Abstract
Background
The neutrophil‐to‐lymphocyte ratio (NLR) has been identified as a biomarker in several inflammatory and autoimmune diseases. Multiple sclerosis (MS) has been found to be associated with changes in the NLR in humans.
Objectives
To examine the diagnostic value of the NLR in meningoencephalitis of unknown etiology (MUE) in dogs.
Animals
Thirty‐eight MUE dogs, 20 hydrocephalic dogs, 10 brain tumor (BT) dogs, 32 idiopathic epilepsy (IE) dogs, and 41 healthy dogs.
Methods
Retrospective study. Medical records were reviewed to identify dogs with a diagnosis of neurologic disease. The NLR was determined in all dogs.
Results
The median NLR was significantly higher in MUE dogs (6.08) than in healthy (1.78, P < .001), IE (2.50, P < .05), and hydrocephalic dogs (1.79, P < .05). The area under the receiver operating characteristic curve of the NLR for differentiation between MUE and healthy dogs was 0.96, and between the MUE dogs and dogs with other forebrain diseases was 0.86. An optimal cutoff of 4.16 for the NLR had a sensitivity of 71.1% and specificity of 83.9% to differentiate the MUE dogs from the dogs with other forebrain diseases.
Conclusions and Clinical Importance
The NLR could be a biomarker for diagnosing MUE and distinguishing it from other intracranial diseases in dogs.
Keywords: canine, MUE, neuronal inflammation, NLR
Abbreviations
- AUC
area under the receiver operator characteristic curve
- CI
confidence interval
- CNS
central nervous system
- CSF
cerebrospinal fluid
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- MUE
meningoencephalitis of unknown etiology
- NLR
neutrophil to lymphocyte ratio
- ROC
receiver operator characteristic
1. INTRODUCTION
Meningoencephalitis of unknown etiology (MUE) is a common inflammatory condition of the central nervous system (CNS) in dogs. 1 Although the specific pathophysiology of MUE is unknown, aggressive immunosuppressive treatment is used for its management. 2 The prognosis of MUE is poor and previously reported survival time for dogs receiving glucocorticoids only was 28 to 602 days. 3 Because the definitive diagnosis of MUE is obtained by postmortem histopathological examination or biopsy which is obtained infrequently, the diagnosis of MUE is challenging. Antemortem diagnosis of MUE typically is achieved by evaluation of history, signalment, laboratory test results, neurological signs, magnetic resonance imaging (MRI) findings, and cerebrospinal fluid (CSF) analysis. 4
Previous studies have shown that MUE is a naturally‐occurring dog model of immune‐mediated CNS diseases, such as multiple sclerosis (MS), in humans. 5 , 6 The pathogenesis of MS is not completely understood, but the involvement of CD4+ myelin‐reactive T cells has been recognized. 7 In humans with MS, autoreactive activated T cells migrate across the blood‐brain barrier (BBB) and cause demyelination associated with inflammation and axonal loss. Similarly, MUE in dogs is characterized by lymphocyte infiltration in the CNS and it has been suggested that T lymphocytes mediate the disease. 1
The neutrophil‐to‐lymphocyte ratio (NLR) is calculated by dividing the neutrophil count by the lymphocyte count, and has been used to determine the prognosis of inflammatory reactions. 8 Moreover, it is considered to be comparatively more stable than the absolute counts. 8 The NLR has been investigated as a useful diagnostic marker for various diseases. It has been studied as a diagnostic marker in acute bacterial meningitis of humans, and in chronic enteropathy and soft tissue sarcoma in dogs. 8 , 9 , 10 An increased NLR can distinguish between MS and normal controls in humans. 11 Additionally, the NLR is an inexpensive marker that can be easily obtained from the CBC.
Despite widespread use of the NLR, to the best of our knowledge, no specific studies of the NLR in MUE dogs have been performed. In dogs, studies have been conducted on inflammatory bowel disease (IBD), septic peritonitis, and lymphoma, but studies on neurological diseases are not available. 12 , 13 , 14 , 15 Therefore, our aim was to evaluate the diagnostic value of the NLR in dogs with MUE. We hypothesized that the NLR could be used to differentiate MUE from healthy controls and dogs with other neurological diseases.
2. MATERIALS AND METHODS
2.1. Animals
We retrospectively reviewed the medical records of dogs with a diagnosis of intracranial diseases, including MUE, hydrocephalus, idiopathic epilepsy (IE), and brain tumor (BT) at our institution from January 2014 to February 2021. The study group included 141 dogs, with 41 healthy control dogs and 100 dogs with neurologic diseases. Healthy dogs without inflammatory diseases and neurological signs were recruited to be part of the healthy group, and the healthy control group consisted of 27 client‐owned healthy dogs and 14 healthy beagle dogs. The neurologic disease group consisted of dogs diagnosed with MUE (n = 38), IE (n = 32), hydrocephalus (n = 20), and BT (n = 10) that did not have any exclusion criteria. The exclusion criteria were as follows: (1) dogs with incomplete medical records, (2) dogs that had received anti‐inflammatory, immunosuppressive, anticonvulsant drugs, or antibiotics, in the past 30 days, and (3) dogs with concurrent disease such as hyperadrenocorticism, lymphoma, or other inflammatory diseases that could affect the white blood cell count.
A minimum database for MUE dogs consisted of the following: CBC, serum biochemistry profile, CSF analysis, MRI, and neurologic examination. Diagnosis of MUE was based on histopathological confirmation, or it was presumptively diagnosed based on the following criteria 4 : (1) dogs older than 6 months, (2) single or multifocal neurologic signs, (3) hyperintense lesions observed on T2‐weighted and fluid‐attenuated inversion recovery (FLAIR) images, (4) hypercellularity on CSF analysis, with >50% mononuclear (monocytes and lymphocytes) cells, and (5) the presence of infectious diseases was ruled out.
Idiopathic epilepsy was defined as dogs with chronic recurrent seizures for which no abnormality was identified on MRI, neurologic examination, hematological examination, and biochemistry profile. 16
Dogs with ventricular enlargement and clinical signs of forebrain deficits were diagnosed as having hydrocephalus. The MRI findings of hydrocephalus were expansion of the third ventricle, disruption of the internal capsule adjacent to the caudate nucleus, periventricular edema, narrowing of cerebral sulci, and dilatation of olfactory recesses. 17 In addition, dogs with a heterogeneous parenchymal lesion or meningeal enhancement were excluded.
Diagnosis of BT was based on histological findings at necropsy or a tentative diagnosis was made according to the following features 18 : (1) >1 neurologic sign; (2) MRI changes characterized by solitary, regular‐shaped T2‐hyperintense, FLAIR‐hyperintense, and T1‐hypo to iso‐intense lesions, (3) postcontrast T1‐hyperintense lesions, (4) dural tail or dural contact, (5) mass effect on MRI, and (6) negative blood and CSF infectious disease titers.
2.2. NLR measurement
For each dog, a 250‐μL blood sample was collected from a jugular or cephalic vein into an ethylenediaminetetraacetic acid tube. The CBC was determined using a blood cell count machine (IDEXX ProCyte Dx, IDEXX Laboratories, Inc) with <3% coefficient of variation for the CBC. 19 The NLR was measured based on CBC data at the first visit. Neutrophil and lymphocyte counts were extracted and the NLR was calculated as the neutrophil count divided by the lymphocyte count. 8
2.3. Grouping and data analysis
To examine differences in the NLR among neurologic diseases, dogs with neurological deficiencies were classified into 2 groups: the IE group (n = 32) and the structural group (n = 68); these groups were compared with the healthy group. The IE group consisted of epileptic dogs with no structural changes in the brain whereas the structural group included dogs with MUE, hydrocephalus, and BT.
The MRI examinations were performed using a 0.3‐Tesla unit (Airis II, Hitachi, Japan) or 1.5‐Tesla unit (Signa Creator, GE Healthcare, Milwaukee, Wisconsin). The T2‐weighted images (T2‐WI), T2‐FLAIR images, and T1‐weighted images (T1‐WI) images before and after paramagnetic contrast injection were acquired in transverse and sagittal planes.
2.4. Statistical analysis
Data were analyzed using Prism 6 software (GraphPad Software, San Diego, California). Results are expressed as median and interquartile range. A P‐value <.05 was considered significant. Normality was assessed using the Shapiro‐Wilk test. The Mann‐Whitney U‐test was used to compare data between MUE and healthy groups. The comparison of age and bodyweight between the healthy and disease groups was conducted using the Kruskal‐Wallis test with posttests (Dunn's multiple comparison test). It also was used for comparison of the NLR for ≥3 independent groups. Receiver operating characteristic (ROC) curve analysis was used to assess diagnostic utility of the NLR to differentiate between MUE and healthy and MUE and other forebrain disease dogs. The area under the curve (AUC) of the ROC curve was obtained and the diagnostic accuracy was classified according to the AUC value as follows: sufficient (0.6‐0.7), good (0.7‐0.8), very good (0.8‐0.9), and excellent (0.9‐1.0). 20 Sensitivity and specificity were calculated and the optimal cutoff with the highest Youden index ([sensitivity + specificity] − 1) was selected. 20
3. RESULTS
3.1. Study population
The healthy dogs consisted of 2 Maltese, 1 Pomeranian, 6 Poodles, 5 Bichon Frise, 5 mixed breed dogs, 1 Jindo dog, 3 Welsh Corgis, 14 healthy beagle dogs, and 1 each of Spitz, Shih Tzu, Shiba Inu, and Miniature Schnauzer. The MUE dogs consisted of 20 Maltese, 5 Yorkshire Terriers, 4 Chihuahuas, 3 Pomeranians, 2 Poodles, 2 mixed breed dogs, and 1 each of Miniature Pinscher Pekingese, and Shih Tzu. The IE dogs consisted of 7 Maltese, 3 Yorkshire Terriers, 2 Chihuahuas, 6 Pomeranians, 3 Poodles, 2 mixed breed dogs, 2 Japanese Chins, and 1 each of Miniature Pinscher, Pekingese, Golden Retriever, Bichon Frise, Labrador Retriever, Jindo dog, and Shiba Inu. The hydrocephalus dogs consisted of 13 Maltese, 4 Chihuahua, and 1 each of Yorkshire Terrier, Poodle, and Spitz. The BT dogs consisted of 1 Maltese, 2 Yorkshire Terriers, 3 Poodles, and 1 each of Miniature Pinscher, Pekingese, Golden Retriever, and Doberman Pinscher. The demographic characteristics of the dogs are presented in Table 1. The BT dogs were significantly older than the IE (P = .004), hydrocephalus (P < .001), and healthy dogs (P < .0001), but not MUE dogs (P = .08). Dogs with MUE and hydrocephalus had significantly lower body weight than other dogs (P < .05). Sex was not different among the 5 groups. The highest frequency of seizures was in dogs with IE, followed by BT, MUE and hydrocephalus dogs.
TABLE 1.
Characteristics of healthy dogs and dogs with neurologic diseases
| Healthy (n = 41) | MUE (n = 38) | IE (n = 32) | Hydrocephalus (n = 20) | BT (n = 10) | |
|---|---|---|---|---|---|
| Age (y) | 2 (1–3) | 5a (3–8) | 4 (2‐5.5) | 2.5 (1–5) | 11a,c,d (7.5‐12.8) |
| Body weight (kg) | 6.2b,d (4.2‐9.4) | 2.8 (2.0‐3.9) | 4.2b,d (2.9‐6.9) | 2.3 (1.8‐2.5) | 5.3b,d (3.6‐5.6) |
| Sex (number) | |||||
| Male | 14 (34.1%) | 21 (55.3%) | 16 (50%) | 7 (35%) | 5 (50%) |
| Female | 27 (65.9%) | 17 (44.7%) | 16 (50%) | 13 (65%) | 5 (50%) |
| Seizure (number) | – | 23 (60.5%d) | 32 (100%b,d) | 4 (20%) | 8 (80%d) |
Note: Age and body weight are expressed as median and interquartile range. The values of sex and seizure are expressed as the number of dogs (percentage of total instances). The value is significantly higher compared to aHealthy, bMUE, cIE, or dhydrocephalus (P < .05).
Abbreviations: BT, brain tumor; IE, idiopathic epilepsy; MUE, meningoencephalitis of unknown etiology.
3.2. Comparison of the NLR between healthy dogs and MUE dogs
The NLR of MUE dogs (median, 6.08 [interquartile range, 3.48‐8.84]) was significantly higher than that of healthy dogs (1.78 [1.36‐2.20]; P < .0001; Figure 1A). The neutrophil count of MUE dogs (8.41 [5.49‐12.25] × 103/μL) was significantly higher than that of healthy dogs (5.19 [4.57‐6.23] × 103/μL; P < .0001; Figure 1B). The MUE dogs (1.43 [1.00‐2.29] × 103/μL) had significantly lower lymphocyte counts than healthy dogs (3.09 [2.59‐3.75] × 103/μL; P < .001; Figure 1C).
FIGURE 1.

Scatterplot comparing the NLR (A), neutrophil count (B), and lymphocyte count (C) of the MUE (n = 38) and healthy groups (n = 41). (A) the NLR of MUE dogs was significantly higher than that of healthy control dogs. (B) the neutrophil count of MUE dogs was significantly higher than that of healthy dogs. (C) the lymphocyte count of MUE dogs was significantly lower than that of healthy dogs. The horizontal bars show the medians and interquartile ranges from the first to the third quartile. Mann‐Whitney U test. ***P < .001; ****P < .0001. MUE, meningoencephalitis of unknown etiology; NLR, neutrophil to lymphocyte ratio
3.3. Comparison of the NLR among neurologic diseases
Multiple comparisons were conducted to identify differences in the NLR between dogs with neurologic diseases and healthy dogs. The NLR of the structural group (3.86 [2.43‐6.76]) was significantly higher than that of the IE group (2.50 [1.58‐3.43]; P = .01) and healthy group (1.78 [1.36‐2.20]; P < .0001). The IE group had a relatively higher NLR compared to the healthy group (P = .04). When the IE group was compared to dogs with 3 types of structural diseases, only MUE dogs (6.08 [3.48‐8.84]; P < .0001) had significantly higher NLR compared to the IE group. The IE group showed no significant difference in terms of the NLR compared to hydrocephalus dogs (1.79 [1.32‐2.83]; P > .99) and BT dogs (4.27 [2.85‐4.86]; P = .26).
In the structural group, hydrocephalus dogs had a significantly lower NLR than MUE dogs (P < .0001) and BT dogs (P = .02), but the difference between the MUE and BT groups was not significant (P > .99). A significant difference in the NLR was found between BT dogs and healthy dogs (P = .001), whereas no difference was found between hydrocephalic dogs and healthy dogs (P > .99). Figure 2A presents a scatterplot of the NLR in all of the groups. The NLR of the MUE dogs (6.08 [3.48‐8.84]) was significantly higher than that of dogs suffering from other forebrain diseases (IE, hydrocephalus, and BT; 2.61 [1.51‐3.41]; P < .0001; Figure 2B).
FIGURE 2.
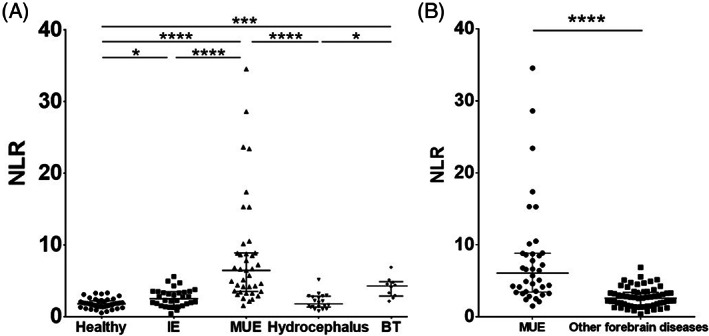
Scatterplot of the NLR in all the groups (healthy, n = 41; IE, n = 32; MUE, n = 38; hydrocephalus, n = 20; and BT, n = 10) (A) and in MUE dogs vs dogs with other forebrain diseases (IE, hydrocephalus, and BT, n = 62) (B). (A) There were significant differences in the NLR between MUE dogs and healthy, IE, and hydrocephalus dogs, whereas no difference was noted between MUE dogs and BT dogs. In addition, there were significant differences between BT dogs and hydrocephalus dogs, BT dogs and healthy dogs, and IE dogs and healthy dogs. Statistical significance was assessed with the Kruskal‐Wallis test with Dunn's multiple comparison test. (B) There was a significant difference in the NLR between MUE dogs and dogs with other forebrain diseases. Mann‐Whitney U test. The horizontal bars show the medians and interquartile ranges from the first to the third quartile. The asterisk indicates a statistically significant difference. *P < .05; ***P < .001; ****P < .0001. BT, brain tumor; IE, idiopathic epilepsy; MUE, meningoencephalitis of unknown etiology; NLR, neutrophil to lymphocyte ratio
3.4. AUC of the NLR in neurologic diseases
The ROC curve illustrates the sensitivity and specificity of use of the NLR to distinguish MUE dogs from healthy, IE, hydrocephalus, and all the forebrain diseases combined (IE, hydrocephalus, and BT; Figure 3). The AUCs were as follow: (1) healthy dogs and MUE dogs, 0.96 (95% confidence interval [CI] = 0.92‐1.00), (2) IE dogs and MUE dogs, 0.87 (95% CI = 0.78‐0.95), (3) hydrocephalus dogs and MUE dogs, 0.93 (95% CI = 0.86‐0.99), and (4) MUE dogs and forebrain disease dogs, 0.86 (95% CI = 0.78‐0.93). The corresponding optimal cutoffs (sensitivity and specificity) for the NLR were as follow: (1) healthy dogs and MUE dogs, 2.98 (86.8% [95% CI = 71.9%‐95.6%] and 90.2%, [95% CI = 76.9%‐97.3%]), (2) IE dogs and MUE dogs, 3.96 (71.1% [95% CI = 54.1%‐84.6%] and 87.50% [95% CI = 71.0%‐96.5%]), (3) hydrocephalus dogs and MUE dogs, 3.23 (95.0% [95% CI = 75.1%‐99.9%] and 84.2% [95% CI = 68.8%‐94.0%]), and (4) MUE dogs and forebrain disease dogs, 4.16 (71.1% [95% CI = 54.1%‐84.6%] and 83.9% [95% CI = 72.3%‐92.0%]), respectively.
FIGURE 3.

Receiver operating characteristic (ROC) curve analysis of predicting the NLR between (A) healthy and MUE dogs, (B) IE and MUE dogs, (C) hydrocephalus and MUE dogs, and (D) MUE dogs and dogs with other forebrain diseases (IE, hydrocephalus, and BT). The thick diagonal line shows a 50% chance. The AUCs were 0.96 (95% CI = 0.92‐1.00) (A), 0.87 (95% CI = 0.78‐0.95) (B), 0.93 (95% CI = 0.86‐0.99) (C), and 0.86 (95% CI = 0.78‐0.93) (D), respectively. The point of intersection in all ROC curves represents the optimal cutoff value (sensitivity and specificity) of 2.98 (86.8% [95% CI = 71.9%‐95.6%] and 90.2%, [95% CI = 76.9%‐97.3%]) (A), 3.96 (71.1% [95% CI = 54.1%‐84.6%] and 87.5% [95% CI = 71.0%‐96.5%]) (B), 3.23 (95.0% [95% CI = 75.1%‐99.9%] and 84.2% [95% CI = 68.8%‐94.0%]) (C), and 4.16 (71.1% [95% CI = 54.1%‐84.6%] and 83.9% [95% CI = 72.3%‐92.0%]) (D), respectively. AUC, area under the receiver operating characteristic curve; BT, brain tumor; CI, confidence interval; IE, idiopathic epilepsy; MUE, meningoencephalitis of unknown etiology; NLR, neutrophil to lymphocyte ratio; ROC, receiver operating characteristic
4. DISCUSSION
In our study, dogs with MUE had higher NLR than healthy dogs. In addition, the NLR of MUE dogs was significantly higher than that of IE and hydrocephalus dogs. These results are consistent with previous studies that showed increased NLR in the relapse phase of MS in human patients. 21 Our data also suggest that the NLR could be used as a diagnostic tool to identify MUE and differentiate it from other neurological diseases in dogs. To the best of our knowledge, ours is the first study to identify the potential role of the NLR in dogs with MUE.
The NLR is known as an inexpensive and easily accessible supplemental marker of several inflammatory diseases. 11 A previous study indicated that an increased NLR is associated with increased inflammatory activity in human patients with MS. 22 Because MUE is considered an animal model of MS, a similar result for the NLR was predicted and the NLR was found to be significantly increased in MUE dogs in our study. 5 , 6 The cause of an increased NLR in CNS autoimmune disease and whether increased neutrophil counts contribute to the development of CNS autoimmunity remains unclear. 23 Nevertheless, some studies suggest that neutrophils contribute to neuronal regulation of the immune system. 24 Studies on experimental autoimmune encephalomyelitis (EAE) have indicated a promoting role of peripheral blood neutrophils in the pathogenesis of EAE, and depletion of neutrophils delayed disease onset. 24 Neutrophils have been suggested to be particularly important in the early stage of disease and involved in lesion formation by promoting the infiltration of inflammatory cells. 25 One previous study also showed that the neutrophils of MS patients are increased because neutrophils are found more in a primed state than in a resting state, which leads to decreased apoptosis. 26 In our study, the neutrophil count was increased in MUE, similar to MS, presumably because the pathophysiology of MUE resembles that of MS. Therefore, it was suspected that the role of neutrophils may be similar to that in MS. 5 In MS patients, pathogenic lymphocytes are triggered in the periphery to infiltrate the CNS and cause local inflammation and demyelination. 27 Similarly, MUE features perivascular cuffs that include lymphocytes, plasma cells, and histiocytic cells and it is presumed to cause decreased peripheral lymphocytes. 28 Therefore, the NLR is associated with neuronal inflammation and could be used as an indicator of inflammatory conditions in MUE.
Our study showed that the NLR was increased in the IE group compared to the healthy group. Our results are consistent with a previous report that showed the NLR to be significantly higher in acute epileptic seizures than in subacute epileptic seizures. 29 Several recent studies also have shown that neuroinflammatory indicators increase after epilepsy. 30 This finding suggests that epilepsy in dogs may involve neuronal inflammation. Therefore, additional investigation of the relationship between the NLR in IE dogs and treatment may identify the influence of anticonvulsant drugs on neuronal inflammation.
The NLR was significantly higher in both the IE and structural groups compared with the healthy group. The additional analysis suggested that a difference exists in the NLR within the structural group. In particular, the NLR of MUE dogs was significantly higher than that of the hydrocephalic dogs. In a study of acute community‐acquired meningitis, it was suggested that the NLR can serve as an additive biomarker for the differential diagnosis of bacterial and viral meningitis. 8 This investigation suggested that the NLR could be influenced by the difference in neuronal inflammation caused by different immune responses. Another study indicated that reactive astrocytosis and microgliosis are associated with progressive ventriculomegaly in hydrocephalus. In MUE, both gliosis and neuronal necrosis are characterized by perivascular cuffing. 31 , 32 Thus, these pathophysiologic variations may contribute to the differences between the NLRs of dogs with MUE and hydrocephalus. In our study, no significant difference was found in the NLR between MUE and BT dogs. It is probable that despite different pathogenesis, brain tumor and autoimmune CNS disease both have pathogenic cells that proliferate excessively and are accompanied by chronic immune system dysfunction. 33 Also, a previous study showed that the NLR is increased in cancer‐associated inflammation and accumulation of inflammatory cells around tumors. 34
Because the antemortem diagnosis of MUE requires MRI and CSF analysis which are expensive and require anesthesia, it can be frustrating for both clients and veterinarians. Substantial differences in treatment methods and prognosis are required for various neurologic diseases and therefore it is important to differentiate these diseases. 2 , 4 , 17 , 35 In our study, the NLR was significantly higher in dogs with MUE than in dogs with IE or hydrocephalus. Therefore, the NLR could be used to differentiate dogs with MUE or BT from dogs with IE or hydrocephalus and it could be a useful marker in determining whether MRI is needed for the early diagnosis of MUE or BT. In addition, the AUC for differentiation between the MUE dogs and dogs suffering from other forebrain diseases showed very good diagnostic accuracy, 20 suggesting that the NLR could be used in the diagnosis of MUE. Furthermore, the NLR can easily be measured by routine blood examination, and it is inexpensive.
Our study had several limitations. First, the small size of each group may not be representative of the remainder of the population, and a larger study could find other associations. Further study of a larger sample size would be beneficial in eliminating or minimizing this limitation. Second, different inclusion criteria were applied to the healthy control group and MUE group; healthy dogs were included in the healthy control group and diseased dogs were included in the MUE group. Studies using healthy controls are at a risk of overestimating the test accuracy. 36 Considering the possibility of overestimating accuracy, comparisons between the MUE group and alternative diagnosis control groups were made in our study. Lastly, MUE is very heterogeneous and has different histological findings for each type, but histopathology was only performed in 2 dogs in our study. Because there is a possibility of various effects on the NLR depending on the MUE type, additional studies to investigate differences in the NLRs according to the type of MUE would be desirable.
In conclusion, the NLR potentially could be used in the diagnosis of MUE. Because the NLR of MUE was higher than that of IE and hydrocephalus, it could be used as a diagnostic tool for distinguishing MUE from other intracranial diseases. Additional prospective investigations are necessary to identify the potential role of the NLR in neuroinflammation of dogs.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Chungbuk National University Animal Care Committee and carried out according to the Guide for Care and Use of Animals (Chungbuk National University Animal Care Committee, CBNUA‐1633‐21‐01), and all the owners were informed about the study.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT), number 2021R1A2C1012058.
Park J, Lee D, Yun T, et al. Evaluation of the blood neutrophil‐to‐lymphocyte ratio as a biomarker for meningoencephalitis of unknown etiology in dogs. J Vet Intern Med. 2022;36(5):1719‐1725. doi: 10.1111/jvim.16512
Funding information National Research Foundation of Korea, Grant/Award Number: 2021R1A2C1012058
REFERENCES
- 1. Uchida K, Park E, Tsuboi M, Chambers JK, Nakayama H. Pathological and immunological features of canine necrotising meningoencephalitis and granulomatous meningoencephalitis. Vet J. 2016;213:72‐77. [DOI] [PubMed] [Google Scholar]
- 2. Mercier M, Barnes Heller HL. Efficacy of glucocorticoid monotherapy for treatment of canine meningoencephalomyelitis of unknown etiology: a prospective study in 16 dogs. Vet Med Sci. 2015;1:16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paušová TK, Tomek A, Šrenk P, Belašková S. Clinical presentation, diagnostic findings, and long‐term survival time in 182 dogs with meningoencephalitis of unknown origin from Central Europe that were administered glucocorticosteroid monotherapy. Top Companion Anim Med. 2021;44:100539. [DOI] [PubMed] [Google Scholar]
- 4. Granger N, Smith PM, Jeffery ND. Clinical findings and treatment of non‐infectious meningoencephalomyelitis in dogs: a systematic review of 457 published cases from 1962 to 2008. Vet J. 2010;184:290‐297. [DOI] [PubMed] [Google Scholar]
- 5. Greer KA, Wong AK, Liu H, et al. Necrotizing meningoencephalitis of pug dogs associates with dog leukocyte antigen class II and resembles acute variant forms of multiple sclerosis. Tissue Antigens. 2010;76:110‐118. [DOI] [PubMed] [Google Scholar]
- 6. Moon JH, Jung HW, Lee HC, et al. A study of experimental autoimmune encephalomyelitis in dogs as a disease model for canine necrotizing encephalitis. J Vet Sci. 2015;16:203‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg N, Smith TW. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015;5:e00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mentis AF, Kyprianou MA, Xirogianni A, et al. Neutrophil‐to‐lymphocyte ratio in the differential diagnosis of acute bacterial meningitis. Eur J Clin Microbiol Infect Dis. 2016;35:397‐403. [DOI] [PubMed] [Google Scholar]
- 9. Macfarlane L, Morris J, Pratschke K, et al. Diagnostic value of neutrophil‐lymphocyte and albumin‐globulin ratios in canine soft tissue sarcoma. J Small Anim Pract. 2016;57:135‐141. [DOI] [PubMed] [Google Scholar]
- 10. Becher A, Suchodolski JS, Steiner JM, Heilmann RM. Blood neutrophil‐to‐lymphocyte ratio (NLR) as a diagnostic marker in dogs with chronic enteropathy. J Vet Diagn Invest. 2021;33:516‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demirci S, Demirci S, Kutluhan S, et al. The clinical significance of the neutrophil‐to‐lymphocyte ratio in multiple sclerosis. Int J Neurosci. 2016;126:700‐706. [DOI] [PubMed] [Google Scholar]
- 12. Benvenuti E, Pierini A, Gori E, Lucarelli C, Lubas G, Marchetti V. Neutrophil‐to‐lymphocyte ratio (NLR) in canine inflammatory bowel disease (IBD). Vet Sci. 2020;7:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henriques J, Felisberto R, Constantino‐Casas F, Cabeçadas J, Dobson J. Peripheral blood cell ratios as prognostic factors in canine diffuse large B‐cell lymphoma treated with chop protocol. Vet Comp Oncol. 2021;19:242‐252. [DOI] [PubMed] [Google Scholar]
- 14. Hodgson N, Llewellyn EA, Schaeffer DJ. Utility and prognostic significance of neutrophil‐to‐lymphocyte ratio in dogs with septic peritonitis. J Am Anim Hosp Assoc. 2018;54:351‐359. [DOI] [PubMed] [Google Scholar]
- 15. Conway EA, Pizarro Del Valle C, Waugh EM, et al. Retrospective investigation of the neutrophil‐to‐lymphocyte ratio in dogs with pneumonia: 49 cases (2011‐2016). J Vet Emerg Crit Care (San Antonio). 2021;31:490‐497. [DOI] [PubMed] [Google Scholar]
- 16. De Risio L, Bhatti S, Muñana K, et al. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet Res. 2015;11:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laubner S, Ondreka N, Failing K, Kramer M, Schmidt MJ. Magnetic resonance imaging signs of high intraventricular pressure—comparison of findings in dogs with clinically relevant internal hydrocephalus and asymptomatic dogs with ventriculomegaly. BMC Vet Res. 2015;11:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bentley RT. Magnetic resonance imaging diagnosis of brain tumors in dogs. Vet J. 2015;205:204‐216. [DOI] [PubMed] [Google Scholar]
- 19. Goldmann F, Bauer N, Moritz A. Evaluation of the IDEXX ProCyte Dx analyzer for dogs and cats compared to the Siemens Advia 2120 and manual differential. Comp Clin Pathol. 2014;23:283‐296. [Google Scholar]
- 20. Simundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19:203‐211. [PMC free article] [PubMed] [Google Scholar]
- 21. D'Amico E, Zanghì A, Romano A, et al. The neutrophil‐to‐lymphocyte ratio is related to disease activity in relapsing remitting multiple sclerosis. Cell. 2019;8:1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yetkin MF, Mirza M. Neutrophil to‐lymphocyte ratio as a possible predictor of prognosis in recently diagnosed multiple sclerosis patients. J Neuroimmunol. 2020;346:577307. [DOI] [PubMed] [Google Scholar]
- 23. Pierson ER, Wagner CA, Goverman JM. The contribution of neutrophils to CNS autoimmunity. Clin Immunol. 2018;189:23‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kanashiro A, Hiroki CH, da Fonseca DM, et al. The role of neutrophils in neuro‐immune modulation. Pharmacol Res. 2020;151:104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steinbach K, Piedavent M, Bauer S, Neumann JT, Friese MA. Neutrophils amplify autoimmune central nervous system infiltrates by maturing local APCs. J Immunol. 2013;191:4531‐4539. [DOI] [PubMed] [Google Scholar]
- 26. Naegele M, Tillack K, Reinhardt S, Schippling S, Martin R, Sospedra M. Neutrophils in multiple sclerosis are characterized by a primed phenotype. J Neuroimmunol. 2012;242:60‐71. [DOI] [PubMed] [Google Scholar]
- 27. van Langelaar J, Rijvers L, Smolders J, van Luijn MM. B and T cells driving multiple sclerosis: identity, mechanisms and potential triggers. Front Immunol. 2020;11:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park ES, Uchida K, Nakayama H. Comprehensive immunohistochemical studies on canine necrotizing meningoencephalitis (NME), necrotizing leukoencephalitis (NLE), and granulomatous meningoencephalomyelitis (GME). Vet Pathol. 2012;49:682‐692. [DOI] [PubMed] [Google Scholar]
- 29. Güneş M, Büyükgöl H. Relationship between generalized epileptic seizure and neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and neutrophil mediated inflammation. Int J Neurosci. 2020;130:1095‐1100. [DOI] [PubMed] [Google Scholar]
- 30. Kostic D, Carlson R, Henke D, Rohn K, Tipold A. Evaluation of IL‐1β levels in epilepsy and traumatic brain injury in dogs. BMC Neurosci. 2019;20:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McAllister JP 2nd. Pathophysiology of congenital and neonatal hydrocephalus. Semin Fetal Neonatal Med. 2012;17:285‐294. [DOI] [PubMed] [Google Scholar]
- 32. Miller JM, McAllister JP 2nd. Reduction of astrogliosis and microgliosis by cerebrospinal fluid shunting in experimental hydrocephalus. Cerebrospinal Fluid Res. 2007;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitchell D, Shireman J, Sierra Potchanant EA, Lara‐Velazquez M, Dey M. Neuroinflammation in autoimmune disease and primary brain tumors: the quest for striking the right balance. Front Cell Neurosci. 2021;15:716947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ashwath KG, Aggarwal A, Praneeth K, Singla N, Gupta K. Neutrophil‐to‐lymphocyte ratio: can it be used as an adjunct tool to predict histopathological grade of brain tumor? J Neurosci Rural Pract. 2019;10:648‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. José‐López R, Gutierrez‐Quintana R, de la Fuente C, et al. Clinical features, diagnosis, and survival analysis of dogs with glioma. J Vet Intern Med. 2021;35:1902‐1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rutjes AW, Reitsma JB, Vandenbroucke JP, et al. Case‐control and two‐gate designs in diagnostic accuracy studies. Clin Chem. 2005;51:1335‐1341. [DOI] [PubMed] [Google Scholar]


