Abstract
Penile cancer is a rare malignancy, particularly in industrialized nations. In the United States, rates are approximately less than 1 per 100,000 men per year with just over 2000 new cases per year. However, there is significantly increased prevalence in developing nations, with limited treatment expertise and reduced access to care, further driving an unmet clinical need. The most noteworthy risk factor for penile cancer is the association with human papillomavirus infection, which may be present in up to 50% of all penile carcinomas. In addition to local primary tumor approaches, multimodality treatment strategies are vital to patients with clinical regional nodal disease, locally advanced disease. Presence and degree of lymph node involvement remains the most important prognostic factor and patients may benefit from multiple treatment strategies. Interim analysis data from the first randomized clinical trial is expected to yield results in mid/late 2024–early 2025. These treatment approaches include neoadjuvant chemotherapy, adjuvant therapy, including chemotherapy and radiation. Systemic therapy for distant recurrent or metastatic disease is primarily a platinum-based chemotherapy, however with poor overall response. As poor outcomes remain high, particularly in indigent populations, there remains an unmet need for these patients, particularly for high level randomized trials and novel therapeutics. In this review, we will highlight treatment updates for penile cancer. In addition to standard of care, we will review novel lines of therapies including immunotherapies and targeted therapies as well as sequencing approaches.
Keywords: HPV, immunotherapy, penile cancer, penile squamous cell carcinoma, therapeutics
Introduction
Penile cancer is a rare malignancy seen in industrialized nations, for example, with just over 2000 new cases annually in the United States and estimated 3100 annual cases in Europe.1,2 Globally, penile cancer accounts for 26,000 cases per year. 3 However, in other parts of the world including Africa, South America, and Asia, rates can be as high as 10–20% of all malignancies in men. 4 In addition, cultural differences including counties that practice neonatal circumcision have decreased rates. 5 Per population-based data, the rates of penile cancer tend to increase with age. Hispanic males have been noted to have a lower median age at diagnosis as well as an increased incidence rate (rate ratio 1.72 per 100,000 males), compared to White and Black males (0.81 and 0.82 per 100,000 males) and Asian-American males (rate ratio 0.45 per 100,000 males). 6
Additional risk factors for penile cancer include human papillomavirus (HPV) infection in up to 50% of patients.7–9 Presence of phimosis, lack of circumcision with a history of phimosis [odds ratio (OR): 11.4, 95% confidence interval (CI): 5.0–25.9], tobacco exposure, poor hygiene, and obesity are associated with increased risk. Medical conditions of the penis are also associated with increased risk, including genital warts (OR: 7.6), penile tear, urinary tract infection, and urethral stricture.10,11
Penile squamous cell carcinoma (PSCC) is the most common form of penile cancer typically arising from the glanular and preputial skin. 5 The World Health Organization 2016 classification of penile carcinomas has been widely accepted and includes non-HPV-related penile squamous cell carcinomas (SCCs), HPV-related penile SCCs, and other relative frequency (Table 1). 12
Table 1.
WHO classification and distribution of penile carcinomas (%).
| Non-HPV-related penile SCCs | HPV-related penile SCCs | Other |
|---|---|---|
| SCC | Basaloid carcinoma (7%) | Unclassified carcinoma (2%) |
| Usual carcinoma (44%) | Papillary-basaloid (rare) | |
| Pseudohyperplastic carcinoma (3%) | Warty carcinoma (7%) | |
| Pseudoglandular carcinoma | Warty-basaloid carcinoma (4%) | |
| Verrucous carcinoma (3%) | Clear cell carcinoma (rare) | |
| Pure verrucous carcinoma (rare) | Lymphoepithelioma-like carcinoma (rare) | |
| Carcinoma cuniculatum (rare) | ||
| Papilary carcinoma, NOS (2%) | ||
| Adenosquamous carcinoma (rare) | ||
| Sarcomatoid squamous carcinoma (7%) | ||
| Mixed carcinoma (21%) |
HPV, human papillomavirus; NOS, not otherwise specified; SCC, squamous cell carcinoma; WHO, World Health Organization.
Typical clinical presentation of penile cancer is a local lesion with mass or ulceration. In approximately 50% of cases, inguinal adenopathy may be present on initial presentation. 13 Distant metastasis at initial presentation is lower at approximately 1–10%.14,15 Staging for penile cancer includes the 8th edition of the American Joint Committee on Cancer, tumor, node, metastasis staging system. Notable updates to the 8th edition include presence or absence of perineural invasion as a division between T1a and 1b tumors, as well as pN1 defined as <2 unilateral inguinal metastasis, without extranodal extension and pN2 defined as >3 unilateral inguinal metastasis or bilateral metastasis. 16 Pathologic features for the tumor locally include histology, presence of lymphovascular invasion, as well as perineural invasion however regional nodal assessment is critical as nodal involvement is the most important prognostic factor influencing survival currently. A published literature review reported 5-year cancer-specific survival for patients without nodal metastasis at 85–100% contrasted with pelvic node metastasis at 0–17%. 17
Diagnostic evaluation for patients with clinical negative inguinal exam in lower risk disease includes surveillance, including pTis, pTa, or T1 lesions. For patients with higher risk disease greater than pT1b, recommendation is for superficial inguinal lymph node dissection (ILND) versus dynamic sentinel node biopsy. For clinically suspicious inguinal nodes, an FNA can be utilized and if positive can aid with definitive surgical management. In patients with clinically suspicious inguinal nodes who are otherwise low risk with negative FNA, an excision biopsy is recommended, and in high-risk (HR) patients with negative FNA, a superficial ILND with frozen section is suggested. These diagnostic evaluations are supported by international guidelines including European Association of Urology (EAU) and National Comprehensive Cancer Network (NCCN) guidelines.18,19
In cases with proven nodal metastasis, imaging systemic modalities including computed tomography (CT) and magnetic resonance imaging (MRI) have become routine for staging and risk stratification, particularly in picking up adverse nodal features such as >3 positive lymph nodes, extranodal extension, and pelvic metastasis. CT/positron emission tomography (PET) may also be considered to assess nodal burden in patients with clinically nodal positive disease, pre-/post-neoadjuvant chemotherapy (NAC), or prior to salvage therapy to assess treatment response primarily. A small trial from the Netherlands with 18 patients with unilateral or bilateral cytologic positive inguinal disease with CT/PET sensitivity of 91%, specificity of 100%, and diagnostic accuracy of 96%. In five patients, they were also able to detect distant metastasis.20,21 Cross-sectional imaging of the chest/abdomen/pelvis via CT/MRI or CT/PET is also supported by both EAU and NCCN guidelines. Patients with bulky inguinal lymph nodes, >4 cm or fixed, are strong candidates for cross-sectional imaging as well as multimodal treatment strategies (i.e. NAC, chemoradiation).18,19
Tumor biology
It is vital to have a strong understanding of the underlying biologic mechanisms and microenvironment in PSCC to help identify effective therapeutic targets.
Penile intraepithelial neoplasia (PeIN) is a risk factor as a precancerous lesion with an undifferentiated (HPV related) and differentiated (non-HPV related) subtype. 22 In a trial from the Netherlands, it was reported that patients with grade 1 mild dysplasia which progressed in 2% of cases versus grade 3 PeIN, severe dysplasia progressed to malignancy in 7%. 23
At this time, there is limited information for familial inheritance pattern in PSCC and family history of PSCC is not considered a major risk factor, although could be a potential risk factor, as it is in many cancer types. Given the rarity of the disease, there are no classical twin studies. There is one large long-term follow-up study of Nordic twins, Nordic Twin Study of Cancer, which evaluated 23 types of cancer including PSCC with follow-up over an average of 32 years with reported discordance in monozygotic twins (n = 15; PSCC cases) and dizygotic twins (n = 34 cases). Despite the limitations, including numbers and population, it appears inheritance has limited influence in PSCC.24,25
HPV infection remains an important driver for oncogenesis in PSCC. Data from recent meta-analysis in 2019 revealed that more than 50% of penile carcinoma was positive for HPV DNA. Basaloid squamous cell carcinoma and warty-basaloid carcinoma had the highest prevalence, 84% and 75.7%, respectively. 7 Particular HPV strains that have commonly been identified include 16, 18, 31, and 33. Incorporation of the HPV virus into host DNA leads to overproduction of oncoproteins E6, E7 which leads to cell cycle dysregulation via interactions with p53 and pRb.26,27 In addition, HPV-associated cancers express increased p16 (INK4A) in response to E7 oncoprotein expression. Studies have shown immunohistochemical (IHC) overexpression of p16 (INK4a) can be a surrogate for HR HPV infection, with p16 overexpression correlating with improved survival.28–33 Based on these mechanisms and percentage of HPV positivity, there remains significant interest in HPV-directed therapies. 34
Penile carcinoma is also driven by an HPV-independent pathway. Primary drivers are thought to be chronic inflammation and somatic gene alterations. 26 Many chronic conditions increase risk including phimosis, balanitis, lichen sclerosis, obesity, lack of circumcision, smoking, and psoralen UV-A phototherapy. 35 A shared mechanism in chronic inflammation is induction of cyclooxygenase 2 (COX2) expression and has been associated with PeIN as well as invasive and distant penile cancer. Mechanistically, it is likely that COX2 overexpression drives prostaglandins and thromboxanes promoting angiogenesis and invasion.36,37 In a detailed evaluation of somatic genomic alterations from cancer tissue of 43 patients, the most mutated genes included TP53, CDKN2A, and HRAS.38,39 Moreover, new driver genes are being considered including RB1 overexpression, AR downregulation, and BIRC5 overexpression based on multidimensional, integrative analysis of accumulation frequency alterations in passenger genes.38,40 TP53 tumor suppressor gene has also been studied, particularly in the development of metastatic penile cancer as the main genetic pathway driving progression as HPV-dependent mechanisms decline over time. 41 Epigenetic changes including hypermethylation may also drive oncogenesis in addition to genetic alterations. 38 Overall, there appears to be multiple mechanisms which may drive new targetable treatment approaches.
From an infection standpoint, penile microbiota from HPV-positive samples has also provided additional insight. A study from Cape Town examining penile microbiota of 238 men from Onywera et al. 42 documented greater abundances of anaerobic bacterial vaginosis-associated bacteria including Prevotella, Peptinophilus, and Dialister, particularly in HR HPV-infected men. Given the causal relationship between persistent HR HPV infection and penile cancer, it is conceivable that these bacterial taxa can promote conditions for persistent infection and oncogenic risk. This would require additional longitudinal study. 42
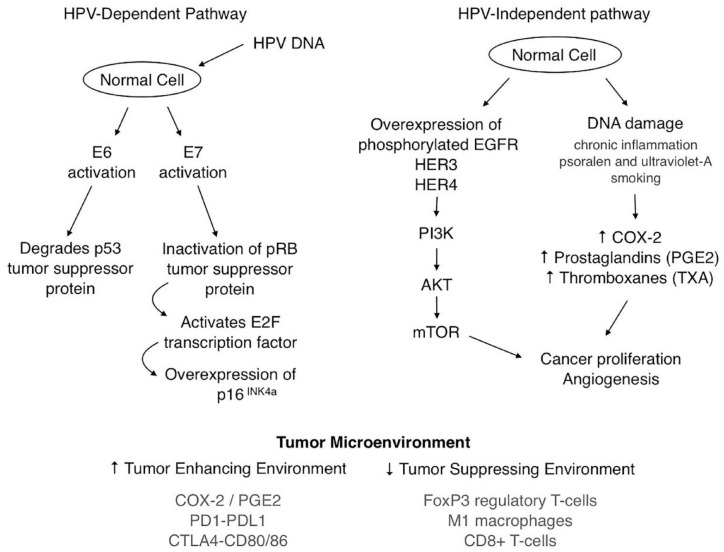
Understanding the tumor microenvironment (TME) remains critical to effectively treat and personalize care for patients with PSCC. There has been studied unique differences between HPV-positive PSCC and HPV-negative PSCC, including increased tumor-infiltrating lymphocytes (TILs), stronger polarization toward T-helper 1 cells, and overall more significant cytotoxic immune response in the HPV-positive SCC subset. 43 Based on a retrospective cohort study, diffuse programmed death-ligand 1 (PD-L1) expression, CD163+ macrophage infiltration, and low stromal CD8+ T-cell infiltration were all associated with lymph node metastasis. 44 Moreover, an additional study in tumoral inflammation in penile cancer from Vassallo et al. 45 demonstrated a high presence of tumor infiltrating FoxP3-positive Tregs was linked to unfavorable outcomes, and worse disease-free survival probability (hazard ratio = 2.50, p = 0.02). A primary illustration of the HPV dependent, HPV independent, and TME is shown in Figure 1.
Figure 1.
Illustrative diagram of HPV-dependent, HPV-independent carcinogenesis and TME.
HPV, human papillomavirus; TME, tumor microenvironment.
Additional research on PD-L1 expression continues to support increased levels in PSCC. One study in particular revealed up to 69.2% of lymph node metastasis as being PD-L1 positive. 46 Correlation with lymph node metastasis has been seen in other studies including Udager et al. 47 showing PD-L1 expression in the primary tumor had notable correlation with lymph node metastasis and shorter cancer-specific survival. These findings appear to provide a rational basis for anti-PD-1, PD-L1 blockade. Consideration for immune checkpoint inhibitors is also supported by tumor mutation burden (TMB), for which the TMB of PSCC was similar to that for other SCCs including head and neck, esophagus, bladder, and lung cancer.48,49
Whole exome sequencing in PSCC has shed additional light on the molecular landscape in PSCC. Chahoud et al. 50 sequenced 34 patients prospectively and noted enrichment for mutation signatures including the Notch pathway (70.6%), comparable with head and neck squamous cell carcinoma (HNSC). Additional enrichment of two distinct mutational signatures, mutation pattern 1 (MP1), and mutation pattern 2 (MP2) were noted. MP1 was associated with oncogenic activity of AID/APOBEC, and MP2 was associated with defective DNA mismatch repair and microsatellite instability (MSI). MP1 enrichment was positively correlated with increased TMB and correlated with significantly worse survival than MP2. 50
Additional studies have confirmed comparability between PSCC and other HPV-related SCCs including gains and amplifications on chromosomes 3q, 8q, 11p, and 5p, as well as losses on 11q, 3p, and 4q.51,52 Furthermore, utilizing The Cancer Genome Atlas data, comparison of PSCC with SCC from other anatomic sites revealed convergence of mutations with increased alterations including TP53, NOTCH1, CKDN2A, PIK3CA, CASP8, and FAT1.48,50
Whole exome sequencing analysis has reported the most common mutations in PSCC including TP53 (35%), NOTCH1 (35%), CDKN2A (23%), PIK3CA (21%), and DDR genes (20%). 53 Additional genomic profiling has outlined potential targetable alterations in metastatic PSCC including mTOR pathway alterations (11%), DNA repair pathway (including BRCA2/ATM at 14%), tyrosine kinase [epidermal growth factor receptor (EGFR) alterations at 6%], and FGFR3 and ERBB2 genomic alterations (4% each). 41 Of note, in a retrospective cohort of 43 PSCC cases, poorer outcomes were observed in cases with lack of p16 expression, MYC, and CCND1 amplifications. 39
Data from HNSC have revealed therapeutic consideration for NOTCH1 loss of function mutations with PI3K/mTOR inhibitors. This remains significant given the current application for these inhibitors in clinical practice, (i.e. metastatic breast cancer) and potential for PSCC trial exploration. 54 In addition, EGFR is significantly expressed by PSCC tumors and metastasis and can be an additional consideration for targeted treatment. 55 The HER/PTEN/Akt pathway also appears to be disrupted in PSCC suggesting possible benefit from therapies to target HER receptors. 56 Given the percentage of DDR gene mutations in PSCC as describe above, consideration for Poly (ADP-ribose) polymerase inhibitor (PARPi)-based therapy should be evaluated further as deleterious mutations in DNA damage responsive genes are frequently associated with response to both PARPi and platinum chemotherapy.53,57–59
In describing the genomic landscape of PSCC, additional studies utilizing the latest high-resolution methodologies including single-cell RNAseq, single-cell ATACseq, and single-cell TCRseq remains critical. These technologies may elucidate cell types and pathways involved in cancer immunology, which can further personalize therapeutic strategies in rare tumors.
Standard of care local therapeutic approaches
Local treatment approaches may include penile sparing techniques. For PeIN, topical chemotherapy is an effective frontline approach including 5-fluorouracil (5-FU) and Imiquimod. There is little randomized data for these treatments; however, complete response up to 57% has been reported in the literature with limited serious adverse events. 60 Low recruitment has limited standardizing protocols for topical therapy.
For PeIN and pT1a, laser ablation therapy is an option for superficial lesions with good functional outcomes. Typically, laser ablation is performed with neodymium-doped yttrium aluminum garnet or carbon dioxide. A multi-center, tertiary referral center retrospective review did note an inguinal/pelvic nodal recurrence rate for pT1b of 18%, and pT2 of 22%. Based on this data, laser ablation coupled with diagnostic nodal staging should be indicted in patients with pT1b or higher. 61 In addition, for PeIN and pT1 tumors confined to the prepuce, circumcision is a common procedure and can be the primary treatment for these lesions. 62 From a preventative standpoint, circumcision reduces chronic inflammatory producing milieu. It is recommended to supplement local treatments such as laser therapy with radical circumcision for additional safety and hygiene in patients who have not been previously circumcised. 63 Moh’s micrographic surgery is another technique for PeIN and pT1a penile cancers, particularly superficial lesions. This technique allows for maximal tissue preservation. However, this technique remains very time-consuming and technically difficult. An observational study reported local recurrence rate for PeIN of 5% and PSCC between 0% and 32%. Glans resurfacing is another technique for PeIN and pT1 which includes the excision of epithelial and subepithelial glans tissue followed by replacement with partial thickness skin graft. 64
For patients with more invasive tumors (pT1-pT2), additional options are available. Glansectomy represents a technique of removing tumors involving the glans via complete removal of glans spongiosum. For smaller tumors, involving less than half of the glans, a partial glansectomy and wide local excision can be considered. 65 Of note, perineural invasion, PeIN, positive margins, lymphovascular invasion, high-grade SCC, and pT3 stage have been identified as risk factors for local recurrence after glansectomy and can guide further treatment.66,67
An alternative organ preserving technique for patients with localized tumors includes radiotherapy such as external beam radiation therapy and brachytherapy (BT). Major international guidelines, including NCCN and ESMO, support radiotherapy as an organ preserving strategy in early stage PSCC.18,68 There is a lack of randomized, prospective trials comparing radiotherapy to surgery; thus, the decision between treatment modalities requires shared decision-making. Data from a meta-analysis including 2178 patients revealed no statistical difference in 5-year local control or overall survival (OS) rates between surgery versus BT groups. 69 Based on published series of patients followed prospectively, patients who were treated with primary radiotherapy were successfully salvaged with surgery. 70
Although patients with T1b and T2 tumors can be treated with organ sparing surgeries. A partial or total penectomy (TP) may be required for T3 disease with corpus cavernosum involvement. Per NCCN guidelines, partial penectomy (PP) should be considered in high-grade primary tumors, provided a functional penile stump can be preserved and negative surgical margins are obtained. If a PP is not possible, a TP should be performed. Intraoperative frozen sections are recommended for evaluating margin status. 18 There is not a consensus regarding negative margins. Historically, a 2 cm margin was standard of care however having a surgical margin greater than 1 mm has been shown to be quite sufficient and have not affected long-term oncologic outcomes. 71 As previously discussed, TP is recommended if a functional penile stump is not possible, and typically indicated in T3 or T4 stage PSCC. Radical penectomy involves penile removal via corporal body to the level of pubic bone. In addition, the urethra is brought through the perineum forming a perineal urethrostomy. 64 Oncologic outcomes in a North American study utilizing National Cancer Data Base in patients with pT1-2 PSCC noted a 5-year and 10-year OS rate of 85% and 72% for PP and 79% and 63% for TP. It is worth noting clinical and pathologic factors likely impacted choice of surgical procedure. In patients who underwent PP, a tumor size <3 cm was noted in 59.7%, pT2 in 46.4%, nodal metastasis in 8.1%, and chemotherapy was given during the course of their disease in 6.5% of patients. In patients who underwent TP, a large size tumor >4 cm was noted 50.5%, pT2 in 61.3%, nodal metastasis in 12.6%, and chemotherapy was given during the course of their disease in 11.2% of the patients. 72
Standard of care for locally advanced PSCC multidisciplinary treatment approaches
Neoadjuvant therapy
For patients with locally advanced PSCC, multidisciplinary care is optimal for disease management. Lymph node status remains pivotal, marking the boundary between curable and incurable disease, and is the strongest predictor for survival in PSCC.73,74 In patients with concern for clinical nodal disease, determining the extent of disease via clinical exam, imaging, and percutaneous biopsy is critical. 75 Patients, with biopsy-proven inguinal lymph nodes that are fixed, bulky >4 cm, bilateral, and/or positive pelvic lymph nodes, are recommended for NAC followed by surgical management of lymph nodes including ILND/pelvic lymph node dissection (PLND). The preferred NAC approach is four cycles of TIP (paclitaxel, ifosfamide, and cisplatin). These recommendations are supported by the NCCN and EAU guidelines.18,19
The recommendation for TIP is based on a single-arm, non-randomized, phase II trial. This was a prospective trial led by Pagliaro et al. with 30 patients at a single institution with clinical N2/N3 PSCC. NAC was administered for four cycles consisting of paclitaxel 175 mg/m2 (administered over 3 h on day 1); ifosfamide 1200 mg/m2 on days 1–3; and cisplatin 25 mg/m2 on days 1 and 2. Key findings included objective response rate (ORR) of 50%, pathologic complete response (pCR) in 10% of patient and 30% alive and recurrence free at median follow-up 34 months (range: 14–59 months). The estimated median time to progression (TTP) was 8.1 months (95% CI: 5.4–50 months), and median OS was 17.1 months (95% CI: 10.3–60 months). Improved TTP and OS were significantly associated with a response to chemotherapy (p < 0.001 and p = 0.001, respectively), absence of bilateral residual tumor (p = 0.002 and p = 0.017, respectively), and absence of extranodal extension (p = 0.001 and p = 0.004, respectively) or skin involvement (p = 0.009 and p = 0.012, respectively). Therapy was overall well tolerated with grade 3 infections (16.7%). 76 This was the first prospective study to evaluate multimodality therapy outcomes in PSCC with regional lymph node involvement, establishing NAC with TIP followed by surgical consolidation as the preferred treatment for PSCC with bulky lymphadenopathy. 75 Of note, there has been limited molecular or imaging biomarkers for early assessment of the benefit of NAC, there is limited but promising results with fluorine-18 ( 18 F-FDG PET/CT).75,77 Biomarkers or alternative prognostic indicators would be beneficial to further risk stratify patients to additional adjuvant-based therapies. In a small retrospective study, the adjuvant chemoradiation setting, HPV positivity predicted for improved locoregional control in pathologic node positive disease compared to HPV-negative patients. These results would require larger, prospective trials for validation; however, HPV remains intriguing as up to 50% of PSCC are HPV positive.7–9,78
Results from Pagliaro et al. 76 remain consistent with published literature despite being a single institution study with a relatively small number of study patients (N = 30). A large retrospective systemic review of neoadjuvant studies, published by Azizi et al. 79 suggested 50% of patients with bulky regional lymph node metastasis in PSCC achieve response to platinum-based NAC, with approximately 16% achieving pCR. In this systematic review, 10 studies met inclusion criteria and the pooled ORR was 53%. A stratified sub-analysis revealed an ORR of 55% and 49%, a pCR of 9% and 20%, a toxicity rate of 26% and 49%, and an overall mortality of 54% and 58% for non-taxane-platinum versus taxane-platinum regimens, respectively. Limitations of this study include 9/10 studies being retrospective with majority being single institution. Additional variables including heterogeneity between prior treatments, extent of disease, dosing of NAC, and follow-up time. 79
To gain further clarity in the neoadjuvant space, the International Penile Advanced Cancer Trial (InPACT: NCT02305654) was started in 2017 internationally, conducted in the United Kingdom, United States, Columbia, and Canada in 2017. This trial recruited patients with cTany/cN1-3/M0 PSCC randomized to either ILND alone, NAC followed by ILND, or neoadjuvant chemoradiation followed by ILND. Primary outcome measure is OS, with key secondary outcome measures including disease-free survival, toxicity, surgical complications, and quality of life. A secondary question this trial addresses is among patients whose inguinal node histology predicts HR of recurrence, does prophylactic PLND plus chemoradiation to the inguinal and pelvic fields improve survival compared with chemoradiation alone. 80 In further detail, the randomizations include InPACT-neoadjuvant and InPACT-pelvis. For InPACT-neoadjuvant, patients are stratified by disease burden including nodal status, radiographic features, and Glomerular Filtration Rate (GFR). Patients with high disease burden are randomized to NAC or neoadjuvant chemoradiotherapy. Patients with intermediate disease burden are randomized to either surgery alone, NAC, or neoadjuvant chemoradiotherapy. Low disease burden patients proceed to surgery alone. In patients whose postoperative findings are concerning for HR features, they proceed to InPACT-pelvis, where patients are randomized to adjuvant chemoradiotherapy with or without PLND (if no previous neoadjuvant chemoradiotherapy) and PLND or observation (in patients who previously received NAC). 81 Overall, the InPACT trial will provide much needed randomized, level 1 evidence, for sequencing therapy based on disease burden in patients with locally advanced PSCC, and should yield results in mid/late 2024–early 2025. This trial will also be a rich resource for biomarker and molecular testing.
Adjuvant therapy
The data for adjuvant chemotherapy remain limited currently as studies are mainly retrospective with heterogenous study size and regimens utilized. Per NCCN guidelines, adjuvant chemotherapy is recommended for patients with 2 or greater positive unilateral inguinal lymph nodes >4 cm (mobile) after lymph node dissection, or with extranodal extension and previously did not receive NAC (pN2, pN3). Given the limited prospective data, TIP chemotherapy over four cycles has been extrapolated from the neoadjuvant setting, other recommended regimen includes 5-FU plus cisplatin. 18 Retrospective data supporting adjuvant chemotherapy include Sharma et al. 82 in 36 patients with positive pelvic lymph nodes, of note eight patients received 5-FU + cisplatin and one patient received TIP, notable findings included significant improvement in OS (21.7 months versus 10.1 months) in responders versus non-responders, respectively. Additional retrospective data from Necchi et al. including over 170 patients, who received lymph node dissection and adjuvant chemotherapy, yielding significant improvement in OS in patients with pN3 (pelvic nodal) disease.
Adjuvant chemoradiotherapy per NCCN guidelines can be considered for palpable bulky inguinal lymph nodes, large pelvic lymph nodes and are considered category 2B recommendation for non-bulky inguinal lymph nodes pN2-3. There has been clinical benefit reported, however sparse and retrospective, including a study from Johnstone et al. 83 with 93 patients with N3 nodal disease revealing survival benefit in the extranodal extension negative patients who received postoperative chemotherapy and inguinopelvic radiation. Additional data on adjuvant chemoradiation will come from the second randomization of the InPACT trial (InPACT Pelvis). 80
Standard of care in relapsed/metastatic disease
Systemic therapy
Platinum-based chemotherapy has been the primary frontline approach in relapsed/metastatic PSCC. The rationale for platinum-based chemotherapy in the metastatic PSCC setting is based on multiple single arm, phase II trials.76,84–89 In a 40-patient trial combining cisplatin with bleomycin and methotrexate, a response rate of 32.5% was reported; however five treatment-related deaths were noted as well as six patients with one or more life-threatening toxic episodes. This trial illustrates the importance of unmanageable toxicities in the setting of incurable disease, thus placing emphasis on safety and quality of life. 84 The mainstay of treatment in the metastatic setting remains TIP per Pagliaro et al. 76 based on prospective neoadjuvant data for locally advanced disease. Another active combination includes cisplatin and 5-FU, with a retrospective analysis revealing an ORR of 32%, noting grade 3 or 4 neutropenia observed in 20% of patients. 85 . These regimens are supported by the NCCN guidelines, with TIP as the preferred regimen. 18 It is worth noting the phase II VinCaP trial, a single-arm study of non-platinum chemotherapy, vinflunine, in locally advanced/metastatic PSCC as the primary endpoint of clinical benefit rate of 40% was exceeded at 45.5%. Neutropenia was the most common adverse effect at 23%, other toxicity profile was in keeping for vinflunine. 90
Unfortunately, patients who have progressed through or recurred after frontline cisplatin chemotherapy have dismal prognosis, with an emphasis for clinical trial evaluation. There is no consensus regarding second-line salvage regimens. In a retrospective study of 30 patients with metastatic penile cancer after first-line chemotherapy, 17 patients received one or more salvage therapies with a median survival from first treatment failure of 5.7 months (range: 1.4–30.3 months). 91 In a single-arm, phase II study, multicenter, patients were treated with 175 mg/m2 paclitaxel at 3-week intervals, ORR was 20% and the primary endpoint with grades 3 and 4 neutropenia of 28%, notable for moderate anticancer activity along with being generally tolerable. 92
Given the overall prognosis and limited systemic data, therapies should be tailored to performance status, comorbidities, with a focus on quality of life. 53 Following platinum chemotherapy, a retrospective analysis revealed poor prognostic factors including visceral metastasis and anemia <10 g/dL associated with reduced OS. In these patients, analysis revealed 1-year OS at 6.7%. 93
Novel therapeutic and sequencing approaches
Targeted therapies including Tyrosine kinase inhibitors (TKI)
Given the limitations of platinum-based chemotherapy, efforts have been made to explore different targeted treatment modalities to personalize care and optimize outcomes. Multiple studies have established that PSCC highly expresses EGFR protein via IHC and harbors gene amplifications despite the difficulty of targeting activating EGFR mutations. 94 Necchi et al. 95 prospectively evaluated panitumumab (anti-EGFR) in patients with unresectable or metastatic PSCC after at least one line of chemotherapy, 11 patients were treated with a median OS of 9.5 months, and one case of grade 3 cutaneous toxicity and diarrhea each. Data from another second-line salvage trial incorporated anti-EGFR agent cetuximab, these patients were retrospectively reviewed receiving taxane therapy alone or in combination as well as cetuximab alone or in combination, there was an ORR of 27% with trend for improved response rate in the cetuximab including regimens compared to other agents (OR = 5.05, p = 0.077). 93 In a phase II study by Necchi et al. 95 , the HER/PTEN/Akt pathway was examined with the use of dacomitinib, a pan-HER TKI able to inhibit EGFR, HER2, and HER4 in a single-arm study of 28 chemo-naïve patients with cN2-3 or M1 disease. The ORR was reported at 32% for the entire group with 12-month OS of 55%, including a 12-month OS of 64% in the locally advanced group. 89 Of note, the ORR was notably reduced compared to TIP data from Pagliaro and colleagues which was 50%. 76 Despite the modest efficacy, grade 3 adverse effects were only seen in three patients (10.7%) and upon further analysis of studies translational results, patient selection plays a significant role as dacomitinib was found to be clinically beneficial in patients that have mutations in downstream effectors of HER receptors as well as with TERT mutations.64,89 Another study that reported out includes a phase I basket trial from National Cancer Institute combining anti-c-Met, VEGFR2, AXL, RET inhibitor, cabozantinib with combination immunotherapy. Three patients with advanced PSCC were enrolled with two patients having stable disease and one partial response. 96 Overall, it remains tough to make cross-trial comparisons and draw definitive conclusions based on these studies given their small sample size, patient heterogeneity, and retrospective nature. It appears anti-EGFR, and pan-HER TKI agents are tolerable treatments and can be options for patients who are not candidates for standard of care combination chemotherapy (i.e. TIP) or following platinum chemotherapy. Further prospective randomized controlled trials in this space are needed. Moreover, additional targetable pathways as discussed in previous section may represent emerging targets including inhibition of mTOR, NOTCH1, DDR, and ERBB2 pathways.53,41
HPV-directed therapies
Given the high prevalence of HPV DNA in PSCC (approximately 50%), research should also be focused on HPV-directed therapies. The pathology primarily relies on carcinogenesis of HPV proteins E6 and E7.26,27 In addition to the consideration for prophylactic vaccines, early vaccine studies have demonstrated correlation between induction of cytotoxic T-cell responses and clearance of HPV-associated precancerous lesions via immune response against proteins E6 and E7.97,98 To date, there is no prospective data on therapeutic vaccines in the setting of PSCC; however, there remains encouraging data and efficacy in both the prophylactic and therapeutic settings in other solid tumors, that is, cervical cancer.97,98 Therapeutic HPV vaccines are being testing in multiple ongoing/recruiting basket trials, that include penile cancer, including monotherapy vaccine versus in combination with immunotherapies including anti-PD-1, anti-PD-L1, and adoptive T-cell therapy (ACT). (Table 2).
Table 2.
HPV-directed basket trials including penile cancer.
| Study | Patient eligibility | HPV target | Other therapy | Number of patients | Primary endpoint | Study status |
|---|---|---|---|---|---|---|
| Phase I/II | ||||||
| NCT02379520 | Recurrent HPV+ disease/HPV+ disease ineligible for SOC treatment | HPV-16/18 E6/E7-specific T lymphocytes | Cytoxan, fludarabine, and nivolumab (anti-PD-1) | 32 | Incidence of DLT | Not recruiting |
| NCT02858310 | Recurrent/metastatic HPV+ disease | HPV-16 E7-targeting TCR T cells (E7 TCR) | Aldesleukin, fludarabine, cyclophosphamide | 180 | Phase II dosing | Recruiting |
| NCT04180215 | Relapsed/metastatic disease | HB-201 +/- HB-202 | None | 200 | Incidence of DLT and phase II dose | Recruiting |
| NCT04432597 | Recurrent/metastatic HPV+ disease | PRGN-2009 (HPV vaccine) | Anti-PD-L1/TGF-Beta Trap (M7824) | 76 | Phase II dose and safety | Recruiting |
| NCT03439085 | Recurrent/metastatic HPV+ disease | INO-3112 | Durvalumab | 77 | ORR | Recruiting |
DLT, dose-limiting toxicity; HPV, human papillomavirus; ORR, objective response rate; PD-L1, programmed death-ligand 1; SOC, standard of care.
Moreover, a promising combination, including binatrafusp alfa (transforming growth factor-beta) and PDL-1, was used in a phase I/II trial with HPV-positive cancers. This dual targeted therapy had an ORR of 30.5% including five complete responses, although no patients on trial had PSCC. 99
Immunotherapies (immune checkpoint blockade, ACT)
Immunotherapies including immune checkpoint blockade (ICB) and ACTs are novel treatment options, primarily being investigated in ongoing clinical trials. Much of the published data currently include small case series, including data from Hahn et al. 100 which reported three cases of PSCC receiving salvage Pembrolizumab as part of a phase II clinical trial for rare tumors. Of note, one of the patients who was MSI high (MSI-H) experienced a durable partial response, underwent consolidative surgery, and remained disease free 38.7 months later. 100 Another published case series from Chahoud et al. 101 revealed ongoing treatment response with Pembrolizumab for one patient with a complete response maintained for 38 months with TMB high status (TMB-H) of 14 (>10) and a second patient with partial response maintained for 18 months with a positive PD-L1 expression (combined positive score 130). In addition to significant responses from PD-1 and PD-L1 blockade, the consideration for combination ICB using PD-(L)1 and CTLA-4 inhibition has shown promise despite limited data, including a case report in a patient with metastatic penile cancer refractory to TIP, who had near resolution of large inguinal mass after two treatment cycles of nivolumab and ipilimumab. 102 An important ICB approval from a genomic standpoint was KEYNOTE 158, a multicohort phase II study of Pembrolizumab for advanced non-colorectal unresectable or metastatic cancers, that are MSI-H or mismatch repair-deficient (dMMR) tumors, and that have progressed following prior treatment without satisfactory alternative. Among the 233 enrolled patients, 27 tumor types were represented. At median follow-up 13.4 months, the ORR was 34.3% with median OS 23.5 months and 14.6% grades 3–5 adverse events. 103 Additional biomarker analysis was explored with TMB-H > 10 mutations per megabase, by Marabelle et al. 104 in 10 tumor-type-specific cohorts from KEYNOTE 158. Based on these data, the NCCN guidelines in penile cancer do endorse the use of Pembrolizumab under subsequent-line systemic therapy for metastatic/recurrent disease patients who are MSI-H, dMMR, or TMB-H.18,103,104 However, despite the biomarker work reviewed above, a strong consideration to study ICB in all patients with metastatic penile cancer is still warranted. As we know, many tumor types are currently managed with ICB independent of certain molecular characteristics (i.e. MSI-H, dMMR, or TMB-H). Furthermore, TMB remains heterogeneous across tumor types, and may not necessarily clearly predict responders versus non responders. For example in renal cell carcinoma, higher TMB does not appear to improve or predict benefit from ICB. 105
The majority of data for ICB and other novel therapies have been limited to the relapsed/refractory setting. It should be noted that sequencing alternative therapies earlier in the disease course remains critical as there is unmet need to optimize therapy and improve upon the standard of care in earlier stages of the disease. In other solid tumors, we have seen Food and Drug Administration (FDA) approvals for ICB in the neoadjuvant space including triple negative breast cancer, utilizing four cycles of NAC in combination with Pembrolizumab, per KEYNOTE 522. 106 In non-small-cell lung cancer (NSCLC), a recent FDA approval for NAC in combination with nivolumab for up to three cycles prior to surgery revealed a significantly improved event-free survival over 10 months, per CheckMate 816. In addition, in NSCLC, there are multiple phase III studies which have confirmed survival benefit with chemo-immunotherapy (ICB) in the frontline metastatic setting.107,108 At this time for PSCC, NCT04224740 is a phase II clinical trial combining pembrolizumab with cisplatin-based chemotherapy in the frontline metastatic setting. 109 Unique in its design, the clinical trial, NCT03774901, is evaluating avelumab in the frontline maintenance setting in patients with locally advanced or metastatic PSCC who have responded or have stable disease after frontline platinum polychemotherapy. 110 Although the trial failed to meet its primary endpoint of PFS, PERICLES (NCT03686332) recruited a cohort of patients with locally advanced PSCC and combined ICB with or without radiotherapy to locoregional lymph nodes. The concept of current multimodality therapy, with novel agents, will benefit from additional study given the potential synergy between radiation and immunotherapy. 111
In addition to sequencing chemo-immunotherapy (ICB) to earlier in the disease course to improve cancer control, this approach has added benefits. From a logistical standpoint, chemo-immunotherapy with ICB is an outpatient regimen for the community, commonly administered with familiar systemic agents. The current standard of care, TIP, requires inpatient chemotherapy that limits community/rural access, reducing access to care. In addition, significant expertise and experience is required to monitor triplet cytotoxic chemotherapy and handle treatment-related toxicities.
ACTs also have increased interest given its novel approach. These therapies utilize ex vivo expansion of TILs as well as engineering of T cells with targeted tumor antigens, that is, T-cell receptor (TCR) and chimeric antigen receptor therapy. A small pilot feasibility study by Aydin et al. 112 was the first to demonstrate expansion of TIL from penile cancer patients. In this study, tumor samples were collected from metastatic lymph nodes and TIL were expanded from 11 out of the 12 samples. TIL expansion and phenotype were independent of previous HPV infection and treatment with NAC and may represent clinical and therapeutic application. 112 In addition, the viability of engineering T cells, particularly with targeting HPV was published in a landmark study from Doran et al. 113 This study was a first-in-human, phase I/II trial in which patients with refractory disease from any site, received autologous genetically engineered T cells expressing a TCR directed against HPV16 E6 (E6 TCR T cells), a conditioning regimen, and systemic aldesleukin. There were no dose-limiting toxicities observed in phase I portion, and two patients experienced objective tumor responses. 113 In a similar trial design, Nagarsheth et al. 114 conducted first-in-human clinical trial targeting HPV-16 E7 in patients with metastatic HPV-associated epithelial cancers and documented an objective clinical response in 6 of 12 patients, including 4 of 8 patients with anti-PD-1 refractory disease. Finally, an innovative, currently active basket trial, NCT02379520, looks to combine TCR-based approach against HPV, E6, and E7, and is also evaluating a combination with nivolumab (ICB). 115
Conclusion
Overall, the treatment of penile cancer represents a complex landscape, particularly in developing nations as there is an unfulfilled need for high-level, multidisciplinary therapeutic options.
Currently, there are no validated screening guidelines for PSCC. This represents an opportunity for a screening process in the future, particularly for HR patients.
Preventative strategies to control modifiable risk factors remain critically important. As mentioned, smoking cessation, safe sex practices (including reducing risk of HPV), circumcision, improving local hygiene, limiting immunosuppression, and controlling obesity may lower the risk for developing PSCC.
Optimizing treatment strategies via basket trials as well as improving upon the current landscape (InPACT) represent practical approaches to help bridge the gap and improve clinical outcomes. Furthermore, including novel therapeutic options not only the metastatic refractory setting, however earlier in the disease course (NAC) is another important consideration to improve the clinical outcomes of our PSCC patients. Ongoing collaboration and clinical trial enrollment remain critical and will continue to transform standard of care.
Acknowledgments
None.
Footnotes
ORCID iD: Juskaran Chadha  https://orcid.org/0000-0003-0974-0147
https://orcid.org/0000-0003-0974-0147
Contributor Information
Juskaran Chadha, Department of Genitourinary Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Jad Chahoud, Department of Genitourinary Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Philippe E. Spiess, Department of Genitourinary Oncology, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive, Tampa, FL 33612, USA.
Declarations
Ethics approval and consent to participate: Not Applicable.
Consent for publication: Not Applicable.
Author contribution(s): Juskaran Chadha: Conceptualization; Writing – original draft; Writing – review & editing.
Jad Chahoud: Conceptualization; Writing – review & editing.
Philippe E Spiess: Conceptualization; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: JAC has been a consultant for Exelixis, Aveo, and Pfizer. PS is vice-chair of the NCCN bladder and penile cancer panel, present of the Global Society of Rare GU Tumors, and member of the EAU/ASCO penile cancer panel.
Availability of data and materials: Not Applicable.
References
- 1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA: Cancer J Clin 2022; 72: 7–33. [DOI] [PubMed] [Google Scholar]
- 2. Visser O, Adolfsson J, Rossi S, et al. Incidence and survival of rare urogenital cancers in Europe. Eur J Cancer [Internet] 2012; 48: 456–464. [DOI] [PubMed] [Google Scholar]
- 3. Cardona CEM, García-Perdomo HA. Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Pública [Internet] 2017; 41: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ornellas AA. GUEST EDITORIAL management of penile cancer. J Surg Oncol [Internet] 2008; 97: 199–200. [DOI] [PubMed] [Google Scholar]
- 5. Thomas A, Necchi A, Muneer A, et al. Penile cancer. Nat Rev Dis Primers 2021 [Internet]. 2021; 7: 1–24. [DOI] [PubMed] [Google Scholar]
- 6. Hernandez BY, Barnholtz-Sloan J, German RR, et al.Burden of invasive squamous cell carcinoma of the penis in the United States. 2008; www.interscience.wiley.com [DOI] [PMC free article] [PubMed]
- 7. Olesen TB, Sand FL, Rasmussen CL, et al. Prevalence of human papillomavirus DNA and p16 INK4a in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol [Internet] 2019; 20: 145–158. [DOI] [PubMed] [Google Scholar]
- 8. Alemany L, Cubilla A, Halec G, et al. Role of human papillomavirus in penile carcinomas worldwide. Eur Urol [Internet] 2016; 69: 953–961. [DOI] [PubMed] [Google Scholar]
- 9. Miralles-Guri C, Bruni L, Cubilla AL, et al. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol [Internet] 2009; 62: 870–878. [DOI] [PubMed] [Google Scholar]
- 10. Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer 2005; 116: 606–616. [DOI] [PubMed] [Google Scholar]
- 11. Barnes KT, McDowell BD, Button A, et al. Obesity is associated with increased risk of invasive penile cancer. BMC Urol [Internet] 2016; 16: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cubilla AL, Velazquez EF, Amin MB, et al. The world health organisation 2016 classification of penile carcinomas: a review and update from the international society of urological pathology expert-driven recommendations. Histopathology [Internet] 2018; 72: 893–904. [DOI] [PubMed] [Google Scholar]
- 13. Heyns CF, Mendoza-Valds A, Pompeo ACL. Diagnosis and staging of penile cancer. Urology 2010; 76: S15–S23. [DOI] [PubMed] [Google Scholar]
- 14. Johnson DE, Fuerst DE, Ayala AG. Carcinoma of the penis. Experience with 153 cases Urology [Internet] 1973; 1: 404–408. [DOI] [PubMed] [Google Scholar]
- 15. Kossow JH, Hotchkiss RS, Morales PA. Carcinoma of penis treated surgically. Analysis of 100 cases. Urology [Internet] 1973; 2: 169–172. [DOI] [PubMed] [Google Scholar]
- 16. Pettaway CA, et al. AJCC cancer staging manual. 8th ed. Springer, 2017, pp. 701–715. [Google Scholar]
- 17. Ficarra V, Akduman B, Bouchot O, et al. Prognostic factors in penile cancer. Urology [Internet] 2010; 76: S66–S73. [DOI] [PubMed] [Google Scholar]
- 18. NCCN guidelines. https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf. 2022. (accessed 16 March 2022)
- 19. Hakenberg OW, Compérat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015; 67: 142–50. [DOI] [PubMed] [Google Scholar]
- 20. Graafland NM, Leijte JAP, Valdés Olmos RA, et al. Scanning with 18F-FDG-PET/CT for detection of pelvic nodal involvement in inguinal node-positive penile carcinoma. Eur Urol [Internet] 2009; 56: 339–345. [DOI] [PubMed] [Google Scholar]
- 21. Graafland NM, Teertstra HJ, Besnard APE, et al. Identification of high risk pathological node positive penile carcinoma: value of preoperative computerized tomography imaging. J Urol [Internet] 2011; 185: 881–887. [DOI] [PubMed] [Google Scholar]
- 22. Diorio GJ, Giuliano AR. The role of human papilloma virus in penile carcinogenesis and Preneoplastic lesions: a potential target for vaccination and treatment strategies. Urol Clin North Am 2016; 43: 419–425. [DOI] [PubMed] [Google Scholar]
- 23. Hoekstra RJ, Trip EJ, Jw Ten, Kate F, et al. Penile intraepithelial neoplasia: nomenclature, incidence and progression to malignancy in the Netherlands. Int J Urol 2018; 26: 353–357. [DOI] [PubMed] [Google Scholar]
- 24. Mucci LA, Hjelmborg JB, Harris JR, et al. Familial risk and heritability of cancer among twins in Nordic Countries. JAMA [Internet] 2016; 315: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chahoud J, Pickering CR, Pettaway CA. Genetics and penile cancer: recent developments and implications. Curr Opin Urol [Internet] 2019; 29: 364–370. [DOI] [PubMed] [Google Scholar]
- 26. Emmanuel A, Nettleton J, Watkin N, et al. The molecular pathogenesis of penile carcinoma-current developments and understanding. Virchows Archiv 2019; 475: 397–405. [DOI] [PubMed] [Google Scholar]
- 27. Yugawa T, Kiyono T. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Rev Med Virol [Internet] 2009; 19: 97–113. [DOI] [PubMed] [Google Scholar]
- 28. Steinestel J, al Ghazal A, Arndt A, et al. The role of histologic subtype, p16(INK4a) expression, and presence of human papillomavirus DNA in penile squamous cell carcinoma. BMC Cancer [Internet] 2015; 15: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cubilla AL, Lloveras B, Alejo M, et al. Value of p16INK4a in the pathology of invasive penile Squamous cell carcinomas: a report of 202 cases. Am J Surg Pathol [Internet] 2011; 35: 253–261. [DOI] [PubMed] [Google Scholar]
- 30. Sand FL, Rasmussen CL, Frederiksen MH, et al. Prognostic significance of HPV and p16 status in men diagnosed with penile cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev [Internet] 2018; 27: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 31. de Andrade Martins V, Pinho JD, Júnior AALT, et al. P16INK4a expression in patients with penile cancer. PLOS ONE [Internet] 2018; 13: e0205350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gunia S, Erbersdobler A, Hakenberg OW, et al. p16INK4a is a marker of good prognosis for primary invasive penile squamous cell carcinoma: a multi-institutional study. J Urol [Internet] 2012; 187: 899–907. [DOI] [PubMed] [Google Scholar]
- 33. Ferrándiz-Pulido C, Masferrer E, de Torres I, et al. Identification and genotyping of human papillomavirus in a Spanish cohort of penile squamous cell carcinomas: correlation with pathologic subtypes, p16INK4a expression, and prognosis. J Am Acad Dermatol [Internet]. 2013; 68: 73–82. [DOI] [PubMed] [Google Scholar]
- 34. Dillner J, Krogh G, von Horenblas S, et al. Etiology of squamous cell carcinoma of the penis. Scand J Urol Nephrol 2000; 34: 189–193. [DOI] [PubMed] [Google Scholar]
- 35. Douglawi A, Masterson TA. Penile cancer epidemiology and risk factors: A contemporary review. Curr Opin Urol [Internet] 2019; 29: 145–149. [DOI] [PubMed] [Google Scholar]
- 36. de Paula AAP, Motta ED, Alencar RDC, et al. The impact of cyclooxygenase-2 and vascular endothelial growth factor C immunoexpression on the prognosis of penile carcinoma. J Urol 2012; 187: 134–140. [DOI] [PubMed] [Google Scholar]
- 37. Greenhough A, Smartt HJM, Moore AE, et al. The COX-2/PGE 2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis [Internet] 2009; 30: 377–386. [DOI] [PubMed] [Google Scholar]
- 38. Aydin AM, Chahoud J, Adashek JJ, et al. Understanding genomics and the immune environment of penile cancer to improve therapy. Nat Rev Urol [Internet] 2020; 17: 555–570. [DOI] [PubMed] [Google Scholar]
- 39. McDaniel AS, Hovelson DH, Cani AK, et al. Genomic profiling of penile squamous cell carcinoma reveals new opportunities for targeted therapy. Cancer Res [Internet] 2015; 75: 5219–5227. [DOI] [PubMed] [Google Scholar]
- 40. Marchi FA, Martins DC, Barros-Filho MC, et al. Multidimensional integrative analysis uncovers driver candidates and biomarkers in penile carcinoma. Sci Rep [Internet]. 2017; 7(1): 6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jacob JM, Ferry EK, Gay LM, et al. Comparative genomic profiling of refractory and metastatic penile and nonpenile cutaneous squamous cell carcinoma: implications for selection of systemic therapy. J Urol 2019; 201: 541–548. [DOI] [PubMed] [Google Scholar]
- 42. Onywera H, Williamson AL, Cozzuto L, et al. The penile microbiota of Black South African men: relationship with human papillomavirus and HIV infection. BMC Microbiol [Internet] 2020; 20 (1): 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lohneis P, Boral S, Kaufmann AM, et al. Human papilloma virus status of penile squamous cell carcinoma is associated with differences in tumour-infiltrating T lymphocytes. Virchows Archiv 2015; 466: 323–331. [DOI] [PubMed] [Google Scholar]
- 44. Roszik J, Rödel F, Lorenzo G di, et al. The prognostic value of immune factors in the tumor microenvironment of penile squamous cell carcinoma. Front Immunol 2018; 9: 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vassallo J, Rodrigues AFF, Campos AHJFM, et al. Pathologic and 21 mmunohistochemical characterization of tumoral inflammatory cell infiltrate in invasive penile squamous cell carcinomas: fox-P3 expression is an independent predictor of recurrence. Tumour Biol [Internet] 2015; 36: 2509–2516. [DOI] [PubMed] [Google Scholar]
- 46. de Bacco MW, Carvalhal GF, MacGregor B, et al. PD-L1 and p16 Expression in penile squamous cell carcinoma from an endemic region. Clin Genitourin Cancer [Internet] 2020; 18: e254–e259. [DOI] [PubMed] [Google Scholar]
- 47. Udager AM, Liu TY, Skala SL, et al. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: potential opportunities for immunotherapeutic approaches. Ann Oncol [Internet] 2016; 27: 1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Campbell JD, Yau C, Bowlby R, et al. Genomic, pathway network, and immunologic features distinguishing squamous carcinomas. Cell Rep [Internet] 2018; 23: 194–212.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hanna GJ, Kacew A, Chau NG, et al. Improved outcomes in PI3K-pathway-altered metastatic HPV oropharyngeal cancer. JCI Insight [Internet] 2018; 3(17): e122799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chahoud J, Gleber-Netto FO, McCormick BZ, et al. Whole-exome sequencing in penile squamous cell carcinoma uncovers novel prognostic categorization and drug targets similar to head and neck squamous cell carcinoma. Clin Cancer Res [Internet]; 27: 2560–2570. [DOI] [PubMed] [Google Scholar]
- 51. Busso-Lopes AF, Marchi FA, Kuasne H, et al. Genomic profiling of human penile carcinoma predicts worse prognosis and survival. Cancer Prev Res [Internet] 2015; 8: 149–156. [DOI] [PubMed] [Google Scholar]
- 52. La-Touche S, Lemetre C, Lambros M, et al. DNA copy number aberrations, and human papillomavirus status in penile carcinoma. Clinico-pathological correlations and potential driver genes. PLoS ONE [Internet] 2016; 11: e0146740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chahoud J, Tamil M, Necchi A. Second line salvage systemic therapy for advanced penile cancer. Urol Oncol: Semin Orig Investig 2020; 40: 229–234. [DOI] [PubMed] [Google Scholar]
- 54. Sambandam V, Frederick MJ, Shen L, et al. PDK1 Mediates NOTCH1-mutated head and neck squamous cell carcinoma vulnerability to therapeutic PI3K/mTOR inhibition. Clin Cancer Res 2019; 25: 3329–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gu W, Zhu Y, Ye D. Beyond chemotherapy for advanced disease–the role of EGFR and PD-1 inhibitors. Transl Androl Urol [Internet] 2017; 6: 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stankiewicz E, Prowse DM, Ng M, et al. Alternative HER/PTEN/Akt pathway activation in HPV positive and negative penile carcinomas. PloS One [Internet] 2011; 6(3): e17517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brown JS, O’Carrigan B, Jackson SP, et al. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov [Internet] 2017; 7: 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Romero D. DDR signature to predict response to ICI. Nat Rev Clin Oncol [Internet] 2018; 15: 346. [DOI] [PubMed] [Google Scholar]
- 59. Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol [Internet] 2018; 36: 1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Manjunath A, Brenton T, Wylie S, et al. Topical therapy for non-invasive penile cancer (Tis)-updated results and toxicity. Transl Androl Urol 2017; 6: 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tang DH, Yan S, Ottenhof SR, et al. Laser ablation as monotherapy for penile squamous cell carcinoma: a multi-center cohort analysis. Urol Oncol: Semin Orig Investig [Internet] 2018; 36: 147–152. [DOI] [PubMed] [Google Scholar]
- 62. Wilkstrom A, Hedblad MA, Johansson B, et al. The acetic acid test in evaluation of subclinical genital papillomavirus infection: a comparative study on penoscopy, histopathology, virology and scanning electron microscopy findings. Genitourin Med [Internet] 1992; 68: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schlenker B, Tilki D, Seitz M, et al. Organ-preserving neodymium-yttrium-aluminium-garnet laser therapy for penile carcinoma: a long-term follow-up. BJU Int [Internet] 2010; 106: 786–790. [DOI] [PubMed] [Google Scholar]
- 64. Spiess PE, Necchi A. (Eds). Penile carcinoma. Springer, Switzerland, 2021. https://link.springer.com/10.1007/978-3-030-82060-2 [Google Scholar]
- 65. Lont AP, Gallee MPW, Meinhardt W, et al. Penis conserving treatment for T1 and T2 penile carcinoma: clinical implications of a local recurrence. J Urol 2006; 176: 575–580. [DOI] [PubMed] [Google Scholar]
- 66. Roussel E, Peeters E, Vanthoor J, et al. Predictors of local recurrence and its impact on survival after glansectomy for penile cancer: time to challenge the dogma? BJU Int [Internet] 2021; 127: 606–613. [DOI] [PubMed] [Google Scholar]
- 67. Albersen M, Parnham A, Joniau S, et al. Predictive factors for local recurrence after glansectomy and neoglans reconstruction for penile squamous cell carcinoma. Urol Oncol 2018; 36: 141–146. [DOI] [PubMed] [Google Scholar]
- 68. van Poppel H, Watkin NA, Osanto S, et al. Penile cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol [Internet] 2013; 24: vi115–vi124. [DOI] [PubMed] [Google Scholar]
- 69. Hasan S, Francis A, Hagenauer A, et al. The role of brachytherapy in organ preservation for penile cancer: a meta-analysis and review of the literature. Brachytherapy 2015; 14: 517–524. [DOI] [PubMed] [Google Scholar]
- 70. Pietrzak P, Corbishley C, Watkin N. Organ-sparing surgery for invasive penile cancer: early follow-up data. BJU Int [Internet] 2004; 94: 1253–1257. [DOI] [PubMed] [Google Scholar]
- 71. Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int [Internet] 2005; 96: 1040–1043. [DOI] [PubMed] [Google Scholar]
- 72. Kamel MH, Tao J, Su J, et al. Survival outcomes of organ sparing surgery, partial penectomy, and total penectomy in pathological T1/T2 penile cancer: report from the national cancer data base. Urol Oncol 2018; 36: 82.e7–82.e15. [DOI] [PubMed] [Google Scholar]
- 73. Djajadiningrat RS, Graafland NM, van Werkhoven E, et al. Contemporary management of regional nodes in penile cancer-improvement of survival? J Urol [Internet] 2014; 191: 68–73. [DOI] [PubMed] [Google Scholar]
- 74. Bandini M, Pederzoli F, Necchi A. Neoadjuvant chemotherapy for lymph node-positive penile cancer: current evidence and knowledge. Curr Opin Urol [Internet] 2020; 30: 218–222. [DOI] [PubMed] [Google Scholar]
- 75. Chahoud J, Kohli M, Spiess PE. Management of advanced penile cancer. Mayo Clin Proc [Internet] 2021; 96: 720–732. [DOI] [PubMed] [Google Scholar]
- 76. Pagliaro LC, Williams DL, Daliani D, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: A phase II study. J Clin Oncol 2010; 28: 3851–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Salazar A, Júnior EP, Salles PGO, et al. 18 F-FDG PET/CT as a prognostic factor in penile cancer. Eur J Nucl Med Mol Imaging [Internet] 2019; 46: 855–863. [DOI] [PubMed] [Google Scholar]
- 78. Yuan Z, Naghavi AO, Tang D, et al. The relationship between HPV status and chemoradiotherapy in the locoregional control of penile cancer. World J Urol [Internet] 2018; 36: 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Azizi M, Aydin AM, Hajiran A, et al. Systematic review and meta-analysis–is there a benefit in using neoadjuvant systemic chemotherapy for locally advanced penile squamous cell carcinoma? J Urol [Internet] 2020; 203: 1147–1155. [DOI] [PubMed] [Google Scholar]
- 80. Canter DJ, Nicholson S, Watkin N, et al. The international penile advanced cancer trial (InPACT): rationale and current status. Eur Urol Focus 2019; 5: 706–709. [DOI] [PubMed] [Google Scholar]
- 81. Pettaway CA, Nicholson S, Spiess PE, et al. The international penile advanced cancer trial (InPACT): the first phase III trial for squamous carcinoma of the penis with regional lymph node metastases. J Clin Oncol 2022; 40: TPS7. [Google Scholar]
- 82. Sharma P, Djajadiningrat R, Zargar-Shoshtari K, et al. Adjuvant chemotherapy is associated with improved overall survival in pelvic node–positive penile cancer after lymph node dissection: a multi-institutional study. Urol Oncol 2015; 33: 496.e17–496.e23. [DOI] [PubMed] [Google Scholar]
- 83. Johnstone PAS, Boulware D, Djajadiningrat R, et al. Primary penile cancer: the role of adjuvant radiation therapy in the management of extranodal extension in lymph nodes. Eur Urol Focus [Internet] 2019; 5: 737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Haas GP, Blumenstein BA, Gagliano RG, et al. Cisplatin, methotrexate and bleomycin for the treatment of carcinoma of the penis: a southwest oncology group study. J Urol 1999; 161: 1823–1825. [PubMed] [Google Scholar]
- 85. di Lorenzo G, Buonerba C, Federico P, et al. Cisplatin and 5-fluorouracil in inoperable, stage IV squamous cell carcinoma of the penis. BJU Int [Internet] 2012; 110: E661–E666. [DOI] [PubMed] [Google Scholar]
- 86. Zhang S, Zhu Y, Ye D. Phase II study of docetaxel, cisplatin, and fluorouracil in patients with distantly metastatic penile cancer as first-line chemotherapy. Oncotarget [Internet] 2015; 6: 32212–32219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Theodore C, Skoneczna I, Bodrogi I, et al. A phase II multicentre study of irinotecan (CPT 11) in combination with cisplatin (CDDP) in metastatic or locally advanced penile carcinoma (EORTC PROTOCOL 30992). Ann Oncol [Internet] 2008; 19: 1304–1307. [DOI] [PubMed] [Google Scholar]
- 88. Bermejo C, Busby JE, Spiess PE, et al. Neoadjuvant chemotherapy followed by aggressive surgical consolidation for metastatic penile squamous cell carcinoma. J Urol 2007; 177: 1335–1338. [DOI] [PubMed] [Google Scholar]
- 89. Necchi A, lo Vullo S, Perrone F, et al. First-line therapy with dacomitinib, an orally available pan-HER tyrosine kinase inhibitor, for locally advanced or metastatic penile squamous cell carcinoma: results of an open-label, single-arm, single-centre, phase 2 study. BJU Int [Internet] 2018; 121: 348–356. [DOI] [PubMed] [Google Scholar]
- 90. Pickering LM, Tovey H, Elliott T, et al. VinCaP: a phase II trial of vinflunine chemotherapy in locally-advanced and metastatic carcinoma of the penis (CRUK/12/021). J Clin Oncol 2018; 36 :4514–4514. [Google Scholar]
- 91. Wang J, Pettaway CA, Pagliaro LC. Treatment for metastatic penile cancer after first-line chemotherapy failure: analysis of response and survival outcomes. Urol [Internet] 2015; 85: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 92. di Lorenzo G, Federico P, Buonerba C, et al. Paclitaxel in pretreated metastatic penile cancer: final results of a phase 2 study. Eur Urol 2011; 60: 1280–1284. [DOI] [PubMed] [Google Scholar]
- 93. Giaccone G, Lu J, Kok VC, et al. Prognostic and predictive factors in patients with advanced penile cancer receiving salvage (2nd or Later Line) systemic treatment: a retrospective, multi-center study. Front Pharmacol [Internet] 2016; 7: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chahoud J, Pham R, Sonpavde G. Innovative systemic therapies for penile cancer. Curr Opin Urol [Internet] 2022; 32: 8–16. [DOI] [PubMed] [Google Scholar]
- 95. Necchi A, Giannatempo P, lo Vullo S, et al. Panitumumab treatment for advanced penile squamous cell carcinoma when surgery and chemotherapy have failed. Clini Genitourin Cancer [Internet] 2016; 14: 231–236. [DOI] [PubMed] [Google Scholar]
- 96. Apolo AB, Nadal R, Girardi DM, et al. Phase I study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J Clin Oncol [Internet] 2020; 38: 3672–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Morrow MP, Yan J, Sardesai NY. Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev Vaccines [Internet] 2013; 12: 271–283. [DOI] [PubMed] [Google Scholar]
- 98. Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015; 386: 2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Strauss J, Gatti-Mays ME, Cho BC, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. J Immunother Cancer [Internet] 2020; 8(2): e001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hahn AW, Chahoud J, Campbell MT, et al. Pembrolizumab for advanced penile cancer: a case series from a phase II basket trial. Invest New Drugs [Internet] 2021; 39: 1405–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chahoud J, Skelton WP, Spiess PE, et al. Case report: two cases of chemotherapy refractory metastatic penile squamous cell carcinoma with extreme durable response to pembrolizumab. Front Oncol [Internet] 2020; 10: 615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Baweja A, Mar N. Metastatic penile squamous cell carcinoma with dramatic response to combined checkpoint blockade with ipilimumab and nivolumab. J Oncol Pharm Pract [Internet] 2021; 27: 212–215. [DOI] [PubMed] [Google Scholar]
- 103. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol [Internet] 2020; 38: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020; 21: 1353–1365. [DOI] [PubMed] [Google Scholar]
- 105. Yakirevich E, Patel NR. Tumor mutational burden and immune signatures interplay in renal cell carcinoma. Ann Trans Med [Internet] 2020; 8: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020; 382: 810–821. [DOI] [PubMed] [Google Scholar]
- 107. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med [Internet] 2018; 378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 108. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med 2018; 379: 2040–2051. [DOI] [PubMed] [Google Scholar]
- 109. Pembrolizumab combined with cisplatin-based chemotherapy as first-line systemic therapy in advanced penile cancer. ClinicalTrials.gov [Internet]. https://clinicaltrials.gov/ct2/show/NCT04224740 (accessed 17 March 2022)
- 110. Gassian N, Frontczak A, Mouillet G, et al. Activity and tolerability of maintenance avelumab immunotherapy after first line polychemotherapy including platinum in patients with locally advanced or metastatic squamous cell penile carcinoma: PULSE. Bull Cancer 2020; 107: eS16–eS21. [DOI] [PubMed] [Google Scholar]
- 111. Penile cancer radio- and immunotherapy clinical exploration study. ClinicalTrials.gov [Internet]. https://clinicaltrials.gov/ct2/show/NCT03686332 (accessed 17 March 2022)
- 112. Aydin AM, Hall M, Bunch BL, et al. Expansion of tumor-infiltrating lymphocytes (TIL) from penile cancer patients. Int Immunopharmacol 2021; 94: 107481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Doran SL, Stevanovic´ S, Adhikary S, et al. T-cell receptor gene therapy for human papillomavirus–associated epithelial cancers: a first-in-human, phase I/II study. J Clin Oncol [Internet] 2019; 37: 2759–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nagarsheth NB, Norberg SM, Sinkoe AL, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med [Internet] 2021; 27: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. HPV-16/18 E6/E7-specific T lymphocytes, relapsed HPV-associated cancers, HESTIA. ClinicalTrials.gov [Internet]. https://clinicaltrials.gov/ct2/show/NCT02379520 (accessed 17 March 2022)