Abstract
Introduction
Chronic musculoskeletal pain causes a significant burden on health and quality of life and may result from inadequate treatment of acute musculoskeletal pain. The emergency department (ED) represents a novel setting in which to test non-pharmacological interventions early in the pain trajectory to prevent the transition from acute to chronic pain. Acupuncture is increasingly recognised as a safe, affordable and effective treatment for pain and anxiety in the clinic setting, but it has yet to be established as a primary treatment option in the ED.
Methods and analysis
This pragmatic clinical trial uses a two-stage adaptive randomised design to determine the feasibility, acceptability and effectiveness of acupuncture initiated in the ED and continued in outpatient clinic for treating acute musculoskeletal pain. The objective of the first (treatment selection) stage is to determine the more effective style of ED-based acupuncture, auricular acupuncture or peripheral acupuncture, as compared with no acupuncture. All arms will receive usual care at the discretion of the ED provider blinded to treatment arm. The objective of the second (effectiveness confirmation) stage is to confirm the impact of the selected acupuncture arm on pain reduction. An interim analysis is planned at the end of stage 1 based on probability of being the best treatment, after which adaptations will be considered including dropping the less effective arm, sample size re-estimation and unequal treatment allocation ratio (eg, 1:2) for stage 2. Acupuncture treatments will be delivered by licensed acupuncturists in the ED and twice weekly for 1 month afterward in an outpatient clinic.
Ethics and dissemination
This study has been reviewed and approved by the Duke University Health System Institutional Review Board. Informed consent will be obtained from all participants. Results will be disseminated through peer-review publications and public and conference presentations.
Trial registration number
Keywords: Pain management, ACCIDENT & EMERGENCY MEDICINE, COMPLEMENTARY MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Two-stage adaptive design balances improved implementation with statistical power to measure effectiveness of acupuncture.
Two types of acupuncture, (battlefield) auricular and peripheral, enable efficient emergency department treatment and are compared with control.
Pragmatic design better replicates real-world conditions but limits assessment of specific versus non-specific effects of acupuncture on pain outcomes.
Includes longitudinal delivery of acupuncture treatments in both the emergency department and outpatient clinic for 1 month to treat acute musculoskeletal pain.
Breadth of biopsychosocial outcomes to assess how acupuncture works and help bridge the gap between eastern and western medicine.
Introduction
Over 40 million adults in the USA suffer from chronic pain, which is pain lasting 3 months or longer.1 Musculoskeletal pain, one of the largest subsets of chronic pain conditions, leads to high rates of healthcare utilisation, increased opioid use, and poor physical, psychological and cognitive health.1 Musculoskeletal pain often results from an acute injury, and if not adequately treated, can transition to a chronic pain condition.2 3 Significant challenges exist for adequately managing musculoskeletal pain due to the heterogenous nature of its causes and pain symptomatology, and standard treatments are often ineffective.4 5 Additionally, numerous biological (eg, inflammatory mediators), psychological (eg, pain catastrophising) and social (eg, social support) factors (referred to collectively as ‘biopsychosocial’ factors) contribute to the complexity of musculoskeletal pain development, severity, progression and disability.6 Recent studies have begun to explore the role of biopsychosocial factors in the transition from acute to chronic pain and how they may serve as targets for intervention.3 7 One strategy to prevent the transition from acute to chronic pain is early intervention using non-pharmacological strategies that influence these biopsychosocial factors. The emergency department (ED) represents a novel setting in which to test non-pharmacological interventions early in the pain trajectory with the goal of preventing the transition from acute to chronic musculoskeletal pain.
Acupuncture is a safe and cost-effective treatment for acute and chronic pain, particularly of the back, neck and shoulder.8 9 Furthermore, acupuncture has shown benefit in treating both pain and anxiety,8 and acts on numerous neural, endogenous opioid and inflammatory pathways,10 thereby representing a broader biopsychosocial intervention than other single pain treatment modalities. However, data on the use of acupuncture for pain management in the ED is limited since acupuncture practitioners are not currently standard or commonplace in US EDs.11 12 A recent meta-analysis of ED studies has shown acupuncture to be superior to sham/placebo and equivalent or better than medications for pain reduction.11 13 Only three small pilot studies have compared acupuncture combined with usual care to usual care alone, with results favouring acupuncture.11 14 No study has compared different acupuncture protocols (eg, battlefield/auricular acupuncture (AA) and peripheral acupuncture (PA)) to determine which is more efficacious, feasible or acceptable in the ED. Moreover, despite evidence that acupuncture is more effective with multiple sessions,15 prior ED studies have not included a longitudinal outpatient acupuncture component for post-ED pain management or longer-term outcomes.
Therefore, the purpose of this study is to determine the effectiveness, feasibility and acceptability of acupuncture initiated in the ED and continued in a group clinic setting for treating acute musculoskeletal pain. The ED population is largely heterogeneous in sociodemographic composition and comprises populations previously excluded from acupuncture studies.16 A pragmatic randomised controlled trial can determine the extent to which ED patients will attend and derive benefit from the full acupuncture experience, while extending the scope and assessment of treatment effectiveness to a more broadly representative US patient population. We hypothesise that, when added to usual care, acupuncture initiated in the ED and continued in an outpatient setting for 1 month is more effective than usual care alone at reducing acute musculoskeletal pain at 1 hour while in the ED and at 1 month after ED visit.
Methods and analysis
Study design
This pragmatic clinical trial uses a two-stage adaptive randomised design to determine the feasibility, acceptability and effectiveness of acupuncture initiated in the ED for treating acute musculoskeletal pain. The objective of the first (treatment selection) stage is to determine the more effective style of ED-based acupuncture, that is, AA based on the Battlefield Acupuncture protocol, or PA, as compared with no acupuncture (NA). The objective of the second (effectiveness confirmation) stage is to confirm the impact of the selected acupuncture arm on pain reduction. An interim analysis is planned at the end of stage 1 based on probability of being the best treatment,17 after which adaptations will be considered including dropping the less effective arm, sample size re-estimation and unequal treatment allocation ratio such as 1:2 ratio for effectiveness in stage 2. Acupuncture treatments will be delivered by licensed acupuncturists in the ED and up to two times per week for 1 month afterward in an outpatient clinic. In this pragmatic design, all study arms will receive usual care for pain management at the discretion of the ED provider who will be blinded to treatment arm.
Study setting and recruitment
Trial participants will be recruited from the Duke University Hospital ED, an urban academic tertiary care referral centre in North Carolina with 80 000 ED visits per year. All study screening, recruitment, informed e-consent (online supplemental appendix 1), and enrolment procedures will be performed by trained clinical research coordinators. Study acupuncturists will be available during enrolment to explain acupuncture treatment to eligible patients. Outpatient acupuncture visits will be scheduled with the study acupuncturists using secure HIPAA-compliant scheduling software and take place at the Duke Integrative Medicine Clinic. Patient recruitment began in February 2020 and is ongoing. The study start date is 10 February 2020, and planned end date is 28 February 2023.
bmjopen-2022-061661supp001.pdf (155.2KB, pdf)
Eligibility criteria
Inclusion criteria
Participants must be adult (age 18 years or older) ED patients with pain in the neck, back, arms and/or legs and a clinical diagnosis of acute (≤7 days) musculoskeletal pain as determined by an ED provider, and able to read and understand the consent form in English. Participants with acute exacerbation of chronic pain in which the acute component is ≤7 days will be included, as this is a common ED presentation.18
Exclusion criteria
Patients will be excluded if they are: (1) suspected to have a non-musculoskeletal cause of pain, (2) unable to receive acupuncture due to injury, infection or other contraindication to the use of needles at acupuncture sites; (3) not possible to attend outpatient clinic (eg, visiting from out-of-state); (4) unable to provide informed consent or to comprehend or complete study measures or procedures due to cognitive impairment, including evidence of drug, medication or alcohol intoxication, or due to severe hearing or speech impairment; (5) unable to safely participate due to critical illness, obvious bony deformity, other serious medical condition (including active COVID-19 infection) and/or based on ED provider judgement.
Randomisation and blinding
Subjects will be randomised 1:1:1 to one of three treatment groups: (1) AA, (2) PA or (3) the control group with NA. The randomisation code will be computer generated by the Biostatistics, Epidemiology and Research Design Methods Core of the Department of Biostatistics and Bioinformatics, Duke University Medical Center. An unstratified block randomisation method will be used to generate the random allocation sequence, which will be stored in a secure electronic file accessible only by the acupuncturists to ensure allocation concealment. The research coordinator enrolling eligible patients will be blinded to the allocation sequence and randomisation assignments. After participant baseline measures and acupuncturist initial clinical assessment, the treating acupuncturist will open the randomisation file and assign the participant to the treatment group corresponding to the next sequential entry.
The participants and the acupuncturists will not be blinded to their treatment allocation. All other members of the ED clinical and research teams will be blinded. Round stickers will be applied to the ears of all participants at the battlefield protocol sites to blind these members to patient assignment while in the ED. If the participant reports an adverse event (AE), the research coordinator may become unblinded to record and address the event. Due to the adaptive nature of the statistical design, the statisticians and data safety monitoring committee (DSMC) will be unblinded to the control treatment arm in stage 1 to perform the interim analysis; the statistician analysing the data will remain blinded to the treatment arms. All other study investigators will remain blinded.
Interventions
Acupuncture will only be performed by licensed acupuncturists. For this study, two styles of acupuncture designed to increase feasibility in the ED will be employed: (1) AA will involve the placement of pyonex needles in up to five sites on each ear based on the previously developed battlefield acupuncture protocol to treat pain.19 20 (2) PA will involve the placement of needles in head, neck, arms, legs, hands and feet sites selected at the clinical discretion of the treating acupuncturist based on acupuncture diagnosis as the primary mode of therapy.21 22 Acupuncture sites on the torso (ie, chest, back and abdomen) will not be used, as accessing these sites is often logistically challenging in a busy ED environment. Both acupuncture groups will receive acupuncture while in the ED. Afterwards, both groups will receive information and free access to acupuncture in an outpatient clinic for up to two times a week for 1 month after their ED visit. Our outpatient acupuncture clinic is designed as a group-based clinic modified for COVID-19-related social distancing to enhance access and affordability. All post-ED outpatient acupuncture treatments for both acupuncture groups will involve either PA, AA or both at the clinical discretion of the treating acupuncturist. The specific components of each acupuncture treatment will be recorded in details according to the revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) recommendations.23 (3) The control group (NA) will not receive acupuncture in the ED and will be asked not to seek acupuncture treatment for 1 month after their ED visit. Control group participants will complete assessments only, with the timing and content of their assessments matching those completed by the intervention groups.
All participants in the control group and in both acupuncture groups will receive usual care for acute pain management at the discretion of their ED provider who will remain blinded to study arm. Usual care may include but is not limited to medications/analgesics, non-pharmacological strategies (eg, ice, heat, walking), and referrals to outpatient specialists and/or other non-pharmacological treatment providers (eg, physical therapy).
Outcomes
Outcome measures and biopsychosocial factors will be collected before randomisation (ED baseline), 1 hour after randomisation, and at 2 weeks and 1 month post-ED visit.
Primary outcome measures
The primary ED effectiveness endpoint will be the change in current pain score based on the 0–10 pain Numeric Rating Scale (NRS) from ED baseline to 1 hour post-treatment. The primary combined ED-outpatient clinic effectiveness endpoint will be change in 24-hour average NRS pain score from ED baseline to 1-month post-ED visit.
Feasibility will be assessed based on patient recruitment and retention rates both in the ED and with subsequent 1-month follow-up. Acceptability will be assessed based on patient-reported satisfaction as well as outpatient acupuncture clinic attendance rates, with attention to reasons for attrition or differential acceptance rates for different groups of participants. Safety will be evaluated by recording any AEs, with common reasons to include bleeding, bruising, or pain at the needle sites. Serious AEs (SAEs) are expected to be extremely rare given previous highly favourable safety data on acupuncture, and include infections, hospitalisations and deaths.21 24
Secondary outcome measures
Secondary outcomes will include patient function, quality of life and biopsychosocial factors.
Function and quality-of-life will be measured at ED baseline, 2 weeks and 1 month across several different domains, including pain interference, fatigue, depression, anxiety, sleep disturbance, physical function, social function and cognitive function, using the validated PROMIS-29 and Neuro-QoL instruments.25 26 Given the acute time course of pain (7 days or less) for eligible participants, the timeframe for the PROMIS-29 questions will be modified from ‘over the past 7 days’ to ‘over the past 24 hours (1 day)’ for the ED baseline assessment only. We will also measure patient-reported medication use, including opioid and non-opioid medications. Opioid use will be assessed through patient report in the past 24 hours and in the past 7 days, as well as by electronic medical record (EMR) data extraction of prescriptions written during and up to 1 year after the ED visit.
A comprehensive set of biopsychosocial factors will be measured at ED baseline and 1-month follow-up. These include:
Patient demographics including age, sex, race, ethnicity, employment, marital status, education, income and insurance status.
Pain characteristics including anatomical location of pain, duration of current episode of musculoskeletal pain, and history of prior episodes of musculoskeletal pain.
Degree and type of social support, including instrumental, informational and emotional support, will be measured using the PROMIS Social Support four-item scales.27
All non-medical substance use, including opioid misuse, through the validated ASSIST tool.28
Presence and severity of chronic pain using a recently validated simplified version of the graded chronic pain scale derived from the three-item Pain, Enjoyment and General activity (PEG) score and degree of activity limitations.29 30
Symptoms of systemic pathology will be measured using the validated Optimal Screening for Prediction of Referral and Outcome Review of Systems (OSPRO) tool, which predicts pain outcomes after musculoskeletal care in outpatient physical therapy settings.31 32
Pain-related psychological distress using the validated concise OSPRO Yellow Flag tool, an assessment tool for measuring psychological response to pain including pain coping, catastrophising, fear-avoidance and mood.32 33
Pain-related emotional distress based on the Perceived Stress Scale tool.34
Pain coping skills using the Coping Skills Questionnaire two-item form.35
Pain self-efficacy using the Pain Self-Efficacy Questionnaire.36
Two additional measures will be collected in-person in the ED at baseline and 1 hour:
Pressure pain threshold, a non-invasive quantitative test of pain sensitivity that measures the lowest applied pressure needed to evoke to an individual’s perception of pain, will be performed on the bilateral trapezius muscles using slow progressive pressure (1 kg/cm2/s) with a standard hand-held algometer (Wagner Digital Force Gauge, Wagner Instruments, Greenwich, Connecticut, USA).37 38
Blood samples will be collected and stored in a secure repository for future biomarker analysis of biochemical and genetic pathways involved in pain and response to acupuncture. Participants may opt out of the blood draw and still participate in the clinical trial.
Additional EMR data will be extracted by trained analysts and include medications administered and prescribed from the ED, opioids prescribed up to 1 year following index ED visit, return ED visits and hospitalisations up to 3 months following index ED visit, and ICD-10 codes for pain conditions and co-occurring diagnoses (eg, medical and psychiatric comorbidities).
Data collection and management
Data will be collected at ED baseline, 1-hour post-treatment, and at 2 weeks and 1-month post-ED visit, with data entered directly into a REDCap secure electronic database.39 All in-person biological measures including pressure pain threshold and blood draws for biomarkers will be obtained during the ED visit at baseline and 1 hour by research coordinators embedded in the ED. Pressure pain threshold will be recorded in REDCap. Blood samples will be coded with a unique study identifier and deidentified for storage.
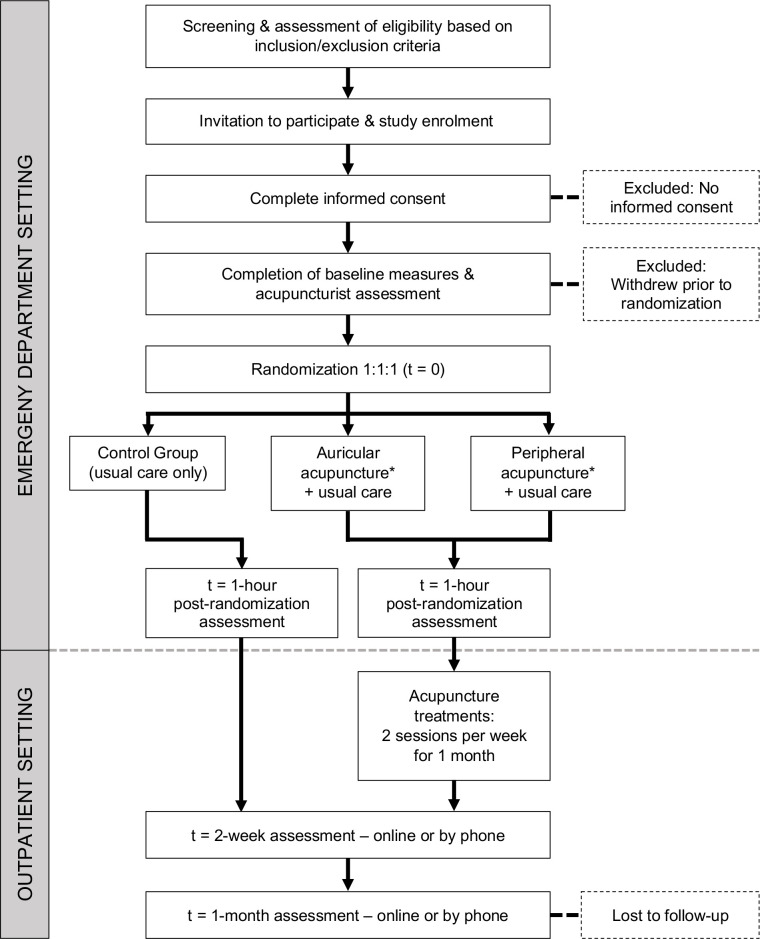
ED baseline and 1-hour questionnaires to collect biopsychosocial factors and outcome measures will be completed by participants independently and entered directly into REDCap while they are in the ED (figure 1). Research coordinators will be available for assistance with data entry if requested by participants. Participants will be contacted at 2 weeks and 1-month post-ED visit to complete online REDCap follow-up surveys (figure 1) using follow-up procedures to facilitate maximum study retention. These procedures include up to three automated electronic follow-up surveys sent via email, automated text message links to the surveys, follow-up phone calls by ED research assistants with the option to complete surveys by phone, and compensation for 1-month follow-up assessment completion.
Figure 1.
Trial flow chart. *After interim analysis in stage 1, the less effective acupuncture arm will be dropped.
Acupuncturist assessments and treatment details in the ED and outpatient clinic will be entered directly into REDCap forms by the treating acupuncturist. ED medication data will be entered into REDCap forms by research coordinators. Acupuncture AEs and SAEs will be entered into REDCap forms by the study personnel notified of the event, and follow-up of the events will be completed within the same forms by the personnel completing follow-ups. Additional EMR data will be extracted by trained analysts and stored in a protected analytics environment. All data will be stored for at least 5 years after study completion for primary and ancillary studies, with access restricted to study staff, and is included in patient consent. To protect confidentiality, no personal information will be shared.
Sample size
For stage 1, a total of 90 subjects (30 subjects per arm) will be used to (1) assess feasibility based on patient recruitment and retention rates, and (2) determine the more effective arm at the ED 1-hour time point for moving forward to stage 2 for effectiveness confirmation. Sample size calculation for achieving stage 2 study objectives assumed17 40 41: (1) a stage 2 two-arm parallel design with 1:2 control:treatment allocation; (2) the primary endpoint is normally distributed; (3) with NA, a mean pain score of 6.5 with an SD of 2.542; (4) a minimally clinically meaningful difference in pain score of 1.342;; (5) a power of 90.0% and (6) a 5% level of significance. This yielded a total sample size of 198 subjects (table 1) at the end of stage 2. To account for a possible 10% drop-out rate requires increasing the total number enrolled to 220 subjects.
Table 1.
Sample size estimation and allocation
| Stage | Randomisation | Sample size | Total |
| Stage 1 | 1:1:1 | 30 (NA), 30 (AA), 30 (PA) | 90 |
| Stage 2 | 1:2 | 36 (NA), 72 (AA or PA) | 108 |
| Total | 198 |
AA, auricular acupuncture; NA, no acupuncture; PA, peripheral acupuncture.
Data analysis and statistical methods
Population: The primary analysis will be performed based on the intention-to-treat population, which is defined as all randomised subjects who have at least one follow-up evaluation regardless of their compliance with the protocol. In case of a substantial number of protocol violations, additional per-protocol analyses may be performed to determine whether they influence the conclusions.
General analysis conventions/rules: Descriptive statistics for continuous variables will be provided as: number of subjects, means and SD, medians and IQRs, and minima and maxima. Descriptive statistics for discrete (categorical) variables will be provided as the number and percentage of subjects in each category. Time-to-event variables will be provided using Kaplan-Meier survival curve estimates. Unless otherwise noted, any tests of hypotheses are two sided, and the nominal level of significance will be 5%.
Handling of missing data: Imputation of missing data will be handled depending on the missingness mechanism.
Baseline comparability: Number of subjects randomised, completing the study and reasons for discontinuation will be summarised by treatment group. Patient demographics and baseline characteristics including biopsychosocial factors will be tabulated and compared for treatment group differences. All comparisons will be performed by using the Cochran-Mantel-Haenszel test for categorical variables and two-way analysis of variance for continuous variables.
Primary analysis: The primary variable, change in pain score from ED baseline to 1-hour post-treatment, will be evaluated and compared between treatment groups. The corresponding 95% CI for the difference in mean response rate between treatment groups will be obtained using analysis of variance (ANOVA).
Secondary analyses: Responder analysis will also be performed using a logistic regression analysis that incorporates potential risk factors identified for response. Point estimate and the corresponding 95% CI of ORs for the identified risk factors will also be obtained. In addition, the Stuart-Maxwell test may be performed to examine changes (or shifts) from baseline to follow-ups after ED discharge.
Exploratory analyses: Exploratory analyses such as biomarker changes pre to 1-hour post-treatment, subgroup analyses based on patient demographics and/or patient characteristics, and predictive model building, validation and/or generalisability may be conducted as deemed appropriate by the principal investigator(s), biostatistician or as recommended by an internal established DSMC. These include secondary analyses of the impact on outcomes of the number of acupuncture needle sites or number of acupuncture pathways used, number of clinic visits attended, within treatment pain and/or anxiety reductions, among others. If possible, additional exploratory models predicting response to acupuncture based on biopsychosocial factors will be examined.
Safety analysis: Table will show the AEs ordered by decreasing frequency for all participants. Separate tabulations will summarise the AEs by seriousness, severity, and possible association with study drug. If appropriate, the incidence rate of AEs will be compared by Fisher’s exact test. Special attention will be given to those subjects who have discontinued due to AEs and those subjects who experienced an SAE.
Interim analysis: There will be one planned interim analysis which will take place when two-thirds of subjects (ie, 20 of 30 subjects per arm) have completed stage 1. At interim analysis, the feasibility will be assessed based on patient recruitment and retention rates, and the more effective arm will be determined based on change in pain score at the 1-hour ED time point based on probability of being the more effective treatment.17 Some adaptations such as modifying current treatment arm, different randomisation scheme, additional interim analyses and/or sample size re-estimation may be applied as recommended by the DSMC.
Data monitoring
A DSMC comprised an independent biostatistician, emergency medicine physician–researcher and a medical acupuncturist-pain medicine clinician will meet at least two times per year with ad hoc reports as needed, to monitor the safety and performance quality of the trial. The DSMC will also evaluate the interim analysis to make recommendations on adaptations for stage 2 to the study investigators.
AEs and SAEs and their follow ups will be recorded in a secure REDCap file. AEs will be reviewed weekly, and SAEs will be reviewed immediately by the principal investigator and lead research coordinator and addressed as needed. All SAEs will be reported within 24 hours to the DSMC, IRB and study sponsor.
Ethics and dissemination
The Duke University Health System Institutional Review Board has reviewed and approved this study (Protocol # Pro00104140). This trial was registered on 7 February 2020 with clinicaltrials.gov (registration # NCT04290741) and released to the public on 28 February 2020. We used the Standard Protocol Items: Recommendations for Interventional Trials checklist when writing our report.43 On completion of the trial, the results will be disseminated through peer-review publications as well as presentations at professional organisation conferences and to the public including healthcare organisations.
Patient and public involvement
Patients and the public were not involved in the original design of the study. However, patient participants will be interviewed for feedback on their acupuncture and research experience to potentially inform future adaptations.
Discussion
Adaptive design: The innovative adaptive design of this study enables findings from the first stage to be used to increase the likelihood of success in the second stage for measuring the true effectiveness of acupuncture in an ED population. Adaptations to drop the least effective arm and/or mitigate issues that arise in stage 1 are designed to optimise implementation of acupuncture for both the study and its broader applicability to other settings.44 Efforts will be made to limit potential bias from adaptations and maintain trial integrity and validity by using an independent DSMC to decide on any adaptations.
Novel care pathways: This will be one of the first randomised trials to integrate acupuncture into ED care and to establish linkages to outpatient acupuncture treatment. It will also be one of the first acupuncture studies to include a diverse sociodemographic population and to use a group-based clinic to enhance access and affordability of this treatment option.16 Patient knowledge of, access to, and availability of non-pharmacological therapies are frequent barriers to use.16 45 Post-ED follow-up can be particularly challenging among ED patients due to cost and time constraints, so lowering these barriers are key to improving access to care. Furthermore, initial therapeutic experience with acupuncture has been shown to increase patient follow-through with continued acupuncture,46 highlighting the benefit of combining ED with outpatient care. Goals of the study include reducing patient need for pain medications, particularly opioids, and return ED visits for pain control through use of acupuncture. Our findings will inform future ED and follow-up acupuncture treatment recommendations.
Tailoring acupuncture to the ED environment: Comparing AA to PA in the ED setting is a key component of our study, as it allows further exploration of the feasibility and acceptability of ED-based acupuncture through two different patient experiences. AA can be delivered quickly, easily and without removal of clothing, thus fitting well within the space and time constraints of the ED environment.19 Furthermore, specific types of auricular needles can be left in place for later self-stimulation by patients to provide additional pain relief for an additional 1–5 days.19 47 Use of AA can be limited by patient discomfort with needles in the ear, potential compatibility issues with obtaining CT and MRI imaging while needles are in place, and lack of clinical guidelines for its use.
PA allows for greater personalisation of treatment than AA by offering a much larger number of meridians or channels that the acupuncturist can access for pain relief.48 49 PA also offers the flexibility to adapt treatments for very anxious or needle-sensitive patients because needle depth and level of stimulation can be modified to suit individual needs. While tight clothing may limit the number of accessible acupuncture points, this is typically not a major barrier for experienced acupuncturists.
While PA can take longer for the patient, 20–45 min for PA compared with 10–20 min for AA, spending more time with the acupuncturist can contribute to their increased sense of support and better anxiety relief. In addition, PA can be more efficient for the acupuncturist than AA when treating multiple patients, as the acupuncturist can leave one patient while needles are in place to tend to the next patient, returning later for needle removal and session completion. By contrast, AA involves frequent patient reassessments between each needle insertion requiring the acupuncturist’s full attention until session completion before proceeding to the next patient.
Usual care for ED pain management: The choice of usual care for all treatment arms was based on the goal of developing a practical and feasible intervention in the ED setting where medications are expected by patients but can be variably prescribed among providers.50 51 Therefore, restriction of medications from any one arm could be perceived as undesirable or unethical by ED patients seeking care. In addition, choice of medication can depend on many factors, including provider and patient preferences, and allergies, adverse reactions or contraindications to specific medications. Therefore, in order to increase the applicability of our findings, the decision was made to allow provider judgement to dictate medication choice as well as dosing. This has the added benefit of managing breakthrough pain through usual ED provider reassessment and repeat dosing as deemed clinically appropriate. ED providers were kept blinded to treatment arm so that their usual clinical judgement determined usual care treatment. Thus, the results of this trial will reflect the results expected in actual clinical practice in an academic ED.
The choice of NA for the control group as compared with sham or other placebo was based on the goal of studying the effect of acupuncture in a pragmatic setting. Given the high volume, high throughput environment of most US EDs, there is not a typical usual care option that would equate to a placebo or sham intervention. For instance, most EDs do not have the time or resources to routinely provide another non-pharmacological practitioner or additional ED staff member who could devote extra time for patient support. Thus, the alternative to acupuncture in most settings would simply be NA, with a focus on medication prescriptions, supportive care, and/or, less commonly, outpatient referrals (eg, primary care, physical therapist and orthopedist) for further management.
Breadth of study outcomes: This study will also generate data on biopsychosocial factors to better characterise the population of patients seen in the ED for acute musculoskeletal pain. Exploration of these factors may also identify mediators of the patient response to acupuncture. These mediators may help identify patients more likely to improve with acupuncture and/or better elucidate potential mechanisms of acupuncture’s therapeutic effects. Findings from this study will further our understanding of acute pain and its non-pharmacological management through acupuncture, as well as their associations with the comprehensive set of biopsychosocial factors.
Supplementary Material
Acknowledgments
The authors are grateful to Tara BiancaRadofor herinputas a licensed acupuncturiston the design and acupuncture treatment details of this study. We are also grateful to Andrew Bouffler, Lauren McGowan and Tedra Porter for informing the study design through their experience and insight as clinical research coordinators.
Footnotes
Contributors: SAE drafted the study protocol manuscript. OG, CAS and MRK participated in the design of the study and revising the protocol manuscript. SC, MK and AG were responsible for the statistical design of the study and revising the protocol manuscript. CDL, MM, AD, AMWM and AL provided clinical advice and made critical revisions to the protocol and manuscript. EW, AO'R, OT and JCD were involved as clinical research coordinators in revising and editing the protocol and manuscript. SAE is principal investigator of the study and is responsible for making final decisions on the trial design and manuscript preparation. All authors approved the final manuscript.
Funding: This project is supported by The Substance Abuse and Mental Health Services Administration (SAMHSA) Emergency Department Alternatives to Opioids Demonstration Program (ED-ALT), grant number H79TI083109. This project is included as part of the Duke School of Medicine Opioid Collaboratory which is administered through the Duke Department of Population Health Sciences and supported by grant funding from the Duke Endowment. The Collaboratory's mission is to save lives and reduce the harmful impact of opioids in North Carolina through the development, implementation, and/or evaluation of system-level interventions. The Duke Biostatistics, Epidemiology, and Research Design Core’s support was made possible by the CTSA Grant (UL1TR002553) from the National Center for Advancing Translational Sciences (NCATS) of the NIH and the NIH Roadmap for Medical Research.
Disclaimer: The study funders had no role in the study design, collection or analysis of data. This report’s contents are solely the responsibility of the authors and do not represent the official views of NCATS or NIH.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Pitcher MH, Von Korff M, Bushnell MC, et al. Prevalence and profile of high-impact chronic pain in the United States. J Pain 2019;20:146–60. 10.1016/j.jpain.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman BW, Conway J, Campbell C, et al. Pain one week after an emergency department visit for acute low back pain is associated with poor three-month outcomes. Acad Emerg Med 2018;25:1138–45. 10.1111/acem.13453 [DOI] [PubMed] [Google Scholar]
- 3.Stevans JM, Delitto A, Khoja SS, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open 2021;4:e2037371. 10.1001/jamanetworkopen.2020.37371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, Deyo R, Friedly J, et al. Systemic pharmacologic therapies for low back pain: a systematic review for an American College of physicians clinical practice guideline. Ann Intern Med 2017;166:480–92. 10.7326/M16-2458 [DOI] [PubMed] [Google Scholar]
- 5.Busse JW, Sadeghirad B, Oparin Y, et al. Management of acute pain from Non–Low back, musculoskeletal injuries. Ann Intern Med 2020;173:730–8. 10.7326/M19-3601 [DOI] [PubMed] [Google Scholar]
- 6.Edwards RR, Dworkin RH, Sullivan MD, et al. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain 2016;17:T70–92. 10.1016/j.jpain.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kent ML, Tighe PJ, Belfer I, et al. The ACTTION-APS-AAPM pain taxonomy (AAAPT) multidimensional approach to classifying acute pain conditions. J Pain 2017;18:479–89. 10.1016/j.jpain.2017.02.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan AY, Miller DW, Bolash B, et al. Acupuncture's role in solving the opioid epidemic: evidence, cost-effectiveness, and care availability for acupuncture as a primary, Non-Pharmacologic method for pain relief and Management-White paper 2017. J Integr Med 2017;15:411–25. 10.1016/S2095-4964(17)60378-9 [DOI] [PubMed] [Google Scholar]
- 9.Chow SC. Quantitative methods for traditional Chinese medicine development. New York: Chapman and Hall/CRC Press, Taylor & Francis, 2015. [Google Scholar]
- 10.McDonald JL, Cripps AW, Smith PK. Mediators, receptors, and signalling pathways in the anti-inflammatory and antihyperalgesic effects of acupuncture. Evid Based Complement Alternat Med 2015;2015:1–10. 10.1155/2015/975632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jan AL, Aldridge ES, Rogers IR, et al. Review article: does acupuncture have a role in providing analgesia in the emergency setting? A systematic review and meta-analysis. Emerg Med Australas 2017;29:490–8. 10.1111/1742-6723.12832 [DOI] [PubMed] [Google Scholar]
- 12.Chia KL, Lam RPK, Lam CK, et al. Acupuncture in the emergency department: a systematic review of randomised controlled trials. Acupunct Med 2018;36:183–92. 10.1136/acupmed-2017-011547 [DOI] [PubMed] [Google Scholar]
- 13.Grissa MH, Baccouche H, Boubaker H, et al. Acupuncture vs intravenous morphine in the management of acute pain in the ED. Am J Emerg Med 2016;34:2112–6. 10.1016/j.ajem.2016.07.028 [DOI] [PubMed] [Google Scholar]
- 14.Goertz CMH, Niemtzow R, Burns SM, et al. Auricular acupuncture in the treatment of acute pain syndromes: a pilot study. Mil Med 2006;171:1010–4. 10.7205/MILMED.171.10.1010 [DOI] [PubMed] [Google Scholar]
- 15.Kligler B, Nielsen A, Kohrherr C, et al. Acupuncture therapy in a group setting for chronic pain. Pain Med 2018;19:393–403. 10.1093/pm/pnx134 [DOI] [PubMed] [Google Scholar]
- 16.Baker K, McDonald J, Steel A. Tackling health inequity: a commentary on the potential of acupuncture to improve health outcomes of marginalised populations. Acupunct Med 2021;39:533-537. 10.1177/0964528420961404 [DOI] [PubMed] [Google Scholar]
- 17.Zheng J, Chow S-C. Criteria for dose-finding in two-stage seamless adaptive design. J Biopharm Stat 2019;29:908–19. 10.1080/10543406.2019.1657130 [DOI] [PubMed] [Google Scholar]
- 18.McLeod D, Nelson K. The role of the emergency department in the acute management of chronic or recurrent pain. Australas Emerg Nurs J 2013;16:30–6. 10.1016/j.aenj.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 19.Niemtzow RC. Battlefield acupuncture. Med Acupunct 2007;19:225–8. 10.1089/acu.2007.0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Federman DG, Zeliadt SB, Thomas ER, et al. Battlefield acupuncture in the Veterans health administration: effectiveness in individual and group settings for pain and pain comorbidities. Med Acupunct 2018;30:273–8. 10.1089/acu.2018.1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hempel S, Taylor SL, Solloway MR. Evidence map of acupuncture. Washington (DC): Department of Veterans Affairs, 2014. [PubMed] [Google Scholar]
- 22.North Carolina Acupuncture Licensing Board . General information: fundamental information about acupuncture. North Carolina acupuncture licensing board website. Available: https://www.ncalb.com/general-information/ [Accessed 31 Mar 2021].
- 23.MacPherson H, Altman DG, Hammerschlag R, et al. Revised standards for reporting interventions in clinical trials of acupuncture (stricta): extending the CONSORT statement. J Evid Based Med 2010;3:140–55. 10.1111/j.1756-5391.2010.01086.x [DOI] [PubMed] [Google Scholar]
- 24.Weidenhammer W, Streng A, Linde K, et al. Acupuncture for chronic pain within the research program of 10 German Health Insurance Funds--basic results from an observational study. Complement Ther Med 2007;15:238–46. 10.1016/j.ctim.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 25.Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63:1179–94. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella D, Lai J-S, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology 2012;78:1860–7. 10.1212/WNL.0b013e318258f744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn EA, DeWalt DA, Bode RK, et al. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol 2014;33:490–9. 10.1037/hea0000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humeniuk R, Henry-Edwards S, Ali R. The alcohol, smoking and substance involvement screening test (assist): manual for use in primary care, 2010. Available: https://www.who.int/substance_abuse/publications/assist/en/ [Accessed 2 Dec 2019].
- 29.Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med 2009;24:733–8. 10.1007/s11606-009-0981-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Von Korff M, DeBar LL, Krebs EE, et al. Graded chronic pain scale revised: mild, bothersome, and high-impact chronic pain. Pain 2020;161:651–61. 10.1097/j.pain.0000000000001758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George SZ, Beneciuk JM, Bialosky JE, et al. Development of a Review-of-Systems screening tool for orthopaedic physical therapists: results from the optimal screening for prediction of referral and outcome (OSPRO) cohort. J Orthop Sports Phys Ther 2015;45:512–26. 10.2519/jospt.2015.5900 [DOI] [PubMed] [Google Scholar]
- 32.George SZ, Beneciuk JM, Lentz TA, et al. Optimal screening for prediction of referral and outcome (OSPRO) for musculoskeletal pain conditions: results from the validation cohort. J Orthop Sports Phys Ther 2018;48:460–75. 10.2519/jospt.2018.7811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lentz TA, Beneciuk JM, Bialosky JE, et al. Development of a yellow flag assessment tool for orthopaedic physical therapists: results from the optimal screening for prediction of referral and outcome (OSPRO) cohort. J Orthop Sports Phys Ther 2016;46:327–43. 10.2519/jospt.2016.6487 [DOI] [PubMed] [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 35.Jensen MP, Keefe FJ, Lefebvre JC, et al. One- and two-item measures of pain beliefs and coping strategies. Pain 2003;104:453–69. 10.1016/S0304-3959(03)00076-9 [DOI] [PubMed] [Google Scholar]
- 36.Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain 2007;11:153–63. 10.1016/j.ejpain.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 37.Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med 2014;15:61–72. 10.1111/pme.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persson AL, Brogårdh C, Sjölund BH. Tender or not tender: test-retest repeatability of pressure pain thresholds in the trapezius and deltoid muscles of healthy women. J Rehabil Med 2004;36:17–27. 10.1080/16501970310015218 [DOI] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow SC, Chang M. Adaptive design methods in clinical trials. 2nd ed. New York: Chapman and Hall/CRC Press, Taylor & Francis, 2011. [Google Scholar]
- 41.Chow S-C, Wang H, Shao J. Sample size calculations in clinical research. Chapman and Hall/CRC, 2007. [Google Scholar]
- 42.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med 2001;38:633–8. 10.1067/mem.2001.118863 [DOI] [PubMed] [Google Scholar]
- 43.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chow S-C. Adaptive clinical trial design. Annu Rev Med 2014;65:405–15. 10.1146/annurev-med-092012-112310 [DOI] [PubMed] [Google Scholar]
- 45.Becker WC, Dorflinger L, Edmond SN, et al. Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Fam Pract 2017;18:41. 10.1186/s12875-017-0608-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas ER, Zeliadt SB, Coggeshall S, et al. Does offering battlefield acupuncture lead to subsequent use of traditional acupuncture? Med Care 2020;58 Suppl 2 9S:S108–15. 10.1097/MLR.0000000000001367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Council of Colleges of Acupuncture and Oriental Medicine . Clean needle technique manual. 7th ed. United States: 2020. Available: https://www.ccaom.org/ccaom/CNT_Manual.asp [Accessed 6 Apr 2021].
- 48.Dorsher PT. Myofascial referred-pain data provide physiologic evidence of acupuncture meridians. J Pain 2009;10:723–31. 10.1016/j.jpain.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 49.Nielsen A, Anderson B, Citkovitz C, et al. Developing and employing a 'responsive manualization' in the 'Acupuncture Approaches to Decrease Disparities in Outcomes of Pain Treatment' comparative effectiveness study. Acupunct Med 2019;37:184–91. 10.1177/0964528419834015 [DOI] [PubMed] [Google Scholar]
- 50.Lee WW, Burelbach AE, Fosnocht D. Hispanic and non-Hispanic white patient pain management expectations. Am J Emerg Med 2001;19:549–50. 10.1053/ajem.2001.28038 [DOI] [PubMed] [Google Scholar]
- 51.Hoppe JA, McStay C, Sun BC, et al. Emergency department attending physician variation in opioid prescribing in low acuity back pain. West J Emerg Med 2017;18:1135–42. 10.5811/westjem.2017.7.33306 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061661supp001.pdf (155.2KB, pdf)