Lanser BJ, Sindher SB, Teng JM, Leung DY, Venter C, et al. Early intervention and prevention of allergic diseases. Allergy. 2021 doi: 10.1111/all.15006. Epub ahead of print. PMID: 34255344. [DOI] [PubMed] [Google Scholar]
- 20.Rosário Filho NA, Urrutia-Pereira M, D'Amato G, Cecchi L, Ansotegui IJ, Galán C, et al. Air pollution and indoor settings. World Allergy Organ J. 2021;14 doi: 10.1016/j.waojou.2020.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox J, Stone T, Ryan P, Burkle J, Jandarov R, Mendell MJ, et al. Associations of observed home dampness and mold with the fungal and bacterial dust microbiomes. Environ Sci Process Impacts. 2021;23:491–500. doi: 10.1039/d0em00505c. [DOI] [PubMed] [Google Scholar]
- 22.Fakunle AG, Jafta N, Naidoo RN, Smit LAM. Association of indoor microbial aerosols with respiratory symptoms among under-five children: a systematic review and meta-analysis. Environ Health. 2021;20:77. doi: 10.1186/s12940-021-00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakunle AG, Jafta N, Okekunle AP, Naidoo RN. Indoor microbiome and risk of lower respiratory tract infections among children under-five years: A meta-analysis. Indoor Air. 2020;30:795–804. doi: 10.1111/ina.12698. [DOI] [PubMed] [Google Scholar]
- 24.Karvonen AM, Kirjavainen PV, Täubel M, Jayaprakash B, Adams RI, Sordillo JE, et al. Indoor bacterial microbiota and development of asthma by 10.5 years of age. J Allergy Clin Immunol. 2019;144:1402–1410. doi: 10.1016/j.jaci.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Dannemiller KC, Gent JF, Leaderer BP, Peccia J. Indoor microbial communities: Influence on asthma severity in atopic and nonatopic children. J Allergy Clin Immunol. 2016;138:76–83. doi: 10.1016/j.jaci.2015.11.027. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dannemiller KC, Gent JF, Leaderer BP, Peccia J. Influence of housing characteristics on bacterial and fungal communities in homes of asthmatic children. Indoor Air. 2016;26:179–192. doi: 10.1111/ina.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharpe RA, Thornton CR, Tyrrell J, Nikolaou V, Osborne NJ. Variable risk of atopic disease due to indoor fungal exposure in NHANES 2005-2006. Clin Exp Allergy. 2015;45:1566–1578. doi: 10.1111/cea.12549. [DOI] [PubMed] [Google Scholar]
- 28.Lai PS, Kolde R, Franzosa EA, Gaffin JM, Baxi SN, Sheehan WJ, et al. The classroom microbiome and asthma morbidity in children attending 3 inner-city schools. J Allergy Clin Immunol. 2018;141:2311–2313. doi: 10.1016/j.jaci.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavaleiro Rufo J, Madureira J, Paciência I, Aguiar L, Pereira C, Silva D, et al. Indoor fungal diversity in primary schools may differently influence allergic sensitization and asthma in children. Pediatr Allergy Immunol. 2017;28:332–339. doi: 10.1111/pai.12704. [DOI] [PubMed] [Google Scholar]
- 30.Tiotiu AI, Novakova P, Nedeva D, Chong-Neto HJ, Novakova S, Steiropoulos P, et al. Impact of air pollution on asthma outcomes. Int J Environ Res Public Health. 2020;17:6212. doi: 10.3390/ijerph17176212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 32.von Mutius E. Environmental microorganisms and lung health. Ann Am Thorac Soc. 2014;11:S13–S15. doi: 10.1513/AnnalsATS.201306-155MG. [DOI] [PubMed] [Google Scholar]
- 33.Dubowski K, Kaali S, Jack D, Prah RKD, Clemente JC, Tawiah T, et al. Infant nasopharyngeal microbiota subphenotypes and early childhood lung function: evidence from a rural Ghanaian pregnancy cohort. Int J Environ Res Public Health. 2021;18:7276. doi: 10.3390/ijerph18147276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruokolainen L, Paalanen L, Karkman A, Laatikainen T, von Hertzen L, Vlasoff T, et al. Significant disparities in allergy prevalence and microbiota between the young people in Finnish and Russian Karelia. Clin Exp Allergy. 2017;47:665–674. doi: 10.1111/cea.12895. [DOI] [PubMed] [Google Scholar]
- 35.Hussey SJK, Purves J, Allcock N, Fernandes VE, Monks PS, Ketley JM, et al. Air pollution alters Staphylococcus aureus and Streptococcus pneumoniae biofilms, antibiotic tolerance and colonisation. Environ Microbiol. 2017;19:1868–1880. doi: 10.1111/1462-2920.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu J, Bao Y, Zhu Y, Osman R, Shen M, Zhang Z, et al. The preliminary study on the association between PAHs and air pollutants and microbiota diversity. Arch Environ Contam Toxicol. 2020;79:321–332. doi: 10.1007/s00244-020-00757-4. [DOI] [PubMed] [Google Scholar]
- 37.Harrod KS, Jaramillo RJ, Berger JA, Gigliotti AP, Seilkop SK, Reed MD. Inhaled diesel engine emissions reduce bacterial clearance and exacerbate lung disease to Pseudomonas aeruginosa infection in vivo. Toxicol Sci. 2005;83:155–165. doi: 10.1093/toxsci/kfi007. [DOI] [PubMed] [Google Scholar]
- 38.Suraju MO, Lalinde-Barnes S, Sanamvenkata S, Esmaeili M, Shishodia S, Rosenzweig JA. The effects of indoor and outdoor dust exposure on the growth, sensitivity to oxidative-stress, and biofilm production of three opportunistic bacterial pathogens. Sci Total Environ. 2015;538:949–958. doi: 10.1016/j.scitotenv.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 39.Niemeier-Walsh C, Ryan PH, Meller J, Ollberding NJ, Adhikari A, Reponen T. Exposure to traffic-related air pollution and bacterial diversity in the lower respiratory tract of children. PLoS One. 2021;16 doi: 10.1371/journal.pone.0244341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosario NA, D'Amato G, Ansotegui I. Global warming and warning. Clinics. 2019;74:e1219. doi: 10.6061/clinics/2019/e1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cecchi L, D'Amato G. Annesi-Maesano I. External exposome and allergic respiratory and skin diseases. J Allergy Clin Immunol. 2018;141:846–857. doi: 10.1016/j.jaci.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Haahtela T, Holgate S, Pawankar R, Akdis CA, Benjaponpitak S, Caraballo L, et al. The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J. 2013;6:3. doi: 10.1186/1939-4551-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haahtela T, von Hertzen L, Anto JM, Bai C, Baigenzhin A, Bateman ED, et al. Helsinki by nature: the nature step to respiratory health. Clin Transl Allergy. 2019;9:57. doi: 10.1186/s13601-019-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Nethery RC, Sabath BM, Braun D, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. medRxiv [Preprint]. 2020 doi: 10.1101/2020.04.05.20054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoang T, Tran TTA. Ambient air pollution, meteorology, and COVID-19 infection in Korea. J Med Virol. 2021;93:878–885. doi: 10.1002/jmv.26325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciencewicki J, Jaspers I. Air pollution and respiratory viral infection. Inhal Toxicol. 2007;19:1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- 47.Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Annesi-Maesano I, Maesano CN, D'Amato M, D'Amato G. Pros and cons for the role of air pollution on COVID-19 development. Allergy. 2021;76:2647–2649. doi: 10.1111/all.14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Setti L, Passarini F, De Gennaro G, Barbieri P, Perrone MG, Borelli M, et al. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ Res. 2020;188 doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Card SD, Pearson MN, Clover GR. Plant pathogens transmitted by pollen. Austral Plant Pathol. 2007;36:455–461. [Google Scholar]
- 51.Beggs PJ. Impacts of climate change on aeroallergens: past and future. Clin Exp Allergy. 2004;34:1507–1513. doi: 10.1111/j.1365-2222.2004.02061.x. [DOI] [PubMed] [Google Scholar]
- 52.Ravindra K, Goyal A, Mor S. Does airborne pollen influence COVID-19 outbreak? Sustain Cities Soc. 2021;70 doi: 10.1016/j.scs.2021.102887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoogeveen MJ, van Gorp ECM, Hoogeveen EK. Can pollen explain the seasonality of flu-like illnesses in the Netherlands? Sci Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.143182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoogeveen MJ. Pollen likely seasonal factor in inhibiting flu-like epidemics. A Dutch study into the inverse relation between pollen counts, hay fever and flu-like incidence 2016-2019. Sci Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138543. [DOI] [PMC free article] [PubMed] [Google Scholar]