Abstract
Recently, genes for two copper-responsive regulatory systems were identified in the Escherichia coli chromosome. In this report, data are presented that support a hypothesis that the putative multicopper oxidase CueO and the transenvelope transporter CusCFBA are involved in copper tolerance in E. coli.
Copper is required for aerobic life and yet, paradoxically, is highly toxic even at low concentrations. Intracellular copper concentrations therefore need to be regulated within very narrow limits (14). Previous attempts to elucidate copper homeostasis in Escherichia coli have been incomplete. Genes such as cutC, cutF, and ndh have been suggested to be involved in copper homeostasis (9, 15, 17), but their exact roles have not been determined. Recently, two copper-responsive regulatory systems were identified. One is a two-component signal transduction system designated the Cu-sensing locus (cus locus). The cusRS genes form a sensor-regulator pair that activates the adjacent but divergently transcribed genes cusCFBA (10). The cusCBA genes are homologous to a family of proton-cation antiporter complexes involved in export of metal ions, xenobiotics, and drugs. CusF is a putative periplasmic copper-binding protein (5). The other system is regulated by CueR, a copper-activated homologue of MerR. CueR has been shown to regulate two genes, copA and cueO (formerly yacK) (13). CopA is a Cu(I)-translocating P-type ATPase, while CueO is a putative multicopper oxidase (6, 13, 16).
CueO is involved in copper tolerance.
CueO and CopA are both regulated by CueR (13). To determine the role of CueO in copper tolerance, the cueO gene was disrupted. Chromosomal deletions were performed as described by Datsenko and Wanner (4), and the gene of interest was replaced by a chloramphenicol cassette. The ΔcueO::cm cassette was transduced into E. coli W3110 by P1 transduction. The resulting strain, E. coli GR1 (ΔcueO::cm), was slightly more copper sensitive on complex medium than wild-type strain E. coli W3110 (Table 1; Fig. 1). At high copper concentrations the cueO-disrupted strain exhibited a distinctive colony morphology: the colonies were small, colorless, and often mucoid. The copper sensitivity of a cueO deletion-containing strain could be complemented by the presence of the cueO gene on plasmid pTYB2::cueO in trans (Table 1). Other metals tested did not have any effect on a cueO-disrupted strain (data not shown).
TABLE 1.
MICsa of copper for different genetic constructs of E. coli W3110
| Strain | Genotype | MIC of CuCl2 (mM) | Source |
|---|---|---|---|
| W3110 | Wild type | 3.5 | Lab stock |
| GR1 | W3110 ΔcueO::cm | 2.75b | This work |
| GR5 | W3110 ΔcusA::cm | 3.5 | This work |
| GR6 | W3110 ΔcusCFBA::cm | 3.5 | This work |
| GR7 | W3110 ΔcueO::cm/pTYB2::cueO | 3.0 | This work |
| DW3110 | W3110 copA::km | 2.5b | Rensing et al. (16) |
| GR8 | W3110 copA::km ΔcueO::cm | 2.25b | This work |
| GR10 | W3110 ΔcueO ΔcusCFBA::cm | 1.3c | This work |
| GR12 | W3110 copA::km ΔcusCFBA::cm | 2.5b | This work |
| GR13 | W3110 copA::km ΔcusA::cm | 2.5b | This work |
| GR15 | W3110 ΔcueO ΔcusA::cm | 1.3c | This work |
| GR16 | W3110 copA::km ΔcueO cusA::cm | 1.3c | This work |
| GR17 | W3110 copA::km ΔcueO cusCFBA::cm | 1.3c | This work |
| GR18 | W3110 ΔcueO ΔcusCFBA::cm/pTYB2::copA (R. metallidurans CH34) | 3.0 | This work |
| GR19 | W3110 ΔcueO ΔcusCFBA::cm/pACYC::cueO | 3.0 | This work |
| GR20 | W3110 ΔcueO::cm/pACYC::cueO | 3.5 | This work |
| GR21 | W3110 ΔcueO::cm/pTYB2::copA (R. metallidurans CH34) | 3.5 | This work |
The MIC is defined as the concentration at which no growth was observed. Overnight cultures were diluted 1:500 into fresh Luria-Bertani medium, and after 2 h of growth at 37°C, they were streaked out on Luria-Bertani plates with different concentrations of CuCl2. Growth was monitored after 16 h at 37°C.
Colonies were small, and colorless, and sometimes mucoid at concentrations well below the MIC.
Colonies were small and mucoid at 0.5 mM CuCl2.
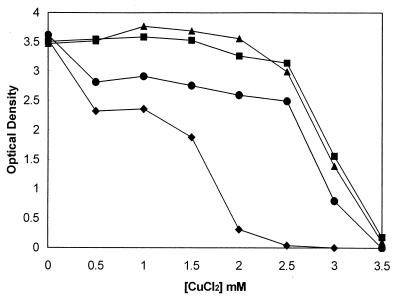
FIG. 1.
Effect of several mutations on copper tolerance. Growth curves with different CuCl2 concentrations are shown. Overnight cultures were diluted 1:500 into fresh Luria-Bertani medium, and after 2 h of growth at 37°C, cells were diluted 1:500 into fresh Luria-Bertani medium with the indicated concentrations of CuCl2. Cell growth was monitored as the optical density at 600 nm after 15 h of incubation at 37°C with shaking. Strains tested include E. coli GR1 ΔcueO::cm (●), E. coli GR6 ΔcusCFBA::cm (▴), E. coli GR10 ΔcueO ΔcusCFBA::cm; (♦), and E. coli W3110 (▪).
The cus determinant encodes a copper efflux system.
There are at least two chromosomal copper-responsive determinants responsible for copper homeostasis. One is the cus determinant, regulated by a two-component signal transduction system encoded by the cusRS genes (10). However, deletion of the cus determinant failed to result in decreased copper tolerance (Table 1; Fig. 1). Disruption of both cueO and cusCFBA::cm in the mutant E. coli GR10 (ΔcueO ΔcusCFBA::cm) resulted in a substantial decrease in copper tolerance. E. coli GR10 was mucoid even at very low copper concentrations, indicating a stress response, and ceased growth at 1.3 mM CuCl2, compared with 2.75 mM for GR1 (ΔcueO::cm) and 3.5 mM for the wild-type E. coli W3110, E. coli GR5(ΔcusA::cm), and E. coli GR6 (ΔcusCFBA::cm) (Table 1). This effect was observed in the presence of copper but not with cadmium, zinc, or cobalt (data not shown). The structural genes cusCFBA resemble genes of proton-cation antiporter complexes such as CzcCBA or SilCFBA involved in heavy metal resistance (8, 11). CusA is a member of the RND superfamily of proteins (18) and the central component of the multicomponent efflux pump. No difference was observed whether cusA or cusCFBA was deleted (Table 1), indicating that cusA is essential for function. These results strongly suggest that the cus determinant encodes a copper efflux system. The copper sensitivity conferred by a copA disruption is not additive when transferred into a ΔcueO ΔcusCFBA::cm double mutant, since the triple mutant E. coli GR16 (ΔcueO ΔcusCFBA::cm copA::km) did not exhibit a further decrease in copper tolerance.
CusCFBA may not transport Cu(I) from the cytoplasm.
The CzcCBA transenvelope transporter from “Ralstonia metallidurans” CH34 is thought to function as a proton-cation antiporter expelling cations from the cytoplasm across the inner and outer membranes. Two channels in CzcA are proposed to form a charge-relay system, where proton transport generates an electrical field that drives the transport of cations into the periplasm (7). However, the cus determinant probably does not transport cytoplasmic Cu(I), since E. coli GR13 (copA::km ΔcusA::cm) was no more sensitive than strain DW3110 (ΔcopA) (Table 1). If CusCFBA extrudes copper from the cytosol, deletion of the cus determinant would be expected to have an additive effect on the phenotype of a copA::km mutant. Other transporters of the RND superfamily, such as AcrB and MexB, can efflux substrates that do not cross the cytoplasmic membrane (12). It was therefore suggested that binding of the substrate might occur on the periplasmic side of the transporter (19). CusA contains multiple methionine residues in the second large periplasmic domain. These residues, which are not present in CzcA, could be involved in copper binding. Furthermore, CusF is a putative periplasmic protein with potential copper binding sites. Thus, it is possible that the CusCFBA transenvelope transporter binds and transports periplasmic copper. Since the cus determinant is also responsible for a small increase in Ag(I) resistance (5), it is likely that the transported copper species is Cu(I).
CopA from “R. metallidurans” can functionally substitute for CueO.
CueO is homologous to the putative multicopper oxidases PcoA (E. coli), CopA (“R. metallidurans”; accession no. CAC07979) and CopA (Pseudomonas syringae), which are encoded by genes present in the plasmid-borne copper resistance operon pco in E. coli and the cop operons of “R. metallidurans” and P. syringae, respectively (3, 1). It should be pointed out that the “R. metallidurans” and P. syringae CopA proteins are not P-type ATPases, although PcoA, P. syringae CopA, and probably “R. metallidurans” CopA are essential components of their plasmid-encoded copper resistance determinants. PcoA, CopA (P. syringae), and CopA (“R. metallidurans”) are largely identical to each other and probably have similar functions. The degree of similarity between CueO and PcoA, CopA (P. syringae), and CopA (“R. metallidurans”) is much smaller, suggesting that they are distantly related. However, all four putative multicopper oxidases have leader sequences including a twin-arginine motif for export into the periplasm by the Tat pathway (13). CueO has a methionine-rich region that is also observed in PcoA and the CopA proteins from “R. metallidurans” and P. syringae. CopA (P. syringae) has been shown to be a periplasmic protein (2). CopA from R. metallidurans is functionally similar to CueO, since copA on plasmid pTYB2::copA can complement the single mutant E. coli GR1 (ΔcueO::cm) (Table 1) and the double mutant E. coli GR10 (ΔcueO ΔcusCFBA::cm) (Table 1). Likewise, the double mutant E. coli GR10 (ΔcueO ΔcusCFBA::cm) can also be complemented by cueO on a plasmid (Table 1). By analogy, we suggest that CueO and CopA (“R. metallidurans”) are periplasmic proteins. Possibly, CueO prevents uptake of Cu(I) into the cytoplasm by oxidizing it to Cu(II). Additionally, by oxidizing Cu(I) to Cu(II), CueO might confer copper tolerance by preventing oxidative damage in the periplasm. The functions of PcoA, CopA (P. syringae), and CopA (“R. metallidurans”) might be similar.
Another possibility is that the putative multicopper oxidases are secreted into the periplasm with their copper atoms already incorporated, consistent with the presence of the twin-arginine motifs. The synthesis and secretion of these proteins could result in a net efflux of copper from the cytoplasm, as was measured for the pco system (1). However, E. coli strain GR8, where both copA and cueO were disrupted, exhibited only a slight decrease in copper tolerance compared to strain DW3110, which has only copA disrupted (Table 1). One molecule of the homologous CopA protein (P. syringae) was shown to bind approximately 11 atoms of copper (2). It seems unlikely that the amounts of copper transported by CopA (P. syringae) and possibly by CueO would be sufficient to confer copper resistance.
Conclusions.
In this report we show that CueO and CusCFBA are involved in copper tolerance. CueO is homologous to multicopper oxidases such as ceruloplasmin, ascorbate oxidase, PcoA, and CopA (P. syringae) and is probably a periplasmic protein. CueO might oxidize Cu(I) to Cu(II) and can be functionally replaced by CopA from “R. metallidurans”. The cus determinant encodes a copper efflux system that might transport periplasmic Cu(I) [and Ag(I)] across the outer membrane. This suggests that the two determinants provide alternate fates for periplasmic Cu(I): either oxidation by CueO or transport into the extracellular medium by CusCFBA. These studies are a starting point to further elucidate the molecular mechanisms of copper homeostasis in prokaryotes. Further biochemical studies are necessary to understand the function of CusCFBA, CueO, and other, yet-unidentified components.
Acknowledgments
This work was supported by hatch project 136713 to C.R.
We thank Barry Rosen for providing P1 lysate and for helpful suggestions and Dietrich Nies and Barry Wanner for the generous gift of strains.
REFERENCES
- 1.Brown N L, Barrett S R, Camakaris J, Lee B T, Rouch D A. Molecular genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coliplasmid pRJ1004. Mol Microbiol. 1995;17:1153–1166. doi: 10.1111/j.1365-2958.1995.mmi_17061153.x. [DOI] [PubMed] [Google Scholar]
- 2.Cha J, Cooksey D A. Copper resistance in Pseudomonas syringaemediated by periplasmic and outer membrane proteins. Proc Natl Acad Sci USA. 1991;88:8915–8919. doi: 10.1073/pnas.88.20.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooksey D A. Molecular mechanisms of copper resistance and accumulation in bacteria. FEMS Microbiol Rev. 1994;14:381–386. doi: 10.1111/j.1574-6976.1994.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 4.Datsenko K A, Wanner B L. One-step inactivation of chromosomal genes in Escherichia coliK12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franke, S., G. Grass, and D. H. Nies. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology, in press. [DOI] [PubMed]
- 6.Gatti D, Mitra B, Rosen B P. Escherichia colisoft metal ion-translocating ATPases. J Biol Chem. 2000;275:34009–34012. doi: 10.1074/jbc.R000012200. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg M, Pribyl T, Juhnke S, Nies D H. Energetics and topology of CzcA, a cation/proton antiporter of the resistance-nodulation-cell division protein family. J Biol Chem. 1999;274:26065–26070. doi: 10.1074/jbc.274.37.26065. [DOI] [PubMed] [Google Scholar]
- 8.Gupta, Matsui A K, Lo J F, Silver S. Molecular basis for resistance to silver cations in Salmonella. Nat Med. 1999;5:183–188. doi: 10.1038/5545. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S D, Lee B T, Camakaris J, Wu H C. Identification of cutC and cutF(nlpE) genes involved in copper tolerance in Escherichia coli. J Bacteriol. 1995;177:4207–4215. doi: 10.1128/jb.177.15.4207-4215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munson G P, Lam D L, Outten F W, O'Halloran T V. Identification of a copper-responsive two-component system on the chromosome of Escherichia coliK-12. J Bacteriol. 2000;182:5864–5871. doi: 10.1128/jb.182.20.5864-5871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nies D H. Microbial heavy-metal resistance. Appl Microbiol Biotechnol. 1999;51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- 12.Nikaido H, Basina M, Nguyen V, Rosenberg E Y. Multidrug efflux pump AcrAB of Salmonella typhimuriumexcretes only those β-lactam antibiotics containing lipophilic side chains. J Bacteriol. 1998;180:4686–4692. doi: 10.1128/jb.180.17.4686-4692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Outten F W, Outten C E, Hale J, O'Halloran T V. Transcriptional activation of an E. colicopper efflux regulon by the chromosomal MerR homologue, CueR. J Biol Chem. 2000;275:31024–31029. doi: 10.1074/jbc.M006508200. [DOI] [PubMed] [Google Scholar]
- 14.Pena M M O, Lee J, Thiele D J. A delicate balance: homeostatic control of copper uptake and distribution. J Nutr. 1999;129:1251–1260. doi: 10.1093/jn/129.7.1251. [DOI] [PubMed] [Google Scholar]
- 15.Rapisarda V A, Montelongo L R, Farias R N, Massa E M. Characterization of an NADH-linked cupric reductase activity from the Escherichia colirespiratory chain. Arch Biochem Biophys. 1999;370:143–150. doi: 10.1006/abbi.1999.1398. [DOI] [PubMed] [Google Scholar]
- 16.Rensing C, Fan B, Sharma R, Mitra B, Rosen B P. CopA: an Escherichia coliCu(I)-translocating P-type ATPase. Proc Natl Acad Sci USA. 2000;97:652–656. doi: 10.1073/pnas.97.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouch D, Camakaris J, Lee B T O. Copper transport in E. coli, In: Hamer D H, Winge D R, editors. Metal ion homeostasis: molecular biology and chemistry. New York, N.Y: Alan R. Liss, Inc.; 1989. pp. 469–477. [Google Scholar]
- 18.Saier M H, Jr, Tam R, Reizer A, Reizer J. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol Microbiol. 1994;11:841–847. doi: 10.1111/j.1365-2958.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 19.Zgurskaya H I, Nikaido H. Multidrug resistance mechanisms: drug efflux across two membranes. Mol Microbiol. 2000;37:219–225. doi: 10.1046/j.1365-2958.2000.01926.x. [DOI] [PubMed] [Google Scholar]