To the Editor: Mucosal IgA can provide immunity against respiratory viruses.1 Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) boosts mucosal IgA responses,2 and neutralizing IgA, including neutralizing IgA against the B.1.1.529 (omicron) variant of SARS-CoV-2, has been detected after infection with wild-type SARS-CoV-2.3 However, the potential role of mucosal IgA in protection against SARS-CoV-2 infection is still largely unknown.
We evaluated SARS-CoV-2–specific mucosal antibody responses in 338 triple-vaccinated health care workers (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org) at the time of their enrollment in a 4-week quantitative polymerase-chain-reaction screening study in January and February 2022.4 Mucosal antibody responses were then evaluated over time in 57 participants who became infected with the omicron variant during the screening period (Figure 1A). Mucosal IgA and IgG responses were analyzed in relation to previously obtained serologic and viral data.4
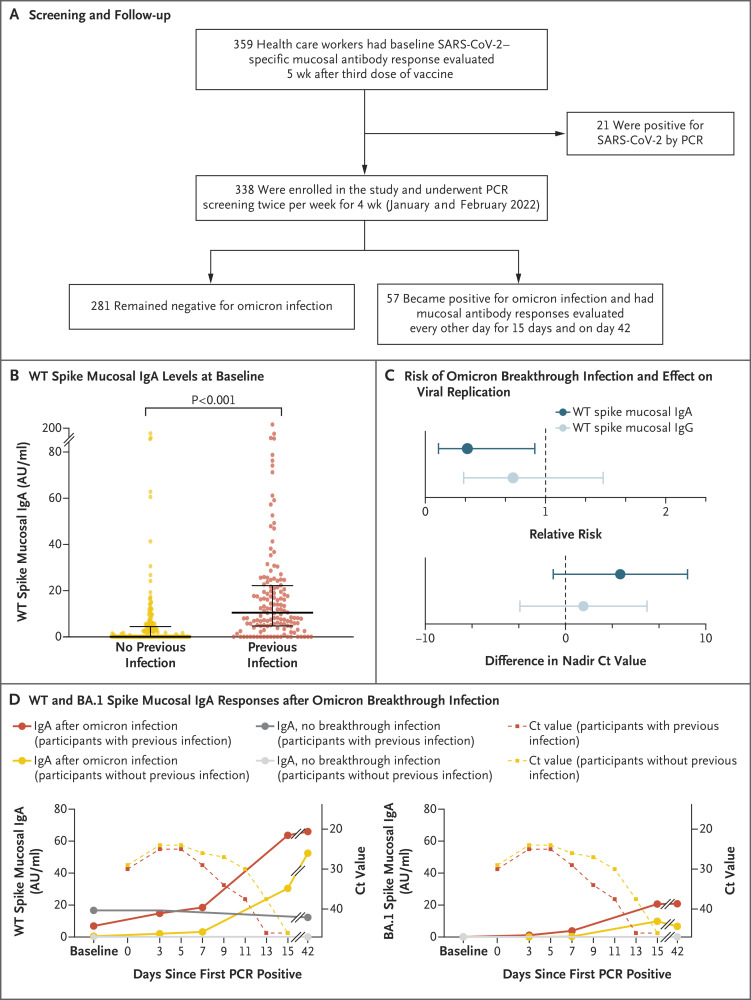
Figure 1. Spike-Specific Mucosal IgA and Omicron Infection.
Panel A shows the screening and follow-up of health care workers in this study. Participants were screened by polymerase-chain-reaction (PCR) testing of nasal, oropharyngeal, and saliva swab specimens twice weekly for 4 weeks. Mucosal antibody levels were determined from nasal swab specimens obtained at baseline (defined as 5 weeks after the booster dose) in all participants, as well as during and after subsequent omicron breakthrough infections (57 participants). Panel B shows levels of wild-type (WT) spike-specific mucosal IgA at baseline. Thick horizontal bars indicate the median, and thin horizontal bars the 25th and 75th percentiles. (For the participants with no previous infection, the median and the 25th percentile were both 0.1 arbitrary units [AU] per milliliter.) Participants who were PCR-positive for SARS-CoV-2 at baseline are not included in the plot. Panel C shows the relative risk of omicron breakthrough infection and the difference in viral replication (measured as the nadir cycle threshold [Ct]) among participants with high levels of WT spike-specific mucosal IgA or IgG (defined as those in the ≥75th percentile) at baseline as compared with participants with lower levels (<75th percentile). Error bars indicate the 97.5% confidence interval. Panel D shows median WT and omicron sublineage BA.1 spike-specific mucosal IgA responses after omicron breakthrough infection.
Wild-type SARS-CoV-2 spike-specific mucosal IgA and IgG were detected in 210 participants (62%) and 337 participants (>99%), respectively (Fig. S1A and S1B). Levels of spike-specific mucosal IgA (Figure 1B) but not IgG (Fig. S1C) were higher among participants with previous SARS-CoV-2 infection than among those without previous infection (P<0.001). The primary vaccine regimen, the time between the third vaccine dose and the time of sampling, age, and sex did not significantly affect the levels of wild-type spike-specific mucosal IgA (Table S2).
Next, we assessed the potential protective effects of mucosal antibodies against omicron infection and viral replication. Participants who had high levels of wild-type spike-specific mucosal IgA (defined as those in the ≥75th percentile) at enrollment had a significantly lower risk of subsequent omicron breakthrough infection than did those with lower levels (relative risk, 0.35; 97.5% confidence interval [CI], 0.11 to 0.91) (Figure 1C and Table S3); this effect was not found among participants who had high levels of IgG at enrollment. The results were similar among participants with and those without previous infection (Fig. S2 and Table S4). At baseline, levels of omicron sublineage BA.1 spike-specific mucosal IgA were generally lower than levels of wild-type spike-specific mucosal IgA (Fig. S1). However, a slightly but nonsignificantly lower risk of subsequent omicron infection was noted among participants with high levels of BA.1 spike-specific mucosal IgA at baseline than among those with low levels at baseline (relative risk, 0.63; 97.5% CI, 0.22 to 1.49). We also observed nonsignificantly lower levels of viral replication among infected participants who had high baseline levels of wild-type spike-specific mucosal IgA (difference in the nadir cycle threshold value, 3.91; 97.5% CI, −0.87 to 8.70) (Figure 1C and Table S5); this effect was not found among participants who had high baseline levels of IgG.
We analyzed the kinetics of mucosal antibody responses after omicron breakthrough infection. Levels of spike-specific, receptor-binding domain–specific, and nucleocapsid-specific mucosal IgA increased over time after infection, in both previously infected participants and previously uninfected participants (Figure 1D and Fig. S3). This finding is in contrast to findings in recent studies by our group4 and Reynolds et al.,5 which showed omicron-induced boosting of systemic spike-specific IgG responses predominantly in participants who had not previously been infected. Levels of wild-type spike-specific mucosal IgA were not correlated with levels of wild-type spike-specific mucosal or serum IgG (Fig. S4A and S4B). However, a strong correlation was seen between levels of spike-specific serum and mucosal IgG (Spearman’s r=0.7, P<0.001) (Fig. S4C), a finding that corroborates an IgG “spillover” from the circulation to the mucosa.1
Taken together, these findings suggest that wild-type SARS-CoV-2 spike-specific mucosal IgA is protective against omicron infection. Further studies are warranted to determine whether vaccines that induce a combination of mucosal and systemic immune responses would confer stronger protection than intramuscular vaccines.
Supplementary Appendix
Disclosure Forms
This letter was published on September 14, 2022, at NEJM.org.
Footnotes
Supported by grants from the Jonas and Christina af Jocknick Foundation (to Dr. Thålin), Region Stockholm (to Dr. Thålin), the Knut and Alice Wallenberg Foundation (to Drs. Thålin, Åberg, and Klingström), the Leif Lundblad Family Foundation (to Dr. Thålin), the Swedish Research Council (to Dr. Smed-Sörensen), the Swedish Heart and Lung Foundation (to Dr. Smed-Sörensen), the Bill and Melinda Gates Foundation (to Dr. Smed-Sörensen), and the Center for Innovative Medicine (to Drs. Blom and Klingström).
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Focosi D, Maggi F, Casadevall A. Mucosal vaccines, sterilizing immunity, and the future of SARS-CoV-2 virulence. Viruses 2022;14:187-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cagigi A, Yu M, Österberg B, et al. Airway antibodies emerge according to COVID-19 severity and wane rapidly but reappear after SARS-CoV-2 vaccination. JCI Insight 2021;6(22):e151463-e151463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planchais C, Fernández I, Bruel T, et al. Potent human broadly SARS-CoV-2-neutralizing IgA and IgG antibodies effective against omicron BA.1 and BA.2. J Exp Med 2022;219(7):e20220638-e20220638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blom K, Marking U, Havervall S, et al. Immune responses after omicron infection in triple-vaccinated health-care workers with and without previous SARS-CoV-2 infection. Lancet Infect Dis 2022;22:943-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds CJ, Pade C, Gibbons JM, et al. Immune boosting by B.1.1.529 (omicron) depends on previous SARS-CoV-2 exposure. Science 2022;377(6603):eabq1841-eabq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.