Abstract
Background:
Myeloproliferative neoplasms (MPNs) are a rare yet important clinical cause of portal hypertension, which may cause recurrent gastroesophageal variceal bleeding (GVB). MPN-associated variceal bleeding lacks specific guidelines and clinical consensus and desiderates cohort studies. We performed a multicenter retrospective study to investigate the efficacy of endoscopic management of bleeding in MPNs.
Methods:
We included consecutive MPN patients with gastroesophageal varices in eight tertiary university hospitals between January 2007 and March 2020. The clinical characteristics of participants were summarized. MPN patients with a history of GVB were followed up for the rebleeding and death, compared with controls suffering from schistosomiasis-associated portal hypertension who received endoscopic treatment for variceal bleeding at the same period.
Results:
A total of 62 MPN patients with gastroesophageal varices were identified, and 37 had a history of GVB. Of these, 24 patients received endoscopic variceal ligation and endoscopic injection of cyanoacrylate for the prophylaxis of variceal rebleeding. Endoscopic treatment significantly reduced the rebleeding rate in MPN patients with a history of GVB (28.2% versus 68.3%, p = 0.0269). Multivariable Cox regression indicated that endoscopic treatment (HR = 0.10, 95% CI: 0.02–0.54, p = 0.008) was the independent protective factor for decreasing the 3-year rebleeding rate, while the use of non-selective beta-blockers (NSBB) (HR = 13.41, 95% CI: 2.15–83.42, p = 0.005) was the risk factor for increasing the 3-year rebleeding rate. As for the efficacy of endoscopic management, 3-year rebleeding rate was significantly lower in MPN patients in contrast to 46 controls with schistosomiasis-associated variceal bleeding (32.9% versus 59.0%, p = 0.0346).
Conclusion:
Endoscopic treatment might be a feasible and potent approach in the management of gastroesophageal variceal rebleeding in MPNs, while NSBB might be ineffective.
Keywords: chronic hematologic malignancy, endoscopy, gastroesophageal varices, Janus kinase 2, non-cirrhotic portal hypertension, rebleeding prophylaxis
Introduction
Non-cirrhotic portal hypertension (PHT) includes a group of diseases characterized by splenomegaly, gastric or esophageal varices, ascites, and preserved liver functions. 1 Myeloproliferative neoplasm (MPN) is an uncommon but important clinical cause of PHT, which might lead to massive splenomegaly, gastroesophageal variceal bleeding (GVB), and ascites. MPNs comprise a heterogeneous group of chronic hematologic malignancies, including polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), and so on. 2 Patients with MPN are characterized by erythrocytosis, thrombocytosis, and splenomegaly, which result from the clonal proliferation of myeloid lineages. 3 The life quality and prognosis of MPN patients mainly depend on disease-related symptoms, especially thrombotic and bleeding events. 4
Janus kinase 2 (JAK2) V617 F mutation is most prevalent in MPN 5 and found in 95% of PV patients while around 50% of ET and PMF cases. 6 JAK2 V617 F test combined with positive findings in bone marrow is highly predictive of MPN diagnosis. 7 Previous studies have shown that JAK2 V617 F has a prominent role in vascular risk and MPN-associated PHT.8,9 The mechanisms underlying PHT in MPNs remain unclear. Splanchnic vein thrombosis (SVT) is a studied contributing force and extramedullary hematopoiesis within the liver and spleen also plays a pivotal role.10,11 Over the past decades, MPN has emerged as the primary systemic cause of non-cirrhotic SVT. 12
Complications of PHT, such as ascites and GVB, usually appear as the first manifestation in MPN patients. 9 A previous study demonstrated that 8.2% of all MPN patients suffered from major bleeding events, of which 55.6% were upper gastrointestinal bleeding. 12 Hemorrhage due to underlying diseases, MPN-specific treatment, or both resulted in the recurrence of complications or even death in MPN patients.4,13 Meanwhile, considering the lethality of thrombosis or thromboembolism, antithrombotic agents such as acetylsalicylic acid are frequently used in MPN patients for prophylaxis.12,14 Yet, these measures lead to the risk of bleeding events and generate difficulties with stopping hemorrhage, thus further complicating the management of MPN. 15 Previous studies indicated that transjugular intrahepatic portosystemic shunt (TIPS) was used to alleviate bleeding, but TIPS-derived thrombosis is a frequent complication in MPN patients. 8 Endoscopic treatment, including esophageal variceal ligation (EVL) and endoscopic injection of cyanoacrylate (EIC), is the recommended treatment selection for variceal bleeding. In MPN patients with gastroesophageal varices, it might be necessary to perform monitoring and treatment via endoscopy, considering the possibility of lethal hemorrhage. The clinical presentation of schistosomiasis-associated PHT is similar to non-cirrhotic PHT, and the utilization of endoscopic management for variceal bleeding is accepted. 16 However, only several case reports pinpointed the utilization of endoscopic management of gastroesophageal varices in MPN patients with a recurrent bleeding history. Research on the long-term outcomes of endoscopic management of this patient population is limited.17–19 Thus, patients with schistosomiasis-associated PHT were recruited as the control group to compare the efficacy of endoscopic management of gastroesophageal varices in MPN patients.
In this study, we aimed to summarize the characteristics of MPN patients with gastric or esophageal varices. We also aimed to evaluate the efficacy of endoscopic treatment in managing variceal bleeding by comparing the outcomes among patients with MPN who underwent endoscopy, with MPN who did not undergo endoscopy, with schistosomiasis-associated liver cirrhosis, and with alcoholic-associated liver cirrhosis.
Methods
Study design
This retrospective study screened all consecutive patients diagnosed with MPNs at eight China tertiary university hospitals between January 2007 and March 2020. We included consecutive patients with MPN-associated gastric or esophageal varices who were (1) diagnosed as PHT and gastroesophageal varices by contrast-enhanced computed tomography (CT) and upper digestive endoscopy; and (2) diagnosed as MPNs via blood or bone test according to World Health Organization (WHO) 2016 criteria. Patients combined with known etiologies of chronic liver disease, including alcoholic liver disease (ALD), hepatitis B or C, primary biliary cirrhosis, autoimmune liver disease, schistosomiasis, and non-alcoholic fatty liver disease and hepatic carcinoma, were excluded from the MPN group. Inclusion criteria for schistosomiasis-related PHT were (1) patients with a history of schistosomiasis infection confirmed by detection of eggs in stool or positive Schistosoma spp. serology in combination with clinical and CT presentation; and (2) endured GVB and received endoscopic treatment for the secondary prevention of rebleeding. Inclusion criteria for alcoholic-related PHT were (1) ALD diagnosed by excluding other liver diseases and alcohol abuse of >40 g/d in women and > 60 g/d in men for > 5 years; and (2) endured GVB and received endoscopic treatment for the secondary prevention of rebleeding. We excluded patients who (1) had hepatic carcinoma or other malignant trumor, (2) had other etiology of chronic liver disease, and (3) had a history of abdominal surgery, including splenectomy, liver transplantation, portosystemic shunts, and so on. The medical data of the included patients were recorded in a predesigned case report form. All the patients were followed up via phone calls, outpatient clinic visits, and chart reviews.
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The institute’s Ethics Committee approval was obtained (B2020-125). Written informed consent was obtained from all individual participants included in the study.
Definitions and study endpoints
The primary outcome of this study was the 3-year rebleeding rate after the first GVB episode. Rebleeding was defined as bleeding from the gastrointestinal tract with a decrease in hemoglobin ⩾20 g/L, causing hematemesis, hematochezia, or melena according to the guidelines and previous studies.20,21 The secondary outcome was the 3-year all-cause mortality rate.
Endoscopic treatment of varices
All gastroscopies were performed using the electronic endoscope GIF-XQ260 (Olympus, Tokyo, Japan) to determine the severity of gastroesophageal varices and treatment selection. EVL is the primary choice for esophageal varices20,22 and gastric varices were treated with EIC using the sandwich method as previously described.21,23 All endoscopy and treatment procedures were performed by experienced endoscopists (S.C., M.Z., and M.X.), with more than 10 years of endoscopic experience. Follow-up was performed at an interval of 8–12 weeks. Endoscopy was performed, and treatment was repeated until complete obliteration of varices was achieved. All eight hospitals specialized in endoscopic treatment of gastroesophageal varices. All the patients were routinely followed up by phone every 6 months until death or study termination on 30 June 2020.
Statistical analysis
All categorical variables are reported as n (proportion). Continuous variables are reported as mean ± standard error (SE) for normally distributed data and median with interquartile range (IQR) for non-normally distributed data. Normally distributed continuous variables are calculated using Student’s unpaired t test, and all the other variables are calculated using the Mann–Whitney U test. Rebleeding rate and overall mortality rate are compared using Kaplan–Meier analysis. Independent factors were identified using Cox proportional hazards regression model. Covariates with a p-value less than 0.100 were included simultaneously in the multivariable Cox proportional hazards regression. Two-tailed test was used for statistical testing significance. A p-value of less than 0.05 was considered statistically significant. SPSS 24.0 (SPSS Inc., IL, USA) and STATA 16.0 (StataCorp LP, Texas) were used for calculations.
Results
MPN-associated gastroesophageal varices were identified in a total of 62 patients (mean age 53.0 ± 13.6; 30 males: 48.4%) from eight hospitals. Among these 62 patients, 39 (62.9%) suffered from ET, 10 (16.1%) from PV, and 13 (21.0%) from PMF. Among them, 37 MPN patients had a history of GVB (Figure 1). Of these, 24 were diagnosed with MPN before GVB and 13 patients were diagnosed after the bleeding episode. The characteristics of patients grouped by different types of MPN are shown in Table 1. In all, 49 patients underwent JAK2 mutation test, and 46 (93.9%) of them carried JAK2 V617 F mutation, while 1 patient had JAK2 EXON12 mutation. In addition, 42 patients (67.7%) had portal vein thrombosis (PVT) and 31 combined with portal cavernoma (Figure 2(a) and (b)). Among 42 patients with PVT, 29 received antithrombotic treatment. Thereinto, 31 (51.7%) patients had severe esophageal varices, and diffuse gastric varices were observed in 29 (61.7%) patients with gastric varices (Figure 2(c)–(h)). Furthermore, there were only two patients combined with Budd Chiari in our cohort, and none of them presented variceal bleeding.
Figure 1.
Flowchart of the study.
Table 1.
The baseline characteristics among different types of myeloproliferative neoplasms with gastroesophageal varices.
| ET (N = 39) | PV (N = 10) | PMF (N = 13) | Total (n = 62) | |
|---|---|---|---|---|
| Sex (male, %) | 18 (46.2) | 5 (50.0) | 7 (48.4) | 30 (48.4) |
| Age (years) | 51.4 ± 2.2 | 54.1 ± 3.5 | 56.8 ± 4.2 | 53.0 ± 1.7 |
| PVT, n (%) | 31 (79.5) | 6 (60.0) | 5 (38.5) | 42 (67.7) |
| Caver cavernoma | 23 (59.0) | 4 (40.0) | 4 (30.8) | 31 (73.8) |
| Portal-systemic shunt | 5 (12.8) | 1 (10.0) | 2 (15.4) | 8 (12.9) |
| Child grade, n (%) | ||||
| A | 22 (56.4) | 4 (40.0) | 7 (53.8) | 29 (51.8) |
| B | 17 (43.6) | 4 (40.0) | 5 (38.5) | 24 (42.9) |
| C | 0 | 2 (20.0) | 1 (7.7) | 3 (5.4) |
| Child score | 6.4 ± 0.2 | 7.4 ± 0.6 | 6.6 ± 0.4 | 6.6 ± 0.2 |
| Ascites, n (%) | ||||
| Absent | 15 (38.5) | 2 (20.0) | 3 (23.1) | 20 (32.3) |
| Mild | 12 (30.8) | 5 (50.0) | 6 (46.2) | 23 (37.1) |
| Severe | 12 (30.8) | 3 (30.0) | 6 (46.2) | 19 (30.6) |
| GVB, n (%) | 22 (56.4) | 6 (60.0) | 9 (69.2) | 37 (59.7) |
| Esophageal varices, n (%) | ||||
| Absent | 6 (15.4) | 0 | 2 (15.4) | 8 (12.9) |
| Mild | 3 (7.7) | 2 (20.0) | 2 (15.4) | 7 (11.3) |
| Moderate | 10 (25.6) | 2 (20.0) | 2 (15.4) | 14 (22.6) |
| Severe | 20 (51.3) | 6 (60.0) | 7 (53.8) | 33 (53.2) |
| Gastric varices, n (%) | 32 (82.1) | 8 (80.0) | 9 (69.2) | 49 (79.0) |
| Diffuse gastric varices | 20 (62.5) | 7 (77.8) | 4 (50.0) | 31 (63.3) |
| RBC (×109/L) | 3.8 ± 0.2 | 5.1 ± 0.7 | 3.3 ± 0.2 | 3.9 ± 0.2 |
| Hemoglobin (g/L) | 103.9 ± 5.4 | 131.0 ± 18.7 | 88.9 ± 9.9 | 105.1 ± 5.1 |
| Platelet (×109/L) | 372 (254–518) | 240 (188–291) | 280 (105–351) | 326 (219–451) |
| WBC (×109/L) | 7.8 (5.7–12.8) | 11.8 (9.8–12.7) | 7.5 (3.9–9.8) | 8.1 (5.8–12.7) |
| Total bilirubin (U/L) | 15.2 (9.8–20.1) | 24.4 (17.3–52.2) | 23.8 (18.3–31.1) | 5.7 (4.1–10.5) |
| Direct bilirubin (U/L) | 15.2 (9.8–20.1) | 24.4 (17.3–52.2) | 23.8 (18.3–31.1) | 17.9 (10.4–26) |
| ALT (U/L) | 23 (17–37) | 23 (18–28) | 18 (9–28) | 22 (17–36) |
| AST (U/L) | 29 (22–35) | 29 (24–34.1) | 25 (21–31) | 29 (22–35) |
| Albumin (g/L) | 38.0 ± 1.0 | 33.8 ± 2.3 | 34.8 ± 1.6 | 36.6 ± 0.8 |
| Creatine (μmol/L) | 63.1 ± 2.8 | 69.0 ± 6.8 | 77.6 ± 8.9 | 67.3 ± 2.9 |
| MPN treatment, n (%) | 22 (56.4) | 8 (80.0) | 9 (69.2) | 39 (62.9) |
| JAKi | 7 (17.9) | 0 | 4 (30.8) | |
| Hydroxyurea | 13 (33.3) | 8 (80.0) | 4 (30.8) | 25 (40.3) |
| Antithrombotic treatment, n (%) | 30 (76.9) | 3 (30.0) | 3 (23.1) | 36 (58.1) |
| Splenectomy, n (%) | 10 (25.6) | 0 | 1 (7.7) | 11 (17.7) |
| NSBB, n (%) | 5 (12.8) | 2 (20.0) | 4 (30.8) | 11 (17.7) |
| Endoscopic treatment, n (%) | 15 (38.5) | 5 (50.0) | 7 (53.8) | 27 (43.5) |
ALT, alanine transaminase; AST, aspartate transaminase; ET, essential thrombocythemia; JAKi, JAK inhibitor; NSBB, non-selective beta-blockers; PMF, primary myelofibrosis; PV, polycythemia vera; PVT, portal vein thrombosis; RBC, red blood cell; WBC, white blood cell.
Figure 2.
(a) and (b) The portal venous phase of CT shows portal vein thrombosis and portal cavernoma. (c) and (d) Endoscopic image shows diffuse or absence of esophageal varices. (e–g) Diffuse gastric varices and thrombus on the varices and (h) endoscopic injection of cyanoacrylate.
Overall survival
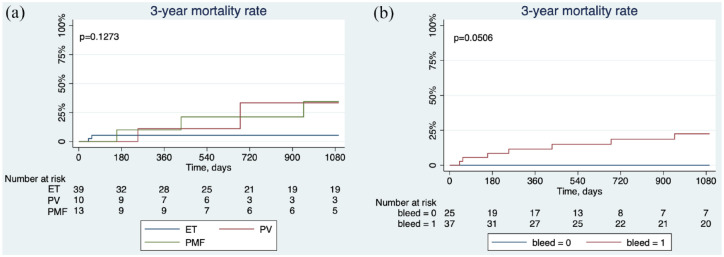
During the 3-year follow-up from the first diagnosis of gastroesophageal varices, seven patients died of variceal bleeding (n = 4), septic shock (n = 2), and intestinal necrosis (n = 1). The 3-year overall survival rate of patients with ET, PV, or PMF was shown in Figure 3(a). MPN patients without a variceal bleeding history seemed to have a better outcome, and MPNs with a variceal bleeding history showed a poor prognosis (Figure 3(b)).
Figure 3.
Kaplan–Meier curves showing (a) the 3-year overall survival rate from the first diagnosis of gastroesophageal varices in patients with ET, PV, and PMF. (b) The 3-year overall survival rate in MPN patients with or without a history of variceal bleeding.
Bleeding episodes in MPN patients
There were 37 MPN patients with a history of GVB, and 14 patients underwent variceal bleeding before the diagnosis of MPN. The comparison of characteristics of MPN patients with and without bleeding episodes is listed in Table 2. About 86.5% of patients had esophageal varices, and 91.9% of patients had gastric varices in the bleeding group. Patients presenting gastric varices, especially diffuse gastric varices, were more likely to bleed.
Table 2.
Characteristics of myeloproliferative neoplasms patients had a history of variceal bleeding and no bleeding.
| Bleeding (N = 37) | Non-bleeding (N = 25) | p | |
|---|---|---|---|
| Sex (male, %) | 16 (43.2) | 14 (56.0) | 0.438 |
| Age (years) | 51.4 ± 2.2 | 54.1 ± 3.5 | |
| PVT, n (%) | 27 (73.0) | 15 (60.0) | 0.407 |
| Caver cavernoma | 21 (56.8) | 10 (40.0) | 0.300 |
| Portal-systemic shunt, n (%) | 7 (18.9) | 1 (4.0) | 0.128 |
| Child grade, n (%) | 0.633 | ||
| A | 20 (54.1) | 13 (52.0) | |
| B | 16 (43.2) | 10 (40.0) | |
| C | 3 (4.8) | 2 (8.0) | |
| Child score | 6.6 ± 0.2 | 6.6 ± 0.3 | 0.962 |
| Ascites, n (%) | 0.419 | ||
| Absent | 10 (27.0) | 10 (40.0) | |
| Mild | 16 (43.2) | 7 (28.0) | |
| Severe | 11 (29.7) | 8 (32.0) | |
| Esophageal varices, n (%) | 0.089 | ||
| Absent | 5 (13.5) | 3 (12.0) | |
| Mild | 3 (8.1) | 4 (16.0) | |
| Moderate | 5 (13.5) | 9 (36.0) | |
| Severe | 24 (64.9) | 9 (36.0) | |
| Gastric varices, n (%) | 34 (91.9) | 15 (60.0) | 0.002 |
| Diffuse gastric varices | 26 (76.5) | 5 (33.3) | 0.004 |
| MPN treatment, n (%) | 24 (64.9) | 15 (60.0) | 0.791 |
| JAKi | 7 (18.9) | 4 (16.0) | 0.768 |
| Hydroxyurea | 14 (37.8) | 11 (44.0) | 0.628 |
| Antithrombotic treatment | 18 (48.6) | 18 (72.0) | 0.068 |
| NSBB, n (%) | 8 (21.6) | 3 (12.0) | 0.331 |
JAKi, JAK inhibitor; NSBB, non-selective beta-blockers; PVT, portal vein thrombosis.
Risk factors of rebleeding
Among 37 patients with a history of GVB, 24 patients received endoscopic treatment, including EVL and EIC, for the prophylaxis of rebleeding, while other 13 patients received partial splenic embolization (n = 1), TIPS (n = 1), splenectomy (n = 2), and no treatment (n = 9). The Kaplan–Meier analysis demonstrated that the rebleeding rate significantly declined in patients who received endoscopic treatment compared to others (28.2% versus 68.3%, p = 0.0269) (Figure 4). The multivariable Cox regression analysis for age, MPN type, PVT, endoscopic treatment, MPN treatment, antithrombotic treatment, splenectomy, and the use of non-selective beta-blockers (NSBB) indicated that endoscopic treatment [hazard ratio (HR) = 0.10, 95% confidence interval (CI): 0.02–0.54, p = 0.008] was the independent protective factor while the use of NSBB (HR = 13.41, 95% CI: 2.15–83.42, p = 0.005) was the risk factor for 3-year rebleeding. Similarly, antithrombotic treatment might be the protective factor for rebleeding (HR = 0.51, 95% CI: 0.15–1.69, p = 0.270), yet with no statistical significance (Table 3).
Figure 4.
Kaplan–Meier curves showing the cumulative incidence of 3-year rebleeding rate after first bleeding episode in MPNs who underwent endoscopic treatment and those who did not undergo endoscopic treatment (p = 0.0269).
Table 3.
Univariable and multivariable cox proportional hazards regression analysis of 3-year rebleeding.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| Age | 1.03 | 0.99–1.07 | 0.208 | |||
| MPN type | 1.51 | 0.81–2.81 | 0.193 | |||
| PVT | 0.53 | 0.17–1.68 | 0.280 | |||
| Endoscopic treatment | 0.30 | 0.09–0.93 | 0.037 | 0.10 | 0.02–0.54 | 0.008 |
| MPN treatment | 1.33 | 0.36–4.95 | 0.671 | |||
| Antithrombotic treatment | 0.51 | 0.15–1.69 | 0.270 | |||
| Splenectomy | 0.23 | 0.03–1.80 | 0.161 | |||
| NSBB | 3.54 | 1.00–12.56 | 0.050 | 13.41 | 2.15–83.42 | 0.005 |
CI, confidence interval; HR, hazard ratio; MPN, myeloproliferative neoplasms; NSBB, non-selective beta-blockers; PVT, portal vein thrombosis.
Comparison of endoscopic efficacy
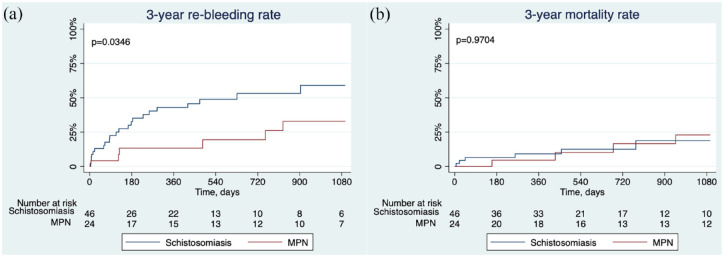
Schistosomiasis-associated PHT exhibits non-cirrhotic pathological manifestations and clinical presentation, and the utilization of endoscopic management for variceal bleeding is accepted. Patients with schistosomiasis-associated PHT were chosen as the controls to evaluate the endoscopic efficacy of non-cirrhotic portal hypertensive bleeding. Patients with schistosomiasis-associated PHT who received endoscopic treatment for variceal bleeding at the same period were compared to evaluate the efficacy of endoscopic treatment for variceal bleeding. The characteristics of patients with MPN- and schistosomiasis-associated PHT are manifested in Supplemental Table 1. The Kaplan–Meier analysis delineated that the cumulative incidence of 3-year rebleeding rate significantly dropped in the MPN group compared with the schistosomiasis group (32.9% versus 59.0%, p = 0.0346), and the all-cause mortality rate was similar in both groups (Figure 5).
Figure 5.
Kaplan–Meier curves showing the cumulative incidence of mortality rate and 3-year rebleeding rate in MPN and schistosomiasis-associated portal hypertension patients who underwent endoscopic treatment for the prevention of variceal rebleeding: (a) 3-year mortality and (b) 3-year rebleeding rate.
We also used a control group of patients with alcoholic cirrhosis to analyze the efficacy difference in endoscopic treatment between non-cirrhotic MPN-associated variceal bleeding and cirrhotic variceal bleeding. Patients who had alcoholic cirrhosis and PHT and received endoscopic treatment for variceal bleeding at the same period were included for the comparison of the efficacy of endoscopic treatment, and their characteristics compared with MPN patients are shown in Supplemental Table 2. The Kaplan–Meier analysis delineated that the cumulative incidence of 3-year rebleeding rate and mortality rate was similar in both groups. (Supplemental Figure 1)
Discussion
Erythrocytosis, thrombocytosis, and platelet dysfunction result in thrombotic events and eventually PHT and varices. 24 Due to the slow progression of MPNs and lack of symptoms, some patients may not be aware of the development of varices until occasional gastroscopy or even recurrent attack of variceal bleeding. This study included the largest number of patients with MPNs and PHT who received endoscopy treatment for GVB. In our study, about half of the included patients came to hospitals because of gastric or esophageal varices or suffered from variceal bleeding before being diagnosed with MPNs. Attention is required to patients with gastric or esophageal varices with the unexplained reason for PHT, especially in those with PVT, diffuse gastric varices, or whose red blood cell or platelet count is inconsistent with splenomegaly.
In our cohort, ET was the most common type of MPNs with gastroesophageal varices, followed by PMF and PV. PHT in MPNs may attribute to thrombosis of the portal venous system and infiltration of myeloid cells in the spleen. 17 Massive splenomegaly, portal thrombosis, portal cavernoma, ascites, and relatively normal liver functions were usually found in these patients. MPN patients had different extents of splanchnic thrombosis, and clinically significant PVT was observed in about 67.7% of patients. The JAK2 V617 F mutation rate was about 93.9% in this population. Constitutive phosphorylation of JAK2 was caused by JAK2 V617 F mutation, thus promoting clonal proliferation through the activation of the JAK/STAT pathway, which in turn augmented the risk for splanchnic circulation thrombosis or extramedullary hematopoiesis. 25 About half of the patients were first diagnosed with varices and were later confirmed with MPNs by bone biopsy. The test of JAK2 V617F mutation in peripheral blood might be the most effective method for rapid screening of the underlying blood disease in non-cirrhotic PHT with unknown etiologies populations.
The development of PHT in MPNs is progressive, and the occurrence of GVB might be lethal. Although recent guidelines including European Association for the Study of Liver disease (EASL) highlight the importance and prevalence of MPN as an etiological factor in SVT, they only put emphasis on the necessity of examining MPN in SVT patients and MPN’s impact on thrombosis management but fail to discuss specific treatment for MPN-related variceal bleeding.26,27 Endoscopic treatment, including esophageal ligation, sclerotherapy, and gastric cyanoacrylate obliteration, is recommended in managing variceal bleeding in cirrhotic and non-cirrhotic PHT.20,22 Thus, endoscopic treatment might also be useful for MPN-related bleeding but lacks systematic investigation on its efficacy despite several case reports that declared successful application of rubber band ligation, EVL, or TIPS for hemostasis in PMF patients.28–30 We provided evidence for the utilization of endoscopic treatment in managing variceal bleeding in MPNs. Our multicenter cohort study investigated the effect of endoscopic treatment on gastroesophageal varices in MPN patients and uncovered that endoscopy had a satisfying efficacy in preventing recurrent bleeding and improving the overall survival in these non-cirrhotic MPN patients. The 3-year rebleeding rate after the first episode of variceal bleeding was significantly lower in patients who underwent endoscopic treatment compared to those who did not. The efficacy of endoscopic treatment in MPNs patients was even better than in patients with schistosomiasis-associated variceal bleeding. Endoscopic therapies should be considered at the diagnosis of gastroesophageal varices in MPN patients for primary or secondary prophylaxis of variceal bleeding.
The combination of NSBB and endoscopic treatment was recommended in cirrhotic patients with recurrent variceal bleeding.22,31 However, there is insufficient data on the effect of NSBB in non-cirrhotic patients with MPNs. 31 Our results showed that the use of NSBB was a risk factor for rebleeding. NSBB is commonly used to prevent variceal bleeding attributed to its function in reducing cardiac output and splanchnic vasoconstriction, but its contribution to decreased portal vein inflow velocity is a pivotal risk factor for PVT. A prospective longitudinal study reported that NSBB (HR = 10.56, 95% CI: 1.35–82.73, p = 0.025) was associated with PVT development. 32 A retrospective study also proved that NSBB was significantly associated with PVT progression (OR = 4.400, 95% CI: 1.107–17.482, p = 0.035). 33 Besides, a meta-analysis demonstrated that use of NSBB progressed PVT (OR = 4.62, 95% CI: 2.50–8.53, p < 0.00001) and subgroup meta-analyses also manifested a consistent association between NSBB and PVT in both cohort studies (RR = 2.57, 95% CI: 1.46–4.51, p = 0.001) and case–control studies (OR = 8.17, 95% CI: 2.46–27.06, p = 0.0006). 34 Therefore, increased risk of PVT development brought by NSBB application might put management of variceal bleeding complicated with PVT into jeopardy.
For MPN-specific therapy, hydroxycarbamide is frequently used as a cytoreductive method, and JAK1/2 inhibitor ruxolitinib is increasingly administered, 35 even though the clinical use of ruxolitinib has only been approved for PMF. A case report showed that the intake of ruxolitinib immediately decreased the hepatic venous pressure gradient (HVPG) from 11 to 6 mmHg and increased the portal venous flow in 30 min, indicating the decline of hepatic resistance. 10 In our study, ruxolitinib was applied to 11 patients; however, no effect on rebleeding reduction was observed.
Prothrombotic disorders that possibly account for PVT and gastroesophageal varices are problematic in these patients. The progression and extension of PVT could amplify the portal venous pressure. 36 Due to the hypercoagulable states, antithrombotic treatment might be necessary to prevent PHT complications in MPNs. About 60% of the involved MPN patients received antithrombotic treatment and had a relatively lower rate of variceal bleeding (HR = 0.51, 95% CI: 0.15–1.69). The antithrombotic treatment helped to stop the progression of SVT and reduced gastroesophageal variceal pressure, contributing to the improvement in rebleeding. 37
Our study has several limitations. First, some patients with underlying hematological diseases might be misdiagnosed and undiagnosed in clinical practice due to the rareness of this disease. Second, the sample size of this study is still relatively small. Third, as the retrospective nature of this study, the severity and changes of PVT and the use of NSBB might affect the efficacy of endoscopic treatment. Further prospective studies are needed to confirm the risk factor in the endoscopic management of bleeding in MPNs.
Conclusion
In conclusion, this study uncovered that MPN patients might have PHT and recurrent variceal bleeding. Endoscopic treatment might be applied as the treatment of choice to prevent rebleeding in MPN patients, while NSBB might be ineffective.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221125691 for Characteristics of myeloproliferative neoplasm-associated portal hypertension and endoscopic management of variceal bleeding by Xiaoquan Huang, Ming Zhang, Yingjie Ai, Siyu Jiang, Mei Xiao, Lifen Wang, Yourong Jian, Yuzheng Zhuge, Chunqing Zhang and Shiyao Chen in Therapeutic Advances in Chronic Disease
Supplemental material, sj-tiff-2-taj-10.1177_20406223221125691 for Characteristics of myeloproliferative neoplasm-associated portal hypertension and endoscopic management of variceal bleeding by Xiaoquan Huang, Ming Zhang, Yingjie Ai, Siyu Jiang, Mei Xiao, Lifen Wang, Yourong Jian, Yuzheng Zhuge, Chunqing Zhang and Shiyao Chen in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors thank collaboration centers, the Second People’s Hospital of Lanzhou, Renmin Hospital of Wuhan University, and Mengchao Hepatobiliary Hospital of Fujian Medical University for the review of patients with portal hypertension and myeloproliferative neoplasms. They thank the center of evidence-based medicine of Fudan University for the assistance of data collection and management. They thank Yichen Wang (Mercy Internal Medicine Service, Trinity Health of New England, Springfield, MA, USA) for language assistance.
Footnotes
ORCID iD: Yingjie Ai  https://orcid.org/0000-0001-6309-1892
https://orcid.org/0000-0001-6309-1892
Contributor Information
Xiaoquan Huang, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai, China.
Ming Zhang, Department of Gastroenterology, Nanjing Drum Tower Hospital Clinical College of Nanjing Medical University, Nanjing, China.
Yingjie Ai, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai, China.
Siyu Jiang, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai, China.
Mei Xiao, Department of Gastroenterology, Anhui Provincial Hospital, Hefei, China.
Lifen Wang, Department of Gastroenterology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China.
Yourong Jian, Department of Gastroenterology and Hepatology, Minhang Hospital, Fudan University, Shanghai, China.
Yuzheng Zhuge, Department of Gastroenterology, Nanjing Drum Tower Hospital Clinical College of Nanjing Medical University, Nanjing, China.
Chunqing Zhang, Department of Gastroenterology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China.
Shiyao Chen, Department of Gastroenterology and Hepatology, Endoscopy Center and Endoscopy Research Institute, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai 200032, China; Center of Evidence-based Medicine, Fudan University, Shanghai, China; Department of Gastroenterology and Hepatology, Minhang Hospital, Fudan University, Shanghai, China.
Declarations
Ethics approval and consent to participate: All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The institute’s Ethics Committee approval was obtained (B2020-125). Informed consent was obtained from all individual participants included in the study.
Consent for publication: Not applicable.
Author contributions: Xiaoquan Huang: Conceptualization; Data curation; Formal analysis; Investigation; Visualization; Writing – original draft; Writing – review & editing.
Ming Zhang: Data curation; Formal analysis; Investigation; Methodology.
Yingjie Ai: Data curation; Formal analysis; Writing – original draft; Writing – review & editing.
Siyu Jiang: Data curation; Formal analysis; Investigation; Visualization.
Mei Xiao: Data curation; Formal analysis; Investigation; Methodology.
Lifen Wang: Data curation; Formal analysis; Investigation.
Yourong Jian: Data curation; Formal analysis; Investigation.
Yuzheng Zhuge: Data curation; Formal analysis; Investigation.
Chunqing Zhang: Conceptualization; Data curation; Writing – review & editing.
Shiyao Chen: Conceptualization; Funding acquisition; Methodology; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Advanced Appropriate Technology Promotion Project of Shanghai Health Commission (Grant No. 2019SY028), the National Natural Science Foundation of China (Grant No. 81900511), and Shanghai Sailing Program (Grant No. 19YF1406500). The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The article has been read and approved by all the authors.
Availability of data and material: Raw data and derived data supporting the findings of this study are available from the corresponding author Shiyao Chen on request.
References
- 1. Khanna R, Sarin SK. Non–cirrhotic portal hypertension – diagnosis and management. J Hepatol 2014; 60: 421–441. [DOI] [PubMed] [Google Scholar]
- 2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127: 2391–2405. [DOI] [PubMed] [Google Scholar]
- 3. Zhao G, Wu ZY, Zhang B, et al. Diagnosis and treatment of portal hypertension secondary to myeloproliferative disorders: a report of three cases. J Dig Dis 2011; 12: 312–316. [DOI] [PubMed] [Google Scholar]
- 4. Kander EM, Raza S, Zhou Z, et al. Bleeding complications in BCR-ABL negative myeloproliferative neoplasms: prevalence, type, and risk factors in a single-center cohort. Int J Hematol 2015; 102: 587–593. [DOI] [PubMed] [Google Scholar]
- 5. Foucar CE, Stein BL. JAK2 V617F mutation testing in patients presenting with hepatic and portal vein thrombosis. JAMA 2017; 317: 2228–2229. [DOI] [PubMed] [Google Scholar]
- 6. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005; 365: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 7. Bayraktar Y, Harmanci O, Buyukasik Y, et al. JAK2V617F mutation in patients with portal vein thrombosis. Dig Dis Sci 2008; 53: 2778–2783. [DOI] [PubMed] [Google Scholar]
- 8. Reilly CR, Babushok DV, Martin K, et al. Multicenter analysis of the use of transjugular intrahepatic portosystemic shunt for management of MPN-associated portal hypertension. Am J Hematol 2017; 92: 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yan M, Geyer H, Mesa R, et al. Clinical features of patients with Philadelphia-negative myeloproliferative neoplasms complicated by portal hypertension. Clin Lymphoma Myeloma Leuk 2015; 15: e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tan HK, Leow WQ, Chang PE. Ruxolitinib for the treatment of portal hypertension in a patient with primary myelofibrosis. Gastroenterology 2019; 157: e26–e27. [DOI] [PubMed] [Google Scholar]
- 11. Leonardi F, Maria N, Villa E. Anticoagulation in cirrhosis: a new paradigm? Clin Mol Hepatol 2017; 23: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaifie A, Kirschner M, Wolf D, et al. Bleeding, thrombosis, and anticoagulation in myeloproliferative neoplasms (MPN): analysis from the German SAL-MPN-registry. J Hematol Oncol 2016; 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Appelmann I, Kreher S, Parmentier S, et al. Diagnosis, prevention, and management of bleeding episodes in Philadelphia-negative myeloproliferative neoplasms: recommendations by the Hemostasis Working Party of the German Society of Hematology and Medical Oncology (DGHO) and the Society of Thrombosis and Hemostasis Research (GTH). Ann Hematol 2016; 95: 707–718. [DOI] [PubMed] [Google Scholar]
- 14. Sant’Antonio E, Guglielmelli P, Pieri L, et al. Splanchnic vein thromboses associated with myeloproliferative neoplasms: an international, retrospective study on 518 cases. Am J Hematol 2020; 95: 156–166. [DOI] [PubMed] [Google Scholar]
- 15. Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012; 366: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang S, Huang X, Ni L, et al. Positive consequences of splenectomy for patients with schistosomiasis-induced variceal bleeding. Surg Endosc 2021; 35: 2339–2346. [DOI] [PubMed] [Google Scholar]
- 17. Tamaki K, Otaka M, Sakamoto N, et al. Acute variceal bleeding in a patient with idiopathic myelofibrosis successfully treated with endoscopic variceal band ligation and chemotherapy: a case report. J Med Case Reports 2010; 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang JB, Gao Y, Liu JW, et al. Gastroesophageal varices in a patient presenting with essential thrombocythemia: a case report. World J Clin Cases 2021; 9: 1871–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsumoto M, Matsumoto H, Miyake T, et al. Endoscopic injection sclerotherapy for esophageal variceal hemorrhage in myeloproliferative disorder: case report. J Gastroenterol 1995; 30: 244–247. [DOI] [PubMed] [Google Scholar]
- 20. de Franchis R. and Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63: 743–752. [DOI] [PubMed] [Google Scholar]
- 21. Huang X, Li F, Wang L, et al. Endoscopic treatment of gastroesophageal variceal bleeding after oxaliplatin-based chemotherapy in patients with colorectal cancer. Endoscopy 2020; 52: 727–735. [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017; 65: 310–335. [DOI] [PubMed] [Google Scholar]
- 23. Huang X, Ma L, Zeng X, et al. Endoscopic approaches to the treatment of variceal hemorrhage in hemodialysis-dependent patients. Gastroenterol Res Pract 2016; 2016: 9732039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wanless IR, Peterson P, Das A, et al. Hepatic vascular disease and portal hypertension in polycythemia vera and agnogenic myeloid metaplasia: a clinicopathological study of 145 patients examined at autopsy. Hepatology 1990; 12: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 25. Klein S, Rick J, Lehmann J, et al. Janus-kinase-2 relates directly to portal hypertension and to complications in rodent and human cirrhosis. Gut 2017; 66: 145–155. [DOI] [PubMed] [Google Scholar]
- 26. de Franchis R. and Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010; 53: 762–768. [DOI] [PubMed] [Google Scholar]
- 27. European Association for the Study of the Liver. EASL clinical practice guidelines: vascular diseases of the liver. J Hepatol 2016; 64: 179–202. [DOI] [PubMed] [Google Scholar]
- 28. Goh BK, Chen JJ, Tan HK, et al. Acute variceal bleed in a patient with idiopathic myelofibrosis successfully treated with endoscopic variceal band ligation. Dig Dis Sci 2007; 52: 173–175. [DOI] [PubMed] [Google Scholar]
- 29. Ghidirim G, Corchmaru I, Mishin I, et al. Endoscopic rubber band ligation for bleeding oesophageal varices in portal hypertension due to idiopathic myelofibrosis. J Gastrointestin Liver Dis 2006; 15: 322. [PubMed] [Google Scholar]
- 30. Tanaka N, Yamakado K, Kihira H, et al. Transjugular intrahepatic portosystemic shunt for intractable esophageal-gastric variceal hemorrhage in a patient with idiopathic myelofibrosis. Cardiovasc Intervent Radiol 2000; 23: 491–492. [DOI] [PubMed] [Google Scholar]
- 31. de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII – renewing consensus in portal hypertension. J Hepatol 2021; 76: 959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nery F, Correia S, Macedo C, et al. Non-selective beta-blockers and the risk of portal vein thrombosis in patients with cirrhosis: results of a prospective longitudinal study. Aliment Pharmacol Ther 2019; 49: 582–588. [DOI] [PubMed] [Google Scholar]
- 33. Xu X, Xu S, Primignani M, et al. Non-selective β-blockers may progress the thrombosis of portal venous system in cirrhotic patients: a retrospective observational study. Adv Ther 2020; 37: 1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu X, Guo X, De Stefano V, et al. Non-selective beta-blockers and development of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis. Hepatol Int 2019; 13: 468–481. [DOI] [PubMed] [Google Scholar]
- 35. Pieri L, Paoli C, Arena U, et al. Safety and efficacy of ruxolitinib in splanchnic vein thrombosis associated with myeloproliferative neoplasms. Am J Hematol 2017; 92: 187–195. [DOI] [PubMed] [Google Scholar]
- 36. Rodriguez-Castro KI, Simioni P, Burra P, et al. Anticoagulation for the treatment of thrombotic complications in patients with cirrhosis. Liver Int 2012; 32: 1465–1476. [DOI] [PubMed] [Google Scholar]
- 37. De Santis A, Moscatelli R, Catalano C, et al. Systemic thrombolysis of portal vein thrombosis in cirrhotic patients: a pilot study. Dig Liver Dis 2010; 42: 451–455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221125691 for Characteristics of myeloproliferative neoplasm-associated portal hypertension and endoscopic management of variceal bleeding by Xiaoquan Huang, Ming Zhang, Yingjie Ai, Siyu Jiang, Mei Xiao, Lifen Wang, Yourong Jian, Yuzheng Zhuge, Chunqing Zhang and Shiyao Chen in Therapeutic Advances in Chronic Disease
Supplemental material, sj-tiff-2-taj-10.1177_20406223221125691 for Characteristics of myeloproliferative neoplasm-associated portal hypertension and endoscopic management of variceal bleeding by Xiaoquan Huang, Ming Zhang, Yingjie Ai, Siyu Jiang, Mei Xiao, Lifen Wang, Yourong Jian, Yuzheng Zhuge, Chunqing Zhang and Shiyao Chen in Therapeutic Advances in Chronic Disease