Abstract
Background
The safety and immunogenicity of the bivalent omicron-containing mRNA-1273.214 booster vaccine are not known.
Methods
In this ongoing, phase 2–3 study, we compared the 50-μg bivalent vaccine mRNA-1273.214 (25 μg each of ancestral Wuhan-Hu-1 and omicron B.1.1.529 [BA.1] spike messenger RNAs) with the previously authorized 50-μg mRNA-1273 booster. We administered mRNA-1273.214 or mRNA-1273 as a second booster in adults who had previously received a two-dose (100-μg) primary series and first booster (50-μg) dose of mRNA-1273 (≥3 months earlier). The primary objectives were to assess the safety, reactogenicity, and immunogenicity of mRNA-1273.214 at 28 days after the booster dose.
Results
Interim results are presented. Sequential groups of participants received 50 μg of mRNA-1273.214 (437 participants) or mRNA-1273 (377 participants) as a second booster dose. The median time between the first and second boosters was similar for mRNA-1273.214 (136 days) and mRNA-1273 (134 days). In participants with no previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the geometric mean titers of neutralizing antibodies against the omicron BA.1 variant were 2372.4 (95% confidence interval [CI], 2070.6 to 2718.2) after receipt of the mRNA-1273.214 booster and 1473.5 (95% CI, 1270.8 to 1708.4) after receipt of the mRNA-1273 booster. In addition, 50-μg mRNA-1273.214 and 50-μg mRNA-1273 elicited geometric mean titers of 727.4 (95% CI, 632.8 to 836.1) and 492.1 (95% CI, 431.1 to 561.9), respectively, against omicron BA.4 and BA.5 (BA.4/5), and the mRNA-1273.214 booster also elicited higher binding antibody responses against multiple other variants (alpha, beta, gamma, and delta) than the mRNA-1273 booster. Safety and reactogenicity were similar with the two booster vaccines. Vaccine effectiveness was not assessed in this study; in an exploratory analysis, SARS-CoV-2 infection occurred in 11 participants after the mRNA-1273.214 booster and in 9 participants after the mRNA-1273 booster.
Conclusions
The bivalent omicron-containing vaccine mRNA-1273.214 elicited neutralizing antibody responses against omicron that were superior to those with mRNA-1273, without evident safety concerns. (Funded by Moderna; ClinicalTrials.gov number, NCT04927065.)
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines are safe and effective against coronavirus disease 2019 (Covid-19). In the Coronavirus Efficacy (COVE) trial, the mRNA-1273 vaccine (Moderna) had an acceptable safety profile and 93.2% efficacy against Covid-19 at a median of 5.3 months after the two-dose 100-μg primary series immunization.1,2
Early in the SARS-CoV-2 pandemic, variants such as beta (B.1.351) and delta (B.1.617.2) emerged that conferred immunologic escape or enhanced transmissibility. In 2022, omicron (B.1.1.529 [BA.1]) and omicron subvariants (BA.2, BA.2.12.1, BA.4, and BA.5), the most antigenically divergent variants to date, outcompeted other variants in the context of substantial preexisting population immunity from vaccination, infection, or both.3-7 Omicron variants continue to cause substantial numbers of illnesses and deaths.8-10
Booster immunization with 50-μg mRNA-1273 improves neutralizing antibody responses against variants and vaccine effectiveness against Covid-19.11-13 Nonetheless, the vaccine effectiveness against omicron is lower than that against other variants,14-17 and second booster doses of omicron-containing vaccines have been authorized in the United States.18,19
Vaccination strategies that can induce more potent, more durable, and broader immune responses are important to enhance protection. We previously reported that a modified, bivalent booster vaccine20 containing equal amounts of messenger RNAs (mRNAs) encoding the ancestral SARS-CoV-2 and beta variant spike proteins elicited superior and more durable neutralizing antibody responses against the beta, delta, and omicron variants as compared with mRNA-1273.20 Here, we present interim analysis results of an omicron-containing bivalent booster candidate, 50-μg mRNA-1273.214, from an ongoing safety and immunogenicity phase 2–3 study.
Methods
Study Oversight and Participants
This open-label, ongoing phase 2–3 study evaluates the immunogenicity, safety, and reactogenicity of bivalent booster vaccine mRNA-1273.214 as compared with the previously authorized mRNA-1273 booster vaccine in adults who had received a two-dose primary series (100 μg) and first booster dose (50 μg) of mRNA-1273 in the COVE trial1,2 or under U.S. emergency use authorization (EUA) at least 3 months earlier. Participants were enrolled and administered single second booster doses of 50-μg mRNA-1273 (part F, cohort 2) or 50-μg bivalent mRNA-1273.214 (part G) in a sequential, nonrandomized manner. The mRNA-1273 group serves as a noncontemporaneous within-study comparator. Adults with a known history of SARS-CoV-2 infection within 3 months before screening were excluded. (Details on inclusion and exclusion criteria, study design, study oversight, and author contributions are provided in the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org.)
Authors who were employees of the sponsor (Moderna) contributed to the design of the study; the collection, analysis, or interpretation of the data; and the drafting of the manuscript. All the authors critically reviewed and provided input to manuscript drafts and made the decision to submit the manuscript for publication. The authors vouch for the completeness and accuracy of the data and for the fidelity of the study to the protocol, which is available at NEJM.org.
Study Vaccines
The 50-μg bivalent mRNA-1273.214 vaccine contains two mRNAs (1:1 ratio, 25 μg each) encoding the prefusion-stabilized spike glycoproteins of ancestral SARS-CoV-2 (Wuhan-Hu-1) and the omicron variant (BA.1). The 50-μg monovalent mRNA-1273 vaccine contains a single mRNA strand encoding the spike glycoprotein of ancestral SARS-CoV-2 (Wuhan-Hu-1). The mRNA-1273.214 and mRNA-1273 vaccines were administered intramuscularly at a dose of 50 μg in a 0.5-ml volume.
Safety Assessment
The primary safety objective was to evaluate the safety and reactogenicity of 50-μg mRNA-1273.214 and 50-μg mRNA-1273 when administered as second booster doses (see the Methods section and Table S1 in the Supplementary Appendix). Safety assessments included solicited local and systemic adverse reactions within 7 days after booster administration; unsolicited adverse events within 28 days after booster administration; and serious adverse events, adverse events leading to discontinuation from study vaccine or participation, medically attended adverse events, and adverse events of special interest from day 1 through the entire study period (approximately 12 months).
Immunogenicity Assessment
The prespecified primary immunogenicity objectives were to show neutralizing antibody responses that were noninferior to 50-μg mRNA-1273 (on the basis of geometric mean titer ratio and difference in the percentage of participants with a seroresponse) or superior to 50-μg mRNA-1273 (on the basis of geometric mean titer ratio) against omicron and noninferior to 50-μg mRNA-1273 (on the basis of geometric mean titer ratio) against ancestral SARS-CoV-2 with the D614G mutation (ancestral SARS-CoV-2 [D614G]) 28 days after second boosters (day 29) of 50-μg mRNA 1273.214. (Details are provided in the Statistical Analysis section below, the Methods section in the Supplementary Appendix, and the statistical analysis plan, available with the protocol.) The prespecified key secondary objective was to show noninferiority (on the basis of the difference in the percentage of participants with a seroresponse) against ancestral SARS-CoV-2 (D614G) 28 days after the second booster of 50-μg mRNA-1273.214 as compared with 50-μg mRNA-1273.
Neutralizing antibody titers (geometric mean titers) at a 50% inhibitory dilution (ID50) were assessed with the use of validated SARS-CoV-2 spike-pseudotyped lentivirus neutralization assays against pseudoviruses containing the SARS-CoV-2 full-length spike proteins of ancestral SARS-CoV-2 (D614G) or omicron BA.1 variant and a research-grade pseudovirus assay containing full-length spike protein for omicron subvariants BA.4 and BA.5 (BA.4/5). Geometric mean levels of spike-binding antibody were also assessed (Meso Scale Discovery) against ancestral SARS-CoV-2 (D614G), gamma (P.1), alpha (B.1.1.7), beta (B.1.351), delta (B.1.617.2 and AY.4), and omicron (BA.1) variants. Immunogenicity assays are further described in the Supplementary Appendix.
Incidence of SARS-CoV-2 Infection
The incidences of symptomatic and asymptomatic SARS-CoV-2 infections were exploratory objectives. (For details, see the Methods section in the Supplementary Appendix.)
Statistical Analysis
Statistical analysis methods are detailed in the Supplementary Appendix, and the analysis sets are described in Table S2 and Figure S1. Safety was evaluated in the safety set, consisting of all the participants who received second boosters; solicited adverse reactions were assessed in the solicited safety set. The per-protocol immunogenicity set consists of all the participants in the full analysis set who received the planned booster doses, had prebooster and day 29 antibody data available, and had no major protocol deviations. Primary immunogenicity objectives were assessed in the per-protocol immunogenicity–SARS-CoV-2–negative set (primary analysis set). Analyses were also performed in participants who had evidence of previous SARS-CoV-2 infection before the booster.
The primary immunogenicity objectives were tested with the use of a prespecified hierarchical approach (see the Methods section and Fig. S2 in the Supplementary Appendix). Two interim analyses were planned at days 29 and 91 with a two-sided alpha level (0.025) allocated at each time point to preserve the family-wise type I error rate (0.05, two-sided) for immunogenicity hypothesis testing. The superiority of the antibody response against omicron after a second booster dose of 50-μg mRNA-1273.214 as compared with 50-μg mRNA-1273 was tested only after the meeting of noninferiority criteria for the three primary objectives21: antibody response against omicron after the second booster doses of 50-μg mRNA-1273.214 as compared with 50-μg mRNA-1273 on the basis of the geometric mean titer ratio, antibody response against omicron after the second booster doses of 50-μg mRNA-1273.214 as compared with 50-μg mRNA-1273 on the basis of the difference in the percentage of participants with a response, and antibody response against ancestral SARS-CoV-2 (D614G) after the second booster doses of 50-μg mRNA-1273.214 as compared with 50-μg mRNA-1273 on the basis of the geometric mean titer ratio; all tests were based on a two-sided alpha level of 0.025 at day 29.
The key secondary objective, noninferiority of the antibody response after second booster doses of 50-μg mRNA-1273.214 as compared with 50-μg mRNA-1273 against ancestral SARS-CoV-2 (D614G) on the basis of the difference in the percentage of participants with a seroresponse, was tested (two-sided alpha level, 0.025) if the primary objectives were met. Noninferiority is considered to be shown when the lower boundary of the 97.5% confidence interval of the geometric mean titer ratio is 0.67 or more and the difference in the percentage of participants with a seroresponse is greater than −10 percentage points. Superiority is considered to be shown when the lower boundary of the 97.5% confidence interval of the geometric mean titer ratio is greater than 1.22,23 Interim analysis results at day 29 are presented.
Observed geometric mean titers and 95% confidence intervals calculated on the basis of the t-distribution of log-transformed antibody titers are presented. Differences in antibody responses between the mRNA-1273.214 and mRNA-1273 groups were assessed with the use of an analysis of covariance (ANCOVA) model (with antibody titers after the booster as the dependent variable and study vaccine as the fixed effect) that was adjusted for age groups (<65 or ≥65 years) and prebooster antibody titers. The geometric mean titers and 95% confidence intervals estimated according to the geometric least-squares means from the model are presented, as are the differences in antibody responses (geometric mean titer ratio) between groups estimated according to the ratio of geometric least-squares means and 97.5% confidence intervals. Also assessed were seroresponses (change from below the lower limit of quantification [LLOQ] to ≥4 times the LLOQ, or an increase by a factor of ≥4 if the baseline value was greater than or equal to the LLOQ) with 95% confidence intervals (Clopper–Pearson) and between-group differences in the percentage of participants with a response and 97.5% confidence intervals (Miettinen–Nurminen) with adjustment for age groups. For the primary and key secondary objectives, 97.5% confidence intervals are provided.
Additional analyses included assessment of the primary immunogenicity end points in the per-protocol set for immunogenicity, an analysis involving participants with evidence of previous SARS-CoV-2 infection, and an analysis excluding participants with evidence of SARS-CoV-2 infection by day 29, performed with the use of an ANCOVA model (see the Methods section in the Supplementary Appendix). Observed geometric mean levels of spike-binding antibody against variants and differences between the mRNA-1273.214 and mRNA-1273 groups based on geometric mean titer ratios and 95% confidence intervals assessed by means of ANCOVA are provided.
The numbers and percentages of participants with asymptomatic or symptomatic SARS-CoV-2 infections and Covid-19 cases are summarized. All analyses were conducted with the use of SAS software, version 9.4 or higher (SAS Institute).
Results
Study Population
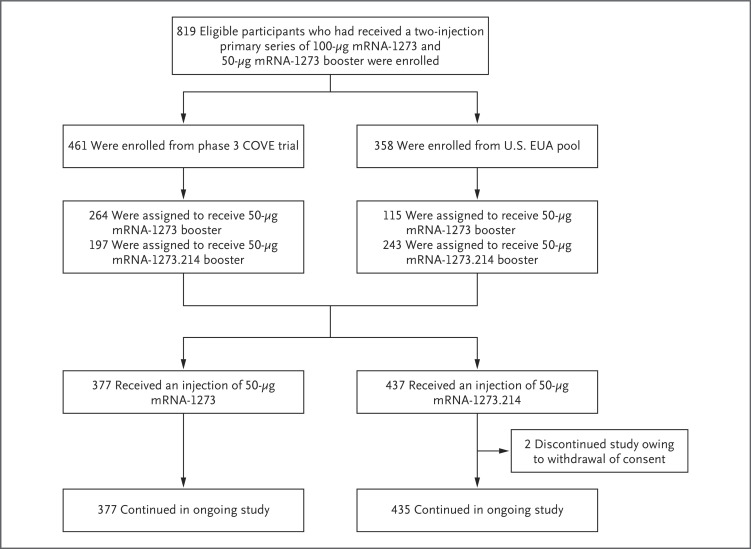
Between February 18 and March 8, 2022 (part F, cohort 2), and between March 8 and March 23, 2022 (part G), 819 participants were enrolled who had previously received the primary series of 100-μg mRNA-1273 and a first booster dose of 50-μg mRNA-1273, at least 3 months before enrollment (Figure 1). Of these, 197 of the COVE participants (44.8%) and 243 of the U.S. EUA participants (55.2%) were assigned to receive second booster doses of 50-μg mRNA-1273.214 (440 participants), and 264 participants (69.7%) and 115 participants (30.3%), respectively, were assigned to receive 50-μg mRNA-1273 (379 participants). A total of 437 participants (53.7%) in the 50-μg mRNA-1273.214 group and 377 participants (46.3%) in the 50-μg mRNA-1273 group received second boosters. Two participants (0.5%) withdrew consent and discontinued the study after receiving mRNA-1273.214.
Figure 1. Study Profile.
Eligible participants who received a previous two-injection primary series of 100-μg mRNA-1273 and a 50-μg mRNA-1273 booster dose either in the Coronavirus Efficacy (COVE) trial or under the U.S. emergency use authorization (EUA) were enrolled to receive a second booster dose of 50-μg mRNA-1273 (administered between February 18 and March 8, 2022) or mRNA-1273.214 (administered between March 8 and March 23, 2022). A total of 379 participants received a second booster dose of 50-μg mRNA-1273; 1 participant had previously received the primary series but not a first booster dose, and another participant had a major protocol deviation. These 2 participants were excluded from all analysis sets. A total of 437 participants received a second booster dose of mRNA-1273.214; 3 participants had discontinued the study before they received the second booster and were excluded from all analysis sets. The data-cutoff date was April 27, 2022.
The demographic and clinical characteristics of the participants were similar in the two groups (Table 1). The mean ages were 57.3 in the 50-μg mRNA-1273.214 group and 57.5 in the 50-μg mRNA-1273 group, and 59.0% and 50.7% of the participants, respectively, were female. Most participants were White (87.2% in the mRNA-1273.214 group and 85.4% in the mRNA-1273 group), and 10.5% and 9.8%, respectively, were Hispanic or Latinx. Black participants were underrepresented. The percentages of participants with evidence of previous SARS-CoV-2 infection were 22.0% in the mRNA-1273.214 group and 26.8% in the mRNA-1273 group. The median time between the second dose of mRNA-1273 in the primary series and the first booster of mRNA-1273 was similar in the two groups (245 days [interquartile range, 224 to 275] in the mRNA-1273.214 group and 242 days [interquartile range, 225 to 260] in the mRNA-1273 group), as was the median time between the first booster dose of mRNA-1273 and the second booster dose (136 days [interquartile range, 118 to 150] and 134 days [interquartile range, 118 to 150], respectively).
Table 1. Demographic and Clinical Characteristics of the Participants (Safety Set).*.
| Characteristic | 50-μg mRNA-1273.214 (N=437) |
50-μg mRNA-1273 (N=377) |
|---|---|---|
| Mean age at screening (range) — yr | 57.3 (20–88) | 57.5 (20–96) |
| Age subgroup — no. (%) | ||
| 18 to <65 yr | 263 (60.2) | 227 (60.2) |
| ≥65 yr | 174 (39.8) | 150 (39.8) |
| Sex — no. (%) | ||
| Male | 179 (41.0) | 186 (49.3) |
| Female | 258 (59.0) | 191 (50.7) |
| Hispanic or Latinx ethnic group — no. (%)† | ||
| Yes | 46 (10.5) | 37 (9.8) |
| No | 390 (89.2) | 340 (90.2) |
| Not reported or unknown | 1 (0.2) | 0 |
| Race or ethnic group other than Hispanic or Latinx — no. (%)† | ||
| White | 381 (87.2) | 322 (85.4) |
| Black | 31 (7.1) | 29 (7.7) |
| Asian | 14 (3.2) | 16 (4.2) |
| American Indian or Alaska Native | 0 | 1 (0.3) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (0.3) |
| Multiracial | 7 (1.6) | 2 (0.5) |
| Other | 3 (0.7) | 2 (0.5) |
| Not reported or unknown | 1 (0.2) | 4 (1.1) |
| Time between second injection of mRNA-1273 in the primary series and the first booster of mRNA-1273 | ||
| No. of participants evaluated | 435 | 374 |
| Median (IQR) — days | 245 (224–275) | 242 (225–260) |
| Time between first booster injection of mRNA-1273 and the second booster | ||
| No. of participants evaluated | 435 | 374 |
| Median (IQR) — days | 136 (118–150) | 134 (118–150) |
| Prebooster RT-PCR assay for SARS-CoV-2 — no. (%) | ||
| Negative | 434 (99.3) | 367 (97.3) |
| Positive | 2 (0.5) | 2 (0.5) |
| Missing | 1 (0.2) | 8 (2.1) |
| Prebooster antibody to SARS-CoV-2 nucleocapsid — no. (%)‡ | ||
| Negative | 341 (78.0) | 276 (73.2) |
| Positive | 95 (21.7) | 100 (26.5) |
| Missing | 1 (0.2) | 1 (0.3) |
| Prebooster SARS-CoV-2 status — no. (%)§ | ||
| Negative | 340 (77.8) | 267 (70.8) |
| Positive | 96 (22.0) | 101 (26.8) |
| By both RT-PCR assay and SARS-CoV-2 nucleocapsid‡ | 1 (0.2) | 1 (0.3) |
| By RT-PCR assay only | 1 (0.2) | 1 (0.3) |
| By SARS-CoV-2 nucleocapsid only‡ | 94 (21.5) | 99 (26.3) |
| Missing | 1 (0.2) | 9 (2.4) |
Percentages may not total 100 because of rounding. IQR denotes interquartile range, RT-PCR reverse-transcriptase polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Race and ethnic group were reported by the participant.
The Elecsys assay for binding antibody to SARS-CoV-2 nucleocapsid was used.
Prebooster SARS-CoV-2 status was positive if there was evidence of previous SARS-CoV-2 infection, defined as positive binding antibody against the SARS-CoV-2 nucleocapsid or positive RT-PCR assay at day 1; negative SARS-CoV-2 status was defined as negative binding antibody against the SARS-CoV-2 nucleocapsid and a negative RT-PCR assay at day 1. The data-cutoff date was April 27, 2022.
Safety
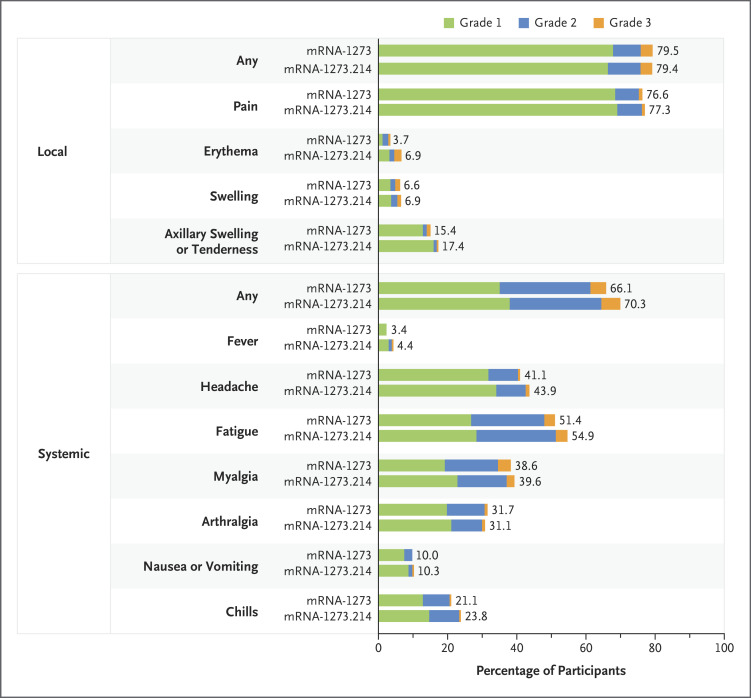
The median durations of follow-up were 43 days (interquartile range, 41 to 45) for the mRNA-1273.214 booster and 57 days (interquartile range, 56 to 62) for the mRNA-1273 booster. Occurrences of solicited adverse reactions within 7 days after the second booster dose were similar for mRNA-1273.214 and mRNA-1273 (Figure 2 and Table S3). The most frequent local adverse reaction after administration of both second boosters was injection-site pain, and the most frequent systemic reactions were fatigue, headache, myalgia, and arthralgia in both groups. The majority of solicited adverse reactions were mild to moderate (grades 1 and 2) for both boosters. Incidences of grade 3 events were similar in the two groups, and the most common such events were fatigue and myalgia. No grade 4 events occurred in either group.
Figure 2. Solicited Local and Systemic Adverse Reactions, According to Grade.
Shown are the percentages of participants in whom solicited local or systemic adverse reactions occurred within 7 days after the booster dose in the solicited safety set (351 participants in the mRNA-1273 group and 437 participants in the mRNA-1273.214 group). For some systemic adverse reactions, data were available for 350 participants in the mRNA-1273 group.
Unsolicited adverse events regardless of the relationship to vaccination at least 28 days after the second booster doses occurred in 18.5% of the participants in the mRNA-1273.214 group and in 20.7% of those in the mRNA-1273 group (Table S4). The overall incidences of adverse events that were considered by the investigator to be related to study vaccination were 5.7% and 5.8% in the respective groups. Serious adverse events were observed in two participants in the mRNA-1273.214 group (prostate cancer and traumatic fracture) and in one participant in the mRNA-1273 group (spinal osteoarthritis); none were considered to be related to study vaccination. Medically attended adverse events occurred in 9.8% of mRNA-1273.214 participants and in 13.8% of mRNA-1273 participants. Medically attended adverse events that were considered to be related to study vaccination occurred in two participants (0.5%) in the mRNA-1273.214 group (grade 2 fatigue and grade 1 dermatitis) and in two participants (0.5%) in the mRNA-1273 group (hypertension and urticaria, both grade 1). No fatal events or adverse events leading to study discontinuation were observed. At the data-cutoff date, no deaths and no events of myocarditis or pericarditis occurred, and one additional serious adverse event (grade 3 nephrolithiasis), considered to be unrelated to study vaccination, was reported in the mRNA-1273.214 group.
Immunogenicity
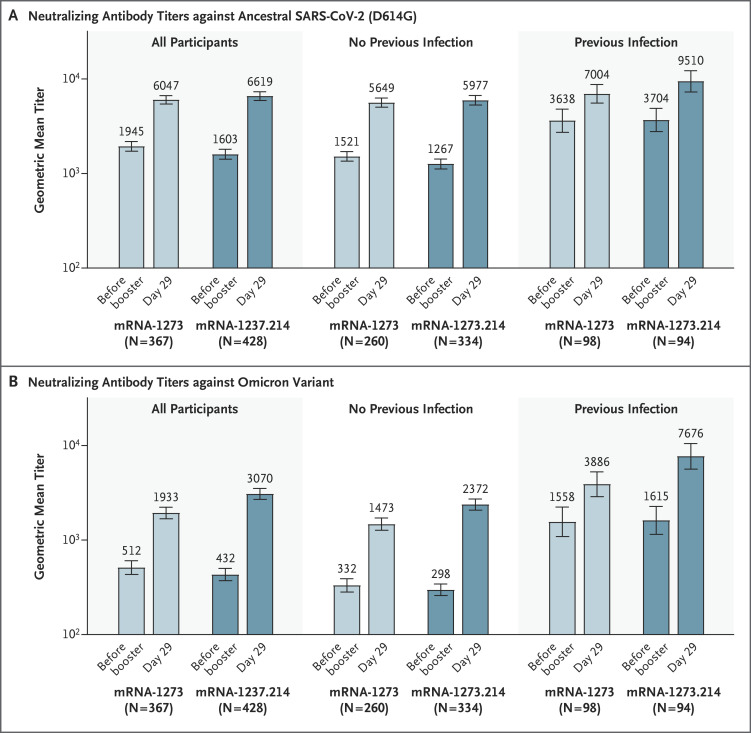
In the primary analysis set of participants without evidence of previous SARS-CoV-2 infection, the observed geometric mean titers of neutralizing antibodies against ancestral SARS-CoV-2 (D614G) were 5977.3 (95% confidence interval [CI], 5321.9 to 6713.3) and 5649.3 (95% CI, 5056.8 to 6311.2) and against omicron were 2372.4 (95% CI, 2070.6 to 2718.2) and 1473.5 (95% CI, 1270.8 to 1708.4) 28 days after the mRNA-1273.214 and mRNA-1273 boosters, respectively (Table 2). Estimated geometric mean titers after adjustment for age groups and prebooster titers were 6422.3 (95% CI, 5990.1 to 6885.7) and 5286.6 (95% CI, 4887.1 to 5718.9) against ancestral SARS-CoV-2 (D614G) 28 days after the mRNA-1273.214 and mRNA-1273 boosters, respectively, with a geometric mean titer ratio of 1.22 (97.5% CI, 1.08 to 1.37), which met the prespecified criterion for noninferiority. Estimated geometric mean titers against omicron were 2479.9 (95% CI, 2264.5 to 2715.8) and 1421.2 (95% CI, 1283.0 to 1574.4) 28 days after the mRNA-1273.214 and mRNA-1273 booster doses, respectively, with a geometric mean titer ratio of 1.75 (97.5% CI, 1.49 to 2.04), which met the prespecified superiority criterion.
Table 2. Primary Immunogenicity Analysis of Ancestral SARS-CoV-2 (D614G) and Omicron after 50 μg of mRNA-1273.214 or mRNA-1273 as a Second Booster Dose in Participants with No Previous SARS-CoV-2 Infection.*.
| Variable | Ancestral SARS-CoV-2 (D614G) | Omicron | ||
|---|---|---|---|---|
| 50-μg mRNA-1273.214 (N=334) |
50-μg mRNA-1273 (N=260) |
50-μg mRNA-1273.214 (N=334) |
50-μg mRNA-1273 (N=260) |
|
| Before booster | ||||
| No. of participants evaluated† | 334 | 260 | 334 | 260 |
| Observed geometric mean titer (95% CI)‡ | 1266.7 (1120.2 to 1432.5) |
1521.0 (1352.8 to 1710.2) |
298.1 (258.8 to 343.5) |
332.0 (282.0 to 390.9) |
| Day 29 | ||||
| No. of participants evaluated† | 334 | 260 | 334 | 260 |
| Observed geometric mean titer (95% CI)‡ | 5977.3 (5321.9 to 6713.3) |
5649.3 (5056.8 to 6311.2) |
2372.4 (2070.6 to 2718.2) |
1473.5 (1270.8 to 1708.4) |
| Factor change in geometric mean titer (95% CI)‡ | 4.7 (4.4 to 5.1) | 3.7 (3.4 to 4.0) | 8.0 (7.2 to 8.8) | 4.4 (4.0 to 5.0) |
| Estimated geometric mean titer (95% CI)§ | 6422.3 (5990.1 to 6885.7) |
5286.6 (4887.1 to 5718.9) |
2479.9 (2264.5 to 2715.8) |
1421.2 (1283.0 to 1574.4) |
| Geometric mean titer ratio (97.5% CI)§ | 1.22 (1.08 to 1.37) | — | 1.75 (1.49 to 2.04)¶ | — |
| Seroresponse at day 29‖ | ||||
| No. of participants/total no. | 334/334 | 260/260 | 333/333 | 256/258 |
| Percentage of participants (95% CI) | 100 (98.9 to 100) | 100 (98.6 to 100) | 100 (98.9 to 100) | 99.2 (97.2 to 99.9) |
| Difference (97.5% CI) — percentage points** | 0 | — | 1.5 (−1.1 to 4.0)†† | — |
Antibody values assessed by means of pseudovirus neutralizing antibody assay that were reported as being below the lower limit of quantification (LLOQ; 18.5 for ancestral SARS-CoV-2 [D614G] and 19.9 for omicron) were replaced by 0.5 times the LLOQ. Values greater than the upper limit of quantification (ULOQ; 45,118 for ancestral SARS-CoV-2 [D614G] and 15,502.7 for omicron) were replaced by the ULOQ if actual values were not available. Included are participants with no previous SARS-CoV-2 infection (primary analysis set).
Shown is the number of participants with nonmissing data at the time point (at or after baseline).
The 95% confidence intervals were calculated on the basis of the t-distribution of log-transformed values or difference in the log-transformed values for geometric mean titer and factor change in geometric mean titer, respectively, then back-transformed to the original scale.
Log-transformed antibody levels were analyzed with the use of an analysis of covariance model, with the study vaccine as a fixed effect and with adjustment for age group (<65 or ≥65 years) and prebooster titers. The resulting least-squares means and 95% confidence intervals, and the difference in least-squares means and 97.5% confidence intervals, were back-transformed to the original scale.
The value exceeded noninferiority criteria and met superiority criteria, including meeting noninferiority criteria for the three primary objectives in the prespecified hypothesis testing sequence and the superiority criterion of a lower boundary of the confidence interval for the geometric mean titer ratio greater than 1.
Seroresponse at a participant level was defined as a change from below the LLOQ to at least 4 times the LLOQ, or an increase by a factor of at least four if the baseline value was greater than or equal to the LLOQ; the comparison was with the prevaccination baseline value. Percentages were based on the number of participants with nonmissing data at baseline and the corresponding time point; 95% confidence intervals were calculated with the use of the Clopper–Pearson method.
The difference in the percentage of participants with a seroresponse is a calculated common risk difference that uses inverse-variance stratum weights and the middle point of Miettinen–Nurminen confidence limits of each one of the stratum risk differences. The stratified Miettinen–Nurminen estimate and the confidence interval cannot be calculated when the percentage of participants with a seroresponse in both groups is 100%; the absolute difference is reported.
The 97.5% confidence interval was calculated by means of a stratified Miettinen–Nurminen method, with adjustment according to age group.
The percentages of participants with a seroresponse against ancestral SARS-CoV-2 (D614G) were 100% (95% CI, 98.9 to 100) for mRNA-1273.214 and 100% (95% CI, 98.6 to 100) for mRNA-1273 at 28 days after the booster doses, with an estimated difference of 0, which met the noninferiority criterion. The percentages of participants with a seroresponse against omicron were 100% (95% CI, 98.9 to 100) for mRNA-1273.214 and 99.2% (95% CI, 97.2 to 99.9) for mRNA-1273 at 28 days after the booster doses, with an estimated difference of 1.5 percentage points (97.5% CI, −1.1 to 4.0), which met the noninferiority criterion. Therefore, the criteria for all primary and key secondary immunogenicity end points were met according to the prespecified testing sequence. The criteria for all immunogenicity end points were also met in the study participants overall, regardless of SARS-CoV-2 infection before the booster (Table S5).
In participants with previous SARS-CoV-2 infection, geometric mean titers were higher after the mRNA-1273.214 booster than after the mRNA-1273 booster against both ancestral SARS-CoV-2 (D614G) and omicron, with geometric mean titer ratios of 1.27 (95% CI, 1.07 to 1.51) and 1.90 (95% CI, 1.50 to 2.40), respectively (Figure 3 and Tables S6 and S7). For both boosters, the percentage of participants with a seroresponse was 100% for ancestral SARS-CoV-2 (D614G) and omicron, and the difference was 0.
Figure 3. Observed Neutralizing Antibody Titers against Ancestral SARS-CoV-2 (D614G) and Omicron after 50 μg of mRNA-1273.214 or mRNA-1273 Administered as a Second Booster Dose.
Pseudovirus neutralizing antibody geometric mean titers are provided for all participants regardless of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection before the booster (per-protocol immunogenicity set) and for those with or without previous SARS-CoV-2 infection before the booster. Data are from participants with nonmissing data at the time point. Nine participants in the mRNA-1273 group had missing data on prebooster SARS-CoV-2 status. Antibody values that were reported as below the lower limit of quantification (18.5 for ancestral SARS-CoV-2 [D614G] and 19.9 for omicron) were replaced by 0.5 times the lower limit of qualification. Values greater than the upper limit of quantification (45,118 for ancestral SARS-CoV-2 [D614G] and 15,502.7 for omicron) were replaced by the upper limit of qualification if actual values were not available. The 95% confidence intervals (indicated by 𝙸 bars) were calculated on the basis of the t-distribution of the log-transformed values for geometric mean titer, then back-transformed to the original scale for presentation. Data for observed neutralizing antibody geometric mean titers according to previous SARS-CoV-2 infection are provided in Table S7.
In participants without evidence of previous SARS-CoV-2 infection, the observed geometric mean titer of neutralizing antibodies against omicron BA.4/5 subvariants at 28 days after the mRNA-1273.214 booster (727.4 [95% CI, 632.8 to 836.1]) was higher than that after the mRNA-1273 booster (492.1 [95% CI, 431.1 to 561.9]), and the model-based geometric mean titer ratio was 1.69 (95% CI, 1.51 to 1.90) (Fig. S3 and Table S8). Similarly, geometric mean titers against the subvariants were higher after the mRNA-1273.214 booster than after the mRNA-1273 booster in participants with previous SARS-CoV-2 infection (2337.4 [95% CI, 1825.5 to 2992.9] vs. 1270.8 [95% CI, 987.3 to 1635.8]) and also in all participants regardless of previous SARS-CoV-2 infection (940.6 [95% CI, 826.3 to 1070.6] vs. 645.4 [95% CI, 570.1 to 730.6]), with corresponding geometric mean titer ratios of 1.60 (95% CI, 1.34 to 1.91) and 1.68 (1.52 to 1.84), both having lower boundaries of the confidence interval greater than 1.
In participants without evidence of previous SARS-CoV-2 infection, geometric mean levels of spike-binding antibody were higher (nominal alpha level, 0.05) after the mRNA-1273.214 booster than after the mRNA-1273 booster, and geometric mean titer ratios ranged from 1.11 (95% CI, 1.03 to 1.19) to 1.24 (95% CI, 1.14 to 1.35) across the ancestral SARS-CoV-2 (D614G) and omicron (BA.1), alpha, beta, gamma, and delta variants (Fig. S4 and Table S9). Similar geometric mean titer ratios were seen in all participants regardless of previous SARS-CoV-2 infection (Table S10). Observed geometric mean levels of spike-binding antibody are summarized in Table S11.
Incidence of SARS-CoV-2 Infection
Among all participants, starting 14 days after the booster and regardless of prebooster SARS-CoV-2 infection status, SARS-CoV-2 infection occurred in 11 participants (2.5%) in the mRNA-1273.214 group and in 9 participants (2.4%) in the mRNA-1273 group. Asymptomatic infection occurred in 6 participants (1.4%) in the mRNA-1273.214 group and in 7 participants (1.9%) in the mRNA-1273 group; Covid-19 according to the COVE trial definition occurred in 4 participants (0.9%) and in 2 participants (0.5%), respectively, and Covid-19 according to the Centers for Disease Control and Prevention (CDC) definition occurred in 5 participants (1.1%) and in 2 participants (0.5%), respectively.
In participants with no previous SARS-CoV-2 infection, infections occurred in 11 of 339 participants (3.2%) in the mRNA-1273.214 group and in 5 of 266 participants (1.9%) in the mRNA-1273 group after the booster (Table S12). Asymptomatic infection occurred in 6 participants (1.8%) in the mRNA-1273.214 group and in 4 participants (1.5%) in the mRNA-1273 group; Covid-19 according to the COVE trial definition occurred in 4 participants (1.2%) and in 1 participant (0.4%), respectively, and Covid-19 according to the CDC definition occurred in 5 participants (1.5%) and in 1 participant (0.4%), respectively.
There were three SARS-CoV-2 reinfections in the mRNA-1273 group. No emergency department visits or hospitalizations due to Covid-19 were seen.
Discussion
When administered as a second booster, a 50-μg dose of the bivalent omicron BA.1–containing mRNA-1273.214 vaccine had a safety and reactogenicity profile that was similar to that of the prototype 50-μg mRNA-1273 booster vaccine. The frequency of adverse events after a second booster dose of 50-μg mRNA-1273.214 was similar to or lower than the frequencies previously reported for the first booster dose of 50-μg mRNA-1273 and the second dose of the 100-μg mRNA-1273 primary series.1,2,12 Overall, the safety and reactogenicity of mRNA-1273.214 after three previous mRNA-1273 doses administered at intervals of at least 3 months are reassuring and are similar to those previously reported for the bivalent beta-containing candidate, mRNA-1273.211, which had a safety profile similar to that of mRNA-1273 through 6 months after vaccination.20
Neutralizing antibody responses have been used to infer Covid-19 vaccine efficacy.24,25 The mRNA-1273.214 vaccine elicited a superior neutralizing antibody response against omicron, as compared with mRNA-1273, 28 days after the booster dose, and the magnitude of the difference in the responses exceeded the recommended superiority criteria.23 The neutralizing antibody response against ancestral SARS-CoV-2 (D614G) was also higher with mRNA-1273.214 than with mRNA-1273, which indicates no decrement in the ancestral SARS-CoV-2 (D614G) antibody responses. Neutralizing antibody responses were consistently higher with mRNA-1273.214 than with mRNA-1273, irrespective of previous SARS-CoV-2 infection. On the basis of epidemiologic data suggesting decreasing vaccine effectiveness against omicron infection and suggesting that breakthrough infections can occur in vaccinated persons, including those with previous SARS-CoV-2 infection,8-10,14,26 having the capability to boost immune responses in persons with previous infection is important, given the potential for enhanced protection against Covid-19.24,25 Mechanisms of increased antibody responses with bivalent vaccines have yet to be elucidated but could include generation of new memory immune responses.27
Cross-reactivity against multiple variants is highly desirable given the continuous viral evolution and emergence of escape variants. We previously reported that the bivalent beta-containing mRNA-1273.211 vaccine induced consistently higher neutralizing antibody responses and spike-binding antibody responses against multiple variants than mRNA-1273.20 In the present study, the bivalent omicron-containing mRNA-1273.214 vaccine also elicited higher neutralizing antibody responses than mRNA-1273 against omicron BA.4/5 subvariants and higher spike-binding antibody responses against alpha, beta, gamma, delta, and omicron variants regardless of previous SARS-CoV-2 infection. The longevity of antibody responses is also important to future vaccination strategies. Enhanced durability of the antibody responses at 6 months after immunization was observed for mRNA-1273.211,20 and monitoring of the persistence of mRNA-1273.214 antibody responses continues in this ongoing clinical study.
Study limitations include the lack of randomization. Although the enrollment dates of the booster groups were within weeks of each other, representing similar epidemiologic environments of circulating variants, variant sequences were not ascertained. Furthermore, the temporal distances between primary vaccination and first and second boosters were similar in the two groups, despite the sequential study design. The study assessed only humoral immune responses, and future work is needed to characterize cellular responses. Given the public health importance of these data, longer-term follow-up is under way to further evaluate the safety and antibody persistence of the bivalent vaccine. The incidence of infections was numerically higher in the mRNA-1273.214 group than in the mRNA-1273 group among participants with no previous SARS-CoV-2 infection and balanced between the two groups among all participants owing to reinfections in the mRNA-1273 group. However, the study was not designed to evaluate vaccine effectiveness, and the follow-up time of infection after the booster is limited, which precludes conclusions about protection.
In this study, the 50-μg bivalent omicron-containing mRNA-1273.214 vaccine, when administered as a booster dose, had a safety and reactogenicity profile that was similar to that of 50-μg mRNA-1273 and elicited superior neutralizing antibody responses against omicron at 28 days after immunization. Neutralizing antibody responses were also higher against omicron subvariants BA.4/5 and ancestral SARS-CoV-2 (D614G). The mRNA-1273.214 booster also elicited higher spike-binding antibody responses against omicron, alpha, beta, gamma, and delta variants than mRNA-1273. These results are consistent with the evaluation of our bivalent beta-containing vaccine, which induced enhanced and durable antibody responses.20 Together, these findings indicate that bivalent vaccines may be a new tool in the response to emerging variants.
Acknowledgments
We thank the participants for their dedication and contributions to the study, the Immune Assay Team at Duke University Medical Center for analyses of pseudovirus neutralizing antibody assays, and Frank J. Dutko, Ph.D. (Moderna consultant), for figure development and editorial support with an earlier version of the manuscript.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on September 16, 2022, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by Moderna.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021;385:1774-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Yisimayi A, Jian F, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by omicron infection. Nature 2022;608:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CoVariants. Overview of variants in countries. 2021. (https://covariants.org/per-country).
- 5.CoVariants. Overview of variants/mutations. 2022. (https://covariants.org/variants).
- 6.Centers for Disease Control and Prevention. COVID data tracker: variant proportions. 2021. (https://covid.cdc.gov/covid-data-tracker/#variant-proportions).
- 7.UK Health Security Agency. Investigation of SARS-CoV-2 variants: technical briefings. September 9, 2022. (https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-technical-briefings).
- 8.Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of omicron in South Africa. Science 2022;376(6593):eabn4947-eabn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suryawanshi RK, Chen IP, Ma T, et al. Limited cross-variant immunity from SARS-CoV-2 omicron without vaccination. Nature 2022;607:351-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J, Novak T, Hecker J, et al. Cross-reactive immunity against the SARS-CoV-2 omicron variant is low in pediatric patients with prior COVID-19 or MIS-C. Nat Commun 2022;13:2979-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pegu A, O’Connell SE, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021;373:1372-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu L, Vrbicky K, Montefiori D, et al. Immune response to SARS-CoV-2 after a booster of mRNA-1273: an open-label phase 2 trial. Nat Med 2022;28:1042-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med 2022;386:1088-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med 2022;28:1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad N, Derado G, Nanduri SA, et al. Effectiveness of a COVID-19 additional primary or booster vaccine dose in preventing SARS-CoV-2 infection among nursing home residents during widespread circulation of the omicron variant — United States, February 14–March 27, 2022. MMWR Morb Mortal Wkly Rep 2022;71:633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. August 31, 2022. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use).
- 19.Centers for Disease Control and Prevention. Stay up to date with COVID-19 vaccines including boosters. September 8, 2022. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html).
- 20.Chalkias S, Eder F, Essink B, et al. Safety, immunogenicity and antibody persistence of a bivalent beta-containing booster vaccine against COVID-19: a phase 2/3 trial. Nature Med (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FDA Center for Drug Evaluation and Research. Multiple endpoints in clinical trials. Guidance for industry. January 2017. (https://www.fda.gov/files/drugs/published/Multiple-Endpoints-in-Clinical-Trials-Guidance-for-Industry.pdf).
- 22.Emergency use authorization for vaccines to prevent COVID-19. Guidance for industry. Rockville, MD: Food and Drug Administration, May 25, 2021. [Google Scholar]
- 23.FDA Center for Biologics Evaluation and Research. Emergency use authorization for vaccines to prevent COVID-19. Guidance for industry. March 2022. (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-vaccines-prevent-covid-19).
- 24.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022;375:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205-1211. [DOI] [PubMed] [Google Scholar]
- 26.Carazo S, Skowronski DM, Brisson M, et al. Protection against omicron re-infection conferred by prior heterologous SARS-CoV-2 infection, with and without mRNA vaccination. May 3, 2022. (https://www.medrxiv.org/content/10.1101/2022.04.29.22274455v2). preprint.
- 27.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021;591:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.