To the Editor: Mounting concern about the long-term efficacy of messenger RNA (mRNA) booster vaccines against coronavirus disease 2019 (Covid-19)1 has been exacerbated by the recent emergence of the B.1.1.529 (omicron) subvariants BA.2.12.1 and BA.4 and BA.5 (hereafter, BA.4/5) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which have high degrees of immune escape.2 To address this concern, we used our previously reported pseudotyped lentivirus neutralization assay (see the Supplementary Appendix, available with the full text of this letter at NEJM.org) to examine neutralizing-antibody titers against major SARS-CoV-2 variants in a longitudinal cohort of health care workers from the Ohio State University Wexner Medical Center in Columbus who had received homologous vaccine and booster doses of an mRNA vaccine. A total of 24 participants received the mRNA-1273 vaccine (Moderna), and 22 received the BNT162b2 vaccine (Pfizer–BioNTech). We classified participant samples into three groups according to the timing of booster-dose administration: 1 to 3 months, 4 to 6 months, and 7 to 9 months before the sample was obtained (Table S1 in the Supplementary Appendix). Over the course of the study, 14 participants had a breakthrough infection; 9 cases occurred during the omicron waves.
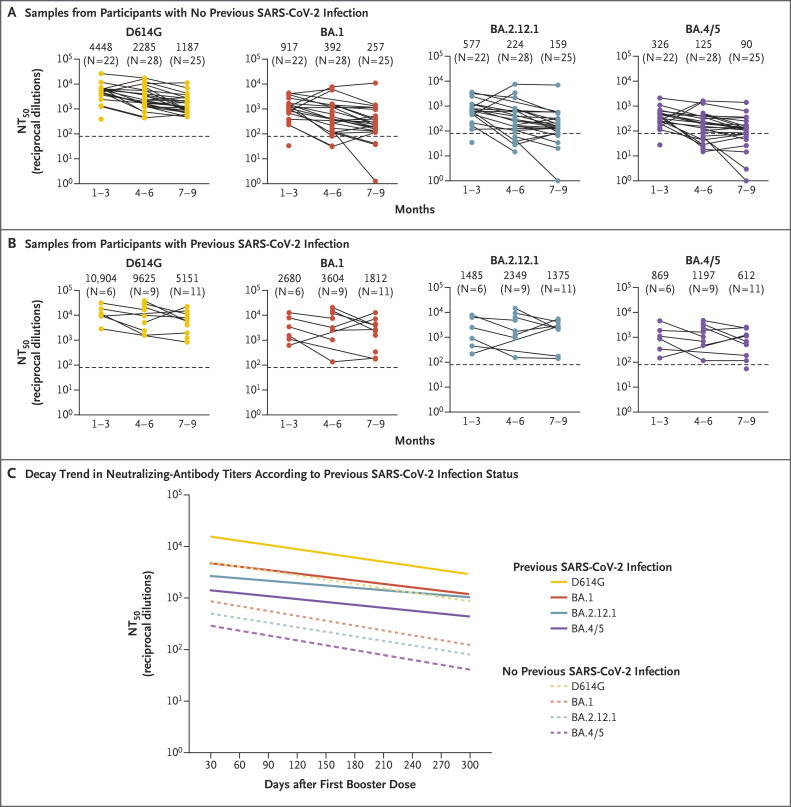
Booster durability waned more substantially in participants who did not have breakthrough infection than in those who had breakthrough infection, with neutralizing-antibody titers, presented as 50% neutralization titers (NT50), against all variants at 1 to 3 months after the booster dose being approximately 1.7 times (95% confidence interval [CI], 1.4 to 2.2) as high as those observed at 7 to 9 months after the booster dose (Figure 1 and Fig. S1). Linear modeling showed a mean 30-day rate of decay in neutralizing-antibody titers of 17.53% (95% CI, 11.87 to 22.79) against virus with the D614G mutation, 19.50% (95% CI, 9.82 to 28.10) against the omicron BA.1 subvariant, 18.44% (95% CI, 9.24 to 26.68) against BA.2.12.1, and 19.55% (95% CI, 10.54 to 27.66) against BA.4/5 (Figure 1C). Participants with previous SARS-CoV-2 infection, including infection with the omicron variant, had a somewhat less steep rate of decay in neutralizing-antibody titers (Figure 1B and 1C and Fig. S2), with 30-day rates of 17.07% (95% CI, 2.70 to 29.29) against virus with the D614G mutation, 14.22% (95% CI, −6.87 to 31.13) against the BA.1 subvariant, 9.97% (95% CI, −11.95 to 27.64) against BA.2.12.1, and 12.12% (95% CI, −7.14 to 27.94) against BA.4/5 (Figure 1C). At all time points tested, all the omicron subvariants, especially BA.4/5, had lower neutralizing-antibody titers than virus with the D614G mutation.
Figure 1. Durability of mRNA Booster–Induced Neutralizing Antibodies According to Previous SARS-CoV-2 Infection.
Panel A shows neutralizing-antibody titers against virus pseudotyped with spike protein from the ancestral severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with the D614G mutation and the B.1.1.529 (omicron) subvariants BA.1, BA.2.12.1, and BA.4 and BA.5 (hereafter, BA.4/5) in serum samples obtained from health care workers with no previous SARS-CoV-2 infection. Samples were obtained from participants who had received a booster dose of messenger RNA (mRNA) vaccine 1 to 3 months, 4 to 6 months, or 7 to 9 months previously. Neutralizing-antibody titers against virus with the D614G mutation and the omicron subvariants BA.1, BA.2.12.1, and BA.4/5 in serum samples obtained from participants with previous SARS-CoV-2 infection are shown in Panel B; samples were obtained according to the same time categories as in Panel A. In both panels, dots represent individual samples, and the horizontal dashed lines represent the limit of detection. Solid lines connect samples that were obtained from the same participant. Geometric mean values for the 50% neutralization titers (NT50) are shown at the top of the plots for each time point. Panel C shows the trend lines in neutralizing-antibody decay over the study period as determined by linear mixed-effects modeling with accounting for repeated measures in participants with no previous SARS-CoV-2 infection against virus with the D614G mutation (slope, −0.0028; 95% CI, −0.0037 to −0.0018) or subvariants BA.1 (slope, −0.0031; 95% CI, −0.0048 to −0.0015), BA.2.12.1 (slope, −0.0030; 95% CI, −0.0045 to −0.0014), or BA.4/5 (slope, −0.0032; 95% CI, −0.0047 to −0.0016) and in participants with previous SARS-CoV-2 infection against virus with the D614G mutation (slope, −0.0027; 95% CI, −0.0050 to −0.0004) or subvariants BA.1 (slope, −0.0022; 95% CI, −0.0054 to −0.0010), BA.2.12.1 (slope, −0.0015; 95% CI, −0.0047 to −0.0016), or BA.4/5 (slope, −0.0019; 95% CI, −0.0047 to −0.0010). The 95% confidence intervals for each trend-line slope have not been adjusted for multiplicity and should not be used in place of hypothesis testing.
Two participants received a second booster dose of mRNA vaccine (Table S2). After a substantial decrease in neutralizing-antibody titers was observed approximately 4 months after receipt of the initial booster dose in these participants, the administration of a second booster dose led to recovered neutralizing-antibody titers (Fig. S3).
The decrease in booster durability appeared to be slower than the decrease that was previously reported for two doses of mRNA vaccine alone.3 Although the rate of booster neutralizing-antibody decay was similar among variants, the omicron subvariants, especially BA.4/5, had substantial neutralization resistance. Our observed trends are consistent with the waning of vaccine protection and natural immunity,4,5 and our data suggest that both SARS-CoV-2 variant evolution and waning neutralizing-antibody titers reduce booster-induced immune protection. Our anecdotal experience in two participants indicates that a fourth dose of vaccine may be effective. A variant-specific booster may become necessary as new variants evolve.
Supplementary Appendix
Disclosure Forms
The content of this letter is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This letter was published on September 7, 2022, at NEJM.org.
Footnotes
Supported by a fund provided by a private donor to Ohio State University (to Dr. Liu), an award (U54CA260582, to Drs. Lozanski, Saif, Oltz, Gumina, and Liu) from the National Cancer Institute of the National Institutes of Health (NIH), a Glenn Barber Fellowship (to Mr. Evans) from the Ohio State University College of Veterinary Medicine, grants (to Dr. Gumina) from the Robert J. Anthony Fund for Cardiovascular Research and the JB Cardiovascular Research Fund, and a grant (R01 HD095881, to Dr. Saif) from the NIH.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qu P, Faraone J, Evans JP, et al. Neutralization of the SARS-CoV-2 omicron BA.4/5 and BA.2.12.1 subvariants. N Engl J Med 2022;386:2526-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans JP, Zeng C, Carlin C, et al. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med 2022;14:eabn8057-eabn8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun 2022;13:3082-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA 2021;326:1930-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.