Abstract
Objective
Endocrine disrupting chemicals (EDCs) are present in many areas and materials of the common life, and exposure to these chemicals can occur from products to personal care, from air and food. This review aims to summarize the more recent epidemiological findings for the impact of EDCs on endocrine system health in children, including effects in growth, metabolism, sexual development, and reproduction.
Sources
The MEDLINE database (PubMed) was searched on August 24th, 2021, filtering for EDCs, endocrine disruptors, children, and humans.
Summary of the findings
Intrauterine exposure of EDCs can have transgenerational effects, thus laying the foundation for disease in later life. The dose-response relationship may not always be predictable as even low-level exposures that may occur in everyday life can have significant effects on a susceptible individual. Although individual compounds have been studied in detail, the effects of a combination of these chemicals are yet to be studied to understand the real-life situation where human beings are exposed to a “cocktail effect” of these EDCs. Epidemiological studies in humans suggest EDCs’ effects on prenatal growth, thyroid function, glucose metabolism, obesity, puberty, and fertility mainly through epigenetic mechanisms.
Conclusions
EDCs cause adverse effects in animals, and their effects on human health are now known and irrefutable. Because people are typically exposed to multiple endocrine disruptors, assessing public health effects is difficult. Legislation to ban EDCs and protect especially pregnant women and young children is required and needs to be revised and adjusted to new developments on a regular basis.
Keywords: Endocrine disruptors, Children, Adolescents
Introduction
Thousands of exogenous chemicals or a mixture of chemicals were defined as endocrine-disrupting chemicals (EDCs) due to their ability to mimic or interfere with the endocrine system and metabolism, causing side effects on health. 1
EDCs mostly interfere with endocrine and metabolic pathways during very sensitive periods of human development and, over the last decade, our knowledge on their pathogenic mechanisms of action from wildlife to humans,1,2 and their effects on health from pregnancy to adulthood greatly improved.3, 4, 5, 6, 7, 8, 9
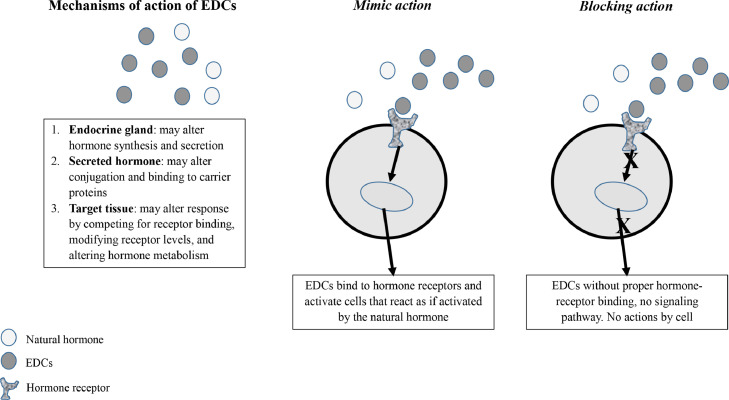
EDCs may alter endogenous hormones levels by interfering with synthesis, secretion, transport, action, and degradation through mechanisms involving the same hormones receptors and signaling pathways (Figure 1). They may act via classical nuclear receptors, nonnuclear steroid hormone receptors, non-steroid receptors, orphan receptors, enzymatic pathways involved in steroid biosynthesis and/or metabolism, and other mechanisms involved in endocrine system function.2
Figure 1.
Mechanism of action of EDCs.
The most studied EDCs with potential effects on human health are plastics and plasticizers [bisphenol A (BPA) and phthalates]. BPA is the most produced substance, and in some consumer products, it is substituted by bisphenol F (BPF) and bisphenol S (BPS).
Other common EDCs are industrial chemicals [dioxins, dioxin-like polychlorinated biphenyls (PCBs)], pesticides [dichloro-diphenyl-trichloroethane (p,p’-DDT) and its metabolite dichloro-diphenyl-dichloroethylene (p,p’-DDE), and hexachlorobenzene (HCB)], perfluoroalkyl and polyfluoroalkyl substances [PFASs - perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA)], brominated flame retardants [BFRs - polybrominated diphenyl ethers (PBDEs)], and polycyclic aromatic hydrocarbons (PAHs).1
Characteristics of the most commonly used EDCs are briefly reported in Table 1.
Table 1.
Characteristics of the most common used EDCs.
| EDCs/Characteristics | Factsheet | Exposure |
|---|---|---|
| Bisphenol A (BPA) | Used to make polycarbonate plastics and epoxy resins, which are found in many plastic products including food storage containers. Use of this plasticizer for production of baby bottles was banned in EU and US since 2011 and 2012, respectively. |
Daily human exposure to BPA is through the diet, but air, dust, and water are other possible sources of exposure. Exposure at low levels comes from eating food or drinking water stored in containers that have BPA. Infants and young children may be exposed by hand-to-mouth and direct mouth contact with materials containing BPA. Workers who manufacture products that contain BPA can be exposed. |
| Phthalates | Used to make plastics more durable and flexible, they are also found in some food packaging, cosmetics, children's toys, and medical devices. | Eating and drinking foods that have contacted products containing phthalates. Some exposure can occur from breathing phthalate particles in the air. Phthalate particles in dust might be a greater risk for children than for adults, because they touch many things and then put their hands in their mouths. |
| Dioxins | Not manufactured or produced intentionally. They are created when other chemicals or products are made (i.e. incineration processes). Being mainly by-products of industrial practices (i.e. herbicide production and paper bleaching) they are also released into the environment during waste burning and wildfires. | People are exposed primarily by eating food, in particular animal products. They are absorbed and stored in fat tissue and, therefore, accumulate in the food chain. |
| Dioxin-like polychlorinated biphenyls (PCBs) | PCBs were used to make electrical equipment like transformers, in hydraulic fluids, heat transfer fluids, lubricants, and plasticizers. Banned in 1979 |
Eating or drinking contaminated food, through the breathed air, or by skin contact. All these chemicals remain in the environment even though they are no longer manufactured. They enter the food chain and build up in larger animals. |
|
Pesticides Dichloro-diphenyl-trichloroethane (p,p’-DDT) Dichloro-diphenyl-dichloroethylene (p,p’-DDE) Hexachlorobenzene (HCB) |
Include herbicides for destroying weeds and other unwanted vegetation, insecticides for controlling a wide variety of insects, fungicides used to prevent the growth of molds and mildew, disinfectants for preventing the spread of bacteria, and compounds used to control mice and rats. p,p’-DDT is an insecticide used in agriculture. US banned its use in 1972, but in some countries it is still used to control mosquitoes that spread malaria. HCB is formed as a byproduct during the manufacture of other chemicals. Widely used as a pesticide until 1965, concerns are due to its persistence in the environment, potential to bioaccumulate, and toxicity to humans and the environment. |
Because of the widespread use of agricultural chemicals in food production, people are exposed to low levels of pesticide residues through their diets. People may also be exposed to pesticides used in a variety of settings including homes, schools, hospitals, and workplaces. Exposure to p,p’-DDT likely occurs by eating foods, including meat, fish, and dairy products. p,p’-DDT exposure can also occur by breathing or touching contaminated products. p,p’-DDT and p,p’-DDE persist in body (fatty tissues store) and environment.Exposure to HCB is via inhalation and dermally, through proximity to industries site where it is produced as a byproduct. Exposure can also occur through consuming foods tainted with HCB. |
|
Per- and polyfluoroalkyl substances (PFASs) Perfluorooctanoic acid (PFOA) Perfluorooctane sulfonate (PFOS) |
A large, complex, and ever-expanding group of man-made chemicals widely used to make various types of everyday products including clothing, furniture, adhesives, food packaging, heat-resistant non-stick cooking surfaces, and the insulation of electrical wire. | Concerns arise because they do not break down in the environment, can move through soils and contaminate drinking water sources, bioaccumulate in fish and wildlife. So, they persist in the environment and people are most likely exposed by consuming contaminated water or food, using products made with PFASs, or breathing air containing PFASs. PFOA and PFOS were manufactured for the longest time, are the most widespread in the environment and the most well-studied. They are no longer made in the US, but they have been replaced with alternative PFAS, such as GenX. |
|
Brominated flame retardants (BFRs) Polybrominated diphenyl ethers (PBDEs) |
Used in many consumer and industrial products since the 1970s, to decrease the ability of materials to ignite. PBDEs do not chemically bind with products to which they are added (furniture, electronics, etc.), so they easily release from these products and enter air and dust. |
These chemicals can get into the air, water, and soil during their manufacture; they can leak from products that contain them or escape when the products that contain them break down. People can be exposed to flame retardants through a variety of ways, including diet, consumer products in the home, car, airplane, and workplace. People can be exposed to PBDEs by eating contaminated foods, especially those with a high fat content. Another source of exposure results from breathing contaminated air or swallowing contaminated dust. Working in industries that make these chemicals or that make, repair, or recycle products containing these chemicals flame retardants can result in exposure. |
| Polycyclic aromatic hydrocarbons (PAHs) | Large family of environmental pollutants found in high amounts as air pollutants in megacities. Chemicals that occur naturally in coal, crude oil, and gasoline. They are also produced when coal, oil, gas, wood, garbage, and tobacco are burned. High-temperature cooking will form PAHs in meat and in other foods. | People are usually exposed to mixtures of PAHs. Breathing air contaminated (motor vehicle exhaust, cigarette smoke, wood smoke, or fumes from asphalt roads) are common ways of exposure. People take in PAHs when they eat grilled or charred meats or foods or foods on which PAHs particles have settled from the air. |
This manuscript aims to review the mainly positive albeit detrimental epidemiological findings for EDCs effects, mainly on growth and pubertal development. Studies evaluating EDCs’ impacts on the male reproduction system, thyroid function, and metabolism are also reported.
Methods
The authors of the present study retrieved studies investigating associations between exposure to the most common EDCs and endocrine system health in children and adolescents. Selection criteria for literature research were text availability (full text), article type (books and documents, clinical trial, meta-analysis, randomized controlled trial, review, and systematic review), language (English), publication date (5 years), species (humans), and age child (birth-18 years).
Articles were systematically searched on August 24th, 2021 in the MEDLINE database (PubMed) using the following search terms: endocrine-disrupting chemicals OR EDCs OR endocrine disrupt* adding (AND) prenatal growth OR postnatal growth OR growth OR pubert* OR thelarche OR pubarche OR reproductive system OR thyroid OR glucose metabolism OR obesity OR type 1 diabetes (T1D) to previous search terms. A hand-screening of all reference lists included in papers was performed to identify studies missed in the primary search process. Papers were reviewed and were included in this review if ECDs exposure levels were examined in relation to auxological outcomes, pubertal development characteristics, male reproductive system alterations, thyroid dysfunction, and impairment of glucose metabolism.
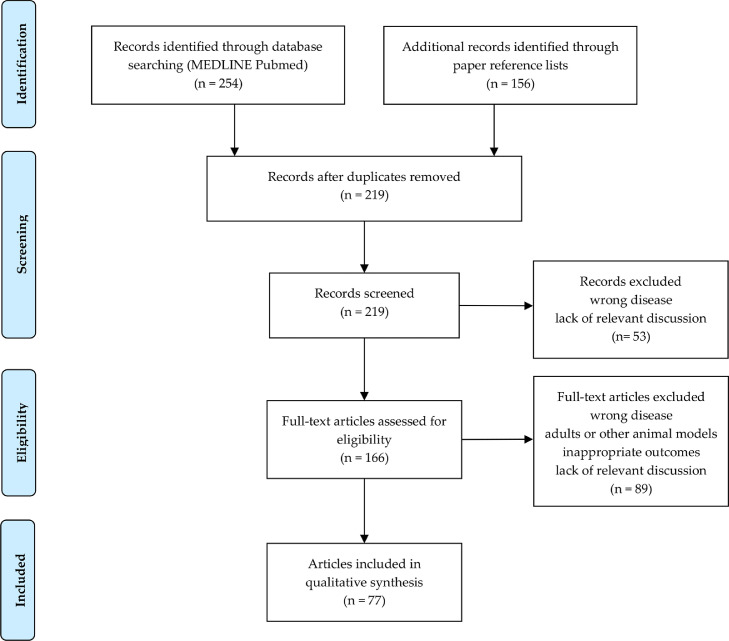
Overall, 219 papers were initially identified. After the screening of titles and abstracts, a total of 142 articles were discarded, leaving 77 articles to be analyzed (Figure 2).
Figure 2.
PRISMA flow diagram. (From: Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. For more information, visit www.prisma-statement.org).
Results
Pre- and post-natal growth
Intrauterine life and early post-natal life represent the most critical windows for child development. Fetal growth is regulated by complex interactions between maternal, placental, and fetal factors, which are still partially known.10 Insulin-like growth factor (IGF) system is a critical regulator of growth and EDCs are able to interfere with IGF system.11
Exposure to EDCs during the early life stage can cause epigenetic shifts besides other effects on protein content, cell number, cell size, organ size, and function. These changes can be transmitted over several generations as "non-transmissible diseases".10
Epidemiological studies reported correlations between in utero exposure to major EDCs and birth outcomes, but sometimes results were contradictory. EDCs and mixtures of chemical substances were found in both mothers and placenta, so exposure to EDCs can be associated with fetal growth restriction and premature birth.12
Pregnant women can be exposed to EDCs at work, and a large-scale prospective study from 13 European cohorts suggested that exposure to one or more EDCs groups was associated with a higher risk of term low birth weight (LBW) newborns [odds ratio (OR)=1.25]. The most specific EDCs were PAHs, pesticides, phthalates, alkyl phenolic compounds, BFRs, and metals. The risk increased with the increasing number of EDCs (OR=2.11 for exposure to ≥4 EDCs groups), suggesting a possible exposure-response relationship.4 Conversely, higher concentrations of persistent organic pollutants (POPs) measured in newborn dried blood spots were found to be positively associated with a slightly higher risk of large for gestational age and higher birth weight.13
A wide range of in utero exposure scenarios was revealed. Combinations exhibiting higher levels of PBDEs and p,p’-DDE were associated with lower birth weight, while combinations with higher levels of PCBs and PFAS were associated with increased birth weight.14 It was suggested that maternal exposure to environmental contaminants (phthalates, PFASs, PCB-153, and p,p’-DDE) might be independently associated with fetal growth. Mono(2-ethyl-5-hydroxyhexyl) and mono(2ethyl-5-oxyhexyl) phthalates, PFOA, and p,p’-DDE were found to be predictors for lower birth weight, while mono(oxo-isononyl) phthalate was associated with higher birth weight.4 PCBs and p,p’-DDT/DDE serum levels were measured in a German cohort of 324 pregnant women. The birth length of newborns was negatively correlated with increased maternal PCBs levels. Positive associations between different maternal PCBs levels and post-natal weight gain were observed. These results suggested that maternal PCBs and p,p’-DDT exposure likely affects different aspects of offspring's pre- and post-natal growth.15 Studies reported conflicting results for PCBs, because an association with LBW was demonstrated by some authors but not by others.5
BPA exposure during pregnancy has huge impacts on the fetus, causing changes in epigenetic programming, resulting in disease onsets during childhood as well as adulthood.16 Combined exposures to BPA from dietary and non-dietary sources during pregnancy may contribute to a tendency towards fetal growth restriction.17 High levels of BPA in maternal blood, urine, or amniotic fluid were associated with lower birth weight newborns. Exposure to unconjugated BPA during the 1st-trimester and the end of gestation was associated with a gender-specific reduction in birth weight. Moreover, it was demonstrated that exposure to BPA was negatively correlated with intrauterine linear growth since the increase of one logarithmic-transformed unit of BPA/creatinine in the maternal urine concentration of BPA during the 3rd-trimester was associated with a reduced length of the femur. Furthermore, the increase of one logarithmic-transformed unit of BPA/creatinine in the prenatal concentration of BPA was correlated with increased birth weight.4
The effects of BPF and BPS on fetal growth in humans are still unclear. Urinary concentrations of BPF and BPS during some trimesters of the pregnancy were associated with significantly lower values of birth weight, length, or ponderal index. Newborns in the 10th percentile of each birth anthropometry measure had higher BPF, and BPS exposures during pregnancy than newborns in the 90th percentile of each outcome.18 Urinary BPA and BPF concentrations of pregnant women were negatively related to birth length and positively related to ponderal index in female infants.19 Conversely, in pregnant women, higher BPS urinary concentrations were related to larger fetal head circumference, higher weight, and lower risk of being small for gestational age (SGA) at birth.20 Evidence on BPA-influence on pubertal height growth are still lacking. An inverse association between urinary BPA levels and height was observed in children. Height z-score significantly decreased by 0.49 for the highest BPA exposure levels compared with the lowest ones.21
Phthalate exposure during pregnancy may be associated with increased odds of prematurity. Possible mechanisms are interference with placental function and steroidogenesis, mainly in subjects with certain genetic mutations, highlighting the gene-environment interaction.5 Differential DNA methylation may link phthalate exposure in utero to fetal growth, having a predictive value for offspring's obesity.22 Phthalates may have trimester-specific effects on fetal growth and birth outcomes. It was demonstrated that, among males, the 1st-trimester urinary bis-2-ethylhexyl phthalate (DEHP) level was negatively related to fetal growth, the 2nd-trimester DEHP was negatively related to birth weight and length, and the 3rd-trimester DEHP was positively associated with birth weight. Among females, the 1st-trimester DEHP was associated with increased birth length.23
Genetic effects of pesticides were shown to result in increased prematurity and preterm birth. Pesticide exposure during the 2nd-trimester of pregnancy was negatively associated with weight, length, and head circumference at birth. Increased incidence of SGA newborns was reported in mothers who were exposed to pesticides.1
Conversely, it was found that overall frequencies of exposure to household pesticides had no effects on birth weight and length.24 Prenatal exposure to pesticides may differently influence birth outcomes.25
Pesticide exposure could be a risk factor for growth disorders in children living in agricultural areas. In children with stunting (height for age z-score <-2 SDS), median IGF-1 levels were significantly lower compared to controls, and both high levels of pesticide exposure and low IGF-1 levels were significantly associated with stunting.26
Fetus is exposed to PFASs via active or passive placenta transfer, while newborns might be exposed via breastfeeding or PFASs in the home environment. Human epidemiological findings suggested possible associations with fetal and postnatal growth, but data are still controversial.5
A systematic review evaluated results from 14 studies. In utero exposure to PFOA was associated with decreased mean birth weight in most studies, but only some results were statistically significant.4 High concentrations of PFOS and perfluorohexanesulfate measured in newborn dried blood spots were demonstrated to be related to lower birth weight z-scores compared to those with low concentrations.27 Increased serum maternal PFOS, PFOA, perfluorononanoic acid, perfluorodecanoic acid, and perfluoroundecanoic acid levels during pregnancy were associated with lower birth weight and SGA at birth. However, associations were significant only in girls.28
PBDEs interfere with the IGF-1 system secretion. A positive association was demonstrated between BDE-196 in breast milk and IGF-I levels in cord serum, and negative correlations between IGF-1 and BDE-99 and 86 other compounds.4
Epidemiological studies reported negative correlations between PBDEs and birth weight in male newborns and positive correlations in female ones.4 A total of 19 PBDEs were detected in maternal serum samples collected during the 3rd-trimester of pregnancy, and a negative association was found with placental size and birth outcomes. Concentrations of BDE-207, -208, -209, and the sum of 19 PBDEs were higher in fetal growth restriction newborns compared with healthy ones.29 Evidence supporting that changes in placental DNA methylation might be part of the underlying biological pathway between prenatal PBDEs exposure and adverse fetal growth were reported.30
Data on post-natal height growth are still lacking. Studies reported data related to the possible association between exposure to EDCs during pregnancy and both birth weight and fast weight gain in early childhood, underlining the risk to develop overweight and obesity and other diseases and dysfunctions later in life due to altered “programming” in utero, according to the Barker hypothesis of the “Developmental Origins of Health and Disease”.5
Studies suggested that in utero exposure to p,p’-DDT, p,p’-DDE, and HCB, may increase the risk for rapid weight gain in infancy and high body mass index (BMI) later in childhood and adolescence.31
Association between in utero POPs exposures and major risk factors for the adult cardio-metabolic syndrome was reported. HCB exposure in the 3rd-tertile was associated with higher BMI, and weight-to-height ratio z-score and a continuous increase in HCB levels were associated with higher body fat %, systolic and diastolic blood pressure z-score, cardio-metabolic-risk score, and lipid biomarkers.32
Prenatal exposure to mixtures of persistent EDCs (PFASs, PCBs, and pesticides) may inversely affect post-natal body size. EDCs mixture at the 75th percentile compared to the 50th percentile was associated with a 0.15 lower weight-for-age z-score. Weakly inverse associations were also seen for height-for-age and BMI-for-age z-scores.33
Gender-specific and trimester-specific relationships between DEHP exposure and fetal and birth offspring growth data were longitudinally confirmed at 6, 12, and 24 months.23
Obesity in children was reported to be induced by phthalate exposure during pregnancy. Some maternal urinary phthalate's metabolites were strongly associated with BMI z-scores, waist circumference z-scores, and body fat % in children of different ages. In the 12-year-old population, in utero levels of diethyl-phthalate, dibutyl-phthalate, and DEHP metabolites were positively associated with overweight or obesity.34
Associations between maternal phthalate levels and changes of auxological data were longitudinally measured. During infancy, females with detectable levels of mono-(3-hydroxybutyl) phthalate or mono-carboxy-iso-octyl phthalate (MCiOP) grew on average 0.73 cm and 0.66 cm less than those with undetectable levels, respectively. Participants in the middle tertile of mono-ethyl phthalate (MEP) grew 0.57 cm less than those in the lowest tertile. Analyzing data from 2 to 10 years of age, females with detectable mono-benzyl phthalate and middle/upper tertile of mono-(2-ethyl-5-carboxypentyl) phthalate levels were 1.19 and 0.99 cm taller than those with undetectable and in the lowest tertile, respectively. At 20-years of follow-up, associations between maternal serum phthalate metabolite levels and deviation from mid-parental height were not found. Similar results were reported for weight and BMI z-score.35
Maternal serum PFASs concentrations, particularly PFOA, resulted inversely associated with longitudinal measures of infant/child anthropometry from 4 weeks to 2 years of age,36 but these data were not found by others.27 Considering discrepancies between studies, the impact of PFAS on health is not yet clear, but it deserves further investigation.
Umbilical cord serum BDE-153 and -154 concentrations were related to reduced adiposity measures at seven years of age.37
Exposure during critical periods of development may have consequences. Future studies should confirm these findings, and it is important to evaluate EDCs that were never studied in experimental and epidemiological research. Current knowledge on placental and fetal growth, as well as in utero programming of metabolism and endocrine function, highlights the need for the implementation of preventive measures for exposure.8
Pubertal development
Pubertal development is characterized by activation of the hypothalamus-pituitary-gonadal and hypothalamus-pituitary-adrenal axis, which are regulated by inhibitory and stimulatory factors.38 As 20–40% of the variation of pubertal timing and progression are not genetically dependent, and other key factors are involved in its regulation, concerns rose on the potential role of EDCs (Table 2). 5,6
Table 2.
| EDC | Possible mechanism | Possible clinical condition |
|
|---|---|---|---|
| Males | Females | ||
| BPA |
|
|
|
| Phthalates |
|
|
|
| Dioxins | Mechanism of action not fully clarified.
|
|
|
| PCBs |
|
|
|
|
Pesticides p,p’-DDT p,p’-DDE |
|
|
|
| PFASs |
|
|
|
|
BFRs PBDEs |
|
|
|
AGD, anogenital distance; AhR, aryl hydrocarbon receptor; AR, androgen receptor; BFRs, brominated flame retardants; BPA, bisphenol A; CPP, central precocious puberty; p,p’-DDE, dichloro-diphenyl-dichloroethylene; p,p’-DDT, dichloro-diphenyl-trichloroethane; ER, estrogen receptor; ERK1/2, extracellular signal-regulated kinase 2; PBDEs, polybrominated diphenyl ethers; PCBs, dioxin-like polychlorinated biphenyls; PFASs, perfluoroalkyl and polyfluoroalkyl substances.
In humans, it is difficult to provide evidence on causal relationships between EDCs exposure and changes in pubertal timing, but they seem to play an important role in pubertal dysregulation through central actions on the hypothalamus or peripheral actions on breast and gonads. Other problems are the concomitant exposure to low doses of tens or hundreds of chemicals and the delay between exposure to EDCs during early childhood and observation of potential consequences on pubertal timing.8
Several EDCs act as agonists of estrogen receptors (ERs) or antagonists of androgen receptors, while progestin receptors represent potential targets for many chlorinated EDCs. EDCs can mimic the physiological effect of estrogens and androgens and can cause hyper-stimulation of hormonal pathways. Moreover, they can bind to intracellular receptors and block the function of endogenous hormones, having anti-estrogenic or anti-androgenic effects.8
Epidemiological researches were conducted in geographical areas where accidental exposure to specific chemicals occurred and suggested that EDCs act by determining an advance but also a delay in puberty.
The ubiquitous use of BPA results in great exposure to its known estrogenic-like action. Most of the cross-sectional studies have shown that serum and urinary BPA levels were higher in girls with central precocious puberty (CPP) than in controls, suggesting a possible role of BPA in the disease onset.8
Exposure to BPA might be one of the underlying factors of early breast development in pre-pubertal girls. Urinary concentrations of BPA were significantly higher in girls with premature thelarche compared to the healthy ones, and weak positive correlations with uterus volume, estradiol, and luteinizing hormone were found in girls with premature thelarche.6,8
The association between the age of menarche and urinary BPA levels was analyzed. Girls with intermediate BPA levels were less likely to develop early menarche than those with lower levels (OR=0.57). Association between BPA exposure and delayed menarche was also suggested. Girls with intermediate and high levels of BPA, compared to those with undetectable levels, were more likely to have delayed menarche.8 Conflicting results from studies do not allow to clearly define the role of BPA in alterations of pubertal development timing.
Although mechanisms by which phthalates act as EDCs are not yet well known. Clinical studies suggested anti-androgenic, agonist and antagonist action on ERs. Low and high exposure to phthalates could alter pubertal development in both genders, and effects were either early or delayed puberty.39
A possible correlation between exposure to phthalates and early breast development in girls was suggested in the early 1980s due to a progressive trend towards premature thelarche found in Puerto Rico. Higher blood levels of phthalates were demonstrated in girls with premature thelarche, and measurable values of phthalates were found in 68% of girls with premature thelarche compared to 14% of healthy controls.6,8
A potential anti-androgenic effect of phthalates was suggested because the highest quartile of urinary phthalates excretion was associated with pubarche delay. Although some studies did not find a difference in phthalate metabolites urinary levels between girls with CPP and controls, in others, plasma and urinary levels of phthalates were demonstrated to be significantly higher in girls with CPP compared to those with precocious pseudo-puberty and the healthy ones.8
In young girls, it was demonstrated that menarche age was much earlier the higher were levels of urinary phthalates measured several years before.40
In utero exposure to some phthalate's metabolites and BPA was associated with delayed puberty in females, especially in normal-weight ones, and with early puberty in males, especially in overweight/obese ones, underlining that body weight may interfere in such associations.41
It was found that the age at menarche was slightly delayed in girls with higher prenatal exposure to phthalate metabolites. According to the sum of phthalate metabolites, significantly later age at menarche was found in subjects at the middle tertile concentration compared to those at the lowest tertile.35 Higher phthalate concentrations were also reported to be associated with earlier menarche among overweight or obese girls.42 These results show discrepancies between different phthalates and different analyzed pubertal outcomes. The role of childhood phthalate exposure on early breast development requires further study, and it is difficult to carry out human studies and to interpret results.
Dioxins act through aryl hydrocarbon receptors (AhR). Exposure to dioxins was associated with delayed puberty in boys and delayed thelarche in girls because of anti-estrogenic effects. The slow progression of breast development towards the adult stage was demonstrated in girls, and it was associated with the high activity of dioxins.1,6,8
Data concerning pesticides derives from findings of precocious or early puberty in children migrated because of international adoptions and previously exposed to p,p’-DDT insecticide in their origin country during pregnancy and post-natal period. Migration can stop the exposure to p,p’-DDT, and precocious puberty can develop indirectly, following the suspension of sex steroids and their environmental analogs negative feedback, and directly, as a consequence of accelerated hypothalamic maturation secondary to sex steroids action.6,8
Studies demonstrated that the earlier was the onset of menarche in girls, the higher was the in utero exposure to p,p’-DDE.8
Possible mechanisms of action of the p,p’-DDE include both anti-androgenic and estrogenic-like effects and the induction of aromatase enzyme. Published results are conflicting because no association was also demonstrated between p,p’-DDE exposure, neither intrauterine nor post-natal through breast milk, and timing of pubertal development, Tanner staging, and age of menarche.1
The authors need further information on the relationship between exposure to multiple pesticides and the onset of idiopathic premature thelarche in girls, mainly from areas of intensive agriculture practice.
Evidence on the potential impact of prenatal PFASs exposure on long-term reproductive health is still lacking, but gender-specific alterations of pubertal timing with different prenatal exposure to PFASs were suggested.43 Data on later age of menarche with higher levels of prenatal PFOA exposure were reported,1 but no association between prenatal PFASs exposures and age at menarche was also demonstrated.44 These results need to be confirmed because the role of these compounds as complex mixtures remains largely unknown.45
Exposure to PBDEs during the peripubertal period seems to interfere with pubertal development. An association between high serum PBDEs concentrations and earlier menarche age was demonstrated. From 1st- to 4th-quartile of total PBDEs concentrations, the rate of menarche occurrence before 12 years of age was higher in girls with the greater PBDEs exposure.6,8
Effects of BFRs on pubertal development were evaluated in girls exposed to polybrominated biphenyls (PBBs) in utero and through breastfeeding. Menarche was found to be one year earlier in girls who were exposed in utero to high concentrations and who were breastfed than in girls not exposed or exposed only in utero but not through breastfeeding (11.6 vs. 12.2-12.7 years, respectively). Perinatal exposure was associated with early pubarche in breastfed girls.6,8 These results support the hypothesis that pubertal development may be influenced by pre- and post-natal exposure to organo-halogen compounds. Since menarche and breast development are estrogen-dependent, while pubic hair development is independent of estrogen levels, these data suggest that PBBs may act through different pathways.
Relationships between prenatal and childhood exposure to PBDEs and alterations in pubertal development timing were also studied. Serum concentrations of four PBDEs (BDE-47, -99, -100, -153) were measured in blood collected from mothers during pregnancy, and pubertal onset was evaluated in their 9-years old children followed until 13 years of age. Prenatal PBDEs concentrations were associated with delayed menarche in females [relative risk (RR) early menarche = 0.5] and early pubarche in males (RR early pubarche = 2.0).6,8 Data are contradictory but suggest that BFRs have estrogenic and androgenic properties and that exposure to these chemicals can have an impact on pubertal development.
A possible link between apparently innocuous topical use of essential oils and onset of pre-pubertal male gynecomastia and premature thelarche was suggested due to their estrogenic and anti-androgenic properties demonstrated in vitro.46
The onset of puberty can be disrupted by exposure to EDCs, mainly phthalates and BPA, and delayed or precocious onset of puberty was described. EDCs can affect processes underlying pubertal development, but the authors still need to better understand which are times in life when EDCs exposure is crucial to determine changes in pubertal physiology and how this may affect fertility later in life.
Male reproductive system
EDCs can affect the male reproductive system by anti-androgenic and estrogenic effects (Table 2).
Hypospadias and cryptorchidism were related to EDCs since the first studies led to the hypothesis of the testicular dysgenesis syndrome. EDCs seem to act on the tubular part of the testicle, which does not develop regularly and is at subsequent risk of cancer, and the endocrine part with consequent lower production of testosterone and other endocrine factors necessary to ensure the normal testicular descent in the scrotum and the normal penile formation.47 Clinically, it is important to evaluate the anogenital distance (AGD), which is a sensitive index of prenatal androgens action, influenced by exposure to chemicals with anti-androgenic activity during the critical developmental period of the fetal testis.48
Maternal serum BPA concentration at 10-17 weeks of gestation was positively associated with congenital or post-natal acquired cryptorchidism.49
The authors still need more powerful evidence to confirm the effects of prenatal phthalate exposure on hypospadias. Its increased prevalence may be the result of exposure to these EDCs having estrogenic or anti-androgenic properties. A high rate of hypospadias was reported in children whose mothers were exposed to phthalate during the 1st-trimester of pregnancy.50
Concentrations of di-isononyl phthalate metabolites in amniotic fluid samples during the 2nd-trimester of pregnancy were associated with an increased likelihood of hypospadias (OR = 1.69).51
An inverse association between maternal urinary phthalate metabolite levels and AGD was demonstrated in boys but not in girls.1,52
Dioxins may have estrogenic effects through the interaction of the dioxin-AhR nuclear translocator complex with ERs. Higher dioxin levels in breast milk and dibutyltin concentrations in the placenta were associated with cryptorchidism.1
Levels of chlorinated pesticides were found to be higher in the breast milk of mothers with cryptorchid boys, and high prenatal exposure to p,p’-DDE and PCBs has a higher risk of cryptorchidism.1 An increased risk of hypospadias was found among children of women with serum p,p’-DDE concentrations at the highest quartile (OR=1.65) compared to women in the 1st-quartile during the 14th week of pregnancy.53
Maternal exposure to PFASs was associated with shorter AGD in boys, providing evidence that they can affect male genital development.54
Male reproductive disorders are increasing in prevalence, which may reflect environmental influences on fetal testicular development. Epidemiological studies generated conflicting results and were often limited by the small sample size and/or measurement of chemical exposures outside the most relevant developmental window.
Thyroid function
Thyroid hormones are essential for normal brain development and regulation of metabolism. EDCs can affect this process, and thyroid disruption can occur at any level of the hypothalamus-pituitary–thyroid axis, including thyroid hormones synthesis (BPA, phthalates, PCBs, PBDEs, and perchlorate), release (phthalates, PCBs, and PBDEs), transport (phthalates, PCBs, and dioxins), and metabolism (BPA, PCBs, and dioxins). The action of thyroid hormones on target tissues can also be disrupted by EDCs (PCB and PBDEs). 4,55,56
In vitro and in vivo studies reported the ability of bisphenols to disrupt thyroid function.57 Antagonism with thyroid receptors (TRs) affecting the TRs-mediated transcriptional activity, direct action of bisphenols on gene expression at the thyroid and the pituitary levels, competitive binding with thyroid transport proteins, and induction of toxicity in several cell lines are main mechanisms leading to thyroid dysfunction.58
A higher late pregnancy maternal BPA exposure was associated with higher thyroid-stimulating hormone (TSH) levels in female newborns and higher free thyroxine (FT4) levels during childhood in males.59 The impact of BPA on thyroid hormone levels in newborns was also reported as uncertain, so this remains an important field of research in the next years.5
In newborns exposed to multiple phthalate metabolites during pregnancy, levels of cord serum TSH and FT4 were found to be significantly negatively associated with cord serum phthalate levels.5,60 Effects of cord blood phthalates and prenatal exposure on thyroid hormones in newborns remain unclear, but the fall of TSH levels may potentially delay their development.
Maternal serum 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) concentrations at higher quartiles were associated with lower free triiodothyronine in children, compared to the lowest quartile. A positive association between maternal serum TCDD and TSH concentrations in children with high thyroid antibody status was also found. Prenatal exposure to TCDD may alter thyroid function later in life, and populations with additional thyroid stress may be particularly susceptible for in utero exposure to thyroid disrupting chemicals.61
Exposure to PFASs can alter circulating thyroid hormone levels. In infants, higher concentrations of PFASs mixture were associated with lower total thyroxine levels. Combined effects of prenatal exposure to multiple PFAS on maternal and neonatal thyroid function was supposed, but the direction and magnitude of these effects may vary across each PFASs.62
PBDEs structurally resemble thyroid hormones. Children with high exposure to BDE-47 during the prenatal period or toddler age had significantly lower mean TSH levels compared to children with low BDE-47 exposure throughout early life.63
EDCs are able to alter the normal thyroid homeostasis. If this occurs in the most critical period of fetal development, damage to normal psycho-intellectual maturation can occur.
Metabolic diseases
Metabolic diseases [i.e. insulin resistance (IR), obesity, diabetes, metabolic syndrome, etc.) are among the most prominent health outcomes of human exposure to EDCs.3,4 Prenatal exposure to low concentrations of EDCs has an impact on cardio-metabolic risk factors in preschool children.64
EDCs act on cellular function via interaction with steroid receptors and nuclear transcription factors, impairment of endocrine signaling transduction, and epigenetic mechanisms. Mechanisms through which “obesogens” EDCs contribute to the etiology of obesity are the direct promotion of adipogenesis increasing both the number and the size of adipocytes with proliferator-activated receptor gamma (PPARγ) activation, promotion of the signal of adipose cell lines to the detriment of other cell lines, greater differentiation of the pre-adipose tissue towards the adipose tissue through activation of PPARγ, and promotion of a greater deposition of fat and potential epigenetic mechanisms that favor activation of transcription factor of adipogenic genes. Many EDCs accumulate in adipose tissue, and this can lead to interactions and changes in the endocrine activity of adipose tissue and homeostatic systems underlying weight control.3,4
“Obesogens” EDCs are risk factors for type 2 diabetes and lead to the dangerous combination of obesity and diabetes. Certain EDCs may directly cause IR and defects in insulin (INS) production and secretion without significantly affecting the weight. EDCs can disrupt body glucose homeostasis by affecting both INS- and glucagon-secretory cells.65 Human studies evaluating EDCs effects on the pathogenesis of T1D are controversial, but this is one of the fields requiring further studies due to the increasing incidence of T1D worldwide.7
In utero exposure to POPs seems to determine permanent physiological changes influencing birth weight, predisposing to subsequent weight gain. POPs have direct effects on INS signaling leading to IR that causes adipose tissue inflammation.3
A relationship between gestational levels of BPA and central adiposity during early childhood was found.66 Urinary BPA levels were associated with a greater risk of central obesity67 and a positive association of dietary exposure to BPA and total bisphenols with being overweight/obese was found in adolescent girls.68
Studies have shown that acute treatment with BPA causes temporary hyperinsulinemia, whereas long-term exposure suppresses adiponectin release and aggravates IR, obesity-related syndromes, and the development of diabetes.4,5
BPA exposure during pre-natal period was associated with increased blood pressure in girls and plasma glucose in boys.69 Adolescents with polycystic ovary syndrome were found to have significantly higher BPA levels when compared with the control group.70
A systematic review and meta-analysis demonstrated a significant association between phthalates and their metabolites concentrations with BMI, BMI z-score, waist circumference, and low-density lipoprotein cholesterol, triglyceride, and glycaemia.71
Maternal urinary concentrations of MEP, MCiOP, and propylparaben during pregnancy were associated with increased BMI z-score and overweight/obesity status. Higher pre-natal exposures to cumulative biomarker mixtures also trended with greater childhood adiposity.72
PFOA and PFOS exposure increased the risk of cardiovascular diseases more than other PFASs.73 In children, both BMI-for-age-and-sex z-score and triceps skinfold z-score were found to increase per logarithmic-unit increase in maternal serum PFOS concentrations. Increased odds for child overweight/obesity for each logarithmic-unit increase in maternal serum PFOS levels (OR=2.04) was found. Similar associations were also detected between maternal serum PFOA concentrations and child overweight/obesity.74
In T1D, the autoimmune process involving ß-cells could be potentially triggered by environmental contaminants,7 such as PFASs. In children and adolescents, PFOS levels were reported to be higher in patients at T1D onset compared to controls.75 High pre-natal exposure to PFASs was found to alter lipid profiles in newborns which may increase the risk of islet autoimmunity and T1D. Interaction between human leukocyte antigens risk genotype and pre-natal PFAS exposure was highlighted as to play a potential role in altered lipid profiles in newborn infants at-risk of developing T1D.76
Obesity is a multifactorial disease caused by an altered balance between food intake and physical activity and influenced by genetic predisposition and environmental factors. Evidence indicate that exposures to EDCs during pre-natal, early infancy, and pubertal times are able to cause abnormal distribution of adipose tissue, its excess, and subsequent metabolic complications. The authors need more conclusive data on relationships between EDCs and metabolism.
Conclusions
EDCs represent an emerging global health problem requiring urgent attention and action, and several challenges remain in the understanding of their role in the development of endocrine diseases at any age. Despite difficulties to translate what happens in wildlife to humans and limitations due to conflicting results from studies related to confounding factors, EDCs’ effects on human health are now irrefutable, and this topic is of particular interest for pediatricians. Identifying a direct relationship between EDCs exposure and disease outcomes is complex because of the exposure to low doses of hundreds of EDCs since in utero. Moreover, the years-lag time between exposure and appearance of diseases must be considered to interpret studies.
A debate is still open on how to classify a chemical as an endocrine disruptor, how to obtain biomarkers of exposure, and how to confirm its possible negative effect on human health. To better understand the reasons for this debate and difficulties encountered in researches on EDCs, it is useful to keep in mind several limitations (Table 3).1,5
Table 3.
| It is difficult to translate what happens in wildlife to humans |
| A direct relationship between EDCs exposure and a disease outcome cannot be established, especially when the lag time is of years |
| Age and gender groups react differently; intrauterine life is the most “critical windows” period |
| Exposure to low doses of EDCs could induce disrupting effects that may be not dose dependent |
| Humans are exposed to different EDCs over time, to a mixture rather than to single compound |
| EDCs may have transgenerational effects probably mediated by epigenetic mechanisms that transfer these effects to the next generations that had not direct exposure to chemicals |
| EDCs are not pure agonists or antagonists of a single hormone receptor or pathway |
| Bioavailability differs among EDCs and depends on their property to bind binding proteins, metabolism, excretion, storage in specific tissues (i.e. fat), and slow release over time |
Humans are usually exposed to a mixture of chemicals and the authors need to keep in mind their active metabolites and the “cocktail effects” on the complex endocrine milieu. Exposure to low-dose mixtures of EDCs characterizes environmental conditions in humans and wildlife, inducing disrupting effects.4 The authors need more information on potential new chemical compounds exposures for which the authors have limited information and that were not previously of concern for environmental health scientists and regulators. Probably, the suspect screening must be increased to identify the presence of poorly characterized EDCs in maternal and umbilical cord blood samples. Further investigations are needed to better understand where these chemicals might be coming from, how exposures may affect human health outcomes, and how to eventually prevent the development of diseases.77
Regulatory measures were taken in the EU, US, and member states restricted the use of certain EDCs. Substituents to regulated compounds were used, but there are still uncertainties concerning the safety of these substituents and more generally concerning new or poorly studied compounds.
EDCs can be identified in human biological fluids like serum, urine, and breast milk, but their quantification is still difficult. There is a lack of tests predicting metabolic outcomes useful to evaluate the impact of EDCs exposure on health, particularly in infants. Exposure to EDCs could induce disrupting effects that may not be dose-dependent, so endogenous hormonal actions at one dosage do not necessarily predict effects at another. Why some EDCs produce non-traditional dose-response curves is still not understood. The authors need reliable chemical analyzes on different biological matrices and valid, reproducible, efficient, and sensitive techniques to quantify specific EDCs and their metabolites in human biological fluid.
Lastly, EDCs are not pure agonists or antagonists of a single hormone receptor or pathway. This translates to complex and sometimes seemingly inconsistent actions of EDCs in experimental models when trying to compare results to endogenous hormone or pharmaceutical actions.
A lot of data has arisen relative to the effects of EDC exposure on growth, puberty, reproductive system, thyroid function, obesity, and its metabolic complications. Many of the reviewed studies present significant limitations, including lack of replication, limited sample sizes, retrospective design, publication biases, and inadequate matching of cases and controls. Further long-term studies performed on the wide number of subjects are necessary to address questions on which EDCs mainly affect each endocrine system and how the authors can reduce relevant exposures. Phenomena such as bio-accumulation and trans-generational inheritance are clear obstacles to research, and new strategies in these regards should be pursued. Research areas in progress should include the development of new models and tools to better understand how EDCs work, new high throughout assays to identify substances with endocrine-disrupting activity, and new assessments and biomarkers of exposure and toxicity.
In conclusion, animals and in vitro research over the past decade improved the understanding of EDCs’ actions on endocrine physiology and pathophysiology. Despite much more information is now available on mechanisms of action, and the authors know the importance of the critical windows of exposure, it is difficult to assess the full impact of human exposure to EDCs because adverse effects develop latently and manifest at later ages. Although the overall evidence on a pathogenic role for EDCs in the modulation of some endocrine diseases is compelling, data related to pre- or post-natal exposure are still scarce, so it is difficult to draw definitive conclusions. The challenge is to understand which these EDCs are, how they change, which are the doses that, interfering with the body with synergistic effects, can trigger illnesses that continue throughout life. There is an urgent need for novel biomarkers, detectors, or assays using novel technologies for the early detection of EDCs. Integrated together, all interdisciplinary studies and researches (experimental methods, high throughput omics technologies, epidemiology and human biomonitoring studies, and advanced computational models) could provide useful insights to regulatory efforts to better characterize suspected EDCs, and their connection to health outcomes.
In this review, the most common EDCs and their main adverse effects on the endocrine system during childhood and adolescence have been summarized. Further studies are needed to clarify which EDCs can mainly act on epigenetic processes, and a better knowledge of EDCs effects on health is crucial for a future regulatory strategy for prevention of EDCs’ exposure to ensure good health in children today, in future generations, and in the environment.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:E1–150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol. 2020;16:45–57. doi: 10.1038/s41574-019-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iughetti L, Lucaccioni L, Predieri B. Childhood obesity and environmental pollutants: a dual relationship. Acta Biomed. 2015;86:5–16. [PubMed] [Google Scholar]
- 4.Street ME, Angelini S, Bernasconi S, Burgio E, Cassio A, Catellani C, et al. Current knowledge on endocrine disrupting chemicals (EDCs) from animal biology to humans, from pregnancy to adulthood: highlights from a National Italian Meeting. Int J Mol Sci. 2018;19:1647. doi: 10.3390/ijms19061647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iughetti L, Lucaccioni L, Street ME, Bernasconi S. Clinical expression of endocrine disruptors in children. Curr Opin Pediatr. 2020;32:554–559. doi: 10.1097/MOP.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 6.Lucaccioni L, Trevisani V, Marrozzini L, Bertoncelli N, Predieri B, Lugli L, et al. Endocrine-disrupting chemicals and their effects during female puberty: a review of current evidence. Int J Mol Sci. 2020;21:2078. doi: 10.3390/ijms21062078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Predieri B, Bruzzi P, Bigi E, Ciancia S, Madeo SF, Lucaccioni L, et al. Endocrine disrupting chemicals and type 1 diabetes. Int J Mol Sci. 2020;21:2937. doi: 10.3390/ijms21082937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Street ME, Bernasconi S. Endocrine-disrupting chemicals in human fetal growth. Int J Mol Sci. 2020;21:1430. doi: 10.3390/ijms21041430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucaccioni L, Trevisani V, Passini E, Righi B, Plessi C, Predieri B, et al. Perinatal exposure to phthalates: from Endocrine to neurodevelopment effects. Int J Mol Sci. 2021;22:4063. doi: 10.3390/ijms22084063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rager JE, Bangma J, Carberry C, Chao A, Grossman J, Lu K, et al. Review of the environmental prenatal exposome and its relationship to maternal and fetal health. Reprod Toxicol. 2020;98:1–12. doi: 10.1016/j.reprotox.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talia C, Connolly L, Fowler PA. The insulin-like growth factor system: a target for endocrine disruptors? Environ Int. 2021;147 doi: 10.1016/j.envint.2020.106311. [DOI] [PubMed] [Google Scholar]
- 12.Santos S, Sol CM, van Zwol-Janssens C, Philips EM, Asimakopoulos AG, Martinez-Moral MP, et al. Maternal phthalate urine concentrations, fetal growth and adverse birth outcomes. A population-based prospective cohort study. Environ Int. 2021;151 doi: 10.1016/j.envint.2021.106443. [DOI] [PubMed] [Google Scholar]
- 13.Bell GA, Perkins N, Buck Louis GM, Kannan K, Bell EM, Gao C, et al. Exposure to persistent organic pollutants and birth characteristics: the upstate KIDS Study. Epidemiology. 2019;30(2):S94–100. doi: 10.1097/EDE.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce JL, Neelon B, Bloom MS, Buckley JP, Ananth CV, Perera F, et al. Exploring associations between prenatal exposure to multiple endocrine disruptors and birth weight with exposure continuum mapping. Environ Res. 2021;200 doi: 10.1016/j.envres.2021.111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krönke AA, Jurkutat A, Schlingmann M, Poulain T, Nüchter M, Hilbert A, et al. Persistent organic pollutants in pregnant women potentially affect child development and thyroid hormone status. Pediatr Res. 2021 doi: 10.1038/s41390-021-01488-5. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zulkifli S, Rahman AA, Kadir SHSA, NSM Nor. Bisphenol A and its effects on the systemic organs of children. Eur J Pediatr. 2021;180:3111–3127. doi: 10.1007/s00431-021-04085-0. [DOI] [PubMed] [Google Scholar]
- 17.Vrachnis N, Loukas N, Vrachnis D, Antonakopoulos N, Zygouris D, Kólialexi A, et al. A systematic review of bisphenol A from dietary and non-dietary sources during pregnancy and its possible connection with fetal growth restriction: investigating its potential effects and the window of fetal vulnerability. Nutrients. 2021;13:2426. doi: 10.3390/nu13072426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, Zhao H, Braun JM, Zheng T, Zhang B, Xia W, et al. Associations of trimester-specific exposure to bisphenols with size at birth: a Chinese prenatal cohort study. Environ Health Perspect. 2019;127 doi: 10.1289/EHP4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang P, Lin BG, Zhou B, Cao WC, Chen PP, Deng YL, et al. Sex-specific associations of prenatal exposure to bisphenol A and its alternatives with fetal growth parameters and gestational age. Environ Int. 2021;146 doi: 10.1016/j.envint.2020.106305. [DOI] [PubMed] [Google Scholar]
- 20.Sol CM, van Zwol-Janssens C, Philips EM, Asimakopoulos AG, Martinez-Moral MP, Kannan K, et al. Maternal bisphenol urine concentrations, fetal growth and adverse birth outcomes: a population-based prospective cohort. Environ Health. 2021;20:60. doi: 10.1186/s12940-021-00747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Liang H, Tu X, Yuan W, Zhou Z, Jin L, et al. Bisphenol A and pubertal height growth in school-aged children. J Expo Sci Environ Epidemiol. 2019;29:109–117. doi: 10.1038/s41370-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miura R, Ikeda-Araki A, Ishihara T, Miyake K, Miyashita C, Nakajima T, et al. Effect of prenatal exposure to phthalates on epigenome-wide DNA methylations in cord blood and implications for fetal growth: the hokkaido study on environment and children's health. Sci Total Environ. 2021;783 doi: 10.1016/j.scitotenv.2021.147035. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Qian X, Zhou Y, Li Y, Xu S, Xia W, et al. Trimester-specific and sex-specific effects of prenatal exposure to di(2-ethylhexyl) phthalate on fetal growth, birth size, and early-childhood growth: a longitudinal prospective cohort study. Sci Total Environ. 2021;777 doi: 10.1016/j.scitotenv.2021.146146. [DOI] [PubMed] [Google Scholar]
- 24.Matsuki T, Ebara T, Tamada H, Ito Y, Yamada Y, Kano H, et al. Association between prenatal exposure to household pesticides and neonatal weight and length growth in the Japan Environment and Children's Study. Int J Environ Res Public Health. 2020;17:4608. doi: 10.3390/ijerph17124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Béranger R, Hardy EM, Binter AC, Charles MA, Zaros C, Appenzeller BM, et al. Multiple pesticides in mothers' hair samples and children's measurements at birth: results from the French national birth cohort (ELFE) Int J Hyg Environ Health. 2020;223:22–33. doi: 10.1016/j.ijheh.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Kartini A, Subagio HW, Hadisaputro S, Kartasurya MI, Suhartono S, Budiyono B. Pesticide exposure and stunting among children in agricultural areas. Int J Occup Environ Med. 2019;10:17–29. doi: 10.15171/ijoem.2019.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross RS, Ghassabian A, Vandyousefi S, Messito MJ, Gao C, Kannan K, et al. Persistent organic pollutants exposure in newborn dried blood spots and infant weight status: a case-control study of low-income Hispanic mother-infant pairs. Environ Pollut. 2020;267 doi: 10.1016/j.envpol.2020.115427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wikström S, Lin PI, Lindh CH, Shu H, Bornehag CG. Maternal serum levels of perfluoroalkyl substances in early pregnancy and offspring birth weight. Pediatr Res. 2020;87:1093–1099. doi: 10.1038/s41390-019-0720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin YT, Deng XK, Zhao YY, Li JL, Song Q, Zhang YH, et al. Concentrations of polybrominated diphenyl ethers in maternal blood, placental size, and risk for fetal growth restriction: a nested case-control study. Biomed Environ Sci. 2020;33:821–828. doi: 10.3967/bes2020.112. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Song Q, Ge W, Jin Y, Chen S, Zhao Y, et al. Associations between in utero exposure to polybrominated diphenyl ethers, pathophysiological state of fetal growth and placental DNA methylation changes. Environ Int. 2019;133 doi: 10.1016/j.envint.2019.105255. [DOI] [PubMed] [Google Scholar]
- 31.Warner M, Ye M, Harley K, Kogut K, Bradman A, Eskenazi B. Prenatal DDT exposure and child adiposity at age 12: the CHAMACOS study. Environ Res. 2017;159:606–612. doi: 10.1016/j.envres.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Güil-Oumrait N, Valvi D, Garcia-Esteban R, Guxens M, Sunyer J, Torrent M, et al. Prenatal exposure to persistent organic pollutants and markers of obesity and cardiometabolic risk in Spanish adolescents. Environ Int. 2021;151 doi: 10.1016/j.envint.2021.106469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks KJ, Howards PP, Smarr MM, Flanders WD, Northstone K, Daniel JH, et al. Prenatal exposure to mixtures of persistent endocrine disrupting chemicals and postnatal body size in British girls. Early Hum Dev. 2021;161 doi: 10.1016/j.earlhumdev.2021.105450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harley KG, Berger K, Rauch S, Kogut K, Claus Henn B, Calafat AM, et al. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatr Res. 2017;82:405–415. doi: 10.1038/pr.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berman YE, Doherty DA, Main KM, Frederiksen H, Hickey M, Keelan JA, et al. Associations between prenatal exposure to phthalates and timing of menarche and growth and adiposity into adulthood: a twenty-years birth cohort study. Int J Environ Res Public Health. 2021;18:4725. doi: 10.3390/ijerph18094725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoaff J, Papandonatos GD, Calafat AM, Chen A, Lanphear BP, Ehrlich S, et al. Prenatal exposure to perfluoroalkyl substances: infant birth weight and early life growth. Environ Epidemiol. 2018;2:e010. doi: 10.1097/EE9.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo J, Miao W, Wu C, Zhang J, Qi X, Yu H, et al. Umbilical cord serum PBDE concentrations and child adiposity measures at 7 years. Ecotoxicol Environ Saf. 2020;203 doi: 10.1016/j.ecoenv.2020.111009. [DOI] [PubMed] [Google Scholar]
- 38.Iughetti L, Predieri B, Ferrari M, Gallo C, Livio L, Milioli S, et al. Diagnosis of central precocious puberty: endocrine assessment. J Pediatr Endocrinol Metab. 2000;13:S709–S715. doi: 10.1515/jpem.2000.13.s1.709. [DOI] [PubMed] [Google Scholar]
- 39.Golestanzadeh M, Riahi R, Kelishadi R. Association of phthalate exposure with precocious and delayed pubertal timing in girls and boys: a systematic review and meta-analysis. Environ Sci Process Impacts. 2020;22:873–894. doi: 10.1039/c9em00512a. [DOI] [PubMed] [Google Scholar]
- 40.Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez M, Rybak M, et al. Associations of urinary phthalate and phenol biomarkers with menarche in a multiethnic cohort of young girls. Reprod Toxicol. 2017;67:56–64. doi: 10.1016/j.reprotox.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger K, Eskenazi B, Kogut K, Parra K, Lustig RH, Greenspan LC, et al. Association of prenatal urinary concentrations of phthalates and bisphenol A and pubertal timing in boys and girls. Environ Health Perspect. 2018;126:97004. doi: 10.1289/EHP3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binder AM, Corvalan C, Calafat AM, Ye X, Mericq V, Pereira A, et al. Childhood and adolescent phenol and phthalate exposure and the age of menarche in Latina girls. Environ Health. 2018;17:32. doi: 10.1186/s12940-018-0376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ernst A, Brix N, Lauridsen LL, Olsen J, Parner ET, Liew Z, et al. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the Danish National Birth Cohort. Environ Health Perspect. 2019;127:17004. doi: 10.1289/EHP3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marks KJ, Howards PP, Smarr MM, Flanders WD, Northstone K, Daniel JH, et al. Prenatal exposure to mixtures of persistent endocrine disrupting chemicals and early menarche in a population-based cohort of British girls. Environ Pollut. 2021;276 doi: 10.1016/j.envpol.2021.116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YJ, Jung HW, Kim HY, Choi Y-J, Lee YA. Early-life exposure to per- and poly-fluorinated alkyl substances and growth, adiposity, and puberty in children: a systematic review. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.683297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsey JT, Li Y, Arao Y, Naidu A, Coons LA, Diaz A, et al. Lavender products associated with Premature thelarche and prepubertal gynecomastia: case reports and endocrine-disrupting chemical activities. J Clin Endocrinol Metab. 2019;104:5393–5405. doi: 10.1210/jc.2018-01880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xing JS, Bai ZM. Is testicular dysgenesis syndrome a genetic, endocrine, or environmental disease, or an unexplained reproductive disorder? Life Sci. 2018;194:120–129. doi: 10.1016/j.lfs.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 48.Nelson W, Liu DY, Yang Y, Zhong ZH, Wang YX. Ding YB. In utero exposure to persistent and nonpersistent endocrine-disrupting chemicals and anogenital distance. a systematic review of epidemiological studies. Biol Reprod. 2020;102:276–291. doi: 10.1093/biolre/ioz200. [DOI] [PubMed] [Google Scholar]
- 49.Fisher BG, Thankamony A, Mendiola J, Petry CJ, Frederiksen H, Andersson AM, et al. Maternal serum concentrations of bisphenol A and propyl paraben in early pregnancy are associated with male infant genital development. Hum Reprod. 2020;35:913–928. doi: 10.1093/humrep/deaa045. [DOI] [PubMed] [Google Scholar]
- 50.Ormond G, Nieuwenhuijsen MJ, Nelson P, Toledano MB, Iszatt N, Geneletti S, et al. Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: case-control study. Environ Health Perspect. 2009;117:303–307. doi: 10.1289/ehp.11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen MS, Anand-Ivell R, Nørgaard-Pedersen B, Jönsson BA, Bonde JP, Hougaard DM, et al. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology. 2015;26:91–99. doi: 10.1097/EDE.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 52.Qian Y, Shao H, Ying X, Huang W, Hua Y. The endocrine disruption of prenatal phthalate exposure in mother and offspring. Front Public Health. 2020;8:366. doi: 10.3389/fpubh.2020.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rignell-Hydbom A, Lindh CH, Dillner J, Jonsson BA, Rylander L. A nested case-control study of intrauterine exposure to persistent organochlorine pollutants and the risk of hypospadias. PLoS One. 2012;7:e44767. doi: 10.1371/journal.pone.0044767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian Y, Liang H, Miao M, Yang F, Ji H, Cao W, et al. Maternal plasma concentrations of perfluoroalkyl and polyfluoroalkyl substances during pregnancy and anogenital distance in male infants. Hum Reprod. 2019;34:1356–1368. doi: 10.1093/humrep/dez058. [DOI] [PubMed] [Google Scholar]
- 55.Köhrle J, Frädrich C. Thyroid hormone system disrupting chemicals. Best Pract Res Clin Endocrinol Metab. 2021 doi: 10.1016/j.beem.2021.101562. [DOI] [PubMed] [Google Scholar]
- 56.Zoeller RT. Endocrine disrupting chemicals and thyroid hormone action. Adv Pharmacol. 2021;92:401–417. doi: 10.1016/bs.apha.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Kim MJ, Park YJ. Bisphenols and thyroid hormone. Endocrinol Metab. 2019;34:340–348. doi: 10.3803/EnM.2019.34.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorini F, Bustaffa E, Coi A, Iervasi G, Bianchi F. Bisphenols as environmental triggers of thyroid dysfunction: clues and evidence. Int J Environ Res Public Health. 2020;17:2654. doi: 10.3390/ijerph17082654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derakhshan A, Philips EM, Ghassabian A, Santos S, Asimakopoulos AG, Kannan K, et al. Association of urinary bisphenols during pregnancy with maternal, cord blood and childhood thyroid function. Environ Int. 2021;146 doi: 10.1016/j.envint.2020.106160. [DOI] [PubMed] [Google Scholar]
- 60.Huang PC, Kuo PL, Chang WH, Shih SF, Chang WT, Lee CC. Prenatal phthalates exposure and cord thyroid hormones: a birth cohort study in southern Taiwan. Int J Environ Res Public Health. 2021;18:4323. doi: 10.3390/ijerph18084323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warner M, Rauch S, Ames J, Mocarelli P, Brambilla P, Signorini S, et al. Prenatal dioxin exposure and thyroid hormone levels in the Seveso second generation study. Environ Res. 2020;183 doi: 10.1016/j.envres.2020.109280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Preston EV, Webster TF, Claus Henn B, McClean MD, Gennings C, Oken E, et al. Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the Project Viva Cohort: a mixtures approach. Environ Int. 2020;139 doi: 10.1016/j.envint.2020.105728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cowell WJ, Sjödin A, Jones R, Wang Y, Wang S, Whyatt RM, et al. Pre- and postnatal polybrominated diphenyl ether concentrations in relation to thyroid parameters measured during early childhood. Thyroid. 2019;29:631–641. doi: 10.1089/thy.2018.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gutiérrez-Torres DS, Barraza-Villarreal A, Hernandez-Cadena L, Escamilla-Nuñez C, Romieu I. Prenatal exposure to endocrine disruptors and cardiometabolic risk in preschoolers: a systematic review based on cohort studies. Ann Glob Health. 2018;84:239–249. doi: 10.29024/aogh.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7:346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- 66.Braun JM, Li N, Arbuckle TE, Dodds L, Massarelli I, Fraser WD, et al. Association between gestational urinary bisphenol a concentrations and adiposity in young children: the MIREC study. Environ Res. 2019;172:454–461. doi: 10.1016/j.envres.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mustieles V, Casas M, Ferrando-Marco P, Ocón-Hernández O, Reina-Pérez I, Rodríguez-Carrillo A, et al. Bisphenol A and adiposity measures in peripubertal boys from the INMA-Granada cohort. Environ Res. 2019;173:443–451. doi: 10.1016/j.envres.2019.03.045. [DOI] [PubMed] [Google Scholar]
- 68.Robles-Aguilera V, Gálvez-Ontiveros Y, Rodrigo L, Salcedo-Bellido I, Aguilera M, Zafra-Gómez A, et al. Factors associated with exposure to dietary bisphenols in adolescents. Nutrients. 2021;13:1553. doi: 10.3390/nu13051553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ouyang F, Zhang GH, Du K, Shen L, Ma R, Wang X, et al. Maternal prenatal urinary bisphenol A level and child cardio-metabolic risk factors: a prospective cohort study. Environ Pollut. 2020;265 doi: 10.1016/j.envpol.2020.115008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akgül S, Sur Ü, Düzçeker Y, Balcı A, Kızılkan MP, Kanbur N, et al. Bisphenol A and phthalate levels in adolescents with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35:1084–1087. doi: 10.1080/09513590.2019.1630608. [DOI] [PubMed] [Google Scholar]
- 71.Golestanzadeh M, Riahi R, Kelishadi R. Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2019;26:35670–35686. doi: 10.1007/s11356-019-06589-7. [DOI] [PubMed] [Google Scholar]
- 72.Berger K, Hyland C, Ames JL, Mora AM, Huen K, Eskenazi B, et al. Prenatal exposure to mixtures of phthalates, parabens, and other phenols and obesity in five-year-olds in the CHAMACOS cohort. Int J Environ Res Public Health. 2021;18:1796. doi: 10.3390/ijerph18041796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdullah Soheimi SS, Abdul Rahman A, Abd Latip N, Ibrahim E, Sheikh Abdul Kadir SH. Understanding the impact of perfluorinated compounds on cardiovascular diseases and their risk factors: a meta-analysis study. Int J Environ Res Public Health. 2021;18:8345. doi: 10.3390/ijerph18168345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lauritzen HB, Larose TL, Øien T, Sandanger TM, Odland JØ, van de Bor M, et al. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: a prospective cohort study. Environ Health. 2018;17:9. doi: 10.1186/s12940-017-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Predieri B, Iughetti L, Guerranti C, Bruzzi P, Perra G, Focardi SE. High levels of perfluorooctane sulfonate in children at the onset of diabetes. Int J Endocrinol. 2015;2015 doi: 10.1155/2015/234358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGlinchey A, Sinioja T, Lamichhane S, Sen P, Bodin J, Siljander H, et al. Prenatal exposure to perfluoroalkyl substances modulates neonatal serum phospholipids, increasing risk of type 1 diabetes. Environ Int. 2020;143 doi: 10.1016/j.envint.2020.105935. [DOI] [PubMed] [Google Scholar]
- 77.Wang A, Abrahamsson DP, Jiang T, Wang M, Morello-Frosch R, Park JS, et al. Suspect screening, prioritization, and confirmation of environmental chemicals in maternal-newborn pairs from San Francisco. Environ Sci Technol. 2021;55:5037–5049. doi: 10.1021/acs.est.0c05984. [DOI] [PMC free article] [PubMed] [Google Scholar]