Abstract
Background
A considerable proportion of stroke patients have unfavorable outcomes despite substantial reperfusion during mechanical thrombectomy for large vessel occlusion. This study aimed to determine predictors of unfavorable outcomes despite substantial reperfusion (modified thrombolysis in cerebral infarction score of ≥2b).
Methods
We conducted a post hoc analysis of Analysis of Revascularization in Ischemic Stroke With EmboTrap, a prospective, multicenter study on the efficacy of the EmboTrap revascularization device. We included patients with anterior large vessel occlusion, substantial reperfusion within three passes, and 3-month follow-up. Univariate and multivariate logistic regression analyses were performed to determine independent predictors of dependency or death (modified Rankin Score 3–6) at 90 days.
Results
Of the 176 patients included in the study, 124 (70.45%) achieved modified Rankin Score of 0–2 at 90 days and 52 (29.6%) had modified Rankin Score of 3–6. On univariate analysis, patient age and initial National Institutes of Health Stroke Scale score were significantly higher in the modified Rankin Score of 3–6 groups (71.4 ± 11.3 years vs. 66.0 ± 13.1 years, 0.01; 18.9 ± 4.13 vs. 14.6 ± 4.36, p < 0.01, respectively). Mean number of passes and symptomatic intracranial hemorrhage were also higher in patients with modified Rankin Score of 3–6 (2.46 ± 1.42 vs. 1.65 ± 0.9, p < 0.01; 13.5% vs. 2.4%, p = 0.008). On multivariate analysis, initial National Institutes of Health Stroke Scale score and mean number of passes and were independent predictors of modified Rankin Score of 3–6 at 90 days.
Conclusion
More severe initial neurologic deficit and higher number of passes in patients with substantial reperfusion were independent predictors of dependency or death. These findings highlight a reduction in the number of passes required to achieve reperfusion as a therapeutic target to improve the outcome after thrombectomy.
Keywords: Stroke, thrombectomy, cerebral infrction, multivariate analysis
Introduction
Futile reperfusion (no improved functional outcome despite reperfusion) and frustrating reperfusion (improved, suboptimal functional outcome despite reperfusion) are not uncommon among acute ischemic stroke patients with large vessel occlusions (LVOs) undergoing endovascular reperfusion therapy. To improve therapeutic strategies, it is important to understand the determinants of poor patient outcomes despite blood flow restoration.
Analysis of Revascularization in Ischemic Stroke With EmboTrap (ARISE II) was a single-arm, prospective, multicenter, international study evaluating the safety and effectiveness of the EmboTrap revascularization device for patients with LVO against a composite performance goal, which was derived using a Bayesian meta-analysis of the SWIFT and TREVO 2 trials. 1 Imaging reperfusion outcomes of the study were adjudicated by an independent core laboratory. The study included 227 patients treated with EmboTrap. Key eligibility criteria included National Institute of Health Stroke Scale (NIHSS) of 8–25 and Alberta Stroke Program Early CT Score (ASPECTS) ≥ 6. Substantial reperfusion, defined as modified thrombolysis in cerebral infarction (mTICI) score of ≥ 2b, was achieved in 9 of every 10 patients, with modified first-pass effect (mFPE) (≥2b) in nearly half of the cases. Despite a high overall rate of revascularization, almost one-third (32.7%) of the patients had unfavorable outcomes (modified Rankin Score (mRS): 3–6). 1
Few studies have explored the predictors of unfavorable outcomes despite successful revascularization, sometimes called futile revascularization.2–5 Most of the factors identified in the literature as predictors of unfavorable outcomes are non-modifiable; however, identification of potentially modifiable factors would help improve the management of LVO. Therefore, we studied the predictors of unfavorable 90-day outcomes despite substantial reperfusion in stroke patients in ARISE II.
Methods
Entry criteria and methods for the ARISE II study have been described in detail in the ARISE II primary results publication. 1 ARISE II was an international multicenter trial with 11 European and 8 US sites. The inclusion/exclusion criteria were in line with criteria typically used in mechanical thrombectomy trials: patients with radiographically confirmed LVO, age: 18–85 years, pre-stroke mRS 0–1, ASPECTS ≥ 6, and NIHSS score of 8–25 were eligible for inclusion and assigned to receive EmboTrap as the first attempted reperfusion device. Study protocol mandated the use of EmboTrap in the first three passes. Inclusion criteria for the current post hoc analysis were target LVO in the anterior circulation (posterior circulation stroke was considered a confounding factor due to the known association with poor outcomes), attainment of substantial reperfusion (mTICI 2b-3) within the first three passes of EmboTrap (based on ARISE II primary endpoint), and follow-up disability assessment at 90 ± 30 days.
A descriptive analysis was performed for the demographics, presentation findings, and radiological and functional outcomes. Percentages and proportions were used to present categorical data. Baseline characteristics included demographics, comorbidities, site of occlusion, initial NIHSS score, and adjudicated entry ASPECTS and American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) collateral grade. Other variables included mTICI score, symptomatic intracranial hemorrhage (sICH) within 24 h, number of passes, use of intravenous tissue plasminogen activator (IV t-PA), general anesthesia, use of a balloon guide catheter (BGC), interhospital transfer, time from stroke onset (or last known well time point) to the first device deployment, and time from treatment start to reperfusion.
Logistic regression was used to evaluate baseline and procedural factors for association with 90-day functional independence (mRS 0–2) and dependency or death (mRS 3–6). A univariate analysis was carried out for age, sex, site of LVO, initial NIHSS score, entry ASPECTS score, entry collateral grade, use of IV t-PA, general anesthesia, mTICI score after up to three passes, number of passes, use of BGC, time from symptom onset to treatment start, direct admission versus transfer from another center, and time from treatment start to recanalization. Models were fitted using the lasso regression technique where the Akaike information criterion (AIC) statistic was used in determining the model choice. Collinearity was explored for the initial set of covariates and verified in the final model using standard statistical measures (e.g. condition indices, proportion of variation, etc.).
For continuous variables, p-values were generated with a t-test, using a Satterthwaite in cases of unequal variances. For categorical variables, p-values were generated using a χ2 test or a Fisher's exact test, as appropriate. A p-value ≤ 0.05 was considered significant. All analyses were performed using SAS version 9.4 software.
Results
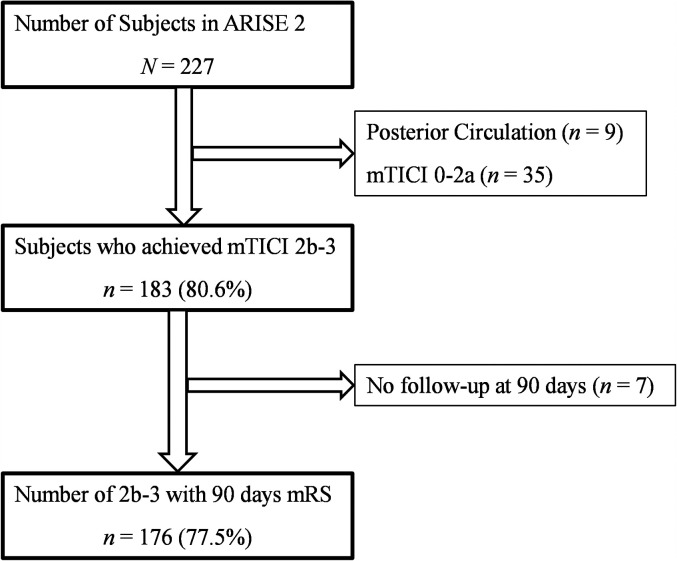
Of the 227 patients in ARISE II, 9 were not eligible due to posterior circulation stroke, 35 were excluded due to mTICI grade 0–2a after the first 3 passes, and 7 were further excluded due to missing 90-day outcome. Figure 1 shows the selection of patients. In the resulting 176 patients fully eligible for the current analysis, dependency or death (mRS 3–6) at 3 months occurred in 52 (29.6%; Table 1 ). Females constituted 54.9% of the total population; mean age was 67.2 ± 12.7 years. Mean NIHSS score was 15.9 ± 4.7. M1 was the most common site of occlusion (54.6%), followed by M2 (25.0%) and internal carotid artery (ICA) (15.9%). Delay from last known well time point to the first deployment of EmboTrap was 3.97 ± 1.44 h. Modified first-pass effect (mFPE, defined as mTICI 2b-3 on the first pass) was seen in 63.6% of patients; mTICI 3 revascularization within the first three passes were seen in 54.5%. The rate of sICH within 24 h was 5.7%.
Figure 1.
Flowchart of patient selection for inclusion in post hoc analysis.
Table 1.
Univariate analysis of baseline clinical variables in patients with anterior circulation large vessel occlusion (LVO).
| mTICI 2b-3 (n = 176) | mTICI 2c-3 (n = 142) | |||||
|---|---|---|---|---|---|---|
| Variable | mRS 0–2 n = 124 | mRS 3–6 n = 52 | p-value | mRS 0–2 n = 103 | mRS 3–6 n = 39 | p-value |
| Age (mean ± SD) | 66.0 (13.1) | 71.4 (11.3) | 0.01* | 65.65 ± 13.29 | 73.33 ± 9.93 | 0.003* |
| Female n (%) | 66 (53.2) | 28 (53.8) | 0.94 | 56 (54.4) | 20 (51.3) | 0.74 |
| Transfer from another hospital n (%) | 67 (54.0) | 36 (69.2) | 0.062 | 58 (56.3) | 30 (76.9) | 0.023* |
| Time from stroke onset to groin puncture (min) | 199.2 (74.9) | 217.3 (85.0) | 0.22 | 202.40 ± 74.88 | 229.82 ± 85.42 | 0.22 |
| IV tPA | 82 (66.1) | 33 (63.5) | 0.73 | 71 (68.9) | 25 (64.1) | 0.58 |
| ASITN collateral grade | ||||||
| Poor | 77 (69.2) | 36 (83.7) | 0.19 | 58 (67.4) | 26 (81.3) | 0.19 |
| Moderate | 28 (26.9) | 7 (16.3) | 24 (27.9) | 6 (18.8) | ||
| Good | 4 (3.8) | 0 | 4 (4.7) | 6 (18.8) | ||
| Baseline NIHSS score | 14.6 (±4.36) | 18.9 (4.13) | < 0.001* | 14.53 ± 4.61 | 18.38 ± 4.08 | < 0.001* |
| Occlusion site | ||||||
| ICA | 16 (13.4) | 12 (24.5) | 0.21 | 15 (15.3) | 8 (22.2) | 0.64 |
| MCA, M1 | 71 (59.7) | 12 (24.5) | 56 (57.1) | 19 (52.8) | ||
| MCA, M2 | 32 (26.9) | 12 (24.5) | 27 (27.6) | 9 (25.0) | ||
ASITN/SIR: American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; ICA: internal carotid artery; IV t-PA: intravenous tissue plasminogen activator; M1: first segment of middle cerebral artery; M2: second segment of middle cerebral artery; mRS: modified Rankin score; mTICI: modified thrombolysis in cerebral infarct; N: number; NIHSS: National Institutes of Health Stroke Scale; SD: standard deviation.
*Statistically significant.
Clinical variables and study outcomes
In the full population with substantial reperfusion, there was no difference between patients who achieved mRS 0–2 in comparison with mRS 3–6 with regard to the following variables: proportion of female patients (53.2% vs. 53.8%, p = 0.94), transfer from other hospitals (54.0% vs. 69.2%, p = 0.06), tPA administration (66.1% vs. 63.5%, p = 0.73), and mean time from stroke onset to groin puncture (199.2 ± 74.9 min vs. 217.3 ± 85.0 min, p = 0.22). There was also no significant difference between the two groups with regard to occlusion site and collateral grade ( Table 1 ). However, patients with mRS 3–6 were older (71.4 ± 11.3 vs. 66.0 ± 13.1 years, p = 0.01) and had higher baseline NIHSS score (18.9 ± 4.13 vs. 14.6 ± 4.36, p < 0.01).
When considering only patients with mTICI ≥2c within the first three passes (n = 142), patients with poor outcomes were also older (73.33 ± 9.93 vs. 65.65 ± 13.29 years, p < 0.01), had higher initial NIHSS (18.38 ± 4.08 vs. 14.53 ± 4.61, p < 0.01), and were more frequently transferred from another hospital (76.9 vs. 56.3%, p = 0.02; Table 1 ).
Procedure-related variables and study outcomes
Among those who achieved substantial reperfusion, there was no significant difference between patients with favorable (mRS 0–2) and unfavorable outcomes with regard to the proportions of patients who were administered general anesthesia (35.5% vs. 23.1%, p = 0.11) or the use of a balloon guide catheter (73.4% vs. 65.4%, p = 0.28). There was also no significant difference with regard to mTICI grade within three passes (p = 0.40; Table 2 ). The mean number of passes in the unfavorable outcome group was higher compared to favorable outcome group (2.46 ± 1.42 vs. 1.65 ± 0.91, p < 0.01). The rate of sICH was also higher in patients with unfavorable outcomes (13.5% vs. 2.4%, p < 0.01).
Table 2.
Univariate analysis of procedural and technical variables in patients with anterior circulation large vessel occlusion (LVO).
| mTICI 2b-3 (n = 176) | mTICI 2c-3 (n = 142) | |||||
|---|---|---|---|---|---|---|
| mRS 0–2 n = 124 | mRS 3–6 n = 52 | p-value | mRS 0–2 n = 103 | mRS 3–6 n = 39 | p-value | |
| General anesthesia n (%) | 44 (35.5) | 12 (23.1) | 0.11 | 39 (37.9) | 11 (28.2) | 0.28 |
| Balloon guide used n (%) | 91 (73.4) | 34 (65.4) | 0.28 | 77 (74.8) | 28 (71.8) | 0.72 |
| Mean number of passes (±SD) | 1.65 (0.91) | 2.46 (1.42) | <0.001* | 1.58 ± 0.83 | 2.28 ± 1.39 | 0.005* |
| mTICI score (one–three passes) | ||||||
| 2b | 21 (16.9) | 13 (25.0) | 0.40 | n/a | n/a | 0.58 |
| 2c | 32 (25.8) | 14 (26.9) | 32 (31.1) | 14 (35.9) | ||
| 3 | 71 (57.3) | 25 (48.1) | 71 (68.9) | 25 (64.1) | ||
| sICH within 24 h n (%) | 3 (2.4) | 7 (13.5) | 0.008* | 1 (1.0) | 6 (15.4) | 0.002* |
mRS: modified Rankin score; mTICI: modified thrombolysis in cerebral infarct; n/a: not applicable; sICH: symptomatic intracranial hemorrhage.
*Statistically significant.
In the subpopulation with mTICI ≥ 2c after up to three passes, poor outcomes were also significantly associated with a higher mean number of passes (2.28 ± 1.39 vs. 1.58 ± 0.83, p < 0.01) and higher rate of sICH (15.4 vs. 1.0%, p < 0.01).
Independent predictors of unfavorable outcomes despite successful reperfusion
Multivariate analysis of the complete study cohort showed that stroke deficit severity at presentation (initial NIHSS score) and more than one pass were independent predictors of unfavorable outcomes at 90 days (OR, 1.24, 95% confidence interval (CI): 1.12–1.38, p < 0.01; and OR, 1.73, 95% CI: 1.12–2.65, p = 0.01, respectively; Table 3 ).
Table 3.
Independently fitted logistic regression analysis of clinical outcome.
| Population with successful reperfusion (mTICI 2b-3) | ||||
|---|---|---|---|---|
| Parameter estimates | ||||
| Effect | Level | Estimate | p-value | Odds ratio (95% CI) |
| Intercept | −4.96 (1.234) | < 0.0001* | ||
| Age at enrollment (years) | ≥ 75 | 0.34 (0.245) | 0.16 | 1.97 (0.75−5.15) |
| Number of passes recorded at study site | > 1 | 0.55 (0.219) | 0.012* | 1.73 (1.12−2.65) |
| NIHSS at presentation to study/treatment hospital | 0.22 (0.054) | < 0.0001* | 1.24 (1.12−1.38) | |
| Balloon guide deployed and inflated? | Yes | 0.04 (0.265) | 0.88 | 1.08 (0.38−3.05) |
| Baseline ASPECTS | ASPECTS 0–8 | 0.08 (0.312) | 0.81 | 1.17 (0.34−3.95) |
| Baseline collateral grade (ASITN) | −0.50 (0.342) | 0.14 | 0.61 (0.31−1.19) | |
| Population with successful reperfusion (mTICI 2c-3) | ||||
| Intercept | −4.26 (1.278) | < 0.001* | ||
| Age at enrollment (years) | ≥ 75 | 0.60 (0.279) | 0.031* | 3.33 (1.11−9.94) |
| Number of passes recorded at study site | > 1 | 0.63 (0.275) | 0.023* | 1.87 (1.09−3.21) |
| NIHSS at presentation to study/treatment hospital | 0.20 (0.059) | < 0.001* | 1.22 (1.08−1.36) | |
| Baseline ASPECTS | ASPECTS 0–8 | 0.13 (0.339) | 0.69 | 1.30 (0.35−4.92) |
| Baseline collateral grade (ASITN) | −0.79 (0.398) | 0.047* | 0.45 (0.21−0.99) | |
ASITN/SIR: American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; ASPECTS: Alberta Stroke Program Early CT Score; CI: confidence interval; mTICI: modified thrombolysis in cerebral infarct; N: number; NIHSS: National Institutes of Health Stroke Scale.
*Statistically significant.
In a sensitivity analysis, we looked at the predictors of unfavorable outcome in those with excellent reperfusion (mTICI 2c-3) within three passes of the device; multivariate analysis showed that age ≥ 75 years, higher NIHSS score at presentation, > 1 pass, and lower collateral grade were independent predictors of unfavorable outcomes.
Discussion
In this international, multicenter study, dependency or death at 3 months occurred in 3 of every 10 patients with acute ischemic stroke due to LVO in whom endovascular thrombectomy yielded substantial reperfusion. As a result, although final substantial reperfusion was achieved in four-fifths of enrolled patients, functional independence outcomes were less frequent. Independent predictors of futile/frustrating reperfusion were more severe neurologic deficits at presentation and higher total number of passes with the device, where substantial reperfusion was achieved within the first three mechanical thrombectomy passes.
The current study is consonant with and extends prior investigations. In a meta-analysis of eight clinical trials on the use of mechanical thrombectomy for LVO stroke, the rate of favorable outcomes was 44.6%, despite substantial reperfusion being achieved in 75.8% of patients. 6 Why some patients do not have favorable outcomes despite successful revascularization is a critical question. Our post hoc analysis of ARISE II data suggests NIHSS score and the number of passes as independent predictors of unfavorable outcomes. Of these two factors, only the number of passes constitutes a potentially modifiable factor, as it carried higher odds for unfavorable outcomes (OR, 1.73, 95% CI: 1.12–2.65, p = 0.01 vs. OR, 1.24, 95% CI: 1.12−1.38, p < 0.01). We additionally explored the predictors of unfavorable outcomes in patients with complete or near-complete reperfusion (i.e. mTICI 2c-3, n = 142). Higher number of passes remained the only potentially modifiable variable that independently predicted unfavorable outcomes (OR, 1.87, 95% CI: 1.09–3.21, p = 0.02). Other independent predictors within this subgroup included older age, NIHSS, and collateral grade.
We have previously shown that complete recanalization with a single pass of the device is associated with significantly higher rates of favorable outcomes. 7 A multicenter study of 467 patients showed that 5 or more passes were associated with unfavorable functional outcomes and the outcomes were not significantly different from among patients with failed recanalization. 8 Another study by Kharouba et al. showed that the number of passes needed to achieve target vessel recanalization modifies outcome after thrombectomy and successful recanalization. 9 Our cohort is different from these studies since it looks at a subgroup of patients who had successful recanalization within three passes. Significant odds for the number of passes within this subgroup highlight the importance of the first-pass effect.
The number of passes is also the only modifiable factor identified in our analysis. First-pass efficacy has been explored in a number of studies. In a large cohort of direct aspiration (ADAPT) first-pass effect patients, non-tandem occlusion, use of large-bore aspiration catheter (Penumbra ACE 64–68), and intravenous thrombolysis were independent predictors of the first-pass effect. 10 Clot composition, source (cardioembolic vs. noncardioembolic), and underlying intracranial atherosclerosis are among factors associated with increasing number of passes.11–13 In another retrospective study of 230 patients with M1 occlusion authors found larger diameter of M1, decreased rate of vessel tapering along the intracranial ICA, and collateral status to be predictors of the first-pass effect. 14 However, the findings need to be validated in an independent, prospective cohort with independent adjudication. Moreover, all the factors described in the study are non-modifiable. Di Maria et al. analyzed data of 1832 patients from a prospective multicenter registry and demonstrated that the combined use of aspiration and stent retriever, increasing age among factors predictive of the first-pass effects. 15 Both of these studies reported much better clinical outcomes with the first-pass effect. Understanding the reasons for clot resistance and reducing the number of passes, therefore, warrants an understanding of clot properties and improvement in design of stent retrievers that could be effective across a variety of clots and in the setting of atherosclerosis.
A multicenter retrospective study from North American centers presented a multivariate analysis of 256 successfully recanalized patients and found age, occlusion site, high NIHSS score, diabetes, no IV tPA, ≥ 3 passes, and use of rescue therapy to be predictors of unfavorable outcomes at 90 days. 5 The overall rate of favorable outcomes was 50.4%. Solitaire was the only stent retriever used in that study. 5 Our study is different as we only included patients who had successful recanalization within three passes. Shi et al. reviewed data from three different clinical trials (MERCI, TREVO, and TREVO 2) to assess factors predictive of functional dependence after successful revascularization. 4 Patients were treated with either MERCI or TREVO stent retrievers. According to their analysis, 50% of patients did not have favorable outcomes despite successful recanalization. Age, severe neurological deficits at presentation, and delayed endovascular treatment were associated with functional dependence despite successful revascularization. The effect of number of passes on outcomes was not analyzed in that study.
In another study, Wang et al. proposed the PREDICT score to predict unfavorable outcomes which included five variables: lymphocyte–neutrophil ratio, blood glucose, baseline NIHSS score, collateral grade, and prior intravenous thrombolysis with an area under the curve (AUC) of 0.74 in the validation cohort. Notably, the score has not been externally validated and all the variables in the scoring model are non-modifiable. Another analysis of a single-center prospective registry demonstrated that increasing age and severity of stroke symptoms were predictive of poor clinical outcomes despite successful reperfusion. However, the authors did not include number of passes in their regression analysis and recognized this as a limitation of their study. 16
While finding an association between a greater number of passes and adverse outcomes is compelling, one must investigate whether this relationship is causal or not. Since the grade of collaterals determines the size of the penumbra with salvageable tissue that survives when reperfused, the amount of salved tissue may be causal for the number of passes needed to reopen the occluded vessel. Dead tissue does not provide outflow of blood from the occluded and recanalized vessel, unlike the reperfused and salved tissue, analogous to an occluded pipe that would be difficult or impossible to recanalize because the outflow is blocked. Therefore, one could hypothesize that penumbra size determines not only clinical outcomes but also the ease with which the occluded vessel can be recanalized. A small penumbra may require a greater number of attempts/passes to fully recanalize the occlusion, thus linking more passes and worse clinical outcomes. Nevertheless, it would not be advisable to abandon further attempts at recanalization if the first attempts fail.
Finally, it is important to set realistic expectations for the reduction of thrombectomy maneuvers required for reperfusion. The number of passes is a function of clot characteristics, devices, and techniques, among other factors, highlighting the need for insight and better diagnostic and therapeutic approaches across these areas. EmboTrap was specifically designed to be effective in a broader range of thrombi with respect to fibrin content compared to prior stent retrievers, and more targeted technologies for tough clots are also becoming available. A number of prospective studies, including a large post-market registry of EmboTrap for AIS focusing on clot collection and analysis, are underway and will provide better insight into the implications of clot characteristics and techniques.
Although study design based on post hoc analysis is a limitation, the results of this study are based on prospectively collected, Core Lab adjudicated data and provide an insight into the predictors of unfavorable outcomes despite substantial revascularization. A systematic review of the literature may be helpful in providing more context for the findings. Since ARISE II was a single-arm study, we did not have information about devices other than EmboTrap. We were also unable to include an analysis of clot length, density, and composition.
Conclusions
NIHSS score at presentation and the mean number of passes are independent predictors of unfavorable outcomes at 90 days in patients with procedural success. Number of passes is the only potentially modifiable factor predictive of unfavorable outcomes with odds higher than NIHSS score; stroke technology developments should focus on achieving recanalization in as few passes as possible.
Acknowledgments
The authors have no acknowledgments to report.
Footnotes
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT02488915.
Declaration of conflicting interests: A. Siddiqui: Co-investigator NIH/NINDS 1R01NS091075; Amnis Therapeutics, Boston Scientific, Canon Medical Systems USA Inc, Cerebrotech Medical Systems, Cerenovus, Corindus Inc., Endostream Medical Ltd, Guidepoint Global Consulting, Imperative Care, Integra LifeSciences Corp, Medtronic, MicroVention, Q'Apel Medical Inc, Rapid Medical, Rebound Therapeutics Corp., Serenity Medical Inc, Silk Road Medical, StimMed, Stryker, Three Rivers Medical, VasSol, W.L. Gore & Associates; C; Amnis Therapeutics, Apama Medical, Blink TBI Inc, Buffalo Technology Partners Inc, Cardinal Consultants, Cerebrotech Medical Systems, Cognition Medical, Endostream Medical Ltd, Imperative Care, International Medical Distribution Partners, Neurovascular Diagnostics Inc, Q'Apel Medical Inc, Rebound Therapeutics Corp, Rist Neurovascular In, Serenity Medical, Silk Road Medical, StimMed, Synchron, Three Rivers Medical Inc, Viseon Spine. T. Andersson: Neuravi, Ablynx, Amnis Therapeutics, Medtronic, Rapid Medical, Stryker.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Muhammad Waqas https://orcid.org/0000-0003-4500-7954
References
- 1.Zaidat OO, Bozorgchami H, Ribo M, et al. Primary results of the multicenter ARISE II study (analysis of revascularization in ischemic stroke with EmboTrap). Stroke 2018; 49. doi: 10.1161/STROKEAHA.117.020125 [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Zhang M, Hao Y, et al. Early prediction of poor outcome despite successful recanalization after endovascular treatment for anterior large vessel occlusion stroke. World Neurosurg 2018; 115: e312–ee21. [DOI] [PubMed] [Google Scholar]
- 3.Hussein HM, Saleem MA, Qureshi AI. Rates and predictors of futile recanalization in patients undergoing endovascular treatment in a multicenter clinical trial. Neuroradiology 2018; 60: 557–563. [DOI] [PubMed] [Google Scholar]
- 4.Shi ZS, Liebeskind DS, Xiang B, et al. Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke 2014; 45: 1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linfante I, Starosciak AK, Walker GR, et al. Predictors of poor outcome despite recanalization: a multiple regression analysis of the NASA registry. J Neurointerv Surg 2016; 8: 224–229. [DOI] [PubMed] [Google Scholar]
- 6.Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA 2015; 314: 1832–1843. [DOI] [PubMed] [Google Scholar]
- 7.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke 2018; 49: 660–666. [DOI] [PubMed] [Google Scholar]
- 8.Baek JH, Kim BM, Heo JH, et al. Number of stent retriever passes associated With futile recanalization in acute stroke. Stroke 2018; 49: 2088–2095. [DOI] [PubMed] [Google Scholar]
- 9.Kharouba R, Gavriliuc P, Yaghmour NE, et al. Number of stentriever passes and outcome after thrombectomy in stroke. J Neuroradiol 2019; 46: 327–330. [DOI] [PubMed] [Google Scholar]
- 10.Anadani M, Alawieh A, Vargas J, et al. First attempt recanalization with ADAPT: rate, predictors, and outcome. J Neurointerv Surg 2019; 11: 641–645. [DOI] [PubMed] [Google Scholar]
- 11.Nikoubashman O, Dekeyzer S, Riabikin A, et al. True first-pass effect. Stroke 2019; 50: 2140–2146. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Hong JM, Lee KS, et al. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke 2016; 18: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrocky T, Piechowiak E, Cianfoni A, et al. Thrombectomy of calcified emboli in stroke. Does histology of thrombi influence the effectiveness of thrombectomy? J Neurointerv Surg 2018; 10: 345–350. [DOI] [PubMed] [Google Scholar]
- 14.Srivatsa S, Duan Y, Sheppard JP, et al. Cerebral vessel anatomy as a predictor of first-pass effect in mechanical thrombectomy for emergent large-vessel occlusion. J Neurosurg 2020; 134: 1–9. doi: 10.3171/2019.11.JNS192673 [DOI] [PubMed] [Google Scholar]
- 15.Di Maria F, Kyheng M, Consoli A, et al. Identifying the predictors of first-pass effect and its influence on clinical outcome in the setting of endovascular thrombectomy for acute ischemic stroke: results from a multicentric prospective registry. Int J Stroke 2020; 16: 20–28. doi: 10.1177/1747493020923051. [DOI] [PubMed] [Google Scholar]
- 16.van Horn N, Kniep H, Leischner H, et al. Predictors of poor clinical outcome despite complete reperfusion in acute ischemic stroke patients. J Neurointerv Surg 2020; 13: 14–18. doi: 10.1136/neurintsurg-2020-015889. [DOI] [PubMed] [Google Scholar]