Abstract
Metformin, a biguanide hypoglycemic agent that is safe and effective for treating acne in women with the polycystic ovarian syndrome (PCOS), has shown growing evidence of improving insulin resistance, hyperandrogenism, dyslipidemia, overall cardiovascular health, quality of life, psychological wellbeing, and general health outcomes. This study aims to identify and summarize the effects of metformin in patients with PCOS-associated acne. This systematic review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic search was done on PubMed, PubMed Central, Cochrane, Google Scholar, and Science Direct databases from 2011 up to 23 February 2022. Randomized controlled trials (RCTs), cross-sectional studies, observational studies, literature reviews, systematic reviews, and meta-analyses published in English were selected. The data was extracted to a predefined template. Each study was individually checked by using a quality assessment. The initial search generated a total of 218 studies. Nine studies were included in the final selection: two RCTs, one hospital-based longitudinal study, one hospital-based clinical trial, three cross-sectional studies, three systematic reviews with meta-analyses, and one narrative review. Metformin is generally effective and safe for improving PCOS-associated acne and the quality of life. More clinical trials are required to determine the indications for prescribing metformin in patients with PCOS-associated acne.
Keywords: polycystic ovarian syndrome, antiandrogen, hyperandrogenism, metformin, nodulocystic acne, acne
Introduction and background
In 1935, Stain and Leventhal first described polycystic ovarian syndrome (PCOS) in a series of seven women who presented with bilateral cystic ovaries, amenorrhea, hirsutism, and obesity [1]. Rotterdam criteria have been used to diagnose PCOS over the last 10 years.
PCOS affects a considerable proportion of women, particularly South Asians residing in the United Kingdom [2-4]. Fifty-two percent of women residing in the Indian subcontinent have polycystic ovaries, the highest reported prevalence [5]. PCOS is associated with interference in reproductive hormones like luteinizing hormone (LH), follicle-stimulating hormone (FSH), estrogen, and testosterone which result in menstrual cycle irregularities like oligomenorrhea or amenorrhea. Acne is the most common manifestation of hyperandrogenism among other cutaneous manifestations like acanthosis nigricans, androgenic alopecia, and hirsutism. Acne vulgaris, characterized by chronic inflammation of the pilosebaceous unit, is one of the most common skin diseases for routine dermatology outpatient visits among patients aged 15 to 40 years [6]. Hyperandrogenism should be considered in female patients with sudden onset of severe acne that is associated with hirsutism or irregular menstrual periods. It is a risk for reproductive complications like infertility or subfertility, metabolic complications, cardiovascular risks, and psychological disturbances, along with type 2 diabetes and endometrial dysplasia [7-8]. Metformin is a biguanide that is effective and safe for improving insulin resistance, menstrual cycle irregularities, acne, and symptoms of hyperandrogenism like hirsutism [9]. Metformin’s mechanism of action is still not clear. While it is known to be an insulin-sensitizing agent which acts by inhibiting gluconeogenesis and lipogenesis, thus enhancing glucose uptake in the liver, skeletal muscles, adipose tissue, and ovaries. Metformin also decreases ovarian androgen production by 20% to 25% [10].
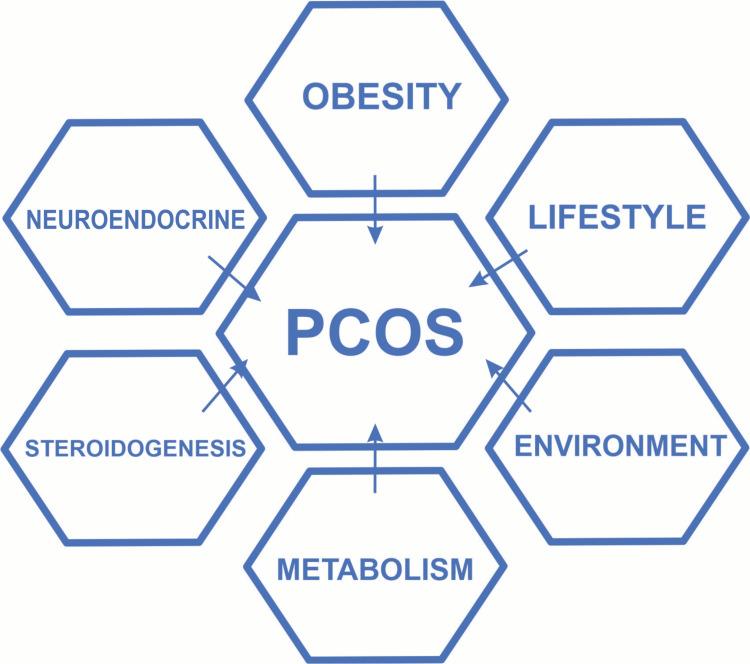
The role of metformin in acne and PCOS has been mentioned in 11 RCTs and nine cohort studies. All are shown to describe the remarkable improvement of PCOS-associated acne, except for three studies [11-12]. PCOS has a genetic predisposition and other multifactorial causes which include neuroendocrine, lifestyle, environmental, and obesity.
Hyperandrogenism, insulin resistance, compensatory hyperinsulinemia, and an imbalanced ratio of LH to FSH produce a metabolic disturbance and affect the ovaries and endometrium [11]. The exact etiology is an enigma.
The management of PCOS is a multidisciplinary approach that includes modification of general lifestyle by endocrinologists, gynecologists, dermatologists, and psychologist consults. Patients with PCOS are commonly seen first by a dermatologist [11]. The underlying hyperandrogenism that characterizes polycystic ovarian syndrome clinically manifests also as hirsutism and menstrual irregularities. Metformin also leads to clinical improvement in PCOS-associated acne. The pathophysiology of acne vulgaris is related to chronic inflammation of pilosebaceous follicles, increased sebum secretion, and colonization of Propionibacterium acnes [11]. This study aims to explore the role of metformin in PCOS-associated acne.
Review
Materials and methods
This study is done using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) to design and describe the findings of this systematic review. Search strategy: A primary literature search was conducted using PubMed, Google Scholar, Cochrane, and Science Direct. Articles from the last 10 years on females with PCOS-associated acne aged 19 to 44 years treated with Metformin were selected if they also met the criteria, for example, full-text articles in English and conducted from 2011 up to 23 February 2022. Using the MeSH search function, the keywords “polycystic ovarian syndrome”, “metformin”, “hyperandrogenism”, and “acne” were used.
The PubMed was explored with Medical Subjects Headings [MeSH] controlled vocabulary or search items as follows "Polycystic Ovary Syndrome/diet therapy"[MeSH] OR "Polycystic Ovary Syndrome/drug therapy"[MeSH] OR "Polycystic Ovary Syndrome/metabolism"[MeSH] OR "Polycystic Ovary Syndrome/physiopathology"[MeSH] OR "Polycystic Ovary Syndrome/statistics and numerical data" [MeSH] OR "Polycystic Ovary Syndrome/therapy" [MeSH] OR PCOS AND ANDROGEN ANTAGONISTS"[ALL FIELDS] OR "ANDROGEN ANTAGONISTS"[MESH TERMS] OR ANTIANDROGENS [TEXT WORD] OR METFORMIN [TEXT WORD] OR HYPERANDROGENISM AND ACNE OR ["acne vulgaris"] [MeSH Terms] OR acne vulgaris [Text Word] OR ["polycystic ovary syndrome"] [MeSH Terms] OR NODULOCYSTIC ACNE OR polycystic ovarian syndrome [Text Word] We applied the Boolean method to combine the keywords and MeSH terms and synthesize a uniform through searching various databases.
A total of 218 articles were included, which included 190 from PubMed, 25 from Google Scholar, two from the Cochrane database, and one from ScienceDirect. PubMed included 190 articles after MeSH searching - 166 records were irrelevant, and eight duplications were removed. Finally, 14 reports were evaluated for eligibility and quality.
The inclusion and exclusion criteria used in this review are presented in Table 1 below.
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
| English language | The presence of any dermatological disease besides acne. The Presence of any systematic disease. Pregnancy and lactation. Females of non-reproductive age group. Acne due to other causes like the use of steroid hormones, antidepressants, or Alcohol. |
| Articles free full text | Animal studies. Non-English language articles Study Selection 218 articles from the last 10 years were taken into consideration, including traditional reviews, systematic reviews, meta-analyses, bibliography, books and documents, case reports, classical articles, clinical studies, clinical studies, and clinical trials. |
| Female patients of PCOS in reproductive (age group: 19-44 years) | Two researchers independently screened all full free text articles titles and abstracts to be included and extracted the relevant studies, animal studies, and articles not written in the English language were excluded. The two researchers resolved disagreements over eligibility by studying the study design, intervention implemented, outcome measured, and the relevance to our inclusion and exclusion criteria. The final decision on study selection was reached by discussion. |
Study quality appraisal
We studied each article separately for the potential risk of bias. A comprehensive analysis of quality was conducted. The systematic reviews and meta-analyses were subjected to assessment using the multiple systematic reviews (AMSTAR) 2 tool [12]. The clinical trials and randomized control trials (RCT) were critically evaluated with the Cochrane Risk of Bias 2 (RoB2) tool. Each study was scrutinized based on seven criteria to find potential biases. The Newcastle Ottawa Scale (NOS) was used to assess the quality of cohort studies [13]. Each criterion was scored as either high quality, low quality, or unclear. Initially, 218 articles over the last 10 years were identified through search terms across four literature databases.
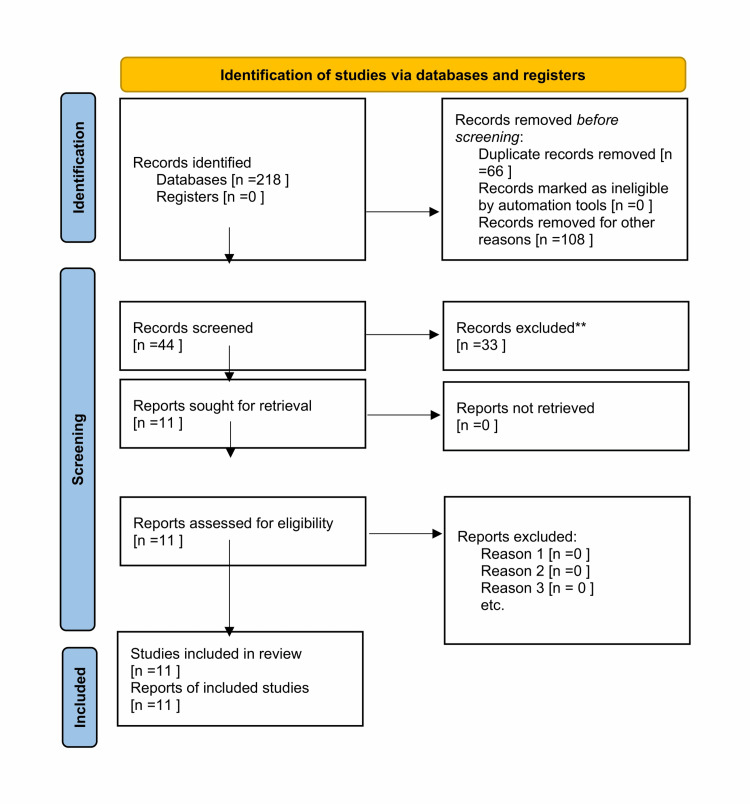
The final 11 articles were short-listed, as mentioned in the PRISMA chart [14].
Figure 1 shows the PRISMA chart.
Figure 1. PRISMA chart.
Discussion
Acne, hirsutism, androgenic alopecia, acanthosis nigricans, and seborrheic dermatitis are common cutaneous manifestations of PCOS. Insulin resistance plays a pivotal role in polycystic ovarian syndrome [15,16]. The biguanide metformin (N, N-dimethyl biguanide) is a hypoglycemic drug that improves insulin sensitivity, decreases insulin levels, and corrects ovarian and functional adrenal hyperandrogenism in PCOS. Metformin also leads to clinical improvement in PCOS-associated acne [17,18].
Dermatologists encounter acne vulgaris in patients of age groups ranging from 14 to 40 years of age [19-20]. Metformin decreases hepatic glucose output and increases glucose availability for muscles and adipose tissues by reducing insulin resistance. Other commonly used treatment modalities in acne vulgaris are oral tetracyclines, azithromycin, isotretinoin, and topical agents like benzoyl peroxide, adapalene, topical tretinoins, and miscellaneous other topical agents. In addition, hormonal imbalance plays a determining role in the clinical development of acne. Androgens increase sebum production by activating the activity of sebaceous glands. Colonization of Propionibacterium acne plays a pivotal role in the pathogenesis of acne formation, which may present as papules, pustules, nodules, cysts, and scarring [21].
Major sources of androgens
Major sources of androgens are ovaries and adrenal glands. Ovaries produce androstenedione and testosterone. Adrenal glands produce dehydroepiandrosterone sulfate [DHEA], androstenedione, and testosterone [19]. Clinical assessment of acne is usually graded by using Acne Global Severity Scale.
The US FDA Acne Global Severity Scale categorizes acne according to the following five categories:
"1) Clear, with no inflammatory signs. 2) Almost clear, with no more than one pustule or papule. 3) Mild, some non-inflammatory lesions with no more than a few papules or pustules but no evidence of any nodule. 4) Moderate, up to many non-inflammatory lesions, may have some inflammatory lesions but no more than one small nodule. 5) Severe, up to many non-inflammatory and inflammatory lesions, but no more than a few nodules."
Acne vulgaris is common in almost 80% of teenage females and may or may not be associated with PCOS. When acne vulgaris is associated with PCOS, the following changes in the serum may be seen.
Hormonal serum analysis
Raised DHEAS, raised total free testosterone, raised androstenedione, reduced sex hormone binding globulin SHBG, raised LH, raised FSH, raised serum prolactin, raised insulin, high insulin-like growth factor binding protein IGFBP-1 and insulin-like growth factor IGF-1.
Psychological effects of PCOS
Patients with PCOS likely suffer from a wide range of psychological problems like low self-esteem, mood swings, depression, anxiety, and even psychosis. In PCOS, acne and hirsutism are the most common markers of affecting the quality of life by worsening the emotional well-being of women [21].
Cutaneous manifestations of PCOS
The cutaneous features of PCOS are the first to develop clinically. Early diagnosis is possible by screening the suspected patients, and prompt treatment to reduce complications and improve quality of life. These cutaneous features, like acanthosis nigricans, are closely related to insulin resistance. The raised levels of insulin act through insulin-like growth factor receptors to cause acanthosis nigricans, a skin condition characterized by thick and dark velvety skin in the axilla, groins, neck, and frictional surfaces [20,21]. Hyperandrogenism has clinical cutaneous findings of acne, hirsutism, androgenic alopecia, and seborrhea.
Reproductive and neoplastic complications
Obesity, infertility, hirsutism, and acne negatively affect the quality of life of women with PCOS. This latter is characterized by amenorrhea or anovulation, which is associated with infertility. Among the other gynecological complications, miscarriage, endometrial hyperplasia, and endometrial carcinoma have been documented.
Raised LH levels are involved in the pathogenesis of PCOS as a result of increased pulse frequency of hypothalamic gonadotropic hormone (GnRH) or low levels of progesterone resulting from oligo-or anovulation [19,22]. These patients can develop subfertility or infertility. The altered FSH- to LH ratio stimulates the ovarian theca cells to produce androstenedione. High levels of insulin also play a key role in PCOS pathogenesis.
PCOS manifests as hyperinsulinemia and hyperandrogenism and raises the level of the luteinizing hormone LH [19,22]. This alters the normal pituitary ovarian axis, which leads to the clinical presentation of oligomenorrhea, infertility, obesity, hirsutism, acne, hypertension, and hyperlipidemia [22]. The use of metformin leads to significant improvement of hyperandrogenism and its clinical manifestations.
Figure 2 explains the etiology of PCOS [23].
Figure 2. Etiology of PCOS.
Figure modified by Shamim H.
Table 2 explains different diagnostic criteria of PCOS [23].
Table 2. Diagnostic criteria of PCOS.
ESHRE/ASRM. European Society for Human Reproduction and Embryology/American Society for Reproductive Medicines; NIH/NICH National institutes of Health/National institute of Child Health and Human Disease
| NIH/NICHD 1992 | ESHRE/ASRM (Rotterdam Criteria) 2004 | Androgen Excess Society 2006 |
| Exclusion of other causes of androgen excess or related disorders includes all of the following: | Exclusion of other androgen excess or related disorder includes two of the following: | Exclusion of other androgen excess and related disorders include all of the following: |
| 1) Clinical or biochemical Hyperandrogenism | 1) Clinical or biochemical Hyperandrogenism | 1) Clinical or biochemical Hyperandrogenism |
| 2) Menstrual Dysfunction | 2) Oligo-ovulation or anovulation | 2) Ovarian dysfunction or polycystic ovaries |
Cardiovascular complications of PCOS
Insulin is produced by the pancreas. It helps to maintain the blood sugar level within the normal range. Hyperinsulinemia is usually linked to type 2 diabetes mellitus. However, symptoms of hyperinsulinemia include sugar cravings, weight gain, anxiety, sweating, frequent hunger, and fatigue leading to hypoglycemia. Metformin helps in ameliorating the symptoms of hyperinsulinemia in patients with PCOS. Metformin is reported to decrease hepatic glucose output, thereby decreasing the insulin requirement [24]. Women with PCOS are prone to hypertension, coronary vascular disease, and ischemic vascular disease [24]. Along with other common comorbidities, young women with PCOS are at risk of early-onset acute coronary vascular disease and endothelial dysfunction [25].
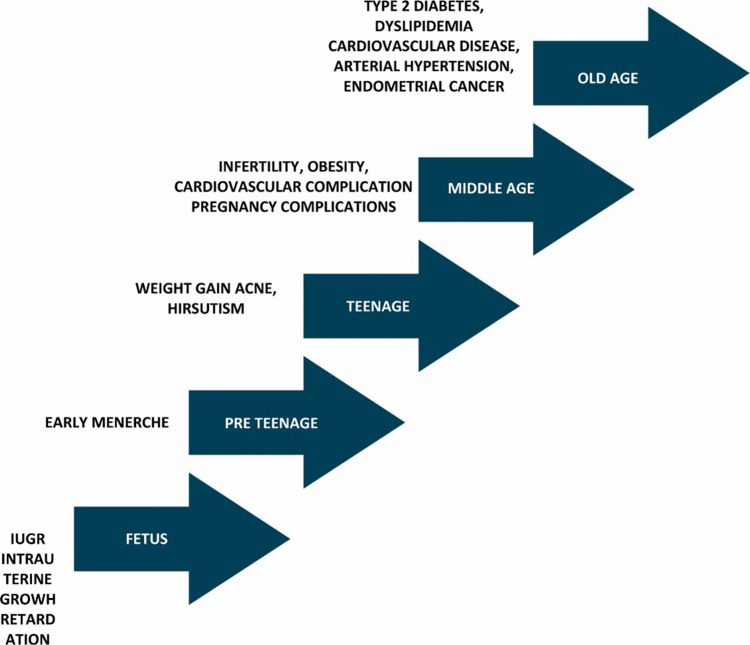
Figure 3 demonstrates the effects of PCOS at different stages of life [26].
Figure 3. The effects of PCOS at different stages of life.
Figure modified by Shamim H.
Mechanism of action of metformin
The exact mechanism is unclear. Metformin is proposed to act by inhibiting mitochondrial function [27]. Creating an adenosine triphosphate-deficient environment causes an activation of adenosine monophosphate-activated protein kinase (AMPK) and upregulation of catabolic metabolism. Metformin diminishes the proinflammatory response by inhibiting nuclear factor kappa B (NF-kB) through both liver-related AMPK dependent and independent pathways, thus suppressing the production of nitric oxide (NO), prostaglandin E2 (PGE2), and acute inflammatory markers like tumor necrosis factor-alpha, interleukin-1, interleukin-6 [28].
Table 3 explains the study synthesis.
Table 3. Study synthesis table.
DHEA: Dehydroandrosterone; PCOS: polycystic ovarian syndrome
| Sr. | Author | Year of publication | Journal published | Type of study | Number of female patients with PCOS/number of articles | Purpose of study | Results | Conclusion |
| 1 | Timothy h. Schmidt et al. [17] | 2016 | JAMA Dermatology | Retrospective cross-sectional study 401 women with PCOS included | 401 patients | To identify cutaneous findings in PCOS. | Hirsutism, acanthosis nigricans, and acne were associated with cutaneous findings in PCOS. | PCOS association with acne, hirsutism, and multiple other skin conditions. |
| 2 | John K Lee ms et al. [18] | 2017 | Dermatology Online Journal | A systematic review | Three clinical trials with 144 patients included. | To assess the role of metformin as an adjuvant for the treatment of acne vulgaris. | Reduction in acne after 12 weeks of metformin use. | Metformin may be an effective and safe adjunct treatment in the treatment of moderate to severe acne vulgaris. |
| 3 | G. Franik et al. [19] | 2018 | European Review for Medical and Pharmacological Sciences | Hospital-based clinical trial | 110 patients with PCOS | To investigate the impact of hormonal and metabolic disorders of PCOS-associated acne vulgaris. | The severity of acne in PCOS is related to serum testosterone, DHEA dihydroando sterone | Acne global severity scale in PCOS women is associated with high total testosterone and dihydrotestosterone sulfate DHEA |
| 4 | M Artani et al. [20] | 2018 | Cureus Journal of Medical Science | Cross-sectional study 50 women PCOS included. | To determine the effects of metformin in polycystic ovarian syndrome. | Metformin improved acne, and menstrual irregularities, and hirsutism | Metformin has a significant role in PCOS women. | |
| 5 | S Mukkamala et al. [21] | 2018 | Journal of Pakistan Association of the Dermatologist | A cross-sectional study | 50 patients | To document various cutaneous manifestations in PCOS. | Acanthosis nigricans and acne are the most common cutaneous manifestation of PCOS. | Acanthosis nigricans to be the most common cutaneous finding in PCOS. |
| 6 | Shipli Sharma et al. [22] | 2019 | J Clin Aesthet Dermatol | Original research hospital-based interventional longitudinal study. | 40 patients | To evaluate the efficacy of metformin therapy in women with PCOS-associated acne in terms of acne load. | Using the disease severity index DSI acne severity was reduced with metformin. | Suggested efficacy in PCOS. |
| 7 | Calvin t. Sung et al. [23] | 2020 | Journal of Drugs in Dermatology | A systematic review | 26 articles were shortlisted | To assess evidence related to the use of metformin for treating primary cutaneous disorders. | Metformin is safe and efficacious in treating various cutaneous disorders, including acne and PCOS. | Metformin is safe and efficacious in PCOS and multiple other dermatological conditions. |
| 8 | Donna Vine et al. [24] | 2021 | Journal of the Endocrine Society | Randomized control trial | 38 patients | To determine the effect of 12 weeks of high dose fish oil and metformin with metabolic syndrome and PCOS. | Fish oil and metformin significantly lowered fasting plasma triglycerides. | High fish oil and fish oil, metformin therapy tends to lower fasting and postprandial triglyceride levels. |
| 9 | J Bulsara et al. [25] | 2021 | Elsevier Journal endocrine and Metabolic Science. | Literature review article | More studies required for PCOS and lifestyle modification can improve quality of life in PCOS. | |||
| 10 | A Bahadur et al. [26] | 2021 | Cureus Journal of Medical Science | Randomized controlled trial | 72 patients | To compare the efficacy of metformin alone and metformin combined with myoinositol plus d chiro-inositol in women with polycystic ovary syndrome. | 72 patients were randomly allocated into two groups combined treatment has better results than metformin alone in these patients. | Combined therapy may have better results. |
| 11 | Jane l et al. [27] | 2021 | Diabetes ther 2021 | A systematic review and meta-analysis | Equal efficacy of longer-acting versus metformin formulation but long-acting formulations being superior |
Tolerability of metformin and potential adverse effects
Gastrointestinal disturbance is the most common adverse effect that limits patient compliance. Lactic acidosis is rarely seen. Metformin is frequently prescribed by endocrinologists. Metformin helps patients with PCOS in weight reduction. It also helps with metabolic comorbidities like dyslipidemia. Metformin acts centrally via suppressing hypothalamic neurons potentially and growth differentiation factor GDF-15 by reducing neuropeptide Y or by reducing appetite stimulation. There is also the involvement of gut-based mechanisms that increase the secretion of glucagon-like peptide GLP-1. Metformin improves lipid profile by inhibiting the activity of lipid synthesis [29].
The dose of metformin ranges from 750 to 2000mg. Metformin also helps the induction of ovulation in patients with PCOS. The side effects of metformin include nausea, diarrhea, flatulence, abdominal pain, and bloating. The small intestine has a concentration of metformin 30 to 300 times notably higher than plasma concentration [30]. The intestinal collection can be the reason for gastrointestinal side effects.
Limitations
This review was limited by the types of papers found in the PubMed, Google Scholar, Cochrane, and Science Direct databases. Only papers in English from 2011 to 2021, and papers from Grey literature were excluded. The small number of articles directly studying the drug metformin in association with PCOS and small sample sizes.
Conclusions
Metformin can have promising results in patients with PCOS. It may also potentially have positive effects on the quality of life. Further clinical trials are necessary. This summary of metformin in acne patients with PCOS recommends adequate efficacy, a high index of safety, and good tolerance. Thus, metformin can have a beneficial role in PCOS-associated acne.
Acknowledgments
Dr. Hassaan Tohid, Dr.Syeda Sidra Hasnain, Dr. Lubna Mohammed, and my family for their motivation.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Amenorrhea associated with bilateral polycystic ovaries. Stein IF. https://www.sciencedirect.com/science/article/abs/pii/S0002937815306426 Am J Obstet Gynecol. 1935:181–191. [Google Scholar]
- 2.Preliminary indication of a high prevalence of polycystic ovary syndrome in indigenous Australian women. Davis SR, Knight S, White V, Claridge C, Davis BJ, Bell R. https://www.tandfonline.com/doi/abs/10.1080/gye.16.6.443.446. Gynecol Endocrinol. 2002;16:443–446. [PubMed] [Google Scholar]
- 3.The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G. Oncotarget. 2017;8:96351–96358. doi: 10.18632/oncotarget.19180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: is there a difference? Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clin Endocrinol (Oxf) 2002;57:343–350. doi: 10.1046/j.1365-2265.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 5.Polycystic ovaries and associated metabolic abnormalities in Indian subcontinent Asian women. Rodin DA, Bano G, Bland JM, Taylor K, Nussey SS. Clin Endocrinol (Oxf) 1998;49:91–99. doi: 10.1046/j.1365-2265.1998.00492.x. [DOI] [PubMed] [Google Scholar]
- 6.Medication and medical service utilization for acne 1995-1998. Stern RS. J Am Acad Dermatol. 2000;43:1042–1048. doi: 10.1067/mjd.2000.110901. [DOI] [PubMed] [Google Scholar]
- 7.Poly cystic ovarian syndrome: an updated overview. El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Front Physiol. 2016;7:124. doi: 10.3389/fphys.2016.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 9.Effects of metformin therapy on hyperandrogenism in women with polycystic ovarian syndrome. Kazerooni T, Dehghan-Kooshkghazi M. Gynecol Endocrinol. 2003;17:51–56. [PubMed] [Google Scholar]
- 10.Ovulation induction in perspective. Homburg R, Insler V. Hum Reprod Update. 2002;8:449–462. doi: 10.1093/humupd/8.5.449. [DOI] [PubMed] [Google Scholar]
- 11.Full investigation of patients with polycystic ovary syndrome (PCOS) presenting to four different clinical specialties reveals significant differences and undiagnosed morbidity. Sivayoganathan D, Maruthini D, Glanville JM, Balen AH. Hum Fertil (Camb) 2011;14:261–265. doi: 10.3109/14647273.2011.632058. [DOI] [PubMed] [Google Scholar]
- 12.AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Shea BJ, Reeves BC, Wells G, et al. BMJ. 2017;358:0. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. 2013. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonradomised Studies in Meta-analyses. [Google Scholar]
- 14.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. BMJ. 2021;372:0. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polycystic ovary syndrome in adolescents: Challenges in diagnosis and treatment. Ramezani Tehrani F, Amiri M. Int J Endocrinol Metab. 2019;17:0. doi: 10.5812/ijem.91554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effects of metformin on symptoms of polycystic ovarian syndrome among women of reproductive age. Artani M, Iftikhar MF, Khan S. Cureus. 2018;10:0. doi: 10.7759/cureus.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. Schmidt TH, Khanijow K, Cedars MI, et al. JAMA Dermatol. 2016;152:391–398. doi: 10.1001/jamadermatol.2015.4498. [DOI] [PubMed] [Google Scholar]
- 18.Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris. Lee JK, Smith AD. Dermatology Online Journal. 2017 [PubMed] [Google Scholar]
- 19.Androgen excess: Investigations and management. Lizneva D, Gavrilova-Jordan L, Walker W, Azziz R. Best Pract Res Clin Obstet Gynaecol. 2016;37:98–118. doi: 10.1016/j.bpobgyn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Cutaneous manifestations of polycystic ovary syndrome: A cross-sectional clinical study. Keen MA, Shah IH, Sheikh G. Indian Dermatol Online J. 2017;8:104–110. doi: 10.4103/2229-5178.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutaneous manifestation of polycystic ovary syndrome. Abusailik MA, Muhanna AM, Almuhisen AA, Alhasanat AM, Alshamaseen AM, Bani Mustafa SM, Nawaiseh MB. Dermatol Reports. 2021;13:8799. doi: 10.4081/dr.2021.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polycystic ovary syndrome and risk of type 2 diabetes, coronary heart disease, and stroke. Zhu T, Cui J, Goodarzi MO. Diabetes. 2021;70:627–637. doi: 10.2337/db20-0800. [DOI] [PubMed] [Google Scholar]
- 23.Bulsara J. Patel P. Soni A, Acharya S. 2021. A review: Brief insight into polycystic ovarian syndrome. Endocrine and Metabolic Science. [Google Scholar]
- 24.Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. Cussons AJ, Watts GF, Mori TA, Stuckey BG. J Clin Endocrinol Metab. 2009;94:3842–3848. doi: 10.1210/jc.2009-0870. [DOI] [PubMed] [Google Scholar]
- 25.A pilot trial: Fish oil and metformin effects on ApoB-remnants and triglycerides in women with polycystic ovary syndrome. Vine D, Proctor E, Weaver O, Ghosh M, Maximova K, Proctor S. J Endocr Soc. 2021;5:0. doi: 10.1210/jendso/bvab114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polycystic ovary syndrome throughout a woman's life. Bellver J, Rodríguez-Tabernero L, Robles A, et al. J Assist Reprod Genet. 2018;35:25–39. doi: 10.1007/s10815-017-1047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comparison of clinical, metabolic and hormonal effects of metformin versus combined therapy of metformin with Myoinositol Plus D-Chiro-Inositol in women with polycystic ovary syndrome (PCOS): A randomized controlled trial. Bahadur A, Arora H, Ravi AK, et al. Cureus. 2021;13:0. doi: 10.7759/cureus.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metformin use in women with polycystic ovary syndrome. Johnson NP. Ann Transl Med. 2014;2:56. doi: 10.3978/j.issn.2305-5839.2014.04.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Osibogun O, Ogunmoroti O, Michos ED. Trends Cardiovasc Med. 2020;30:399–404. doi: 10.1016/j.tcm.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Metformin--mode of action and clinical implications for diabetes and cancer. Pernicova I, Korbonits M. Nat Rev Endocrinol. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]