Abstract
Background
The Spanish registry of α1-antitrypsin deficiency (AATD) integrated in the European Alpha-1 Research Collaboration (EARCO) provides information about the characteristics of patients, in particular those with the PI*SZ genotype, which is frequent in Spain.
Method
Individuals with severe AATD defined as proteinase inhibitor (PI) genotypes PI*ZZ, PI*SZ and other rare deficient variants were included from February 1, 2020, to February 1, 2022. The analysis focused on a comparison of the characteristics of PI*ZZ and PI*SZ patients.
Results
409 patients were included (53.8% men) with a mean±sd age of 53.5±15.9 years. Genotypes were PI*ZZ in 181 (44.7%), PI*SZ in 163 (40.2%), PI*SS in 29 (7.2%) and other in 32 (7.9%). 271 (67.4%) had lung disease: 175 chronic obstructive pulmonary disease (43.5%), 163 emphysema (40.5%) and 83 bronchiectasis (20.6%). Patients with the PI*SZ genotype were younger, more frequently non-index cases and had a lower frequency of respiratory diseases except asthma compared with PI*ZZ patients. Among patients with respiratory diseases, PI*SZ individuals were significantly older both at onset of symptoms and at diagnosis; only asthma was more frequent in PI*SZ than in PI*ZZ individuals. Twelve PI*SZ patients (15.4%) received augmentation therapy compared with 94 PI*ZZ patients (66.2%; p<0.001).
Conclusions
There is a high prevalence of PI*SZ in Spain. Patients with the PI*SZ genotype were older at symptom onset and diagnosis and had less severe lung disease compared with PI*ZZ patients. The prevalence of asthma was higher in PI*SZ, and up to 15% of PI*SZ patients received augmentation therapy.
Short abstract
Patients with the PI*ZZ genotype have more severe pulmonary disease than those with the PI*SZ genotype. However, asthma is as frequent in PI*SZ as in PI*ZZ. https://bit.ly/3m10MNN
Introduction
α1-antitrypsin deficiency (AATD) is considered the most frequent potentially severe rare disease affecting adults, and its prevalence is around two in 10 000 in Europe [1]. AATD may predispose to liver disease in children and adults and pulmonary emphysema in adults [2].
The majority of patients with severe AATD are homozygous carriers of the Z mutation of the gene coding for the α1-antitrypsin (AAT) protein (proteinase inhibitor (PI*ZZ)), but hundreds of variants have been identified, some of which are associated with low serum AAT levels [3, 4]. Among these variants, the most frequent is the S variant, which is particularly frequent in Western Spain [5]. The risk of lung disease associated with the heterozygous PI*SZ genotype is controversial. Some carriers have serum levels below those considered protective and may be candidates for augmentation therapy with intravenous AAT, if emphysema develops [6, 7], while some studies suggest that the risk of lung disease associated with PI*SZ is significantly less than that associated with PI*ZZ [8].
The low prevalence of AATD and the extreme variability of its respiratory clinical manifestations make it likely that a single centre will never see enough patients to acquire experience in the management of this condition. Therefore, the European Council [9] and the European Respiratory Society (ERS) [1] recommend organising the care of AATD patients in reference centres and developing prospective registries to better understand the natural history of the disease and the potential impact of different treatments [10]. The Spanish registry of AATD was founded in 1992 [11] and in 2020 it was merged into the new European Alpha-1 Research Collaboration (EARCO) International Registry [12]. The EARCO registry is a prospective international registry developed by the EARCO Clinical Research Collaboration of the ERS [13], and the Spanish registry consists of the Spanish investigators, centres and participants included in the EARCO registry [14].
The current study presents the demographic and clinical characteristics of the participants in the Spanish registry in EARCO and, because Spain is one of the countries with the highest prevalence of the S allele, it provides a unique opportunity to investigate the differential characteristics of individuals with the PI*SZ genotype [15] and compare them with those of PI*ZZ individuals.
Method
Structure of the Spanish registry of AATD
The Spanish registry of patients with AATD is constituted by the Spanish investigators and patients registered in the EARCO International Registry [14]. The registry is open to every clinician who manages AATD patients in Spain. The registry is observational and, therefore, patients are treated according to the attending physicians’ criteria. The Spanish registry has one national coordinator (MT-D) designated by the Spanish Society of Pneumology and Thoracic Surgery (SEPAR), and a steering committee formed by clinicians and researchers with expertise in the disease. According to the data processor agreement signed between the national coordinator, representing the SEPAR and the Spanish registry, and the data controller of EARCO (Vall d'Hebron Research Institute, Barcelona, Spain), it is free to access the fully anonymised data of the Spanish patients in the Spanish registry for analysis and evaluation.
The study protocol received central ethics approval by the research ethics committee of the Vall d'Hebron University Hospital of Barcelona, Spain (PR(AG)480/2018) and was subsequently approved by all the participating centres. All participants provided written informed consent. The EARCO registry protocol has been registered at www.clinicaltrials.gov (ID: NCT04180319) and is hosted by www.earco.org. The personal data of the patients are kept under strict confidentiality in compliance with the provisions of the Spanish Organic Law 3/2018 of December 5, 2018, Protection of Personal Data and Guarantee of Digital Rights (LOPDGDD) and its development regulations, and in accordance with the provisions of the General Data Protection Regulation (GDPR) 2016/679 of the European Parliament and of the European Council of April 27, 2016, regarding the protection of personal data.
Objectives of the Spanish AATD registry
The objectives of the Spanish AATD registry are linked to those of EARCO and have been described in detail in a previous publication [16]. Briefly, the main objectives are 1) to generate longitudinal long-term, high-quality clinical data of individuals with AATD; 2) to understand the natural history and prognosis of AATD; 3) to investigate the effect of AAT augmentation and other therapies on the progression of lung disease; and 4) to learn more about the course of the disease in patients with severe AATD with genotypes other than PI*ZZ.
In the current publication, we analyse the baseline data of patients included in the Spanish registry in the first 2 years of EARCO, from February 1, 2020, to February 1, 2022.
Population and measurements
The protocol of the study has been published previously [16]. The inclusion criteria are 1) individuals with diagnosed severe AATD; 2) deficiency defined as AAT serum levels <11 μM (50 mg·dL−1) and/or proteinase inhibitor genotypes PI*ZZ, PI*SZ and compound heterozygotes or homozygotes of other rare deficient variants. The only exclusion criterion is lack of patient consent.
The data collected include the following domains: demographics, proteinase inhibitor genotype and other laboratory analyses, comorbidities, lung function, respiratory symptoms, ultrasound-based elastography of the liver, exacerbations of respiratory disease, quality of life, physical activity, chest computed tomography (as applicable) and treatment.
Data are entered into a secure database through an electronic case report form hosted by the EARCO website (www.earco.org). Data are centrally monitored, and queries are sent for missing or invalid data.
Statistical analysis
Qualitative variables are described with absolute frequencies and percentages. Quantitative variables are described as mean±sd or median and quartiles. The Kolmogorov–Smirnov test was used to assess the normality of distributions. The sociodemographic and clinical characteristics were compared according to the genotypes PI*ZZ and PI*SZ. In the case of quantitative variables, a t-test was carried out (Mann–Whitney U-test if normality was not assumed). A Chi-squared test (Fisher test for frequencies <5) was used to compare categorical variables. Cigarette smoking pack-years were transformed into an ordinal variable (0, <10, 10–20, 20–30 and >30) to compare the per cent predicted forced expiratory volume in 1 s (FEV1 % pred) between genotypes according to the degree of smoking consumption. For all the tests, p<0.05 was considered statistically significant. The statistical package RStudio v2.5.1 (www.rstudio.com) was used for the analyses.
Results
Population included
The Spanish registry included 409 individuals with severe AATD from 17 centres distributed throughout the country; 218 participants (53.8%) were men, mean±sd age was 53.5±15.9 years and only 33 (8%) were active smokers. The distribution of genotypes was PI*ZZ in 181 patients (44.7%), PI*SZ in 163 patients (40.2%), PI*SS in 29 patients (7.2%) and others in 32 patients (7.9%). Up to 70.6% were index cases and the mean age at diagnosis was 46.4±17.3 years.
Characteristics of lung disease in participants in the Spanish registry
Lung disease was present in 271 participants (67.4%); the most frequent pulmonary disease was chronic obstructive pulmonary disease (COPD) in 175 patients (43.5%), followed by emphysema in 163 (40.5%) and bronchiectasis in 83 (20.6%). The most frequent respiratory symptoms at presentation were dyspnoea in 217 (53%), cough in 96 (23.5%) and sputum production in 68 (16.6%). Up to 77 patients (19.3%) had a history of pneumonia and spirometry showed a mean±sd FEV1 % pred of 81.7±31.3% and a mean±sd per cent predicted transfer coefficient of the lung for carbon monoxide (KCO % pred) of 76.8±23.4%.
Comparison of demographic and clinical characteristics between PI*ZZ and PI*SZ patients
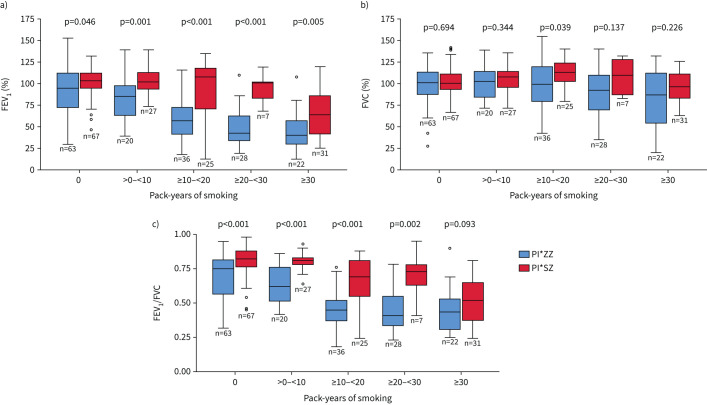
Patients with the PI*SZ genotype were younger, more frequently non-index cases and had a lower frequency of most respiratory diseases (COPD 25.9%, emphysema 24.7% and bronchiectasis 10% versus 60.3%, 59.8% and 32.4% in the PI*ZZ patients; p<0.001 for all comparisons). However, there was no difference in the prevalence of asthma (13.6% versus 13.4%, p=0.96). Among PI*SZ patients there were more active smokers (23.2% versus 2.8%, p<0.001), but they had better lung function and a lower frequency of exacerbations and previous pneumonia (table 1). Never-smokers showed no differences in FEV1 % pred, but significant differences in FEV1 % pred were observed with increased accumulated tobacco consumption in both genotypes (p for trend<0.001 for PI*ZZ and PI*SZ), with smoking having a higher impact at lower cumulative doses in PI*ZZ patients (figure 1a). Significant differences between both genotypes were also observed for per cent predicted forced vital capacity (FVC % pred) but not for FEV1/FVC (figure 1b, c).
TABLE 1.
Demographic and clinical characteristics of the participants in the Spanish registry of the European Alpha-1 Research Collaboration and comparison between PI*ZZ and PI*SZ genotypes
| Variables | Global | ZZ | SZ | p-value |
| Subjects (n) | 405 | 181 | 163 | |
| Age (years) | 53.5±15.9 | 55.4±14.7 | 51.0±17.3 | 0.015 |
| Sex, male | 218 (53.8) | 95 (52.5) | 87 (53.4) | 0.869 |
| BMI (kg·m−2) | 26.4±4.8 | 26.2±4.9 | 26.3±4.9 | 0.730 |
| Smokers | 33 (8) | 3 (2.8) | 22 (23.2) | <0.001 |
| Ex-smokers | 222 (54.4) | 111 (61.3) | 3 (44.9) | |
| Never-smokers | 149 (36.8) | 66 (36.7) | 68 (41) | |
| Tobacco exposure (pack-years) | 24.5±21.9 | 20.3±13.8 | 25.2±25.1 | 0.953 |
| Chronic respiratory disease | 267 (66.4) | 146 (81.6) | 82 (50.6) | <0.001 |
| COPD | 175 (43.5) | 108 (60.3) | 42 (25.9) | <0.001 |
| Emphysema | 163 (40.5) | 107 (59.8) | 40 (24.7) | <0.001 |
| Bronchiectasis | 83 (20.6) | 58 (32.4) | 16 (10) | <0.001 |
| Asthma | 55 (13.7) | 24 (13.4) | 22 (13.6) | 0.963 |
| Liver disease | 42 (10.6) | 24 (13.4) | 16 (10) | 0.322 |
| Age at onset of symptoms (years) | 46.1±17.2 | 44.2±16.3 | 47.9±17.5 | 0.063 |
| Age at diagnosis of AATD (years) | 46.4±17.4 | 44.5±17.2 | 46.8±18 | 0.354 |
| Index case | 286 (70.6) | 135 (74.6) | 106 (65) | 0.053 |
| Family screening | 98 (24) | 39 (21.5) | 45 (27.6) | 0.121 |
| Charlson index (age corrected) | 3.1±2.1 | 3.2±2 | 2.9±2 | 0.347 |
| Cardiovascular disease# | 89 (22.5) | 47 (26.4) | 25 (15.7) | 0.017 |
| FVC % pred | 99.4±22.6 | 96.1±26.2 | 103±17.4 | 0.011 |
| FEV1 % pred | 81.7±31.4 | 69.8±32.1 | 93.3±26 | <0.001 |
| FEV1/FVC % pred | 65±19 | 57±19 | 72±18 | <0.001 |
| KCO % pred | 76.8±23.4 | 67.6±22.5 | 84.7±20.8 | <0.001 |
| Augmentation therapy | 114 (28.9) | 95 (54) | 13 (8.2) | 0.001 |
| AAT (mg·dL−1) | 44.5±24.4 | 24.9±17.6 | 56.7±11.8 | <0.001 |
| Number of exacerbations previous year | 0.44±1 | 0.66±1.2 | 0.28±0.8 | <0.001 |
| History of pneumonia | 77 (19.3) | 44 (24.6) | 21 (13.1) | 0.007 |
| CAT | 7.4±7.2 | 9.4±7.8 | 5.6±6.2 | <0.001 |
| BODEx | 1.2±1.8 | 1.8±2 | 0.6 (1.3) | <0.001 |
Data presented as mean±sd or n (%), unless otherwise indicated. BMI: body mass index; COPD: chronic obstructive pulmonary disease; AATD: α1-antitrypsin deficiency; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; KCO: transfer coefficient of the lung for carbon monoxide; AAT: α1-antitrypsin; CAT: COPD Assessment Test; BODEx: BMI, Obstruction, Dyspnoea, Exacerbations index. #: cardiovascular disease includes hypertension, ischaemic heart disease, congestive heart failure and peripheral vascular disease.
FIGURE 1.
Comparison of per cent predicted forced expiratory volume in 1 s (FEV1 % pred) (a), forced vital capacity (FVC) (b) and FEV1/FVC (c) between participants in the Spanish registry with the PI*ZZ and PI*SZ genotypes. In each box plot, the median value is indicated by the centre horizontal line, and the 25th and 75th percentiles are indicated by the lower and upper box horizontal lines. Circles indicate the outliers. p-values determined using a t-test. There was a statistically significant difference between pack-years of smoking groups for FEV1 % pred and FEV1/FVC for both the PI*ZZ genotype (p-value for trend <0.001) and PI*SZ genotype (p-value for trend <0.001). For FVC % pred, the p-value for trend was p=0.004 for PI*ZZ and p=0.29 for PI*SZ.
Comparison of characteristics and severity of lung disease between PI*ZZ and PI*SZ patients
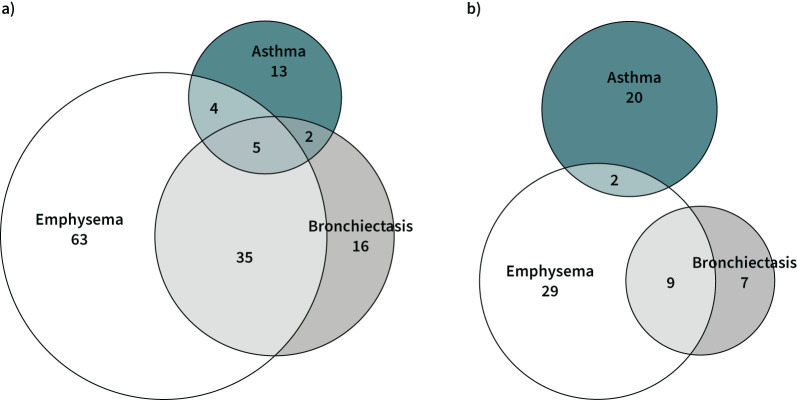
The comparison of the 82 PI*SZ and 146 PI*ZZ patients with respiratory disease is shown in table 2. The distributions of age and sex were no different between the two genotypes, but PI*SZ patients were more frequently active smokers. The proportions of index cases, patients identified by family screening and patients with cardiovascular comorbidity did not differ between the two groups; however, PI*SZ patients had a significantly older age than PI*ZZ patients both at symptom onset (49.9±18.1 years versus 45.6±15 years, p=0.008) and diagnosis (52.5±18 years versus 47.4±14.9 years, p=0.003). The distribution of respiratory diseases was different between the two genotypes, with more emphysema, COPD and bronchiectasis in PI*ZZ patients and more frequent asthma in PI*SZ patients (table 2 and figure 2a, b). Both FEV1 % pred and KCO % pred were significantly lower in Pi*ZZ than PI*SZ patients (FEV1 % pred: 61.1±28.2% versus 78.7±28.5%; KCO % pred: 62.9±20.6% versus 84.7±20.8%; p<0.001 for both comparisons). The COPD Assessment Test score was also higher (worse) for PI*ZZ compared to PI*SZ patients (10.9±7.9 versus 8.6±7, p<0.036). A total of 12 PI*SZ patients (15.4%) received augmentation therapy compared with 94 PI*ZZ patients (66.2%; p<0.001).
TABLE 2.
Comparison of demographic and clinical characteristics of the participants in the Spanish registry of European Alpha-1 Research Collaboration with chronic lung disease and PI*ZZ and PI*SZ genotypes
| Variables | ZZ | SZ | p-value |
| Subjects (n) | 146 | 82 | |
| Age (years) | 58.6±12.3 | 57.6±17.2 | 0.692 |
| Sex, male | 75 (51.4) | 47 (57.8) | 0.386 |
| BMI (kg·m−2) | 26.4±4.8 | 27.6±5.3 | 0.067 |
| Smokers | 2 (1.4) | 14 (17.1) | <0.001 |
| Ex-smokers | 105 (71.9) | 48 (58.5) | |
| Never-smokers | 39 (26.7) | 20 (24.4) | |
| Tobacco exposure (pack-years) | 18.8±9.7 | 20.7±13.5 | 0.460 |
| COPD | 108 (74) | 42 (51.2) | 0.001 |
| Emphysema | 107 (73.3) | 40 (48.8) | <0.001 |
| Bronchiectasis | 58 (39.7) | 16 (19.5) | 0.002 |
| Asthma | 24 (16.4) | 22 (26.8) | 0.061 |
| Liver disease | 17 (11.8) | 8 (9.8) | 0.637 |
| Age at onset of symptoms (years) | 45.6±15 | 49.9±18.1 | 0.008 |
| Age at diagnosis of AATD (years) | 47.4±14.9 | 52.5±18 | 0.003 |
| Index case | 25 (17.1) | 15 (18.3) | 0.824 |
| Family screening | 21 (14.4) | 12 (14.6) | 0.959 |
| Charlson index (age corrected) | 3.5±1.9 | 4±2.2 | 0.105 |
| Cardiovascular comorbidity# | 43 (30.1) | 22 (28.2) | 0.771 |
| FVC % pred | 93.4±26.6 | 100.4±20.7 | 0.046 |
| FEV1 % pred | 61.1±28.2 | 78.7±28.5 | <0.001 |
| FEV1/FVC % pred | 51±17 | 62±19 | <0.001 |
| KCO % pred | 62.9±20.6 | 84.7±20.8) | <0.001 |
| Augmentation therapy | 94 (66.2) | 12 (15.4) | <0.001 |
| AAT (mg·dL−1) | 25.8±19.4 | 57.5±12.2 | <0.001 |
| Number of exacerbations previous year | 0.80±1.3 | 0.43±1 | 0.018 |
| History of pneumonia | 39 (26.9) | 10 (12.7) | 0.014 |
| CAT | 10.9±7.9 | 8.6±7 | 0.036 |
| BODEx | 2.2±2.1 | 1.1±1.7 | <0.001 |
Data presented as mean±sd or n (%), unless otherwise indicated. BMI: body mass index; COPD: chronic obstructive pulmonary disease; AATD: α1-antitrypsin deficiency; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; KCO: transfer coefficient of the lung for carbon monoxide; AAT: α1-antitrypsin; CAT: COPD Assessment Test; BODEx: BMI, Obstruction, Dyspnoea, Exacerbations index. #: cardiovascular disease includes hypertension, ischaemic heart disease, congestive heart failure and peripheral vascular disease.
FIGURE 2.
Distribution of lung diseases in participants of the Spanish registry with the PI*ZZ (a) and PI*SZ (b) genotypes. The size of each circle is proportional to the number of individuals in each group.
Characteristics of lung diseases in patients with AATD
Patients with AATD and asthma had different characteristics from those with emphysema or bronchiectasis. Patients with asthma were younger and more frequently female, with a higher proportion of never-smokers. They also had better lung function and a lower prevalence of comorbidities but a similar frequency of previous pneumonia compared to patients with emphysema. The demographic and clinical characteristics of patients with the most frequent chronic respiratory diseases are shown in table 3. No statistical comparisons were performed because some patients could be included in more than one category due to the coexistence of respiratory diseases in some individuals.
TABLE 3.
Demographic and clinical characteristics of the participants in the Spanish registry of European Alpha-1 Research Collaboration with different pulmonary diseases
| Variables | Emphysema | Bronchiectasis | Asthma |
| Subjects (n) | 163 | 83 | 55 |
| Age (years) | 61.2±11.7 | 62.5±11.5 | 45.8±16.4 |
| Sex, male | 96 (58.9) | 41 (49.4) | 24 (43.6) |
| BMI (kg·m−2) | 26.7±5.1 | 26.8±4.4 | 26.4±5.3 |
| Smokers | 11 (6.7) | 4 (4.8) | 6 (10.9) |
| Ex-smokers | 129 (79.1) | 52 (62.7) | 25 (45.5) |
| Never-smokers | 23 (14.1) | 27 (32.5) | 24 (43.6) |
| Tobacco exposure (pack-years) | 29.4±22.5 | 25.5±22 | 18.7±19 |
| COPD | 128 (78.5) | 53 (63.9) | 14 (25.5) |
| Emphysema | 163 (100) | 53 (63.9) | 13 (23.6) |
| Bronchiectasis | 53 (32.5) | 83 (100) | 8 (14.5) |
| Asthma | 13 (8) | 8 (9.6) | 55 (100) |
| Liver disease | 18 (11.2) | 8 (9.6) | 5 (9.1) |
| Age at onset of symptoms (years) | 48.7±15.4 | 48.7±14.9 | 31.4±17.3 |
| Age at diagnosis of AATD (years) | 51.7±14.1 | 52.9±12.9 | 38.8±19 |
| Index case | 139 (85.3) | 75 (90.4) | 41 (74.5) |
| Family screening | 21 (12.9) | 8 (9.6) | 10 (18.2) |
| Charlson index (age corrected) | 3.9±1.7 | 3.9±1.6 | 2.3±2.1 |
| Cardiovascular comorbidity # | 58 (36.7) | 28 (34.1) | 9 (17) |
| FVC % pred | 94.3±27.7 | 90.7±25.5 | 101±4 |
| FEV1 % pred | 59.6±27.5 | 67.7±28.7 | 86.3±27.9 |
| FEV1/FVC % pred | 49±15 | 58±16 | 69±17 |
| KCO % pred | 60.9±19.9 | 70.4±21.7 | 81.8±20.9 |
| Augmentation therapy | 95 (59.7) | 38 (47.5) | 8 (16) |
| AAT (mg·dL−1) | 38.2±26.9 | 37.4±22.3 | 45.8±21.9 |
| Number of exacerbations previous year | 0.7±1.2 | 0.7±1.3 | 0.5±1.2 |
| History of pneumonia | 34 (21) | 27 (32.5) | 12 (23.1) |
| CAT | 11.5 (7.7) | 11 (7.8) | 7 (6.4) |
| BODEx | 2.3 (2) | 1.7 (2) | 1 (1.5) |
Data presented as mean±sd or n (%), unless otherwise indicated. The different subgroups are not mutually exclusive. BMI: body mass index; COPD: chronic obstructive pulmonary disease; AATD: α1-antitrypsin deficiency; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; KCO: transfer coefficient of the lung for carbon monoxide; AAT: α1-antitrypsin; CAT: COPD Assessment Test; BODEx: BMI, Obstruction, Dyspnoea, Exacerbations index. #: cardiovascular disease includes hypertension, ischaemic heart disease, congestive heart failure and peripheral vascular disease.
Discussion
The implementation of the EARCO International Registry has been an opportunity for the Spanish AATD registry to join an international initiative and upgrade the database and take advantage of the structure and monitoring system of EARCO to improve the quality of data collection [14]. During the first 2 years of recruitment, and despite the COVID-19 pandemic, a total of 17 centres included more than 400 patients in the EARCO registry, which constitutes the new Spanish AATD registry.
The characteristics of the patients included have changed from the previous reports of the Spanish registry. The first report made in 1998 included 223 patients: 73% male, mean age 46 years and mean FEV1 % pred of 53% [11]. The second report in 2007 included 462 individuals: 63% male, mean age 51 years and mean FEV1 % pred of 53% [17]. Interestingly the percentage of males is now reduced to 54%, while the mean age has increased to 53 years and mean FEV1 to 81% predicted. This increase in the mean FEV1 % pred may, in part, be due to the improvement in population and family screening, earlier detection of carriers and early diagnosis of lung diseases in individuals affected [18–21]. The inclusion of genotypes other than PI*ZZ may also explain the better lung function in the current analysis, although the influence is very limited because the mean FEV1 % pred in PI*ZZ patients was 69.8%, which is still much better than in previous reports [11, 17].
Several registries have evaluated the characteristics of individuals with the most common deficient PI*ZZ genotype [22–25], but there is limited information about the characteristics of patients with the PI*SZ genotype. Because the S allele is especially frequent in Spain [5, 15], the Spanish registry provides a unique opportunity to investigate the clinical manifestations of PI*SZ individuals and to compare with PI*ZZ carriers. In general, patients with the PI*SZ genotype were younger, more frequently non-index cases and had a lower frequency of most respiratory diseases, despite a higher prevalence of active smokers, but there were no differences in the prevalence of asthma. The possible influence of the S alleles in the prevalence and severity of asthma has been previously reported [26] and justifies the investigation of AATD in all adult asthmatic patients with non-fully reversible airflow obstruction, as indicated in guidelines. It is of note that we did not observe significant differences in lung function between PI*SZ and PI*ZZ never-smokers. However, with increasing intensity of smoking, patients with PI*ZZ presented more severely impaired lung function, while PI*SZ patients only showed deteriorated lung function at smoking consumptions of >20 pack-years, highlighting again the increased susceptibility of homozygous Z patients to the deleterious effect of tobacco smoking [27–29].
Considering only the individuals with diagnosed lung disease, we observed that PI*SZ carriers were older, both at onset of symptoms and at diagnosis, than PI*ZZ patients and the severity of lung disease was milder in terms of FEV1 and KCO % pred.
Augmentation therapy was administered to two thirds of patients with the PI*ZZ genotype and lung disease. It is not possible to ascertain why the remaining third of patients was not treated. Two patients were active smokers and treatment was therefore not indicated, but other reasons, including the patient refusing treatment, could explain the lack of augmentation for PI*ZZ patients with lung disease. Although the indications for augmentation are clear, the opinions and attitudes about augmentation may vary greatly among specialists [30], and some may not indicate augmentation for patients with very mild or very severe disease and others may not initiate augmentation if patients do not show clinical, functional or radiological deterioration during follow-up [30, 31].
By contrast, 15.4% of PI*SZ patients with lung disease received augmentation. Treatment for PI*SZ patients is included in the indications described in the summary of product characteristics of both brands of augmentation therapy available in Spain [32, 33]; nevertheless, there is no consensus among specialists about the efficacy of augmentation in PI*SZ patients with lung disease. While some studies demonstrated that PI*SZ patients have a significantly increased risk of lung disease and augmentation would help to prevent its progression [7, 29, 34], others suggest that the risk of lung disease associated with the PI*SZ genotype is similar to that associated with PI*MZ and much less than the homozygous form of PI*ZZ [8, 35].
An interesting observation derived from the data is the different distribution of lung diseases in PI*SZ and PI*ZZ patients. Emphysema and bronchiectasis were more frequent in PI*ZZ, but asthma was relatively more frequent in PI*SZ. Among the PI*ZZ patients, only a minority had lung disease without emphysema, with 16 cases of bronchiectasis (11%), 13 of asthma (9%) and two cases of both diseases combined (1.3%). In contrast, 23.2% of PI*SZ patients had asthma and no evidence of emphysema. We can compare our results with those of a similar cohort of 424 unrelated PI*ZZ patients in the UK. In this study, Wood et al. [36] reported a higher prevalence of emphysema (65.8% versus 59.8% in our series) and lower prevalence of bronchiectasis (19.5% versus 32.4% in Spain). It is of note that 10% of their patients with bronchiectasis had no emphysema, very similar to the 12% observed in our patients. Data from Wood et al. [36] were published 16 years ago and it is not clear whether differences observed are due to real differences in phenotypes or due to a higher implementation of AATD screening and more frequent use of high-resolution computed tomography scans during the last years.
The demographic and clinical profiles of patients with emphysema or bronchiectasis in our group were quite similar, but those of asthmatic patients were clearly different. Patients with asthma were younger and diagnosed at a younger age, there was a lower prevalence of smokers, more women and a higher frequency of patients diagnosed by family screening, and patients had better lung function, lower symptom burden and higher serum AAT concentrations. History of pneumonia was also more frequent in PI*ZZ than PI*SZ patients, both in the global population and in individuals with lung disease. These differences could in part be explained by the higher severity of lung disease in PI*ZZ patients, while other risk factors for pneumonia, such as age, were no different between the genotypes.
Spain is only one of the 16 countries currently participating in the EARCO International Registry. Some of the participating countries have joined EARCO through a unique national reference centre, but others, such as Portugal [37] and the UK, have followed the same structure as the Spanish registry with multiple centres contributing directly to the database and designating a national coordinator who has direct access to the anonymised national data. The EARCO International Registry is the main research project of the EARCO initiative, but there are other research projects under development derived from the survey of unmet needs developed by academics, patients and caregivers’ representatives. The follow-up of patients included in the registry will provide relevant information about the natural history of the disease [38].
Conclusions
The Spanish registry has shown the evolution of the baseline characteristics of the patients included, who now have an older age and more preserved lung function, suggesting an improvement in early diagnosis and better management of pulmonary disease. In general, patients with the PI*SZ genotype had milder respiratory disease and were less susceptible to tobacco smoking but had a similar prevalence of asthma. Longitudinal follow-up is necessary to better understand the natural history of the AATD.
Acknowledgements
The Spanish registry would like to acknowledge the support of the EARCO Steering committee: Christian Clarenbach and Marc Miravitlles (co-chairs), Robert Bals, Jan Stolk, Joanna Chorostowska-Wynimko, Karen O'Hara, Marion Wilkens, José Luis López-Campos, Alice M. Turner, Ilaria Ferrarotti, Gerry McElvaney and Robert A. Stockley.
The following investigators have contributed to the Spanish registry of AATD: Maria Torres-Durán (national coordinator), José Luis López-Campos, Juan Luis Rodríguez-Hermosa, Myriam Calle, Cristina Esquinas (data manager), Cristina Martinez, José María Hernández-Pérez, Carlota Rodríguez, Ana Bustamante, Francisco Casas-Maldonado, Miriam Barrecheguren, Cruz González, Marc Miravitlles, Eva Tabernero, Mar Fernández-Nieto, Francisco Javier Michel, Virginia Almadana, Lourdes Lázaro, Carlos Martínez-Rivera and María Isabel Parra.
We wish to acknowledge Elise Heuvelin from the European Respiratory Society (Laussane, Switzterland) for her support in the management of EARCO, and Gemma Vilagut and Christina Founti (Bioclever, Barcelona, Spain) for their support in EARCO data monitoring. We also acknowledge the participation of Beatriz Lara (Coventry, UK) and Eduardo Loeb (Barcelona, Spain) in the development of the database and the monitoring of data.
Footnotes
Provenance: Submitted article, peer reviewed.
Availability of data and materials: Information on the EARCO data sharing commitments and requesting access to anonymised aggregated participant data and associated documents can be found at www.earco.org.
Authors’ contributions: Study concept and design by M. Torres-Durán, C. Esquinas and M. Miravitlles. Data acquisition by M. Torres-Durán, J.L. López-Campos, J.L. Rodríguez-Hermosa, C. Martínez-González, J.M. Hernández-Pérez, C. Rodríguez, A. Bustamente, F. Casas-Maldonaldo, M. Barrecheguren, C. González and M. Miravitlles. Statistical analysis by C. Esquinas. Drafting of the manuscript by M. Miravitlles, C. Esquinas and M. Torres-Durán. Critical revision and approval for submission by M. Torres-Durán, J.L. López-Campos, J.L. Rodríguez-Hermosa, C. Esquinas, C. Martínez-González, J.M. Hernández-Pérez, C. Rodríguez, A. Bustamente, F. Casas-Maldonaldo, M. Barrecheguren, C. González and M. Miravitlles. All authors have read and approved the final manuscript.
Competing interests: M. Torres-Durán has received speaker fees from Chiesi, CSL Behring, Grifols and Resmed, and consulting fees from CSL Behring and Grifols. J.L. López-Campos has received honoraria during the last 3 years for lecturing, scientific advice, participation in clinical studies or writing for publications for AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Esteve, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Novartis, Rovi and Teva. J.L. Rodríguez-Hermosa has received speaker fees from Zambon, Bial, Gebro Pharma, GlaxoSmithKline, Chiesi, Boehringer Ingelheim, CSL Behring and Grifols. J.M. Hernández-Pérez has received speaker fees from Grifols, CSL Behring, AstraZeneca, GlaxoSmithKline, Bial laboratory and Teva laboratory, support for attending meetings from Grifols and CSL Behring, and consulting fees from CSL Behring. C. Rodríguez has received speaker fees from GlaxoSmithKline, AstraZeneca, Grifols, Chiesi, Ferrer, Menarini and Boehringer Ingelheim, has provided expert testimony for Chiesi, and has received support for attending meetings from FAES. A. Bustamante has received speaker fees from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, Novartis and Ferrer, and funding for travelling or attending meetings from CSL Behring, AstraZeneca and Chiesi. F. Casas-Maldonado has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Gebro Pharma, GlaxoSmithKline, Laboratorios Esteve, Laboratorios Ferrer, Menarini, Novartis, Rovi, Teva, Vertex, Zambon, CSL Behring and Grifols, and consulting fees from AstraZeneca, Chiesi, GlaxoSmithKline, CSL Behring and Grifols. M. Barrecheguren has received speaker fees from Grifols, Menarini, CSL Behring, GlaxoSmithKline and Boehringer Ingelheim, and consulting fees from GlaxoSmithKline, Novartis, CSL Behring and Boehringer Ingelheim. C. González has received speaker fees from Menarini, GlaxoSmithKline, Novartis, Boehringer Ingelheim and Chiesi. M. Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, Teva, Spin Therapeutics, pH Pharma, Novartis, Sanofi and Grifols, and research grants from GlaxoSmithKline and Grifols. The remaining authors report no conflicts of interest.
Support statement: The Spanish registry is funded by an unrestricted grant from Grifols. The EARCO International Registry is funded by unrestricted grants of Grifols, CSL Behring, Kamada, pH Pharma and Takeda to the European Respiratory Society. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Blanco I, Bueno P, Diego I, et al. α1-antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an update. Int J Chron Obstruct Pulmon Dis 2017; 12: 561–569. doi: 10.2147/COPD.S125389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur Respir J 2017; 50: 1700610. doi: 10.1183/13993003.00610-2017 [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Frias F, Miravitlles M, Vidal R, et al. Rare α1-antitrypsin variants: are they really so rare? Ther Adv Respir Dis 2012; 6: 79–85. doi: 10.1177/1753465811434320 [DOI] [PubMed] [Google Scholar]
- 4.López-Campos JL, Casas-Maldonado F, Torres-Duran M, et al. Results of a diagnostic procedure based on multiplex technology on dried blood spots and buccal swabs for patients with suspected α1-antitrypsin deficiency. Arch Bronconeumol 2021; 57: 42–50. [DOI] [PubMed] [Google Scholar]
- 5.Blanco I, de Serres FJ, Fernandez-Bustillo E, et al. Estimated numbers and prevalence of PI*S and PI*Z alleles of α1-antitrypsin deficiency in European countries. Eur Respir J 2006; 27: 77–84. doi: 10.1183/09031936.06.00062305 [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Granda L, Cabero-Perez MJ, Bustamante-Ruiz A, et al. PI SZ phenotype in chronic obstructive pulmonary disease. Thorax 1997; 52: 659–661. doi: 10.1136/thx.52.7.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seersholm N, Kok-Jensen A. Intermediate α1-antitrypsin deficiency PiSZ: a risk factor for pulmonary emphysema? Respir Med 1998; 92: 241–245. doi: 10.1016/S0954-6111(98)90102-0 [DOI] [PubMed] [Google Scholar]
- 8.McElvaney GN, Sandhaus RA, Miravitlles M, et al. Clinical considerations in individuals with α1-antitrypsin PI*SZ genotype. Eur Respir J 2020; 55: 1902410. doi: 10.1183/13993003.02410-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rare Diseases Task Force . Centres of Reference for Rare Diseases in Europe: State-of-the-art in 2006 and Recommendations of the Rare Diseases Task Force. www.ec.europa.eu/health/archive/ph_threats/non_com/docs/contribution_policy.pdf. Date last accessed: April 19, 2022.
- 10.Miravitlles M, Nuñez A, Torres-Durán M, et al. the importance of reference centers and registries for rare diseases: the example of α1-antitrypsin deficiency. COPD 2020; 17: 346–354. doi: 10.1080/15412555.2020.1795824 [DOI] [PubMed] [Google Scholar]
- 11.Miravitlles M, Vidal R, Barros-Tizón JC, et al. Usefulness of a national registry of α1-antitrypsin deficiency. The Spanish experience. Respir Med 1998; 92: 1181–1187. doi: 10.1016/S0954-6111(98)90418-8 [DOI] [PubMed] [Google Scholar]
- 12.Miravitlles M, Chorostowska-Wynimko J, Ferrarotti I, et al. The European Alpha-1 Research Collaboration (EARCO): a new ERS Clinical Research Collaboration to promote research in α1-antitrypsin deficiency. Eur Respir J 2019; 53: 1900138. doi: 10.1183/13993003.00138-2019 [DOI] [PubMed] [Google Scholar]
- 13.Brightling C, Genton C, Bill W, et al. ERS Clinical Research Collaborations: underpinning research excellence. Eur Respir J 2018; 52: 1801534. doi: 10.1183/13993003.01534-2018 [DOI] [PubMed] [Google Scholar]
- 14.Barrecheguren M, Torres-Duran M, Casas-Maldonado F, et al. Spanish implementation of the new international α1-antitrypsin deficiency international registry: The European Alpha-1 Research Collaboration (EARCO). Arch Bronconeumol (Engl Ed) 2021; 57: 81–82. doi: 10.1016/j.arbr.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 15.Blanco I, Bueno P, Diego I, et al. α1-antitrypsin Pi*SZ genotype: estimated prevalence and number of SZ patients worldwide. Int J Chron Obstruct Pulmon Dis 2017; 12: 1683–1694. doi: 10.2147/COPD.S137852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greulich T, Altraja A, Barrecheguren M, et al. Protocol for the EARCO Registry: a pan-European observational study in patients with α1-antitrypsin deficiency. ERJ Open Res 2020; 6: 00181-2019. doi: 10.1183/23120541.00181-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lara B, Blanco I, Martínez MT, et al. Spanish registry of patients with α1-antitrypsin deficiency: database evaluation and population analysis. Arch Bronconeumol 2017; 53: 13–18. doi: 10.1016/j.arbres.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 18.Torres-Durán M, Lopez-Campos JL, Barrecheguren M, et al. α1-antitrypsin deficiency: outstanding questions and future directions. Orphanet J Rare Dis 2018; 13: 114. doi: 10.1186/s13023-018-0856-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrecheguren M, Monteagudo M, Simonet P, et al. Diagnosis of α1-antitrypsin deficiency: a population-based study. Int J Chron Obstruct Pulmon Dis 2016; 11: 999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esquinas C, Barrecheguren M, Sucena M, et al. Practice and knowledge about diagnosis and treatment of α1-antitrypsin deficiency in Spain and Portugal. BMC Pulm Med 2016; 16: 64. doi: 10.1186/s12890-016-0222-4 [DOI] [Google Scholar]
- 21.López-Campos JL, Carrasco Hernandez L, Marquez-Martín E, et al. Diagnostic performance of a lateral flow assay for the detection of α1-antitrypsin deficiency. Arch Bronconeumol 2020; 56: 124–126. [DOI] [PubMed] [Google Scholar]
- 22.Survival and FEV1 decline in individuals with severe deficiency of α1-antitrypsin. The Alpha-1 Antitrypsin Deficiency Registry Study Group. Am J Respir Crit Care Med 1998; 158: 49–59. doi: 10.1164/ajrccm.158.1.9712017 [DOI] [PubMed] [Google Scholar]
- 23.Piitulainen E, Tanash HA. The clinical profile of subjects included in the Swedish National Register on individuals with severe α1-antitrypsin deficiency. COPD 2015; 12: Suppl. 1, 36–41. doi: 10.3109/15412555.2015.1021909 [DOI] [PubMed] [Google Scholar]
- 24.Stockley RA. Antitrypsin deficiency assessment and programme for treatment (ADAPT): The United Kingdom registry. COPD 2015; 12: Suppl. 1, 63–68. doi: 10.3109/15412555.2015.1021911 [DOI] [PubMed] [Google Scholar]
- 25.Chorostowska-Wynimko J, Struniawski R, Sliwinski P, et al. The national α1-antitrypsin deficiency registry in Poland. COPD 2015; 12: Suppl. 1, 22–26. doi: 10.3109/15412555.2015.1021915 [DOI] [PubMed] [Google Scholar]
- 26.Miravitlles M, Vilà S, Torrella M, et al. Influence of deficient α1-antitrypsin phenotypes on clinical characteristics and severity of asthma in adults. Respir Med 2002; 96: 186–192. doi: 10.1053/rmed.2001.1237 [DOI] [PubMed] [Google Scholar]
- 27.Piras B, Ferrarotti I, Lara B, et al. Clinical phenotypes of Italian and Spanish patients with α1-antitrypsin deficiency. Eur Respir J 2013; 42: 54–64. doi: 10.1183/09031936.00104712 [DOI] [PubMed] [Google Scholar]
- 28.Choate R, Mannino DM, Holm KE, et al. Comparing patients with ZZ versus SZ α1-antitrypsin deficiency: findings from AlphaNet's disease management program. Chronic Obstr Pulm Dis 2019; 6: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green CE, Vayalapra S, Hampson JA, et al. PiSZ α1-antitrypsin deficiency (AATD): pulmonary phenotype and prognosis relative to PiZZ AATD and PiMM COPD. Thorax 2015; 70: 939–945. doi: 10.1136/thoraxjnl-2015-206906 [DOI] [PubMed] [Google Scholar]
- 30.Greulich T, Albert A, Cassel W, et al. Opinions and attitudes of pulmonologists about augmentation therapy in patients with α1-antitrypsin deficiency. A survey of the EARCO Group. Int J Chron Obst Pulm Dis 2022; 17: 53–64. doi: 10.2147/COPD.S346051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockley RA, Miravitlles M, Vogelmeier C. Augmentation therapy for α1-antitrypsin deficiency: towards a personalised approach. Orphanet J Rare Dis 2013; 8: 149. doi: 10.1186/1750-1172-8-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Respreeza . Summary of Product Characteristics (SmPC). www.ec.europa.eu/health/documents/community-register/2015/20150820132594/dec_132594_es.pdf. Date last accessed: April 11, 2022.
- 33.Prolastin . Summary of Product Characteristics (SmPC). www.grifols.com/documents/10192/27754341/ft-prolastin-es-en/786cce6a-effb-41b6-867f-59d7a34ca086. Date last accessed: April 11, 2022.
- 34.Dahl M, Hersh CP, Ly NP, et al. The protease inhibitor PI*S allele and COPD: a meta-analysis. Eur Respir J 2005; 26: 67–76. doi: 10.1183/09031936.05.00135704 [DOI] [PubMed] [Google Scholar]
- 35.Franciosi AN, Carroll TP, McElvaney NG. SZ α1-antitrypsin deficiency and pulmonary disease: more like MZ, not like ZZ. Thorax 2021; 76: 298–301. doi: 10.1136/thoraxjnl-2020-215250 [DOI] [PubMed] [Google Scholar]
- 36.Wood AM, Simmonds MJ, Bayley DL, et al. The TNF-α gene relates to clinical phenotype in α1-antitrypsin deficiency. Respir Res 2008; 9: 52. doi: 10.1186/1465-9921-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sucena M, Gomes J, Guimarães C, et al. Implementation of European Alpha-1 Research Collaboration (EARCO) in Portugal: the future starts now. Pulmonology 2020; 26: 181–183. doi: 10.1016/j.pulmoe.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 38.Bilton D, Caine N, Cunningham S, et al. Use of a rare disease patient registry in long-term post-authorisation drug studies: a model for collaboration with industry. Lancet Respir Med 2018; 6: 495–496. doi: 10.1016/S2213-2600(18)30192-9 [DOI] [PubMed] [Google Scholar]