Abstract
Vitamins were closely associated with non-alcoholic fatty liver disease (NAFLD) development, but no study had explored the association of serum multivitamin levels with NAFLD risk. We assessed the association between serum levels of both single-vitamin and multivitamins (VA, VB6, VB9, VB12, VC, VD, and VE) and the risk of NAFLD, using the database of National Health and Nutrition Examination Survey (NHANES) (cycles 2003–2004 and 2005–2006). We employed multivariable logistic regression and weighted quantile sum (WQS) regression models to explore the association of serum multivitamin levels with NAFLD. Among all 2,294 participants, 969 participants with NAFLD were more likely to be male, older, less educated, or have hypertension/high cholesterol/diabetes. After adjustment of covariates, serum VC/VD/VB6/VB9 levels were negatively correlated with NAFLD risk, while serum VA/VE levels were positively correlated with NAFLD risk. In the WQS model, elevated serum VA/VE levels and lowered serum VC/VD/VB6 levels were linearly associated with increased NAFLD risk. There was a non-linear relationship between serum VB9/VB12 levels and NAFLD risk. There were evident associations between serum multivitamin levels and reduced NAFLD risk, which was mainly driven by VD/VB9/VC. In conclusion, our findings suggested that serum multivitamin levels were significantly associated with the risk of NAFLD.
Keywords: multi-vitamins, NAFLD, weighted quantile sum, dose-response relationship, serum level
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a syndrome characterized by excessive deposition of fat in hepatocytes other than factors from alcohol or other definite liver damage, encompassing a spectrum of non-alcoholic fatty liver, non-alcoholic steatohepatitis, related cirrhosis, liver cancer, and others. NAFLD has become the most common chronic liver disease worldwide and exerts influence on the health of 25.24% adults (1). With increasing living standards and obese population, the prevalence of NAFLD will rise continuously. In 2030, the prevalence of NAFLD is expected to reach 33.5% in population aged over 15 years and 28.4% in all ages, with 100.9 million newly increased cases (2). The increasing population with NAFLD imposes a growing societal and economic burden to the world. Presently, there is a lack of safe and effective drug for NAFLD, and the most effective way is lifestyle improvement (3). It is noteworthy that further research studies should focus on the prevention and relief of NAFLD development.
As a class of organic compounds necessary for maintaining the normal physiological function, vitamins play an important role in the growth, metabolism, and development of human body. More and more evidences suggest that vitamins are closely associated with the occurrence and development of NAFLD (4–7). However, some results are controversial. For example, individuals with elevated serum VB9 and VB12 levels have lower risk of NAFLD (8, 9), while those with elevated serum VA level possibly have higher risk of NAFLD (10). Most studies currently focus on the relationship between serum level of single-vitamin, instead of multivitamins, and the development of NAFLD. Previous studies demonstrate that some vitamins may affect the biological functions by interacting with other vitamins (11). So it is significant to explore the potential association between serum multivitamin levels and NAFLD risk.
The absorption of vitamins is affected by various factors, including gender, age, and BMI (12). And also there is vitamin loss during food storage, processing, and cooking. Therefore, the serum vitamin level is a more representative and persuasive indicator to evaluate vitamin circulation, comparing with vitamin intake from food.
Based on the data from 2003 to 2006 in the National Health and Nutrition Examination Survey (NHANES), this study aims to elaborate the relationship between the serum levels of 7 vitamins (VA, VB6, VB9, VB12, VC, VD, and VE) and the risk of NAFLD in American adults, as well as to explore the association between serum levels of both single-vitamin and multivitamins and the prevalence of NAFLD.
Materials and methods
Study design and participants
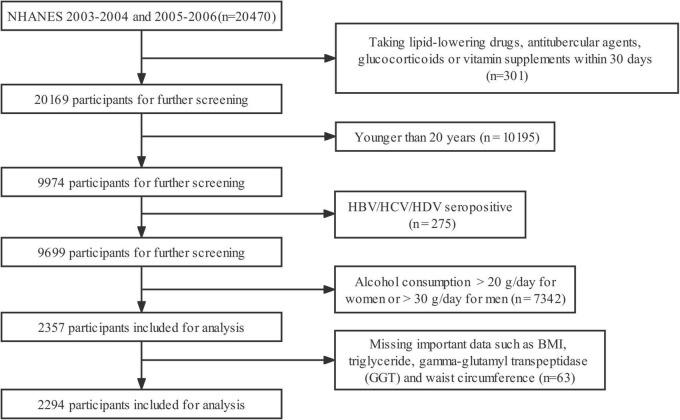
This study included all participants ≥ 20 years old from the 2003–2004 and 2005–2006 cycles of the NHANES in the United States. Profiles of the NHANES were introduced by other researchers (13). Employing a complex, multi-stage, and probability sampling design, the NHANES collected a representative sample from non-institutionalized American population. In those two cycles of NHANES, all baseline data were collected by household interviews, mobile physical examinations, and laboratory tests. Figure 1 showed the flow chart of participant screening. Of all the 20,470 participants, we excluded those taking lipid-lowering drugs, antitubercular agents, glucocorticoids, or vitamin supplements within 30 days (n = 301), those < 20 years old (n = 10,195), those with positive serology for hepatitis B, C, and D (n = 275), those consuming alcohol > 20 g/day for female or > 30 g/day for male (n = 7,342), and those missing important data such as BMI, triglyceride, gamma-glutamyl transpeptidase (GGT), and waist circumference (n = 63) (Figure 1). At last, a total of 2,294 participants were included for the statistical analysis. The protocol of NHANES was reviewed and approved by the Research Ethics Review Board of the National Center for Health Statistics. All participants signed written informed consent before their participation.
FIGURE 1.
Flow chart for the selection of participants in the cohort study. HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus.
Definition of non-alcoholic fatty liver disease
NAFLD was a condition of excessive fat accumulation in the liver, excluding fatty liver due to other causes by liver disease and/or excessive alcohol consumption. Fatty liver index (FLI) was considered a simple and accurate predicting index for hepatic steatosis in the general population and was widely used in NAFLD research, with an accuracy of 0.84 (95% CI: 0.81–0.87) (14). So we used FLI to predict the risk of NAFLD. FLI was calculated with the following Equation (15):
where triglycerides were in mg/dl, BMI in kg/m2, GGT in mmol/L, and waist circumference in cm. This equation of FLI generated scores ranging from 0 to 100. An FLI < 30 suggested no presence of NAFLD, while an FLI ≥ 60 suggested the probable presence of fatty liver (15).
Measurement of serum vitamin levels
All serum samples were processed and delivered to the Division of Environmental Health Laboratory Sciences, National Center for Environmental Health, and Centers for Disease Control and Prevention (CDC) for analysis. Serum levels of VA (retinol), VC (ascorbic acid), and VE (α-tocopherol) were measured with high-performance liquid chromatography (HPLC) and quantitatively determined with spectrophotometric methods. Serum 25-hydroxyvitamin D [25(OH)D] levels were first determined with the DiaSorin RIA kit. NHANES employed regression to convert equivalently all 25(OH)D measurements obtained from standardized liquid chromatography–tandem mass spectrometry (LC-MS/MS) methods, to integrate the laboratory test data from different cycles. Serum VB9 and VB12 levels were measured with the radio-assay kit from Bio-Rad Laboratories. Serum VB6 level was measured with reversed-phase HPLC methods. All the laboratory assay data were accessible to the public on the NHANES website.
Covariates
Demographic variables were extracted from the baseline household questionnaires as covariates, including age, gender, ethnicity (Mexican American, non-Hispanic white, non-Hispanic black, and others), family income—poverty ratio (FIPR), and education level (below high school, high school or equivalent, and college or above). Histories of hypertension, high cholesterol, and diabetes were also collected. More details of the above-mentioned covariates were accessible to the public on the NHANES website.
Statistical analysis
According to the analytic guidelines for the NHANES survey, we employed sampling weights in our analyses and estimated variances by considering clustering and stratification. Binary or categorical variables were presented with number (%), while continuous variables were presented with median (interquartile range, IQR). The differences of population characteristics within the survey cycle were analyzed with Rao–Scott Chi-square test and Wilcoxon rank-sum test for categorical and continuous variables, respectively.
Multivariate-adjusted logistic regression models were employed to explore the relationship between the serum level of single vitamin and NAFLD. Serum levels of vitamins were analyzed as both continuous and categorical variables (classified into four groups according to quartiles, with the first quartile as reference). We computed the odds ratio (OR) and corresponding 95% confidence intervals (95% CIs) in three models. Model 1 included only independent variables. Model 2 was additionally adjusted for gender, age, ethnicity, FIPR, and education level. Model 3 was further adjusted for the disease history (hypertension, high cholesterol, and diabetes).
Weighted Quantile Sum (WQS) regression, a weighted quartile sum approach combined with logistic regression, was employed to examine the associations between both multivitamins/single-vitamin and the risk of NAFLD. The WQS regression integrated the serum levels of multivitamins into one index. The contribution of a single vitamin level was weighted according to its relevance to the overall association with the outcome. The weights are constrained to sum to 1, with higher numbers indicating a larger contribution (16, 17). The serum levels of vitamins were highly correlated, so there would be misleading results when employing linear regression models to examine the association of a single vitamin level with NAFLD while adjusting for other levels, due to the problem of collinearity. Therefore, we proposed WQS regression to examine the association of single serum vitamin level with NAFLD, while addressing the highly positive correlations among multivitamin levels.
All statistical analyses were completed with R 3.6.2, employing packages “lme4”. p < 0.05 for a two-tailed test denoted statistical significance.
Results
Characteristics of the study participants
There were 969 individuals with NAFLD (42.24%) among the 2,294 participants (Table 1). The mean ± SD age was 51 (36, 67) years [54 (40, 67) in participants with NAFLD and 49 (34, 67) in those without]. The proportions of men and women were 56.8 and 43.2%, respectively. Except for ethnicity and FIPR, there were statistical differences between participants with and without NAFLD in the basic characteristics. Participants with NAFLD were more likely to be male, older, less educated, or have hypertension/high cholesterol/diabetes. Most serum vitamin levels were lower in participants with NAFLD than in those without, except for VA and VE (Table 1).
TABLE 1.
Basic characteristics of participants by non-alcoholic fatty liver disease (NAFLD) in NHANES 2003–2006.
| Variables | Non-NAFLD (n = 1325) | With NAFLD (n = 969) | Total (n = 2294) | P-value |
| Sex, n (%) | <0.001 | |||
| Female | 650 (49.1) | 341 (35.2) | 991 (43.2) | |
| Male | 675 (50.9) | 628 (64.8) | 1303 (56.8) | |
| Age (years) | 49 (34, 67) | 54 (40, 67) | 51 (36, 67) | <0.001 |
| Race, n (%) | 0.431 | |||
| Mexican American | 178 (13.4) | 154 (15.9) | 332 (14.5) | |
| Non-Hispanic black | 225 (17) | 158 (16.3) | 383 (16.7) | |
| Non-Hispanic white | 847 (63.9) | 603 (62.2) | 1450 (63.2) | |
| Other | 75 (5.7) | 54 (5.6) | 129 (5.6) | |
| FIPR | 3.3 (1.7, 5) | 3 (1.6, 5) | 3.2 (1.7, 5) | 0.082 |
| Missing | 49 (3.7%) | 37 (3.8%) | 86 (3.7%) | |
| Education, n (%) | 0.006 | |||
| College or above | 828 (62.5) | 542 (55.9) | 1370 (59.7) | |
| High school or equivalent | 277 (20.9) | 236 (24.4) | 513 (22.4) | |
| Less than high school | 219 (16.5) | 191 (19.7) | 410 (17.9) | |
| Missing | 1 (0.1%) | 0 (0%) | 1 (0.0%) | |
| Hypertension, n (%) | <0.001 | |||
| No | 980 (74.2) | 518 (53.8) | 1498 (65.6) | |
| Yes | 341 (25.8) | 444 (46.2) | 785 (34.4) | |
| Missing | 4 (0.3%) | 7 (0.7%) | 11 (0.5%) | |
| High cholesterol, n (%) | <0.001 | |||
| No | 620 (62.2) | 389 (48.9) | 1009 (56.3) | |
| Yes | 376 (37.8) | 407 (51.1) | 783 (43.7) | |
| Missing | 329 (24.8%) | 173 (17.9%) | 502 (21.9%) | |
| Diabetes, n (%) | <0.001 | |||
| No | 1259 (95.8) | 806 (85.3) | 2065 (91.4) | |
| Yes | 55 (4.2) | 139 (14.7) | 194 (8.6) | |
| Missing | 11 (0.8%) | 24 (2.5%) | 35 (1.5%) | |
| VA (μmol/L) | 2.0 (1.7, 2.4) | 2.2 (1.8, 2.6) | 2.1 (1.7, 2.5) | <0.001 |
| Missing | 11 (0.8%) | 8 (0.8%) | 19 (0.8%) | |
| VE (μmol/L) | 27.9 (22.3, 35.8) | 30.1 (24.1, 40.6) | 28.7 (22.9, 37.8) | <0.001 |
| Missing | 11 (0.8%) | 16 (1.7%) | 27 (1.2%) | |
| VD (μmol/L) | 62.4 (48.3, 75.3) | 56.8 (42.2, 68.9) | 59.2 (45.9, 72.9) | <0.001 |
| Missing | 3 (0.2%) | 1 (0.1%) | 4 (0.2%) | |
| VC (μmol/L) | 63.0 (48.3, 77.2) | 51.7 (34, 67.7) | 59.1 (41.4, 73.8) | <0.001 |
| Missing | 7 (0.5%) | 5 (0.5%) | 12 (0.5%) | |
| VB6 (μmol/L) | 53.3 (29.2, 93.1) | 43.6 (26.9, 77) | 49.5 (27.9, 85.7) | <0.001 |
| Missing | 41 (3.1%) | 26 (2.7%) | 67 (2.9%) | |
| VB12 (μmol/L) | 366.0 (279.3, 493.0) | 331.0 (250.2, 439.3) | 352.0 (265.7, 467.3) | <0.001 |
| Missing | 17 (1.3%) | 13 (1.3%) | 30 (1.3%) | |
| VB9 (μmol/L) | 30.8 (22, 41.7) | 26.7 (19, 39.2) | 29 (20.6, 40.6) | <0.001 |
| Missing | 8 (0.6%) | 11 (1.1%) | 19 (0.8%) |
FIPR, family income-poverty ratio; VA, vitamin A; VE, vitamin E; VC, vitamin C; VD, vitamin D; VB6, vitamin B6, VB12, vitamin B12; VB9, vitamin B9. P-values were calculated by Rao–Scott chi-square test and Wilcoxon rank-sum test for categorical and continuous variables, respectively.
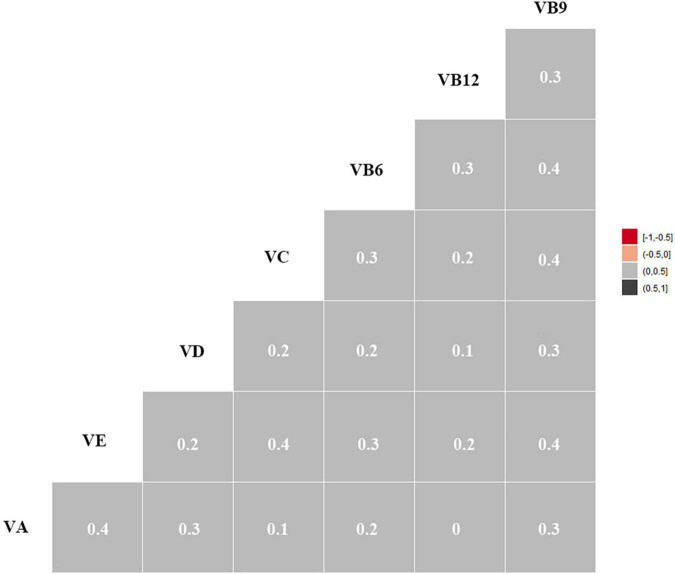
Pearson correlations (rs) of all studied vitamins are shown in Figure 2. Most of the vitamins were positively correlated with each other (rs = 0.1–0.4). The correlation coefficients between VB6 and VB9, VC and VB9, VE and VC, VE and VB9, and VA and VE were 0.4.
FIGURE 2.
Heat Map for Spearman correlation between serum vitamins. Correlation intensity between two vitamins was indicated by square color. In the heat map, the black color shows a strong positive expression correlation, whereas the gray color depicts a weak expression correlation. VA, vitamin A; VE, vitamin E; VC, vitamin C; VD, vitamin D; VB6, vitamin B6, VB12, vitamin B12; VB9, vitamin B9.
Serum level of single vitamin and the risk of non-alcoholic fatty liver disease
Table 2 showed the multi-variate adjusted OR and 95% CIs for the relationship between the risk of NAFLD and increment of serum level of seven studied vitamins. After adjusting for all covariates, negative associations with the risk of NAFLD were found for VC (OR = 0.985, 95% CI = 0.981–0.989), VD (OR = 0.981, 95% CI = 0.975–0.987), VB6 (OR = 0.995, 95% CI = 0.994–0.997), and VB9 (OR = 0.988, 95% CI = 0.979–0.997), while positive associations were found for VA (OR = 1.361, 95% CI = 1.045–1.772) and VE (OR = 1.020, 95% CI = 1.012–1.028). Moreover, we found that the risk of NAFLD increased by 36.1% (95% CI: 4.5%, 77.2%) with each unit increment of serum VA level in model 3.
TABLE 2.
Multi-variate adjusted odds ratio (95% CIs) for the relationship between the risk of NAFLD and increment of serum level of vitamins among participants in NHANES 2003–2006.
| Variables | Model l | Model 2 | Model 3 |
| VA | 1.768 (1.427, 2.189) | 1.551 (1.226, 1.964) | 1.361 (1.045, 1.772) |
| VE | 1.017 (1.011, 1.024) | 1.019 (1.011, 1.027) | 1.020 (1.012, 1.028) |
| VC | 0.981 (0.977, 0.985) | 0.982 (0.978, 0.985) | 0.985 (0.981, 0.989) |
| VD | 0.985 (0.980, 0.990) | 0.981 (0.976, 0.985) | 0.981 (0.975, 0.987) |
| VB6 | 0.996 (0.994, 0.998) | 0.995 (0.994, 0.997) | 0.995 (0.994, 0.997) |
| VB12 | 0.999 (0.998, 1.000) | 0.999 (0.998, 1.000) | 0.999 (0.998, 1.000) |
| VB9 | 0.987 (0.979, 0.996) | 0.985 (0.977, 0.994) | 0.988 (0.979, 0.997) |
All estimates accounted for complex survey designs. Model 1 included only independent variables; model 2 was additionally adjusted for gender, age, ethnicity, FIPR and education level; and model 3 was further adjusted for the disease history (hypertension, high cholesterol and diabetes). Those results in bold had statistical significance.
We repeated the above-mentioned statistical analyses by using vitamins as categorical variables (quartiles) and acquired similar results (Table 3). In addition, the risk of NAFLD showed a stepwise decrease with the quartiles of VB9 (P for trend < 0.05) and was at the lowest point in the highest VB9 quartile group (Q4) (OR, 0.537; 95% CI, 0.364-0.793) after adjusting for all covariates. Similar trends were found for VC and VB6. After adjusting for all covariates in model 3, the risk of NAFLD was higher in Q4 group than that in the Q1 group for both serum VA (OR, 1.661 (1.093, 2.526)) and serum VE (OR, 1.976 (1.377, 2.836)).
TABLE 3.
Multi-variate adjusted odds ratios (95% CIs) of risks of NAFLD in relation to serum vitamins levels among participants in NHANES 2003–2006.
| Variables | The range of serum vitamin levels (μ mol/L) | Model 1 | P-value | Model 2 | P-value | Model 3 | P-value |
| VA | 0.34–4.78 | ||||||
| Q1 | 0.34–1.72 | 1 | 1 | 1 | |||
| Q2 | 1.72–2.07 | 1.614 (1.239, 2.103) | 0.001 | 1.441 (1.097, 1.892) | 0.017 | 1.35 (0.932, 1.955) | 0.132 |
| Q3 | 2.07–2.45 | 1.619 (1.217, 2.155) | 0.003 | 1.248 (0.882, 1.766) | 0.226 | 1.083 (0.715, 1.64) | 0.712 |
| Q4 | 2.45–4.78 | 2.409 (1.761, 3.296) | 0.000 | 1.934 (1.359, 2.752) | 0.002 | 1.661 (1.093, 2.526) | 0.030 |
| p for trend | <0.001 | 0.010 | 0.102 | ||||
| VE | 2.04–89.12 | ||||||
| Q1 | 2.04–22.87 | 1 | 1 | 1 | |||
| Q2 | 22.89–28.68 | 1.185 (0.868, 1.619) | 0.295 | 1.248 (0.893, 1.745) | 0.210 | 1.393 (0.929, 2.09) | 0.128 |
| Q3 | 28.72–37.76 | 1.397 (1.049, 1.861) | 0.030 | 1.447 (1.043, 2.009) | 0.039 | 1.5 (1.014, 2.219) | 0.059 |
| Q4 | 37.83–89.12 | 1.736 (1.303, 2.314) | 0.001 | 1.905 (1.361, 2.666) | 0.001 | 1.976 (1.377, 2.836) | 0.002 |
| p for trend | <0.001 | 0.002 | 0.003 | ||||
| VC | 0.60–195.90 | ||||||
| Q1 | 0.60–41.40 | 1 | 1 | 1 | |||
| Q2 | 42.00–59.10 | 0.585 (0.431, 0.795) | 0.002 | 0.602 (0.437, 0.829) | 0.006 | 0.713 (0.498, 1.021) | 0.083 |
| Q3 | 59.60–73.80 | 0.35 (0.262, 0.469) | 0.000 | 0.374 (0.277, 0.505) | 0.000 | 0.463 (0.338, 0.632) | 0.000 |
| Q4 | 74.40–195.90 | 0.301 (0.229, 0.396) | 0.000 | 0.314 (0.246, 0.4) | 0.000 | 0.377 (0.282, 0.503) | 0.000 |
| p for trend | <0.001 | <0.001 | <0.001 | ||||
| VD | 9.10–166.00 | ||||||
| Q1 | 9.10–45.90 | 1 | 1 | 1 | |||
| Q2 | 47.10–59.20 | 0.951 (0.688, 1.314) | 0.763 | 0.873 (0.603, 1.263) | 0.479 | 0.987 (0.65, 1.499) | 0.953 |
| Q3 | 60.60–72.90 | 0.747 (0.562, 0.993) | 0.055 | 0.641 (0.475, 0.867) | 0.009 | 0.823 (0.552, 1.226) | 0.352 |
| Q4 | 73.80–166.00 | 0.496 (0.372, 0.661) | 0.000 | 0.411 (0.31, 0.546) | 0.000 | 0.426 (0.286, 0.635) | 0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | ||||
| VB6 | 3.80–400.00 | ||||||
| Q1 | 3.80–27.90 | 1 | 1 | 1 | |||
| Q2 | 28.00–49.50 | 1.136 (0.904, 1.427) | 0.285 | 0.993 (0.786, 1.256) | 0.955 | 0.871 (0.627, 1.211) | 0.424 |
| Q3 | 49.70–85.60 | 0.868 (0.672, 1.121) | 0.288 | 0.741 (0.558, 0.985) | 0.053 | 0.68 (0.472, 0.979) | 0.055 |
| Q4 | 85.80–400.00 | 0.632 (0.486, 0.823) | 0.002 | 0.528 (0.406, 0.688) | 0.000 | 0.529 (0.383, 0.73) | 0.001 |
| p for trend | 0.001 | <0.001 | 0.002 | ||||
| VB12 | 25.09–2952.00 | ||||||
| Q1 | 25.09–265.68 | 1 | 1 | 1 | |||
| Q2 | 266.40–352.00 | 0.726 (0.511, 1.032) | 0.086 | 0.714 (0.502, 1.017) | 0.077 | 0.75 (0.472, 1.189) | 0.239 |
| Q3 | 352.80–467.10 | 0.654 (0.49, 0.872) | 0.008 | 0.656 (0.487, 0.885) | 0.012 | 0.738 (0.513, 1.062) | 0.121 |
| Q4 | 467.90–2952.00 | 0.445 (0.324, 0.611) | 0.000 | 0.456 (0.324, 0.64) | 0.000 | 0.54 (0.36, 0.809) | 0.009 |
| p for trend | <0.001 | <0.001 | 0.005 | ||||
| VB9 | 5.40–144.30 | ||||||
| Q1 | 5.40–20.60 | 1 | 1 | 1 | |||
| Q2 | 20.80–29.00 | 0.683 (0.522, 0.894) | 0.010 | 0.665 (0.503, 0.879) | 0.010 | 0.668 (0.485, 0.92) | 0.025 |
| Q3 | 29.20–40.50 | 0.538 (0.395, 0.733) | 0.001 | 0.535 (0.381, 0.753) | 0.002 | 0.559 (0.357, 0.878) | 0.022 |
| Q4 | 40.80–144.30 | 0.52 (0.377, 0.716) | 0.000 | 0.492 (0.359, 0.676) | 0.000 | 0.537 (0.364, 0.793) | 0.006 |
| p for trend | 0.002 | 0.004 | 0.023 |
All estimates accounted for complex survey designs. Model 1 contains only independent variables; model 2 was additionally adjusted for gender, age, ethnicity, FIPR and education level; and model 3 was further adjusted for the disease history (hypertension, high cholesterol and diabetes). Those results in bold had statistical significance. Q1, Q2, Q3, and Q4 indicated the 1st, 2nd, 3rd, and 4th quartiles across the lowest to the highest concentrations of circulating vitamins.
Dose-response relationships between serum vitamin levels and non-alcoholic fatty liver disease
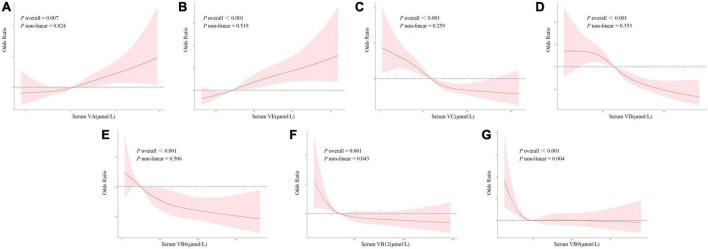
Dose–response curves for the relationships between all studied vitamins and the risk of NAFLD were presented in Figure 3, which showed similar directions to those curves in the single-vitamin logistic regression model.
FIGURE 3.
(A–G) Dose–response relationships between serum vitamin levels and the risk of NAFLD with restricted cubic spline model. The model was adjusted for gender, age, ethnicity, FIPR, education level, and disease history (hypertension, high cholesterol, and diabetes).
Figure 3A showed the dose–response relationship between serum VA level and NAFLD. There was a positive linear correlation between NAFLD and VA (p overall = 0.007, p non-linear = 0.824). Lower serum VA level was uncorrelated with NAFLD risk. However, the risk of NAFLD increased linearly as serum VA levels elevated when the latter exceeded a certain threshold.
Figure 3B showed the dose–response relationship between serum VE level and NAFLD. There was a positive linear correlation between NAFLD and VE (p overall < 0.001, p non-linear = 0.519). Lower serum VE level could be protective against NAFLD (OR < 1), but its protective effect weakened with elevated levels. The risk of NAFLD increased as serum VE levels elevated when the latter exceeded a certain threshold (OR > 1).
Figure 3C showed the dose–response relationship between serum VC level and NAFLD. There was a negative linear correlation between NAFLD and VC (p overall < 0.001, p non-linear = 0.259). A lower serum VC level could be a risk factor for NAFLD (OR > 1), but its risk effect weakened with an elevated level. The risk of NAFLD decreased as serum VC levels elevated when the latter exceeded a certain threshold (OR < 1). However, serum VC was uncorrelated with NAFLD risk when it was above a limited level.
Figure 3D showed the dose–response relationship between serum VD level and NAFLD. There was a negative linear correlation between NAFLD and VD (p overall < 0.001, p non-linear = 0.353). Lower serum VD level could be a risk factor for NAFLD (OR > 1), but its risk effect weakened with elevated levels. The risk of NAFLD decreased consistently as serum VD levels elevated when the latter exceeded a certain threshold (OR < 1).
Figure 3E showed the dose–response relationship between serum VB6 level and NAFLD. There was a negative linear correlation between NAFLD and VB6 (p overall < 0.001, p non-linear = 0.506). Its dose–response trend was similar to that of VD.
Figure 3F showed the dose–response relationship between serum VB12 level and NAFLD. There was a non-linear correlation between NAFLD and VB12 (p overall = 0.001, p non-linear = 0.043). Lower serum VB12 level could be a risk factor for NAFLD (OR > 1), but its risk effect weakened with elevated levels. However, serum VB12 was uncorrelated with NAFLD risk when it was above a limited level.
Figure 3G showed the dose–response relationship between serum VB9 level and NAFLD. There was a non-linear correlation between NAFLD and VB9 (p overall <0.001, p non-linear = 0.004). Its dose–response trend was similar to that of VB12.
Serum multivitamin levels and the risk of non-alcoholic fatty liver disease
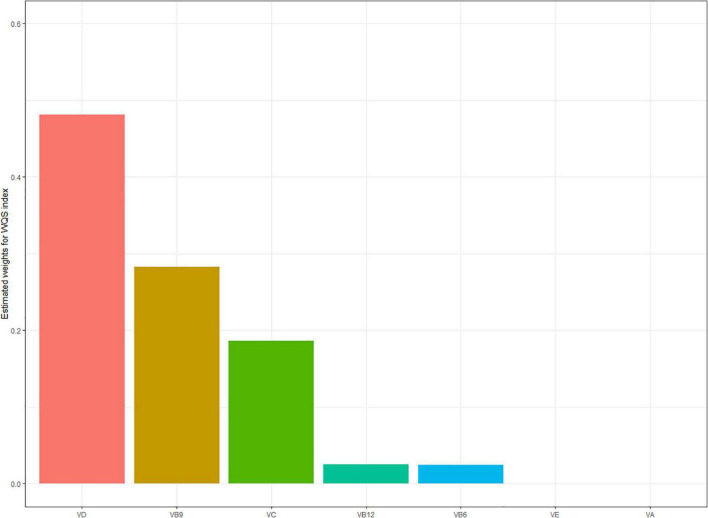
Table 4 showed the results from rough and fully adjusted models with WQS regression. We found the indices in WQS were significantly associated with NAFLD. In the fully adjusted models, each increment of the index was associated with 41.5% lower odds of NAFLD (OR = 0.585, 95% CI: 0482–0.712). We also observed the highest weights in WQS model: 0.48 for VD, 0.28 for VB9, and 0.19 for VC in negative correlation. VE and VA were observed in positive correlation (Figure 4).
TABLE 4.
Overall effects of the multivitamins estimates and 95% confidence interval.
| Model | OR | P |
| Model 1 | 0.580 (0.508, 0.662) | <0.001 |
| Model 2 | 0.527 (0.447, 0.622) | <0.001 |
| Model 3 | 0.585 (0.482, 0.712) | <0.001 |
All estimates accounted for complex survey designs. Model 1 included only independent variables; model 2 was additionally adjusted for gender, age, ethnicity, FIPR, and education level; and model 3 was further adjusted for the disease history (hypertension, high cholesterol, and diabetes).
FIGURE 4.
Estimated weights assigned to each vitamin level with the WQS regression model in a negative direction. Model adjusted for gender, age, ethnicity, FIPR, education level, and disease history (hypertension, high cholesterol, and diabetes).
Discussion
To date, most population-based research studies focused on the relationship between single vitamin and the risk of NAFLD (9, 18, 19). A few research studies explored their dose–response relationship. There was no reported evidence for the correlations between multivitamins and NAFLD risk. People normally encounter mixed exposures rather than a single exposure due to multivitamins contained in daily food. Unlike previous studies, in this cross-sectional study of a nationally representative sample, we explored for the first time the associations between serum multivitamin levels and the risk of NAFLD as well as their dose–response relationships through comprehensive analyses with multiple novel statistical approaches. We found that serum levels of VC, VD, VB6, and VB9 were negatively correlated with the risk of NAFLD, while serum levels of VA and VE were positively correlated with the risk of NAFLD. We also observed linear correlations of those five vitamins with the risk of NAFLD from the dose–response curves. WQS regression suggested significant associations between serum multivitamin levels and reduced risk of NAFLD, with major contributors of VD, VB9, and VC. Our findings suggested that serum multivitamin levels were significantly associated with the risk of NAFLD.
Vitamin A and the risk of non-alcoholic fatty liver disease
As a lipid-soluble vitamin and an antioxidant, VA (retinol) existed abundantly in animals, especially in fish. Interestingly enough, VA intake might not guarantee any antioxidative and/or protective effects (20). Instead, long-term intake of VA would elevate the levels of alkaline phosphatase, triacylglycerol, and cholesterol (21), which were somehow associated with the risk of NAFLD. Based on the dose–response relationship curves, we suggested that there was a linear positive correlation between serum VA level and the risk of NAFLD. VA played an important role in both metabolic regulation and hepatic stellate cell activation, so it might be a critical factor during the exploration of NAFLD pathogenesis (22). Previous studies proposed some evidence for the association between VA and NAFLD. Similar to our findings, Bahcecioglu et al. suggested that patients with non-alcoholic steatohepatitis (NASH) or simple hepatic steatosis have elevated serum VA levels compared with healthy individuals (23). According to a prospective longitudinal study including 2,658 participants (24), higher serum VA level was associated with the progression of NAFLD, which was possibly attributed to increased triglycerides, insulin resistance, serum retinol-binding protein 4, and BMI. However, some studies proposed different conclusions. Botella-Carretero et al. suggested that serum VA level was negatively correlated with BMI for morbidly obese people as well as the level of serum transaminase for NAFLD patients (25). A study suggested that there was a significant association between low retinol levels and insulin resistance (26). Those discrepancies might be due to the differences in overall serum VA levels in the included population. According to the dose–response curves, a lower serum VA level was protective against NAFLD, while higher serum VA level became a risk factor for NAFLD. Therefore, we suggested that maintaining a proper serum VA level might be good for health.
Vitamin C and the risk of non-alcoholic fatty liver disease
We found that serum VC level was negatively correlated with the risk of NAFLD, as well as the most important contributor to the prevalence of NAFLD in U.S. adults. Vitamin C, also known as ascorbic acid, was a water-soluble vitamin and existed in a variety of vegetables and fruits. Wei et al. (27) found that the risk of NAFLD was decreased by 0.71 times in the highest quartile of dietary vitamin C intake compared with the lowest quartile, which was similar to our findings. More and more epidemiologic studies proposed that serum VC levels had a significant negative correlation with hepatic steatosis and hepatic fibrosis (28, 29). According to a prospective double-blinded randomized controlled trial, oral VC supplements could significantly improve liver function and glucose metabolism as well as guarantee intestinal microbial diversity and adiponectin concentration for patients with NAFLD (30). A suitable intake of VC could alleviate hepatic fibrosis for patients with NASH (31). And as an antioxidant, VC could scavenge free radicals, enhance the activity of manganese superoxide dismutase (SOD) and glutathione peroxidase (GPx), improve the secretion and expression of adiponectin, and lower the levels of low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG) (32–35), which provided possible explanations for that VC could reduce the risk of NAFLD.
Vitamin D and the risk of non-alcoholic fatty liver disease
Vitamin D, a secosteroid, had an influence not only on calcium homeostasis but also on cell differentiation and proliferation, immune modulation, and inflammatory response. We found that there was a negative linear correlation between serum VD level and the risk of NAFLD. According to a cross-sectional study including 16,190 participants (36), serum VD level had a significant negative correlation with liver enzyme, insulin resistance, and the components of metabolic syndrome, which was similar to our findings. The elevated serum VD level might alleviate inflammation/steatosis of the liver and improve insulin sensitivity by activating liver macrophage vitamin D receptors (VDR) (37). However, some pieces of evidence for the association between VD deficiency (VDD) and the risk of NAFLD were still controversial. Animal studies suggested that VDD would exacerbate NAFLD by activating Toll-like receptors (TLRs). Moreover, VDD would result in insulin resistance, up-regulation of hepatic inflammatory and oxidative stress genes, and higher hepatic resistin gene expression (38). On the contrary, a 16-week RCT study (39) reported that VD supplements in non-Western VDD immigrants with prediabetes failed to improve insulin sensitivity or β-cell function or change the incidence of metabolic syndrome. One possible explanation would be that the change in insulin sensitivity after the VD supplement was influenced by the single nucleotide polymorphisms (SNPs) of the VDR gene (40). Therefore, SNPs of the VDR gene should be considered when concluding that the VD supplement was beneficial to NAFLD.
Vitamin B6 and the risk of non-alcoholic fatty liver disease
VB6 was a key cofactor in the metabolism of amino acids, glucose, and fat. Similar to VC and VD, VB6 was also negatively correlated with the risk of NAFLD. Federico et al. found that patients with NASH had a less daily intake of VB6 than those in the control group (41). A prospective clinical study suggested that an oral VB6 supplement could significantly ameliorate liver fat accumulation in patients with NAFLD (42). Moreover, Lin et al. proposed that a borderline VB6 deficit was associated with the increased risk of dyslipidemia and coronary artery disease (43). The deficiency of VB6 resulted in homocysteine (Hcy) accumulation. Hcy would induce protein misfolding in the endoplasmic reticulum (ER), triggering a stress response in the ER. Then ER stress drives de novo lipogenesis by activating the transcription factor sterol response element-binding protein 1c (SREBP-1c), resulting in NAFLD development (44). VB6 could prevent insulin resistance, endothelial dysfunction, and liver fat accumulation (45), which were possible underlying mechanisms of VB6 to reduce the risk of NAFLD.
Vitamin B9 and the risk of non-alcoholic fatty liver disease
VB9, also known as folate, is a water-soluble vitamin widely distributed in green leafy vegetables such as spinach, beet, and kale. The dose–response curve showed that serum VB9 was non-linearly correlated with NAFLD risk and would increase the incidence of NAFLD when staying at a lower level. Similarly, Xia et al. (8) suggested that serum folate level was negatively correlated with the grade of hepatic steatosis and liver fat content, and low serum folate level was an independent risk factor for NAFLD. The liver was an essential organ for storage and metabolism of folate. More and more research studies confirmed that folate was closely associated with lipid metabolism. Folate deficiency might depress phospholipid N-methylation in the liver and reduce de novo phosphatidylcholine synthesis, resulting in TG accumulation in the liver (46), which was further verified in animal experiments by Pogribny et al. (47). Moreover, folate deficiency would accelerate the synthesis of hepatic lipid by inducing related genes, resulting in hepatic steatosis (48). It was noteworthy that excessive high serum folate levels might increase the risk of cognitive disorder and insulin resistance (49). Maintaining a proper serum folate level might be good for health.
Vitamin E and the risk of non-alcoholic fatty liver disease
In this study, serum VE level had a significant positive correlation with the risk of NAFLD. With each increment of the VE level, the risk of NAFLD elevated by approximately 1.9% (95% CI: 1.1–1.2%). Jeon et al. suggested that serum VE level was positively associated with the prevalence of NAFLD, which was similar to our findings (50). Alpha-tocopherol (VE) was identified as one of the predictors of MRI-determined liver fat (51). A cross-sectional study on Swedish adults proposed that serum VE level was positively associated with serum cholesterol and obesity (52). Waniek et al. (53) suggested that alpha-tocopherol level was positively associated with high triglycerides and low high-density lipoprotein cholesterol (HDL-C) levels. Moreover, serum VE levels played important roles in visceral adipose tissue and metabolic syndrome. Animal experiments indicated that mice with NAFLD had impaired liver metabolism and gene response of alpha-tocopherol (54). All these studies provided evidence that serum VE level was positively associated with the risk of NAFLD.
Multi-vitamins and the risk of non-alcoholic fatty liver disease
People were exposed to a variety of vitamins in daily life due to different dietary structures or nutritional components in food. It was necessary to explore the effect of multi-vitamins on health. In this study, we employed the WQS regression model to explore the association between serum levels of multivitamins and the risk of NAFLD. Results indicated that higher serum levels of multivitamins are associated with reduced NAFLD risk, despite different influences and weights of a single vitamin. There was no study exploring the relationship between multivitamins and NAFLD. It was incorrect to include all studied vitamins in a single generalized linear regression model, which might distort the results due to the correlation that exists among vitamins (55). The WQS was a novel model to explore the influences of multivitamins on health, by considering highly correlated vitamins. Based on the weights empirically determined by bootstrap sampling, we used WQS to examine the whole-body burden with serum levels of multivitamins. The WQS enabled us to encompass the complex serum levels of multivitamins in the real world. In this study, VD, VB9, and VC were weighted highly. More prospective cohort and large-population studies are necessary to determine the contributions and mechanism of serum multivitamin levels to NAFLD.
Strengths and limitations
Strengths: We employed, in this study, for the first time both logistic regression and WQS regression models that enabled us to systematically assess the association between serum multivitamin levels and the risk of NAFLD, as well as to explore the dose–response relationship between single vitamin and the risk of NAFLD. Moreover, this study covered a wide range of participants with good sample representativeness. We also adjusted important covariates in our models, including demographic characteristics and disease history.
Limitations: First, serum levels of vitamins were tested only once at baseline, which might not represent a long-term status. Second, the diagnosis of NAFLD was based on the FLI model, instead of a gold standard in histology. Third, although we adjusted multiple covariates in our study, there might be other potential confounders such as dietary intakes, physical activities, and metabolic syndrome. And last, our work was based on a cross-sectional study and could not confirm the cause-and-effect relationships between the serum vitamin level and the risk of NAFLD. More large-scale and prospective cohort studies should be encouraged in the future.
Conclusion
This cross-sectional study explored the association between serum levels of 7 common vitamins and the risk of NAFLD. Among U.S. adults, high serum levels of VC, VD, VB6, and VB9 and low serum levels of VA/VE were associated with reduced risk of NAFLD. Among all the 7 kinds of vitamins, VD was weighted highest and the most important. Proper serum levels of multivitamins contributed to a lower risk of NAFLD. Our findings suggested a novel perspective to explore the association between multivitamins and NAFLD risk. Further studies are still required to reveal the underlying mechanisms of multivitamins to NAFLD.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The protocol of NHANES was reviewed and approved by the Research Ethics Review Board of the National Center for Health Statistics. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HP and WL conceived of the study. HP, MW, and LP conducted the data analysis and drafted the manuscript. ZC, ZY, and QC drafted the manuscript. YL and YW visualized the result. All authors edited the manuscript, read and approved the final manuscript.
Funding
This research was funded by the Scientific and Technological Innovation Project of China, Academy of Chinese Medical Sciences (CI2021A00801 and CI2021A00802).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.962705/full#supplementary-material
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 2.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. (2018) 67:123–33. 10.1002/hep.29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. (2020) 323:1175–83. 10.1001/jama.2020.2298 [DOI] [PubMed] [Google Scholar]
- 4.Barchetta I, Cimini FA, Cavallo MG. Vitamin D and metabolic dysfunction-associated fatty liver disease (Mafld): an update. Nutrients. (2020) 12:3302. 10.3390/nu12113302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeed A, Dullaart RPF, Schreuder T, Blokzijl H, Faber KN. Disturbed vitamin a metabolism in non-alcoholic fatty liver disease (NAFLD). Nutrients. (2017) 10:29. 10.3390/nu10010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagashimada M, Ota T. Role of vitamin E in nonalcoholic fatty liver disease. IUBMB Life. (2019) 71:516–22. 10.1002/iub.1991 [DOI] [PubMed] [Google Scholar]
- 7.Fang H, Li Z, Graff EC, McCafferty KJ, Judd RL. Niacin increases diet-induced hepatic steatosis in B6129 mice. Biochim Biophys Acta Mol Cell Biol Lipids. (2020) 1865:158731. 10.1016/j.bbalip.2020.158731 [DOI] [PubMed] [Google Scholar]
- 8.Xia MF, Bian H, Zhu XP, Yan HM, Chang XX, Zhang LS, et al. Serum Folic Acid Levels Are Associated with the Presence and Severity of Liver Steatosis in Chinese Adults. Clin Nutr. (2018) 37:1752–8. 10.1016/j.clnu.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 9.Li L, Huang Q, Yang L, Zhang R, Gao L, Han X, et al. The association between non-alcoholic fatty liver disease (Nafld) and advanced fibrosis with serological vitamin B12 markers: results from the Nhanes 1999-2004. Nutrients. (2022) 14:1224. 10.3390/nu14061224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeed A, Bartuzi P, Heegsma J, Dekker D, Kloosterhuis N, de Bruin A, et al. Impaired hepatic vitamin a metabolism in Nafld mice leading to vitamin a accumulation in hepatocytes. Cell Mol Gastroenterol Hepatol. (2021) 11:309–325.e3. 10.1016/j.jcmgh.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renner S, Kuçi Z, d’Cruze H, Niethammer D, Bruchelt G. Isotachophoretic analysis of the dihydrofolate reductase reaction in the presence of methotrexate and ascorbic acid. Electrophoresis. (2000) 21:2828–33. 10.1002/1522-2683(20000801)21:143.0.Co;2-r [DOI] [PubMed] [Google Scholar]
- 12.Galan P, Viteri FE, Bertrais S, Czernichow S, Faure H, Arnaud J, et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general french adult population. Eur J Clin Nutr. (2005) 59:1181–90. 10.1038/sj.ejcn.1602230 [DOI] [PubMed] [Google Scholar]
- 13.Wolffenbuttel BHR, Heiner-Fokkema MR, Green R, Gans ROB. Relationship between serum B12 concentrations and mortality: experience in Nhanes. BMC Med. (2020) 18:307. 10.1186/s12916-020-01771-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cueto-Galán R, Barón FJ, Valdivielso P, Pintó X, Corbella E, Gómez-Gracia E, et al. Changes in fatty liver index after consuming a mediterranean diet: 6-year follow-up of the predimed-malaga trial. Med Clin. (2017) 148:435–43. 10.1016/j.medcli.2016.11.032 [DOI] [PubMed] [Google Scholar]
- 15.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. 10.1186/1471-230x-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. (2015) 20:100–20. 10.1007/s13253-014-0180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czarnota J, Gennings C, Colt JS, De Roos AJ, Cerhan JR, Severson RK, et al. Analysis of environmental chemical mixtures and non-hodgkin lymphoma risk in the nci-seer nhl study. Environ Health Perspect. (2015) 123:965–70. 10.1289/ehp.1408630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Liu Y, Wan B, Zhang H, Wu S, Zhu Z, et al. Association between vitamin D status and non-alcoholic fatty liver disease: a population-based study. J Nutr Sci Vitaminol. (2019) 65:303–8. 10.3177/jnsv.65.303 [DOI] [PubMed] [Google Scholar]
- 19.Wan B, Gao Y, Zheng Y, Chen R. Association between serum 25-hydroxy vitamin D level and metabolic associated fatty liver disease (Mafld)-a population-based study. Endocr J. (2021) 68:631–7. 10.1507/endocrj.EJ20-0758 [DOI] [PubMed] [Google Scholar]
- 20.Petiz LL, Girardi CS, Bortolin RC, Kunzler A, Gasparotto J, Rabelo TK, et al. Vitamin a oral supplementation induces oxidative stress and suppresses Il-10 and Hsp70 in skeletal muscle of trained rats. Nutrients. (2017) 9:353. 10.3390/nu9040353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cartmel B, Moon TE, Levine N. Effects of long-term intake of retinol on selected clinical and laboratory indexes. Am J Clin Nutr. (1999) 69:937–43. 10.1093/ajcn/69.5.937 [DOI] [PubMed] [Google Scholar]
- 22.Blaner WS. Vitamin a signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. (2019) 197:153–78. 10.1016/j.pharmthera.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahcecioglu IH, Yalniz M, Ilhan N, Ataseven H, Ozercan IH. Levels of serum vitamin a, alpha-tocopherol and malondialdehyde in patients with non-alcoholic steatohepatitis: relationship with histopathologic severity. Int J Clin Pract. (2005) 59:318–23. 10.1111/j.1742-1241.2004.00312.x [DOI] [PubMed] [Google Scholar]
- 24.Xiao ML, Zhong HL, Lin HR, Liu CY, Yan Y, Ke YB, et al. Higher serum vitamin a is associated with a worsened progression of non-alcoholic fatty liver disease in adults: a prospective study. Food Func. (2022) 13:970–7. 10.1039/d1fo03119h [DOI] [PubMed] [Google Scholar]
- 25.Botella-Carretero JI, Balsa JA, Vázquez C, Peromingo R, Díaz-Enriquez M, Escobar-Morreale HF. Retinol and alpha-tocopherol in morbid obesity and nonalcoholic fatty liver disease. Obes Surg. (2010) 20:69–76. 10.1007/s11695-008-9686-5 [DOI] [PubMed] [Google Scholar]
- 26.Villaça Chaves G, Pereira SE, Saboya CJ, Ramalho A. Non-alcoholic fatty liver disease and its relationship with the nutritional status of vitamin a in individuals with class iii obesity. Obes Surg. (2008) 18:378–85. 10.1007/s11695-007-9361-2 [DOI] [PubMed] [Google Scholar]
- 27.Wei J, Lei GH, Fu L, Zeng C, Yang T, Peng SF. Association between dietary vitamin C intake and non-alcoholic fatty liver disease: a cross-sectional study among middle-aged and older adults. PLoS One. (2016) 11:e0147985. 10.1371/journal.pone.0147985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Shen H, Chen M, Shao J. Clinical relevance of vitamins and carotenoids with liver steatosis and fibrosis detected by transient elastography in adults. Front Nutr. (2021) 8:760985. 10.3389/fnut.2021.760985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie ZQ, Li HX, Tan WL, Yang L, Ma XW, Li WX, et al. Association of serum vitamin C with Nafld and Mafld among adults in the United States. Front Nutr. (2021) 8:795391. 10.3389/fnut.2021.795391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He Z, Li X, Yang H, Wu P, Wang S, Cao D, et al. Effects of oral vitamin C supplementation on liver health and associated parameters in patients with non-alcoholic fatty liver disease: a randomized clinical trial. Front Nutr. (2021) 8:745609. 10.3389/fnut.2021.745609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. (2003) 98:2485–90. 10.1111/j.1572-0241.2003.08699.x [DOI] [PubMed] [Google Scholar]
- 32.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. (1993) 300:535–43. 10.1006/abbi.1993.1074 [DOI] [PubMed] [Google Scholar]
- 33.Rose FJ, Webster J, Barry JB, Phillips LK, Richards AA, Whitehead JP. Synergistic effects of ascorbic acid and thiazolidinedione on secretion of high molecular weight adiponectin from human adipocytes. Diabetes Obes Metab. (2010) 12:1084–9. 10.1111/j.1463-1326.2010.01297.x [DOI] [PubMed] [Google Scholar]
- 34.McRae MP. Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: a meta-analysis of 13 randomized controlled trials. J Chiropr. Med. (2008) 7:48–58. 10.1016/j.jcme.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valdecantos MP, Pérez-Matute P, Quintero P, Martínez JA. Vitamin C, resveratrol and lipoic acid actions on isolated rat liver mitochondria: all antioxidants but different. Redox Rep. (2010) 15:207–16. 10.1179/135100010x12826446921464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong HC, Lee JS, Choi HY, Yang SJ, Yoo HJ, Seo JA, et al. Liver enzymes and vitamin d levels in metabolically healthy but obese individuals: Korean national health and nutrition examination survey. Metabolism. (2013) 62:1305–12. 10.1016/j.metabol.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 37.Dong B, Zhou Y, Wang W, Scott J, Kim K, Sun Z, et al. Vitamin D receptor activation in liver macrophages ameliorates hepatic inflammation, steatosis, and insulin resistance in mice. Hepatology. (2020) 71:1559–74. 10.1002/hep.30937 [DOI] [PubMed] [Google Scholar]
- 38.Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and toll-like receptor activation. Hepatology. (2012) 55:1103–11. 10.1002/hep.24737 [DOI] [PubMed] [Google Scholar]
- 39.Oosterwerff MM, Eekhoff EM, Van Schoor NM, Boeke AJ, Nanayakkara P, Meijnen R, et al. Effect of moderate-dose vitamin d supplementation on insulin sensitivity in vitamin D-deficient non-western immigrants in the netherlands: a randomized placebo-controlled trial. Am J Clin Nutr. (2014) 100:152–60. 10.3945/ajcn.113.069260 [DOI] [PubMed] [Google Scholar]
- 40.Jain R, von Hurst PR, Stonehouse W, Love DR, Higgins CM, Coad J. Association of vitamin D receptor gene polymorphisms with insulin resistance and response to vitamin D. Metabolism. (2012) 61:293–301. 10.1016/j.metabol.2011.06.018 [DOI] [PubMed] [Google Scholar]
- 41.Federico A, Dallio M, Caprio GG, Gravina AG, Picascia D, Masarone M, et al. Qualitative and quantitative evaluation of dietary intake in patients with non-alcoholic steatohepatitis. Nutrients. (2017) 9:1074. 10.3390/nu9101074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi T, Kessoku T, Ozaki A, Iwaki M, Honda Y, Ogawa Y, et al. Vitamin B6 efficacy in the treatment of nonalcoholic fatty liver disease: an open-label, single-arm, single-center trial. J Clin Biochem Nutr. (2021) 68:181–6. 10.3164/jcbn.20-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin PT, Cheng CH, Liaw YP, Lee BJ, Lee TW, Huang YC. Low pyridoxal 5’-phosphate is associated with increased risk of coronary artery disease. Nutrition. (2006) 22:1146–51. 10.1016/j.nut.2006.08.013 [DOI] [PubMed] [Google Scholar]
- 44.Ai Y, Sun Z, Peng C, Liu L, Xiao X, Li J. Homocysteine induces hepatic steatosis involving er stress response in high methionine diet-fed mice. Nutrients. (2017) 9:346. 10.3390/nu9040346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, Li P, Zhao ZH, Zhang Y, Ma ZM, Wang SX. Vitamin B6 prevents endothelial dysfunction, insulin resistance, and hepatic lipid accumulation in apoe (-/-) mice fed with high-fat diet. J Diabetes Res. (2016) 2016:1748065. 10.1155/2016/1748065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akesson B, Fehling C, Jägerstad M, Stenram U. Effect of experimental folate deficiency on lipid metabolism in liver and brain. Br J Nutr. (1982) 47:505–20. 10.1079/bjn19820063 [DOI] [PubMed] [Google Scholar]
- 47.Pogribny IP, Kutanzi K, Melnyk S, de Conti A, Tryndyak V, Montgomery B, et al. Strain-dependent dysregulation of one-carbon metabolism in male mice is associated with choline- and folate-deficient diet-induced liver injury. FASEB J. (2013) 27:2233–43. 10.1096/fj.12-227116 [DOI] [PubMed] [Google Scholar]
- 48.Champier J, Claustrat F, Nazaret N, Fèvre Montange M, Claustrat B. Folate depletion changes gene expression of fatty acid metabolism, dna synthesis, and circadian cycle in male mice. Nutr Res. (2012) 32:124–32. 10.1016/j.nutres.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 49.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. (2008) 87:517–33. 10.1093/ajcn/87.3.517 [DOI] [PubMed] [Google Scholar]
- 50.Jeon D, Son M, Shim J. Dynamics of serum retinol and alpha-tocopherol levels according to non-alcoholic fatty liver disease status. Nutrients. (2021) 13:1720. 10.3390/nu13051720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim U, Turner SD, Franke AA, Cooney RV, Wilkens LR, Ernst T, et al. Predicting total, abdominal, visceral and hepatic adiposity with circulating biomarkers in caucasian and Japanese American women. PLoS One. (2012) 7:e43502. 10.1371/journal.pone.0043502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallström P, Wirfält E, Lahmann PH, Gullberg B, Janzon L, Berglund G. Serum concentrations of beta-carotene and alpha-tocopherol are associated with diet, smoking, and general and central adiposity. Am J Clin Nutr. (2001) 73:777–85. 10.1093/ajcn/73.4.777 [DOI] [PubMed] [Google Scholar]
- 53.Waniek S, di Giuseppe R, Plachta-Danielzik S, Ratjen I, Jacobs G, Koch M, et al. Association of vitamin E levels with metabolic syndrome, and mri-derived body fat volumes and liver fat content. Nutrients. (2017) 9:1143. 10.3390/nu9101143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartolini D, Torquato P, Barola C, Russo A, Rychlicki C, Giusepponi D, et al. Nonalcoholic fatty liver disease impairs the cytochrome p-450-dependent metabolism of α-tocopherol (Vitamin E). J Nutr Biochem. (2017) 47:120–31. 10.1016/j.jnutbio.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 55.Marill KA. Advanced statistics: linear regression, part ii: multiple linear regression. Acad Emerg Med. (2004) 11:94–102. 10.1197/j.aem.2003.09.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.