To the Editor: Nirmatrelvir is an inhibitor of the main protease in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that has been shown to block viral replication and reduce disease severity in unvaccinated persons at risk for the progression of coronavirus disease 2019 (Covid-19).1 Here, we describe the occurrence of rebound symptoms and viral replication after treatment with nirmatrelvir combined with ritonavir.
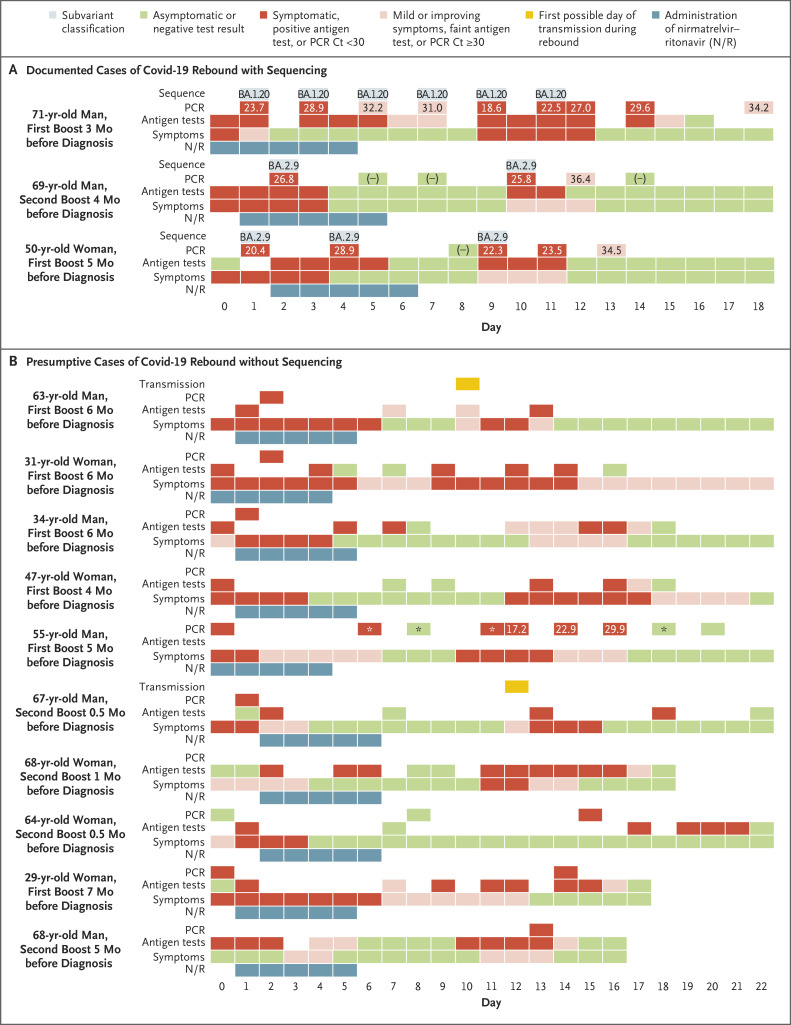
Patient 1, a 71-year-old man with asthma, reported having rhinorrhea, sore throat, congestion, cough, fatigue, malaise, chills, fever (temperature, 38.4°C), and a positive rapid antigen test for SARS-CoV-2. A 5-day course of nirmatrelvir–ritonavir was started on the same day. He was asymptomatic from day 2 through day 8. On days 9 through 12, while the patient was still isolating, he had a return of typical cold symptoms with rhinorrhea, sore throat, and congestion along with increased asthma symptoms. SARS-CoV-2 viral load was determined from anterior nasal swabs according to the cycle threshold (Ct) on quantitative reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay and indirectly from results of antigen testing. Peaks of symptoms and viral load coincided on days 1 and 9 (Figure 1 and Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Whole-genome viral sequencing identified the BA.1.20 subvariant of the SARS-CoV-2 B.1.1.529 (omicron) variant from day 1 through day 11.
Figure 1. Time Course of SARS-CoV-2 Infection and Covid-19 Symptoms in 13 Patients with Rebound.
Shown are data that were obtained between February 10 and May 30, 2022, from the 13 study patients. Day 0 was the first day of positive results on diagnostic testing or symptoms. The time periods are indicated for the administration of nirmatrelvir–ritonavir (N/R), symptoms, antigen tests, PCR (cycle threshold [Ct] values on polymerase-chain-reaction assay, when available), subvariant classification, and transmission. A rapid molecular test (Rapid PCR, Cue Health) was used only in the 55-year-old male patient, for whom PCR results with Ct values were obtained through BioReference Laboratories.
Patient 2, a 69-year-old man, had cold symptoms and positive results on rapid antigen testing and PCR assay on day 0 through day 3. A 5-day course of nirmatrelvir–ritonavir was started on day 1. The patient was asymptomatic with negative results on rapid antigen testing and intermittent PCR assays from day 4 to day 9. Mild cold symptoms and positive results on both rapid antigen testing and RT-PCR assay recurred on day 10 and lasted for 3 days. Patient 3, a 50-year-old woman who lived in the same household with Patient 2, had a similar pattern of rebound symptoms and viral load after treatment with nirmatrelvir–ritonavir. Viral sequencing identified the omicron BA.2.9 subvariant in these two patients. In all three patients who have been described here, there were no mutations in the gene encoding the main SARS-CoV-2 protease that occurred during treatment and no evidence of reinfection with a different variant.
In addition to these three patients with documented infection on sequencing, we also identified 10 presumptive cases of rebound viral replication (for a total of 13 cases in 7 men and 6 women), including 2 family members of Patient 1 (Table S1). The 13 patients ranged in age from 29 to 71 years (median age, 63 years). All the patients had been fully vaccinated and had received at least one dose of a messenger RNA booster 0.5 to 7 months before diagnosis. None of the patients had any known immunocompromising conditions, and all recovered without additional antiviral treatment. Rebound results on rapid antigen testing became strongly positive on days 9 through 15, remained positive for 2 to 7 days, and turned negative as late as day 22. Two patients may have transmitted SARS-CoV-2 infection to close contacts during the rebound period. (Details regarding possible transmission are provided in the Supplementary Appendix.)
In the EPIC-HR (Evaluation of Protease Inhibition for Covid-19 in High-Risk Patients) trial of nirmatrelvir–ritonavir, which was conducted during a period when the B.1.617.2 (delta) variant was predominant in the United States, investigators noted the occurrence of virologic rebound in some patients regardless of whether they had received antiviral treatment.2 In contrast, in a study involving National Basketball Association personnel who were under infection surveillance and who were not treated with nirmatrelvir–ritonavir, Ct values of less than 30 were not detected for the omicron variant between day 11 and day 15.3 Here, our evaluation includes a convenience sample in which 5 of 13 cases occurred within two families, which suggests that rebound after nirmatrelvir–ritonavir therapy is not uncommon. Additional data are needed to determine the cause, frequency, duration, and spectrum of rebound symptoms along with the relation to antiviral treatment. During rebound, the findings of a higher viral load, the ease of viral culture,4,5 and possible viral transmission suggest that patients are likely to be contagious during the rebound period.
The institutional review board at VA Boston Health Care determined that our report could be categorized as quality improvement and thus did not require additional review. Some of the cases described here were part of an observational cohort study that had been approved by the institutional review board at Columbia University Medical Center.
Supplementary Appendix
Disclosure Forms
This letter was published on September 7, 2022, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022;386:1397-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Drug Administration. Emergency use authorization (EUA) for Paxlovid (nirmatrelvir tablets co-packaged with ritonavir tablets). 2021. (https://www.fda.gov/media/155194/download).
- 3.Hay JA, Kissler SM, Fauver JR, et al. Viral dynamics and duration of PCR positivity of the SARS-CoV-2 omicron variant. January 14, 2022. (https://www.medrxiv.org/content/10.1101/2022.01.13.22269257v1). preprint.
- 4.Carlin AF, Clark AE, Chaillon A, et al. Virologic and immunologic characterization of COVID-19 recrudescence after nirmatrelvir/ritonavir treatment. Clin Infect Dis 2022. June 20 (Epub ahead of print).35723411 [Google Scholar]
- 5.Boucau J, Uddin R, Marino C, et al. Characterization of virologic rebound following nirmatrelvir–ritonavir treatment for COVID-19. Clin Infect Dis 2022. June 23 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.