Abstract
The rhizobacterium Pseudomonas brassicacearum forms phenotypic variants which do not show extracellular protease and lipase activity. The operon encoding these enzymes, a serine protease homolog, and a type I secretion machinery was characterized. Transcriptional lacZ gene fusions revealed that the expression of the operon is under the control of phase variation.
Phenotypic variation leads to the division of a bacterial population into subpopulations expressing different surface compounds, thereby creating and maintaining sets of functionally different organisms within the population. Several pathogenic bacteria use this strategy to escape from the immune defense of the host. Phenotypic variation has also been observed in some soil-borne pseudomonads, for example, Pseudomonas tolaasii, the causal agent of brown blotch disease in mushrooms (7). Recently, we described a new Pseudomonas species, Pseudomonas brassicacearum, which was frequently isolated as a major root-colonizing population from Arabidopsis thaliana and Brassica napus (1). In the present study, we demonstrate that the expression of several extracellular enzymes in this species is prone to phase variation, and we characterized the operon that encodes these enzymes with the eventual goal of understanding the mechanism of phase variation and its putative role in root colonization.
Phase variation in P. brassicacearum.
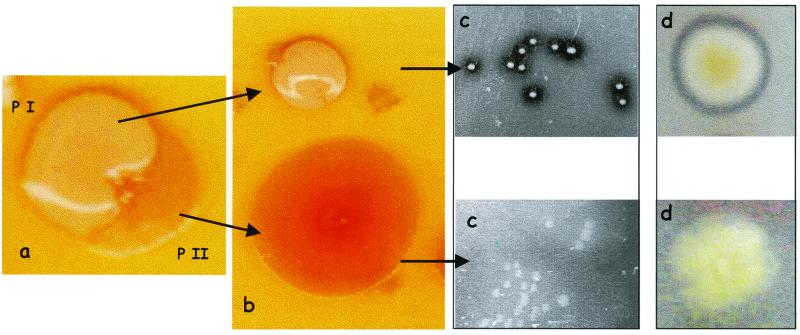
The original isolate of P. brassicacearum strain NFM421 (1) formed small mucoid colonies on rich medium plates (phase I cells). After prolonged growth on these plates, variants appeared on the edges of colonies (Fig. 1a), which formed large, flat, nonmucoid colonies (phase II cells) (Fig. 1b). Occasionally these phase II cells were found to revert to phase I (data not shown), consistent with the idea that phase variation rather than mutation is the basis for this phenotypic variation. In contrast to the phase II cells, the phase I cells were found to produce extracellular protease and lipase, as revealed by the halos formed around the colonies on protease and lipase indicator plates (Fig. 1c and d).
FIG. 1.
Phenotypic variation of P. brassicacearum strain NFM421. (a) Seven-day-old colony grown on PAF medium (Difco Laboratories, Detroit, Mich.) with a sector corresponding to phase II cells. (b) Colony morphology of the two phases. (c and d) Extracellular protease and lipase activity in phase I but not phase II cells was revealed on 10-fold-diluted tryptic soy broth medium (Difco Laboratories) agar plates containing 1% skim milk (c) or 1% tributyrin and 0.8% gum arabic (d). Bacteria were grown at 30°C.
Isolation of a cosmid containing phase-variable genes of P. brassicacearum.
Southern hybridization experiments (27) with a DNA probe encompassing the alkaline protease gene aprA from Pseudomonas aeruginosa identified specific signals within genomic DNA digests of P. brassicacearum NFM421 phase I (data not shown), suggesting the presence of an aprA homolog in this organism. Identical fragments were revealed within DNA from phase II cells. To clone the aprA homolog of P. brassicacearum, a genome library of strain NFM421 phase I was constructed. Genomic DNA was purified using CsCl gradient centrifugation, partially digested with Sau3A, and size fractionated using a 10 to 30% sucrose gradient, and DNA fragments of between 15 and 25 kb were ligated with pLAFR3 (30) digested with BamHI. In vitro packaging with the Gigapack III XL packaging extract (Stratagene, La Jolla, Calif.) was followed by transduction of Escherichia coli S17-1 (28). The genome library was screened with the aprA probe, and a positive clone, designated pAG500, was identified.
Nucleotide sequence analysis.
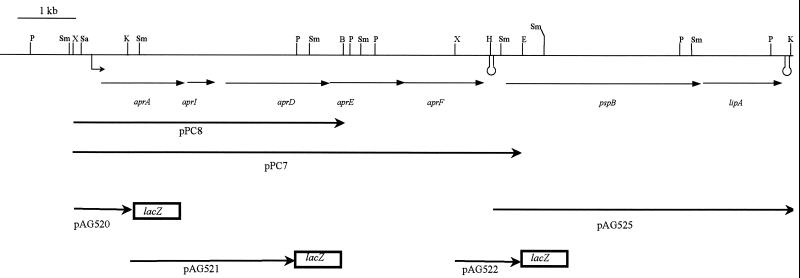
Relevant DNA fragments from pAG500 were subcloned and sequenced. The nucleotide sequence of a fragment spanning a total of 12 kb was determined (GenBank accession number AF286062). Seven complete open reading frames were identified and, based on homologies described below, were tentatively designated aprA, aprI, aprD, aprE, aprF, pspB, and lipA (Fig. 2).
FIG. 2.
Genetic organization and restriction map of the sequenced 12-kb fragment. Restriction sites indicated are BamHI (B), EcoRI (E), HindIII (H), KpnI (K), PstI (P), SacI (Sa), SmaI (Sm), and XhoI (X). Thin arrows indicate the open reading frames. The two putative terminators are indicated by stem-loop structure. Thick arrows represent fragments subcloned in pEMBL19, pPC7, and pPC8 or pUR6500HE (pAG525), in the orientation indicated relative to the lac or tac promoter, or in pMP220, in the orientation indicated relative to the promoterless lacZ gene.
The putative aprA gene product showed high levels of homology to several metalloproteases, including alkaline protease of P. aeruginosa PAO1. A putative Zn2+-binding domain corresponding to the consensus sequence QTLHEIGHxxGLxHP (5) was identified in AprA of P. brassicacearum. A three-times-repeated glycine-rich sequence of nine amino acids (xxxGGxGxD), implicated in Ca2+ binding (5), was present in the C-terminal domain, and the sequence DIVA, complying with the consensus sequence Dhhh, where h stands for any hydrophobic residue, was found at the extreme C terminus. These motifs, as well as the absence of an N-terminal signal sequence, are characteristic of proteins secreted via type I machinery (4, 15) and were shown to be important for recognition of the secreted proteins by the translocator (12). The aprI gene putatively encodes a protein with homology to protease inhibitors. The protein contains an N-terminal hydrophobic segment, which could function as a signal sequence or a membrane anchor. The aprDEF genes code for the three components of a type I secretion system and show high levels of homology with the corresponding components of type I systems involved in the secretion of proteases in other bacteria (2, 8, 11, 21, 22). AprD contains four putative transmembrane segments in the N-terminal region and an ATP-binding consensus sequence (GxxGxGKS) in the C-terminal half. It belongs to the ATP-binding cassette (ABC) protein family, which includes prokaryotic and eukaryotic proteins involved in the export and import of various substrates (19). The AprE protein contains one predicted transmembrane segment, followed by a large periplasmic domain. It is a member of the membrane fusion protein family (10). The aprF gene encodes a protein synthesized with an N-terminal signal sequence, which constitutes the outer membrane component of the type I secretion apparatus (10).
The next gene in the cluster, pspB, putatively encodes a protein of 1,036 amino acids with strong homology to serine proteases (21, 25). In addition, lower but significant homology (24% identity) over the entire length of the protein was observed with the serotype 1-specific antigen of Pasteurella haemolytica (16, 23). Interestingly, these serine proteases are not secreted via a type I pathway, but they belong to the autotransporter family of secreted proteins. Autotransporters possess an overall unifying structure comprising three functional domains: an N-terminal signal peptide, the secreted mature protein (passenger or α-domain), and a C-terminal β-barrel (β-domain), which probably forms a pore in the outer membrane to allow the secretion of the passenger domain (18).
The last gene of the cluster, lipA, putatively encodes a protein of 619 amino acids with homology to lipases secreted via a type I secretion pathway (2, 3, 20). The LipA sequence contains the lipase consensus sequence GxSxG, which comprises the active-site serine residue (20). It does not possess an N-terminal signal sequence but does contain glycine-rich repeats and a motif, EGIA, with similarity to the Dhhh motif in the metalloproteases at the extreme C terminus. Similar gene clusters have been described in Pseudomonas fluorescens strains SIK-W1 (2), CYO91 (22), and 33 (21). However, whereas P. brassicacearum contains one gene for a serine protease homolog, P. fluorescens strains 33 and SIK-W1 contain two and none, respectively. In addition, the lipase of P. brassicacearum is considerably larger than those of P. fluorescens strains SIK-W1 and 33.
Expression and secretion of P. brassicacearum lipase and protease in E. coli.
To investigate whether the genes identified code for functional proteins, various plasmids carrying these genes under the control of the lac or tac promoter on pEMBL19 (9) or pUR6500HE (14), respectively, were constructed (Fig. 2) and introduced into E. coli strain DH5α. With the strain carrying aprAID plasmid pPC8, no protease activity was detected on milk plates at either 30 or 37°C. However, protease activity was observed for the strain carrying plasmid pPC7 (aprAIDEF), but only at 30°C (results not shown). Such temperature dependence was also reported when the apr gene homologs of P. fluorescens were expressed in E. coli (21). Apparently, the aprA gene encodes a functional protease, which requires the type I machinery encoded by aprDEF for its secretion.
When plasmid pAG525 (pspB-lipA in pUR6500HE) was cointroduced with pPC8 carrying aprAID in E. coli, a small halo around the colonies was observed on tributyrin plates (results not shown). This halo was larger when pPC7 carrying all secretion genes was introduced with pAG525, showing that the lipA gene encodes an active enzyme which is secreted by the AprDEF secretion apparatus. The small halo observed in the absence of the ABC transporter may result from overexpression of the lipase or from unspecific leakage.
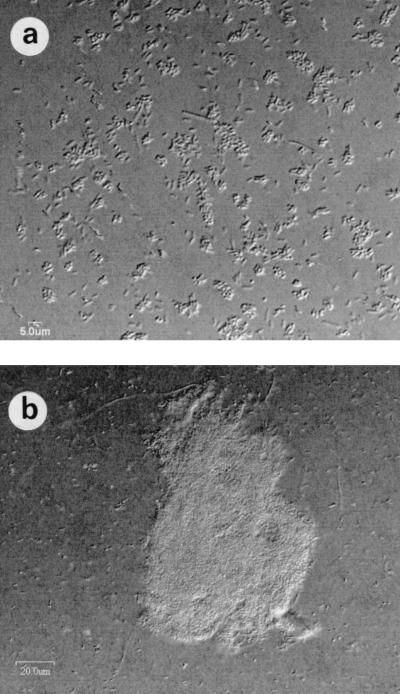
Expression of the serine protease homolog PspB from plasmid PspB in E. coli did not result in the detection of protease activity on milk plates. Similarly, no protease activity was detected for the serine protease homologs PspA and PspB of P. fluorescens (21) and Ssp-h1 and Ssp-h2 of Serratia marcescens (25). Interestingly, expression of pAG525 in E. coli led to massive aggregation (Fig. 3), suggesting that PspB might act as an autoaggregation factor. Moreover, many proteins belonging to the autotransporter family are involved in adhesion or autoaggregation, even though several of them possess the active-site motif of serine proteases (18), and some of them, for example antigen 43 of E. coli, are subject to phase variation (17, 26). Such autoaggregation may be important in the rhizosphere for biofilm formation.
FIG. 3.
Confocal microscopy observation of E. coli DH5α without plasmid (a) and with plasmid pAG525 (b) after growth on LB plates supplemented with 0.5 mM isopropyl-β-d-thiogalactoside. Bars: (a) 5.0 μm; (b) 20.0 μm.
Analysis of promoter activities in the apr-lip gene cluster.
The introduction of pAG500 into P. brassicacearum phase II cells did not restore protease and lipase secretion. Hence, the structural genes for either the enzymes or the ABC transporter or both are not expressed. Promoter activity was investigated by cloning potential promoter-containing fragments of pAG500 upstream of a promoterless lacZ reporter gene on pMP220 (29), yielding pAG520, pAG521, and pAG522 (Fig. 2). Plasmids were transferred from E. coli to P. brassicacearum using the conjugative properties of pRK2013 (13), and β-galactosidase activity was measured (24). In phase I cells, promoter activity was only detected with the aprA-lacZ fusion on pAG520 (950 Miller units), strongly suggesting that the aprA-lipA genes constitute a single operon. In phase II cells, the promoter was not active (95 Miller units). These results indicate that phase II cells do not produce extracellular protease and lipase activity because the aprA-lipA operon is not transcribed in this variant and that regulation of this operon occurs at the transcriptional level. Possibly, phase II cells lack an activator, or, alternatively, phase I cells lack a repressor. Phase variation altering the expression of protease and lipase genes has previously been observed for the soil-borne insect-pathogenic species Xenorhabdus luminescens (6). In that case, the expression of lipase activity appeared to be controlled at the posttranslational level (31). Therefore, the phase variation-controlled regulation of lipase expression in X. luminescens is entirely different from that in P. brassicacearum. The molecular mechanism of the phase variation in P. brassicacearum and its role in root colonization will be investigated in the future. The identification of an operon that is regulated by this mechanism, as described in the present study, will be a valuable tool for future studies.
Acknowledgments
We are grateful to Alain Filloux (CNRS, Marseille) for helpful discussions.
This study was partially funded through the PICS program of CNRS, France, and a Van Gogh project France-Holland.
REFERENCES
- 1.Achouak W, Sutra L, Heulin T, Meyer J-M, Fromin N, Degraeve S, Christen R, Gardan L. Pseudomonas brassicacearum sp. nov. and Pseudomonas thivervalensis sp. nov., two root-associated bacteria isolated from Arabidopsis thaliana and Brassica napus. Int J Syst Bacteriol. 2000;50:9–18. doi: 10.1099/00207713-50-1-9. [DOI] [PubMed] [Google Scholar]
- 2.Ahn J H, Pan J G, Rhee J S. Identification of the tliDEF ABC transporter specific for lipase in Pseudomonas fluorescens SIK W1. J Bacteriol. 1999;181:1847–1852. doi: 10.1128/jb.181.6.1847-1852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akatsuka H, Binet R, Kawai E, Wandersman C, Omori K. Lipase secretion by bacterial hybrid ATP-binding cassette exporters: molecular recognition of the LipBCD, PrtDEF, and HasDEF exporters. J Bacteriol. 1997;179:4754–4760. doi: 10.1128/jb.179.15.4754-4760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binet R, Létoffé S, Ghigo J M, Delepelaire P, Wandersman C. Protein secretion by Gram-negative bacterial ABC exporters—a review. Gene. 1997;192:7–11. doi: 10.1016/s0378-1119(96)00829-3. [DOI] [PubMed] [Google Scholar]
- 5.Boehm D F, Welch R A, Snyder I S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990;58:1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boemare N E, Akhurst R J. Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. (Enterobacteriaceae) J Gen Microbiol. 1988;134:751–761. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- 7.Cutri S S, Macaulay B J, Roberts W P. Characteristic of pathogenic non-fluorescent (smooth) and non-pathogenic fluorescent (rough) form of Pseudomonas tolaasii and Pseudomonas gingeri. J Appl Bacteriol. 1984;57:291–298. [Google Scholar]
- 8.Delepelaire P, Wandersman C. Characterization, localization and transmembrane organization of the three proteins PrtD, PrtE and PrtF necessary for protease secretion by the gram-negative bacterium Erwinia chrysanthemi. Mol Microbiol. 1991;5:2427–2434. doi: 10.1111/j.1365-2958.1991.tb02088.x. [DOI] [PubMed] [Google Scholar]
- 9.Dente L, Cesareni G, Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983;11:1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinh T, Paulsen I T, Saier M H J. A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J Bacteriol. 1994;176:3825–3831. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duong F, Lazdunski A, Cami B, Murgier M. Sequence of a cluster of genes controlling synthesis and secretion of an alkaline protease in Pseudomonas aeruginosa: relationships to other secretory pathways. Gene. 1992;121:47–54. doi: 10.1016/0378-1119(92)90160-q. [DOI] [PubMed] [Google Scholar]
- 12.Duong F, Lazdunski A, Murgier M. Protein secretion by heterologous bacterial ABC-transporters: the C-terminus secretion signal of the secreted protein confers high recognition specificity. Mol Microbiol. 1996;21:459–470. doi: 10.1111/j.1365-2958.1996.tb02555.x. [DOI] [PubMed] [Google Scholar]
- 13.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenken L G J, Bos J W, Visser C, Muller W, Tommassen J, Verrips C T. An accessory gene, lipB, required for the production of active Pseudomonas glumae lipase. Mol Microbiol. 1993;9:579–589. doi: 10.1111/j.1365-2958.1993.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 15.Ghigo J M, Wandersman C. A carboxyl-terminal four-amino acid motif is required for secretion of the metalloprotease PrtG through the Erwinia chrysanthemi protease secretion pathway. J Biol Chem. 1994;269:8979–8985. [PubMed] [Google Scholar]
- 16.Gonzalez C T, Maheswaran S K, Murtaugh M P. Pasteurella haemolytica serotype 2 contains the gene for a noncapsular serotype 1-specific antigen. Infect Immun. 1995;63:1340–1348. doi: 10.1128/iai.63.4.1340-1348.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson I R, Meehan M, Owen P. A novel regulatory mechanism for a novel phase-variable outer membrane protein of Escherichia coli. Adv Exp Med Biol. 1997;412:349–355. doi: 10.1007/978-1-4899-1828-4_56. [DOI] [PubMed] [Google Scholar]
- 18.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 19.Higgins C F. ABC transporters—from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 20.Jaeger K-E, Ransac S, Dijkstra B W, Colson C, van Heuvel M, Misset O. Bacterial lipases. FEMS Microbiol Rev. 1997;15:29–63. doi: 10.1111/j.1574-6976.1994.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawai E, Idei A, Kumura H, Shimazaki K, Akatsuka H, Omori K. The ABC-exporter genes involved in the lipase secretion are clustered with the genes for lipase, alkaline protease, and serine protease homologues in Pseudomonas fluorescens no. 33. Biochim Biophys Acta. 1999;1446:377–382. doi: 10.1016/s0167-4781(99)00094-9. [DOI] [PubMed] [Google Scholar]
- 22.Liao C-H, McCallus D E. Biochemical and genetic characterization of an extracellular protease from Pseudomonas fluorescens CY091. Appl Environ Microbiol. 1998;64:914–921. doi: 10.1128/aem.64.3.914-921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo R, Strathdee C A, Shewen P. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987;55:1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 25.Ohnishi Y, Beppu T, Horinouchi S. Two genes encoding serine protease homologues in Serratia marcescens and characterization of their products in Escherichia coli. J Biochem. 1997;121:902. doi: 10.1093/oxfordjournals.jbchem.a021672. [DOI] [PubMed] [Google Scholar]
- 26.Owen P, Meehan M, de Loughry-Doherty H, Henderson I. Phase-variable outer membrane proteins in Escherichia coli. FEMS Immunol Med Microbiol. 1996;16:63–76. doi: 10.1111/j.1574-695X.1996.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Simon M, Priefer U, Pühler A. A broad range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 29.Spaink H P, Okker R J H, Wijffelman C A, Pees E, Lugtenberg B J J. Promoters in the nodulation region of Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol Biol. 1987;9:27–39. doi: 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- 30.Staskawicz B, Dahlbeck D, Keen N, Napoli C. Molecular charaterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Dowds B C A. Phase variation in Xenorhabdus luminescens: cloning and sequencing of the lipase gene and analysis of its expression in primary and secondary phases of the bacterium. J Bacteriol. 1993;175:1665–1673. doi: 10.1128/jb.175.6.1665-1673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]