Abstract
Background
Whether domestic cat hepadnavirus (DCH) infection is associated with clinical disease remains to be determined.
Objectives
To determine the relationship between DCH detection, hematology, serum bichemistry and liver histology in DCH‐positive cats.
Animals
One thousand twenty‐two cats in Thailand without concurrent diseases and not undergoing treatments adversely affecting the liver.
Methods
Retrospective cross‐sectional study. Samples derived from cats with concurrent virus detection were excluded. DCH detection was determined in blood and fresh‐frozen liver by quantitative polymerase chain reaction (qPCR) and further investigated in liver sections showing histological parenchymal disorders (HPD) and normal liver (HNL) using in situ hybridization (ISH). Proliferative/apoptotic activities were determined using immunohistochemistry and ISH panels. Biochemical variables and risk factors for DCH infection were investigated.
Results
Six hundred sixty‐one (557 blood and 119 liver samples) cats were included. DCH was detected in 18.50% (103/557), 13.85% (9/65), and 3.70% (2/54) of the blood, HPD, and HNL groups, respectively. Cats with DCH revealed abnormally high activity of aspartate aminotransferase (AST) (P = .001) and alanine aminotransferase (ALT) (P < .001). Among DCH‐positive HPD case 2/9 an 7/9 were acute and chronic hepatitis, of which 4/7 had hepatitis. Log viral copy number (LVCN) was positively correlated with ALT (P < .001), triglyceride (P < .001), and gamma‐glutamyl transpeptidase (GGT) (P = .022). The LVCN also had a positive association with degree of hepatitis (P < .05). There was hepatocyte proliferation activity in DHC positive cats.
Conclusion and Clinical Importance
Domestic cat hepadnavirus infection was associated with high serum activity of liver enzymes and chronic lymphoplasmacytic hepatitis (LPH).
Keywords: domestic cat hepadnavirus, hepatitis, hepatocyte proliferation, real‐time polymerase chain reaction, viral localization
Abbreviations
- ALB
albumin
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ARRIVE
Animal Research: Reporting of In Vivo Experiments
- AST
aminotransferase
- CBC
complete blood count
- CBoV‐2
canine bocavirus‐2
- DCH
domestic cat hepadnavirus
- DIG
digoxigenin
- DSH
domestic short hair
- EDTA
ethylenediaminetetraacetic acid
- FCoV
feline coronavirus
- FCV
feline calicivirus
- FeLV
feline leukemia virus
- FFPE
formalin‐fixed paraffin‐embedded
- FIV
feline immunodeficiency virus
- GGT
gamma‐glutamyl transpeptidase
- HBV
hepatitis B virus
- HNL
histologically normal liver
- HPD
histologically parenchymal disorder
- IHC
immunohistochemistry
- ISH
in situ hybridization
- LPH
lymphoplasmacytic hepatitis
- LVCN
log viral copy number
- MCH
mean corpuscular hemoglobin
- MCV
mean corpuscular volume
- PCNA
proliferating cell nuclear antigen
- qPCR
quantitative polymerase chain reaction
- RT
reverse‐transcription
- Rt
room temperature
- SSC
saline‐sodium citrate
- TP
total protein
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick‐end labeling
1. INTRODUCTION
Hepadnaviruses are blood‐borne viral members of the Hepadnaviridae family, which are associated with hepatobiliary diseases in various vertebrates. Aside from a well‐known mammalian hepadnavirus that infects humans, namely hepatitis B virus (HBV), the other novel mammalian hepadnavirus, called domestic cat hepadnavirus (DCH), was first discovered in an Australian domestic cat in 2018 during a metagenomic study. 1 The emergence of DCH was subsequently reported in several countries including Italy, United States, United Kingdom, New Zealand, Thailand, and Malaysia. 1 , 2 , 3 , 4 , 5 The prevalence of DCH varies across countries, ranging from 10% to 20%, but the prevalence of infection was highest in cats with concomitant infection, especially with feline retroviruses. 3 , 4
Although hepadnaviruses are hepatotropic viruses, the impact of DCH in feline health has not been fully investigated and requires further studies. 2 Investigations of DCH in archival samples of hepatic‐related disease collections using genomic‐based detection such as polymerase chain reaction (PCR) and in situ hybridization (ISH) have indicated the presence of DCH in liver tissues, which presented either chronic hepatitis or hepatocellular carcinoma (HCC) but not in the cases with purely cholangitis or cholangiocarcinoma. 2 In cases positive for DCH detection, the histological features and inflammatory reaction in liver parenchyma were similar to what has been described in HBV‐infected humans. Furthermore, studies have provided compelling evidence of higher cellular proliferative index in the regions of DCH localization in a small number of HCC cases; however, the proliferative indices have been not investigated in DCH‐cases associated with hepatitis, and neither concomitant infection nor other factors that could cause secondary liver inflammation were screened out. 2 Apart from genomic detection, DCH particles were also visualized in hepatocytes of cats showing chronic hepatitis and fibrosis. 3 These findings could indicate an association between DCH infection and parenchymal disorders in cats.
Evidence revealing that DCH has been found in liver samples corresponded with epidemiological data of DCH in cats, indicating that it was more prevalent in cats showing abnormal hepatic biochemical indices suggesting liver damage. 4 , 5 However, most DCH‐positive cats had concomitant infections, which also play a role in alteration in serum activity of liver enzyme. 1 , 3 , 4 , 5 Interpretation of DCH's association with chronic hepatitis in cats is, therefore, cautioned because the present studies did not elucidate the concomitant coinfection. 2 Taken together, under the current situation of lacking in vitro or in vivo investigations for DCH infection to comply with Koch's postulation, the definitive role of DCH in feline liver disease warrants further investigation. Overall, systematic approaches to examining DCH prevalence and the association between infection and serum biochemical parameters associated with viral load, as well as risk factors for infection, are limited. Using molecular‐based and histochemical investigations, we conducted a study on DCH prevalence in domestic cats that tested negative for a variety of common feline viruses causing hepatopathy. This study also aimed to examine the presence of DCH in cats with and without a histologically parenchymal disorder (HPD) 6 and to determine the association between the presence of DCH and liver damage based on proliferative and apoptotic indices. Other clinical variable and possible risk factors for DCH infection were also addressed.
2. MATERIALS AND METHODS
2.1. Research design and sample collection
One thousand twenty‐two cats that (a) had no evidence (based on history taking or information available in medical records) of concurrent diseases (such as hyperthyroidism, pancreatitis, and cardiovascular disorders); (b) had not been treated with corticosteroids, antifungal drugs, or anti‐seizure medication within 3 months before sample collection; and (c) had not undergone an operation within the previous month, to ensure there were no consequences associated with an increase of liver enzyme or impairment of hepatic perfusion, 6 were enrolled in this study. Evidence of concurrent diseases was based on history taking or information available in medical records. Not all cats had measurement of T4 or pancreatic enzymes or completely work up. The cats that were met these initial criteria were included for further sample collection, which included either ethylenediaminetetraacetic acid (EDTA) blood (first cohort) or liver samples (second cohort) based on availability. For the first cohort, the study was conducted by collecting the remaining EDTA blood samples, which were used for health monitoring, of the cats who had been brought to small animal hospitals/clinics in the Bangkok metropolitan area and vicinity from January 2020 to March 2021. The collected EDTA blood samples were kept at −80°C before DNA extraction. Available information of hematological data and liver biochemical parameters including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma‐glutamyl transpeptidase (GGT), total protein (TP), albumin (ALB), triglycerides, cholesterol, and total bilirubin on the day of blood sampling was recorded for further analysis. General signalment such as age, sex, and breed was also recorded (Figure 1).
FIGURE 1.
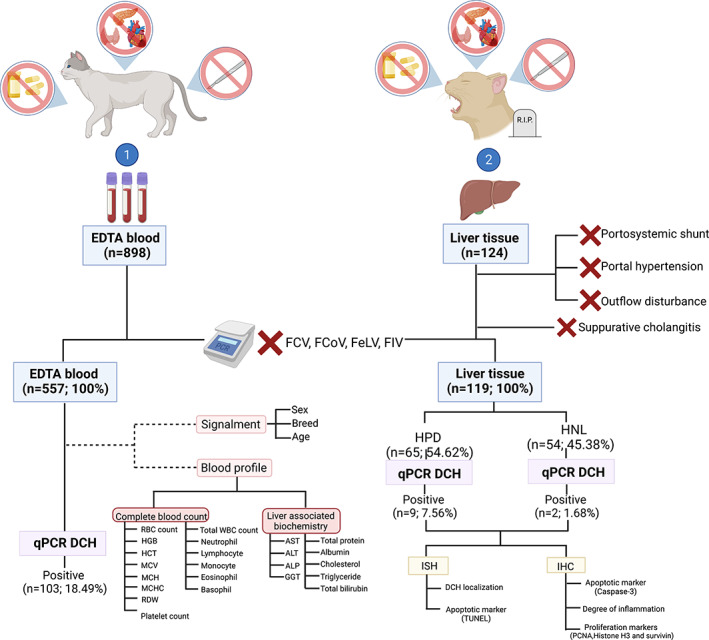
Research design and sample collection for DCH investigations. Two sample cohorts including EDTA blood and liver tissues from cats that (a) had no evidence of concurrent diseases (such as hyperthyroidism, pancreatitis, and cardiovascular disorders); (b) had not been treated with corticosteroids, antifungal drugs, or anti‐seizure medication within 3 months before sample collection; and (c) had not undergone an operation within the previous month were employed for this study. For necropsied cases, the cats that had postmortem evidence of portosystemic shunt, portal hypertension, and hepatic outflow disturbances (such as hepatic veno‐occlusive disease, obstructive bile flow diseases, etc) were discarded. The liver samples that showed histologically present suppurative cholangitis was also ruled out. The blood and liver samples in which FCV, FCoV, FeLV, or FIV was co‐detected by RT‐PCR were also excluded, and the remaining samples were further screened for DCH using qPCR. Information regarding the complete blood count (CBC) and liver biochemical profiles was collected for statistical analysis. Two sets of DCH‐positive liver samples (HPD and HNL) subsequently underwent ISH targeting the DCH partial gene to indicate the tropism of the virus; additionally, the proliferative and apoptotic activities of hepatocytes were investigated using apoptotic (caspase‐3 and terminal deoxynucleotidyl transferase dUTP nick‐end labeling [TUNEL] assays) and proliferative (PCNA, phospho‐histone H3, and survivin) panels
In the second cohort, we collected the liver samples as for fresh and formalin‐fixed paraffin‐embedded (FFPE) tissues deriving from cats that met the initial criteria for sample collection described above, submitted for routine necropsy at the Department of Pathology, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand from June 2020 to July 2021. Cats that presented portosystemic shunts or disorders associated with outflow disturbances and portal hypertension based on postmortem investigation, were excluded from this investigation. The fresh tissues were frozen at −80°C until used. The FFPE samples underwent routine histology and were subsequently examined by veterinary pathologists (S.S., S.T., and T.K.) to categorize the samples according to definition of the Word Small Animal Veterinary Association's (WSAVA) Liver Standardization Group. 6 The sections showing histological indication of suppurative cholangitis according to the WSAVA's guideline for clinical and histological diagnosis of feline liver disease, 6 were excluded from this assessment. The remaining samples were divided into 2 groups: histologically normal liver (HNL) and HPD (Figure 1), which included reversible hepatic injuries, hepatic amyloidosis, hepatocellular death, hepatitis and cirrhosis, hepatic metabolic storage diseases, and neoplasia. Only 1 liver section (sample no. 2, Table 2) from a previous study 3 was included in this study.
TABLE 2.
Determination of DCH quantification with degree of hepatitis, proliferative and apoptotic activities of hepatocytes in infected cats
| Sample No. | Viral copy number (/μL) | Histology | Inflammation grade a | Vacuolation score | ISH b | PCNA | Survivin | Phospho histone‐H3 | Caspase‐3 | TUNEL | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Portal area | Parenchymal area | ||||||||||
| 1 | 4.33 × 103 | HNL | 0 | 0 | 2 | Rare | − | + | − | + | + |
| 2 | 1.74 × 108 | HPD; C, I | 3 | 6 | 3 | Widespread | +++ | +++ | ++ | − | − |
| 3 | 4.35 × 106 | HPD; C, I | 2 | 4 | 2 | Widespread | ++ | +++ | + | − | − |
| 4 | 2.38 × 104 | HPD; A | 1 | 1 | 1 | Rare | + | − | + | − | − |
| 5 | 1.65 × 102 | HNL | 0 | 0 | 3 | No signals | − | − | − | + | + |
| 6 | 4.35 × 104 | HPD; A | 1 | 1 | 2 | Rare | + | − | − | − | − |
| 7 | 1.05 × 105 | HPD; C | 1 | 1 | 0 | Common | + | ++ | + | − | − |
| 8 | 1.22 × 105 | HPD; C | 1 | 3 | 5 | Common | + | + | − | − | − |
| 9 | 2.65 × 109 | HPD; C, I | 3 | 6 | 4 | Widespread | +++ | +++ | ++ | − | − |
| 10 | 1.33 × 105 | HPD; C, I | 3 | 6 | 2 | Widespread | +++ | +++ | ++ | − | − |
| 11 | 2.65 × 104 | HPD; C | 2 | 4 | 5 | Common | ++ | +++ | ++ | − | − |
Abbreviations: Averaged positive immunoreactivity of proliferative/apoptotic indices: −, no immunoreactivity; +, ≤25% positive immunoreactivity; ++, 26%‐50% positive immunoreactivity; +++, ≥50% positive immunoreactivity. A, acute hepatitis; C, chronic hepatitis; HNL, histologically normal liver; HPD, histological parenchymal disorder; I, interface hepatitis; ISH, in situ hybridization; PCNA, proliferating cell nuclear antigen; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Significant association between viral copy number and histological grading of portal inflammation (P = .014) and parenchymal inflammation (P = .03).
Significant correlation between viral copy number and intensity of staining pattern (P = .004).
All investigative procedures were conducted in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines and regulations. The cat owners signed consent approvals for sample collection and data publication. This study was approved by the Chulalongkorn University Animal Care and Use Committee (No. 2131004).
2.2. Nucleic acid extraction and quantification
Ethylenediaminetetraacetic acid blood and frozen liver samples collected from each case were subjected to total viral nucleic extraction. Briefly, 200 μL of EDTA blood samples and an estimated 5 g of frozen liver samples were homogenized using an automatic homogenizer (Bead Ruptor 12, OMNI International, Georgia, USA). The total viral DNA/RNA of the homogenized samples was then extracted using a column extraction kit (Geneaid, Taipei, Taiwan) following the manufacturer's protocols. The extracted nucleic acids were subsequently quantified and qualified using a spectrophotometer (NanoDrop, Thermo Fisher Scientific, Massachusetts, ) and then stored at −80°C.
2.3. Screening for concomitant viral infection
The extracted nucleic acids deriving from the abovementioned blood and liver samples were initially screened for concomitant viral infections that have been associated with liver damage in cats, 7 including feline retroviruses (feline immunodeficiency virus [FIV] and feline leukemia virus [FeLV]), feline calicivirus (FCV), and feline coronavirus (FCoV), using 1‐step reverse‐transcription (RT)‐PCR (QIAGEN OneStep RT‐PCR Kit, Qiagen GmbH, Hilden, Germany). 8 The samples that were PCR‐positive for any screened viruses were discharged from this study (Figure 1).
2.4. Detection and quantification of DCH
Presence of DCH in the EDTA blood and frozen liver samples was detected by quantitative PCR (qPCR) as previously described. 3 Briefly, 2 μL of extracted DNA was added to the 25‐μL reaction of the KAPA SYBR Fast qPCR Master Mix (2X) Universal kit (KAPABIOSYSTEMS, Sigma‐Aldrich, Cape Town, South Africa), which contained 200 nM of each DCH‐specific primer. The reactions were performed in the Rotor‐Gene Q real‐time PCR cycler (Qiagen GmbH, Hilden, Germany) with thermal cycling conditions and settings as previously described. 3 The DCH loads in the tested sample were calculated on the basis of standard curve calculation, which was generated from 10‐fold serial dilutions of the positive control plasmid derived from a previous study. 3
2.5. DCH localization assessment
Domestic cat hepadnavirus tropism in the liver was examined by ISH to define the localization of viral DNA in the FFPE liver sections derived from DCH qPCR‐positive cases. Chromogenic ISH was performed using the constructed DNA probe covering 290 bp of the intersected portion in between the core (C) and polymerase (L) genes. The DNA probe was synthesized using a PCR DIG Probe Synthesis Kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's protocols. The probe construction reaction was performed under the same thermal cycling conditions and Hgap primers described earlier, 1 using digoxigenin (DIG)‐labeled oligonucleotides instead of the normal oligonucleotides. The constructed hybridization probe was determined by size resolution on 1% (wt/vol) agarose gel electrophoresis, compared with a control DNA. The ISH with chromogenic DNA was performed as previously reported, with minor modifications. Briefly, 3‐μm‐thick FFPE slides were deparaffinized, dehydrated, and subsequently treated with citrate buffer (pH 6) at 95°C for 20 minutes. Slides were then washed 3 times in distilled water for 5 minutes each. Thereafter, slides were prehybridized in prehybridization buffer containing 50% (vol/vol) formamide and 4X saline‐sodium citrate (SSC) at 37°C for at least 15 minutes. The prehybridized sections were then incubated with hybridization buffer containing 5X SSC, 5X Denhardt's solution, 100 μg/mL of salmon sperm DNA, 0.5% (wt/vol) sodium dodecyl sulfate, and 10 ng/mL of DCH probe at 55°C overnight in a humidified slide incubator. Meanwhile, hybridization buffer containing a DIG‐labeled canine bocavirus‐2 (CBoV‐2) probe 9 used as an unrelated probe was placed on negative control slides. Liver sections derived from DCH qPCR‐positive 3 and qPCR‐negative cats, incubated with the constructed DCH probe, were used as additional positive and negative controls, respectively. After overnight incubation, slides were subsequently immersed in the series of SSC buffer including 2X SSC at 37°C for 15 minutes and a series of 1X, 0.5X SSC at 42°C for 15 minutes each. To block nonspecific hybridization, the slides were soaked with a blocking solution mixture composed of 5% bovine serum albumin at room temperature (Rt) for 60 minutes. After nonspecific blocking, 200 μL of anti‐DIG‐POD (poly) Fab Fragments (Roche, Basel, Switzerland) (1:200 in 1X blocking solution) was poured onto each slide. Then, slides were placed in a moisture chamber for 60 minutes at Rt. A hybridization detection system using the Vector VIP Substrate Kit (Vector Laboratories, Inc, California, USA) was applied in a dark chamber at Rt for 10 minutes. Slides were then counterstained with methyl green, dried, and mounted with a coverslip. The intensity of the DCH‐specific signals presented in the liver section of each case was further scored as follows: no hybridization signal, rare hybridization signals, common hybridization signals, and widespread staining. 10 The intensity scores were used for further analysis.
2.6. Histological assessment of DCH‐positive liver sections
To determine the degree of inflammation in the livers of DCH qPCR‐positive cats, the sections were further scored as grade 0 to 3 according to a previous description as follows 11 : Grade 0: no inflammation, defined by fewer than 5 inflammatory cells per portal area, with most portal areas lacking inflammatory cells; Grade 1: mild inflammation, defined by small numbers of present inflammatory cells (>5 but <20) within the adventitia of the portal area, and the inflammatory cells not involved within the portal triads; Grade 2: moderate inflammation, defined by intermediate numbers of inflammatory cells (>20 but <75) within the adventitia of the portal area, and mostly Infiltrated into the portal triads; and Grade 3: severe inflammation, defined by large numbers of inflammatory cells (>75) within the adventitia of portal triads and by large involvement of portal triads. Furthermore, the degree of hepatic parenchymal inflammation and hepatocyte vacuolation was scored from 0 to 6 according to previous publications with some modifications. 12 , 13 Briefly, Score 0: no represented lesion localized in hepatic parenchyma; Score 1: represented lesions localized to periportal area; Score 2: represented lesions localized to midzonal area; Score 3: represented lesions to centrilobular area; Score 4: represented lesions involving periportal to midzonal area; Score 5: represented lesions involving midzonal to centrilobular area; and Score 6: represented diffuse lesion involving all zones.
To determine proliferative and apoptotic activities of hepatocytes in FFPE sections obtained from DCH qPCR‐positive cats, immunohistochemistry (IHC) panels were subsequently performed for the sections to assess for proliferative and apoptotic markers using polyclonal anti‐cleaved caspase‐3 (Asp175) antibody, 14 polyclonal anti‐phospho‐histone H3 (Ser10) antibody, monoclonal anti‐proliferating cell nuclear antigen (PCNA; PC10) antibody, and monoclonal anti‐survivin (71B4B7) antibody (SignalStain Proliferation/Apoptosis IHC Sampler Kit, Cell Signaling Technology, Massachusetts, USA). 15 The positive control sections available within the kit and the known feline mammary carcinoma section were used as positive controls. To further determine apoptotic activity in identical sections of DCH qPCR‐positive cats, in situ detection of DNA fragmentation based on a modified terminal deoxynucleotidyl transferase dUTP nick‐end labeling (TUNEL) assay (TACS‐XL In Situ Apoptotic Detection Kit, R&D System, Minnesota) was employed. The DCH qPCR‐negative liver sections were used as controls to compare the proliferative and apoptotic activities. The proliferative and apoptotic activities were graded as the average number of positive staining hepatocytes, as follows: −, no immunoreactivity; +, ≤25% positive immunoreactivity; ++, 26% to 50% positive immunoreactivity; +++, ≥51% positive immunoreactivity.
2.7. Statistical analyses
Statistical analysis was performed using the SAS software package (Version 9.4, SAS Inc, Cary, North Carolina, USA). Descriptive statistics including mean, standard deviation (SD), median, and minimum and maximum number of all continuous data were analyzed using the MEANS procedure of SAS. The continuous data were tested for normal distribution using the Shapiro‐Wilk test. Frequency analyses were conducted to evaluate categorical data. The results for continuous data were expressed as mean (±SD), median and range, and categorical data were expressed as percentage. Hematological and biochemical data were compared between cats with and without DCH infection through Wilcoxon's rank sum test using the NPAR1WAY procedure of SAS. Additionally, Pearson's correlation was conducted to determine the association between the log viral copy number (LVCN) and hematological and biochemical parameters Moreover, multiple ANOVAs were carried out to analyze the effect of the age class and gender of the cat on the quantity of DCH virus as determined by qPCR using the general linear model procedure of SAS. The statistical model included age class (<1.0, 1.0‐1.9, 2.0‐4.9, 5.0‐9.9, and ≥10 years), gender (male and female), and 2‐way interaction. Least square means were obtained from each class of the variables and were compared using the least significant difference test. In addition, logistic regression was conducted to analyze the effect of the cat's age class and gender on the absence or presence (score of 0 or 1, respectively) of DCH using the generalized linear model procedure of SAS. Odds ratios among the cats' age classes were obtained using the ODDSRATIO statement under the LOGISTIC procedure of SAS. The differences were considered significant at P < .05.
For liver samples, the associations between DCH LVCN and variable factors including severity of inflammation, ISH staining signals, and proliferative/apoptotic markers were analyzed using the Kruskal‐Wallis test. The differences were considered significant at P < .05.
3. RESULTS
3.1. Study group and demographic data
A total of 1022 samples, comprising 898 EDTA blood and 124 liver samples obtained from individual cats that met our inclusion criteria for sample collection described above, were collected and subsequently tested to rule out concomitant FCV, FCoV, FeLV, or FIV infection, with additional examination of liver histology. Regarding these investigations, 341 EDTA blood and 5 liver samples were excluded from this investigation. The remained 557 EDTA blood and 119 liver samples were subjected for DCH detection and further analysis (Figure 1).
The 557 EDTA blood samples were collected from 274 (49.19%; 95% CI, 44.96‐53.43) male and 229 (41.11%; 95% CI, 37.10‐45.25) female cats, and the remaining 54 samples (9.69%; 95% CI, 7.51‐12.44) were unrecorded. The mean age of the studied cats was 5.05 years (median = 3.5 years), with a minimum and maximum of 22 days and 20 years old, respectively. Of these cats, domestic short hair (DSH) was the most prevalent breed, accounting for 74.51% (415/557; 95% CI, 70.73‐77.95), followed by Persian at 6.64% (37/557; 95% CI, 4.86‐9.02), Scottish fold at 3.59% (20/557; 95% CI, 2.34‐5.48), and others at 4.85% (27/557; 95% CI, 3.35‐9.96). The remaining 58 samples (10.41%; 95% CI, 8.14‐13.23) were not documented.
The 119 liver samples were collected from 70 (58.82%; 95% CI, 49.84‐67.26) male and 49 (41.18%; 95% CI, 32.74‐50.16) female cats. The mean age was 4.25 years (median = 3.30 years), with a minimum of 2 months and maximum of 17 years. The most prevalent breed was DSH (84.87%, 101/119; 95% CI, 77.35‐90.21), followed by Persian (10.92%, 14/119; 95% CI, 6.50‐17.80) and others (3.36%, 4/119; 95% CI, 1.31‐8.32). Among the 119 liver samples, 54.62% (65/119; 95% CI, 45.67‐63.28) were categorized as HPD based on initial histological assessment. Among the 65 HPD cases, 101 histological lesions were found that included hepatic fibrosis (n = 12), hepatic vacuolation (n = 14), hepatic parenchymal necrosis (n = 10), chronic hepatitis (n = 19), acute hepatitis (n = 31), and hepatic abscess and granuloma (n = 15). Neither hepatic cirrhosis nor hepatocellular carcinoma/adenoma was present in this HPD group. Ceroid‐lipofuscinosis was seen in some HPD cases or otherwise in HNL liver sections.
3.2. DCH quantitation on blood samples and correlation analysis with hepatic enzymes
Among the 557 tested blood samples, 18.49% (103/557; 95% CI, 15.49‐21.93) were positive for DCH using qPCR. The averaged viral copy number of the DCH was 2.13 × 103 viral copies/μL, and 1.33 × 101 and 2.65 × 108 viral copies/μL were the minimum and maximum. We determined the distribution of DCH across different age groups and found that cats aged between 5.0 and 9.9 years had a higher proportion of DCH detection than those younger than 1 year (P = .043; odds ratio [OR], 1.26; 95% CI, 0.602‐2.655) (Table 1).
TABLE 1.
Characteristics of investigated cats
| Sample | Number (%) | Mean ± SD |
|---|---|---|
| EDTA‐blood (n = 557) | ||
| Age (year) | 5.05 ± 4.92 | |
| <1.0 | 88 (15.80) | |
| 1.0–1.9 | 83 (14.90) | |
| 2.0–4.9 | 100 (17.95) | |
| 5.0–9.9 | 97 (17.41) a | |
| >10.0 | 106 (19.03) | |
| Unknown | 83 (14.90) | |
| Sex | ||
| Male | 274 (49.19) | |
| Female | 229 (41.11) | |
| Unknown | 54 (9.69) | |
| Liver tissue (n = 119) | ||
| Age (year) | 4.25 ± 3.45 | |
| <1.0 | 20 (16.81) | |
| 1.0‐1.9 | 37 (31.09) | |
| 2.0‐4.9 | 32 (26.89) | |
| 5.0‐9.9 | 41 (39.42) | |
| >10.0 | 4 (3.85) | |
| Sex | ||
| Male | 70 (58.82) | |
| Female | 49 (41.18) | |
| Histological characteristic | ||
| HNL | 54 (45.38) | |
| HPD | 65 (54.62) | |
The age period is associated with the presence of DCH compared to age lower than 1 year group (P = .043).
Abbreviations: HNL, histologically normal liver; HPD, hepatic parenchymal disorders.
To determine the possible role of DCH infection, we analyzed across different clinical parameters between DCH‐positive and ‐negative cats. We found that cats with DCH showed significant elevation of liver enzymes including AST (P = .001, median = 87 U/L, min‐max = 24‐2425 U/L) and ALT (P < .001, median = 118 U/L, min‐max = 16‐4055 U/L) but not ALP (P = .07, median = 30.5 U/L, min‐max = 2.8‐446) and GGT (P = .21, median = 3.35 U/L, min‐max = 0.1‐9.6 U/L). Other hematological and liver‐related biochemical parameters (TP, ALB, cholesterol, triglyceride, total bilirubin) were not associated with the presence of DCH (P > .05) (Figure 2).
FIGURE 2.
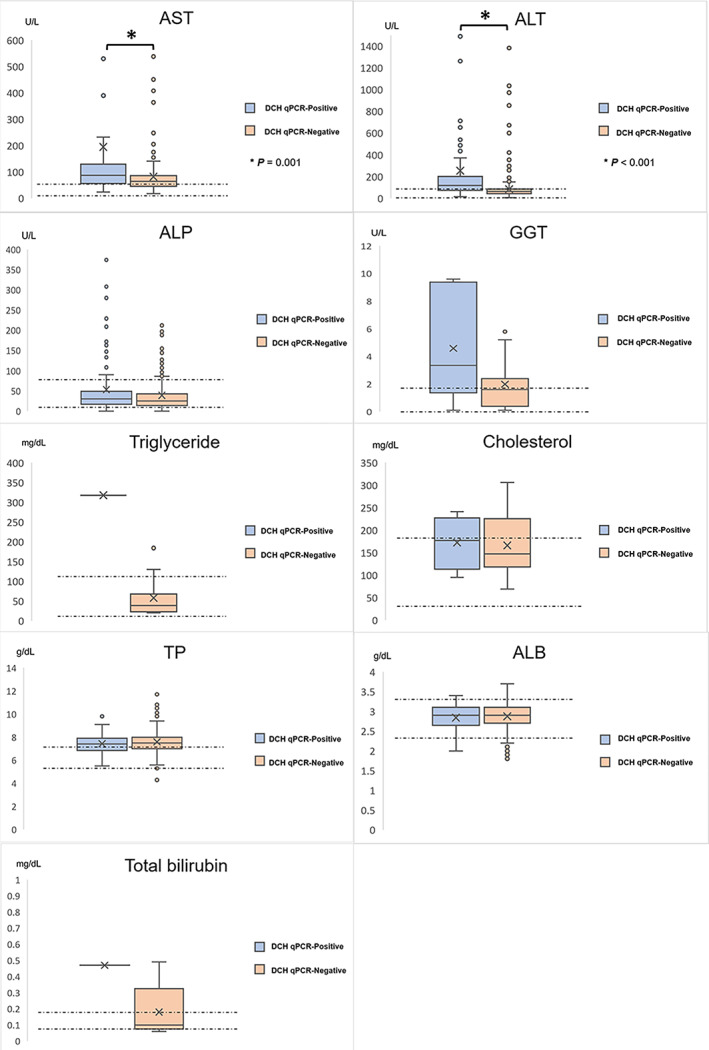
Biochemical variable of DCH qPCR‐positive cats. Box‐and‐whiskers plots indicate the biological parameters of the DCH‐positive cats that revealed statistical significance in elevation of liver enzymes including AST and ALT, whereas ALP, GGT, ALB, TP, total bilirubin, triglyceride, and cholesterol levels were not statistically different from those of the DCH‐negative cats. The “×” in the boxes and circles beyond the whiskers denote mean and outliers, respectively. The normal range of each parameter is marked by a dotted line. The statistical significance of the representative parameters among DCH‐positive and ‐negative cats is indicated by asterisks. ALB, serum albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transpeptidase; TP, total protein
Among DCH‐positive blood samples, we performed the Pearson correlation coefficient analysis, which revealed that the LVCN also had positive correlations with the serum triglyceride (r = .833, P = .0008), GGT (r = .549, P = .022), and ALT (r = .149, P = .0004). The LVCN had negative correlations with some hematological parameters including platelet number (r = −.392, P < .001), mean corpuscular volume (r = −.287, P = .003), lymphocyte number (r = −.278, P = .004), mean corpuscular hemoglobin (r = −.269, P = .006), and hematocrit (r = −.202, P = .040).
3.3. DCH detection and histological determination of liver tissue
Of the 119 liver samples, 9.24% (11/119; 95% CI, 5.24‐15.80) tested positive for DCH using the qPCR. Among positive liver samples, 81.81% (9/11; 95% CI, 52.30‐94.86) were found in cats with histological classification to HPD. The averaged viral copy number of the DCH was 4.13 × 105 viral copies/μL, and 1.65 × 102 and 2.65 × 109 copies/μL were the minimum and maximum values.
In cases of HPD that were positive for DCH qPCR detection, the sections revealed various degrees of both lymphoplasmacytic periportal and parenchymal infiltrations (9/9). Overall (9/9), hepatocytes were diffusely rounded and dissociated, resulting in irregular cords; the hepatic sinusoidal spaces were diffusely variably hypercellular and contained individualized and small clustered circulating mixed leukocytes including lymphocytes, plasma cells, and fewer neutrophils; and Kupffer cells were reactive and often engulfed erythrocytes in many areas. The portal tracts of 7/9 DCH‐positive HPD cases were surrounded by thick bands of fibrosis that bridged between portal structures in some areas and contained high numbers of tortuous and slit‐like bile ducts with associated increased arteriolar profiles and variable infiltrates of numerous lymphocytes and plasma cells, few Mott cells, and rare lymphofollicular aggregates (Figure 3A and Table 2). Individual necrotic hepatocytes are rarely seen. Within most DCH‐positive HPD cases (4/9), interface hepatitis represented by infiltrations of lymphocytes and plasma cells that extended beyond and disrupted the limiting plate, dissected around adjacent hepatocytes, and were accompanied by rare acidophilic bodies was noted (Figure 3A,B and Table 2).
FIGURE 3.
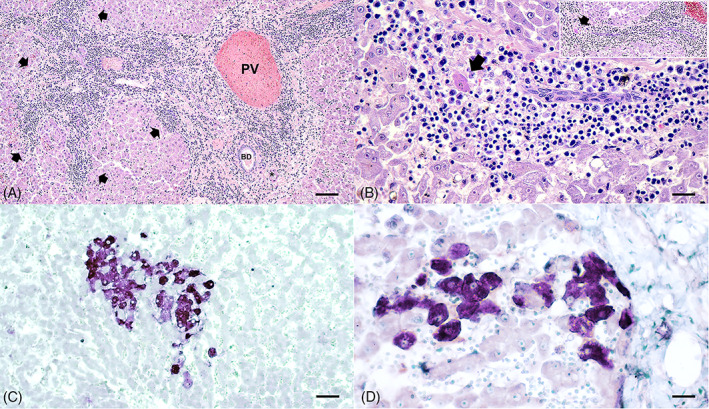
Histopathological features of DCH‐positive liver sections of HPD cats. (A) The portal tracts were surrounded by thick bands of fibrosis that bridged between portal structures in some areas and contained high numbers of infiltrates of numerous lymphocytes and plasma cells. Infiltrates of lymphocytes and plasma cells extended beyond and disrupted the limiting plate (arrows). (B) Infiltrated lymphocytes and plasma cells clustered within the hepatic sinusoid, extended beyond and disrupted the limiting plate (inset), and dissected around adjacent hepatocytes, accompanied by rare acidophilic body (arrows) that represent interface hepatitis. Variable sizes of vacuolated hepatocytes were also presented. (C) Hybridization signals of DCH were present within most of the cytoplasm of hepatocytes in this field. (D) A cluster of DCH‐positive cells was present within the area of inflammation adjacent to the portal tract. An asterisk indicates hepatic artery. BD, bile duct; PV, portal vein. Bars indicates 45 μm for (A), 170 μm for (B, D), and 80 μm for (C)
To illustrate the DCH localization in liver sections, DCH‐specific ISH was performed, revealing that most DCH qPCR‐positive samples were positive for DCH‐specific ISH (Figure 3C), except 1 sample (no. 5) that showed the lowest viral copy number presented in qPCR results. The DCH‐DNA signals were present within the cytoplasm of the hepatocytes, where they were within the area most adjacent to inflammatory lesions (Figure 3D). Some nuclear hybridization signals were seen in some single hepatocyte cells. No evidence of hybridization signals was seen in the negative controls (Figure S1, Supporting Information). Interestingly, the DCH viral copy number in the liver had a positive association with degree of portal hepatitis (P = .014), parenchymal hepatitis (P = .030), and intensity of DCH hybridization signals (P = .004) but not degree of hepatocyte vacuolation (P = .687) (Table 2). For the DCH‐positive HNL, only small degrees of fatty degeneration were observed (Table 2).
Determination of proliferative and apoptotic activities of hepatocytes in HPD cats showing positive qPCR results revealed markedly positive staining signals of hepatocytes in both the nucleus for the PCNA and phospho‐histone H3 antibodies (Figure 4A,B) and in the cytoplasm for the survivin antibody (Figure 4C), whereas the cleaved caspase‐3 antibody and a modified TUNEL assay revealed positive staining within the infiltrated inflammatory cells but not for the hepatocytes (Figure 4D). For the HNL cats, few hybridization signals of the cleaved caspase‐3 and TUNEL were positive within the nucleus of hepatocytes. Neither immunohistochemical nor hybridization signals within the hepatocytes were seen in the DCH‐negative liver sections (Figure S1).
FIGURE 4.
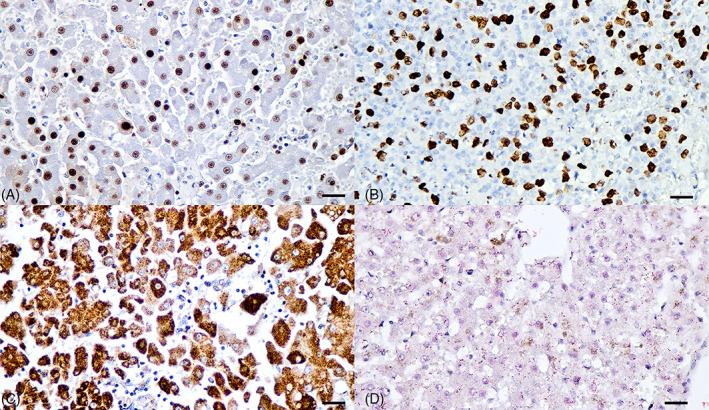
Proliferative and apoptotic features of DCH‐positive liver section of HPD cats. (A) The PCNA and phospho‐histone H3 (B) immunostainings were diffusely presented within the nucleus of hepatocytes (golden brown precipitates), indicating proliferative activity of DCH‐infected liver. (C) Cytoplasmic immunostaining of survivin was positive within the cytoplasm of hepatocytes. (D) No immunostaining of cleaved caspase‐3 was found in the DCH‐positive liver section (red precipitates). Bars indicate 170 μm
4. DISCUSSION
Investigation of DCH localization found the virus located in hepatocytes, 2 , 3 together with the evidence of DCH genome identification in most cats showing either hepatitis or hepatocellular carcinomas. 2 Although the latest studies found that DCH‐infected cats revealed high serum activity of hepatic enzymes, 4 , 5 , 16 most infected cats were coinfected with other viruses, mainly retroviruses, 3 , 4 which are associated with liver damage. 17 Likewise, other factors including systemic diseases and viral coinfection that could be associated with an augmentation in liver enzymes were not fully excluded in cats showing DCH infection, and the association of single DCH infection with high serum activity of liver enzymes and related chemical variable remains obscure. In this study, we investigated the presence of DCH in cats that were ruled out for the most common liver‐associated viruses and had no history of hepatic impairment and secondary liver diseases, as our exclusion criteria, and found that DCH was highly associated with high serum activity of liver‐associated chemical variable. We found that DCH infection was more prevalent in cases of chronic hepatitis and exclusively presented interface hepatitis as the most common pathological feature as found in a previous study. 2 Our findings also extend the information on DCH infection associated with hepatic parenchymal proliferation, but not apoptosis.
In this study, we investigated the presence of DCH in a large retrospective collection of cat sera, which were initially ruled out for other concomitant viral infections and other liver‐associated diseases, indicating that the presence of DCH is likely associated with an increase of liver enzymes including AST and ALT, similar to previous observations 3 , 4 , 5 and extending the observations of other liver‐related biochemical profiles in DCH‐infected cats. Although there was a small increase of AST and ALT in cats, it is considered to be specific to liver injuries due to the limited short half‐life. 18 , 19 Because the alterations of liver parenchyma that lead to increased liver enzymes have been associated with multifactorial causes such as infections or other systemic diseases, 20 even though we attempted to rule out other hepatotropic viruses 7 and other systemic causes, the definitive association of DCH presentation and increased liver enzymes presented in this study still warrants further investigations. We also found that the LVCN had a positive correlation with an increase of ALT and GGT, which precedes increases in ALP and is considered a more sensitive indicator for liver disease in feline species. Although most DCH‐positive cats with a higher LVCN showed higher ALT and GGT levels, some DCH‐positive samples revealed normal limits of these enzymes. This finding might indicate that most of the DCH‐positive cats in this study were in the chronic stage of DCH infection, whereas some of the positive cats that revealed normal liver biochemical values might become acutely infected, as observed in a recent longitudinal observation on chronic DCH infection 16 and chronic HBV infection in humans. 21 , 22 Further research on chronic DCH infection will tentatively support this speculation. Additionally, the LVCN exhibited a positive correlation with hyperlipidemia; however, the exact pathogenesis of this phenomenon could not be explained and requires future observations. In HBV‐infected patients, triglyceride level has been reported to be associated with the viral loads, but the actual pathophysiology remains to be determined. 23 , 24 , 25 Nonetheless, DCH infection in the liver could alter the synthesis of lipids in infected individuals, which is hypothesized as a possible reason. 23 , 26 Furthermore, we also found a negative correlation of the LVCN of DCH with some hematological parameters such as erythrocyte number and its indices and lymphocyte number; however, conclusions about this finding could not be drawn from this study and requires further investigations. HBV infection that localizes in the liver and alters the liver function is usually related to the onset of liver failure and damage, and anemia could occur. 27 So far, the association of HBV in rare cases of anemia has been reported, but the pathophysiology is still questioned. 28
Regarding the intrinsic factors of DCH presence, it appears to be significantly correlated with cats' age but not with sex and breed, in accordance with previous studies. 4 , 5 This finding indicated that DCH detection mostly appeared in adult cats (aged 5.0‐9.9 years), in agreement with a recent study 5 ; however, Lanave et al. reported that DCH was prevalent in younger cats. 4 Thus, the cats' proneness to DCH infection in infective phases is still debatable.
This study identified the hepatotropic nature of DCH using ISH, which indicated potential cellular localization of this virus in hepatocytes, 2 corresponding with a previous study that found the highest viral copy numbers in liver samples. 3 Among the DCH‐positive HPD cases, chronic lymphoplasmacytic hepatitis, but not pyogranulomatous hepatitis, with identification of interface hepatitis characterized by inflammation extended beyond and disrupting the limiting plate and accompanied by rare individual hepatocyte apoptosis, supported possible common lesions of hepadnavirus‐associated hepatitis as found in recently described DCH infection. 2 Hepatocyte vacuolation associated with HBV‐infected patients with fibrosing cholestatic hepatitis, a rapidly progressive form of viral hepatitis B infection, has been reported, 29 , 30 prompting us to determine the association between DCH infection and degree of hepatocyte vacuolation. However, the present study did not find statistical significance between the severity of such vacuolation and viral load.
Preliminary results found that DCH‐positivity in cats significantly correlated with an increase in liver enzymes, which could directly reflect the damage of hepatocytes. This prompted us to investigate the proliferative and apoptotic activities of the hepatocytes in DCH‐positive cats. In this study, we found a higher proliferative activity of hepatocytes in DCH‐positive cats, similar to a previous study describing a higher proliferative index, using Ki‐67 staining, in regions of the liver showing DCH‐positive hybridization. 2 Together, ISH staining pattern and histological severity might indicate a positive correlation of severe infection to a severely histological infected liver. For HBV infection, the pathology is diverse and reflects the natural history of infection in that its severity is associated with the viral loads. 31 , 32 These findings corresponded with the lower numbers of viral loads, which could indicate the latency stage of DCH infection in HNL cats. 33 Although this study pointed out the relationship between severity and numbers of viral loads, the low number of studied samples represents a limitation to this observation.
Several studies have been conducted to determine the relationship between proliferative/apoptotic activities of hepatocytes and HBV infection; however, the obtained results are still contradictory 34 to the majority of the findings indicating the inhibitory effect of apoptosis caused by the HBV, thereby facilitating the virus replication and inducing proliferation of the hepatocytes for the viral factory. 35 , 36 Although the strong intensity of PCNA, phospho‐histone H3, and survivin staining in the DCH‐positive HPD sections indicated the proliferative activity of hepatocytes, the role of DCH in hepatocyte proliferation and regeneration could not be concluded in this study. These findings provide fundamental observations and establish future intensive focuses.
In conclusion, this study demonstrated the potential association of DCH in cats with an increase in the liver biochemical profile, together with other biological parameters, from a large retrospective cohort of domestic cat sera in which the common feline hepatotropic viruses had been ruled out. We were able to indicate the possible role of DCH in association with hepatocyte activities that could refer to the result of infection. Because neither other infection (such as bacteria or parasites or other feline viruses included parvovirus, herpesvirus, or etc) nor other underlying diseases associated with an increase in liver enzymes were investigated in these cohorts, the tentative role of DCH in liver diseases remains to be discussed. This study provides additional information about DCH in feline health.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interests.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by Chulalongkorn University Animal Care and Use Committee (No. 2131004).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1 Immunostaining pattern of DCH‐negative liver sections of LPH cats. No DCH hybridization signals (A) were observed. A few hepatocytes were positive for PCNA immunostaining (B). No PCNA (C) and phospho‐histone H3 (D) immunostaining was present in this liver section. Positive immunostaining of cleaved caspase‐3 marker (E) and TUNEL hybridization (F) were present within the periportal area of inflammatory cells but not within the hepatocytes. Bars indicate 170 μm.
ACKNOWLEDGMENT
Chutchai Piewbang and Sirintra Sirivisoot were supported by the Ratchadapisek Somphot Fund for Postdoctoral Fellowship, Chulalongkorn University. Wichan Dankaona was granted by The Thailand Research Fund through the Royal Golden Jubilee PhD Program (Grant No. PHD/0021/256). Panida Poonsin received a grant from The Thailand Research Fund through the Royal Golden Jubilee PhD Program (Grant No. NRCT5‐RGJ63001‐013) and The Second Century Fund (C2F), Chulalongkorn University. This research is funded by Thailand Science Research and Innovation Fund (TSRI; CU_FRB640001_01_31_2; to Somporn Techangamsuwan) and National Research Council of Thailand (NRCT; N41A640175; to Chutchai Piewbang) and Grant for Joint Funding of External Research Project, Ratchadaphiseksomphot Endowment Fund and Veterinary Science Research Fund (RES_65_005_31_005), Chulalongkorn University (to Chutchai Piewbang). This study also partly supported by National Research Council of Thailand (NRCT): R. Thanawongnuwech NRCT Senior scholar 2022 #N42A650553.
Piewbang C, Dankaona W, Poonsin P, et al. Domestic cat hepadnavirus associated with hepatopathy in cats: A retrospective study. J Vet Intern Med. 2022;36(5):1648‐1659. doi: 10.1111/jvim.16525
Funding information Joint Funding of External Research Project, Ratchadaphiseksomphot Endowment Fund and Veterinary Science Research Fund, Grant/Award Number: RES_65_005_31_005; National Research Council of Thailand, Grant/Award Numbers: N41A640175, N42A650553; Ratchadapisek Somphot Fund for Postdoctoral Fellowship, Chulalongkorn University; Thailand Research Fund, Grant/Award Numbers: NRCT5‐RGJ63001‐013, PHD/0021/256; Thailand Science Research and Innovation Fund (TSRI), Grant/Award Number: CU_FRB640001_01_31_2; The Second Century Fund (C2F), Chulalongkorn University
REFERENCES
- 1. Aghazadeh M, Shi M, Barrs VR, et al. A novel hepadnavirus identified in an immunocompromised domestic cat in Australia. Viruses. 2018;10:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pesavento PA, Jackson K, Hampson T, Munday JS, Barrs VR, Beatty JA. A novel hepadnavirus is associated with chronic hepatitis and hepatocellular carcinoma in cats. Viruses. 2019;11:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piewbang C, Wardhani SW, Chaiyasak S, et al. Insights into the genetic diversity, recombination, and systemic infections with evidence of intracellular maturation of hepadnavirus in cats. PloS One. 2020;15:e0241212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lanave G, Capozza P, Diakoudi G, et al. Identification of hepadnavirus in the sera of cats. Sci Rep. 2019;9:10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anpuanandam K, Selvarajah GT, Choy MMK, et al. Molecular detection and characterisation of domestic cat Hepadnavirus (DCH) from blood and liver tissues of cats in Malaysia. BMC Vet Res. 2021;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cullen J, Van den Ingh T, Bunch S, et al. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Disease. Philadelphia, Pennsylvania: Elsevier Saunders; 2006. [Google Scholar]
- 7. Capozza P, Decaro N, Beikpour F, Buonavoglia C, Martella V. Emerging hepatotropic viruses in cats: a brief review viruses. 2021;13:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piewbang C, Wardhani SW, Chanseanroj J, et al. Natural infection of parvovirus in wild fishing cats (Prionailurus viverrinus) reveals extant viral localization in kidneys. PloS One. 2021;16:e0247266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piewbang C, Wardhani SW, Dankaona W, et al. Canine bocavirus‐2 infection and its possible association with encephalopathy in domestic dogs. PloS One. 2021;16:e0255425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pantin‐Jackwood MJ, Spackman E, Day JM. Pathology and virus tissue distribution of Turkey origin reoviruses in experimentally infected Turkey poults. Vet Pathol. 2007;44:185‐195. [DOI] [PubMed] [Google Scholar]
- 11. Warren A, Center S, McDonough S, et al. Histopathologic features, immunophenotyping, clonality, and eubacterial fluorescence in situ hybridization in cats with lymphocytic cholangitis/cholangiohepatitis. Vet Pathol. 2011;48:627‐641. [DOI] [PubMed] [Google Scholar]
- 12. Sepesy LM, Center SA, Randolph JF, Warner KL, Erb HN. Vacuolar hepatopathy in dogs: 336 cases (1993–2005). J Am Vet Med Assoc. 2006;229:246‐252. [DOI] [PubMed] [Google Scholar]
- 13. Webster CRL, Center SA, Cullen JM, et al. ACVIM consensus statement on the diagnosis and treatment of chronic hepatitis in dogs. J Vet Intern Med. 2019;33:1173‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silva MN, Leite JS, Mello MF, et al. Histologic evaluation of Ki‐67 and cleaved caspase‐3 expression in feline mammary carcinoma. J Feline Med Surg. 2017;19:440‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Preziosi R, Sarli G, Benazzi C, Marcato PS. Detection of proliferating cell nuclear antigen (PCNA) in canine and feline mammary tumours. J Comp Pathol. 1995;113:301‐313. [DOI] [PubMed] [Google Scholar]
- 16. Capozza P, Lanave G, Diakoudi G, et al. A longitudinal observational study in two cats naturally‐infected with hepadnavirus. Vet Microbiol. 2021;254:108999. [DOI] [PubMed] [Google Scholar]
- 17. Reinacher M. Diseases associated with spontaneous feline leukemia virus (FeLV) infection in cats. Vet Immunol Immunopathol. 1989;21:85‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Center SA, Baldwin BH, Dillingham S, Erb HN, Tennant BC. Diagnostic value of serum gamma‐glutamyl transferase and alkaline phosphatase activities in hepatobiliary disease in the cat. J Am Vet Med Assoc. 1986;188:507‐510. [PubMed] [Google Scholar]
- 19. Scott MA, Stockham SL. Fundamentals of Veterinary Clinical Pathology. Iowa, USA: Wiley‐Blackwell; 2008. [Google Scholar]
- 20. Kearns S. Infectious hepatopathies in dogs and cats. Top Companion Anim Med. 2009;24:189‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klair JS, Vancura J, Murali AR. PRO: patients with chronic hepatitis B in immune‐tolerant phase should be treated. Clin Liver Dis. 2020;15:21‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar M, Sarin SK, Hissar S, et al. Virologic and histologic features of chronic hepatitis B virus‐infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376‐1384. [DOI] [PubMed] [Google Scholar]
- 23. Quaye O, Amuzu BG, Adadey SM, Tagoe EA. Effect of hepatitis B virus (HBV) infection on lipid profile in Ghanaian patients. Virology (Auckl). 2019;10:1178122X19827606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu PT, Hwang AC, Chen JD. Combined effects of hepatitis B virus infection and elevated alanine aminotransferase levels on dyslipidemia. Metabolism. 2013;62:220‐225. [DOI] [PubMed] [Google Scholar]
- 25. Chien C‐H, Chen L‐W, Lin C‐L, et al. Unawareness of hepatitis B virus infection confers on higher rate of metabolic syndrome: a community‐based study. Sci Rep. 2017;7:9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su TC, Lee YT, Cheng TJ, Chien HP, Wang JD. Chronic hepatitis B virus infection and dyslipidemia. J Formos Med Assoc. 2004;103:286‐291. [PubMed] [Google Scholar]
- 27. Furtado I, Valadares D, Nery FG. Acute hepatitis B virus infection and severe non‐immune haemolytic anaemia: a rare relationship. BMJ Case Rep. 2017;2017:bcr2017221763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hafeez M, Sarfraz T, Khan RG, Rafe A, Rasool G, Ahmed KN. Hepatitis B leading to megaloblastic anemia and catastrophic peripheral thrombocytopenia. J Coll Physicians Surg Pak. 2016;26:992‐994. [PubMed] [Google Scholar]
- 29. Foo NC, Ahn BY, Ma X, Hyun W, Benedict Yen TS. Cellular vacuolization and apoptosis induced by hepatitis B virus large surface protein. Hepatology. 2002;36:1400‐1407. [DOI] [PubMed] [Google Scholar]
- 30. Nie Y‐Z, Zheng Y‐W, Miyakawa K, et al. Recapitulation of hepatitis B virus–host interactions in liver organoids from human induced pluripotent stem cells. EBioMedicine. 2018;35:114‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biazar T, Yahyapour Y, Hasanjani Roushan MR, et al. Relationship between hepatitis B DNA viral load in the liver and its histology in patients with chronic hepatitis B. Caspian J Intern Med. 2015;6:209‐212. [PMC free article] [PubMed] [Google Scholar]
- 32. Bayram A, Erkilic S, Ozkur A, Bayram M, Sari I. Quantification of intrahepatic total hepatitis B virus DNA in chronic hepatitis B patients and its relationship with liver histology. J Clin Pathol. 2008;61:338‐342. [DOI] [PubMed] [Google Scholar]
- 33. Valsamakis A. Molecular testing in the diagnosis and management of chronic hepatitis B. Clin Microbiol Rev. 2007;20:426‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin S, Zhang Y‐J. Interference of apoptosis by hepatitis B virus. Viruses. 2017;9:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dai R, Peng F, Xiao X, et al. Hepatitis B virus X protein‐induced upregulation of CAT‐1 stimulates proliferation and inhibits apoptosis in hepatocellular carcinoma cells. Oncotarget. 2017;8:60962‐60974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chao CC. Inhibition of apoptosis by oncogenic hepatitis B virus X protein: implications for the treatment of hepatocellular carcinoma. World J Hepatol. 2016;8:1061‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Immunostaining pattern of DCH‐negative liver sections of LPH cats. No DCH hybridization signals (A) were observed. A few hepatocytes were positive for PCNA immunostaining (B). No PCNA (C) and phospho‐histone H3 (D) immunostaining was present in this liver section. Positive immunostaining of cleaved caspase‐3 marker (E) and TUNEL hybridization (F) were present within the periportal area of inflammatory cells but not within the hepatocytes. Bars indicate 170 μm.


