Abstract
Objective
To evaluate a novel parameter, motion segment size (MSS), in stroke patients with upper limb impairment and validate its clinical applicability by correlating results with a standard clinical task-based functional evaluation tool.
Methods
In this cross-sectional study, patients with hemiplegia and healthy controls equipped with multiple inertial measurement unit (IMU) sensors performed Action Research Arm Test (ARAT) and activities of daily living (ADL) tasks. Acceleration of the wrist and Euler angles of each upper limb segment were measured. The average and maximum MSS, accumulated motion, total performance time, and average motion speed (AMS) were extracted for analysis.
Results
Data from nine patients and 10 controls showed that the average MSS of forearm supination/pronation and elbow flexion/extension during full ARAT tasks showed a significant difference between patients and controls and a significant correlation with ARAT scores.
Conclusions
We suggest that MSS may provide clinically relevant information regarding upper limb functional status in stroke patients.
Keywords: Stroke, inertial measurement unit, upper extremity, movement, clinical outcome measure, sensor, motion segment size
Introduction
In the neurorehabilitation of upper extremities impairment, it is essential to assess the functional status of the patients’ activities of daily living or their gait function.1,2 Traditionally, functional status was evaluated by a therapist using verified clinical assessment tools that capture performance of various tasks. For upper-extremity evaluation, the Fugl-Meyer assessment scale, Action Research Arm Test (ARAT) and modified Barthel Index are widely used in stroke rehabilitation.3,4 Well-verified assessment tools provide objective information on patients’ current functional status, which plays a critical role in providing appropriate therapy and serial follow-up. In addition, the information assists physicians in supporting medical insurance applications and social care. However, these outcome measures have limitations and the same score may not always represent the same functional status. 5 Moreover, performing a full clinical evaluation for each patient is time consuming and requires trained personnel. 5
Reports have emerged describing the use of inertial measurement unit (IMU) sensors in the acquisition of quantitative motion data in diseases accompanying neurological impairment.6,7 A framework of measuring individual joint movement angles in major anatomical joints using IMU sensors has been suggested. 8 In stroke rehabilitation, several wearable sensor systems that use accelerometers or IMU sensors, involve monitoring and feedback on movements and posture of the upper extremities. 9 Using various algorithms, these data have been used to predict functional assessment scores. 10 However, studies have tended to focus on signal processing and clinical correlations have only evaluated the difference between predicted and clinical scores. It is our opinion that IMU sensors have not been fully utilised in clinical practice. However, IMU sensors do have drawbacks such as drift and the gimbal-lock phenomenon, 11 which compromises data on position tracking. Therefore, to use the sensor-derived data confidently and take advantage of its simplicity, parameters that are not significantly affected by common IMU errors are essential. A good example of this is the use of measures that minimise jerk. 12 Indeed, previous studies have shown that movement smoothness is important in stroke rehabilitation and demonstrates significant correlation with clinical functional assessments and brain activation.12–14 In addition to jerk, the number of movement units and trajectory errors have also been used to represent smoothness. 15
The aim of this study was to evaluate a novel parameter, motion segment size (MSS), in stroke patients with upper limb impairment and validate its clinical applicability by correlating results with a standard clinical task-based functional evaluation tool. From a clinical perspective, we suggest that MSS may represent motion smoothness.
Methods
This cross-sectional study was performed from October 2016 to February 2017 and involved patients with hemiplegic stroke and healthy volunteers (controls). Inclusion criteria for stroke patients were as follows: hemiplegia due to stroke; Brunnstrom stage ≥3 for the hemiplegic arm 16 ; able to perform visible movement voluntarily following instructions. Patients who were unable to provide informed consent, those with cognitive impairment that prevented them from following instructions, and those with other medical or personal conditions that may have affected their participation were excluded from the study. The reporting of this study conforms to STROBE guidelines. 17 All participants provided written informed consent and the study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 1505-017-668). Patient data were de-identified prior to analysis.
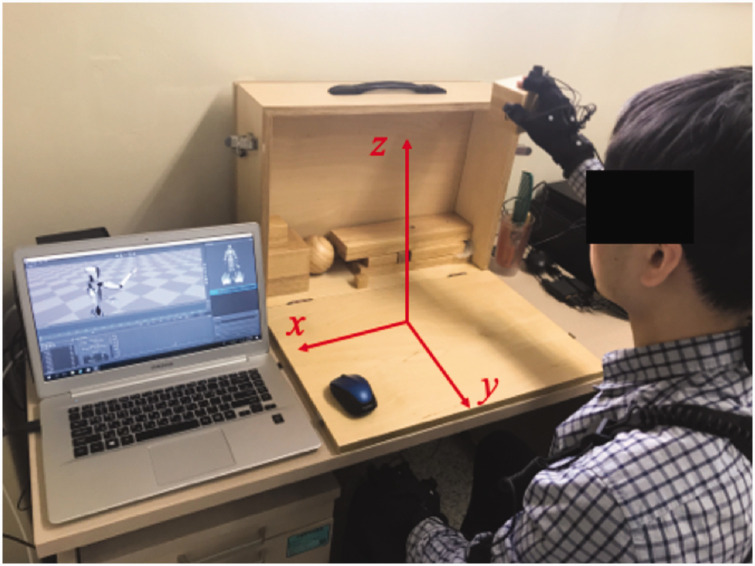
Basic demographic data, Brunnstrom-stage, and ARAT scores without assistance were obtained for all participants. For upper-extremity motion capture, Perception Neuron® (Noitom Ltd., Beijing, China), a wearable multi-IMU based modular motion capture system was used. A user interface software, Axis Neuron (Noitom Ltd., Beijing, China), was used for motion recording and data extraction. The data sampling rate was set to 60 Hz. All subjects wore the IMU sensor-based motion capture system on both upper extremities. In total 11 sensors (three for axial [head and trunk], and four on each upper limb [shoulder-upper arm-forearm-hand]) were attached to each participant. Left-right horizontal, front-back horizontal, and up-down vertical axes were defined as x, y, and z axes, respectively (Figure 1).
Figure 1.
A healthy subject wearing the Perception Neuron® system performing the Action Research Arm Test (ARAT). The orthogonal coordination system used for analysis is indicated.
Sensor calibration was performed as per the manufacturer’s guidelines and consisted of three poses: a steady pose; ‘A’ pose (both arms down on side); ‘T’ pose (both arms abducted horizontally). After sensor calibration, all participants performed the 19 Action Research Arm Test (ARAT) items, followed by six pre-determined activities of daily living (ADL) tasks (i.e., opening a bottle cap; peeling a banana; opening and closing the buttons on a shirt; combing hair; brushing teeth; opening a door knob). The ADL tasks were derived from our previous survey performed on hemiplegic stroke patients. 18 The ARAT was chosen because task movements are standardized with designated equipment and do not involve lower extremity functions. The ARAT has four domains: tasks in domains 1 and 3 consists of grasping and pinching objects of various sizes (e.g., wooden blocks or marbles) and then moving them using a reaching movement. Domain 2 mainly involves moving items on a table, focusing on grip function, and domain 4 involves gross movement tasks that require lifting the arm to the head or face. 19 The total score on the ARAT ranges from 0 to 57. In patients with moderate to severe hemiparesis, a level of active assistive support was provided so that the patients could complete the task.
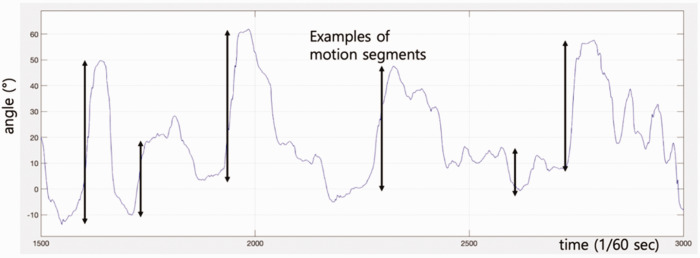
Using Axis Neuron software, acceleration and position data of the wrist and hand sensors from the accelerometer, and the Euler angles for the sensors of all major joints with reference to their proximal segment sensors were extracted during the ARAT and ADL tasks. Accelerometer data were extracted for elbow, wrist, and hand sensors in x, y, and z directions (Figure 1). Euler angle data corresponded to major anatomical joint motions: 1) shoulder flexion/extension; 2) shoulder abduction/adduction; 3) shoulder internal/external rotation; 4) elbow flexion/extension; 5) forearm supination/pronation; 6) wrist dorsiflexion/volar flexion; 7) wrist deviation. Motion segment size (MSS) was defined as the accumulated change of a parameter in one direction until its first derivative becomes zero, which represents changing the direction in the opposite way.
| (1) |
where i is the dataset of timepoints when .
To determine each motion segment, a threshold was set to a minimum of 5 cm for position data and 3° for angle data. Examples of motion segments are shown in Figure 2.
Figure 2.
Some examples of motion segments (not all motion segments are indicated).
For all participants, total performance time, and average and maximum MSS for position and angle data were calculated. Accumulated motion (AM), and average motion speed (AMS) were also extracted and analysed. AM was defined as a sum of all displacements or changes for corresponding measurements and was calculated as following algorithm.
| (2) |
AMS was calculated by dividing the AM by total performance time.
Statistical analyses
Data were analysed using the Statistical Package for Social Sciences (SPSS®) for Windows® release 25.0 (IBM Corp., Armonk, NY, USA). A P-value <0.05 was considered to indicate statistical significance. For all sensor-based parameters, paired t tests were performed between stoke patients and controls. For parameters that showed significant differences between groups, Pearson’s correlation analyses were performed using the ARAT score as the dependent variable. For a sensor-based parameter to be useful as a clinical outcome measure, the value for stroke patients had to be significantly different from that for controls and needed to be distributed in a sufficient spectrum of values to reflect the severity of the disease or degree of functional impairment. 20
Results
Demographic and baseline data
Nine patients (four women, five men) with hemiplegic stroke and 10 healthy controls (four women, six men) were recruited for this study. The mean (±SD) age of the stroke patients was 57.4 (±17.2) years (range: 22–73 years) and mean (± SD) age of the healthy controls was 29.3 (±4.7) years (range: 23–35 years). All healthy controls were right-handed.
Of the stroke patients, four had left hemiplegia and five had right hemiplegia (Table 1). For Brunnstrom-stage 3, 4, 5, and 6, there were 2, 2, 3, and 2 patients, respectively. Mean (±SD) ARAT score for the patients was 34.8 (±21.7) points (range: 2–57). Distribution of Brunnstrom-stage and ARAT scores showed a distinct range, therefore, for further analysis, Brunnstrom-stage 3 (ARAT scores 2) was considered severely impaired, Brunnstrom-stage 4 (ARAT scores 26–27) as moderately impaired, Brunnstrom-stage 5 (ARAT scores 46–48) as mildly impaired, and Brunnstrom-stage 6 (ARAT scores 57) as near ‘normal’.
Table 1.
Basic demographic data of the stroke patients (n = 9).
| Patient number | Age y | Sex | Pre-stroke Dominant hand | Hemiplegic side | Brunnstrom stage 16 | ARAT score |
|---|---|---|---|---|---|---|
| 1 | 58 | Male | Right | Left | 3 | 2 |
| 2 | 73 | Female | Right | Right | 5 | 48 |
| 3 | 71 | Male | Right | Left | 4 | 26 |
| 4 | 63 | Male | Right | Right | 3 | 2 |
| 5 | 70 | Male | Right | Left | 5 | 46 |
| 6 | 22 | Female | Right | Right | 4 | 27 |
| 7 | 64 | Female | Right | Right | 5 | 48 |
| 8 | 36 | Female | Right | Left | 6 | 57 |
| 9 | 60 | Male | Right | Right | 6 | 57 |
Abbreviation: ARAT, Action Research Arm Test.
Correlation of sensor-based parameters with clinical measures
Sensor-based parameters that showed statistically significant differences between stroke patients and controls and a significant correlation with clinical measures (ARAT score) were extracted. For accelerometer data (acceleration, m/s2), average MSS of hand (P = 0.005, 0.002, and 0.006, for x, y, and z axes, respectively) and wrist (P = 0.023 and <0.001 for x and y axes, respectively) during the full ARAT performance was shown to be significantly different between patients and controls. Average MSS of acceleration for all axes for hand (R = 0.765, 0.680, and 0.739, for x, y, and z axes, respectively) and y-axis for wrist sensors (R = 0.736) showed significant correlation with the ARAT scores (P < 0.01).
For the angle data, all AM values for all major joint range of motion (ROM) did not correlate with ARAT scores. For AMS, there were significant differences between patients and controls and significant correlation with ARAT scores for forearm supination/pronation during ADL tasks, elbow flexion/extension during ARAT domain 3, and wrist deviation during ADL tasks (P < 0.001 for comparison vs controls; R > 0.7 for correlation with ARAT scores). Total performance time for the ARAT was negatively correlated with ARAT score (R = –0.914) (Table 2).
Table 2.
Sensor-derived parameters demonstrating significant correlations with clinical measures.
| Task | Domain | Variable | Parameter | Healthy Controls (n = 10) | Brunnstrom Stage
16
|
Pearson’s correlation coefficient Statistical Significancea | Statistical significanceb | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 (n = 2) | 4 (n = 2) | 5 (n = 3) | 6 (n = 2) | ||||||||
| Forearm supination/pronation | |||||||||||
| ARAT | 1 | angle | aMSS | 18.03 | 8.67 | 10.56 | 14.08 | 16.78 | 0.767 | P = 0.016 | P = 0.014 |
| ARAT | 4 | angle | aMSS | 38.22 | 11.40 | 16.14 | 23.06 | 29.92 | 0.725 | P = 0.027 | P = 0.004 |
| ARAT | Full | angle | aMSS | 19.30 | 9.94 | 12.71 | 14.89 | 16.15 | 0.753 | P = 0.019 | P = 0.002 |
| ADL task | Full | angle | AMS | 0.60 | 0.14 | 0.25 | 0.26 | 0.37 | 0.741 | P = 0.022 | P < 0.001 |
| Wrist dorsiflexion/volarflexion | |||||||||||
| ARAT | 2 | angle | aMSS | 13.68 | 7.71 | 7.73 | 9.88 | 12.38 | 0.764 | P = 0.017 | P = 0.001 |
| ARAT | Full | angle | aMSS | 13.57 | 7.23 | 8.62 | 11.61 | 14.31 | 0.877 | P = 0.002 | P = 0.001 |
| Elbow flexion/extension | |||||||||||
| ARAT | 1 | angle | aMSS | 32.91 | 9.12 | 14.49 | 22.29 | 22.23 | 0.745 | P = 0.021 | P<0.001 |
| ARAT | 3 | angle | aMSS | 15.60 | 9.12 | 15.32 | 19.23 | 21.96 | 0.800 | P = 0.010 | P = 0.040 |
| ARAT | Full | angle | aMSS | 28.21 | 10.49 | 14.67 | 19.33 | 19.39 | 0.728 | P = 0.026 | P<0.001 |
| ARAT | 3 | angle | AMS | 0.63 | 0.16 | 0.29 | 0.32 | 0.39 | 0.728 | P = 0.026 | P<0.001 |
| ARAT total performance time (sec) | 122.44 | 353.04 | 268.16 | 223.42 | 179.90 | −0.914 | P <0.001 | P = 0.010 | |||
aPearson’s correlation coefficient for the parameter versus ARAT score.
bComparison between healthy controls and patient using paired t tests.
Abbreviations: ARAT, Action Research Arm Test; aMSS, average motion segment size; AMS, average motion speed.
For forearm supination/pronation and elbow flexion/extension, the average MSS for the full ARAT task was significantly different between patients and controls and was significantly correlated with ARAT scores (P = 0.002 and <0.001 for comparison vs controls; R = 0.753 and 0.728 for correlation with ARAT scores). The average MSS for elbow flexion/extension was 19.4 and 10.5 in near ‘normal’ and severely impaired patients, respectively.
Individual domains of ARAT (domain 1 and 4 for forearm supination/pronation, domain 1 and 3 for elbow flexion/extension) also showed similar results. For wrist dorsiflexion/volarflexion, average MSS for full ARAT task showed significant results (P = 0.001 for comparison vs controls, R = 0.877 for correlation with ARAT scores).
Potential parameters for use as clinical outcome measures
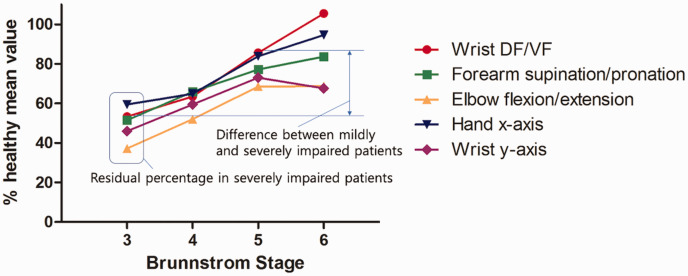
The average MSS of wrist dorsiflexion/volarflexion during the full ARAT task showed the largest difference between severely and mildly impaired patients (52%), whereas the percentage in severely impaired patients was lowest for average MSS of forearm supination/pronation during ARAT domain 4 (30%), elbow flexion/extension during ARAT domain 1 (28%) and domain 3 (28%). Representative data are shown in Figure 3.
Figure 3.
Average motion segment sizes of selected parameters (angle for wrist dorsiflexion/volarflexion [DF/VF], forearm supination/pronation, elbow flexion/extension; acceleration for hand x-axis and wrist y-axis) during full Action Research Arm Test tasks are shown in percentage of mean values compared to healthy controls. These parameters may serve as potential sensor-based clinical outcome measures as they show significant correlation with clinical scores as well as sufficient interval difference across the severity of the impairment.
Brunnstrom stage categorized in this study: 3-severely impaired; 4-moderately impaired; 5-mildly impaired; 6-near ‘normal’.
For the severely impaired group, the average MSS of acceleration in the left-right horizontal (x-axis) and up-down vertical (z-axis) directions of the hand and wrist sensors during full ARAT tasks showed approximately 46–62% value of healthy controls. The difference between severely and mildly impaired patients was 22–35%.
Discussion
In this study, we evaluated a new sensor-based clinical outcome measure (MSS), which we believe represents smoothness from an anatomical perspective. Among the sensor-based parameters that showed significant difference between patients and controls, the average MSS for wrist dorsiflexion/volarflexion for full ARAT task showed the highest correlation with ARAT score, and also demonstrated the largest difference between severely and mildly impaired patients (52%). Parameters representing forearm supination/pronation and elbow flexion/extension also showed high clinical correlations.
The ARAT tasks were pre-specified movements mainly consisting of reaching and overhead motions, whereas the ADL tasks performed in this study did not involve detailed movements but the use of an intact hand was permitted. The movement during ARAT tasks represented ‘capacity’, and the movement during ADL tasks probably represented ‘performance’.21,22 Clinical functional assessments usually represent ‘capacity’, and so this may explain our results in that the most relevant parameters were from the ARAT data. The finding that the performance time for ADL tasks was not significantly different between groups was not surprising since the participants completed the tasks with the support of intact hands. However, AMS, which evaluated the accumulated movement of the impaired limb over time, was clinically meaningful for several parameters in ADL tasks. Because the AMS has the velocity feature, it can be inferred that AMS may represent both the functional capacity and performance in a practical setting, reflecting the natural use of the impaired limb.
We suggest that average MSS may represent smoothness of movement. In previous studies, jerk, the third derivative of position data, was used to assess the smoothness of limb motion.7,23 Results showed that, in chronic neck pain, there was a significant correlation between the jerk index, ROM variability, and the repositioning acuity of neck muscles. 23 Various modifications of jerk, such as root mean square jerk and dimensionless jerk, have also been evaluated but their validity and reliability have not been well verified. 24 The concept of a spectral arc-length metric that uses a movement speed profile's Fourier magnitude spectrum to quantify movement smoothness has shown better reliability and practicality. 25 However, the aforementioned mentioned parameters are dimensionless, and so do not provide intuitive information for the clinical setting. Interestingly, a study on post-stroke patients using a camera-based motion capture system for a drinking task and ARAT tasks, showed that smoothness (defined as number of movement units) along with movement time was correlated with clinically meaningful improvement. 26 Our results are consistent with these findings in that the decreased number of movement units in a given task results in a larger motion segment. In the previous study, the elbow flexion axis in well-recovered patients showed decreased number of movement units from 19.0 to 7.9 and we found an average MSS of 10.5 in severely impaired and 19.4 in near normal patients. 26 Two metrics have been proposed in acceleration data to represent smoothness (i.e., peaks/second and peak ratio) and they have been shown to be significantly different between frail and non-frail persons in performing ADLs. 27 These findings support our results for acceleration data in that the average MSS of the left-right horizontal (x-axis) and up-down vertical (z-axis) in the wrist and hand sensors during the ARAT task was significantly correlated with the ARAT score. In contrast with previous parameters which are dimensionless, we found that the average MSS, especially for angular measurements, corresponded to the respective anatomical joint angle change of the movement unit, which we believe may provide clinicians with clinically useful information on the patients’ function.
This study had several limitations. Firstly, the number of subjects was relatively small and so the generalisability of the results to all hemiplegic stroke patients may not be applicable. Controlled studies using large numbers of participants and a diverse spectrum of diseases are required. Secondly, the data generated from the Perception Neuron® system are based on a human model which may have led to some unintentional biases. However, in previous studies, we verified that the system shows adequate accuracy and consistency for clinical applications.28,29 Finally, the ARAT and ADL tasks were to be completed by all participants and assistance was provided as required. This may have affected the results for some stroke patients, especially those with Brunnstrom-stage 3. However, even with assistance, the patients showed low average MSS values compared with patients at a higher recovery stage, whereas without assistance, the values would have been even lower.
Average MSS for forearm supination/pronation and elbow flexion/extension angles during pre-determined tasks, and the average MSS of acceleration in left-right horizontal and up-down vertical axes were significantly correlated and proportional with the ARAT scores in stroke patients. Although upper-extremity movements may not be accurately measured with IMU sensors, specific parameters such as the average MSS may serve as clinically relevant outcome measures in simple or serial functional evaluation in stroke rehabilitation.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221122750 for Evaluation of Motion Segment Size as a New Sensor-based Functional Outcome Measure in Stroke Rehabilitation by Hyung Seok Nam, Woo Hyung Lee, Han Gil Seo, Matthew W. Smuck and Sungwan Kim in Journal of International Medical Research
Footnotes
Declaration of conflicting interests: The authors declare that there are no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partly supported by a fund from Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1904984).
ORCID iD
Hyung Seok Nam https://orcid.org/0000-0002-2210-7170
References
- 1.Alt Murphy M, Resteghini C, Feys P, et al. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. 2015; 15: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridao-Fernandez C, Pinero-Pinto E, Chamorro-Moriana G. Observational Gait Assessment Scales in Patients with Walking Disorders: Systematic Review. Biomed Res Int. 2019; 2019: 2085039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair. 2002; 16: 232–240. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh CL, Hsueh IP, Chiang FM, et al. Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing. 1998; 27: 107–113. [DOI] [PubMed] [Google Scholar]
- 5.Santisteban L, Teremetz M, Bleton JP, et al. Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review. PLoS One. 2016; 11: e0154792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Repnik E, Puh U, Goljar N, et al. Using Inertial Measurement Units and Electromyography to Quantify Movement during Action Research Arm Test Execution. Sensors (Basel). 2018; 18: 2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpinella I, Cattaneo D, Ferrarin M. Quantitative assessment of upper limb motor function in Multiple Sclerosis using an instrumented Action Research Arm Test. J Neuroeng Rehabil. 2014; 11: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez R, Costa U, Torrent M, et al. Upper limb portable motion analysis system based on inertial technology for neurorehabilitation purposes. Sensors (Basel). 2010; 10: 10733–10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Markopoulos P, Yu B, et al. Interactive wearable systems for upper body rehabilitation: a systematic review. J Neuroeng Rehabil. 2017; 14: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noorkoiv M, Rodgers H, Price CI. Accelerometer measurement of upper extremity movement after stroke: a systematic review of clinical studies. J Neuroeng Rehabil. 2014; 11: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J. A Review of Wearable IMU (Inertial-Measurement-Unit)-based Pose Estimation and Drift Reduction Technologies. Journal of Physics: Conference Series. 2018; 1087: 042003. Available online: https://iopscience.iop.org/article/10.1088/1742-6596/1087/4/042003 [Google Scholar]
- 12.Rohrer B, Fasoli S, Krebs HI, et al. Movement smoothness changes during stroke recovery. J Neurosci. 2002; 22: 8297–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz A, Kanzler CM, Lambercy O, et al. Systematic Review on Kinematic Assessments of Upper Limb Movements after Stroke. Stroke. 2019; 50: 718–727. [DOI] [PubMed] [Google Scholar]
- 14.Buma FE, van Kordelaar J, Raemaekers M, et al. Brain activation is related to smoothness of upper limb movements after stroke. Exp Brain Res. 2016; 234: 2077–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celik O, O'Malley MK, Boake C, et al. Normalized movement quality measures for therapeutic robots strongly correlate with clinical motor impairment measures. IEEE Trans Neural Syst Rehabil Eng. 2010; 18: 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966; 46: 357–375. [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 18.Nam HS, Seo HG, Leigh J-H, et al. External Robotic Arm vs. Upper Limb Exoskeleton: What Do Potential Users Need? Applied Sciences. 2019; 9: 2471. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-85068125862&origin=inward&txGid=45dfd6200d12bf27c737c82115f2d609 [Google Scholar]
- 19.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008; 22: 78–90. [DOI] [PubMed] [Google Scholar]
- 20.Veras M, Kairy D, Rogante M, et al. Scoping review of outcome measures used in telerehabilitation and virtual reality for post-stroke rehabilitation. J Telemed Telecare. 2017; 23: 567–587. [DOI] [PubMed] [Google Scholar]
- 21.Smuck M, Tomkins-Lane C, Ith MA, et al. Physical performance analysis: A new approach to assessing free-living physical activity in musculoskeletal pain and mobility-limited populations. PLoS One. 2017; 12: e0172804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristersson T, Persson HC, Alt Murphy M. Evaluation of a short assessment for upper extremity activity capacity early after stroke. J Rehabil Med. 2019; 51: 257–263. [DOI] [PubMed] [Google Scholar]
- 23.Sjolander P, Michaelson P, Jaric S, et al. Sensorimotor disturbances in chronic neck pain–range of motion, peak velocity, smoothness of movement, and repositioning acuity. Man Ther. 2008; 13: 122–131. [DOI] [PubMed] [Google Scholar]
- 24.Balasubramanian S, Melendez-Calderon A, Roby-Brami A, et al. On the analysis of movement smoothness. J Neuroeng Rehabil. 2015; 12: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balasubramanian S, Melendez-Calderon A, Burdet E. A robust and sensitive metric for quantifying movement smoothness. IEEE Trans Biomed Eng. 2012; 59: 2126–2136. [DOI] [PubMed] [Google Scholar]
- 26.Alt Murphy M, Willen C, Sunnerhagen KS. Responsiveness of upper extremity kinematic measures and clinical improvement during the first three months after stroke. Neurorehabil Neural Repair. 2013; 27: 844–853. [DOI] [PubMed] [Google Scholar]
- 27.Schmidle S, Gulde P, Herdegen S, et al. Kinematic analysis of activities of daily living performance in frail elderly. BMC Geriatr. 2022; 22: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam HS, Lee WH, Seo HG, et al. Inertial Measurement Unit Based Upper Extremity Motion Characterization for Action Research Arm Test and Activities of Daily Living. Sensors (Basel). 2019; 19: 1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HS, Hong N, Kim M, et al. Application of a Perception Neuron((R)) System in Simulation-Based Surgical Training. J Clin Med. 2019; 8: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221122750 for Evaluation of Motion Segment Size as a New Sensor-based Functional Outcome Measure in Stroke Rehabilitation by Hyung Seok Nam, Woo Hyung Lee, Han Gil Seo, Matthew W. Smuck and Sungwan Kim in Journal of International Medical Research