Abstract
Background
Corticosteroids are the mainstay of treatment for immune checkpoint inhibitor-associated acute kidney injury (ICPi-AKI), but the optimal duration of therapy has not been established. Prolonged use of corticosteroids can cause numerous adverse effects and may decrease progression-free survival among patients treated with ICPis. We sought to determine whether a shorter duration of corticosteroids was equally efficacious and safe as compared with a longer duration.
Methods
We used data from an international multicenter cohort study of patients diagnosed with ICPi-AKI from 29 centers across nine countries. We examined whether a shorter duration of corticosteroids (28 days or less) was associated with a higher rate of recurrent ICPi-AKI or death within 30 days following completion of corticosteroid treatment as compared with a longer duration (29–84 days).
Results
Of 165 patients treated with corticosteroids, 56 (34%) received a shorter duration of treatment and 109 (66%) received a longer duration. Patients in the shorter versus longer duration groups were similar with respect to baseline and ICPi-AKI characteristics. Five of 56 patients (8.9%) in the shorter duration group and 12 of 109 (11%) in the longer duration group developed recurrent ICPi-AKI or died (p=0.90). Nadir serum creatinine in the first 14, 28, and 90 days following completion of corticosteroid treatment was similar between groups (p=0.40, p=0.56, and p=0.89, respectively).
Conclusion
A shorter duration of corticosteroids (28 days or less) may be safe for patients with ICPi-AKI. However, the findings may be susceptible to unmeasured confounding and further research from randomized clinical trials is needed.
Keywords: Immunotherapy
Introduction
Immune checkpoint inhibitor-associated acute kidney injury (ICPi-AKI) is an increasingly recognized immune-related adverse event (irAE) that occurs in 2–5% of patients treated with ICPis.1 2 Patients who develop ICPi-AKI often have their ICPi therapy interrupted or permanently discontinued.3 They are also typically treated with immunosuppression, usually in the form of high-dose corticosteroids (CS).3
Despite their efficacy in treating irAEs, including ICPi-AKI, CS can result in hyperglycemia, weight gain, edema, fractures, gastrointestinal bleeds, infection, and other adverse events.4 5 Accordingly, defining the optimal duration of treatment with CS is critical to minimizing its side effect profile, as well as allowing for timely ICPi rechallenge, if indicated. Simultaneously, there is concern that premature discontinuation of CS might increase the risk of ICPi-AKI recurrence. There are few data available to guide clinicians in choosing the duration of CS for ICPi-AKI, and treatment duration varies widely in clinical practice.3
To address this knowledge gap, we used data from an international multicenter cohort study of adults with ICPi-AKI to examine whether shorter duration of CS treatment is associated with a higher risk of recurrent ICPi-AKI as compared with longer duration.
Methods
Study design
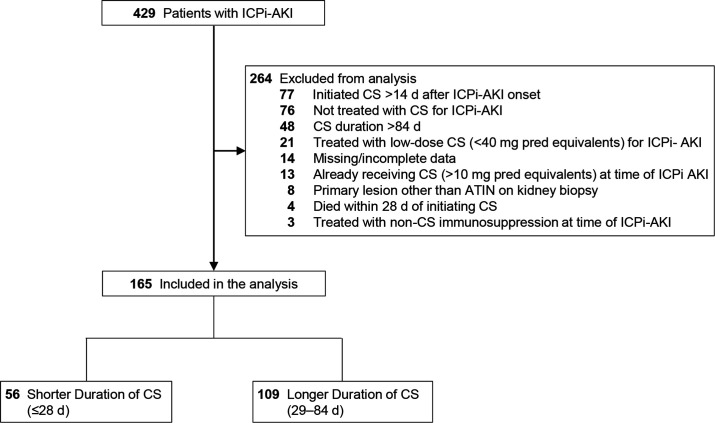
We previously described the clinical features, treatment, and outcomes of 429 adults diagnosed with ICPi-AKI between January 1, 2012, and December 31, 2020, from 30 sites across 10 countries.3 The cohort consisted of patients with AKI directly attributable to ICPi therapy (online supplemental table S1). AKI severity was staged according to the Kidney Disease: Improving Global Outcomes criteria (online supplemental table S2).6 In the current analyses, we included patients who initiated treatment with high-dose CS (≥40 mg daily in prednisone equivalents) within 14 days following ICPi-AKI diagnosis and had their CS tapered to ≤10 mg daily of prednisone equivalents within 12 weeks (84 days) following CS initiation. Eighty-four days was selected as the cut-off for the long duration group based on the distribution of the data, with the vast majority of patients tapered within this time frame (online supplemental figure S1). We excluded the following groups of patients: those already receiving treatment with CS (>10 mg daily of prednisone equivalents) at the time of ICPi-AKI diagnosis; those with a primary histopathologic lesion other than acute tubulointerstitial nephritis (ATIN); those treated with non-CS immunosuppression at the time of ICPi-AKI diagnosis; and, to avoid immortal time bias, those who died within 28 days of initiating CS (figure 1).
Figure 1.
Flowchart. ATIN, acute tubulointerstitial nephritis; CS, corticosteroids; d, days; ICPi-AKI, immune checkpoint inhibitor-associated acute kidney injury; pred, prednisone.
jitc-2022-005646supp001.pdf (105.4KB, pdf)
Primary objective and definition of recurrent ICPi-AKI
The primary objective was to determine the incidence and time to recurrent ICPi-AKI following completion of CS among patients who received a shorter duration (<28 days) versus a longer duration (29–84 days) of CS treatment. CS treatment was considered to be completed once the dose was tapered to ≤10 mg per day of prednisone equivalents. Recurrent ICPi-AKI was defined as meeting each of the following criteria: (1) an increase in serum creatinine (SCr) ≥50% compared with the value at completion of CS, or receipt of kidney replacement therapy; (2) the AKI was directly attributable to the ICPi by the treating provider; and (3) the AKI was treated with re-initiation or escalation of CS. To focus on unprovoked recurrence of ICPi-AKI (as opposed to recurrence of ICPi-AKI following ICPi rechallenge), we limited the assessment of the outcome to the first 30 days following completion of CS treatment. To account for death as a competing risk, we examined a composite outcome of recurrent ICPi-AKI or death in the 30 days following completion of CS treatment.
Statistical analysis
We compared baseline characteristics between patients in the shorter versus longer CS treatment groups. Categorical data were compared using χ2 or Fisher’s exact test, as appropriate. Continuous data were compared using Student’s t-test or Wilcoxon rank-sum test for normally distributed and skewed data, respectively. We compared time to recurrent ICPi-AKI or death between groups using Kaplan-Meier curves and the log-rank test. We compared nadir SCr in the first 14, 28, and 90 days following completion of CS treatment between groups using the Wilcoxon rank-sum test. Finally, in a sensitivity analysis, we compared the incidence of recurrent ICPi-AKI or death between groups only in patients who had received a kidney biopsy during their initial episode of ICPi-AKI. Two-sided p values<0.05 were considered significant. Analyses were performed in SAS V.9.5 (SAS Institute).
Results
Baseline characteristics
The original cohort included 429 patients with ICPi-AKI from 30 sites across 10 countries. After applying the exclusion criteria, the cohort for the current analyses consisted of 165 patients from 29 sites across 9 countries, 56 (34%) of whom received a CS treatment duration of 28 days or less and 109 (66%) of whom received a treatment duration of 29–84 days (figure 1). Patients in the shorter versus longer treatment groups were largely similar with respect to age, sex, race, malignancy type, baseline kidney function, and comorbidities (table 1).
Table 1.
Baseline characteristics
| Variable | Shorter duration of CS (n=56) |
Longer duration of CS (n=109) |
P value |
| Age at ICPi initiation, years, median (IQR) | 68 (59–75) | 69 (61–76) | 0.51 |
| Male, n (%) | 36 (64.3) | 69 (63.3) | 0.99 |
| Race, n (%) | 0.84 | ||
| White | 47 (83.9) | 95 (87.2) | |
| Black | 4 (7.1) | 3 (2.8) | |
| Other/unknown | 5 (8.9) | 11 (10.1) | |
| Comorbidities, n (%) | |||
| Hypertension | 36 (64.3) | 73 (67.0) | 0.74 |
| Diabetes | 10 (17.9) | 22 (20.2) | 0.84 |
| CHF | 3 (5.4) | 4 (3.7) | 0.69 |
| COPD | 0 (0) | 17 (15.6) | <0.01 |
| Cirrhosis | 1 (1.8) | 0 (0) | 0.34 |
| Body mass index, median (IQR) | 26 (23–30) | 28 (24–31) | 0.20 |
| Baseline eGFR,* mL/min per 1.73 m2 | |||
| Median (IQR) | 72 (58–85) | 72 (60–87) | 0.54 |
| eGFR categories, n (%) | 0.61 | ||
| ≥90 | 12 (21.4) | 20 (18.4) | |
| 60–89 | 28 (50.0) | 62 (56.9) | |
| 45–59 | 6 (10.7) | 15 (13.8) | |
| <45 | 10 (17.9) | 12 (11.0) | |
| Extrarenal irAE,† n (%) | 26 (46.4) | 57 (52.3) | 0.51 |
| Malignancy, n (%) | 0.54 | ||
| Lung | 11 (19.6) | 29 (26.6) | |
| Melanoma | 17 (30.4) | 28 (29.4) | |
| Genitourinary | 17 (34.7) | 32 (65.3) | |
| Other | |||
| PPI,‡ n (%) | 28 (50.0) | 67 (61.5) | 0.18 |
| Combo anti-CTLA-4+anti-PD-1/PD-L1 | 15 (26.8) | 27 (24.8) | 0.85 |
| Duration of CS, median (IQR) | 21 (14–25) | 46 (36–59) | <0.01 |
Data are shown as median (IQR) and n (%). All data are complete.
*Baseline eGFR was defined based on the closest SCr prior to ICPi initiation, and was calculated based on Chronic Kidney Disease-Epidemiology Collaboration equation.13
†Extrarenal irAEs were assessed prior to (>14 days) or concomitant (within 14 days before or after) with ICPi-AKI diagnosis.
‡PPIs were assessed in the 14 days preceding ICPi-AKI diagnosis.
AKI, acute kidney injury ; CHF, congestive heart failure; Combo, combination therapy; COPD, chronic obstructive pulmonary disease; CS, corticosteroids; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; eGFR, estimated glomerular filtration rate; ICPi, immune checkpoint inhibitor; irAE, immune-related adverse event; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1; PPI, proton pump inhibitor; SCr, serum creatinine.
Characteristics of initial episode of ICPi-AKI
Characteristics of the initial episode of ICPi-AKI are shown in table 2. The distribution of AKI severity was similar between patients in the shorter versus longer duration of CS treatment groups, as were urinalysis findings and urine protein studies (table 2). A total of 13 of the 56 patients (23.2%) in the shorter duration group, and 38 of the 109 patients (34.9%) in the longer duration group were biopsied, with ATIN found on all biopsies (table 2). Time from ICPi-AKI diagnosis to initiation of CS was also similar between groups. The median initial oral dose of CS was 60 mg daily in prednisone equivalents in both groups (table 2).
Table 2.
Characteristics of initial episode of ICPi-AKI
| Variable | Shorter duration (n=56) |
Longer duration (n=109) |
P value |
| Time to ICPi-AKI, days, median (IQR) | 97 (63–188) | 112 (56–224) | 0.81 |
| ICPi-AKI stage,* n (%) | 0.37 | ||
| Stage 1 | 8 (14.3) | 11 (10.1) | |
| Stage 2 | 20 (35.7) | 37 (33.9) | |
| Stage 3 | 28 (50.0) | 61 (56.0) | |
| KRT, n (%) | 4 (7.1) | 5 (4.6) | 0.49 |
| Hospitalized for AKI, n (%) | 33 (58.9) | 63 (57.8) | 0.99 |
| Nephrologist involved, n (%) | 44 (78.6) | 94 (86.2) | 0.27 |
| Urine studies | |||
| Blood (≥2+) on UA, n (%) | 10 (17.9) | 11 (10.1) | 0.24 |
| Leukocyte esterase (≥2+) on UA, n (%) | 11 (19.6) | 18 (16.5) | 0.77 |
| Pyuria (≥5 WBCs per hpf on UA), n (%) | 25 (44.6) | 57 (51.4) | 0.44 |
| UPCR ≥0.3 g/g, n (%) | 16 (28.6) | 34 (31.2) | 0.87 |
| Biopsied, n (%) | 13 (23.2) | 38 (34.9) | 0.16 |
| ATIN on kidney biopsy, n (%) | 13 (100) | 38 (100) | 0.99 |
| Time to CS Initiation, days, median (IQR) | 3 (0–7) | 2 (0–5) | 0.43 |
| Initial daily oral CS dose (prednisone equivalent units, mg), median (IQR) | 60 (58–60) | 60 (60–88) | 0.78 |
| Received intravenous pulse CS, n (%) | 17 (30.4) | 26 (23.9) | 0.58 |
| Non-CS immunosuppression,† n (%) | 1 (1.8) | 2 (1.8) | 0.99 |
| Rechallenged, n (%) | 13 (23.2) | 15 (13.7) | 0.13 |
| Recurrent ICPi-AKI after rechallenge, n (%) | 1 (1.8) | 2 (1.8) | 0.99 |
A total of 28 patients (50%) were missing data on UPCR, and 8 (14.3%) were missing data on leukocyte esterase, blood, and pyuria on UA in the shorter duration group. A total of 52 patients (47.8%) were missing data on UPCR, and 28 (25.7%) were missing data on leukocyte esterase, blood, and pyuria on UA in the longer duration group.
*AKI stages are defined by Kidney Disease: Improving Global Outcomes criteria.
†One patient in the shorter duration group received tocilizumab. In the longer duration group, one patient received mycophenolate mofetil, and one received infliximab.
ATIN, acute tubulointerstitial nephritis; CS, corticosteroid; hpf, high power field; ICPi-AKI, immune checkpoint inhibitor-associated acute kidney injury; KRT, kidney replacement therapy; SCr, serum creatinine; UA, urinalysis; UPCR, urine protein:creatinine ratio; WBCs, white blood cells.
Recurrent ICPi-AKI or death
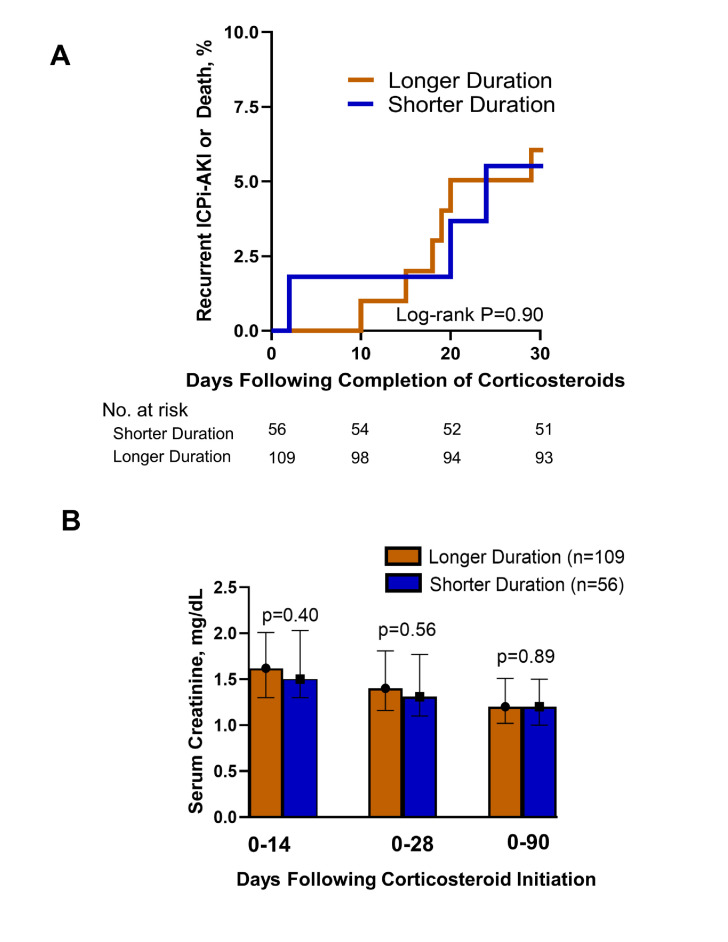
A total of 17 patients (10.3%) developed recurrent ICPi-AKI or death within 30 days following completion of CS treatments, including 5 of 56 patients (8.9%) in the shorter treatment duration group and 12 of 109 (11%) in the longer duration group (figure 2A). In the shorter treatment duration group, 3 of 56 patients developed recurrent ICPi-AKI and 2 of 56 died in the 30 days following completion of treatment with CS. In the longer duration treatment group, 3 of 109 patients developed recurrent ICPi-AKI and 9 died in the 30 days following completion of treatment with CS.
Figure 2.
Recurrent ICPi-AKI or death and longitudinal kidney function following completion of shorter versus longer duration of treatment with corticosteroids. (A) Kaplan-Meier curve showing risk of recurrent ICPi-AKI or death in the 30 days following completion of treatment with corticosteroids. N=56 in the shorter duration group; n=109 in the longer duration group. (B) Nadir serum creatinine in the shorter versus longer duration of corticosteroid therapy groups. Median serum creatinine levels are depicted, with error bars representing IQR. ICPi-AKI, immune checkpoint inhibitor-associated acute kidney injury.
Recurrent ICP-AKI or death occurred at a median of 20 days (IQR, 14–20) and 5 days (IQR, 1–18) in the shorter and longer treatment duration groups, respectively (log-rank p=0.90) (figure 2A). Nadir SCr in the first 14, 28, and 90 days following CS initiation was similar between groups (p=0.40, p=0.56, and p=0.89, respectively) (figure 2B).
When examining the characteristics of the 17 patients who developed recurrent ICPi-AKI or death compared with the 148 who did not, the former tended to be older and to have a lower baseline estimated glomerular filtration rate compared with the latter, but these findings did not reach statistical significance (online supplemental table S3). No characteristic reliably predicted recurrent ICPi-AKI or death (online supplemental table S3).
In a sensitivity analysis limited to patients who were biopsied, none of the 13 patients in the shorter duration group and 4 of the 38 patients (10.5%) in the longer duration group developed recurrent ICPi-AKI or death within 30 days of CS treatment completion (p=0.56).
Discussion
In this international multicenter cohort study of adults with ICPi-AKI, we found no difference in the incidence or timing of recurrent ICPi-AKI or death in patients treated with shorter versus longer durations of CS. These data suggest that shorter durations of CS may be similarly efficacious and safe compared with longer durations.
Guidelines from the National Comprehensive Cancer Network recommend that ICPi-AKI should be treated with CS, gradually tapered over 4–6 weeks and only once the SCr improves to grade 1 toxicity or below.7 However, data supporting these recommendations are scarce. Lee et al examined outcomes among 13 patients with ICPi-AKI treated with a short duration of CS (tapered to ≤10 mg daily of prednisone equivalents within 3 weeks) versus 14 patients treated with a longer duration of CS, and found no significant difference in the time to renal recovery between groups.8 Our data are consistent with these findings and expand on them in a larger and more generalizable cohort.
Data on the impact of CS on cancer outcomes among patients receiving immunotherapy are mixed. Some studies found that administration of CS is not associated with reduced efficacy of immunotherapy,9 10 while others demonstrated an association with decreased progression-free survival.11 12 Irrespective of a potential negative effect on the antitumor efficacy of immunotherapy, prolonged use of high-dose CS can cause numerous adverse effects.4 5 Additionally, longer durations of high-dose CS may preclude early rechallenge with ICPis, which has been shown to be safe in the vast majority of patients with ICPi-AKI.3
We acknowledge several limitations. First, we focused on recurrence of ICPi-AKI or death within the first 30 days following completion of CS treatment, and therefore we cannot exclude the possibility that differences between groups may have been observed with longer follow-up. Second, given the relatively small number of events, we could not study the multivariable-adjusted risk of recurrent ICPi-AKI or death, though notably there were no predictors even in univariate analyses (online supplemental table S3). Third, we did not have data on cancer outcomes.
In summary, we found no difference in the risk of recurrent ICPi-AKI or death among patients who received shorter versus longer durations of treatment with CS. Randomized clinical trials are needed to further investigate the effects of varying durations of CS on renal and extrarenal outcomes in patients with ICPi-AKI.
Footnotes
Twitter: @ShrutiGKidney, @DavidLeaf9
MES, MJS and DEL contributed equally.
Collaborators: ICPi-AKI Consortium Investigators: Assistance Publique-Hôpitaux de Paris (AP-HP), Sorbonne Université, Hôpital Pitié-Salpêtrière: Luca Campedel, Joe-Elie Salem, Corinne Isnard Bagnis Brigham and Women’s Hospital/Dana-Farber Cancer Institute: Shruti Gupta, David E Leaf, Harkarandeep Singh, Shveta S Motwani, Naoka Murakami, Maria C Tio, Suraj S Mothi, Umut Selamet Charité – Universitätsmedizin Berlin: Sebastian Loew, Kai M Schmidt-Ott Chi-Mei Medical Center: Weiting Chang Donald and Barbara Zucker School of Medicine: Kenar D Jhaveri, Rimda Wanchoo, Yuriy Khanin, Jamie S Hirsch, Vipulbhai Sakhiya, Daniel Stalbow, Sylvia Wu Duke University Medical Center: David I Ortiz-Melo Guy’s and St. Thomas NHS Hospital: Marlies Ostermann, Nuttha Lumlertgul, Nina Seylanova, Armando Cennamo, Anne Rigg, Nisha Shaunak Harvard Medical School: Zoe A Kibbelaar Heidelberg University Hospital: Karolina Benesova Icahn School of Medicine at Mount Sinai Hospital: Priya Deshpande Massachusetts General Hospital: Meghan E Sise, Kerry L Reynolds, Harish S Seethapathy, Meghan Lee, Ian A Strohbhen Mayo Clinic: Sandra M Herrmann, Busra Isik Memorial Sloan Kettering Cancer Center: Ilya G Glezerman New York Nephrology Vasculitis and Glomerular Center: Frank B Cortazar Northwestern University: Vikram Aggarwal, Sunandana Chandra Ohio State University: Jason M Prosek, Sethu M Madhavan, Dwight H Owen, Marium Husain Sheba Medical Center: Pazit Beckerman, Sharon Mini Stanford University School of Medicine: Shuchi Anand, Pablo Garcia, Aydin Kaghazchi University of Alabama at Birmingham: Sunil Rangarajan University of California-Los Angeles: Daniel Sanghoon Shin, Grace Cherry University of California-San Francisco: Christopher A Carlos, Raymond K Hsu, Andrey Kisel University Hospitals Cleveland Medical Center: Arash Rashidi, Sheru K Kansal, Nicole Albert, Katherine Carter, Vicki Donley, Tricia Young, Heather Cigoi University Hospital of Geneva: Sophie De Seigneux, Thibaud Koessler University Hospitals Leuven: Ben Sprangers, Els Wauters University of Florida: Chintan V Shah University Medical Center Groningen: Mark Eijgelsheim University of Miami Miller School of Medicine: Zain Mithani, Javier A Pagan University of Pennsylvania Health System: Gaia Coppock, Jonathan J Hogan University of Texas MD Anderson Cancer Center: Ala Abudayyeh, Omar Mamlouk, Jamie S Lin, Valda Page University of Toronto: Abhijat Kitchlu University of Vermont Larner College of Medicine: Samuel AP Short University of Virginia Health System: Amanda D Renaghan, Elizabeth M Gaughan University of Washington: A Bilal Malik Vall d’Hebron University Hospital: Maria Jose Soler, Clara García-Carro, Sheila Bermejo, Enriqueta Felip, Eva Muñoz-Couselo, Maria Josep Carreras.
Contributors: Conceptualization: SG, DEL, MJS, MES, and CG-C. Data curation and original draft preparation: SG and DEL. Visualization and investigation: SG and DEL. Data collection: MES, CG-C, JMP, IG, SMH, PG, AA, NL, ABM, SL, PB, ADR, CAC, AR, ZM, PD, SR, CVS, SDS, LC, AK, DSS, GC, DIO-M, BS, VA, KB, RW, NM, FBC, and KLR. Supervision: DEL and MJS. Writing, reviewing, and editing: SG, MJS, and DEL. All authors approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SG receives research funding from GE Healthcare and BTG International and is President and Founder of the American Society of Onconephrology. CGC has received travel and congress fees support from AstraZeneca, Esteve, NovoNordisk, Boehringer Ingelheim Lilly, Astellas, Otsuka, Novartis, Astellas, and Baxter and has given scientific lectures and participated in advisory boards organized by AstraZeneca, Boehringer Ingelheim Lilly, Mundipharma, and NovoNordisk. DSS participates in the speakers’ bureau at Genentech. FBC is a consultant for ChemoCentryx and Retrophin. AA is supported by the Division of Internal Medicine Immuno-Oncology Toxicity Award Program of the University of Texas MD Anderson Cancer Center. BS is a senior clinical investigator at the Research Foundation Flanders (F.W.O.) (1842919N) and is supported by Stichting tegen Kanker (grant C/2020/1380). AR is a consultant for Otsuka Pharmaceutical, and treasurer of the American Society of Onconephrology. SMH is supported by the Mayo Clinic K2R award. KB receives grant support from Olympia Morata Programme, Foundations Commission of University of Heidelberg, Rheumaliga Baden-Württemberg e.V., AbbVie, and Novartis. KB also serves as a consultant/receives speaker fee/travel reimbursements from AbbVie, BMS, Janssen, MSD, Viatris, Gilead/Galapagos, Lilly, Medac, Mundipharma, Novartis, Pfizer, Roche, and UCB. MES has served on a scientific advisory board for Mallinckrodt. LC serves as a consultant/receives honorarium/travel reimbursements from Pfizer, Bristol Myers Squibb, MSD. The remaining authors have no conflicts of interest or disclosures. MJS reports personal fees from NovoNordisk, Janssen, Mundipharma, AstraZeneca, Esteve, Fresenius, Ingelheim Lilly, Vifor, ICU, Pfizer, Bayer, Travere Therapeutics, GE Healthcare and Boehringer Ingelheim. MJS is a consultant for NovoNordisk, Travere Therapeutics, GE Healthcare, AstraZeneca, and Boehringer. MJS receives grant support form Boehringer Ingelheim, ISCIIII-FEDER and ISCIII-RETICS REDinREN, grant number PI17/00257, PI21/01292, RD16/0009/0030, RICORS RD21/0005/0016, Marató TV3 2020 421/C/2020, Marató TV3 2021 215/C/2021, and EIN2020-112338. MJS is elected Editor-in-Chief of Clinical Kidney Journal.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
ICPi-AKI Consortium collaborators:
Luca Campedel, Joe-Elie Salem, Corinne Isnard Bagnis, Shruti Gupta, David E Leaf, Harkarandeep Singh, Shveta S Motwani, Naoka Murakami, Maria C Tio, Suraj S Mothi, Umut Selamet, Sebastian Loew, Kai M Schmidt-Ott, Weiting Chang, Kenar D Jhaveri, Rimda Wanchoo, Yuriy Khanin, Jamie S Hirsch, Vipulbhai Sakhiya, Daniel Stalbow, Sylvia Wu, David I Ortiz-Melo, Marlies Ostermann, Nuttha Lumlertgul, Nina Seylanova, Armando Cennamo, Anne Rigg, Nisha Shaunak, Zoe A Kibbelaar, Karolina Benesova, Priya Deshpande, Meghan E Sise, Kerry L Reynolds, Harish S Seethapathy, Meghan Lee, Ian A Strohbhen, Sandra M Herrmann, Busra Isik, Ilya G Glezerman, Frank B Cortazar, Vikram Aggarwal, Sunandana Chandra, Jason M Prosek, Sethu M. Madhavan, Dwight H Owen, Marium Husain, Pazit Beckerman, Sharon Mini, Shuchi Anand, Pablo Garcia, Aydin Kaghazchi, Sunil Rangarajan, Daniel Sanghoon Shin, Grace Cherry, Christopher A Carlos, Raymond K Hsu, Andrey Kisel, Arash Rashidi, Sheru K Kansal, Nicole Albert, Katherine Carter, Vicki Donley, Tricia Young, Heather Cigoi, Sophie De Seigneux, Thibaud Koessler, Els Wauters Ben Sprangers, Chintan V Shah, Mark Eijgelsheim, Zain Mithani, Javier A Pagan, Gaia Coppock, Jonathan J Hogan, Ala Abudayyeh, Omar Mamlouk, Jamie S Lin, Valda Page, Abhijat Kitchlu, Samuel AP Short, Amanda D Renaghan, Elizabeth M Gaughan, A Bilal Malik, Maria Jose Soler, Clara García-Carro, Sheila Bermejo, Enriqueta Felip, Eva Muñoz-Couselo, and Maria Josep Carreras
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All protocols were approved by the Mass General Brigham Institutional Review Board (IRB) (Protocol 2017P000501), and by the IRBs of the participating sites.
References
- 1. Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–47. 10.1016/j.kint.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seethapathy H, Zhao S, Chute DF, et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol 2019;14:1692–700. 10.2215/CJN.00990119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta S, Short SAP, Sise ME, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer 2021;9:e003467. 10.1136/jitc-2021-003467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saag KG, Koehnke R, Caldwell JR, et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med 1994;96:115–23. 10.1016/0002-9343(94)90131-7 [DOI] [PubMed] [Google Scholar]
- 5. Del Castillo M, Romero FA, Argüello E, et al. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis 2016;63:1490–3. 10.1093/cid/ciw539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kellum JA, Lameire N, Aspelin P. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138. 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 7. Thompson JA, Schneider BJ, Brahmer J. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Cancer Netw 2019;17:255–89. 10.6004/jnccn.2019.0013 [DOI] [PubMed] [Google Scholar]
- 8. Lee MD, Seethapathy H, Strohbehn IA, et al. Rapid corticosteroid taper versus standard of care for immune checkpoint inhibitor induced nephritis: a single-center retrospective cohort study. J Immunother Cancer 2021;9:e002292. 10.1136/jitc-2020-002292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maher VE, Fernandes LL, Weinstock C, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol 2019;37:2730–7. 10.1200/JCO.19.00318 [DOI] [PubMed] [Google Scholar]
- 10. Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol 2020;6:1952–6. 10.1001/jamaoncol.2020.5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed Death-Ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018;36:2872–8. 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 12. Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–14. 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005646supp001.pdf (105.4KB, pdf)