Abstract
Introduction
Few clinical trials have examined the effects of home-based exercise programmes on health-related quality of life (HR-QoL) in older adults with frailty. Radio-Taiso is the most famous exercise programme in Japan. A home-based Radio-Taiso exercise programme may serve as an accessible, scalable and sustainable care intervention for older adults with frailty. The primary aim of this trial is to test whether older adults with frailty who are prescribed our home-based Radio-Taiso exercise programme will receive greater benefits for HR-QoL compared with those who are not prescribed the exercise programme. Potential mechanisms underlying the effectiveness of the programme and the effects of the programme on daily lifestyle will also be investigated.
Methods and analysis
This assessor-blind randomised controlled trial will be conducted at the Tokyo Metropolitan Institute of Gerontology (TMIG) in Itabashi-ku, Tokyo, Japan. From April to May 2022, 226 older adults with prefrailty or frailty according to the revised Japanese version of the Cardiovascular Health Study criteria will be included from a large database. After a baseline assessment in June 2022, participants will be randomly assigned to the intervention (home-based Radio-Taiso exercise and nutrition programme) or control (nutrition programme) groups at a 1:1 ratio. After intervention completion, a follow-up assessment will be conducted in September 2022. The primary outcome is the change in the mental domain of HR-QoL assessed using SF-36. Secondary outcomes include physical and role/social domains and subscales of HR-QoL, frailty phenotype, physical fitness, posture, cognition, exercise self-efficacy, depressive symptoms, brain-derived neurotrophic factor, social network, habitual energy intake, physical activity and sleep conditions.
Ethics and dissemination
The Research Ethics Committee of TMIG has approved the research protocol. This trial will be conducted in accordance with the principles of the Declaration of Helsinki. The findings will be presented at international academic conferences and published in peer-reviewed international journals.
Trial registration number
UMIN000047229.
Keywords: GERIATRIC MEDICINE, MENTAL HEALTH, PUBLIC HEALTH, SPORTS MEDICINE
Strengths and limitations of this study.
The feasibility of this phase III trial has been enhanced based on the processes, resources, management and scientific data of a pilot trial.
The trial will include older adults with frailty defined using a validated assessment tool.
Random allocation, blinding of assessors and analysts, interpretation of results based on the intention-to-treat principle and disclosure of protocol papers will reduce the risk of selection, measurement, reduction and reporting bias.
The non-blinding of allocation information to participants and care providers may increase the risk of performance bias.
The generalisability of the study findings to non-Japanese populations is unclear.
Introduction
Frailty is defined as vulnerability of homeostatic responses to stressors due to cumulative age-related decline of physiological systems.1 Research suggests that 7% and 47% of older adults have frailty and prefrailty, respectively, and their risk of falls, disabilities, nursing home admission, and death is significantly higher than that of robust older adults.2 Interventions to reduce frailty prevalence and severity may benefit older adults with care needs, their caregivers and the public social insurance system. However, clinical trials involving older adults with frailty defined using validated assessment tools are required to confirm the effectiveness of these interventions.1
Interventions such as exercise and nutritional programmes are useful for reducing the prevalence of frailty, but stakeholders have raised concerns that research on these interventions has focused predominantly on improving physical outcomes.3 Studies focusing on patient-reported outcomes (eg, health-related quality of life (HR-QoL)) may address the gaps between scientific evidence and clinical practice.4 Exercise interventions are reportedly the most effective for improving HR-QoL among older adults with frailty, but this warrants validation in robust clinical trials.5 Nevertheless, evidence for the effects of home-based exercise interventions on HR-QoL remains scarce.6 As older adults with frailty exhibit decreased mobility,7 readily available home-based exercise programmes are feasible and rational interventions.
Radio-Taiso is the most famous Japanese exercise programme, with reports suggesting that approximately 96.9% of the national population are aware of it.8 In 1928, the Postal Life Insurance Bureau of the Ministry of Communications (successor agency: Japan Post Insurance) developed Radio-Taiso as a ‘National Health Exercise’ to improve health among Japanese people.9 Radio-Taiso is customarily practised in numerous settings and is firmly established in Japanese culture (figure 1).10 It is broadcasted daily by the Japan Broadcasting Corporation via public radio and television, thus facilitating home access by Japanese older adults with frailty.
Figure 1.
Pictures of Radio-Taiso as a firmly established, traditional Japanese exercise programme. (A) Community-dwelling children get together to practice Radio-Taiso. (B) Physical education classes. (C) Practice in the workplace. (D) Gathering and practising during community events. All pictures were provided by courtesy of Japan Post Insurance Co., Ltd.
Our recent pilot randomised controlled trial examined the feasibility and potential effectiveness of a home-based Radio-Taiso exercise programme in community-dwelling older adults with prefrailty and frailty. Participants demonstrated high adherence and the results suggested that the programme may lead to clinically important improvements in the mental domain of HR-QoL (under review). However, the effectiveness of the programme remains to be validated by robust clinical trials.
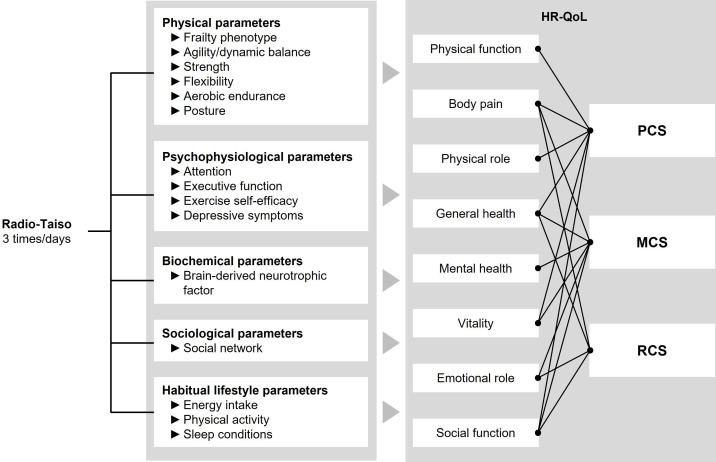
This trial aims to test whether older adults with prefrailty and frailty who undergo the programme will experience greater benefits in the mental domain of HR-QoL compared with those who do not undergo the programme. Furthermore, the trial aims to identify the mechanisms underlying programme effectiveness and the impact of the programme on participants’ lifestyles (figure 2).
Figure 2.
A conceptual model for explaining the mechanisms of the effect of the home-based Radio-Taiso exercise programme. HR-QoL, health-related quality of life; MCS, mental component summary; PCS, physical component summary; RCS, role/social component summary.
Methods and analysis
Study design, setting and procedures
This is a 12-week randomised, assessor-blind, parallel-design, two-arm, phase III trial. The protocol was designed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement (online supplemental material 1) and several aspects of the SPIRIT-PRO Extension.
bmjopen-2022-063201supp001.pdf (68.9KB, pdf)
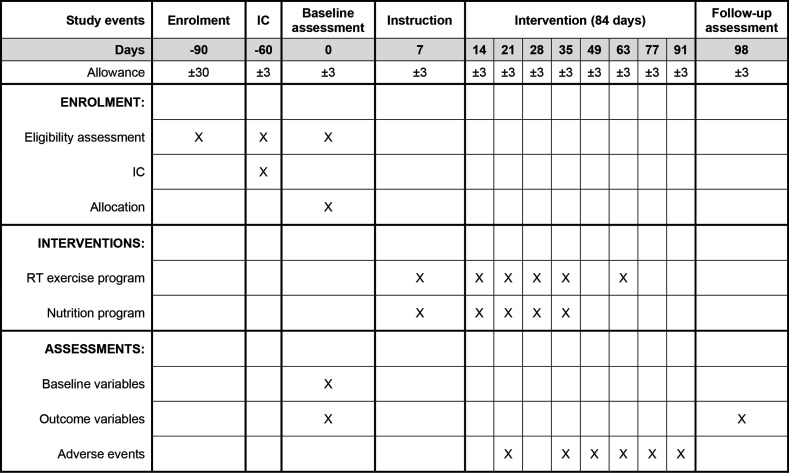
All outcomes will be assessed at the Tokyo Metropolitan Institute of Gerontology (TMIG) in Itabashi-ku, Tokyo, Japan. Participants will practice the programme at home. Six face-to-face group sessions will be conducted at the TMIG or nearby community facilities (figure 3). Recruitment will be conducted in April 2022. Informed consent will be obtained in the subsequent month (May 2022). Baseline and follow-up assessments will be conducted in June and September 2022, respectively. After baseline assessments, participants who meet the eligibility criteria will be included in the trial and randomly assigned to the intervention (home-based Radio-Taiso exercise programme+nutrition programme) or control (nutrition programme) groups. The intervention will begin and end in June and September 2022, respectively.
Figure 3.
Study flow diagram. IC, informed consent; RT, Radio-Taiso.
Participants
Inclusion criteria are: (1) aged 65 years or older and (2) frailty or prefrailty, as defined by the revised Japanese version of the Cardiovascular Health Study (J-CHS) criteria (see below).
Exclusion criteria are: (1) inability to participate in both baseline and follow-up assessments, (2) diagnosed with dementia or prescribed antidementia drugs, (3) self-reports of being unable to eat, excrete, dress, move or bathe independently, (4) not allowed to exercise (except for light-intensity exercise) by the family physician, (5) unstable/severe medical conditions that prevent trial physicians from permitting study participation, (6) angina pectoris, myocardial infarction or cardiac surgery in the past 3 months, having a terminal illness, or receiving palliative care, (7) practising Radio-Taiso exercises for ≥1 day/week for the past month, (8) participating in a specific rehabilitation programme, (9) unable to walk ≥10 m independently, (10) participating or will participate in other clinical trials, (11) lacking a television at home, (12) difficulty communicating in Japanese, (13) deemed by the principal investigator and/or trial physicians to be ineligible and (14) unable to provide consent to participate.
Potential participants will be recruited from a large database of trial-ready cohorts managed by the TMIG. In our pilot trial in 2020, 902 individuals were newly enrolled or had their information updated. Of these, 514 (60%) met the prefrailty or frailty criteria and 186 (recruitment rate: 20.6%) met all eligibility criteria and were willing to participate. Thus, at least 1097 new registrations or updates are required to ensure that the target number of 226 participants is reached (described in more detail in the sample size section).
Invitation letters and eligibility criteria checklists will be sent to individuals diagnosed with frailty or prefrailty between October 2021 and March 2022. Participants who meet all eligibility criteria and intend to participate will be provided a detailed verbal explanation of the study (ie, aims, procedures, confidentiality, possible benefits and disadvantages, anticipated risks and methods to address risks). Only individuals who provide written informed consent will participate. If more individuals are willing to participate than the target sample size, participants will be randomly selected using a computer-generated random sequence. During baseline assessments, trial physicians will assess compliance with exclusion criteria numbered 2, 5 and 6.
Allocation and blinding
After baseline assessments, participants will be randomly allocated to the intervention and control groups at a 1:1 ratio. The sequence for generating the allocation code will be stratified by sex (male or female), age (<75 or ≥75 years) and frailty severity (prefrailty or frailty). Block randomisation will be applied.
The principal investigator (YO) at the TMIG will send identification codes of eligible participants to an allocator (KM) who has no contact with participants. KM will combine the codes with a prescribed randomisation code, which will be generated prior to baseline assessments. An independent research staff member will inform participants of their group based on the allocation code sent by KM. Group labels will not be disclosed to the assessor and statistical analyst until completion of the primary analysis.
Intervention
During the 12-week intervention period, participants will be instructed to avoid starting new exercise or nutrition programmes. To address the ethical disadvantages of the control group, a nutrition programme will be provided to both groups.
Home-based Radio-Taiso exercise programme
Radio-Taiso includes three exercise patterns: Radio-Taiso No. 1 and 2 and Minna no Taiso. These patterns comprise 8–13 rhythmic whole-body movements with music. Each pattern starts with a low-intensity movement, gradually increases in intensity and ends with another low-intensity movement, allowing safe practice. The smooth execution of movements requires fitness in various physical domains (strength, flexibility, endurance and balance), making Radio-Taiso a multicomponent exercise programme (details in online supplemental table 1–3).
bmjopen-2022-063201supp003.pdf (57.5KB, pdf)
The home-based programme comprises six 60 min face-to-face group sessions with a certified instructor from the Japan Radio-Taiso Federation and daily practice in participants’ homes. Before the programme, the intervention group will receive a face-to-face group session on correctly performing the three exercise patterns. In the first 4 weeks of the intervention, participants will receive face-to-face group sessions on effectively performing the exercise programmes. In week 8, a face-to-face group session will be provided to review the key points of each exercise programme.
Participants will be instructed to complete a three-pattern exercise programme once daily at home by themselves via a broadcast by the Japan Broadcasting Corporation or on DVD. If the participants feel unwell while performing any exercise or feel that the exercise intensity is too high, they will be instructed to stop or reduce the number of sessions. Participants will be required to record (1) whether they performed the exercise programme and (2) compliance with key points for effective programme implementation in an exercise diary. On the day of face-to-face group sessions, research staff will check the exercise diary, adverse events and participation in the new rehabilitation programme. In weeks when sessions are not provided, these details will be checked by telephone every fortnight.
The pilot trial confirmed good adherence to the home-based Radio-Taiso exercise programme (retention rate: 100%; median (IQR) practice rate: 97.6% (88.1%–98.8%)). However, no trend towards improvements in physical outcomes was observed. Radio-Taiso may not include properties that significantly improve physical outcomes because it predominately consists of flexibility exercises. However, thoroughly completing the purpose of each movement in Radio-Taiso necessitates quicker and larger movements, which require muscle endurance and whole-body endurance. In this regards, several participants who received face-to-face instructions in the pilot study reported that it is important to understand the purpose of each movement of the Radio-Taiso via face-to-face instructions to improve the quality of practice at home. Thus, to provide participants with a programme that yields better physical outcomes, the number of face-to-face sessions will be increased from three to six and include processes that help participants familiarise with the key points of the programme. An item will be added to the exercise diary to check compliance with these points.
Nutrition programme
The nutrition programme comprises: (1) distribution of a nutrition leaflet, (2) recording of a dietary variety score and (3) telephonic nutrition counselling. One week before intervention onset, participants will receive a face-to-face briefing from a dietitian on programme implementation. A nutrition leaflet will be distributed once weekly during the first 4 weeks of the intervention, detailing the nutritional role and recommended intake amounts of protein, calcium, vitamins/minerals and carbohydrates/fats, alongside specific recipes for the efficient intake of these nutrients. Participants will be instructed to record daily in a nutrition diary the consumption of 10 food groups (meat, seafood, eggs, soya and soya products, milk, green and yellow vegetables, seaweed, potatoes, fruit and oil) using a dietary variety score. Participants will be requested to provide scores on intake of these food groups on a 10-point scale.11 A high dietary variety score has been associated with lower frailty severity.12 Participants will be allowed to call the dietitian 1 day per week to discuss how to proceed with the nutrition programme or to ask any questions.
Outcome measures
The primary outcome changes in the mental component summary (MCS) score of HR-QoL. Secondary outcomes are the physical component summary (PCS) score, role/social component summary (RCS) score and eight subscales of HR-QoL as well as frailty phenotype, agility/dynamic balance, strength, flexibility, aerobic endurance, posture, attention, executive function, exercise self-efficacy, depressive symptoms, brain-derived neurotrophic factor (BDNF), social network, habitual energy intake, physical activity, sleep conditions, safety and adherence. All outcomes excluding habitual physical activity will be assessed at a baseline survey within 2 weeks prior to the start of the intervention and at a follow-up survey within 1 week after the end of the intervention (figure 3).
Baseline information
Baseline information including age, sex, disease history (hypertension, heart disease, diabetes, hyperlipidaemia, osteoporosis and respiratory disease) and low-back and knee pain will be obtained by face-to-face interview.
Assessments
Objective outcomes including physical fitness, posture, cognition, BDNF and physical activity will be assessed by research staff blinded to allocation information.
HR-QoL
HR-QoL will be assessed using the Japanese version of the SF-36, which is a widely used, reliable and validated tool.13 14 The SF-36 measures eight health domains: physical function, physical role, body pain, general health, vitality, social function, emotional role and mental health. These domains are aggregated and scored into the MCS, PCS and RCS (figure 2).15 These scores are standardised as T-scores using the 2017 Japanese national norm.16 A change of three or more points in the MCS or two or more points in the PCS constitutes a minimal clinically important difference, enabling clinical interpretation of changes in scores.17
Frailty phenotype
Frailty phenotype will be assessed using Fried’s frailty criteria, characterised by five limitations: slowness, weakness, exhaustion, low activity and weight loss.2 The trial will use the revised J-CHS criteria to define frailty (three or more limitations) and pre-frailty (one or two limitations).18
Slowness
Slowness will be assessed based on usual gait speed. An 11 m walking path will be used, with a 3 m acceleration/deceleration path at each end. The assessor will measure the time taken between the 3 m and 8 m markers.19 The measurement will be performed once. Slowness will be defined as a gait speed of less than 1.0 m/s.
Weakness
Weakness will be assessed based on grip strength using a handheld Smedley-type dynamometer. Participants will be instructed to grip the device as strongly as possible with their dominant hand in a standing position.19 The measurement will be performed once. Weakness will be defined as less than 28 kg and 18 kg for men and women, respectively.
Exhaustion
Exhaustion will be assessed using question 25 of the Kihon checklist developed by the Ministry of Health, Labour and Welfare, as follows20: ‘In the last 2 weeks, have you felt tired for no reason?’ Exhaustion will be defined as an ‘yes’ response.
Low activity
Low activity will be assessed using two simple questions regarding participation in exercise or physical activity, as follows: (1) ‘How often do you engage in light intensity exercise or calisthenics?’ and (2) ‘How often do you engage in exercise or sports activities’? Low activity will be defined by a response of ‘less than once a week’ to both questions.
Weight loss
Weight loss will be assessed using question 11 of the Kihon checklist developed by the Ministry of Health, Labour and Welfare, as follows20: ‘Have you lost 2 kg or more in the past 6 months?’ Weight loss will be defined as a ‘yes’ response.
Physical fitness
Six physical fitness domains (agility/dynamic balance, lower body strength, upper body strength, lower body flexibility, upper body flexibility and aerobic endurance) will be assessed using Senior Fitness Tests.21
Agility and dynamic balance
Agility and dynamic balance will be assessed using the 8-foot up-and-go test. Participants will be instructed to stand up at the start signal, walk around a cone 8 ft away, turn around and sit down again. This sequence must be performed as quickly as possible. After one practice session, two trials will be performed. The values for the minimum time required will be used in the analysis.
Lower body strength
Lower body strength will be assessed using the chair stand test. At test onset, participants will be instructed to stand up and sit down again with their arms crossed in front of the chest. After 2–3 practice sequences of this exercise, a single trial will be performed, in which the sequence is repeated as quickly as possible for 30 s. The number of sequences achieved will be used in the analysis.
Upper body strength
Upper body strength will be assessed using the arm curl test. Participants will be requested to flex and extend the elbow of the favourite arm while holding a dumbbell (8 lb for men and 5 lb for women) at the start signal, with both upper arms in a natural down position. After 2–3 practice sequences, a single trial will be performed, in which the sequence will be repeated as quickly as possible for 30 s.
Lower body flexibility
Lower body flexibility will be assessed using the chair sit-and-reach test. Participants will be requested to sit in a shallow position on a chair and extend their favourite leg. Participants will then be instructed to place the fingertips of both hands together, slowly flex the upper body towards the toes of the favourite leg until reaching their limit and remain still for 2 s. Participants will be instructed to hold the ankle joint at 90°. The assessor will measure the distance between the toes of the favourite leg and fingertips of both hands using a ruler. After two practice sessions, the measurements will be performed two times. The best values will be used in the analysis.
Upper body flexibility
Upper body flexibility will be assessed using the back scratch test. In an upright position, the participant will be instructed to rotate the favourite hand backwards obliquely upward and the other hand backwards obliquely downward to the posterior region. The assessor will measure the shortest distance between the middle fingers of both hands using a ruler. The measurement will be performed two times. The best values will be used in the analysis.
Aerobic endurance
Aerobic endurance will be assessed using a 2 min step-in-place test. The assessor will mark a wall with masking tape midway between the participant’s patella and iliac crest. The participant will be instructed to stand next to the wall and march in place for 2 min. Participants who face difficulties marching for the 2 min will be allowed to take a break and hold the wall or a stable chair. The assessor will record the number of times the right knee reaches the marker within 2 min. The trial will be conducted once.
Anthropometric indices
Height, body weight and body mass index
Height will be measured using a digital height meter (DSN-70; MURATEC-KDS Corporation, Kyoto, Japan). Body weight is measured using a body composition analyser (InBody770, InBody, Seoul, Korea). Body mass index is calculated by dividing the weight (kg) by the square of the height (m).
Posture
Posture will be assessed by the degree of kyphosis and the range of motion of the spine. The degree of kyphosis is assessed by the thoracic kyphosis angle (KA) and lumbar lordosis angle.22 23
Thoracic KA and lumbar lordosis angles are measured using the Idiag M360 (Idiag AG, Switzerland). The participant is first instructed to stand in a normal position, and then the assessor moves the Idiag M360 along the spinous processes from the vertebra prominens (C7) to the third cervical vertebra (C3). The trial is conducted once, and the smaller the angle of the thoracic KA, the severer the kyphosis, whereas the larger the lumbar lordosis angle, the severer the kyphosis.22 23
The range of motion of the spine is measured using the Idiag M360 (Idiag AG, Switzerland).22 23 The assessor moves the Idiag M360 over the spinous processes from C7 to C3 in the maximum forward flexion and extension positions. Range of motion is assessed as the difference between the thoracic KA and lumbar lordosis angle at maximum forward flexion and maximum extension, based on the angles measured for the thoracic KA and lumbar lordosis angle.
Cognition
Attention and executive function will be assessed using parts A and B of the trail making test.24 In part A, participants must connect the numbers in ascending order, and the time taken for finalising the task is measured (ie, 1-2-3-4…). In part B, the task is to connect the numbers and kana characters in an alternating order (ie, 1-A-2-I…), and the time taken to finalise the task is measured.
Home-based exercise self-efficacy
The participants’ home-based exercise self-efficacy will be assessed using the Home-Exercise Barrier Self-Efficacy Scale.25 Participants will be asked to respond to six exercise situations at home (1: when I am tired, 2: when I am in pain, 3: when I do not feel very good, 4: when I do not have time, 5: when I do not have the equipment or environment to exercise and 6: when I am alone) on a 5-point Likert scale ranging from 1 (not at all confident) to 5 (absolutely confident). Details on items and scoring are described in prior research.25 Total scores range from 6 to 30, with higher scores indicating greater self-efficacy in home-based exercise.
Depressive symptoms
Depressive symptoms will be assessed using the short version of the Geriatric Depression Scale.26 Participants will be asked to answer to 15 yes-or-no questions about their daily mood. Details on items and scoring are described in prior research.26 Total scores range from 0 to 15, with higher scores indicating a more depressed mood.
Brain-derived neurotrophic factor
BDNF is the most widely expressed neurotrophin in the mammalian brain and an important regulator of the development and function of neural circuits in the brain.27 Exercise-induced BDNF mediates neuronal differentiation and growth, synapse formation and plasticity, and may explain how exercise training improves mental health and cognitive function.28
Participants will fast for at least 2 hours and a blood sample will be collected from the anterior elbow vein. To exclude the potential effects of acute exercise, participants will be asked to refrain from engaging in vigorous intensity exercise for 36 hours before the blood sample collection. To control for diurnal variation, participants will have their blood drawn at the same time of day in both the baseline and follow-up assessments. Blood samples will be processed according to the manufacturer’s specifications: plasma is obtained by centrifugation at 3000 g for 10 min at 22°C, aliquoted and stored at −80°C until measurement. Plasma samples will be assessed for BDNF concentrations using a commercially available two-site sandwich ELISA kit (R&D Systems, Minneapolis, Minnesota). The analysis will be conducted centrally in an independent laboratory.
Social network
Social network will be assessed using the Japanese version of the Lubben Social Network Scale-6.29 Participants will answer six questions regarding support, indicating the number of family members and friends who can provide them with emotional and instrumental support from the five given options. Details on items/scoring are described in prior research.29 Total scores range from 0 to 30, with higher scores indicating a larger social network.
Habitual energy intake
Habitual energy intake will be assessed using a reliable and valid, brief, self-administered diet history questionnaire developed to provide a simple estimate of energy intake over the past month.30 31 Participants will be asked to recall their average dietary habits over the past month and indicate the frequency of consumption of each food item. Habitual energy intake will be calculated using special software provided by Gender Medical Research Co. Japan.
Habitual physical activity
Habitual physical activity levels will be assessed based on the average number of daily steps taken. The number of steps will be measured using validated tri-axis accelerometers and algorithms (Active style Pro HJA-750C; Omron Healthcare, Tokyo, Japan).32 33 Participants will be instructed to wear the device around the waist for 7 days from baseline to follow-up assessments during all activities of daily living, from waking to bedtime, except for underwater activities (eg, bathing and swimming). A record is valid if the device is worn for at least 10 hour/day.34 If valid records are collected for more than 3 days, the number of daily steps is calculated.
Habitual sleep conditions
Sleep conditions will be subjectively assessed using the Japanese version of the Pittsburgh Sleep Quality Index,35 36 comprising seven components: sleep quality, time to fall asleep, time stayed asleep, sleep efficiency, difficulty sleeping, use of sleeping pills and difficulty waking up during the day in the past month. Details on items and scoring are described in prior research.35 36 Total scores range from 0 to 21, with higher scores indicating a worse subjective sleep condition.
Adverse events
Adverse events are defined in this study as any undesirable/unintended sign, symptom or disease occurring during the intervention, regardless of causality. The number of adverse events that occur during the intervention period will be assessed. Trial physicians will determine adverse event severity and potential relevance to the intervention. Any adverse events (eg, subjective symptoms, falls and other surgical or medical findings) suspected to be related to the intervention will be recorded. Every fortnight, research staff will ask participants, telephonically or in person, whether they have experienced an adverse event.
Adherence
Adherence will be assessed via the retention rate during the intervention period and the rate of practice of the Radio-Taiso exercise programme. The retention rate will be calculated as the percentage of participants who complete the follow-up assessment. The rate of practice of the programme will be calculated by dividing the number of practice days (days when the exercises are practised at least once a day) by 84 days. The total number of practice sessions of the programme will also be assessed.
Sample size
Based on the pilot trial, the effect size (Cohen’s d) of the programme on the MCS score is 0.395. Using G*Power V.3.1.9.2 for Windows (Heinrich-Heine-Universität Düsseldorf, Germany),37 with the alpha error set at 5%, a power of 80%, and this effect size, the sample required is 204. Considering a 10% drop-out rate, the target sample is 226.
Data management
Data quality control comprises (1) measurements based on established standard operating procedures, (2) manual checks (visual checks) of questionnaires and case reports and (3) data entry using the double-entry method. Manual checks include confirmation of compliance with eligibility criteria, missing measurements and value ranges. After entering all the data, the person responsible for the analysis will perform logical checks (programming checks) and data coding to ensure and fix any issues pertaining to data quality. These checks include examining for outliers.
Statistical analysis
As ‘tests of baseline homogeneity’ in randomised controlled trials do not have any practical value,38 comparison of baseline characteristics will not be performed in this study. At baseline, continuous variables are presented as means (SD) or medians (IQRs) and categorical variables as n (%).
Main analysis
To test programme effectiveness, changes in the primary outcome will be compared between the groups using an analysis of covariance model adjusted for allocation stratification factors and baseline values. Changes in secondary outcomes will be compared using the same model. Differences between groups in the change of each outcome will be expressed as differences in adjusted means (95% CIs). Statistical significance will be set at a p<0.05.
The results of the main analyses will be interpreted based on the intention-to-treat principle. The full analysis set will exclude participants (1) who are subsequently found not to meet the eligibility criteria; (2) who never participated in the intervention programme or (3) for whom no postrandomisation data are available39; the resulting sample will be applied as the main analysis population. The International Council for Harmonisation guideline recommends applying a full analysis set that excludes these participants from the main analyses as a conservative strategy.39
Additional analyses
Additional analyses using a per-protocol set will be conducted to assess the extent to which adherence affects the results. The per-protocol set will include participants who have practised at least 75% of the stipulated home-based Radio-Taiso exercises.40
A sensitivity analysis will be performed to assess heterogeneity owing to missing follow-up data (missing data bias). Missing data will be processed through multiple imputation by applying the chained equation method to generate 20 imputed data sets based on the outcome, allocation and group variables at baseline.
To assess study generalisability, subgroup analyses will be conducted based on participants’ background data (male vs female, <75 vs ≥75 years of age and frailty vs prefrailty).
Retention rates and the incidence of adverse events (one or more for those who report them) will be compared between groups using Fisher’s exact test or the χ2 test. All analyses will be carried out using R V.4.1.2 or higher (The R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
This study will be conducted without participant involvement. Participants will not be invited to comment on study design, define relevant outcomes or interpret the results, nor contribute to the writing or editing of this paper for readability or accuracy.
Ethics and dissemination
The research protocol will be conducted in accordance with the Declaration of Helsinki. The Research Ethics Committee of TMIG approved the protocol on 16 December 2021. The study protocol was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) on 20 March 2022. All amendments to the study protocol will be disclosed to UMIN-CTR.
Study results will be presented at an international scientific conference and reported in a peer-reviewed international journal. After publication, a summary of the results will be published on the TMIG and Japan Post Insurance. websites.
Discussion
Few trials have examined the effect of exercise interventions on HR-QoL in older adults with frailty, diagnosed with validated assessment tools.3 5 6 Thus, this robustly designed trial will strengthen the evidence needed for or against the effectiveness of home-based exercise programmes on HR-QoL. International health policy bodies, regulators and patients are increasingly recognising the importance of patient-reported outcomes, including HR-QoL, for health.41 The results of this study will provide valuable insights that may serve to address the evidence and clinical practice gaps associated with care programmes for older adults with frailty. This study will also contribute to a better understanding of the potential mechanisms by which home-based exercise programmes can improve HR-QoL, by analysing various secondary outcomes.
Researchers have systematically compared HR-QoL between frailty and non-frailty groups, showing a moderate or greater standardised mean difference between the groups and that the frailty group had a worse HR-QoL.42 The measures to curb the COVID-19 pandemic also limit the life-space mobility of older adults with frailty and have a significant impact on QoL.43 Patient-reported outcomes from clinical trials using scientifically robust methodologies are reflected in clinical decision-making and influence health and social care policy.41 Thus, if this study helps to clarify the effectiveness of the proposed programme on HR-QoL, this may enable invested stakeholders to develop a policy framework for care programmes of older adults with frailty.
For a care programme targeting people with frailty to be implemented in public health policy, it must be accessible (ie, low cost and high acceptability/availability) and be delivered equitably and continuously.4 A care programme that draws on existing cultural and social resources may be able to meet these requirements. In Japan, the Radio-Taiso exercise programme has a long history of being customarily watched in different settings and is familiar to many older adults; thus, it is a cultural exercise programme in the country.10 In fact, our pilot trial confirmed that adherence to the programme is very high and that it is highly compatible with daily life. Radio-Taiso is also available at low cost to everyone via television/radio on a daily basis. To date, more than 24 600 certified Radio-Taiso instructors have been trained, and the programme is practised nationwide at more than 2000 regional bases.44 Rich in cultural and social resources, the Radio-Taiso exercise programme may serve as a socially implementable public health strategy.
This study has several limitations. First, although the study addresses measurement, attrition and reporting bias, participants and treatment providers will not be blinded to allocation information; this increases the risk of performance bias and reduces effect estimation accuracy. Second, calisthenic programmes such as Radio-Taiso are popular in some countries, such as China, Korea, and Scandinavian countries, but it remains unclear to what extent the usefulness of the Radio-Taiso exercise programmes is generalisable to non-Japanese older adults with frailty.
bmjopen-2022-063201supp002.pdf (99.6KB, pdf)
Supplementary Material
Acknowledgments
We are grateful to the participants and the staff members of the TMIG.
Footnotes
Contributors: YO: study concept and design, data collection and interpretation, manuscript preparation. NK: study concept and design, data collection and interpretation, manuscript preparation. MS: study concept and design, safety assessment, manuscript preparation. TO: study concept and design, safety assessment, manuscript preparation. KM: study concept and design, data collection and interpretation, manuscript preparation. TU: study concept and design, data collection and interpretation, manuscript preparation. KM: study concept and design, statistical advice, randomisation, data interpretation, manuscript preparation. RO: study concept and design, intervention, data interpretation, manuscript preparation. TA: study concept and design, data interpretation, manuscript preparation. SI: study concept and design, data interpretation, manuscript preparation. HK: study concept and design, data interpretation, manuscript preparation. HS: study concept and design, analysis, data interpretation, manuscript preparation.
Funding: This study will be conducted under a collaborative agreement between four institutions (TMIG, Tokyo Medical University, Japan Post Insurance Co., Ltd., and the Japan Radio-Taiso Federation). Japan Post Insurance Co., Ltd. will financially support the study and will provide the Radio-Taiso DVD free of charge but will not be involved in the design of the study, selection of methods, recruitment of participants, data collection, analysis and interpretation, or writing of the manuscript. The Japan Radio-Taiso Federation will provide certified Radio-Taiso instructors free of charge and will be involved in the design of the study, intervention, data interpretation, and writing of the manuscript.
Competing interests: YO is the principal investigator of a joint research agreement between the four organisations; RO and TA are accredited instructors for the Japan Radio-Taiso Federation. Japan Post Insurance Co., Ltd. and the Japan Radio-Taiso Federation are committed to the promotion and dissemination of Radio-Taiso. The other authors have no conflicts of interest to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Patient consent forms are attached in supplementary material 2.
References
- 1.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752–62. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–57. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 3.Puts MTE, Toubasi S, Andrew MK, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing 2017;46:383–92. 10.1093/ageing/afw247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent E, Martin FC, Bergman H, et al. Management of frailty: opportunities, challenges, and future directions. Lancet 2019;394:1376–86. 10.1016/S0140-6736(19)31785-4 [DOI] [PubMed] [Google Scholar]
- 5.Negm AM, Kennedy CC, Thabane L, et al. Management of frailty: a systematic review and network meta-analysis of randomized controlled trials. J Am Med Dir Assoc 2019;20:1190–8. 10.1016/j.jamda.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 6.Clegg AP, Barber SE, Young JB, et al. Do home-based exercise interventions improve outcomes for frail older people? findings from a systematic review. Rev Clin Gerontol 2012;22:68–78. 10.1017/S0959259811000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarrete-Villanueva D, Gómez-Cabello A, Marín-Puyalto J, et al. Frailty and physical fitness in elderly people: a systematic review and meta-analysis. Sports Med 2021;51:143–60. 10.1007/s40279-020-01361-1 [DOI] [PubMed] [Google Scholar]
- 8.Japan Post Insurance Co. Ltd . Recognition and participation rates for "Radio-Taiso"; 2021. https://www.jp-life.japanpost.jp/information/assets/pdf/211011pr-6-2.pdf [Accessed 10 Mar 2022].
- 9.Public Relations Office . Government of Japan. Higlighting Japan 2019 [14-15]. Available: https://dwl.gov-online.go.jp/video/cao/dl/public_html/gov/book/hlj/20191001/html5.html#page=15137
- 10.British Broadcasting Corporation . The lifelong exercise that keeps Japan moving, 2020. Available: https://www.bbc.com/worklife/article/20200609-the-life-long-exercise-that-keeps-japan-moving [Accessed 10 Mar 2022].
- 11.Kumagai S, Watanabe S, Shibata H, et al. [Effects of dietary variety on declines in high-level functional capacity in elderly people living in a community]. Nihon Koshu Eisei Zasshi 2003;50:1117–24. [PubMed] [Google Scholar]
- 12.Motokawa K, Watanabe Y, Edahiro A, et al. Frailty severity and dietary variety in Japanese older persons: a cross-sectional study. J Nutr Health Aging 2018;22:451–6. 10.1007/s12603-018-1000-1 [DOI] [PubMed] [Google Scholar]
- 13.Fukuhara S, Bito S, Green J, et al. Translation, adaptation, and validation of the SF-36 health survey for use in Japan. J Clin Epidemiol 1998;51:1037–44. 10.1016/S0895-4356(98)00095-X [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara S, Ware JE, Kosinski M, et al. Psychometric and clinical tests of validity of the Japanese SF-36 health survey. J Clin Epidemiol 1998;51:1045–53. 10.1016/S0895-4356(98)00096-1 [DOI] [PubMed] [Google Scholar]
- 15.Suzukamo Y, Fukuhara S, Green J, et al. Validation testing of a three-component model of short Form-36 scores. J Clin Epidemiol 2011;64:301–8. 10.1016/j.jclinepi.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 16.Fukuhara S, Suzukamo Y. Manual of SF-36v2 Japanese version. Kyoto: iHope international Inc 2004, 2019. [Google Scholar]
- 17.Maruish ME. User's manual for the SF-36v2 health survey. Quality Metric Incorporated 2011. [Google Scholar]
- 18.Satake S, Arai H. The revised Japanese version of the cardiovascular health study criteria (revised J-CHS criteria). Geriatr Gerontol Int 2020;20:992–3. 10.1111/ggi.14005 [DOI] [PubMed] [Google Scholar]
- 19.Shinkai S, Watanabe S, Kumagai S, et al. Walking speed as a good predictor for the onset of functional dependence in a Japanese rural community population. Age Ageing 2000;29:441–6. 10.1093/ageing/29.5.441 [DOI] [PubMed] [Google Scholar]
- 20.Sewo Sampaio PY, Sampaio RAC, Yamada M, et al. Systematic review of the kihon checklist: is it a reliable assessment of frailty? Geriatr Gerontol Int 2016;16:893–902. 10.1111/ggi.12833 [DOI] [PubMed] [Google Scholar]
- 21.Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act 1999;7:129–61. 10.1123/japa.7.2.129 [DOI] [Google Scholar]
- 22.Mannion AF, Knecht K, Balaban G, et al. A new skin-surface device for measuring the curvature and global and segmental ranges of motion of the spine: reliability of measurements and comparison with data reviewed from the literature. Eur Spine J 2004;13:122–36. 10.1007/s00586-003-0618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Post RB, Leferink VJM. Spinal mobility: sagittal range of motion measured with the SpinalMouse, a new non-invasive device. Arch Orthop Trauma Surg 2004;124:187–92. 10.1007/s00402-004-0641-1 [DOI] [PubMed] [Google Scholar]
- 24.Adjutant General's Office . Army individual test battery. Washington, DC: War Department, 1944. [Google Scholar]
- 25.Arita N, Takenaka K, Shimazaki T. Development of a home-exercise barrier self-efficacy scale for elderly people requiring support and care. J Jpn Phys Ther Assoc 2014;41:338–46. [Google Scholar]
- 26.Yatomi N. The factor structure and item characteristic of the GDS (Geratric depression scale) short version in a Japanese elderly sample. Jpn J Geriatr 1994;16:29–36. [Google Scholar]
- 27.Park H, Poo M-ming. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 2013;14:7–23. 10.1038/nrn3379 [DOI] [PubMed] [Google Scholar]
- 28.Knaepen K, Goekint M, Heyman EM, et al. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med 2010;40:765–801. 10.2165/11534530-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 29.Kurimoto A, Awata S, Ohkubo T, et al. [Reliability and validity of the Japanese version of the abbreviated Lubben social network scale]. Nihon Ronen Igakkai Zasshi 2011;48:149–57. 10.3143/geriatrics.48.149 [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi S, Murakami K, Sasaki S, et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 D dietary records in Japanese adults. Public Health Nutr 2011;14:1200–11. 10.1017/S1368980011000504 [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi S, Honda S, Murakami K, et al. Both comprehensive and brief self-administered diet history questionnaires satisfactorily RANK nutrient intakes in Japanese adults. J Epidemiol 2012;22:151–9. 10.2188/jea.JE20110075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oshima Y, Kawaguchi K, Tanaka S, et al. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture 2010;31:370–4. 10.1016/j.gaitpost.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 33.Ohkawara K, Oshima Y, Hikihara Y, et al. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br J Nutr 2011;105:1681–91. 10.1017/S0007114510005441 [DOI] [PubMed] [Google Scholar]
- 34.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181–8. 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 35.Doi Y, Minowa M, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the pittsburgh sleep quality index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res 2000;97:165–72. 10.1016/S0165-1781(00)00232-8 [DOI] [PubMed] [Google Scholar]
- 36.Doi Y, Minowa M, Okawa M. Development of the Japanese version of the pittsburgh sleep quality index. Japan Journal of Psychiatry Treatment 1998;13:755–63. [Google Scholar]
- 37.Faul F, Erdfelder E, Lang A-G, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 38.Senn S. Testing for baseline balance in clinical trials. Stat Med 1994;13:1715–26. 10.1002/sim.4780131703 [DOI] [PubMed] [Google Scholar]
- 39.The International Council for Harmonisation . E9 statistical principles for clinical trials: the International Council for harmonisation, 1998. https://database.ich.org/sites/default/files/E9_Guideline.pdf [Google Scholar]
- 40.Hawley-Hague H, Horne M, Skelton DA, et al. Review of how we should define (and measure) adherence in studies examining older adults' participation in exercise classes. BMJ Open 2016;6:e011560. 10.1136/bmjopen-2016-011560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA 2018;319:483–94. 10.1001/jama.2017.21903 [DOI] [PubMed] [Google Scholar]
- 42.Crocker TF, Brown L, Clegg A, et al. Quality of life is substantially worse for community-dwelling older people living with frailty: systematic review and meta-analysis. Qual Life Res 2019;28:2041–56. 10.1007/s11136-019-02149-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saraiva MD, Apolinario D, Avelino-Silva TJ, et al. The impact of frailty on the relationship between life-space mobility and quality of life in older adults during the COVID-19 pandemic. J Nutr Health Aging 2021;25:440–7. 10.1007/s12603-020-1532-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Japan Radio-Taiso Federation . Information of certified instructors and regional bases, 2022. Available: https://www.radio-exercises.org/ [Accessed 10 Mar 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063201supp001.pdf (68.9KB, pdf)
bmjopen-2022-063201supp003.pdf (57.5KB, pdf)
bmjopen-2022-063201supp002.pdf (99.6KB, pdf)