Abstract
Background and aims
Causal research concerning coffee intake and rheumatoid arthritis (RA) risk is controversial. The objective of this study was to further explore the causal relationship between coffee intake and RA risk.
Methods
The 4,310 participants from NHANES 2003–2006 were included in an epidemiological study to assess the association between coffee intake and RA by weighted multivariate logistic regression. The inverse variance weighted (IVW) method of two-sample Mendelian randomization (MR), employing genetic data from UK Biobank (428,860 cases) of coffee intake and MR-Base platform (14,361 cases and 43,923 controls) of RA, was performed to estimate the causal relationship between coffee intake and RA.
Results
Weighted multivariate logistic regression suggested no significant correlation between coffee intake and RA. Compared to the no-coffee group, the odds ratio for RA in the <1, 1–3, ≥4 cups/day group were 1.297, 1.378, and 1.125 (P = 0.204, 0.098, and 0.698, respectively). In the IVW of MR analysis, there was no causal relationship between coffee intake and RA (OR = 1.47, P = 0.218).
Conclusion
Our study did not support a causal association between coffee intake and RA risk. However, it is necessary to consider valid information on coffee intake, including brewing method, type of coffee, and quantity, in further analysis of coffee intake and RA.
Keywords: coffee intake, rheumatoid arthritis, national health and nutrition examination survey, Mendelian randomization, epidemiological study
Introduction
Rheumatoid arthritis (RA) is a common chronic rheumatoid immune disease characterized by synovitis, cartilage, and symmetrical joint damage (1). RA can affect individuals of any age and its prevalence rate is estimated at 0.5–1% worldwide (2). The pathogenic factors of RA are unclear, which may be related to infection, hormone secretion disorder, genetics, etc. (3, 4). Coffee is one of the most popular beverages in the world, with about 85% of Americans reportedly consuming at least one caffeinated beverage per day (5). Because of the widespread popularity and easy availability of coffee, the public and the scientific community are extraordinarily interested in the relationship and interaction between coffee and essential health. Coffee contains hundreds of bioactive compounds, including caffeine, caffeic acid, chlorogenic acid, etc. (6).
At present, a large number of studies have indicated that coffee can affect the risk of some chronic diseases such as depression, type 2 diabetes, Parkinson's disease (7–9), and RA. Among them, we are very interested in the relationship between coffee intake and RA risk. Previous prospective studies have shown that coffee intake was not associated with RA risk (10–12). However, Mikuls et al. found that decaffeinated coffee intake was independently positively correlated with RA risk, while the caffeinated coffee intake was not associated with RA rise (13) and similar results were obtained by a meta-analysis by Asoudeh et al. (14). Overall, their results were not consistent.
Mendelian randomization (MR) is considered a method comparable to the randomized controlled study (15), which uses genetic variation as an instrumental variable (IV) to deduce the causality between the outcome and exposure. Genetic variation is randomly assigned at conception to effectively avoid the confounding bias and reverse causal bias of traditional epidemiological studies (16). Moreover, as the effect of genetic variation is much longer than clinical intervention, the actual causal effect estimated by MR is more significant and more accurate than the clinically observed causal effect, including the meta-analysis of clinical research and prospective studies (17).
Therefore, this study intended to use the extensive database of the National Health and Nutrition Examination Survey (NHANES) to conduct a cross-sectional study on the correlation between coffee intake and RA risk, followed by the MR study to evaluate the causal relationship between coffee intake and RA from the level of genetic variation.
Materials and methods
Observational epidemiological analysis
NHANES is a series of continuous cross-sectional surveys conducted jointly by the National Center For Health Statistics and the Centers for Disease Control And Prevention. We collected two consecutive NHANES two-year cycles (2003–2006) in total since participants responded to the question “Did you drink coffee” in the two cycles (18). The individuals, under 20 years old (n = 11,650), with missing coffee intake data (n = 3,283), unknown diagnosis of arthritis (n = 670), and missing data of related covariates (n = 1,757), were excluded (Supplementary Figure 1). Finally, a total of 4,310 individuals were included in the final analysis. Detailed data is accessible on https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Variables included in observational epidemiological analysis
RA was defined based on the items in the medical conditions questionnaire: “Doctor ever said you had arthritis?” and “Which type of arthritis?”. Respondents who responded only RA were classified as RA. According to the question, “How many cups of coffee, caffeinated or decaffeinated, did you drink?”, we divided coffee intake into four groups: no consumption, <1, 1–3, and ≥4 cups/day. According to the previous study, we used the following variables as covariates: sex, age, race, education level, Body mass index (BMI), smoking status, poverty-income ratio, alcohol, high-density lipoprotein, total cholesterol, hypertension, and diabetes condition (19, 20). In addition, through screening, total calcium, phosphorus, kidney disease, and CRP may also be associated with RA risk (21–23), so they were also included in the covariates.
Sources of two-sample MR
UK Biobank is a large-scale biomedical database and research resource containing in-depth genetic and health information from 500,000 British participants. The genome-wide association study (GWAS) summary data set for coffee intake (ukb-b-5237) based on UK Biobank includes over 428,860 samples of European ancestry. Detailed information regarding the phenotype and quality control process in UK Biobank is available on the website (https://www.ukbiobank.ac.uk/). We also collected the aggregated data on RA (ieu-a-832) from a previous study containing 582,84 participants of European ancestries. All RA cases in this study fulfilled the 1,987 criteria of the American College of Rheumatology for RA diagnosis or were diagnosed as RA by a professional rheumatologist (24).
Statistical analysis
In the observational epidemiological analysis, the baseline characteristics of all study participants were described by average (continuous variables) or proportions (classified variables). We executed weighted multivariate-adjusted logistic regression to calculate the odds ratio (OR), P-value, and 95% confidence interval (CI) between different coffee intakes and RA risk.
MR is a method that accomplishes genetic variation to estimate the causality between exposure and outcome. MR's validity is based on three hypotheses: (1) the genetic IVs are related to the exposure factors, (2) IVs are independent of confounders, and (3) IVs act only through exposure factors to outcomes (25) (Supplementary Figure 2). To construct genetic instruments for coffee intake and RA, we obtained the SNPs (single nucleotide polymorphisms) that are reliable (P <5 × 10−8) and independent (r2 <0.001, kb = 10,000) of coffee intake. We chose not to use the SNP proxy and set the minimum allele frequency (MAF) to 0.01. In addition, we coordinated the effect alleles in the exposure and result data sets, excluding all SNPs with palindromes. The fixed-effect IVW, MR-Egger, weighted median, weighted mode, and simple model were used to examine a causal association and IVW was considered the primary analytical method (26). The MR-Egger tested its horizontal pleiotropic. When the value of the intercept term was close to 0 and P > 0.05, it indicated that there was no horizontal multiplicity (27). If horizontal multiplicity exists, the SNP with horizontal multiplicity was removed with CAUSE (Causal Analysis Using Summary Effect estimates) packages. The IVW and MR-Egger were used to quantify the heterogeneity effect between the genetic instruments and heterogeneous SNP was removed using the MR-PRESSO (Mendelian Randomization Pleiotropy RESidual Sum and Outlier) packages. F-statistics were used to test weak instrumental variables. R2 is the variance of coffee intake explained by genetic instruments, K is the number of genetic variants (Because we calculated the F value of each SNP, which was K = 1), and N is the sample size (28). F > 10 indicates that weak IV deviations are unlikely (29).
R2 was calculated as follows:
The F-statistic for each SNP was calculated as follows:
In addition, the leave-one-out method was used to deal with sensitivity analysis, in which the SNP was removed one by one; the estimated value of the remaining IVs was calculated and compared with the total estimate to test the impact of each SNP (30).
All data were analyzed by packages “TwoSampleMR,” “MR-PRESSO”, and “CAUSE” in R software v.4.0.3, and EmpowerStats software. All images were generated by GraphPad Prism 9.0.0 and Adobe Illustrator 2021. P < 0.05 indicated statistical significance.
Results
Epidemiological observation and analysis
Participants' information was extracted from the NHANES 2003–2006. After inclusion and exclusion criteria screening, we recruited 4,310 participants in the final analysis (Supplementary Figure 1). Table 1 compares the baseline characteristics of participants with RA and without RA in the study sample. The average age of 275 participants with RA was 60.6 years (45.3–75.8 years), and 4,035 participants without RA was 49.0 years (30.4–67.8 years). The prevalence rate of RA was 6.4%.
Table 1.
Baseline characteristics of study participants with or without rheumatoid arthritis in the NHANES 2003–2006.
| Characteristics | Total number of participants (n = 4,310) | P | |
|---|---|---|---|
| RA (n = 275) | Non-RA (n = 4,035) | ||
| Age (years, mean ± SD) | 60.64 ± 15.14 | 49.04 ± 18.69 | <0.001 |
| Race/ethnicity, n (%) | 0.018 | ||
| Mexican American | 46 (16.73%) | 775 (19.21%) | |
| Other Hispanic | 6 (2.18%) | 105 (2.60%) | |
| Non-Hispanic White | 136 (49.45%) | 2,264 (56.11%) | |
| Non-Hispanic Black | 76 (27.64%) | 725 (17.97%) | |
| Other Race | 11 (4.00%) | 166 (4.11%) | |
| Education, n (%) | <0.001 | ||
| Under high school | 101 (36.73%) | 946 (23.44%) | |
| High school or equivalent | 79 (28.73%) | 976 (24.19%) | |
| Some College or AA degree | 69 (25.09%) | 1,176 (29.14%) | |
| College Graduate or above | 26 (9.45%) | 937 (23.22%) | |
| BMI, mean ± SD (kg/m2) | 29.86 ± 6.70 | 28.52 ± 6.61 | 0.052 |
| Smoking, n (%) | <0.001 | ||
| Never | 76 (27.64%) | 912 (22.60%) | |
| Former | 94 (34.18%) | 1,057 (26.20%) | |
| Current | 105 (38.18%) | 2,066 (51.20%) | |
| Had at least 12 alcohol drinks past 1 year? n (%) | 0.068 | ||
| Yes | 175 (63.64%) | 2,822 (69.94%) | |
| No | 100 (36.36%) | 1,213 (30.06%) | |
| Marital status, n (%) | 0.477 | ||
| Live with someone | 161 (58.55%) | 2,628 (65.13%) | |
| Live alone | 114 (41.45%) | 1,407 (34.87%) | |
| PIR, n (%) | <0.001 | ||
| <1.3 | 82 (29.82%) | 977 (24.21%) | |
| 1.3-3.5 | 129 (46.91%) | 1,588 (39.36%) | |
| >3.5 | 64 (23.27%) | 1,470 (36.43%) | |
| Hypertension, n (%) | <0.001 | ||
| Yes | 157 (57.09%) | 1,311 (32.49%) | |
| No | 118 (42.91%) | 2,724 (67.51%) | |
| Diabetes, n (%) | 0.008 | ||
| Yes | 49 (17.82%) | 374 (9.27%) | |
| No | 216 (78.55%) | 3,617 (89.64%) | |
| Borderline | 10 (3.64%) | 44 (1.09%) | |
| Kidney disease, n (%) | 0.007 | ||
| Yes | 18 (6.55%) | 84 (2.08%) | |
| No | 257 (93.45%) | 3,951 (97.92%) | |
| Phosphorus (mmol/L, mean ± SD) | 3.74 ± 0.50 | 3.81 ± 0.54 | 0.772 |
| Total calcium (mmol/L, mean ± SD) | 9.46 ± 0.41 | 9.51 ± 0.37 | 0.021 |
| CRP (mmol/L, mean ± SD) | 0.68 ± 1.19 | 0.45 ± 0.83 | 0.006 |
| HDL-C (mmol/L, mean ± SD) | 53.15 ± 16.22 | 55.22 ± 16.47 | 0.061 |
| TC (mmol/L, mean ± SD) | 196.63 ± 43.67 | 202.06 ± 43.16 | 0.767 |
| Coffee intake, n (%) | 0.057 | ||
| None | 45 (16.36%) | 1,037 (25.70%) | |
| <1 cup/day | 70 (25.45%) | 1,086 (26.91%) | |
| 1–3 cups/day | 141 (51.27%) | 1,638 (40.59%) | |
| ≥4 cups/day | 19 (6.91%) | 274 (6.79%) | |
NHANES, National Health and Nutrition Examination Survey; SD, standard deviation; Values are mean ± SD or n (%); HDL-C, high-density lipoprotein cholesterol; BMI, Body mass index; CRP, C-reactive protein; TC, total Cholesterol; PIR, Poverty Impact Ratio; CI, confidence interval; β, regression coefficient.
Supplementary Table 1 indicates the association between different coffee intakes and RA risk. Only 1-3 cups/day of coffee intake was statistically significant (adjusted for age, sex and race; OR = 1.479, 95%CI:1.027–2.130, P = 0.036). However, Table 2 shows no association between coffee intake and RA in the overall study population after adjustment for all variables.
Table 2.
Weighted multivariable-adjusted a logistic regression of RA risk across coffee intake categories in NHANES 2003–2006.
| Coffee intake(cup/day) | OR | 95%CI | P-value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| 0 | Ref | — | — | — |
| <1 | 1.198 | 0.783 | 1.834 | 0.405 |
| 1–3 | 1.417 | 0.951 | 2.111 | 0.086 |
| >3 | 1.026 | 0.539 | 1.950 | 0.938 |
| P for trend | — | — | — | 0.260 |
| Increase per cup | 1.100 | 0.932 | 1.298 | — |
Age, sex, race/ethnicity, Education, Smoking, Had at least 12 alcohol drinks past one year? Marital status, PIR, Hypertension, Diabetes, BMI (obese, overweight, normal), TC (quartile groups), HDL(quartile groups), Ca (quartile groups), P (quartile groups), CRP (quartile groups) were adjusted in the model.
The results of the two-sample MR analysis
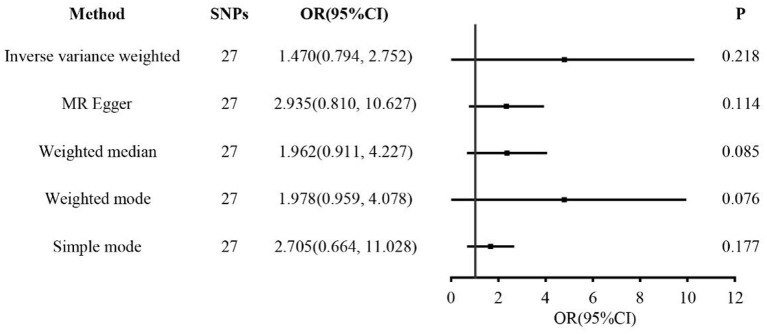
In the two-sample MR analysis, 27 SNPs related to coffee intake were selected (Supplementary Figure 3) (through linkage disequilibrium analysis, P < 5 × 10−8, r2=0.001, Kb=10,000). The F statistics of each SNP was >10 (Table 3), and there was no significant correlation with outcome variables, indicating an absence of weak instrument bias. The fixed effect IVW results showed that coffee intake had no significant effect on RA risk (P = 0.218); similar results were observed in MR-Egger regression, weighted median, weighted mode, and simple mode (Figure 1). The scatter plot of these results is shown in Figure 2. No evidence of horizontal pleiotropic in the MR-Egger regression was detected (regression intercept = −0.013, P = 0.245). The P-values of MR-Egger and IVW methods were 0.059 and 0.049, respectively, indicating no significant heterogeneity between IVs and visualization results indicated in the funnel plot (Supplementary Figure 4). Leave-one-out sensitivity analysis showed that the comprehensive effects of removing any SNP were unchanged or reversed, indicating that the results were credible (Supplementary Figure 5). The forest plots of coffee-RA estimates in each SNP are presented in Supplementary Figure 6.
Table 3.
Characteristics of SNPs associated with coffee consumption.
| SNP | EA | EAF | BETA | SE | P1 | P2 | N | R 2 | F |
|---|---|---|---|---|---|---|---|---|---|
| rs1057868 | T | 0.28 | 0.0200 | 0.0018 | 5.40E−29 | 0.0210 | 428860 | 0.0001187 | 51 |
| rs12514566 | A | 0.34 | −0.0114 | 0.0017 | 2.40E−11 | 0.3400 | 428860 | 0.0000465 | 20 |
| rs12989746 | T | 0.25 | 0.0104 | 0.0019 | 2.80E−08 | 0.4300 | 428860 | 0.0000269 | 12 |
| rs13054099 | C | 0.26 | −0.0108 | 0.0018 | 4.30E−09 | 0.0470 | 428860 | 0.0000310 | 13 |
| rs13163336 | A | 0.16 | 0.0149 | 0.0022 | 1.30E−11 | 0.3600 | 428860 | 0.0000283 | 12 |
| rs1338549 | G | 0.53 | −0.0095 | 0.0016 | 5.60E−09 | 0.0620 | 428860 | 0.0000394 | 17 |
| rs13387939 | A | 0.83 | 0.0166 | 0.0021 | 9.80E−15 | 0.6000 | 428860 | 0.0000397 | 17 |
| rs1421085 | C | 0.40 | 0.0185 | 0.0016 | 1.70E−29 | 0.6700 | 428860 | 0.0001427 | 61 |
| *rs1527961 | C | 0.13 | −0.0133 | 0.0024 | 1.70E−08 | 0.0650 | 428860 | 0.0000173 | 7 |
| *rs17842490 | G | 0.01 | −0.0452 | 0.0068 | 3.30E−11 | 0.5800 | 428860 | 0.0000029 | 1 |
| rs1942965 | C | 0.50 | −0.0089 | 0.0016 | 3.80E−08 | 0.4500 | 428860 | 0.0000352 | 15 |
| rs2465037 | A | 0.34 | −0.0106 | 0.0017 | 4.80E−10 | 0.3100 | 428860 | 0.0000407 | 17 |
| rs2472297 | T | 0.26 | 0.0465 | 0.0018 | 1.10E−142 | 0.4900 | 428860 | 0.0005844 | 251 |
| rs2597805 | T | 0.68 | 0.0099 | 0.0018 | 2.00E−08 | 0.8200 | 428860 | 0.0000318 | 14 |
| rs34060476 | G | 0.13 | 0.0184 | 0.0024 | 7.50E−15 | 0.3800 | 428860 | 0.0000327 | 14 |
| rs4410790 | C | 0.63 | 0.0391 | 0.0017 | 1.20E−120 | 0.0460 | 428860 | 0.0005916 | 254 |
| rs4615895 | A | 0.74 | 0.0122 | 0.0018 | 4.20E−11 | 0.6200 | 428860 | 0.0000390 | 17 |
| rs476828 | C | 0.24 | 0.0173 | 0.0019 | 5.60E−20 | 0.6600 | 428860 | 0.0000707 | 30 |
| rs516636 | A | 0.21 | 0.0117 | 0.0020 | 4.00E−09 | 0.0950 | 428860 | 0.0000267 | 11 |
| rs56113850 | C | 0.58 | 0.0127 | 0.0016 | 8.90E−15 | 0.2000 | 428860 | 0.0000684 | 29 |
| *rs57918684 | A | 0.15 | 0.0129 | 0.0022 | 8.60E−09 | 0.2900 | 428860 | 0.0000202 | 9 |
| rs6062682 | T | 0.46 | 0.0104 | 0.0016 | 2.50E−10 | 0.7800 | 428860 | 0.0000464 | 20 |
| rs6063085 | C | 0.37 | 0.0104 | 0.0017 | 4.50E−10 | 0.4600 | 428860 | 0.0000424 | 18 |
| rs61928609 | C | 0.84 | −0.0147 | 0.0022 | 1.30E−11 | 0.6200 | 428860 | 0.0000294 | 13 |
| rs62064918 | T | 0.24 | −0.0103 | 0.0019 | 4.10E−08 | 0.6800 | 428860 | 0.0000259 | 11 |
| rs630194 | C | 0.34 | −0.0114 | 0.0017 | 2.30E−11 | 0.1000 | 428860 | 0.0000470 | 20 |
| rs6469262 | C | 0.56 | −0.0092 | 0.0016 | 1.90E−08 | 0.6800 | 428860 | 0.0000362 | 16 |
| rs780093 | C | 0.62 | 0.0133 | 0.0017 | 1.00E−15 | 0.4500 | 428860 | 0.0000710 | 30 |
| rs7811609 | T | 0.37 | 0.0091 | 0.0017 | 4.00E−08 | 0.4300 | 428860 | 0.0000329 | 14 |
| rs8056750 | T | 0.36 | 0.0105 | 0.0017 | 1.30E−09 | 0.0030 | 428860 | 0.0000395 | 17 |
SNP, singlE–nucleotide polymorphism; EAF, effect allele frequency; EA, effect allele; BETA, beta.exposure; SE, standard error; P1, pval.coffee; P2, pval. rheumatoid arthritis; R2 was calculated as follows:. The F-statistic for each SNP was calculated as follows:.
Three SNPs (rs17842490, rs1527961, rs57918684) being weak instrumental variables were removed, and 27 SNPs were included for MR analyses.
Figure 1.
Forest plots of MR study using genetically predicted coffee intake with RA. IVW, MR-Egger, weighted median, weighted mode, and simple mode were used in this study.
Figure 2.
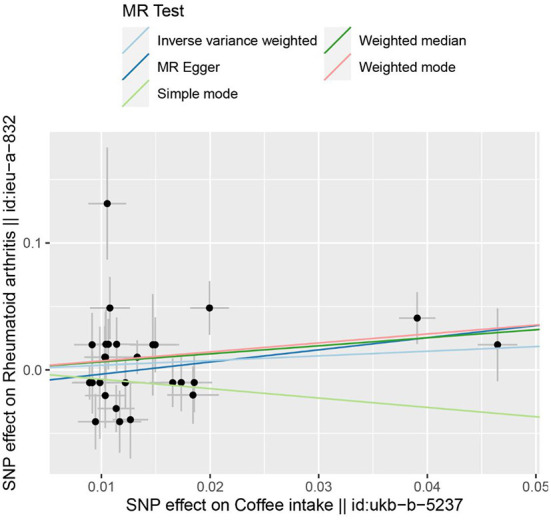
The scatter plot for MR analyses of causal associations between each coffee intake SNP and RA.
Discussion
This present study used epidemiological analysis and two-sample MR analysis to explore the causality between coffee intake and RA risk. Our results did not support a causal association between coffee intake and RA risk.
RA is a chronic autoimmune inflammatory disease. Based on susceptible genes, RA initiates T cell activation and autoimmune reaction by infection, smoking, etc. A large number of inflammatory cytokines, autoantibodies, and oxygen free radicals increase, resulting in the inflammatory injury of joint tissues, synovial hyperplasia, and structural destruction of bone and cartilage (31). Firstly, its occurrence and development are closely related to inflammation. A prospective cohort study of RA in older women showed that caffeine-free coffee intake was independently positive correlated with RA incidence (13). Findings from observational studies further showed that coffee intake was associated with an increased risk of RA. Possible mechanisms include promoting the production of rheumatoid factor (32), antagonizing adenosine receptors, and inhibiting cytokine anti-inflammation (33, 34). Furthermore, Oxidative stress can lead to inflammatory infiltration of neutrophils, and free radicals can be used as oxidants and inflammatory mediators to participate in RA pathological process. Yashin et al. found that coffee contains unique antioxidants such as polyphenols, caffeine alcohol, and melanoid, which play an indispensable role in scavenging free radicals, inducing DNA repair, and activating detoxification enzymes (35–37). Secondly, immune regulation is also a vital influencing factor of RA. Sharif et al. suggested that coffee intake may have a protective effect on autoimmune diseases (38). Oxidative stress can decrease the number of activated peripheral blood mononuclear cells (PBMCs) and down-regulate immune function. The antioxidant activity of caffeic acid and its derivatives can maintain an adequate amount of PBMCs and contribute to maintaining immune function (39). Park et al. verified this theory through experiments on the effects of caffeic acid phenethyl ester on the immune system of mice (40). On the contrary, many animal experiments have proved that caffeine intake will inhibit the proliferation of B cells and T cells (41), affect antibody production and destroy the body's immune function (42).
In addition, a large cross-sectional study in South Korea reported that coffee consumption was not related to the prevalence of RA among Koreans (20). This result was consistent with our NHANES cross-sectional study. We speculate that coffee is a complex mixture, and the biological mechanisms by which it affects disease are multifactorial. On the one hand, caffeine in coffee can antagonize adenosine receptors and inhibit the production of cytokines, thus inhibiting anti-inflammatory effects (33, 34). However, antioxidants such as polyphenols and cafestol in coffee can inhibit the oxidation of the body and produce anti-inflammatory effects (35). On the other hand, the antioxidant activity of caffeic acid and its derivatives can maintain the effective amount of PBMCs and help maintain the body's immune function (39). Conversely, caffeine intake will inhibit the proliferation of T cells and B cells and destroy the body's immune function (41). In conclusion, due to the diversity of the pathogenesis of RA affected by coffee intake, the effects of multiple mediators (positive and negative) may be offset, resulting in coffee intake not being associated with RA. At present, the relationship between coffee intake and RA risk remains controversial. The main reason for this dispute may be that prospective and cross-sectional studies are easily affected by confounding factors such as environment and selection bias, so deterministic causality can not be obtained.
MR has been a popular and effective method for causal inference in recent years. It uses genetic variation as an IV to deduce the causality between outcome and exposure, which can effectively avoid the confounding bias of traditional epidemiological studies (43). Consulting the literature, we found only one MR research report on coffee consumption and RA risk—the result showed no causal relationship between coffee consumption and RA (44). However, only four SNPs in the study explained a small part of the difference in coffee consumption. A weak IV may bias the causal estimation of two-sample MR toward zero. This study selected the GWAS data set with the most prominent coffee intake and RA samples to solve the bias problem and finally screened 27 SNPs. The IVW, MR-Egger, weighted median, weighted mode, and simple mode were used to analyze the causality between the two samples. The results still indicated no causal relationship between coffee intake and RA risk. We speculate that people are an organic whole with a steady-state solid regulatory system, and the effect of coffee intake on inflammation and immunomodulatory function is not enough to cause damage to human tissues and organs. Therefore, coffee intake is unlikely to have a causality with the risk of RA.
The main advantages of this study include a large cross-sectional study based on NHANES and a two-sample MR analysis study. Cross-sectional studies can explore the relationship between coffee consumption and RA risk from the real-world epidemiological level based on population self-question-answering. MR overcomes the inherent effects of residual confusion, reverse causal deviation, and measurement errors in traditional epidemiological studies. The second advantage is that compared with previous MR studies on coffee and RA, we screened more SNPs and reduced the causal estimation bias caused by weak SNPs. However, this study has some inevitable limitations. Firstly, the cross-sectional study may have self-reported recall bias on RA diagnosis and coffee intake. Second, the results did not apply to other populations due to deviations from the data limited to American and European populations. Finally, valid information on coffee intake, including brewing method, type of coffee, and quantity, is very significant in exploring the causality between coffee intake and exposure genes, so it is necessary to consider these factors in further analysis of coffee and RA.
Conclusions
Our study did not support a causal association between coffee intake and RA risk. However, it is necessary to consider valid information on coffee intake, including brewing method, type of coffee, and quantity, in further analysis of coffee intake and RA.
Data availability statement
All National Health and Nutrition Examination Survey files are available from the American Centers for Disease Control and Prevention database (URL https://wwwn.cdc.gov/nchs/nhanes/Default.aspx, accessed on 6 March 2022). All the Mendelian randomization study files are available from GWAS (URL https://gwas.mrcieu.ac.uk, accessed on 17 March 2022). For all the original data, please see the Supplementary material Data Sheet 2.ZIP.
Ethics statement
The studies involving human participants were reviewed and approved by the National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BP and PG designed the study, wrote, reviewed and edited the manuscript. CZ and LM analyzed data. ZZ and XZ reviewed and edited the manuscript. ZZ is the guarantor of this work. All authors approved the final version of the manuscript to be published.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
ZZ will act as the guarantors for the paper.
Glossary
Abbreviations
- MR
Mendelian randomization
- RA
rheumatoid arthritis
- NHANES
National Health and Nutrition Examination Survey
- IVW
inverse variance weighted
- IV
instrumental variable
- GWAS
genome-wide association study
- SNP
- single nucleotide polymorphism
- PBMCs
peripheral blood mononuclear cells
- OR
odds ratio
- HDL-C
high-density lipoprotein cholesterol
- BMI
Body mass index
- CRP
C-reactive protein
- TC
Cholesterol
- PIR
Poverty Impact Ratio
- CI
confidence interval
- β regression coefficient
- SD
standard deviation
- EAF
effect allele frequency
- EA
effect allele
- BETA
beta. exposure
- SE
standard error.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.926190/full#supplementary-material
References
- 1.Williams B, Dharmapatni A, Crotti T. Intracellular apoptotic pathways: a potential target for reducing joint damage in rheumatoid arthritis. Inflamm Res. (2018) 67:219–31. 10.1007/s00011-017-1116-5 [DOI] [PubMed] [Google Scholar]
- 2.Boissier M, Biton J, Semerano L, Decker P, Bessis N. Origins of rheumatoid arthritis. Pre-proof. (2020) 87:301–6. 10.1016/j.jbspin.2019.11.009 [DOI] [PubMed] [Google Scholar]
- 3.George MD, Hsu JY, Hennessy S, Chen L, Xie F, Curtis JR, et al. Risk of serious infection with low-dose glucocorticoids in patients with rheumatoid arthritis. Epidemiology. (2022) 33:65–74. 10.1097/EDE.0000000000001422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padyukov L. Genetics of rheumatoid arthritis. Semin Immunopathol. (2022) 44:47–62. 10.1007/s00281-022-00912-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell DC, Knight CA, Hockenberry J, Teplansky R. Hartman TJ. Beverage caffeine intakes in the US. Food Chem Toxicol. (2014) 63:136–42. 10.1016/j.fct.2013.10.042 [DOI] [PubMed] [Google Scholar]
- 6.Nigra AD, de Almeida Bauer Guimarães D, Prucca CG, Freitas-Silva O, Teodoro AJ, Gil GA. Antitumor effects of freeze-dried robusta coffee (coffea canephora) extracts on breast cancer cell lines. Oxid Med Cell Longev. (2021) 2021:1–16. 10.1155/2021/5572630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall S, Desbrow B, Anoopkumar-Dukie S, Davey AK, Arora D, McDermott C, et al. A review of the bioactivity of coffee, caffeine and key coffee constituents on inflammatory responses linked to depression. Food Res Int. (2015) 76:626–36. 10.1016/j.foodres.2015.07.027 [DOI] [PubMed] [Google Scholar]
- 8.Medeiros MS, Schumacher-Schuh AF, Altmann V, Rieder CRDM. A case–control study of the effects of chimarrão (ilex paraguariensis) and coffee on Parkinson's disease. Front Neurol. (2021) 12:619535. 10.3389/fneur.2021.619535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos RMM, Lima DRA. Coffee consumption, obesity and type 2 diabetes: a mini-review. Eur J Nutr. (2016) 55:1345–58. 10.1007/s00394-016-1206-0 [DOI] [PubMed] [Google Scholar]
- 10.Karlson EW, Mandl LA, Aweh GN, Grodstein F. Coffee consumption and risk of rheumatoid arthritis. Arthritis Rheum. (2003) 48:3055–60. 10.1002/art.11306 [DOI] [PubMed] [Google Scholar]
- 11.Lamichhane D, Collins C, Constantinescu F, Walitt B, Pettinger M, Parks C, et al. Coffee and tea consumption in relation to risk of rheumatoid arthritis in the women's health initiative observational cohort. J Clin Rheumatol. (2019) 25:127–32. 10.1097/RHU.0000000000000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen M, Stripp C, Klarlund M, Olsen SF, Tjønneland AM, Frisch M. Diet and risk of rheumatoid arthritis in a prospective cohort. J Rheumatol. (2005) 32:1249. [PubMed] [Google Scholar]
- 13.Mikuls TR, Cerhan JR, Folsom AR, Saag KG. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis. Arthritis Rheum. (2002). [DOI] [PubMed] [Google Scholar]
- 14.Asoudeh F, Dashti F, Jayedi A, Hemmati A, Fadel A, Mohammadi H. Caffeine, coffee, tea and risk of rheumatoid arthritis: systematic review and dose-response meta-analysis of prospective cohort studies. Front Nutr. (2022) 9:822557. 10.3389/fnut.2022.822557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362:k601. 10.1136/bmj.k601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Q, Burgess S, Turman C, Bolla MK, Wang Q, Lush M, et al. Body mass index and breast cancer survival: a Mendelian randomization analysis. Int J Epidemiol. (2017) 46:1814–22. 10.1093/ije/dyx131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgess S, Butterworth A, Malarstig A, Thompson SG. Use of mendelian randomisation to assess potential benefit of clinical intervention. BMJ. (2012) 345:e7325. 10.1136/bmj.e7325 [DOI] [PubMed] [Google Scholar]
- 18.Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on nhanes dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. (2016) 7:121–34. 10.3945/an.115.009258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Jian Z, Yuan C, Jin X, Li H, Wang K. Coffee consumption and prostate cancer risk: results from national health and nutrition examination survey 1999–2010 and mendelian randomization analyses. Nutrients. (2021) 13:2317. 10.3390/nu13072317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Kang JW, Jeong SM, Song GG, Choi SJ, Jung JH. Is rheumatoid arthritis related to coffee consumption in Korea? A nationwide cross-sectional observational study. Int J Env Res Pub He. (2021) 18:7880. 10.3390/ijerph18157880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu B, Lin J. Characteristics and risk factors of rheumatoid arthritis in the United States: an nhanes analysis. PeerJ. (2017) 5:e4035. 10.7717/peerj.4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Wu Q. Prevalence trend and disparities in rheumatoid arthritis among us adults, 2005–2018. J Clin Med. (2021) 10:3289. 10.3390/jcm10153289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walwadkar SD, Suryakar AN, Katkam RV, Kumbar KM, Ankush RD. Oxidative stress and calcium-phosphorus levels in rheumatoid arthritis. Indian J Clin Biochem. (2006) 21:134–7. 10.1007/BF02912928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. (1988) 31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Sun X, Zhou M. Body shape and Alzheimer's disease: a Mendelian randomization analysis. Front Neurosci-Switz. (2019) 13:1084. 10.3389/fnins.2019.01084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. 10.1002/gepi.21758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. 10.1093/ije/dyx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Xiong Y, Shen S, Yang J, Wang W, Wu T, et al. Causal association between tea consumption and kidney function: a Mendelian randomization study. Front Nutr. (2022) 9:801591. 10.3389/fnut.2022.801591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierce BL, Ahsan H, VanderWeele TJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. 10.1093/ije/dyq151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H, Zhang Y, Liu J, Yang Y, Fang W, Hong S, et al. Education and lung cancer: a Mendelian randomization study. Int J Epidemiol. (2019) 48:743–50. 10.1093/ije/dyz121 [DOI] [PubMed] [Google Scholar]
- 31.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. (2011) 365:2205–19. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 32.Heliovaara M. Coffee consumption, rheumatoid factor, and the risk of rheumatoid arthritis. Ann Rheum Dis. (2000) 59:631–5. 10.1136/ard.59.8.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Moine O, Stordeur P, Schandene L, Marchant A, de Groote D, Goldman M, et al. Adenosine enhances il-10 secretion by human monocytes. J Immunol. (1996) 156:4408–14. [PubMed] [Google Scholar]
- 34.Ullah F, Ali T, Kim MO. Caffeine prevents d galactose induce source neurochem int so 2015 jul 21. Neurochem Int. (2015) 90:114–24. 10.1016/j.neuint.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 35.Islam MT, Tabrez S, Jabir NR, Ali M, Kamal MA Da SAL, et al. An insight into the therapeutic potential of major coffee components. Curr Drug Metab. (2018) 19:544–56. 10.2174/1389200219666180302154551 [DOI] [PubMed] [Google Scholar]
- 36.Yanagimoto K, Ochi H, Lee K, Shibamoto T. Antioxidative activities of fractions obtained from brewed coffee. J Agr Food Chem. (2004) 52:592–6. 10.1021/jf030317t [DOI] [PubMed] [Google Scholar]
- 37.Yashin A, Yashin Y, Wang J, Nemzer B. Antioxidant and antiradical activity of coffee. Antioxidants. (2013) 2:230–45. 10.3390/antiox2040230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharif K, Watad A, Bragazzi NL, Adawi M, Amital H, Shoenfeld Y. Coffee and autoimmunity: more than a mere hot beverage!. Autoimmun Rev. (2017) 16:712–21. 10.1016/j.autrev.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 39.Sy LB, Yang L, Chiu C, Wu W. The immunoregulatory effects of caffeic acid phenethyl ester on the cytokine secretion of peripheral blood mononuclear cells from asthmatic children. Pediatrics Neonatol. (2011) 52:327–31. 10.1016/j.pedneo.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 40.Park JH, Lee JK, Kim HS, Chung ST, Eom JH, Kim KA, et al. Immunomodulatory effect of caffeic acid phenethyl ester in balb/c mice. Int Immunopharmacol. (2004) 4:429–36. 10.1016/j.intimp.2004.01.013 [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal LA, Taub DD, Moors MA, Blank KJ. Methylxanthine-induced inhibition of the antigen- and superantigen-specific activation of t and b lymphocytes. Immunopharmacology. (1992) 24:203. 10.1016/0162-3109(92)90076-O [DOI] [PubMed] [Google Scholar]
- 42.Laux Dc Klesiu Ph. Suppressive effects of caffeine on the immu source proc soc exp biol med 1973 nov 144 2 633 8. Proc Soc Exp Biol Med. (1973) 144:633–8. 10.3181/00379727-144-37651 [DOI] [PubMed] [Google Scholar]
- 43.Larsson SC, Burgess S, Michaëlsson K. Association of genetic variants related to serum calcium levels with coronary artery disease and myocardial infarction. JAMA. (2017) 318:371. 10.1001/jama.2017.8981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bae SC, Lee YH. Coffee consumption and the risk of rheumatoid arthritis and systemic lupus erythematosus: a mendelian randomization study. Clin Rheumatol. (2018) 37:2875–9. 10.1007/s10067-018-4278-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All National Health and Nutrition Examination Survey files are available from the American Centers for Disease Control and Prevention database (URL https://wwwn.cdc.gov/nchs/nhanes/Default.aspx, accessed on 6 March 2022). All the Mendelian randomization study files are available from GWAS (URL https://gwas.mrcieu.ac.uk, accessed on 17 March 2022). For all the original data, please see the Supplementary material Data Sheet 2.ZIP.