Abstract
Background:
The PRIMA phase 3 trial showed niraparib significantly prolongs median progression-free survival (PFS) versus placebo in patients with advanced ovarian cancer (OC) responsive to first-line platinum-based chemotherapy, including those who had tumors with homologous recombination deficiency (HRd). This analysis of PRIMA examined the quality-adjusted PFS (QA-PFS) and quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) of patients on maintenance niraparib versus placebo.
Methods:
Patients were randomized 2:1 to receive once-daily maintenance niraparib (n = 487) or placebo (n = 246). QA-PFS was defined as the PFS of patients adjusted for their health-related quality of life (HRQoL) prior to disease progression, measured using European Quality of Life Five-Dimension (EQ-5D) questionnaire index scores from the PRIMA trial. Q-TWiST was calculated by combining data on PFS, duration of symptomatic grade ⩾2 adverse events (fatigue or asthenia, nausea, vomiting, abdominal pain, and abdominal bloating) prior to disease progression, and EQ-5D index scores. Analyses used data collected up to the last date of PFS assessment (May 17, 2019).
Results:
The restricted mean QA-PFS was significantly longer with niraparib versus placebo in the HRd (n = 373) and overall intention-to-treat (ITT; n = 733) populations (mean gains of 6.5 [95% confidence interval; CI, 3.9–8.9] and 4.1 [95% CI, 2.2–5.8] months, respectively). There were also significant improvements in restricted mean Q-TWiST for niraparib versus placebo (mean gains of 5.9 [95% CI, 3.5–8.6] and 3.5 [95% CI, 1.7–5.6] months, respectively) in the HRd and ITT populations.
Conclusions:
In patients with advanced OC, first-line niraparib maintenance was associated with significant gains in QA-PFS and Q-TWiST versus placebo. These findings demonstrate that niraparib maintenance treatment is associated with a PFS improvement and that treatment benefit is maintained even when HRQoL and/or toxicity data are combined with PFS in a single measure.
Trial registration:
ClinicalTrials.gov: NCT02655016; trial registration date: January 13, 2016
Plain language summary
Background: In a large clinical trial called PRIMA, patients with advanced cancer of the ovary (ovarian cancer) were given either niraparib (a type of cancer medicine) or placebo (a pill containing no medicine/active substances) after having chemotherapy (another type of cancer medicine). Taking niraparib after chemotherapy is called maintenance therapy and aims to give patients more time before their cancer returns or gets worse than if they were not given any further treatment. In the PRIMA trial, patients who took niraparib did have more time before their cancer progressed than if they took placebo. However, it is important to consider patients’ quality of life, which can be made worse by cancer symptoms and/or side effects of treatment. Here, we assessed the overall benefit of niraparib for patients in PRIMA.
Methods: Both the length of time before disease progression (or survival time) and quality of life were considered using two different analyses:
● The first analysis was called quality-adjusted PFS (QA-PFS) and looked at how long patients survived with good quality of life.
● The second analysis was called quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) and looked at how long patients survived without cancer symptoms or treatment side effects.
Results: The PRIMA trial included 733 patients; 487 took niraparib and 246 took placebo. Around half of the patients in both groups had a type of ovarian cancer that responds particularly well to drugs like niraparib – they are known as homologous recombination deficiency (HRd) patients.
● When information on quality of life (collected from patient questionnaires) and survival was combined in the QA-PFS analysis, HRd patients who took niraparib had approximately 6.5 months longer with a good quality of life before disease progression than those who took placebo. In the overall group of patients (including HRd patients and non-HRd patients), those who took niraparib had approximately 4 months longer than with placebo.
● Using the second analysis (Q-TWiST) to combine information on survival with cancer symptoms and treatment side effects, the HRd patients taking niraparib had approximately 6 months longer without cancer symptoms or treatment side effects (such as nausea or vomiting) than patients taking placebo. In the overall group of patients, those taking niraparib had approximately 3.5 months longer without these cancer symptoms/side effects than patients receiving placebo.
Conclusions: These results show that the survival benefits of niraparib treatment remain when accounting for patients’ quality of life. These benefits were seen not only in HRd patients who are known to respond better to niraparib, but in the overall group of patients who took niraparib.
Keywords: maintenance therapy, niraparib, ovarian cancer, quality of life
Introduction
Ovarian cancer (OC) is a rare but frequently fatal cancer, constituting the seventh leading cause of cancer death among women globally. 1 Most cases of OC are diagnosed at an advanced stage where prognosis is poor, and >50% of patients with advanced disease die within 5 years of diagnosis.2,3
Although first-line treatment with platinum-based chemotherapy has high response rates,4,5 a large proportion of patients with advanced OC experience disease recurrence, with duration of progression-free survival (PFS) decreased with each subsequent line of chemotherapy.5–7 Maintenance therapy with poly(adenosine diphosphate-ribose) polymerase inhibitors (PARPis), which aims to delay disease progression, can be used to extend the time between chemotherapy treatments and prolong PFS.8–12 Patients are likely to be asymptomatic at the point of treatment initiation; therefore, maintenance therapies should have a minimal negative impact on quality of life (QoL). 10 The current standard of care for maintenance therapy in advanced OC comprises either an anti-angiogenic monoclonal antibody (e.g., bevacizumab), a PARPi (e.g., niraparib or olaparib), or a combination of bevacizumab and olaparib.9,11–14 Observation with follow-up may be considered in certain patients, such as those with a complete response (CR) to first-line chemotherapy. 14 However, available data suggest that disease recurrence is very common, even among patients with a favorable response to first-line treatment.4,15,16
Niraparib, a potent oral PARPi, is approved in the USA and Europe for the maintenance treatment of advanced OC in patients with a CR or partial response (PR) to first-line platinum-based chemotherapy.17,18 In the phase 3 PRIMA trial, maintenance therapy with niraparib significantly prolonged median PFS compared with placebo in patients with newly diagnosed platinum-sensitive advanced OC. The PFS benefit was observed in both patients who had tumors with homologous recombination deficiency [HRd; 21.9 months versus 10.4 months; hazard ratio (HR): 0.43; p < 0.001] and the overall intention-to-treat (ITT) population (13.8 months versus 8.2 months; HR: 0.62; p <0.001). 9
In recent years, patient-centered outcomes, defined as outcomes important to patients and caregivers, 19 have become increasingly important to clinicians and regulators when assessing the risk/benefit of cancer treatments. 20 Indeed, in order to better define the clinical benefit to patients, regulatory agencies and advisory bodies now recommend the inclusion and evaluation of patient-reported outcome (PRO) measures in clinical trials of new cancer treatments.21–25 This recommendation is also strongly supported by the Gynecological Cancer InterGroup. 26
Quality-adjusted PFS (QA-PFS) and quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) are methods of evaluation that integrate data on both the quality and quantity of survival time.27–29 QA-PFS represents the duration of survival without disease progression, adjusted for the value patients place on their health status by incorporating information on patient-reported health-related quality of life (HRQoL) prior to disease progression.29,30 Q-TWiST represents the time without symptoms of disease or toxicity (TWiST) from treatments, prior to disease progression. Data on toxicity (adverse events [AEs]) and patient-reported HRQoL are combined with PFS data to give a single measure of the quality of survival.27–29
Both QA-PFS and Q-TWiST complement efficacy and safety data from clinical trials and can help to evaluate the net benefit of new therapies across many different cancers.29–33 In this analysis, using data from the PRIMA trial, we assessed the QA-PFS and Q-TWiST of niraparib versus placebo among patients with advanced OC in the maintenance setting following response on first-line platinum-based chemotherapy.
Methods
Data source
This study analyzed data from the PRIMA (PRIMA/ENGOT-OV26/GOG-3012) trial (ClinicalTrials.gov identifier: NCT02655016; trial registration date: January 13, 2016), for which the design and results have been reported previously. 9 In brief, PRIMA was a randomized, double-blind, placebo-controlled, phase 3 trial conducted at 181 sites across 20 countries. Eligible patients were adults (aged ⩾18 years) with advanced OC (stage III or IV) who had completed six to nine cycles of platinum-based chemotherapy with a physician-assessed CR or PR. Tumor samples were tested to identify those with HRd, defined as the presence of a breast cancer gene (BRCA) deleterious mutation, a score of ⩾42 on the myChoice test (Myriad Genetics, Inc., Salt Lake City, UT, USA), or both. Patients whose HRd status was not determined were included in the overall population and not the HRd cohort. The trial was performed in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines, and local regulations. All patients provided written informed consent to participate.
In total, 733 patients were enrolled and underwent randomization between July 2016 and June 2018. Patients were randomized 2:1 to receive oral niraparib or placebo once daily in 28-day cycles for 36 months or until disease progression. Niraparib was administered once daily as either a fixed dose of 300 mg or an individualized starting dose of 200 mg for patients with a baseline body weight of <77 kg, a platelet count of <150,000 per cubic millimeter, or both. The primary endpoint in the PRIMA trial was PFS in patients who had tumors with HRd and in the overall population, as determined with hierarchical testing. PFS was defined as the time from the date of randomization to the date of first documentation of disease progression or death from any cause, whichever occurred first, and was assessed by blinded independent central review (BICR).
Secondary endpoints included overall survival (OS), time to first subsequent therapy, PFS-2 (time from randomization to progression while the patient was receiving a subsequent anticancer therapy), and pharmacokinetic analyses. In addition, PROs were assessed using the Functional Assessment of Cancer Therapy–Ovarian Symptom Index, the European Quality of Life Five-Dimension, Five-Level questionnaire (EQ-5D-5L), the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life questionnaire (EORTC-QLQ-C30), and the EORTC Quality of Life Questionnaire Ovarian Cancer module (EORTC-QLQ-OV28) instruments. Scores were measured at the screening visit, throughout treatment and at 4, 8, 12, and 24 weeks after the last dose of niraparib or placebo. Safety was assessed through the monitoring of AEs (with grading according to the National Cancer Institute Common Terminology for Adverse Events, version 4.03), laboratory testing, vital sign measurements, and physical examination.
Analysis objectives
The primary objective of this analysis was to assess the QA-PFS and Q-TWiST of niraparib versus placebo among patients with advanced OC in the maintenance setting following response on first-line platinum-based chemotherapy using the PRIMA trial data for the overall (ITT) population. Exploratory objectives were to assess the same outcomes in prespecified patient subpopulations from the PRIMA trial: (1) HRd and homologous recombination proficient (HRp [lack of HRd]) patients, and (2) BRCA wild type and BRCA mutant populations. Patients with undetermined HRd status were included in the overall population, but not in the HRd or HRp populations.
Statistical analysis
QA-PFS and Q-TWiST analyses were performed for the ITT population from PRIMA (niraparib, n = 487; placebo, n = 246), as well as in the HRd population (n = 373) and other prespecified genetically defined subgroups of interest. Outcome measures and calculations of all QA-PFS and Q-TWiST parameters are shown in Table 1, and additional details of all analyses are provided in the Supplemental Methods, Supplemental Figure 1, and Supplemental Table 1. In brief, QA-PFS was calculated as the product of the BICR PFS function and the mean EQ-5D-5L index score 34 prior to progression. Q-TWiST was calculated as the sum product of the following two health states and each state’s assigned QoL weight (utility):
Table 1.
Outcome measures of interest.
| Variable | Role | Operational definition |
|---|---|---|
| Mean QA-PFS | Calculated outcome | Product of the PFS function, obtained by restricted mean survival estimation and the mean EQ-5D-5L index score prior to progression |
| TOX time, months | Partitioned survival variable | (Area under the Kaplan–Meier curve for days with AEs)/30.4375 days |
| TWiST time, months | Partitioned survival variable | (Area under the Kaplan–Meier curve for days to PFS event − area under the Kaplan–Meier curve for days with AEs)/30.4375 days |
| UTOX | Utility | Baseline: average EQ-5D-5L utilities collected during TOX state in the PRIMA trial; sensitivity analysis for the overall ITT population using a utility of 0.5 |
| UTWiST | Utility | Assumed to be 1.0a |
| Mean Q-TWiST | Calculated outcome | Q-TWiST = UTWiST × TWiST + UTOX × TOX |
TWiST was considered to have utility equal to 1.0, representing the best possible quality of life for a patient, and consistent with previous Q-TWiST analyses in oncology. 29
AE, adverse event; EQ-5D-5L, European Quality of Life Five-Dimension, Five-Level questionnaire; ITT, intention-to-treat; PFS, progression-free survival; QA-PFS, quality-adjusted progression-free survival; Q-TWiST, quality-adjusted time without symptoms of disease or toxicity; TOX, time before PFS during which patients experienced grade ⩾2 AEs; TWiST, time without symptoms of disease or toxicity; UTOX, utility for TOX; UTWiST, utility for TWiST.
(1) TOX: Time with toxicities, defined as the time prior to PFS, during which patients experienced grade ⩾2 symptomatic AEs of interest (fatigue/asthenia, nausea, vomiting, abdominal pain, and abdominal bloating). Grade 1 AEs were not included as they were considered to be less clinically significant than grade ⩾2 AEs.
AEs of interest were selected based on a targeted literature search to identify AEs included in TWiST and Q-TWiST analyses that evaluated PARPis as maintenance treatment for OC. The most common symptomatic AEs from the PRIMA trial, as well as external expert clinical opinions, were also considered, in order to focus on clinically meaningful specific symptomatic AEs associated with disease progression or recurrence that would be expected to impact most substantially on QoL. The duration of AEs was defined as the time between the start and end (resolution) dates of an AE. For AEs that had not resolved by the time of progression, the date of progression was used as the end date. For AEs with missing resolution dates, the end date was truncated at PFS date/end of follow-up, whichever occurred first. When patients experienced > 1 AE on a given day, the AE with the longest duration was used when counting time to resolution.
(2) TWiST: Time without symptoms of disease or toxicity prior to PFS.
For all analyses, the level of significance was set to 5%, and confidence intervals (CIs) were calculated using a non-parametric bootstrap method. Analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Sensitivity analyses
The following sensitivity analyses were conducted for the overall ITT population: (1) Difference in Q-TWiST between treatment arms when restricting data to different periods of follow-up, and (2) use of a utility value of 0.5 for TOX, instead of values directly elicited from PRIMA trial patients via the EQ-5D-5L instrument. Further details of these sensitivity analyses are presented in the Supplemental Methods.
Results
Patients
A total of 733 patients were randomized to niraparib (n = 487) and placebo (n = 246) in the PRIMA trial. 9 The analyses presented here use data collected up to the last date of PFS assessment (May 17, 2019). Baseline demographic and clinical characteristics were balanced between treatment arms. 9
QA-PFS analysis
Restricted mean PFS and QA-PFS in the HRd and overall ITT populations, comparing patients randomized to niraparib versus placebo, are summarized in Table 2. Restricted mean PFS was significantly longer with niraparib versus placebo in the HRd (19.3 [95% CI, 17.6–20.7] versus 13.4 [11.0–15.1] months; mean difference, 5.9 [3.5–8.7] months) and overall ITT (15.5 [14.3–16.5] versus 11.9 [10.2–13.3] months; mean difference, 3.6 [1.8–5.7] months) populations. The restricted mean QA-PFS was also significantly longer with niraparib than with placebo in both the HRd (17.7 [15.6–19.1] versus 11.2 [9.1–12.6] months; mean difference, 6.5 [3.9, 8.9] months) and overall ITT (14.0 [12.6–15.0] versus 9.9 [8.6–11.0] months; mean difference, 4.1 [2.2–5.8] months) populations. Restricted mean PFS and QA-PFS were also significantly longer with niraparib versus placebo in the BRCA wild type and BRCA mutant subgroups, and numerically longer with niraparib versus placebo in the HRp subgroup (Supplemental Table 2).
Table 2.
Restricted mean duration of PFS and QA-PFS for the HRd and overall ITT populations at last PFS of the treatment group.
| Subgroup | Restricted mean duration (95% CI), months | ||
|---|---|---|---|
| Niraparib | Placebo | Difference c | |
| HRd at 27.8 months a | n = 247 | n = 126 | |
| PFS | 19.3 (17.6–20.7) | 13.4 (11.0–15.1) | 5.9 (3.5–8.7) |
| QA-PFS b | 17.7 (15.6–19.1) | 11.2 (9.1–12.6) | 6.5 (3.9–8.9) |
| Overall ITT at 27.8 months a | n = 487 | n = 246 | |
| PFS | 15.5 (14.3–16.5) | 11.9 (10.2–13.3) | 3.6 (1.8–5.7) |
| QA-PFS b | 14.0 (12.6–15.0) | 9.9 (8.6–11.0) | 4.1 (2.2–5.8) |
Patients without an EQ-5D-5L index score were assigned the mean EQ-5D-5L for their treatment arm.
QA-PFS is a function resulting from the product of quality of life function and the survival function.
Bold type denotes statistically significant differences.
CI, confidence interval; EQ-5D-5L, European Quality of Life Five-Dimension, Five-Level questionnaire; HRd, homologous recombination deficient; ITT, intention-to-treat; PFS, progression-free survival; QA-PFS, quality-adjusted progression-free survival.
Q-TWiST analyses
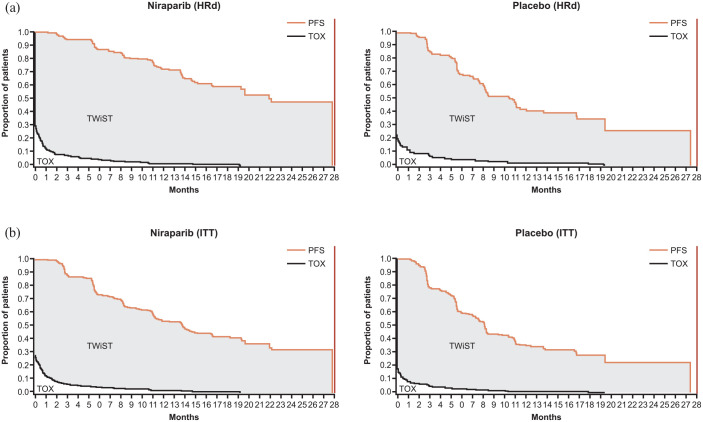
The Q-TWiST analyses for the HRd and overall ITT populations were conducted at the last PFS of patients randomized to niraparib (27.8 months). Partitioned survival curves for the niraparib and placebo groups are shown in Figure 1. For TOX calculations, a total of 140/487 and 42/246 patients in the niraparib and placebo groups, respectively, experienced grade ⩾2 symptomatic AEs of interest prior to PFS or last assessment date. The restricted mean duration of time spent in the TOX state (with grade ⩾2 AEs of interest) was numerically longer but not statistically significant with niraparib versus placebo in both the HRd (difference [95% CI], 0.1 [−0.4, 0.6] months) and overall ITT (difference [95% CI], 0.2 [−0.1, 0.6] months) populations (Table 3).
Figure 1.
Partitioned survival curves for the (a) HRd and (b) overall ITT populations.
TOX included grade ⩾2 AEs of interest (fatigue or asthenia, nausea, vomiting, abdominal pain, and abdominal bloating). HRd and overall ITT populations had a maximum PFS of 27.8 months.
AE, adverse event; HRd, homologous recombination deficient; ITT, intention-to-treat; PFS, progression-free survival; TOX, time before PFS during which patients experienced grade ⩾2 AEs; TWiST, time without symptoms of disease or toxicity.
Table 3.
Restricted mean duration of health states for the HRd and overall ITT populations at maximum PFS of the treatment group.
| Health state | Restricted mean duration (95% CI), months | ||
|---|---|---|---|
| Niraparib | Placebo | Difference b | |
| HRd at 27.8 months | n = 247 | n = 126 | |
| TOX a | 0.7 (0.4–1.0) | 0.6 (0.2–1.0) | 0.1 (−0.4, 0.6) |
| TWiST | 18.6 (16.9–20.0) | 12.8 (10.6–14.6) | 5.8 (3.5–8.4) |
| Overall ITT population at 27.8 months | n = 487 | n = 246 | |
| TOX a | 0.7 (0.5–0.8) | 0.4 (0.2–0.6) | 0.2 (−0.1, 0.6) |
| TWiST | 14.8 (13.6–16.0) | 11.5 (9.8–12.9) | 3.3 (1.5–5.3) |
TOX included grade ⩾2 AEs of interest (fatigue or asthenia, nausea, vomiting, abdominal pain, and abdominal bloating).
Bold type denotes statistically significant differences.
AE, adverse event; CI, confidence interval; HRd, homologous recombination deficient; ITT, intention-to-treat; PFS, progression-free survival; TOX, time before PFS during which patients experienced grade ⩾2 AEs; TWiST, time without symptoms of disease or toxicity.
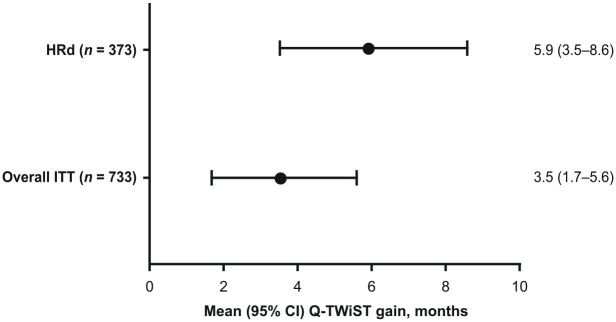
In contrast, restricted mean TWiST time was significantly longer with niraparib compared with placebo in both the HRd (difference [95% CI], 5.8 [3.5–8.4] months) and ITT (difference [95% CI], 3.3 [1.5–5.3] months) populations (Table 3). In the quality-adjusted analysis, setting utility weights for the TOX health state to 0.767, as estimated from EQ-5D-5L data (Supplemental Table 1), patients treated with niraparib had significantly greater mean Q-TWiST gain compared with placebo in both the HRd (difference [95% CI], 5.9 [3.5–8.6] months) and ITT (difference [95% CI], 3.5 [1.7–5.6] months) populations (Figure 2).
Figure 2.
Mean Q-TWiST gain for the HRd and overall ITT populations at maximum PFS of the treatment group.
CI, confidence interval; HRd, homologous recombination deficient; ITT, intention-to-treat; PFS, progression-free survival; Q-TWiST, quality-adjusted time without symptoms of disease or toxicity.
Results from other genetically defined subgroups (HRp, BRCA wild type, and BRCA mutant patients) are shown in Supplemental Figures 2 and 3, and Supplemental Table 3. There were numerical Q-TWiST gains with niraparib versus placebo across these subgroups, and these were statistically significant in BRCA wild type and BRCA mutant patients.
Sensitivity analyses
In Q-TWiST analyses conducted at different periods of follow-up, Q-TWiST gains for niraparib versus placebo consistently and significantly increased with longer follow-up (Supplemental Figure 4). Similarly, when utility weights for the TOX health state were set to 0.5 (rather than the PRIMA trial-derived value of 0.767 that was used in the main analysis), patients treated with niraparib had a significant Q-TWiST gain compared with placebo (difference [95% CI], 3.4 [1.7–5.5] months) (Supplemental Table 4) that was similar to results from the main analysis.
Discussion
This analysis combined data on treatment efficacy, toxicity, and HRQoL to comprehensively assess the treatment benefit with niraparib maintenance therapy following first-line platinum-based chemotherapy in patients with advanced OC. The findings showed that niraparib increased PFS without significantly impacting toxicity, and niraparib-treated patients spent longer time in the TWiST health state (i.e., without symptoms of disease or toxicity) than those who received placebo.
Mean QA-PFS was significantly longer with niraparib versus placebo, both in the HRd and overall ITT populations. These findings demonstrated that the PFS benefit of niraparib shown in the PRIMA trial persisted when adjusted for patients’ perception of their HRQoL, thereby demonstrating a patient-relevant improvement in PFS with niraparib. Results of the Q-TWiST analysis supported and expanded on these findings by showing that: (1) Niraparib increased restricted mean PFS without significantly increasing the duration of symptomatic grade ⩾2 AEs prior to disease progression, and (2) niraparib-treated patients spent longer without symptoms of disease or toxicity than those who received placebo, as evidenced by the significant gain in TWiST observed with niraparib versus placebo. Exploratory analysis of prespecified biomarker subgroups, including BRCA wild type, BRCA mutant, and HRp patients, also showed a consistent trend toward QA-PFS and Q-TWiST benefits.
Our data add to and support the existing evidence for the benefit of PARPi in the maintenance treatment of OC. PRO data from phase 3 clinical trials in both newly diagnosed and recurrent OC have suggested that PARPi therapy does not result in decreased QoL compared with placebo.9,12,35–37 Subsequent analyses have expanded on these data by assessing QA-PFS and TWiST/Q-TWiST in the trial populations. In the SOLO1 trial comparing maintenance olaparib with placebo in women with newly diagnosed platinum-sensitive advanced OC and a BRCA mutation, mean QA-PFS and TWiST gains of 12.17 and 12.92 months, respectively, were reported for olaparib versus placebo. 38 These findings, while in concordance with our own data, are not directly comparable with the QA-PFS and TWiST gains reported for niraparib, owing to differences between the patient populations in the SOLO1 and PRIMA trials. Patients in SOLO1 had a lower risk of disease progression or death than the PRIMA population, based on prognostic factors; the PRIMA trial also enrolled patients with non-mutated BRCA OC, in addition to those with a BRCA mutation.9,12
Other trials (SOLO2/ENGOT-Ov21, ENGOT-OV16/NOVA, and ARIEL3) have also demonstrated QA-PFS and/or TWiST/Q-TWiST gains with other PARPi in patients with recurrent OC,29,39,40 but these findings cannot be directly compared with those from trials of maintenance therapy with PARPi in the first-line setting, owing to differences in the characteristics of patients with newly diagnosed versus recurrent OC. Analyses to date have also differed in the methods and assumptions used to calculate the TWiST, Q-TWiST, and QA-PFS parameters, as well as in the type and severity of AEs included when evaluating treatment toxicity. For example, although previous Q-TWiST analyses have not consistently included abdominal pain/bloating, these were included in the present analysis due to being frequently reported AEs among patients in the PRIMA trial. 9 Consequently, it is difficult to make direct comparisons between studies. Nonetheless, the analysis of PRIMA data reported here demonstrates gains in QA-PFS and Q-TWiST as a result of maintenance treatment with niraparib.
Maintaining good QoL is a key goal of maintenance therapy for OC. Results from a survey of 1400 women with OC showed that adverse effects of treatment are highly important to these patients, with many willing to trade increased duration of PFS for fewer side effects, particularly when treatment is not curative. 41 These data emphasize the importance of directly assessing QoL in clinical trials to provide a more complete assessment of the clinical benefit of treatment to patients, irrespective of clinical efficacy. Conceptually, the Q-TWiST methodology applied in this analysis is similar to other clinical benefit measures, such as the American Society of Clinical Oncology Value Framework’s net health benefit,21,42 which seeks to incorporate patient-centered outcomes when assessing the overall value of new therapies for patients.
Strengths of the present analysis include the use of Q-TWiST methodology, which enabled the incorporation of EQ-5D-5L data, and therefore information on patients’ perceptions of QoL, into TWiST analyses. Additionally, use of a QA-PFS analysis to assess quality-adjusted survival enabled us to combine data on both quality (EQ-5D-5L scores) and quantity (PFS) of life. QA-PFS is an important metric that has been used extensively to evaluate oncology therapies,29,30,43 and enables a more comprehensive assessment of treatment benefit than is permitted by either HRQoL or PFS data alone. The consistency between findings from the QA-PFS and Q-TWiST analyses increases the robustness of the findings. Limitations of this analysis include the small sample sizes for some subgroup analyses and missing EQ-5D-5L index scores for 7% of patients in both treatment arms. Additionally, the PRIMA trial was only powered to show differences in the overall ITT population and HRd subgroup, and not the other genetically defined subgroups. Consequently, findings from the subgroups are exploratory and no firm conclusions can be drawn regarding the impact of niraparib on QA-PFS and Q-TWiST in these patients.
Limitations specific to the Q-TWiST analyses are largely inherent to the methodology. Multiple AEs on the same day were counted as one AE, as in most Q-TWiST analyses.39,40 Average utility of TOX for Q-TWiST can only be calculated among patients with a QoL assessment conducted during the occurrence of the grade ⩾2 AEs of interest. All toxicities were assigned the same utility weight and combined into one continuous time period at the start of therapy, irrespective of the severity or duration of the AEs. This assumption may not be strictly accurate, as AEs of different type or severity could have different effects on utility. Similarly, some drug-related effects that impacted patients’ QoL may not have been reportable as AEs and, as such, would not have been captured. Finally, the OS data for PRIMA were not mature at the time of this analysis and could not be incorporated into the Q-TWiST analysis.
Conclusions
In patients with advanced OC, first-line maintenance therapy with niraparib was associated with longer QA-PFS and Q-TWiST compared with placebo. Significant benefit was seen in both the HRd and overall ITT populations, confirming the benefit of niraparib in genetically diverse patients with OC. Collectively, these findings demonstrate that niraparib maintenance treatment is associated with a PFS improvement and that treatment benefit is maintained even when HRQoL and/or toxicity data are combined with PFS in a single measure.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221126149 for Quality-adjusted time without symptoms of disease or toxicity and quality-adjusted progression-free survival with niraparib maintenance in first-line ovarian cancer in the PRIMA trial by Maria-Pilar Barretina-Ginesta, Bradley J. Monk, Sileny Han, Bhavana Pothuri, Annika Auranen, Dana M. Chase, Domenica Lorusso, Charles Anderson, Sophie Abadie-Lacourtoisie, Noelle Cloven, Elena I. Braicu, Amnon Amit, Andrés Redondo, Ruchit Shah, Nehemiah Kebede, Carol Hawkes, Divya Gupta, Tatia Woodward, David M. O’Malley and Antonio González-Martín in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359221126149 for Quality-adjusted time without symptoms of disease or toxicity and quality-adjusted progression-free survival with niraparib maintenance in first-line ovarian cancer in the PRIMA trial by Maria-Pilar Barretina-Ginesta, Bradley J. Monk, Sileny Han, Bhavana Pothuri, Annika Auranen, Dana M. Chase, Domenica Lorusso, Charles Anderson, Sophie Abadie-Lacourtoisie, Noelle Cloven, Elena I. Braicu, Amnon Amit, Andrés Redondo, Ruchit Shah, Nehemiah Kebede, Carol Hawkes, Divya Gupta, Tatia Woodward, David M. O’Malley and Antonio González-Martín in Therapeutic Advances in Medical Oncology
Acknowledgments
All listed authors meet the criteria for authorship set forth by the International Committee of Medical Journal Editors. Writing and editorial support, including drafting of the manuscript, collating author comments, and fact checking, funded by GSK (Waltham, MA, USA) and coordinated by Michael Sheldon, PhD, of GSK, was provided by Lucy Ambrose, DPhil, of Core Medica (London, UK).
Footnotes
ORCID iDs: Maria-Pilar Barretina-Ginesta  https://orcid.org/0000-0003-0074-6614
https://orcid.org/0000-0003-0074-6614
Antonio González-Martín  https://orcid.org/0000-0001-8376-9576
https://orcid.org/0000-0001-8376-9576
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Maria-Pilar Barretina-Ginesta, GEICO and Medical Oncology Department, Institut Català d’Oncologia, Sant Ponç, Avinguda de França, Girona 17007, SpainGirona Biomedical Research Institute, Girona University, Girona, Spain.
Bradley J. Monk, GOG Foundation and Arizona Oncology (US Oncology Network), University of Arizona, Creighton University, Phoenix, AZ, USA
Sileny Han, BGOG and Department of Gynaecology and Obstetrics,University Hospitals Leuven, Leuven, Belgium.
Bhavana Pothuri, GOG Foundation and Department of Obstetrics/Gynecology, Laura and Isaac Perlmutter Cancer Center, NYU Langone Health, New York, NY, USA.
Annika Auranen, NSGO and Department of Obstetrics and Gynecology and Tays Cancer Centre, Tampere University Hospital, Tampere, Finland.
Dana M. Chase, GOG Foundation and Arizona Oncology (US Oncology Network), University of Arizona, Creighton University, Phoenix, AZ, USA
Domenica Lorusso, MITO and Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy.
Charles Anderson, GOG and Willamette Valley Cancer Institute, Eugene, OR, USA.
Sophie Abadie-Lacourtoisie, GINECO and Oncologie Médicale Gynécologique, Institut de Cancérologie de l’Ouest – Site Paul Papin, Angers, France.
Noelle Cloven, GOG and Division of Gynecologic Oncology, Texas Oncology (US Oncology Network), Fort Worth, TX, USA.
Elena I. Braicu, AGO and Department for Gynaecology, Campus Virchow Clinic, Charité – Universitätsmedizin Berlin, Berlin, GermanyDepartment of Obstetrics and Gynecology, Stanford University, Palo Alto, CA, USA
Amnon Amit, ISGO and Division of Obstetrics and Gynecology, Rambam Medical Centre, Haifa, Israel.
Andrés Redondo, GEICO and Medical Oncology Department, Hospital Universitario La Paz-IdiPAZ, Madrid, Spain.
Ruchit Shah, Open Health Evidence and Access, Bethesda, MD, USAHealth Economics and Outcomes Research, Daiichi Sankyo, Basking Ridge, NJ, USA.
Nehemiah Kebede, Open Health Evidence and Access, Bethesda, MD, USAReal World Evidence Science, Oncology Business Unit, AstraZeneca, Gaithersburg, MD, USA.
Carol Hawkes, GSK, Brentford, UK.
Divya Gupta, GSK, Waltham, MA, USA.
Tatia Woodward, GSK, Philadelphia, PA, USAGlobal Value and Evidence Strategy, Pfizer, Baltimore, MD, USA.
David M. O’Malley, GOG and Division of Gynecologic Oncology, Ohio State University COM – James CCC, Columbus, OH, USA
Antonio González-Martín, GEICO and Medical Oncology Department, Clínica Universidad de Navarra, Madrid, SpainCIMA-University of Navarra, Program in Solid Tumors, Pamplona, Spain.
Declarations
Ethics approval and consent to participate: The PRIMA trial protocol, amendments to the protocol, informed consent form, and other study documents were approved by an Institutional Review Board or Independent Ethics Committee for each study center prior to implementation. Written informed consent was obtained from each study participant according to the regulatory and legal requirements of the participating country.
Consent for publication: Not applicable.
Author contribution(s): Maria-Pilar Barretina-Ginesta: Conceptualization; Writing – review & editing.
Bradley J. Monk: Conceptualization; Writing – review & editing.
Sileny Han: Conceptualization; Writing – review & editing.
Bhavana Pothuri: Conceptualization; Writing – review & editing.
Annika Auranen: Conceptualization; Writing – review & editing.
Dana M. Chase: Conceptualization; Writing – review & editing.
Domenica Lorusso: Conceptualization; Writing – review & editing.
Charles Anderson: Conceptualization; Writing – review & editing.
Sophie Abadie-Lacourtoisie: Conceptualization; Writing – review & editing.
Noelle Cloven: Conceptualization; Writing – review & editing.
Elena I. Braicu: Conceptualization; Writing – review & editing.
Amnon Amit: Conceptualization; Writing – review & editing.
Andrés Redondo: Conceptualization; Writing – review & editing.
Ruchit Shah: Conceptualization; Data curation; Formal analysis; Writing – review & editing.
Nehemiah Kebede: Conceptualization; Data curation; Formal analysis; Writing – review & editing.
Carol Hawkes: Conceptualization; Writing – review & editing.
Divya Gupta: Conceptualization; Writing – review & editing.
Tatia Woodward: Conceptualization; Writing – review & editing.
David M. O’Malley: Conceptualization; Writing – review & editing.
Antonio González-Martín: Conceptualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by GSK (PRIMA/ENGOT-OV26/GOG-3012; ClinicalTrials.gov number: NCT02655016).
Competing interests: MPBG reports receiving lecture fees, advisory board fees, and travel support from AstraZeneca, Clovis Oncology, GSK, Merck, PharmaMar, Roche, and Tesaro. BJM has received honoraria and lecture fees from Tesaro. BP has received institutional grant support from AstraZeneca, Celsion, Clovis Oncology, Genentech/Roche, Merck, Mersana, Tesaro/GSK, Toray, I-Mab, Incyte, and Seagen; and advisory board compensation from Arquer, AstraZeneca, Eisai, Elevar, Lilly, Merck, Mersana, Tesaro/GSK, I-Mab, ImmunoGen, InxMed, Onconova Therapeutics, Seagen, and Toray. AAu reports receiving consulting fees from GSK. DMC reports personal fees from GSK; has received honoraria from Roche; has served as a consultant to AstraZeneca and Mateon Therapeutics; and has received research grants to her institution from Genentech. DL reports receiving advisory board fees from Amgen, AstraZeneca, Clovis Oncology, Genmab, and ImmunoGen; grant support, paid to her institution, and consulting fees from PharmaMar; and grant support, paid to her institution, and advisory board fees from Merck. EIB reports receiving consulting fees, lecture fees, and travel support from AstraZeneca, Clovis, GSK, Tesaro, Roche, Roche Diagnostics, Eisai, and Merck Sharp & Dohme; and grant support to their institution from Roche Diagnostics, Merck Sharp & Dohme, and Bayer. AR has received grant support, advisory fees, and travel support from PharmaMar and Roche; advisory fees and travel support from AstraZeneca and Tesaro/GSK; advisory fees from Clovis; and grant support from Eisai. RS is an employee of Open Health Evidence and Access, which received research funding from GSK for this study. NK was an employee of Open Health Evidence and Access at the time of the study. CH and DG are employees of GSK. TW is a former employee of GSK. DMO reports grants and personal fees from Clovis; has served on advisory boards for Agenus, AstraZeneca, Eisai, Genentech/Roche, ImmunoGen, Iovance Biotherapeutics, Janssen/Johnson & Johnson, Merck, Mersana, Myriad, Novartis Pharmaceuticals, Novocure, Regeneron Pharmaceuticals, Seagen, Tarveda, and Tesaro/GSK; has served on steering committees for Amgen; has served as a consultant to AbbVie, Agenus, Ambry, AstraZeneca, Eisai, Genentech/Roche, GOG Foundation, ImmunoGen, Iovance Biotherapeutics, Merck, Mersana, Novartis Pharmaceuticals, Novocure, Seagen, and Tesaro/GSK; and has received research support to his institution from AbbVie, Agenus, Ajinomoto, Amgen, Array BioPharma, AstraZeneca, Bristol-Myers Squibb, Cerulean Pharma, Eisai, EMD Serono, Ergomed Clinical Research, Genentech/Roche, Genmab, GOG Foundation, ImmunoGen, INC Research, inVentiv Health Clinical, Iovance Biotherapeutics, Janssen/Johnson & Johnson, Ludwig Institute for Cancer Research, Merck, Mersana, New Mexico Cancer Care Alliance, Novocure, PRA International, Regeneron Pharmaceuticals, Seagen, Serono, Stemcentrx, Tesaro/GSK, TRACON Pharmaceuticals, VentiRx, and Yale University. AGM reports receiving consulting fees from Alkermes, Amgen, AstraZeneca, Clovis, Genmab, GSK, Immunogen, Mersana, Merck Sharp & Dohme, Oncoinvent, PharmaMar, Roche, SOTIO, and Takeda; lecture fees from AstraZeneca, Clovis, GSK, Merck Sharp & Dohme, PharmaMar, and Roche; travel support from AstraZeneca, GSK, and PharmaMar; grant support to their institution from Roche Holding and Tesaro. SH, CA, SAL, NC, and AAm have no conflicts to disclose.
Availability of data and materials: Information on GSK’s data sharing commitments and requesting access to anonymized individual participant data and associated documents can be found at www.clinicalstudydatarequest.com.
Prior presentation: Preliminary results from this analysis were presented at the European Society for Medical Oncology virtual congress, September 16–21, 2021.
References
- 1. International Agency for Research on Cancer. Estimated age-standardized mortality rates (World) in 2020, worldwide, females, all ages, https://gco.iarc.fr/today/home (2020, accessed 2 August 2021).
- 2. SEER. Cancer stat facts: ovarian cancer, https://seer.cancer.gov/statfacts/html/ovary.html (2021, accessed 25 June 2021).
- 3. Badgwell D, Bast RC., Jr. Early detection of ovarian cancer. Dis Markers 2007; 23: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996; 334: 1–6. [DOI] [PubMed] [Google Scholar]
- 5. Bruchim I, Jarchowsky-Dolberg O, Fishman A. Advanced (>second) line chemotherapy in the treatment of patients with recurrent epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol 2013; 166: 94–98. [DOI] [PubMed] [Google Scholar]
- 6. Foley OW, Rauh-Hain JA, del Carmen MG. Recurrent epithelial ovarian cancer: an update on treatment. Oncology (Williston Park) 2013; 27: 288–294, 298. [PubMed] [Google Scholar]
- 7. Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol 2012; 23: 2605–2612. [DOI] [PubMed] [Google Scholar]
- 8. Gupta S, Nag S, Aggarwal S, et al. Maintenance therapy for recurrent epithelial ovarian cancer: current therapies and future perspectives – a review. J Ovarian Res 2019; 12: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzalez-Martin A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2019; 381: 2391–2402. [DOI] [PubMed] [Google Scholar]
- 10. DiSilvestro P, Alvarez Secord A. Maintenance treatment of recurrent ovarian cancer: is it ready for prime time? Cancer Treat Rev 2018; 69: 53–65. [DOI] [PubMed] [Google Scholar]
- 11. Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 2019; 381: 2416–2428. [DOI] [PubMed] [Google Scholar]
- 12. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018; 379: 2495–2505. [DOI] [PubMed] [Google Scholar]
- 13. Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann Oncol 2019; 30: 672–705. [DOI] [PubMed] [Google Scholar]
- 14. National Comprehensive Cancer Network. NCCN guidelines version 3.2021, https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (2021, accessed 13 September 2021).
- 15. Giornelli GH. Management of relapsed ovarian cancer: a review. Springerplus 2016; 5: 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hess LM, Rong N, Monahan PO, et al. Continued chemotherapy after complete response to primary therapy among women with advanced ovarian cancer: a meta-analysis. Cancer 2010; 116: 5251–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Medicines Agency. Zejula summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/zejula-epar-product-information_en.pdf (2021, accessed 30 June 2021).
- 18. Food and Drug Administration. Zejula: highlights of prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208447s015s017lbledt.pdf (2020, accessed 30 June 2021).
- 19. Frank L, Basch E, Selby JV; Patient-Centered Outcomes Research Institute. The PCORI perspective on patient-centered outcomes research. JAMA 2014; 312: 1513–1514. [DOI] [PubMed] [Google Scholar]
- 20. Bhat G, Karakasis K, Oza AM. Measuring quality of life in ovarian cancer clinical trials-can we improve objectivity and cross trial comparisons? Cancers (Basel) 2020; 12: 3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J Clin Oncol 2016; 34: 2925–2934. [DOI] [PubMed] [Google Scholar]
- 22. Cherny NI, Sullivan R, Dafni U, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 2015; 26: 1547–1573. [DOI] [PubMed] [Google Scholar]
- 23. Food and Drug Administration. Core patient-reported outcomes in cancer clinical trials: guidance for industry, https://www.fda.gov/media/149994/download (2021, accessed 1 July 2021).
- 24. European Medicines Agency. Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man, https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evaluation-anticancer-medicinal-products-man_en.pdf (2016, accessed 1 July 2021).
- 25. Joly F, Hilpert F, Okamoto A, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer 2017; 78: 133–138. [DOI] [PubMed] [Google Scholar]
- 26. Kurtz JE, Gebski V, Sukhin V, et al. Incorporating patient centered benefits as endpoints in randomized trials of maintenance therapies in advanced ovarian cancer: a position paper from the GCIG symptom benefit committee. Gynecol Oncol 2021; 161: 502–507. [DOI] [PubMed] [Google Scholar]
- 27. Gelber RD, Goldhirsch A, Cole BF, et al. A quality-adjusted time without symptoms or toxicity (Q-TWiST) analysis of adjuvant radiation therapy and chemotherapy for resectable rectal cancer. J Natl Cancer Inst 1996; 88: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 28. Goldhirsch A, Gelber RD, Simes RJ, et al. Costs and benefits of adjuvant therapy in breast cancer: a quality-adjusted survival analysis. J Clin Oncol 1989; 7: 36–44. [DOI] [PubMed] [Google Scholar]
- 29. Oza AM, Lorusso D, Aghajanian C, et al. Patient-centered outcomes in ARIEL3, a phase III, randomized, placebo-controlled trial of rucaparib maintenance treatment in patients with recurrent ovarian carcinoma. J Clin Oncol 2020; 38: 3494–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diaby V, Adunlin G, Ali AA, et al. Using quality-adjusted progression-free survival as an outcome measure to assess the benefits of cancer drugs in randomized-controlled trials: case of the BOLERO-2 trial. Breast Cancer Res Treat 2014; 146: 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zbrozek AS, Hudes G, Levy D, et al. Q-TWiST analysis of patients receiving temsirolimus or interferon alpha for treatment of advanced renal cell carcinoma. Pharmacoeconomics 2010; 28: 577–584. [DOI] [PubMed] [Google Scholar]
- 32. Chen RC, Choueiri TK, Feuilly M, et al. Quality-adjusted survival with first-line cabozantinib or sunitinib for advanced renal cell carcinoma in the CABOSUN randomized clinical trial (Alliance). Cancer. 2020; 126: 5311–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pelzer U, Blanc JF, Melisi D, et al. Quality-adjusted survival with combination nal-IRI+5-FU/LV vs 5-FU/LV alone in metastatic pancreatic cancer patients previously treated with gemcitabine-based therapy: a Q-TWiST analysis. Br J Cancer 2017; 116: 1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pothuri B, Han S, Chase D, et al. 810MO Patient-reported outcomes (PROs) in patients (pts) receiving niraparib in the PRIMA/ENGOT-OV26/GOG-3012 trial. Ann Oncol 2020; 31: S612–S613. [Google Scholar]
- 36. Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Del Campo JM, Matulonis UA, et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. J Clin Oncol 2019; 37: 2968–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Friedlander M, Moore KN, Colombo N, et al. Patient-centred outcomes and effect of disease progression on health status in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation receiving maintenance olaparib or placebo (SOLO1): a randomised, phase 3 trial. Lancet Oncol 2021; 22: 632–642. [DOI] [PubMed] [Google Scholar]
- 39. Matulonis UA, Walder L, Nottrup TJ, et al. Niraparib maintenance treatment improves time without symptoms or toxicity (TWiST) versus routine surveillance in recurrent ovarian cancer: a TWiST analysis of the ENGOT-OV16/NOVA trial. J Clin Oncol 2019; 37: 3183–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Friedlander M, Gebski V, Gibbs E, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. Lancet Oncol 2018; 19: 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Minion LE, Coleman RL, Alvarez RD, et al. Endpoints in clinical trials: what do patients consider important? A survey of the Ovarian Cancer National Alliance. Gynecol Oncol 2016; 140: 193–198. [DOI] [PubMed] [Google Scholar]
- 42. Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol 2015; 33: 2563–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friedlander M, Rau J, Lee CK, et al. Quality of life in patients with advanced epithelial ovarian cancer (EOC) randomized to maintenance pazopanib or placebo after first-line chemotherapy in the AGO-OVAR 16 trial. Measuring what matters-patient-centered end points in trials of maintenance therapy. Ann Oncol 2018; 29: 737–743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221126149 for Quality-adjusted time without symptoms of disease or toxicity and quality-adjusted progression-free survival with niraparib maintenance in first-line ovarian cancer in the PRIMA trial by Maria-Pilar Barretina-Ginesta, Bradley J. Monk, Sileny Han, Bhavana Pothuri, Annika Auranen, Dana M. Chase, Domenica Lorusso, Charles Anderson, Sophie Abadie-Lacourtoisie, Noelle Cloven, Elena I. Braicu, Amnon Amit, Andrés Redondo, Ruchit Shah, Nehemiah Kebede, Carol Hawkes, Divya Gupta, Tatia Woodward, David M. O’Malley and Antonio González-Martín in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359221126149 for Quality-adjusted time without symptoms of disease or toxicity and quality-adjusted progression-free survival with niraparib maintenance in first-line ovarian cancer in the PRIMA trial by Maria-Pilar Barretina-Ginesta, Bradley J. Monk, Sileny Han, Bhavana Pothuri, Annika Auranen, Dana M. Chase, Domenica Lorusso, Charles Anderson, Sophie Abadie-Lacourtoisie, Noelle Cloven, Elena I. Braicu, Amnon Amit, Andrés Redondo, Ruchit Shah, Nehemiah Kebede, Carol Hawkes, Divya Gupta, Tatia Woodward, David M. O’Malley and Antonio González-Martín in Therapeutic Advances in Medical Oncology