Abstract
Background
Serum symmetric dimethylarginine (SDMA) concentrations are considered a biomarker for renal dysfunction in dogs and humans with acute kidney injury (AKI). No studies have assessed SDMA in cats with AKI.
Hypothesis/Objectives
SDMA correctly identifies cats with azotemic AKI.
Animals
Fifteen control cats, 22 with novel AKI, 13 with acute on chronic‐AKI (AoC) and 19 with chronic kidney disease (CKD).
Methods
Retrospective study. Cats with azotemia (serum creatinine concentrations >1.7 mg/dL) were defined as having AKI or CKD based on history, clinical signs, clinicopathological findings and diagnostic imaging, and classified using the International Renal Interest Society (IRIS) grading/staging systems. Serum SDMA concentrations were compared between groups with nonparametric methods, and correlations assessed using Spearman's correlation coefficient. Data are presented as median [range].
Results
SDMA concentrations were 11 (8‐21) μg/dL, 36 (9‐170)μg/dL, 33 (22‐75) μg/dL and 25 (12‐69) μg/dL in control, novel AKI, AoC and CKD cats. SDMA concentrations were significantly higher in cats with novel AKI (P < .001), AoC (P < .001) and CKD (P < .01) compared to controls. SDMA concentrations were significantly higher in cats with more advanced AKI (IRIS grade IV‐V) compared to less severe AKI (IRIS grade II). Serum creatinine and SDMA concentrations had a significant correlation in cats with novel AKI (r s = 0.826, n = 22; P < .001) and a significant correlation when all cats across all 4 groups were considered together (r s = 0.837, n = 69; P < .001).
Conclusions and Clinical Importance
Serum SDMA concentrations are elevated in cats with established AKI (novel and AoC) and CKD, providing evidence for use of SDMA as a biomarker for AKI in cats.
Keywords: feline, renal biomarker, SDMA
Abbreviations
- AKI
acute kidney injury
- AoC
acute on chronic acute kidney injury
- CKD
chronic kidney disease
- DSH
domestic shorthair
- GFR
glomerular filtration rate
- IRIS
International Renal Interest Society
- RCV
reference change interval
- SDMA
symmetric dimethylarginine
- SIRS
systemic inflammatory response syndrome
- USG
urine specific gravity
1. INTRODUCTION
Acute kidney injury (AKI) is defined as an abrupt reduction in renal filtration and function, characterized by hypercreatinemia and altered urine volume. 1 , 2 Case fatality can reach 74% when renal replacement therapy is required in human AKI, 3 , 4 and 53.1% in conservatively‐treated cats with AKI, 1 despite advances in medical care 5 and the reversibility of early AKI. 6 The gold‐standard for diagnosing a decline in renal function in chronic kidney disease (CKD) is measurement of glomerular filtration rate (GFR) through inulin or iohexol clearance, but this is time‐consuming, technically challenging and impractical. 7 , 8 , 9 , 10 , 11 There is currently no gold‐standard for diagnosing AKI. 5 Current recommendations utilize hypercreatinemia as an estimate of GFR (alongside changes in urine output) to diagnose and grade the severity of AKI. 12 , 13 , 14 , 15 However, hypercreatinemia is an insensitive biomarker of decreased GFR, especially in AKI 16 due to the nonlinear relationship with GFR. 17 Hypercreatinemia is only identified when approximately 75% of renal function has been lost, 7 , 18 and is a late marker of disease (increasing 48‐72 hours after injury) 19 affected by extrarenal factors such as diet, hydration status and muscle mass. 8 , 9 , 18 Creatinine production by muscle decreases in AKI, 20 further reducing the sensitivity of creatinine for detecting AKI.
Previously researched novel veterinary renal biomarkers include urinary or serum cystatin C (cysC), 21 , 22 , 23 and other urinary biomarkers (neutrophil gelatinase‐associated lipocalin [NGAL]), 24 , 25 , 26 kidney injury molecule‐1 (KIM‐1), 27 , 28 interleukins 6 and 18 (IL‐6, IL‐18), 29 , 30 alkaline phosphatase (ALP), 30 , 31 heat‐shock protein (HSP), 16 , 30 retinol‐binding proteins (RBP) 26 , 32 and gamma‐glutamyl transpeptidase (GGT); 30 , 31 however, these biomarkers are not readily available to practitioners and do not serve as robust biomarkers of AKI. Furthermore, many of these are markers of renal cell damage rather than being a measure of function (as for creatinine and symmetric dimethylarginine [SDMA]).
Symmetric dimethylarginine (SDMA) is a by‐product of intranuclear protein methylation carried out by all nucleated cells, 9 primarily excreted by renal filtration 9 and less influenced by muscle mass 9 , 10 , 33 , 34 than serum creatinine concentrations. Increases above reference interval occur when there is a smaller reduction in GFR (average 40% reduction in cats and dogs, 24% in cats alone). 9 , 35 In humans, serum SDMA concentrations correlate highly with GFR as measured by inulin clearance 11 ; in cats, a linear relationship has also been observed between GFR and reciprocal serum SDMA concentration. 10 Previous studies have shown that serum SDMA concentration is considered a marker of early kidney disease in cats, 9 dogs, 36 and humans, 37 and it also increases in dogs with AKI. 6 No studies have yet evaluated serum SDMA concentrations as a biomarker of AKI in cats.
The aims of this study were to evaluate serum SDMA concentrations in cats with azotemic AKI (both novel and acute on chronic), CKD and controls to assess its suitability as a biomarker for AKI, and to assess the correlation between serum creatinine and SDMA concentrations in these groups. We hypothesized that serum SDMA concentrations would be increased in cats with azotemic AKI and CKD, and would be correlated with severity of AKI (as defined by International Renal Interest Society [IRIS] grading), thus confirming it as a marker of severity of renal dysfunction in AKI.
2. MATERIALS AND METHODS
2.1. Case selection
Cases were included after a database search of cats attending a University teaching hospital that had serum creatinine concentration measured between 2016 and 2020. Information was recorded for each case: signalment, age, historical signs, reason for biochemistry analysis, serum creatinine concentration, urinalysis (including urine specific gravity [USG]), imaging findings and survival to 30 days after discharge. Cases were excluded from analysis if records were incomplete, or if no stored residual serum samples were available. Cases were also excluded if clinically relevant comorbidities known to influence serum SDMA concentrations were identified, including systemic inflammation 38 , 39 (documented as elevated serum amyloid A concentrations), hyperthyroidism 40 , 41 and neoplasia. 42 , 43 Formal institutional approval was granted by the ethics and welfare committee at the University teaching hospital.
Control cases included cats that had been referred to the University teaching hospital but had no evidence of azotemia (defined as serum creatinine concentration <1.7 mg/dL [upper limit of laboratory‐specific reference interval]) and that had adequate urine concentrating ability (USG >1.035). To be included, cases must have had hematology, biochemistry and urinalysis performed, with no evidence of systemic inflammation (serum amyloid A concentrations within reference interval [<0.5 mg/dL]).
Cats presenting with azotemia were defined as having novel AKI if they met inclusion criteria of (as defined in a previous study assessing serum SDMA concentrations in dogs with AKI and CKD) 6 ; acute history and clinical signs of AKI in a previously healthy cat, history of exposure to nephrotoxic agents or toxins (including general anesthesia), compatible imaging findings (ie, renomegaly with medullary rim sign), compatible laboratory changes (including, but not limited to, a combination of; azotemia, hyperphosphatemia, metabolic acidosis, hypocalcemia and hyperkalemia), USG <1.035 (unless laboratory changes and imaging findings were present that were compatible with AKI) and a marked improvement in azotemia (>50% reduction in serum creatinine concentration, or resolution of azotemia) within 30 days of discharge (in survivors). For inclusion, cats that were anuric at presentation did not require urinalysis. Cats with suspected pyelonephritis that had a history of recent antibiotic administration did not require a positive urine culture result to be included, if other imaging and laboratory findings were consistent with pyelonephritis (ie, pyelectasia, active urine sediment, clinical and clinicopathological improvement after antibiotic administration). Cats deemed to be hypovolemic (azotemic cats with a USG >1.035 showing an improvement in serum creatinine after normalization of hydration status) were differentiated from cats with AKI (azotemic cats with a USG of <1.035), and the hypovolemic cats were excluded. The AKI group was further graded based on current IRIS guidelines, 13 and according to survival to 30 days after discharge vs nonsurvival to 30 days after discharge.
Cats with previously stable CKD that had an acute deterioration in renal function (suggesting an acute exacerbation) with subsequent resolution to previously stable levels were included in the acute on chronic AKI group (AoC). In order to be classified as AoC (and in accordance with previous studies), cats were included in this group if they had acute onset of compatible clinical signs, alongside a previous diagnosis of CKD (based on persistently elevated serum creatinine concentrations) with a concurrent increase in serum creatinine concentration of >25% above the previously documented baseline, and abdominal ultrasonography findings compatible with CKD (including increased renal echogenicity, reduced corticomedullary differentiation, decreased renal size, or irregular renal contour). 44
Cats with stable CKD were included if they had stable azotemia (defined as a <25% increase in serum creatinine concentrations over a 1 year time period). Cats were included if they were managed with medication (including angiotensin converting enzyme inhibitors), a protein‐restricted prescription diet, or a combination of medication and dietary modification. Newly diagnosed CKD cases were included if azotemia was present on at least 2 samples taken >7 days apart without evidence of dehydration on clinical examination, in conjunction with reduced urine concentrating ability (USG <1.035) and imaging findings consistent with CKD (ie, decreased renal size with loss of corticomedullary definition), if abdominal ultrasonography was performed. Cats with a positive urine culture were excluded from the CKD group due to the possibility of pyelonephritis resulting in AoC (rather than stable CKD).
2.2. SDMA analysis
Blood samples were collected from cats during hospitalization, and serum was harvested within 2 hours of sample collection. Residual serum was frozen and stored at −80°C within 24 hours of biochemical analysis. SDMA concentration was measured from 50 μL serum samples using an immunoassay method as previously described 34 (IDEXX Laboratories, Wetherby, UK).
2.3. Statistical analysis
Data are reported as median [range] unless otherwise specified. Statistical analysis was performed using commercially available software (SPSS v27 and GraphPad Prism). Since the data were not normally distributed (based on Shapiro‐Wilk and Kolgomorov‐Smirnov tests), the Kruskal‐Wallis test with Dunn's multiple comparisons test was used to compare serum creatinine and SDMA concentrations between control, AKI, and CKD groups. The correlation between serum SDMA and creatinine concentrations was determined using Spearman's correlation coefficient. Association between serum creatinine and serum SDMA concentrations with survival to 30 days after discharge were investigated using univariable logistic regression analysis. Statistical significance was defined as P < .05.
3. RESULTS
Two hundred ninety‐seven azotemic cat records were initially screened for study inclusion. Of the records with complete medical data and stored serum, 79 cats with suspected AKI and 36 cats with CKD were initially identified. The main reason for exclusion was subsequent reclassification as hypovolemic (n = 30) or final diagnosis of neoplasia (n = 14) in the AKI group, and comorbid disease such as hyperthyroidism (n = 8), or congestive heart failure (n = 5) and concurrent medication unrelated to CKD treatment such as prednisolone (n = 4) in the CKD group. Sixty‐nine cats were included in the study; 15 control cats, 22 cats with novel AKI, 13 cats with AoC, and 19 cats with CKD. There were 1 male entire, 30 male neutered and 38 female neutered cats with a median age of 8 [0.6‐18.5] years. Seventy‐seven percent of cats were Domestic Short or Longhaired, and the remaining 23% were pure breeds (3 British Shorthaired, 2 Ragdoll, 2 Persian, 2 Siamese, and 1 each of the following breeds: Burmese, Birman, Korat, Egyptian Mau, Bengal, Scottish Fold, and Exotic). Baseline clinicopathological data for all cats at inclusion are summarized in Table 1. Cats with CKD were significantly older than both control cats and cats with AKI, but otherwise there were no significant differences in signalment between the groups.
TABLE 1.
Clinical and clinicopathological data of control cats and cats with AKI and CKD
| Control cats median (range) | Novel AKI cats median (range) | AoC‐AKI cats median (range) | CKD cats median (range) | |
|---|---|---|---|---|
| Age (y) |
7.6 (0.6‐13.8) n = 15 |
6.3 (1.0‐14.0)1 n = 22 |
8.0 (1.20‐16.0) n = 13 |
11.0 (4.8‐18.5)1 n = 19 |
| Bodyweight (kg) |
2.8 (1.7‐5.0) n = 10 |
4.3 (3.8‐6.1) n = 6 |
4.6 (2.6‐5.6) n = 9 |
4.4 (2.7‐5.9) n = 9 |
| Creatinine (mg/dL) |
1.17 (0.80‐1.55)1,2,3 n = 15 |
5.17 (1.73‐20.20)1 n = 22 |
3.99 (2.31‐18.39)2 n = 13 |
2.52 (1.79‐12.76)3 n = 19 |
| USG |
1.043 (1.035‐1.050)1,2,3 n = 15 |
1.023 (1.010‐1.050)1 n = 20 |
1.015 (1.010‐1.030)2 n = 13 |
1.019 (1.008‐1.029)3 n = 19 |
| SDMA (μg/dL) |
11 (8‐21)1,2,3 n = 15 |
36 (9‐170)1 n = 22 |
33 (20‐75)2,4 n = 13 |
19 (12‐69)3,4 n = 19 |
Note: Kruskal‐Wallis test with post hoc Dunn's test for multiple comparisons was performed. Values with same superscript numbers are significantly different to one another.
Abbreviations: AKI, acute kidney injury; AoC, acute on chronic acute kidney injury; CKD, chronic kidney disease; SDMA, serum symmetric dimethylarginine; USG, urine specific gravity.
Twenty‐two cats with AKI (63%) had no previous history of CKD (novel AKI group) and 13 cats with AKI (37%) developed AKI as an acute on chronic episode (AoC group). Within the novel AKI group, 36% (n = 8) were classified as IRIS grade II AKI, 9% (n = 2) as IRIS grade III AKI, 32% (n = 7) as IRIS grade IV AKI, and 23% (n = 5) as IRIS grade V AKI. Within the AoC group, 23% (n = 3) were classified as IRIS grade II AKI, 31% (n = 4) as IRIS grade III AKI, 23% (n = 3) as IRIS grade IV AKI, and 23% (n = 3) as IRIS grade V AKI. The underlying cause for AKI (when both groups were considered together) was identified in 30/35 cats, and included ureteral obstruction (n = 11), urethral obstruction (n = 7), urogenital tract trauma (n = 2), pyelonephritis (n = 3), recent general anesthesia (n = 1), NSAID toxicosis (n = 2), sepsis or systemic inflammatory response syndrome (SIRS; n = 2), and renal thromboembolism (n = 2).
Within the CKD group, 52% (n = 10) were classified as IRIS stage 2 CKD, 32% (n = 6) were classified as IRIS stage 3 CKD, and 16% (n = 3) were classified as IRIS stage 4 CKD.
Serum SDMA concentrations were significantly higher in cats with novel AKI (36 [9‐170] μg/dL; P < .001), AoC (33 [20‐75] μg/dL; P < .001) and CKD (25 [12‐69] μg/dL; P < .01) compared to control cats (11 [8‐21]μg/dL). There was no difference in serum SDMA concentrations between cats with novel AKI or AoC (P = .422), or between cats with novel AKI and CKD (P = .132), but there was a significantly higher serum SDMA concentration in cats with AoC than cats with CKD (P = .003). Serum SDMA concentrations were within reference intervals in 4/22 cats with novel AKI and 5/19 cats with CKD, and were elevated in 3/15 control cats. When considering the novel AKI group only (Figure 1), serum SDMA concentrations were significantly higher in cats with IRIS grade IV AKI (45 [13‐69] μg/dL; P = .015) and IRIS grade V AKI (70 [40‐170] μg/dL; P = .003) compared with cats with IRIS grade II AKI (16 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 μg/dL). There were only 2 cats with IRIS grade III AKI, which had SDMA concentrations of 9 μg/dL and 18 μg/dL. Serum SDMA concentrations were significantly higher in cats with IRIS stage 4 CKD compared to cats with IRIS stage 2 CKD (44 [27‐69] μg/dL vs 17 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 μg/dL; P < .05). Serum SDMA concentrations in cats with IRIS stage 3 CKD (27 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 μg/dL) were not significantly different to those of cats with IRIS stage 2 or 4 CKD.
FIGURE 1.
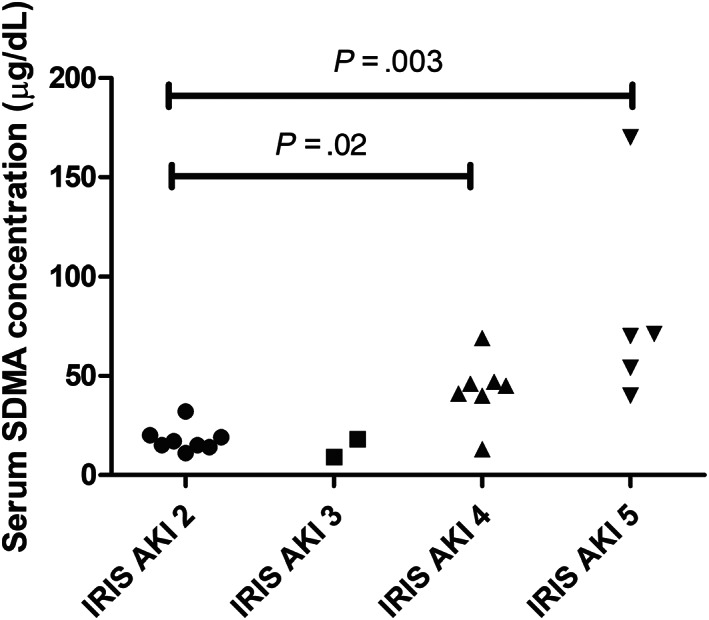
Serum SDMA concentrations in cats with novel AKI, stratified by IRIS grade. The Kruskal‐Wallis test with Dunn's multiple comparisons test was used to compare serum SDMA concentrations between groups. AKI, acute kidney injury; SDMA, serum symmetric dimethylarginine
Serum creatinine and SDMA concentrations showed a strong positive correlation in the novel AKI group (r s = 0.826, n = 22; P < .001) and a strong positive correlation when all cats across all 4 groups were considered together (r s = 0.837, n = 69; P < .001).
Survival data (alive or dead 30 days after discharge) were available for all cats in the novel AKI group. Fifteen cats with novel AKI (68%) survived to 30 days after discharge and 7 cats with novel AKI (32%) did not survive to 30 days after discharge. Serum SDMA concentration, serum creatinine concentration and serum potassium concentration were not associated with nonsurvival to 30 days after discharge (Table 2).
TABLE 2.
Univariable logistic regression analysis of the association between baseline serum SDMA, creatinine and potassium concentrations in cats with novel AKI, with survival to 30 days postdischarge
| Confidence interval (CI) 95% | ||||
|---|---|---|---|---|
| Odds ratio | Lower | Upper | P‐value | |
| SDMA | 1.003 | 0.976 | 1.031 | .82 |
| Creatinine | 1.000 | 0.998 | 1.002 | .83 |
| Potassium | 1.395 | 0.572 | 3.403 | .46 |
Abbreviations: AKI, acute kidney injury; SDMA, serum symmetric dimethylarginine.
4. DISCUSSION
In this study, we retrospectively evaluated serum SDMA concentrations in cats with known azotemic AKI, to assess whether serum SDMA concentration is a useful biomarker of renal dysfunction in AKI. Serum SDMA and creatinine concentrations were significantly higher in cats with renal azotemia (due to both novel AKI and AoC, and CKD) compared to control cats. Both serum creatinine and SDMA concentrations are markers of GFR 9 , 10 although serum creatinine concentration is affected by some extrarenal factors in human and veterinary species 8 which could make it a weaker biomarker. 8 Serum SDMA concentrations are elevated in dogs with AKI 6 and humans with reduced GFR 11 ; however, this is the first study to demonstrate that serum SDMA concentrations are elevated in cats with AKI. Given that many cats in this study with AKI had no prior history of renal dysfunction (or thus reduced GFR), this provides proof of concept for further studies evaluating the utility of serum SDMA concentrations as an early biomarker for diagnosis of nonazotemic AKI (grade I) in cats, before traditional markers of renal dysfunction such as serum creatinine concentrations are elevated.
Serum SDMA concentrations were significantly higher in cats with more severe AKI (demonstrated by higher IRIS AKI grades) compared to less severe AKI (demonstrated by lower IRIS AKI grades). 13 Although this has not been demonstrated beforehand in cats, this is an expected result given that AKI is defined as an abrupt reduction in renal filtration 1 and serum SDMA concentration is a marker of filtration with a linear relationship to GFR. 8 , 9 , 10 In this study serum SDMA concentration was different between cats with IRIS grade IV/V AKI and cats with IRIS grade II AKI. This demonstrates that as well as being elevated in cats with AKI, the degree of elevation in serum SDMA concentrations is proportional to the degree of renal dysfunction present.
In this study, serum SDMA concentration had a strong positive correlation with serum creatinine concentrations in novel AKI cats. Serum concentrations of SDMA and creatinine also showed strong positive correlation in all groups (control, CKD, AoC and novel AKI). The strength of this correlation with serum creatinine concentration in AKI cats suggests that serum SDMA concentration is a biomarker of azotemic AKI in cats. We also postulated that the significant correlation of serum SDMA concentration with serum creatinine concentration seen across all groups supports the correct categorization of cases retrospectively and reduces the chance that cases were misclassified, thus validating the stringent inclusion criteria laid out in the materials and methods. Compared to this study, a previous study found a weaker positive correlation between serum concentrations of SDMA and creatinine (r = 0.4) in dogs with AKI compared to CKD (r = 0.74), despite the fact that this correlation would be expected to be the converse since lower muscle mass in dogs with CKD would be expected to weaken this relationship. 6 It has previously also been suggested that extrarenal factors can influence serum SDMA concentrations in dogs with both AKI and CKD. 6 Furthermore, it could be postulated that the cats with AKI in our study presented at a later stage relative to the Dahlem study, thus allowing time for both markers to equilibrate in the extracellular fluid or total body water after a decline in GFR, which could account for the stronger correlation in our study sample. However, due to the retrospective nature of this study, we cannot be certain of when the insult resulting in AKI occurred and therefore cannot draw any conclusions about whether timing of presentation has affected the correlation between serum concentrations of SDMA and creatinine in cats with AKI in this study.
In this study, some cats with both AKI and CKD had a serum SDMA concentration within the reference interval, despite fulfilling the inclusion criteria of azotemia (4/22 cats in the novel AKI group, 18%; 5/19 cats with CKD; 26%). Some of nonazotemic control cats also had serum SDMA concentrations outside of the reference interval (3/15 control cats, 20%). This has also been reported in a previous study assessing SDMA in dogs with AKI and CKD 6 where the discrepancy was postulated to be due to laboratory error, or normalization of GFR before normalization of serum creatinine concentrations after renal injury (where serum creatinine concentration usually lags behind). 6 In contrast, a previous study identified serum SDMA concentrations as having a sensitivity of 100% and specificity of 91% for CKD in cats, and no cats with high serum creatinine concentrations had normal serum SDMA concentrations. 9 We postulate that the discrepancy between serum SDMA and creatinine concentrations in our study reflects the fact that no test has 100% sensitivity or specificity, and the normal serum SDMA concentrations in some AKI and CKD cats were therefore considered to be false negatives. The cats with normal serum SDMA concentrations in our study had SDMA concentrations near the upper limit of the reference interval (novel AKI 12 [9‐14] μg/dL; CKD 13 [12‐13] μg/dL), which could reflect analytical or biological variability. Equally, the cats with elevated serum SDMA concentrations in our control group had SDMA concentrations close to the upper limit of the reference interval (SDMA 17 [15‐21] μg/dL). Previous studies have documented a biological variability of serum SDMA concentrations in healthy geriatric cats of 1.54 μg/dL, 9 so it is possible that these values within (and outside) the reference interval in our study (for azotemic and control cats respectively) are due to normal biological variability. It is possible that despite a measured serum SDMA concentration within the reference interval, due to intraindividual variability there could have been a clinically relevant increase in serum SDMA concentration. Serum SDMA concentration has lower interindividual variability compared to serum creatinine concentrations (due to effects of age, sex and lean muscle mass on serum creatinine concentrations) but increased intraindividual variability, leading to the limitation that if a serum SDMA concentration falls within the population‐based reference interval but outside of the individual homeostatic setpoint for SDMA, it could be falsely interpreted as normal (ie, a false negative result). 45 In a clinical setting, previous measurements of serum creatinine and SDMA on file from previous health checks can be used to assess what is considered to be normal for an individual animal and can be used to assess clinically relevant changes by use of reference change values (RCVs). Use of RCVs for serum biomarkers (including serum creatinine and SDMA) improves their specificity and sensitivity as markers of GFR in individual animals, thus alleviating some of the inherent inaccuracies of clinical biomarkers in individual animals that are analyzed based on population‐based reference intervals. Our findings could also reflect dispersion, which is the range of possible results that are feasible for a given sample based on a combination of analytical and biological variation. Dispersion is at least 40% for a serum SDMA concentration (both point of care and commercial laboratory measurements) of 14 μg/dL in cats, which means that a measured serum SDMA concentration of greater than or equal to 20 μg/dL is required to have confidence (with a 95% statistical probability) that serum SDMA concentrations are truly increased above the reference interval. 46 Equally a cat with subnormal GFR could have a serum SDMA concentration as low as 8 μg/dL. 46 Dispersion and biological variability applies to azotemic as well as control cats in this study.
No baseline serum biochemical biomarker (serum SDMA, creatinine, potassium concentration) was associated with nonsurvival to 30 days after discharge. This study also reported a higher AKI survival rate (68%) than previous studies (36%‐47%). 47 , 48 Disease etiology is a known prognostic factor in AKI 47 , 49 , 50 , 51 , 52 with obstructive cases having a higher reported survival rate than infectious, hemodynamic and toxic causes. 53 Although we postulated that the higher AKI survival rate seen in our study could be due to the high proportion of cats with AKI secondary to urinary obstruction (ureterolithiasis and urolithiasis—12/22 cats with novel AKI, 55%), no significant association was found when survival to 30 days after discharge was compared between cats with obstructive or nonobstructive AKI (data not shown). We postulated that the lack of significant association between serum biochemical markers and survival documented in our study (which differs to what has been previously reported), 47 , 53 is most likely related to the small study size, thus increasing the likelihood of type 2 error. Serum potassium concentration is a prognostic indicator in cats with AKI, with a 57% decrease in chance of survival seen with every unit increase (mEq/L) in potassium. 47 Oliguria and anuria are negative prognostic indicators in AKI, 47 and decreased urine production leads to hyperkalemia, given that renal excretion is the major mechanism for removing potassium from the body, 54 and thus any AKI etiology that leads to prolonged oliguria/anuria is likely to predispose to hyperkalemia. It is therefore postulated that the improved survival documented in this study related to timely relief of obstructive AKI; given that serum potassium concentration was not significantly elevated in this group of cats when compared to the nonobstructive cohort (data not shown), which may indicate that these cats did not demonstrate clinically relevant oliguria/anuria (especially if unilateral ureteral obstruction was present, which we documented as a common cause of obstructive AKI this cohort of cats). However, timing of obstruction relief and comparison to survival was not specifically evaluated in this study, due to the retrospective nature of the study design. The results from this study are, however, in keeping with 2 previous studies assessing prognostic factors in cats with AKI, which found no association with serum potassium or creatinine concentrations. 48 , 52
Despite finding significant differences in serum SDMA concentrations in cats with AKI, this study has several limitations. The first limitation is the retrospective nature of the study, and the small number of cases included, thus increasing the possibility of type 2 error. Future prospective or multicenter studies are required to recruit a larger number of cases for further analysis. Despite the small sample size, AKI was defined using stringent inclusion criteria to try and reduce the risk of falsely including cases that were hypovolemic/dehydrated (ie, with prerenal causes of azotemia) rather than suffering from renal injury (AKI). In order to differentiate, we defined AKI as an increasing serum creatinine concentration despite fluid therapy and normal hydration status. It is, however, possible that some of the prerenal azotemic cats which were excluded did have AKI. It has previously been documented that serum SDMA concentrations can be influenced by prerenal factors and volume status, 55 thus highlighting the importance of assessing a concurrent USG (where available) in order to exclude cats with prerenal azotemia from our study. Concurrent USG was an inclusion criterion in this study, and cats with concentrated urine were excluded from the AKI group in order to avoid falsely including dehydrated or hypovolemic cats that did not have AKI but might have had an elevated SDMA concentration due to prerenal azotemia alone. Due to the retrospective nature of the study, it was also unknown when serum samples were obtained in relation to the onset of renal injury, which could influence the strength of the correlation between serum SDMA and creatinine concentrations; ideally future prospective studies are required to standardize timing of samples. In addition, AKI cases with concurrent systemic inflammation (defined as an elevated serum amyloid A concentrations) were excluded. Although some underlying etiologies of AKI can be proinflammatory, and this exclusion criteria could therefore have led to less AKI cases being included, these cases were excluded because systemic inflammatory disorders are associated with increased serum SDMA concentrations 38 , 39 even in nonazotemic cats, which could therefore confound our SDMA results. Ideally, future prospective studies are required in order to include cases with AKI due to a proinflammatory etiology.
GFR was also not directly measured in the present study; AKI and CKD were both defined by the presence of azotemia (ie, elevated urea and creatinine) for study inclusion. However, serum creatinine concentrations are known to be an insensitive marker for reduced renal function; gold standard measurement of renal function in CKD includes GFR assessment via inulin or iohexol clearance 7 , 8 , 9 , 10 , 11 but this was not carried out in our cohort of cats. Serum creatinine and SDMA concentrations are not an accurate reflection of GFR in AKI (when there is no steady‐state equilibrium). 6 , 12 This ultimately means that any new renal biomarker that is assessed (such as SDMA in this paper) is compared to serum creatinine concentrations, a suboptimal marker of renal function. It has long been known that serum creatinine concentration is only elevated when greater than 75% of renal function has been lost 7 , 18 ; as such, a large proportion of cats with early AKI will be missed in clinical practice, and will not have been included in this study. However, our study design allowed assessment of serum SDMA concentrations as a novel biomarker in cats with known/advanced AKI, in order to prove that serum SDMA concentrations are correlated with serum creatinine concentration in this cohort of cats. Further studies are required to assess serum SDMA concentrations as an early biomarker of AKI in prospective studies.
Obstructive causes of AKI were included in the study design, in order to evaluate the serum SDMA concentrations in a representative cohort of cases likely to be seen in clinical practice. It is possible that these cases could have hypercreatinemia simply as a result of decreased GFR associated with obstruction rather than AKI per se. However, even in human medicine, there is a lack of a gold standard to define AKI, and GFR measurement is only appropriate in well‐hydrated stable patients. There is difficulty distinguishing between intrinsic renal damage (histological AKI) and physiological decreases in renal perfusion (functional AKI) in a clinical setting. 5 It is likely that a subset of these cats included in our study will have developed intrinsic renal injury secondary to obstructive AKI.
Finally, due to the retrospective nature of this study, euthanasia is likely to have influenced case fatality rates. Unfortunately and due to the often delayed detection of AKI in dogs and cats, prognosis can be guarded, and this is likely to affect client decisions about whether to pursue treatment.
Future studies of serum SDMA concentrations in cats with AKI would appear warranted on the basis of our findings. Serum SDMA concentrations were not sequentially measured during hospitalization or the recovery phase to assess whether this provides any further prognostic information, or indicates cats which are more likely to survive AKI, due to the retrospective nature of the study and the inconsistency of sample timings. This was considered to be outside the scope of this current study; however, this would be interesting to assess in future studies. In addition, given that our study has indicated that serum SDMA concentrations correlate with serum creatinine concentrations in a cohort of cats known to have AKI, further studies are required to analyze serum SDMA concentrations in cats with early AKI (including hospital‐acquired and IRIS grade I AKI) to explore if elevated serum SDMA concentrations are a more sensitive biomarker of AKI.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of Cambridge ethics and welfare committee (CR462), and Royal Canin (AKI_2021_02_V2).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by Royal Canin, PNKH.GAAA, and the University of Cambridge (senior clinical training scholarship funding), PNKI.HAHH.
Loane SC, Thomson JM, Williams TL, McCallum KE. Evaluation of symmetric dimethylarginine in cats with acute kidney injury and chronic kidney disease. J Vet Intern Med. 2022;36(5):1669‐1676. doi: 10.1111/jvim.16497
Funding information Royal Canin; University of Cambridge
REFERENCES
- 1. Legatti S, El Dib R, Legatti E, et al. Acute kidney injury in cats and dogs: a proportional meta‐analysis of case series studies. PLoS One. 2018;13:e0190772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hilton R. Clinical review—acute renal failure. BMJ. 2006;333(7572):786‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Metnitz P, Krenn C, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051‐2058. [DOI] [PubMed] [Google Scholar]
- 4. Metcalfe W, Simpson M, Khan I, et al. Acute renal failure requiring renal replacement therapy: incidence and outcome. QJM Int J Med. 2002;95(9):579‐583. [DOI] [PubMed] [Google Scholar]
- 5. Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in‐depth review of the literature. Nephrol Dial Transplant. 2013;28(2):254‐273. [DOI] [PubMed] [Google Scholar]
- 6. Dahlem DP, Neiger R, Schweighauser A, et al. Plasma symmetric dimethylarginine concentration in dogs with acute kidney injury and chronic kidney disease. J Vet Intern Med. 2017;31(3):799‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Von Hendy‐Willson VE, Pressler BM. An overview of glomerular filtration rate testing in dogs and cats. Vet J. 2011;188(2):156‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yerramilli M, Farace G, Quinn J, Yerramilli M. Kidney disease and the nexus of chronic kidney disease and acute kidney injury—the role of novel biomarkers as early and. Vet Clin North Am Small Anim Pract. 2016;46:961‐993. [DOI] [PubMed] [Google Scholar]
- 9. Hall JA, Yerramilli M, Obare E, Yerramilli M, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med. 2014;28(6):1676‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braff J, Obare E, Yerramilli M, Elliott J, Yerramilli M. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J Vet Intern Med. 2014;28(6):1699‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kielstein JT, Salpeter SR, Bode‐Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—a meta‐analysis. Nephrol Dial Transplant. 2006;21(9):2446‐2451. [DOI] [PubMed] [Google Scholar]
- 12. Bellomo R, Ronco C, Kellum J, Mehta R, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8(4):R204‐R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cowgill LD. IRIS Grading of Acute Kidney Injury . University of California; 2016. http://www.iris-kidney.com/. Accessed October 8, 2021.
- 14. Fliser D, Laville M, Covic A, et al. A European Renal Best Practice (ERBP) position statement on the kidney disease improving global outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast‐induced nephropathy. Nephrol Dial Transplant. 2012;27(12):4263‐4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta R, Kellum J, Shah S, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen H, Avital Y, Bruchim Y, Aroch I, Segev G. Urinary heat shock protein‐72: a novel marker of acute kidney injury and chronic kidney disease in cats. Vet J. 2019;243:77‐81. [DOI] [PubMed] [Google Scholar]
- 17. Finco D, Brown S, Vaden S, Ferguson D. Relationship between plasma creatinine concentration and glomerular filtration rate in dogs. J Vet Pharmacol Ther. 1995;18(6):418‐421. [DOI] [PubMed] [Google Scholar]
- 18. Braun JP, Lefebvre HP, Watson ADJ. Creatinine in the dog: a review. Vet Clin Pathol. 2003;32(4):162‐179. [DOI] [PubMed] [Google Scholar]
- 19. Slocum JL, Heung M, Pennathur S. Marking renal injury: can we move beyond serum creatinine? Transl Res. 2012;159(4):277‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson FP, Sheehan JM, Mariani LH, Berns JS. Creatinine generation is reduced in patients requiring continuous venovenous hemodialysis and independently predicts mortality. Nephrol Dial Transplant. 2012;27(11):4088‐4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghys LFE, Paepe D, Duchateau L, et al. Biological validation of feline serum cystatin C: the effect of breed, age and sex and establishment of a reference interval. Vet J. 2015;204(2):168‐173. [DOI] [PubMed] [Google Scholar]
- 22. Ghys L, Paepe D, Smets P, Lefebvre H, Delanghe J, Daminet S. Cystatin C: a new renal marker and its potential use in small animal medicine. J Vet Intern Med. 2014;28(4):1152‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monti P, Benchekroun G, Berlato D, Archer J. Initial evaluation of canine urinary cystatin C as a marker of renal tubular function. J Small Anim Pract. 2012;53(5):254‐259. [DOI] [PubMed] [Google Scholar]
- 24. Lee YJ, Hu YY, Lin YS, et al. Urine neutrophil gelatinase‐associated lipocalin (NGAL) as a biomarker for acute canine kidney injury. BMC Vet Res. 2012;8:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu PH, Hsu WL, Tsai PSJ, Wu VC, Tsai HJ, Lee YJ. Identification of urine neutrophil gelatinase‐associated lipocalin molecular forms and their association with different urinary diseases in cats. BMC Vet Res. 2019;15(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nabity MB, Lees GE, Cianciolo R, Boggess MM, Steiner JM, Suchodolski JS. Urinary biomarkers of renal disease in dogs with X‐linked hereditary nephropathy. J Vet Intern Med. 2012;26(2):282‐293. [DOI] [PubMed] [Google Scholar]
- 27. Bland SK, Schmiedt CW, Clark ME, DeLay J, Bienzle D. Expression of kidney injury molecule‐1 in healthy and diseased feline kidney tissue. Vet Pathol. 2017;54(3):490‐510. [DOI] [PubMed] [Google Scholar]
- 28. Bland SK, Clark ME, Côté O, Bienzle D. A specific immunoassay for detection of feline kidney injury molecule 1. J Feline Med Surg. 2019;21(12):1069‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen H, Avital Y, Aroch I, Segev G. Interleukin 6 and interleukin 18 as markers of kidney injury in dogs. In: Research communications of the 27th ECVIM‐CA congress. J Vet Intern Med. 2017;32:562‐563. [Google Scholar]
- 30. Nivy R, Chaim N, Hanael E, et al. Prospective evaluation of 5 urinary biomarkers as predictors of acute kidney injury in nonazotemic, hospitalized dogs. J Vet Intern Med. 2021;1–9:2812‐2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nivy R, Avital Y, Aroch I, Segev G. Utility of urinary alkaline phosphatase and γ‐glutamyl transpeptidase in diagnosing acute kidney injury in dogs. Vet J. 2017;220:43‐47. [DOI] [PubMed] [Google Scholar]
- 32. Segev G, Daminet S, Meyer E, et al. Characterization of kidney damage using several renal biomarkers in dogs with naturally occurring heatstroke. Vet J. 2015;206(2):231‐235. [DOI] [PubMed] [Google Scholar]
- 33. Hall JA, Yerramilli M, Obare E, Yerramilli M, Melendez LD, Jewell DE. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J Vet Intern Med. 2015;29(3):808‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sargent HJ, Elliott J, Jepson RE. The new age of renal biomarkers: does SDMA solve all of our problems? J Small Anim Pract. 2020;62(2):71‐81. [DOI] [PubMed] [Google Scholar]
- 35. Relford R, Robertson J, Clements C. Symmetric dimethylarginine: improving the diagnosis and staging of chronic kidney disease in small animals. Veterinary Clinics of North America—Small Animal Practice. W.B. Saunders; 2016;46:941‐60. [DOI] [PubMed] [Google Scholar]
- 36. Hall JA, Yerramilli M, Obare E, Yerramilli M, Almes K, Jewell DE. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J Vet Intern Med. 2016;30(3):794‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dixon J, Lane K, Dalton R, MacPhee I, Philips B. Symmetrical dimethylarginine is a more sensitive biomarker of renal dysfunction than creatinine. Crit Care. 2013;17(Suppl 2):P423. [Google Scholar]
- 38. Koch A, Weiskirchen R, Bruensing J, et al. Regulation and prognostic relevance of symmetric dimethylarginine serum concentrations in critical illness and sepsis. Mediators Inflamm. 2013;2013:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Köster LS, Peda A, Fraites T, Sithole F. A preliminary investigation into the prognostic relevance of symmetric dimethylarginine in critically ill dogs. J Vet Emerg Crit Care. 2018;28(6):527‐531. [DOI] [PubMed] [Google Scholar]
- 40. Peterson ME, Varela FV, Rishniw M, Polzin DJ. Evaluation of serum symmetric dimethylarginine concentration as a marker for masked chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med. 2018;32:295‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Covey H, Chang Y, Elliott J, Syme H. Changes in thyroid and renal function after bilateral thyroidectomy in cats. J Vet Intern Med. 2019;33(2):508‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yerramilli M, Yerramilli M, Farace G, et al. Symmetric dimethylarginine (SDMA) as kidney biomarker in canine and feline cancer. J Vet Intern Med. 2017;31:251. [Google Scholar]
- 43. Abrams‐Ogg A, Rutland B, Levis P, et al. Lymphoma and symmetric dimethylarginine concentration in dogs: a preliminary study. J Vet Intern Med. 2017;31:1584‐1585. [Google Scholar]
- 44. Dunaevich A, Chen H, Musseri D, et al. Acute on chronic kidney disease in dogs: etiology, clinical and clinicopathologic findings, prognostic markers, and survival. J Vet Intern Med. 2020;34(6):2507‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kopke MA, Burchell RK, Ruaux CG, Burton SE, Lopez‐Villalobos N, Gal A. Variability of symmetric dimethylarginine in apparently healthy dogs. J Vet Intern Med. 2018;32(2):736‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baral RM, Freeman KP, Flatland B. Comparison of serum and plasma SDMA measured with point‐of‐care and reference laboratory analysers: implications for interpretation of SDMA in cats. J Feline Med Surg. 2021;23(10):906‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Worwag S, Langston CE. Acute intrinsic renal failure in cats: 32 cases (1997‐2004). J Am Vet Med Assoc. 2008;232(5):728‐732. [DOI] [PubMed] [Google Scholar]
- 48. Lee YJ, Chan JPW, Hsu WL, Lin KW, Chang CC. Prognostic factors and a prognostic index for cats with acute kidney injury. J Vet Intern Med. 2012;26(3):500‐505. [DOI] [PubMed] [Google Scholar]
- 49. Ettinger SJ, Feldman EC, Côté E. Textbook of Veterinary Internal Medicine. 8th ed. St Louis, Missouri: Elsevier; 2017. [Google Scholar]
- 50. Adin CA, Cowgill LD. Treatment and outcome of dogs with leptospirosis: 36 cases (1990‐1998). J Am Vet Med Assoc. 2000;216(3):371‐375. [DOI] [PubMed] [Google Scholar]
- 51. Kyles AE, Hardie EM, Wooden BG, et al. Management and outcome of cats with ureteral calculi: 153 cases (1984‐2002). J Am Vet Med Assoc. 2005;226(6):937‐944. [DOI] [PubMed] [Google Scholar]
- 52. Segev G, Nivy R, Kass PH, Cowgill LD. A retrospective study of acute kidney injury in cats and development of a novel clinical scoring system for predicting outcome for cats managed by hemodialysis. J Vet Intern Med. 2013;27(4):830‐839. [DOI] [PubMed] [Google Scholar]
- 53. Eatroff A, Langston C, Chalhoub S, Poeppel K, Mitelberg E. Long‐term outcome of cats and dogs with acute kidney injury treated with intermittent hemodialysis: 135 cases (1997‐2010). J Am Vet Med Assoc. 2012;241(11):1471‐1478. [DOI] [PubMed] [Google Scholar]
- 54. Chew D, Gieg J. Managing fluid and electrolyte disorders in renal failure. In: DiBartola SP, ed. Fluid, Electrolyte and Acid‐Base Disorders in Small Animal Practice. 3rd ed. St Louis: Saunders Elsevier; 2006:518‐540. [Google Scholar]
- 55. Choi W, Kang JH, Woo S, et al. Serum concentrations of symmetric dimethylarginine in dogs with dehydration. J Vet Intern Med. 2017;31:1324. [Google Scholar]


