Abstract
Background:
Peroneal nerve (PN) palsy is one of the most debilitating sequelae of multiligamentous knee injuries (MLKIs). There is limited research on recovery from complete PN palsy.
Purpose/Hypothesis:
The purpose of this study was to characterize PN injuries and develop a predictive model of complete PN recovery after MLKI using machine learning. It was hypothesized that elevated body mass index (BMI) would be predictive of lower likelihood of recovery.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
The authors conducted a retrospective review of patients seen at 2 urban hospital systems for treatment of MLKI with associated complete PN palsy, defined as the presence of complete foot drop with or without sensory deficits on physical examination. Recovery was defined as the complete resolution of foot drop. A random forest (RF) classifier algorithm was used to identify demographic, injury, treatment, and postoperative variables that were significant predictors of recovery from complete PN palsy. Validity of the RF model was assessed using overall accuracy, F1 score, and area under the receiver operating characteristic curve (AUC).
Results:
Overall, 16 patients with MLKI with associated complete PN palsy were included in the cohort. Among them, 75% (12/16) had documented knee dislocation requiring reduction. Complete recovery occurred in 4 patients (25%). Nerve contusions on magnetic resonance imaging were more common among patients without PN recovery, but there were no other significant differences between recovery and nonrecovery groups. The RF model found that older age, increasing BMI, and male sex were predictive of worse likelihood of PN recovery. The model was found to have good validity, with a classification accuracy of 75%, F1 score of 0.86, and AUC of 0.64.
Conclusion:
The RF model in this study found that increasing age, BMI, and male sex were predictive of decreased likelihood of nerve recovery. While further study of machine learning models with larger patient data sets is required to identify the most superior model, these findings present an opportunity for orthopaedic surgeons to better identify, counsel, and treat patients with MLKIs and concomitant complete PN palsy.
Keywords: complete peroneal nerve palsy, multiligament knee injury, knee dislocation, peroneal nerve injury, reconstruction
Multiligamentous knee injuries (MLKIs) are rare but devastating, accounting for less than 0.02% of all orthopaedic injuries. 33 Peroneal nerve (PN) palsy is a common and often disabling complication of MLKI, with an overall incidence of PN palsy in 14% to 40% of all injuries with and without knee dislocations. 19 However, because of the rarity of MLKIs, there has been minimal investigation into PN injuries. 20,24,33
Because of the high-energy nature of most MLKI injuries, or the rotational component in low- and ultra–low velocity MLKIs from knee dislocations, the PN is particularly at risk because of its circuitous course around the fibular neck. 9,20 PN injuries, which can range from common traction neuropraxias to rare neurotmesis injuries, are associated with worse functional outcomes among patients with MLKI. 24 PN injuries are classified as complete or partial palsies based on the severity of presenting symptoms. Complete palsies are characterized by paralysis of the ankle dorsiflexors (innervated by the deep PN) resulting in an inability to lift the foot at the ankle, commonly referred to as foot drop. 39 Patients with complete PN palsy may also ambulate with a steppage or circumduction gait due to the loss of ankle dorsiflexion. 39
Several studies have identified predictors of PN injury and deficit within MLKIs, including demographic variables such as sex and body mass index (BMI), as well as specific injury patterns including fibular head fracture, posterolateral corner (PLC) injury, anterior cruciate ligament (ACL) injury, and tibiofemoral knee dislocation requiring reduction. 5,9,20,21,38,40 While these studies have examined predictors of PN injury, few studies have investigated factors that may predict PN recovery in the setting of MLKI.
Based on clinical experience and previous literature that shows an increased rate of neurovascular injury in patients with higher BMI, for example, ultra-low-velocity knee dislocation, we hypothesized that BMI as a feature would rank highly in relative importance among the various features for predicting recovery. 10,18,28,37,38 However, other predictive factors have not been clearly identified. The purpose of this study was to characterize PN injuries and develop a predictive model of complete PN recovery after MLKI using patient characteristics, injury, and treatment data.
Methods
Cohort Selection
This multicenter, institutional review board–approved study was conducted as a retrospective review of patients who were initially evaluated at 1 of 2 academic hospital level 1 trauma centers for treatment of MLKI with associated complete PN palsy between 2006 and 2021. Inclusion criteria for patients were a diagnosis of MLKI confirmed on magnetic resonance imaging (MRI), surgical reconstruction or repair of 2 or more ligaments, and clinical diagnosis of complete PN palsy based on the presence of complete foot drop on physical examination with or without sensory deficits. Diagnosis of complete PN palsy could be made at any time between the date of injury and the first episode of surgical intervention. Exclusion criteria included a diagnosis of MLKI without associated complete PN palsy (ie, absence of foot drop on physical examination) or a diagnosis of MLKI with associated partial PN palsy (ie, presence of partial foot drop/extensor hallucis longus deficit on physical examination). Patients with MLKI evaluated with only paresthesia in the PN distribution without concomitant motor symptoms were also excluded.
Predictors Measured
Demographic and perioperative data for each patient were obtained from electronic medical records. Recorded variables included age at the time of injury, sex, BMI, smoking status, date of injury, date of the first surgery, use of external fixation during the first surgery, and date(s) of secondary and tertiary staged surgeries (if applicable). MLKIs were categorized by Schenck classification, 12 mechanism of injury (ultralow velocity [eg, fall from standing], low velocity [eg, sports], and high velocity [eg, motor vehicle accident]), 41 presence of knee dislocation on lateral and anteroposterior radiographs, and presence and type of periarticular fracture (femoral condyle, tibial plateau, or fibular head) at injury requiring surgical repair. MLKIs were also classified as acute (<6 weeks between date of injury and date of first surgery) or chronic (>6 weeks).
Time elapsed between the date of injury and date of identification of PN palsy was recorded. Complete injuries to the common PN initially identified on clinical examination were confirmed using both the preoperative MRI and the intraoperative report for the first surgery. Intraoperative reports were used to identify PN edema, contusion, displacement, and/or discontinuity. Concomitant PN neurolysis, repair, or graft was noted from the intraoperative report. Subsequent readmissions, reoperations, and complications were recorded based on clinical follow-up.
Surgical Technique
The surgical technique for repair or reconstruction of MLKIs depends greatly on the patient’s age, function, ligamentous involvement, associated injuries, vascular status, and knee stability, and thus specific surgical techniques are extremely heterogeneous and beyond the scope of this paper. However, given that every MLKI in our cohort included a PLC injury and PN palsy, the same systematic approach to the PN nerve was taken in each case. A standard posterolateral approach to the knee was performed with the knee in 90° of flexion. Three windows were developed—within the iliotibial band (ITB), between the ITB and biceps femoris, and posterior to the biceps femoris for PN exploration and neurolysis when applicable. In every case, the PN was explored from its course, emerging from posterior to the biceps femoris at least 6 to 7 cm proximal to the joint line, to coursing around the fibular neck, with 1 to 2 cm of peroneal fascia incised to free the nerve distally. A formal PN neurolysis was performed with any PLC exposure intraoperative indication of PN edema, contusion, displacement or kinking, scar, or essentially any case in which the PN did not appear completely intact. In cases of PN discontinuity, a primary repair was performed if the nerve ends appeared to be cleanly transected and able to be approximated without undue tension. No nerve grafting was required in our cohort.
Outcomes Measured
Resolution of PN palsy symptoms was determined using physical examination findings from follow-up notes. Complete resolution was defined as the absence of foot drop and presence of Medical Research Council grade 5 strength in the deep PN–innervated muscles (eg, tibialis anterior, extensor hallucis longus) on physical examination. For patients who experienced complete recovery, time to recovery was defined as the time elapsed between identification of PN palsy and identification of PN recovery. If postinjury electromyography (EMG) was performed, the date of the visit and time elapsed between identification of PN palsy and the EMG visit were recorded. Indications for EMG were patients with MLKI with a PN palsy who did not display any signs of recovery at the 3-month postoperative visit.
Statistical Analysis
Descriptive statistics were performed for the cohort. Continuous variables were assessed for normality using the Shapiro-Wilk test, and Student t tests and Wilcoxon rank-sum tests were used to compare normal and nonnormal distributions, respectively. Categorical variables were compared between groups using chi-square and Fisher exact tests where appropriate.
While logistic regression models are frequently used in the literature to investigate predictors of MLKIs, they lack the ability to learn from complex relationships between multiple inputs and improve when “fed” additional data. 3,22 In contrast, supervised machine learning algorithms are able to study relationships in a training data set and, with experience, are able to independently recognize patterns. These predictions are then compared with the “correct answers” in a testing set to determine the accuracy of the algorithm. Supervised machine learning models, particularly the random forest (RF) classifier, have shown significant promise in their predictive ability compared with other models including logistic regression in the orthopaedic literature, 22 yet their use is limited. In the present study, the RF classifier algorithm was used to develop a model that predicted the likelihood of complete PN recovery from demographic, injury, and treatment variables (henceforth referred to as “features”). The RF classifier consists of a large number of decision trees (so-called forest) created based on feature order and number, that are first generated and then voted for by popularity. 6 The RF classifier was used in this study because multiple studies have demonstrated its robust predictive accuracy, high sensitivity to small changes in data, resistance to overfitting due to model design, and minimal requirements for feature normalization and feature selection. 1,6,22,29
Given the high sensitivity of RF to small changes in data and the sample size, patients with missing values were removed to yield the final data set of 16 patients. Features used to build the model were selected based on clinical significance and existing MLKI literature demonstrating the importance of the following features on clinical outcomes. § Selected features included BMI, age, sex, chronicity of injury, mechanism of injury, laterality of injury, documented dislocation requiring reduction, periarticular fractures (femoral condyle, tibial plateau, or fibular head) requiring surgical fixation, and application of an external fixator. Schenck classification, time between injury and surgery, ACL tear, medial collateral ligament tear, posterior cruciate ligament tear, PLC tear, discontinuity noted intraoperatively, staged reconstruction, manipulation under anesthesia, use of an ankle-foot orthosis (AFO) at any time point postoperatively, and tendon transfer were also variables included. Intraoperative nerve findings including PN edema, contusion, displacement, and procedures performed on the PN, including neurolysis, repair, and graft, were not included as features in the model.
Model Validation
The model was trained using the randomForest and caret packages in R Version 4.1.0 (R Foundation for Statistical Computing). 25,27 Cross-validation of the model was performed using leave-one-out cross-validation (LOOCV). In LOOCV, model training is conducted with all except a single participant’s data, which are used as the test data set. This process is replicated N times (N = sample size). With each subsequent test, a different participant is singled out to be used as the test data set until every participant has been used, allowing for aggregation of results in an otherwise small data set. 1,4,13,32
Model Performance
The performance of LOOCV of our RF model was determined by measuring overall accuracy, F1 score, and area under the receiver operating characteristic curve (AUC). 1,22 Accuracy was calculated as the number of patients accurately classified by the model as patients who recovered or did not recover complete PN function divided by the total number of patients in the sample. The AUC is a measure of separability, representing the model’s ability to distinguish patients who recovered complete PN function from those who did not. An accuracy and AUC >80% were considered excellent; 71% to 80%, good; 51% to 70%, fair; and ≤50%, poor. 1 An F1 score (a harmonic mean of precision and recall) that was close to but not equal to 1 was considered most favorable. 11 This value indicates model accuracy without overfitting. Among the features used to build the model, feature importance in rank order was determined and plotted on a scale of 0 to 1.0 from lowest to highest contribution to recovery using the variable importance function for RF models in the caret package. For the top 3 features in the variable importance plot, partial dependence plots were used to calculate and visualize the partial dependence of the outcome on each of the various features used in the RF model. 14,31
Results
MLKIs with complete PN palsy constituted 8.0% (19/237) of all recorded MLKIs at both investigating institutions. Of the patients with complete PN palsy, 16 had complete data without loss to follow-up and were included in the investigational cohort. Patients who completely recovered PN function (n = 4; 25%) by final follow-up were then compared with those who did not recover any PN function (n = 12; 75%). Of all studied patients with complete PN palsy, 12 (75%) had documented dislocation requiring reduction.
There were no significant differences between the no-recovery and complete-recovery groups in age, sex distribution, BMI, low-velocity mechanism of injury, time to treatment, individual ligaments injured, Schenck classification of injury pattern, dislocation at the time of injury, fracture at the time of injury requiring surgery, external fixation use, time to PN palsy diagnosis, staged reconstructions, or follow-up times (Tables 1 and 2). The minimum follow-up time in our cohort was 3 months, and the maximum time was 104 months.
Table 1.
Patient Characteristics, Injuries, and Operative Management a
| No PN Recovery, n = 12 | Complete PN Recovery, n = 4 | P | |
|---|---|---|---|
| Age, y | 29.5 ± 12.9 | 22.3 ± 5.4 | .30 |
| BMI | 34.7 ± 12.1 | 33.89 ± 7.62 | .90 |
| Male sex | 12 (100) | 2 (50.0) | .08 |
| Injury laterality, right | 4 (33.3) | 2 (50.0) | .99 |
| Injury chronicity, chronic | 1 (8.3) | 1 (25.0) | .99 |
| Mechanism of injury, low velocity | 9 (75.0) | 4 (100) | .71 |
| External fixation required | 6 (50.0) | 1 (25.0) | .77 |
| Fracture at injury requiring surgery | 4 (33.3) | 0 (0) | .21 |
| Dislocation at time of injury | 8 (66.7) | 4 (100) | .51 |
| Staged reconstruction, 2 surgeries | 5 (41.7) | 3 (75.0) | .56 |
| Time to treatment, days | 26.8 ± 48.6 | 23.3 ± 16.2 | .89 |
| Time to PN palsy diagnosis, days | 0.7 ± 1.1 | 0.5 ± 1.0 | .79 |
| Time to complete PN recovery, days | — | 609.5 ± 539.4 | — |
| Required manipulation under anesthesia | 4 (33.3) | 2 (50.0) | .99 |
| Follow-up, mo | 31.8 ± 31.8 | 20.3 ± 17.9 | .51 |
a Data are reported as mean ± SD or n (%). BMI, body mass index; PN, peroneal nerve. Dashes indicate not available.
Table 2.
Schenck Classification and Ligamentous Injuries a
| No PN Recovery, n = 12 | Complete PN Recovery, n = 4 | P | |
|---|---|---|---|
| Schenck classification | .34 | ||
| KD1 | 3 (25.0) | 0 | |
| KD3L | 5 (41.7) | 3 (75.0) | |
| KD4 | 1 (8.3) | 1 (25.0) | |
| KD5 | 3 (25.0) | 0 | |
| Ligaments injured | |||
| ACL | 10 (83.3) | 4 (100) | .99 |
| PCL | 9 (75.0) | 4 (100) | .71 |
| MCL | 1 (8.3) | 1 (25.0) | .99 |
| LCL | 10 (83.3) | 4 (100) | .99 |
| PLC | 12 (100) | 4 (100) | — |
a Data are reported as n (%). ACL, anterior cruciate ligament; KD, knee dislocation; LCL, lateral collateral ligament; MCL, medial collateral ligament; PCL, posterior cruciate ligament; PLC, posterolateral corner; PN, peroneal nerve.
Postoperatively, there were no significant differences between the study groups with respect to use of AFO, EMG, time between PN diagnosis and EMG, or stiffness requiring manipulation under anesthesia (Tables 1 and 3).
Table 3.
PN Intraoperative Findings and Treatment a
| No PN Recovery, n = 12 | Complete PN Recovery, n = 4 | P | |
|---|---|---|---|
| PN intraoperative findings | |||
| Edema | 1 (8.3) | 2 (50.0) | .41 |
| Contusion | 2 (16.7) | 4 (100) | .05 |
| Displacement | 4 (33.3) | 1 (25.0) | .96 |
| Discontinuity | 6 (50.0) | 0 | .23 |
| PN procedures | |||
| Neurolysis | 9 (75.0) | 4 (100) | — |
| Repair | 3 (25.0) | 0 | .55 |
| Used AFO | 11 (91.7) | 3 (75.0) | .99 |
| Underwent EMG | 8 (66.7) | 1 (25.0) | .38 |
| Time from PN diagnosis to EMG, days, mean ± SD | 202.6 ± 172.6 | 133.0 | — |
| Underwent therapeutic tendon transfer | 1 (8.3) | — | — |
a Data are reported as n (%) unless otherwise specified. AFO, ankle-foot orthosis; EMG, electromyography; PN, peroneal nerve.Dashes indicate not available.
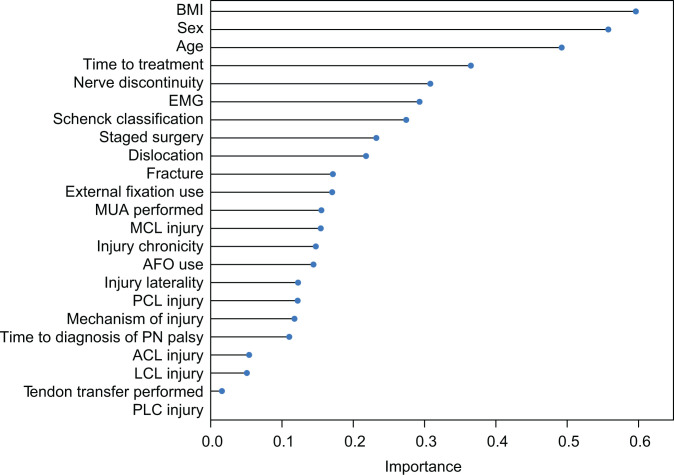
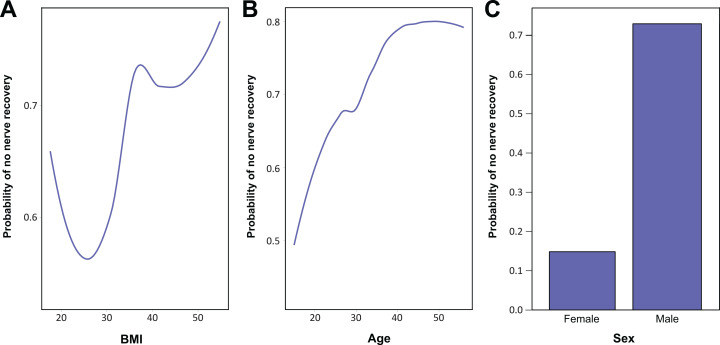
On cross-validation with LOOCV, the RF model was shown to predict complete PN recovery in patients with MLKI based on patient characteristics, injury characteristics, and management, with a classification accuracy of 75%. The AUC predicting complete PN recovery was determined to be 0.64. The F1 score was determined to be 0.86 (Figure 1). Accuracy was considered “good” and the AUC was considered “fair.” Variable importance plotting showed that BMI, sex, and age ranked among the top 3 most important contributors to the model (Figure 2). Subsequent partial dependence plots demonstrated that increasing age and BMI, as well as male sex, increased the probability of poor recovery (Figure 3).
Figure 1.
The receiver operating characteristic curve of a patient with multiligamentous knee injury and complete peroneal nerve palsy for predicting recovery based on patient characteristics, injury characteristics, and management.
Figure 2.
Variables ranked by relative contribution to prediction of recovery. While the variable importance function shows contributions, it does not determine whether these contributions are positive or negative. ACL, anterior cruciate ligament; AFO, ankle-foot orthosis; BMI, body mass index; EMG, electromyography; LCL, lateral collateral ligament; MCL, medial collateral ligament; MUA, manipulation under anesthesia; PCL, posterior cruciate ligament; PLC, posterolateral corner; PN, peroneal nerve.
Figure 3.
Partial dependence plot of probability of not recovering complete peroneal nerve function based on (A) body mass index (BMI), (B) age, and (C) sex.
Discussion
The primary findings of this study were that in patients with MLKI with complete PN palsy and associated foot drop, increasing BMI, male sex, and increasing age were the most important predictors of poor nerve recovery in our machine learning model. Increasing BMI and age contributed to poorer neurological recovery, with plateaus seen at approximately a BMI of 40 and age of 45 years, respectively. BMI was found to be the greatest predictor of poor nerve recovery, which confirmed our primary hypothesis. In addition, an RF classifier model scan can be built to predict complete recovery with a good degree of accuracy and separability without overfitting. While the features used in the model are not individually predictive of the outcome, collectively they predict recovery with a good degree of accuracy, an AUC of 0.64 and an F1 score of 0.86. To date, this is the largest cohort to our knowledge of patients with complete PN palsy studied using machine learning to predict recovery from complete PN palsy. Clinically, our model has the potential to assist orthopaedic surgeons and physical therapists in identifying patients with PN injury after MLKI who are most at risk of long-term disability, allow for improved prognostication and patient counseling, and encourage early intervention if and when appropriate.
Prior studies describing MLKI with concomitant complete PN palsy are limited to small case series. 8,34,35 In the present study, we report on 16 patients with complete foot drop in a multicenter cohort of 237 MLKIs. In the current literature, the largest study of predictors of PN injuries consisted of 26 patients with PN palsy. 38 The aforementioned study found that while sex, BMI, and fibular head fracture were predictive of PN injury, only younger age was a significant predictor of PN recovery. However, this study included only patients with documented knee dislocations and did not investigate other variables that may affect PN recovery, such as having a nondislocated MLKI, the use of external fixation, time to treatment, or any treatment-related variables (eg, staged reconstructions) in their multivariate regression model. 38 In the current study, patient characteristics, injury characteristics, diagnostic tests, and operative management of the MLKI and PN injury were factored into the analysis, resulting in a more robust and informed model. Partial dependence plots demonstrated that the probability of not recovering complete PN function was partially dependent on BMI, increasing until approximately a BMI of 40 and stabilizing thereafter. Similarly, partial dependence on age continued to increase until approximately the age of 45 years, after which the curve began to stabilize. Male sex was also shown to have a poorer probability of recovering complete PN function per partial dependence plots. These findings have the potential to guide prognosis in this patient population, with more realistic expectation setting for patients regarding their recovery.
The incidence of complete PN recovery in the current study was 25%, which is within the range of values reported in the literature, 14% to 75%. 15,30 Similar to Peskun et al, 38 our model and the partial dependence plot interpretation of the model showed that increasing BMI was a predictor of poor recovery. Multiple studies of outcome in patients with MLKI have pointed to obesity as a predictor of poor outcomes in general. 10,38 Studies have shown that patients with obesity are more likely to experience neurovascular injury, largely in the context of ultra–low velocity knee dislocation. 18,28,37 Obesity also has implications in nerve recovery after injury, not just as a risk factor for injury. Whether through metabolic syndrome or diabetes-associated neuroinflammation or slowed conduction velocities, multiple animal model studies have demonstrated worse neural markers and neuroinflammation in obese models. 2,17 In vivo clinical studies have demonstrated worse nerve recovery and conduction velocities in Bell palsy, carpal tunnel syndrome, and PN palsy after total hip arthroplasty in patients with obesity. 7,36,42 Our model also showed that increasing age was a risk factor for poor PN recovery. This is consistent with literature that has shown that the rate of peripheral nerve regeneration decreases with age. 23 Unlike Peskun et al, who found that sex was only a predictor of PN injury, not recovery, our results imply that male sex is a poor predictor of recovery in patients with complete nerve injury.
Notably, all patients with complete PN palsy in our study had PLC injuries, which have previously been demonstrated to be significantly associated with a higher rate of PN injury compared with those without PLC injury. 20 Kahan et al 20,21 identified pathoanatomic risk factors for PN injury among patients with MLKI that included knee dislocations (Schenck grades 3 and 4), PLC injuries, and midsubstance tearing and/or fibular avulsion of the lateral collateral ligament (LCL). However, our analysis found Schenck classification to be a less important predictor of recovery from PN palsy compared with patient characteristics and time to treatment, while PLC and LCL injuries were some of the least important predictors for recovery based on our model. This implies that while anatomic features of the initial injury may predict the likelihood and the specific location of PN injury, these same characteristics may not be as predictive of prognosis compared with known risk factors for surgical outcomes in general (eg, age, sex, BMI, time to treatment) and for neural recovery (eg, EMG findings). While Kahan et al 20 did not identify specific anatomic characteristics that portended recovery, they did find that different patterns of LCL injury (fibular avulsion vs femoral avulsion vs midsubstance tear) were associated with different rates of PN palsy and further speculated that different LCL injury patterns could be associated with different prognoses for nerve recovery. However, our model grouped together all LCL injury types without distinguishing tear pattern, and thus we were unable to determine whether LCL injury pattern was a significant predictor of nerve recovery. Further studies should include consideration of PLC and LCL injuries, along with ligamentous pattern and Schenck classification, in their analysis.
Interestingly, nerve discontinuity was not the best predictor of recovery. It is known that neurotmesis leads to Wallerian degeneration of the axon distal to the site of neurotmesis, and even with aggressive and early nonoperative treatments including AFO use and operative treatments including nerve repair, grafting, and tendon transfers, outcomes remain poor. 26 Given this, one would expect nerve discontinuity seen intraoperatively to be the most important predictive feature in our model. Intuitively, nerve discontinuity should be the strongest predictor of lack of recovery, as a severed PN will not simply “heal.” Surgeons who encounter PN neurotmesis intraoperatively should appropriately counsel patients on recovery and perhaps discuss earlier treatments targeted toward the PN, such as tendon transfer, if nerve repair or grafting is not possible. In our cohort, however, while 6 patients with discontinuity did not recover, 6 patients without discontinuity also did not recover, suggesting that the absence of discontinuity was not the best predictor of recovery in our model. While electromyograms can be useful adjunctive information in the patient with MLKI with PN palsy who does not demonstrate evidence of recovery, we found that EMG results influence treatment less than patient-related factors (such as age, BMI, activity demands, and occupation) and timeline and nature of PN recovery. 23,26,33,38
Limitations
This study has several limitations because of the retrospective nature of data collection. Despite the very large overall size of our cohort, the complete palsy cohort had a relatively small sample size and missing values in the data because of difficulty with follow-up, which were common in the patient population with MLKI at the investigating tertiary care centers and anecdotally are likely an issue throughout the United States, particularly with follow-up at trauma centers. Unfortunately, a majority of intraoperative findings could not be included as features used to train the RF model to build the most robust model possible. Intraoperative findings and nerve procedures at the time of initial MLKI reconstruction should be incorporated in future machine learning and predictor studies. Machine learning algorithms improve with data or experience, as exposure to patterns helps them learn. Here we demonstrated that our model can predict recovery well given reported performance metrics. In order to further increase the model’s predictive ability and to investigate the predictive ability of individual variables at a statistically significant level, a larger training set is required, and sources of bias in the model include differences in surgeon approach to management of PN injury as well as the imbalanced outcome data. In addition, RF is one of many models available, and further research must consider other models that may be superior to the current model. Future studies should build on our work and construct models with larger patient data sets and consider the use of data augmentation techniques in order to build the most robust model that can be used for clinical decision making.
Conclusion
MLKI with complete PN palsy remains a challenging injury to treat and continues to have life-altering impacts on patients. Our model has the ability not only to identify important contributors to recovery (age, sex, BMI), but also to predict with good accuracy whether a patient who is evaluated with complete PN injury will recover. While further study of machine learning models with larger patient data sets is required to identify the most superior model, these findings present an opportunity for orthopaedic surgeons to better identify, counsel, and treat patients with MLKIs and concomitant complete PN palsy.
Footnotes
Final revision submitted June 17, 2022; accepted July 6, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: M.J.M. has received consulting fees and speaking fees from Smith & Nephew. M.J.A. has received education payments from Arthrex, consulting fees from Siemens Medical Solutions, and speaking fees from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from NYU Langone Health.
References
- 1. Ahmadi M, O’Neil M, Fragala-Pinkham M, Lennon N, Trost S. Machine learning algorithms for activity recognition in ambulant children and adolescents with cerebral palsy. J Neuroeng Rehabil. 2018;15(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bekar E, Altunkaynak BZ, Balcı K, et al. Effects of high fat diet induced obesity on peripheral nerve regeneration and levels of GAP 43 and TGF-β in rats. Biotech Histochem. 2014;89(6):446–456. [DOI] [PubMed] [Google Scholar]
- 3. Bini SA. Artificial intelligence, machine learning, deep learning, and cognitive computing: what do these terms mean and how will they impact health care? J Arthroplasty. 2018;33(8):2358–2361. [DOI] [PubMed] [Google Scholar]
- 4. Bishop CM. Pattern Recognition and Machine Learning. Springer; 2006. [Google Scholar]
- 5. Bloom DA, Essilfie AA, Lott A, et al. Distal biceps femoris avulsions: associated injuries and neurological sequelae. Knee. 2020;27(6):1874–1880. [DOI] [PubMed] [Google Scholar]
- 6. Breiman L. Random forests. Machine Learning. 2001;45(1):5–32. [Google Scholar]
- 7. Choi SA, Shim HS, Jung JY, et al. Association between recovery from Bell’s palsy and body mass index. Clin Otolaryngol. 2017;42(3):687–692. [DOI] [PubMed] [Google Scholar]
- 8. Chowdhry M, Burchette D, Whelan D, et al. Knee dislocation and associated injuries: an analysis of the American College of Surgeons National Trauma Data Bank. Knee Surg Sports Traumatol Arthrosc. 2020;28(2):568–575. [DOI] [PubMed] [Google Scholar]
- 9. Essilfie AA, Alaia EF, Bloom DA, et al. Distal posterolateral corner injury in the setting of multiligament knee injury increases risk of common peroneal palsy. Knee Surg Sports Traumatol Arthrosc. 2022;30(1):239–245. [DOI] [PubMed] [Google Scholar]
- 10. Everhart JS, Du A, Chalasani R, et al. Return to work or sport after multiligament knee injury: a systematic review of 21 studies and 524 patients. Arthroscopy. 2018;34(5):1708–1716. [DOI] [PubMed] [Google Scholar]
- 11. Forman G, Scholz M. Apples-to-apples in cross-validation studies: pitfalls in classifier performance measurement. SIGKDD Explor Newsl. 2010;12(1):49–57. [Google Scholar]
- 12. Goebel CP, Domes C. Classifications in brief: the Schenck classification of knee dislocations. Clin Orthop Relat Res. 2020;478(6):1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodlich BI, Armstrong EL, Horan SA, et al. Machine learning to quantify habitual physical activity in children with cerebral palsy. Dev Med Child Neurol. 2020;62(9):1054–1060. [DOI] [PubMed] [Google Scholar]
- 14. Greenwell BM, Boehmke BC, McCarthy AJ. A simple and effective model-based variable importance measure. arXiv. Preprint posted online May 12, 2018. doi:10.48550/arXiv.1805.04755
- 15. Harner CD, Waltrip RL, Bennett CH, et al. Surgical management of knee dislocations. J Bone Joint Surg Am. 2004;86(2):262–273. [DOI] [PubMed] [Google Scholar]
- 16. Irgit KS, Cush G. Tendon transfers for peroneal nerve injuries in the multiple ligament injured knee. J Knee Surg. 2012;25(4):327–333. [DOI] [PubMed] [Google Scholar]
- 17. Jayaraman A, Lent-Schochet D, Pike CJ. Diet-induced obesity and low testosterone increase neuroinflammation and impair neural function. J Neuroinflammation. 2014;11:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson JP, Kleiner J, Klinge SA, et al. Increased incidence of vascular injury in obese patients with knee dislocations. J Orthop Trauma. 2018;32(2):82–87. [DOI] [PubMed] [Google Scholar]
- 19. Johnson ME, Foster L, DeLee JC. Neurologic and vascular injuries associated with knee ligament injuries. Am J Sports Med. 2008;36(12):2448–2462. [DOI] [PubMed] [Google Scholar]
- 20. Kahan JB, Li D, Schneble CA, et al. The pathoanatomy of posterolateral corner ligamentous disruption in multiligament knee injuries is predictive of peroneal nerve injury. Am J Sports Med. 2020;48(14):3541–3548. [DOI] [PubMed] [Google Scholar]
- 21. Kahan JB, Schneble CA, Li D, et al. Increased neurovascular morbidity is seen in documented knee dislocation versus multiligamentous knee injury. J Bone Joint Surg Am. 2021;103(10):921–930. [DOI] [PubMed] [Google Scholar]
- 22. Karnuta JM, Luu BC, Haeberle HS, et al. Machine learning outperforms regression analysis to predict next-season Major League Baseball player injuries: epidemiology and validation of 13,982 player-years from performance and injury profile trends, 2000-2017. Orthop J Sports Med. 2020;8(11):2325967120963046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kovacic U, Sketelj J, Bajrović FF. Chapter 26: age-related differences in the reinnervation after peripheral nerve injury. Int Rev Neurobiol. 2009;87:465–482. [DOI] [PubMed] [Google Scholar]
- 24. Krych AJ, Giuseffi SA, Kuzma SA, Stuart MJ, Levy BA. Is peroneal nerve injury associated with worse function after knee dislocation? Clin Orthop Relat Res. 2014;472(9):2630–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuhn M. Building predictive models in R using the caret package. J Stat Softw. 2008;28(1):1–26.27774042 [Google Scholar]
- 26. Levy BA, Giuseffi SA, Bishop AT, et al. Surgical treatment of peroneal nerve palsy after knee dislocation. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1583–1586. [DOI] [PubMed] [Google Scholar]
- 27. Liaw A, Wiener M. Classification and regression by randomForest. R News. 2002;2(3):18–22. [Google Scholar]
- 28. Marin EL, Bifulco SS, Fast A. Obesity: a risk factor for knee dislocation. Am J Phys Med Rehabil. 1990;69(3):132–134. [PubMed] [Google Scholar]
- 29. Martin RK, Wastvedt S, Pareek A, et al. Predicting anterior cruciate ligament reconstruction revision: a machine learning analysis utilizing the Norwegian Knee Ligament Register. J Bone Joint Surg Am. 2022;104(2):145–153. [DOI] [PubMed] [Google Scholar]
- 30. Meyers MH, Moore TM, Harvey JP, Jr. Traumatic dislocation of the knee joint. J Bone Joint Surg Am. 1975;57(3):430–433. [PubMed] [Google Scholar]
- 31. Molnar C. Interpretable Machine Learning. Lulu.com; 2020. [Google Scholar]
- 32. Montoye AHK, Westgate BS, Fonley MR, Pfeiffer KA. Cross-validation and out-of-sample testing of physical activity intensity predictions with a wrist-worn accelerometer. J Appl Physiol. 2018;124(5):1284–1293. [DOI] [PubMed] [Google Scholar]
- 33. Mook WR, Ligh CA, Moorman CT, III, Leversedge FJ. Nerve injury complicating multiligament knee injury: current concepts and treatment algorithm. J Am Acad Orthop Surg. 2013;21(6):343–354. [DOI] [PubMed] [Google Scholar]
- 34. Niall DM, Nutton RW, Keating JF. Palsy of the common peroneal nerve after traumatic dislocation of the knee. J Bone Joint Surg Br. 2005;87(5):664–667. [DOI] [PubMed] [Google Scholar]
- 35. O’Malley MP, Pareek A, Reardon P, et al. Treatment of peroneal nerve injuries in the multiligament injured/dislocated knee. J Knee Surg. 2016;29(4):287–292. [DOI] [PubMed] [Google Scholar]
- 36. Park JH, Hozack B, Kim P, et al. Common peroneal nerve palsy following total hip arthroplasty: prognostic factors for recovery. J Bone Joint Surg Am. 2013;95(9):e55. [DOI] [PubMed] [Google Scholar]
- 37. Peltola EK, Lindahl J, Hietaranta H, Koskinen SK. Knee dislocation in overweight patients. AJR Am J Roentgenol. 2009;192(1):101–106. [DOI] [PubMed] [Google Scholar]
- 38. Peskun CJ, Chahal J, Steinfeld ZY, Whelan DB. Risk factors for peroneal nerve injury and recovery in knee dislocation. Clin Orthop Relat Res. 2012;470(3):774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poage C, Roth C, Scott B. Peroneal nerve palsy: evaluation and management. J Am Acad Orthop Surg. 2016;24(1):1–10. [DOI] [PubMed] [Google Scholar]
- 40. Schenck RC, Jr. The dislocated knee. Instr Course Lect. 1994;43:127–136. [PubMed] [Google Scholar]
- 41. Vaidya R, Roth M, Nanavati D, Prince M, Sethi A. Low-velocity knee dislocations in obese and morbidly obese patients. Orthop J Sports Med. 2015;3(4):232596711557571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Werner RA, Jacobson JA, Jamadar DA. Influence of body mass index on median nerve function, carpal canal pressure, and cross-sectional area of the median nerve. Muscle Nerve. 2004;30(4):481–485. [DOI] [PubMed] [Google Scholar]