Abstract
Introduction
Health policy leaders recommend screening and referral (S&R) for unmet social needs (eg, food) in clinical settings, and the American Heart Association recently concluded that the most significant opportunities for reducing cardiovascular disease (CVD) death and disability lie with addressing the social determinants of CVD outcomes. A limited but promising evidence base supports these recommendations, but more rigorous research is needed to guide health care-based S&R efforts. Funded by the Veteran Health Administration (VA), the study described in this paper will assess the efficacy of S&R on Veterans’ connections to new resources to address social needs, reduction of unmet needs and health-related outcomes (adherence, utilisation and clinical outcomes).
Methods and analysis
We will conduct a 1-year mixed-methods randomised controlled trial at three VA sites, enrolling Veterans with CVD and CVD-risk. 880 Veterans experiencing one or more social needs will be randomised within each site (n=293 per site) to one of three study arms representing referral mechanisms of varying intensity (screening only, screening and provision of resource sheet(s), screening and provision of resource sheet(s) plus social work assistance). For each Veteran, we will examine associations of unmet social needs with health-related outcomes at baseline, and longitudinally compare the impact of each approach on connection to new resources (primary outcome) and follow-up outcomes over a 12-month period. We will additionally conduct qualitative interviews with key stakeholders, including Veterans to identify potential explanatory factors related to the relative success of the interventions.
Ethics and dissemination
Ethics approval was obtained from the VA Central Internal Review Board on 13 July 2021 (reference #: 20-07-Amendment No. 02). Findings will be disseminated through reports, lay summaries, policy briefs, academic publications, and conference presentations.
Trial registration number
Keywords: health policy, organisation of health services, adult cardiology
Strengths and limitations of this study.
Prior studies have examined cross-sectionally how addressing unmet needs is associated with health outcomes, but we will examine these associations longitudinally, which will allow a better assessment of causality and possible mechanisms for associations.
We will conduct this study within the largest integrated health system in the USA—the Department of Veterans Affairs—which will provide an opportunity for widespread dissemination within this health system.
Often randomised controlled trials end data collection with their outcomes data but for this study, we enhance our findings to understand facilitators, barriers and potential explanatory factors related to the relative success of the interventions.
Introduction
Social determinants of health (SDH) are ‘the structural determinants and conditions in which people are born, grow, live, work and age’.1 These conditions shape the degree to which basic needs are met both at the individual-level (eg, housing, food and social connections) and the community-level (eg, safe neighbourhoods). They also shape health trajectories as recent estimates suggest that clinical care accounts for less than 20% of modifiable health outcomes, whereas other factors, including SDH, are more significant drivers of morbidity and mortality.2 3 Consequently, there is consensus that improving population health will require healthcare delivery systems, including the Veterans Health Administration (VA), to address unmet social and economic needs (hereafter, unmet needs), rather than addressing disease from only a biomedical perspective.
The relationship between unmet needs and health is strikingly evident for patients with or at risk for cardiovascular disease (CVD),4 5 the leading cause of morbidity and mortality in the USA.6 For example, lower socioeconomic status is associated with greater prevalence of CVD risk factors and higher mortality from CVD;7–9 the risk for myocardial infarction is highest in the first year of unemployment and increases with the number of job losses10 and lack of social support is associated with increased CVD mortality.11 Thus, the American Heart Association recently declared that, ‘at present, the most significant opportunities for reducing death and disability from CVD in the US lie with addressing the social determinants of cardiovascular outcomes’.4
The American Heart Association’s recommendations, as well as similar recommendations from other leading health policy groups,1 12 13 rest on limited, yet promising, evidence that implementing systematic screening and referral (S&R) for unmet needs leads to greater receipt of resources that address identified needs14 15 as well as reduction in unmet needs.16 Such a process can potentially improve both proximal outcomes, such as adherence to medications and care appointments,17 as well as more distal outcomes, such as overall health.5 18–20 However, much of the limited evidence on programmes to address unmet needs is based in paediatric or specialised settings (eg, women’s health clinics), or on interventions targeting a single need, such as food insecurity.21 Importantly, as far as we know, there are no randomised controlled trials (RCT) demonstrating the impact of systematic S&R for unmet needs on patients’ connection to resources or other utilisation and health outcomes in the general adult ambulatory care setting nor among a Veteran population. In short, there is no definitive guidance on how best to screen for and address unmet needs in clinical settings, creating a key barrier to implementing this practice in healthcare delivery systems.22
The criteria prioritising access to VA services to those with financial need, in addition to those with service-related health conditions, result in many Veterans using VA healthcare services having low incomes, poor quality of life and multiple comorbidities.23 24 For these reasons, Veterans are at especially high risk of experiencing unmet social needs. For example, up to 24% have been reported to experience food insecurity.25 Given the simultaneously high prevalence of CVD and its risk factors and unmet needs among Veterans enrolled in the VA, Veterans’ outcomes may be improved by comprehensively assessing and addressing unmet needs.
Currently, the VA administers system-wide clinical screens for two unmet needs (housing and food insecurity), yet other unmet needs are not routinely identified. While VA invests in social work (SW) to address a wide range of unmet needs, referral to and staffing of SW is highly variable across and within facilities. Many Veterans who could benefit from VA SW are not systematically identified and referred. Furthermore, there are no comprehensive data on Veterans’ unmet needs, nor on their association with utilisation and clinical outcomes (blood pressure and A1c control), hampering VA’s ability to understand the effects of unmet needs and to target resources to address them. Finally, it is not known whether a SW is required to address all unmet needs; it is plausible that a less resource-intense or personnel-intense process can address identified unmet needs and improve outcomes, as suggested by a recent paediatric S&R intervention.18
Funded by the VA’s Health Services and Research Development division, the aim of this study is to assess the efficacy of comprehensive S&R among Veterans with or at-risk for CVD. Our study objectives are threefold: (1) to describe the prevalence and distribution of unmet needs and identify their associations with baseline sociodemographic characteristics, adherence, utilisation and clinical outcomes; (2) to compare the efficacy of three S&R strategies of increasing intensity on connection to new resources to address unmet needs (primary outcome) and on secondary outcomes of postintervention change in unmet needs, adherence, utilisation and clinical outcomes and (3) to identify barriers and facilitators to Veterans’ connecting with resources to address unmet needs and getting needs met, and explore potential explanatory factors related to the relative success of each study arm.
Methods
Overview
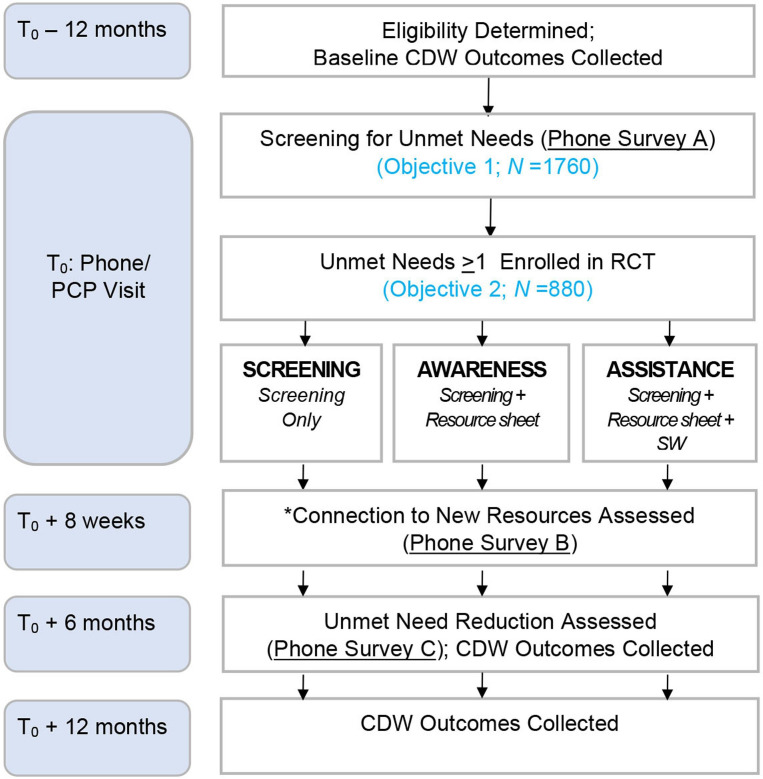
We are conducting a mixed-method RCT (see figure 1). The RCT protocol adheres to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) (see additional file). The study will take place at three VA medical facilities between February 2021 and January 2024. For objective 1, we will use baseline trial data gathered via a survey of Veterans at the study sites about their unmet needs and conduct quantitative analyses of survey, administrative and clinical data to characterise the prevalence of unmet needs and their association with baseline outcomes (adherence, utilisation and clinical). For objective 2, Veterans who screened positive for one or more unmet needs in the survey will be randomised within each site to one of three trial study arms defined by referral approaches of varying intensity. Quantitative analyses will longitudinally compare the effects of the referral approaches on the primary outcome (connections to new resources) and secondary outcome (reduction in unmet needs, adherence, utilisation and health outcomes). Often RCTs end data collection with their outcomes data. For this study, we enhance our findings to qualitatively understand more about the facilitators, barriers and potential explanatory factors related to the relative success of the interventions. Therefore, for objective 3, we will conduct qualitative interviews with a purposeful sample of key stakeholders who participated in the trial, including Veterans. We first describe the methods for the RCT (objectives 1 and 2) followed by the methods for the qualitative inquiry (objective 3).
Figure 1.
On a weekly basis for the 12-month trial, we will identify potentially eligible Veterans with upcoming primary care appointments. Trained Research Assistants (RA) will contact Veterans to explain the research protocol, review the elements of informed consent, and secure verbal consent. During this phone call, if verbal informed consent is obtained, the RA will screen for unmet needs (hereafter, ‘index screen’). If a Veteran reports no unmet needs, their study participation will be restricted to objective 1. If a Veteran reports one or more unmet needs, the RA will randomise them to one of the three trial arms. Trial participants will be re-surveyed 8 weeks after the index screen to assess resource connection and 6 months after the index screen to assess unmet need reduction. CDW, Corporate Data Warehouse; RCT, randomised controlled trials.
Evaluation frameworks and theory of change
The study is guided by the Outcomes from Addressing SDH in Systems (OASIS) framework (see figure 2).26 This framework, developed by the study team is based on Maslow’s Hierarchy of Needs model, which specifies that basic physiological needs (eg, food and shelter) must be met before higher order needs (eg, medication adherence) can be addressed.27 Following OASIS, our theory of change is therefore that S&R will result in more patients connecting to resources to address those needs and that connection to resources will then have multiple downstream effects, including reduced needs and enhanced adherence to medical treatments and care. In turn, better adherence will lead to improved outcomes.
Figure 2.
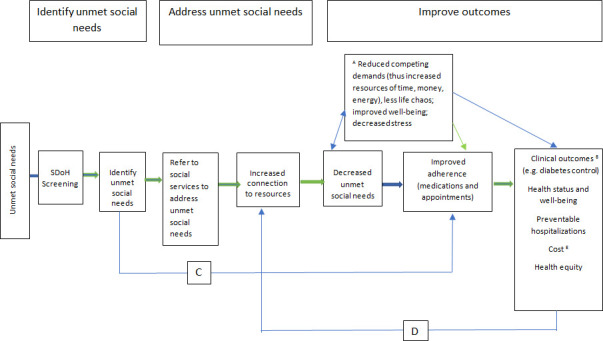
Green links are supported by data; blue links need further investigation. AFor patients with multiple unmet social needs, resolution of one need may enable them to address another. Reduced competing demands include freeing up various resources (money, time and energy) to address other needs, which in turn can affect health outcomes. BClinical outcomes may include but are not limited to conditions where adherence to therapy directly impacts outcomes, such as hypertension, diabetes and asthma. CIdentification of unmet social needs may be beneficial, even without referring to resources. For patients with transportation problems, for example, delivering prescriptions through mail order can bypass the barrier posed by the unmet transportation need without directly addressing it. DImproved outcomes, such as improved well-being, may help patients connect to resources. ECosts may be reduced through improved control of chronic conditions, such as hypertension, which could avert costly future admissions for stroke or target organ damage. But increased costs to address unmet social needs may affect the equation for other conditions.
The qualitative inquiry is additionally guided by Anderson’s model of service utilisation.28 The model posits that a Veteran’s connection to resources is determined by three interacting factors: predisposing factors (eg, belief that available resources can meet their need); enabling factors (eg, accessibility of identified resources) and need (eg, level of perceived unmet needs). We will explore the degree to which these factors help to explain why some participants do or do not connect with resources to address unmet needs.
Randomised controlled trial (objectives 1 and 2)
Study setting and participant eligibility
For objective 1, the study population comprised Veterans with, or at risk for, CVD seen in primary care (PC) clinics of three urban VA medical centres. Veterans must have at least one PC visit in the year prior to the RCT start date to ensure that included study subjects are at least minimally engaged in VA care. Veterans who have impaired decision-making and/or are illiterate or have limited of no English proficiency are excluded from the study. Using data from VA’s Corporate Data Warehouse (CDW), we will identify CVD patients as those with International Classification of Disease 10 diagnoses for coronary artery disease, cerebrovascular disease or peripheral artery disease, and patients with CVD risk as having diagnoses of hypertension, diabetes mellitus (DM) or hyperlipidaemia. For objective 2, the study population comprised the subset of objective 1 participants who have one or more unmet needs.
Study procedures and randomisation
On a weekly basis for the 12-month trial, we will identify potentially eligible Veterans with upcoming PC appointments who will be mailed a recruitment package. The recruitment package will include a description of the study and elements of informed consent (see Appendix A), as well as an opt-out postcard. Trained Research Assistants (RA) at each study site will contact Veterans (who have not opted-out) via telephone to explain the research protocol, review the elements of informed consent, secure verbal consent and enrol the Veteran.
During this phone call, among enrolled Veterans, the RA will administer a brief survey to screen for nine unmet needs (housing, food insecurity, utility insecurity, transportation, legal needs, employment, safety, stress and social isolation), hereafter referred to as the ‘index screen’. As part of the brief survey, each unmet need measure, if endorsed, is followed by a question about whether the Veteran is already receiving assistance for the need. To inform selection of the nine unmet needs, we used similar criteria to what other leading healthcare groups have used: (1) strength of the evidence linking the domain with CVD outcomes, (2) availability of a valid measure of the domain, (3) stakeholder priorities (input from VA providers, operational partners and a Veteran consultant from the VA Veteran Engagement Resource Group) and (4) ability to meet the need with available resources in VA and/or community.22 29 30 This process yielded the final set of nine unmet needs. The unmet need measures themselves were then reproduced or adapted from previously validated measures.
If a Veteran reports no unmet needs, their study participation will be restricted to objective 1. If a Veteran reports one or more unmet needs, the RA will randomise them to one of the three trial arms using the sealed opaque envelope method.31 The Data Analyst will be responsible for randomly generating the treatment allocations within the sealed envelopes. Once an envelope is open, the RA will inform the Veteran of their arm assignment.
The intervention
Following the naming convention used by Centers for Medicare and Medicaid Services for the arms in the Accountable Healthy Communities trial, we have the following study arms: (1) unmet needs screening and provision of a postcard with a list of generic VA resources (hereafter, ‘Screening’ arm), (2) screening and provision of a postcard with a list of generic VA resources plus provision of a tailored Resource Sheet listing available resources in VA and/or the community to address identified unmet needs (hereafter, ‘Awareness’ arm) or (3) screening and provision of generic resources plus provision of a tailored Resource Sheet plus SW-supported referral to assist with connection to resources for unmet needs (hereafter, ‘Assistance’ arm). Administering the intervention will not be blinded to group assignment.
Screening
Veterans in the Screening arm will receive a postcard listing the phone numbers for generic resources available to Veterans including the Veteran’s VA Medical Center, VA Veterans Crisis Line and National Call Center for Homeless Veterans. The postcard will be included in the initial recruitment packet mailed to all potential study participants. The screening arm reflects enhanced usual care. At present, VA systematically screens for only two of the nine unmet social needs (housing and food insecurity) being assessed in this study and while VA refers Veterans to the resources listed on the postcard, it is on an as-needed basis, not as part of usual care. We included the generic resource postcard to address ethical concerns raised by the VA Central Internal Review Board about assessing unmet needs without offering any resources.
Awareness
Veterans in the Awareness arm will receive the postcard listing generic resources as described above. Additionally, for each unmet need identified through the index screen, Veterans will receive by mail a tailored Resource Sheet that will include the names of available resources within VA and/or the local community that can help address the identified need(s). During the index screen, the RA will additionally ask participants if they would like to receive the Resource Sheet(s) as an email attachment. For Veterans who respond affirmatively, the RA will send the Resource Sheets as an email attachment during the index screen phone call and offer to review its content with them. To ensure that the Resource Sheets stay current, the RA will contact listed programmes monthly for current contact information and ability to accept referrals.
Assistance
Veterans in the Assistance arm will receive the postcard listing generic resources and tailored Resource Sheets as described above. Additionally, during the index screen, the RA will offer these Veterans assistance from a SW specifically hired and trained (one per site) to support Veterans with connecting to resources. With Veteran assent, the SW will contact the Veteran by phone within two business days of the in-clinic encounter. During this initial call, the SW will use proven motivational interviewing methods to develop an action plan for the Veteran to connect to needed resources.32 33 The SW will conduct follow-up by phone 1 week after the action plan development, with projected subsequent phone outreach every 2 weeks for up to 7 weeks. At each call, the SW will review progress and as needed, employ motivational interviewing methods to reaffirm the action plan and/or modify the action plan to address unexpected barriers.
Fidelity
The research RAs and SWs responsible for delivering the intervention will be provided written Standard Operation Procedures (SOP) detailing their roles and training on their respective SOPs. The SWs will additionally complete a training module on Motivational Interviewing. We will assess fidelity via data captured in REDCap (detailed below) and monitor the quality of calls with participants as part of regular check-ins (weekly for the first few months of the trial but likely reducing to monthly thereafter).
Data collection and management
All data will be collected by RAs and SWs uninvolved in the medical care of patients. All study participants will be asked to complete a brief telephone-based survey to assess unmet needs during the index screen. We refer to this as Survey A. All RCT participants will be asked to complete two additional brief telephone-based surveys. Survey B will occur 8 weeks after the index screen, when the RA will assess if trial participants connected to any new resources in the intervening time, and if so, to which one(s). Survey C will occur 6 months after the index screen, when the RA will rescreen all trial participants for unmet needs. Participants will receive a $15 gift voucher for each survey they complete. The 12-month recruitment period started on 2 May 2022 and all follow-up is planned to be complete by 28 April 2023. For participants randomised to the Assistance arm, SWs will capture their interactions with participants, including the timing, duration and outcomes of their interactions. Data will be recorded via the REDCap system and will be cleaned and checked for accuracy by the project manager and data analyst. Survey data will be merged with administrative data from the VA CDW within the VA Informatics and Computing Infrastructure. CDW includes demographics, diagnoses, vital signs, laboratory values, prescriptions and data on service use. Only the principal investigator (PI) and study team members conducting data analyses will have access to the data set.
Planned outcomes
Table 1 provides a complete list of planned outcome measures for objectives 1 and 2. The primary outcomes for objective 1 will be various measures of treatment adherence, utilisation and clinical outcomes. The primary outcome for objective 2 will be connection to new resources to address unmet needs. Connection to resources will be defined as a Veteran connecting to one or more new resources since the index screen, as indicated by their responses to the question, ‘Since you completed the unmet need social need screen on (insert date), were you able to connect with any of the programs or resources to help with (insert need(s) identified)?’ Secondary outcomes will be reduction of unmet needs, various measures of treatment adherence, utilisation and clinical outcomes. Our rationale for this ordering of outcomes for objective 2 is the importance of understanding whether S&R leads to connection to new resources, the first step in our conceptual model (see figure 1) that we anticipate will, in turn, lead to improved adherence, utilisation and ultimately, clinical outcomes. As further rationale for considering clinical outcomes as secondary, we posit they may be difficult to change over the study’s time-limited 12-month period. Moreover, while much existing literature demonstrates associations between unmet needs and clinical health outcomes, there is a dearth of preliminary data assessing the impact of interventions (eg, S&R) on these clinical outcomes. This precluded us from reliably estimating effect sizes for comparisons across intervention arms or needed sample size to adequately power such comparisons.
Table 1.
Planned outcomes for the RCT
| Outcome | Data source | Description |
| Primary outcome | ||
| Connection to new resources | Survey B* | Veteran connecting to one or more new resources 8 weeks after index screen. |
| Secondary outcomes | ||
| Unmet need reduction | Surveys A and C* | Measured two ways: (1) one or more of index needs no longer identified as unmet at 6-month rescreen and (2) percentage of index needs not reported as unmet at 6-month rescreen. |
| Preventable hospitalisations | CDW† | Prevention Quality Indicators using Agency for Healthcare Research and Quality criteria.36 |
| Urgent care utilisation | CDW† | Emergency Department and urgent care visits (CDW*). |
| Medication adherence | CDW† | Proportion of days covered of each CVD and CVD risk factors medication.17 37 |
| Clinic visit appointment attendance | CDW† | Proportion of PC and cardiology appointments classified as no-show, relative to the total number of appointments scheduled in both.38 |
| Blood pressure (BP) | CDW† | Controlling for antihypertensive medications treatment intensification, using methods from prior work.39 |
| Haemoglobin A1c (HbA1c) | CDW | To ensure values reflect health status around time of index screen and 12-month follow-up window, we will only include Veterans with DM who have an HbA1c in the 6 months prior to each time point. |
*Described under Data collection.
†VA Corporate Data Warehouse (CDW).
CVD, cardiovascular disease; DM, diabetes mellitus; PC, primary care; RCT, randomised controlled trial.
Sample size calculations
Power analyses were used to determine sample size based on an effect size estimate for our primary outcome from a prior study.14 Our sample size (n=880) ensure adequate power (80%) to detect small-to-medium effect sizes for each of the primary and secondary outcomes even if the attrition rate for survey B and survey C are both as high as 50%. The team’s prior study with a demographically similar Veteran population found only a 35% attrition rate.34
Analysis
Objective 1
We will generate descriptive statistics (eg, proportions, 95% CIs) to characterise the prevalence and distribution of each of the eight unmet needs at baseline across all study sites. We will next conduct inferential analyses to examine associations between unmet needs and sociodemographic characteristics (including race and ethnicity) as well as baseline outcomes (ie, adherence, utilisation and clinical outcomes drawn from CDW data in the 12 months before the index screening for each Veteran). General linear mixed models (GLMM) will be used to control for the nesting of patients within sites, and logistic models will be used as appropriate for binary variables. Variables found to have statistically significant associations with unmet needs will be entered into multivariable models to better understand the correlates of each need. Bonferroni-corrected significance levels will control for multiple comparisons.
Objective 2
We will compare connection to new resources at 8 weeks postindex screen across the study arms. GLMM will be used to control for the nesting of patients within sites. In all regression models, patient-level intercepts and slopes will be treated as random effects. In addition to examining how the intervention conditions influence connection to new SDH resources, we will conduct supplemental exploratory analyses to examine whether there is differential impact between the three intervention arms on connection to new SDH resources among Veterans defined by differing sociodemographic characteristics including race and ethnicity.
Using a difference-in-difference approach, we will compare the secondary outcomes (unmet need reduction, adherence, utilisation and clinical outcomes) across study arms. We will examine whether changes from baseline at 6 months and 12 months postreferral differ across the three arms in a series of GLMM analyses. As with the other analyses, all models will treat patient-level intercepts and predictors as random effects. Similar analyses will be used to examine differences across our three study arms in change from baseline in the proportion of unmet needs among the subsample of participants who complete the rescreening at 6 months postreferral. To the extent that we discover differences across intervention arms in any of our more distal outcomes, we will also conduct exploratory analyses to test appropriate causal mediational paths as proposed by the OASIS framework using a series of GLMM analyses.
Finally, we will conduct additional analyses controlling for connection to SDH resources prior to enrolment in our intervention because it is possible that individuals already connected to resources before enrolling in our intervention may be more likely to seek out additional support/resources (eg, because they already have successful experiences using VA or non-VA resources to meet certain unmet needs) or less likely to seek out additional support/resources (eg, because they feel they already have the support they most need).
For all analyses, we hypothesise that providing a tailored Resource Sheet plus SW support (Assistance arm) will generally have a larger impact on outcomes than providing a tailored Resource Sheet alone (Awareness arm). We do so because navigating the social service delivery system can be challenging and may be especially challenging for Veterans experiencing unmet social needs. This means that simply being made aware of available resources may be an insufficient mechanism for connecting participants to resources. In contrast, being made aware of resources and provided navigation assistance may enable participants to overcome barriers and by extension increase the likelihood of connecting to resources. However, it will be beneficial to know if either the tailored Resource Sheet alone or provision of generic resources alone (Screening arm) is sufficient to produce comparable changes in outcomes among Veterans with certain unmet needs or among Veterans with fewer unmet needs. If true, future implementation research could create tailored interventions that funnel the resources for more time-intensive and cost-intensive referral strategies to only those Veterans who need it most.
Qualitative inquiry (objective 3)
Study setting and participant eligibility
We will recruit for qualitative interviews a purposeful sample of two stakeholder groups: (1) Veterans enrolled in the RCT (n=60), and; (2) representatives of the VA and community programmes to which trial participants are referred (n=15). If thematic saturation is achieved before we reach these targeted sample sizes, we will stop recruiting. For the Veteran interviews, we will seek three Veteran types (20 per type): Veterans who did not connect to new resources; Veterans who connected to at least one new resource but did not have their unmet need(s) met and Veterans who connected to new resources and had one or more needs met. This sampling plan will allow us to understand the conditions that facilitate or impede a Veteran connecting to resources, and the conditions under which resources do or do not address a Veteran’s needs. Veterans who participate in a qualitative interview will receive a $25 gift voucher. For the VA and community programme representatives, we will seek up to five of the most frequently used programmes at each study site. We will first identify all VA-based and community-based programmes that trial participants used because of the intervention based on data derived from survey 2 (see Data collection). We will then seek up to five of the most frequently used programmes at each study site. By concentrating on the most highly used programmes, this sampling plan will allow us to understand the experience of programmes more likely to ‘feel’ the intervention.
Data collection and management
All data will be collected by study RAs who are uninvolved in patient care. Interviews will be conducted by phone using a semistructured interview guide. We will ask Veterans about their experience participating in the trial (eg, being screened and receiving resource sheets); experience with the unmet needs they identified; decision-making around accessing resources and experience connecting to and using resources to address unmet needs. We will ask representatives of VA-based and community-based programmes about their funding structure and services provided; experiences with increased demand for their services during the trial period and the factors that facilitate and impede addressing Veterans’ needs. Interviews will be digitally audiorecorded, with the permission of each respondent. Deidentified audiorecordings will be transcribed by a premier service provider for the VA. The study team will store recordings on a secure VA server and will be password protected. All names and places mentioned will be deleted to protect confidentiality.
Analysis
We will transcribe interviews verbatim and employ both deductive and inductive coding methods. For the former, codes will be informed by the previously described Anderson model of service utilisation.28 Additional emergent codes will be identified, grounded in the data. Coding will be guided by the constant comparative method.35 That is, previously coded material will be constantly compared with the new data to determine whether the same concept is being expressed and, if so, to be sure that all exemplars of that concept are assigned to the most recently refined category. After coding is complete, code output will be analysed to identify themes within and across sample strata.
Patient and public involvement
During the study design process, we engaged a Veteran consultant from VA’s Veteran Engagement in Research Group to provide input on the intervention, including the burden of being screened for multiple unmet needs and receiving facilitated referral services. Veterans will not be involved in the recruitment to and conduct of the study. We will disseminate findings via the Veteran Engagement in Research Group, as well to individual study participants, on request.
Ethics and dissemination
This study protocol was approved by the VA Central Internal Review Board (Reference #: 20-07-Amendment No. 02). A Data and Safety Monitoring Board (DSMB) will oversee the study. DSMB is an independent review board chartered by the VA Health Services Research and Development Service that meets at specified intervals and requires routine reporting from the PI. The PI will follow a specific Data and Safety Monitoring Plan, which has been reviewed and approved by the DSMB. We will conduct monthly assessments with each trial site to monitor serious adverse events. Should we receive any negative feedback from research subjects or have any unexpected serious or adverse events as reported by site staff, the PI will report this information to the DSMB, Central Internal Review Board and R&D immediately.
We are conducting a benign behavioural intervention and while the risks of adverse events are thus minimal, there is the potential that some participants will get upset answering questions about unmet social needs. To protect against this risk, we will train study RAs to be sensitive to the individual needs of each participant and to create an environment that feels safe and nonjudgmental. RAs will also be trained to remind participants that they may decline to answer any survey question or discontinue with the surveys at any time. We will additionally establish procedures for the intervention research staff to connect patients with site staff who can assist and facilitate referrals to services and providers within the VA, as needed.
The study results will be disseminated regardless of effect direction and size through publications in peer-reviewed journal and presentations at conferences. Final data sets underlying all publications resulting from this research will be shared outside the VA. Quantitative data meeting VA standards for discloser to the public will be made available within 1 year of publications. Prior to distribution, a local privacy officer will certify that the data set contains to Protected Health Information, Personal Identifiable Information or VA Sensitive Information prior to release outside VA. Qualitative data (ie, transcripts) will not be shared. The sensitive nature of the study data precludes asking participants to consent and grant authorisation as required by the Health Insurance Portability and Accountability Act for disclosing data outside the VA.
Discussion
This study will provide much-needed evidence to document the prevalence of Veterans’ unmet needs at three large urban VA Medical Centres, inform how best to address unmet needs and assess how such a process can affect adherence, utilisation and clinical outcomes. If any of our intervention study arms demonstrate greater improvements in one or more study outcomes overall or for particular Veteran types (eg, those with certain unmet needs), these findings can be tested and spread through future implementation research and processes. Importantly, the addition of our stakeholder interviews and analysis is unique to most clinical trials and will help to identify barriers and facilitators to future implementation as well as potential needed modifications to the intervention. Doing so will facilitate future uptake of the intervention should it prove effective. Furthermore, our focus on the sentinel condition of CVD may help bridge the substantial sociodemographic gap in life expectancy related to CVD, and our methods can be used to examine the effects of interventions to address unmet needs on other conditions.
bmjopen-2021-058972supp001.pdf (112.7KB, pdf)
Supplementary Material
Footnotes
Contributors: DG, NK, BGB, AL, MED, KJH, GMF and BLN made substantial contributions to the conception and design of the study; DG drafted the work and NK, BGB, AL, MED, KJH, GMF and BLN substantially revised it. All authors have approved the submitted version and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated resolved and the resolution documented in the literature. The authors also appreciate the contributions of Jolie Wormwood, Rory Ostrow and our Veteran consultant.
Funding: This study is funded by Health Services Research and Development (IIR 19-013). The views in this article are those of the authors and do not necessarily reflect the position or policy of the VA or the US government. Dr Fix is a VA HSR&D Career Development awardee at the Bedford VA (CDA 14-156).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Marmot M, Allen J, Bell R, et al. WHO European review of social determinants of health and the health divide. Lancet 2012;380:1011–29. 10.1016/S0140-6736(12)61228-8 [DOI] [PubMed] [Google Scholar]
- 2.McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff 2002;21:78–93. 10.1377/hlthaff.21.2.78 [DOI] [PubMed] [Google Scholar]
- 3.Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med 2016;50:129–35. 10.1016/j.amepre.2015.08.024 [DOI] [PubMed] [Google Scholar]
- 4.Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American heart association. Circulation 2015;132:873–98. 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 5.Berkowitz SA, Hulberg AC, Standish S, et al. Addressing unmet basic resource needs as part of chronic cardiometabolic disease management. JAMA Intern Med 2017;177:244–52. 10.1001/jamainternmed.2016.7691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016;133:447–54. 10.1161/CIR.0000000000000366 [DOI] [PubMed] [Google Scholar]
- 7.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol 2006;16:91–104. 10.1016/j.annepidem.2005.06.053 [DOI] [PubMed] [Google Scholar]
- 8.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993;88:1973–98. 10.1161/01.cir.88.4.1973 [DOI] [PubMed] [Google Scholar]
- 9.Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health 2005;5:7. 10.1186/1471-2458-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupre ME, George LK, Liu G, et al. The cumulative effect of unemployment on risks for acute myocardial infarction. Arch Intern Med 2012;172:1731–7. 10.1001/2013.jamainternmed.447 [DOI] [PubMed] [Google Scholar]
- 11.Kawachi I, Colditz GA, Ascherio A, et al. A prospective study of social networks in relation to total mortality and cardiovascular disease in men in the USA. J Epidemiol Community Health 1996;50:245–51. 10.1136/jech.50.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzau VJ, McClellan MB, McGinnis JM. Vital directions for health and health care: priorities from a National Academy of Medicine initiativeNAM’s vital directions for health and health care initiativeNAM’s vital directions for health and health care initiative. JAMA 2017;317:1461–70. [DOI] [PubMed] [Google Scholar]
- 13.Daniel H, Bornstein SS, Kane GC, et al. Addressing social determinants to improve patient care and promote health equity: an American College of physicians position paper. Ann Intern Med 2018;168:577–8. 10.7326/M17-2441 [DOI] [PubMed] [Google Scholar]
- 14.Garg A, Toy S, Tripodis Y, et al. Addressing social determinants of health at well child care visits: a cluster RCT. Pediatrics 2015;135:e296–304. 10.1542/peds.2014-2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon JA, Emond JA, Camargo CA. The state children's health insurance program: a multicenter trial of outreach through the emergency department. Am J Public Health 2005;95:250–3. 10.2105/AJPH.2003.037242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb LM, Wing H, Adler NE. A systematic review of interventions on patients' social and economic needs. Am J Prev Med 2017;53:719–29. 10.1016/j.amepre.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 17.Zullig LL, Shaw RJ, Crowley MJ, et al. Association between perceived life chaos and medication adherence in a postmyocardial infarction population. Circ Cardiovasc Qual Outcomes 2013;6:619–25. 10.1161/CIRCOUTCOMES.113.000435 [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb LM, Adler NE, Wing H, et al. Effects of in-person assistance vs personalized written resources about social services on household social risks and child and caregiver health: a randomized clinical trial. JAMA Netw Open 2020;3:e200701. 10.1001/jamanetworkopen.2020.0701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb LM, Hessler D, Long D, et al. Effects of social needs screening and in-person service navigation on child health: a randomized clinical trial. JAMA Pediatr 2016;170:e162521. 10.1001/jamapediatrics.2016.2521 [DOI] [PubMed] [Google Scholar]
- 20.Poleshuck E, Wittink M, Crean HF, et al. A comparative effectiveness trial of two patient-centered interventions for women with unmet social needs: personalized support for progress and enhanced screening and referral. J Womens Health 2020;29:242–52. 10.1089/jwh.2018.7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seligman HK, Levi R, Ridberg R, et al. Impact of enhanced food pantry services on food security among adults with diabetes using a crossover study design. Curr Dev Nutr 2022;6:nzac021. 10.1093/cdn/nzac021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billioux A, Verlander K, Anthony S. Standardized screening for health-related social needs in clinic settings: the accountable communities screening tool. Discussion paper, National Academy of Medicine, Washington, DC 2017.
- 23.Fine MJ, Demakis JG. The Veterans health administration’s promotion of health equity for racial and ethnic minorities. Am J Public Health 2003;93:1622–4. 10.2105/ajph.93.10.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurman WA, Harrison T. Social context and value-based care: a capabilities approach for addressing health disparities. Policy Polit Nurs Pract 2017;18:26–35. 10.1177/1527154417698145 [DOI] [PubMed] [Google Scholar]
- 25.Cohen AJ, Rudolph JL, Thomas KS, et al. Food insecurity among veterans: resources to screen and intervene. Fed Pract 2020;37:16–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Gurewich D, Garg A, Kressin NR. Addressing social determinants of health within healthcare delivery systems: a framework to ground and inform health outcomes. J Gen Intern Med 2020;35:1571–5. 10.1007/s11606-020-05720-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maslow AH. A theory of human motivation. Psychol Rev 1943;50:370–96. 10.1037/h0054346 [DOI] [Google Scholar]
- 28.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav 1995;36:1–10. 10.2307/2137284 [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine . Capturing social and behavioral domains and measures in electronic health records: phase 1. Washington, DC: National Academies Press (US), 2014. [PubMed] [Google Scholar]
- 30.National Association of Community Health Centers (NACHC) . PRAPARE. Available: https://www.nachc.org/research-and-data/prapare/ [Accessed 3 Dec 2018].
- 31.Torgerson DJ, Roberts C. Understanding controlled trials. Randomisation methods: concealment. BMJ 1999;319:375–6. 10.1136/bmj.319.7206.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller WR, Rollnick S. Motivational interviewing: preparing people for change. Guilford Press, 1991. [Google Scholar]
- 33.Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychother 2009;37:129–40. 10.1017/S1352465809005128 [DOI] [PubMed] [Google Scholar]
- 34.Kressin NR, Long JA, Glickman ME, et al. A brief, multifaceted, generic intervention to improve blood pressure control and reduce disparities had little effect. Ethn Dis 2016;26:27–36. 10.18865/ed.26.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strauss AL. Basics of qualitative research: grounded theory procedures and techniques. Sage Publications, 2013. [Google Scholar]
- 36.Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. JAMA 1995;274:305–11. [PubMed] [Google Scholar]
- 37.Borne RT, O'Donnell C, Turakhia MP, et al. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the veterans health administration. BMC Cardiovasc Disord 2017;17:236. 10.1186/s12872-017-0671-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teo AR, Forsberg CW, Marsh HE, et al. No-show rates when phone appointment reminders are not directly delivered. Psychiatr Serv 2017;68:1098–100. 10.1176/appi.ps.201700128 [DOI] [PubMed] [Google Scholar]
- 39.Manze M, Rose AJ, Orner MB, et al. Understanding racial disparities in treatment intensification for hypertension management. J Gen Intern Med 2010;25:819–25. 10.1007/s11606-010-1342-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058972supp001.pdf (112.7KB, pdf)