Abstract
Background
In both the general population and people with multiple sclerosis (PwMS), physical exercise is associated with improved mental well-being. Moreover, there is evidence of the possible protection of physical activity against disease progression in multiple sclerosis (MS). However, the question arises if acute or regular exercise has any impact on the immune system in PwMS. To answer this question, we performed a systematic review and meta-analysis on both plasma and serum cytokine levels (IL-6 and TNF-α) before and after acute and regular exercise among PwMS and compared to healthy controls.
Method
We performed an online search via PubMed, EMBASE, SCOPUS, Web of Science, and Cochrane Library till September 2021 to identify original studies on IL-6 and TNF-α changes after acute and regular exercise in PwMS and controls. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), 11 original studies were included in the meta-analysis. Sensitivity analyses were used to identify the origins of heterogeneity. R 4.0.4 was used to perform the meta-analysis of IL-6 and TNF-α levels before and after acute and regular exercise in PwMS, compared to controls. This study does not qualify for a clinical trial number.
Results
IL-6 levels did neither increase nor decrease after acute and regular exercise in PwMS, and compared to controls (pre- vs. post-intervention: Standardized Mean Difference (SMD) -0.09, 95% CI [−0.29; 0.11], p-value = 0.37, PwMS vs. Control: SMD −0.08, 95% CI [−0.33; 0.16], p-value = 0.47). In PwMS, TNF-α levels decreased after regular exercise and when TNF-α levels of both acute and regular exercise were pooled (pre- vs. post-intervention: SMD −0.51, 95% CI [-0.91; 0.11], p-value = 0.01, PwMS vs. Control: SMD −0.23, 95% CI [−0.66; 0.18], p-value = 0.26). TNF-α levels did neither increase nor decrease after acute and regular exercise in PwMS, when compared to controls.
Conclusion
This systematic review and meta-analysis show that exercise does not lead to significant changes in peripheral levels of IL-6 in PwMS in contrast to the observed response in healthy subjects and other medical contexts. However, regular exercise had a specific anti-inflammatory effect on blood TNF-α levels in PwMS. It remains to be investigated why PwMS display this different exercise-induced pattern of cytokines.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-022-00814-9.
Keywords: Multiple sclerosis, Cytokine, Interleukin-6, TNF-α, Exercise
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease estimated to affect 900,000 people in the United States, and 5–300 per 100,000 people worldwide [1]. It is characterized by varying patterns of neuro-inflammation, demyelination, and axonal loss [2]. As a result of neuro-inflammation and neurodegeneration in the central nervous system (CNS), people with MS suffer from a wide range of sensory and motor symptoms that influence their quality of life [3]. People with MS (PwMS) can exhibit lower levels of muscle strength, speed, endurance, and cardiorespiratory fitness compared to healthy individuals [4]. Moreover, PwMS were reported to avoid physical activities believing that elevated body temperature worsens their symptoms [5]. However, recent studies have shown that physical exercises can positively affect the quality of life [6, 7], potentially by playing disease-modifying roles in PwMS, lessening depression [8, 9], fatigue [10, 11], paresthesia [12], and improving sexual dysfunction, emotion regulation, and subjective and objective sleep dimensions [13, 14].
The underlying biological mechanisms of MS are complex and not fully understood [15]. Dysregulation of the CD4+ T cells (T-helpers) remains the main immunological background of this disease [16]. T-helper 1 and T-helper 17 cells are aberrantly found in the CNS lesions, cerebrospinal fluid (CSF), and blood of people with MS [17]. These cells are associated with elevated inflammatory cytokines, such as interleukin-6 (IL-6), interferon-gamma (INF-γ), tumor necrosis factor-alpha (TNF-α), IL-17, and IL-22 in MS, both leading to the blood–brain barrier (BBB) breakdown and astrocyte and microglia activation [18–20]. In contrast, T-helper 2 cells, suppressors of microglial activation, are declined in MS; and reduced T-helper 1/T-helper 2 ratios in the CSF of PwMS enhance the neuro-inflammation in this disease [20, 21].
In multiple sclerosis, the rise in pro-inflammatory cytokines in blood and CSF accelerates the demyelination and axonal damage in the CNS [22, 23]. Among the main pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α [24], the role of IL-1 β is reported to be limited compared to the other two. A study showed that the role of IL-1 signaling in immune cells is redundant for the pathogenesis of experimental autoimmune encephalomyelitis (EAE), a murine model of MS [25]. On the contrary, the blockade of IL-6 and TNF-α in EAE suppresses disease development [26, 27]. Furthermore, high levels of IL-6 in the cerebrospinal fluid correlate with reduced synaptic plasticity with clinical expression of brain damage [28]. Physical exercise is reported to suppress CNS IL-6 production and thus inhibit microglial activation [28, 29]. On the other hand, muscle-derived IL-6 during physical activities stimulates anti-inflammatory cytokines (e.g., IL-10) and, therefore, inhibits the effects of tumor necrosis factor-alpha, the other pro-inflammatory cytokine [30]. TNF-α levels are also elevated in MS patients and are associated with MS severity [31].
The origin of these cytokines is variable in the body, and distinct sources determine different functions in cytokines; for example, it is suggested that the elevated IL-6 levels are mainly due to its production in skeletal muscle, brain, and peri‑tendinous tissues [32]. Contrary to immunologic cell-derived IL-6, muscle-derived IL-6 exhibits anti-inflammatory features, as it suppresses T-helper 1 cells and its interferon-gamma production and induces the production of IL-10 and Il-4 [33]. Furthermore, the elevation of muscle TNF-α is reported to have regenerative effects on muscles through activating satellite cells [34].
Exercise appears to influence the central nervous system (CNS) in a variety of ways. These include boosting cerebral blood flow, modulating endocannabinoids and neurotransmitters, influencing neuroendocrine responses, and CNS structural changes. [35–37] For instance, Prakash et al. [38] showed that aerobic exercise in PwMS decreased gray matter volume while maintaining the integrity of white matter. Several brain regions, including the hippocampus, thalamus, caudate, putamen, and pallidum, have been positively linked to the amount of moderate/vigorous physical exercise [39]. PwMS who completed a six-month, two-day-a-week weight exercise program had no significant reduction in brain atrophy, according to Kjølhede et al. [40]. Reflecting these structural findings, physical therapy and exercise seem to improve cognitive functioning, including memory, learning, and information processing [41–45].
To date, there is no eligible biomarker to assess the effects of exercise on people with MS. Herein, in this systematic review and meta-analysis, we aimed to evaluate the acute and long-term impacts of physical activities on serum IL-6 and TNF-α, the well-known pro-inflammatory cytokines, in people with MS. We will also discuss whether the cytokine changes are related to disease progression and clinical outcomes in these people.
Methods and materials
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines [46] were followed for this meta-analysis.
Information source and search strategy
We performed an online search via PubMed, EMBASE, SCOPUS, Web of Science, and Cochrane Library until September 2021, aiming to identify original studies investigating IL-6 and TNF-α changes after exercise in MS patients and controls. There were no language or date restrictions. Results from PubMed and Embase were retrieved using Medical Subject Headings (MeSH) and Emtree, respectively. Additionally, we searched the reference lists of relevant papers for other publications that met the criteria. Our search keywords are described in the Additional file 1.
Selection criteria
Studies were included if (1) they were peer-reviewed clinical trial studies, (2) IL-6 or TNF-α blood levels were measured quantitatively using enzyme-linked immunoassays (ELISA) or other assays, (3) IL-6 or TNF-α measured before and after an exercise intervention, and (4) the absolute values of the IL-6 or TNF-α markers were either given within the manuscript or provided by the authors of the original study for performing the meta-analysis. Exclusion criteria were (1) pediatric MS and (2) case reports, case series, letters, commentaries, abstracts, protocols, review articles, and animal and in vitro studies. Two authors (P.S and H.S) independently completed the screening and eligibility assessment. In case of disagreement, the two authors discussed and settled the conflict.
Data extraction
Two reviewers independently extracted (1) bibliographic information (study title, year of publication, first author, study type, and country), (2) demographic and clinical features of the sample (number of patients and controls, age, sex, disease duration, mean expanded disability status scale [EDSS] score), (3) methodological details (diagnostic criteria, characteristics of the ELISA or other assay), and (4) levels of the IL-6 or TNF-α before and after the intervention in either MS or control group. We contacted the studies' corresponding authors for more information if the absolute values of the levels of IL-6 or TNF-α were not included in the published article. The inter-rater reliability between reviewers was calculated using the kappa coefficient [47].
Study quality assessment
The methodological quality of the included studies was rated by two reviewers (P.S and H.S) separately, based on the PEDro scale [48]. PEDro is a trustworthy and valid checklist consisting of 11 items as follows: (1) eligibility criteria, (2) random allocation, (3) concealed allocation, (4) baseline comparability, (5) masked participants, (6) masked therapists, (7) masked assessors, (8) adequate follow-up, (9) intention to treat analysis, (10) between-group comparison, and (11) point estimates and variability. As the eligibility criterion item does not count to the overall score, each study gets a score from 0 to 10. We categorized studies based on their PEDro score; below 4 as "poor" quality, a score between 4 and 5 indicating "fair" quality, a score of 6 to 8 regarded to be of "good" quality, and a score of 9 to 10 indicating "excellent" quality. Any differences were addressed by discussion between the reviewers.
Statistical analysis
We calculated a standardized mean difference (SMD) (Hedges' g), and 95% confidence interval (CI) for each between-group comparison as the included studies were done in a 17-year span and probably used assays with different sensitivity. The SMD values ≤ 0.2, 0.2–0.8, and ≥ 0.8 denoted small, moderate, and large effect sizes, respectively. Meta-analyses were done for comparisons for which findings from at least three separate datasets were available.
If the values reported in the manuscript were given as a median and interquartile range (IQR) or median and range, and we were not able to retrieve the mean ± standard deviation (SD) from the authors, we used statistical methods suggested by Luo et al. [49] and Wan et al. [50] to convert these values.
To assess heterogeneity between studies in the between-group meta-analyses, we used Cochrane's Q-test and the I2-index. The I2-indices of ≤ 25%, 26–75%, and 75% ≤ represented low, moderate, and high heterogeneity degrees, respectively. In terms of the heterogeneity tests, the p-value < 0.1 was considered significant. We utilized random effect models according to the DerSimonian and Laird method. Random-effects models are preferred if significant heterogeneity is expected, as they account for variable underlying effects in estimates of uncertainty, including both within- and between-study variance. We visualized the results of the meta-analysis as forest plots.
To further assess the causes of heterogeneity, we conducted a sensitivity analysis to identify influential cases for meta-analyses with significant heterogeneity, including ten or more studies. Each time we omitted one study and recalculated the effect size (leave-one-out Analyses). To reduce the heterogeneity among individual studies, we conducted a subgroup analysis based on the type of intervention used in each study.
Publication bias was initially assessed by visual observation of the degree of funnel plot asymmetry. Then, we used Egger's bias test to objectively confirm the visual perception from the funnel plot. A p-value < 0.1 was considered as evidence of publication bias. Funnel plots and Egger's plots are available. When there was evidence of publication bias, we adjusted the effect sizes using the trim-and-fill method.
All computations and visualizations were carried out using R version 4.0.4 (R Core Team [2020]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria) and STATA 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC) for metaregression and Egger's plots. We used following packages: “meta” (version 4.17–0), “metafor” (version 2.4–0), “dmetar” (version 0.0–9), and “tidyverse” (version 1.3.0). All forest plots and the drapery plot were designed using R. A p-value of < 0.05 was considered statistically significant.
Results
Selection of studies
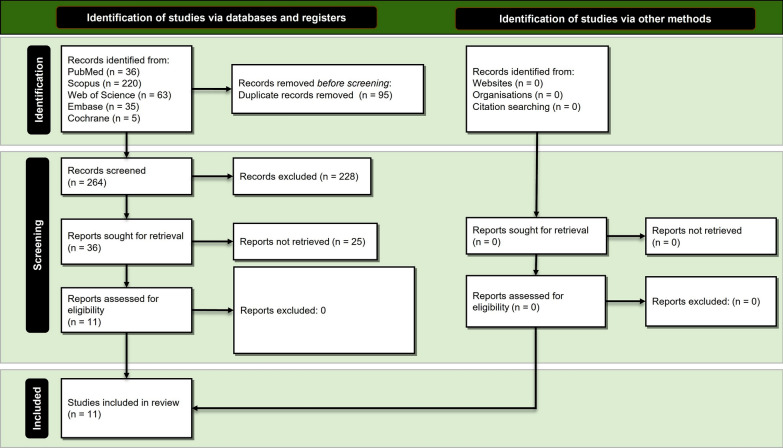
The search strategy retrieved a total yield of 359 studies. After the removal of duplicates, 264 studies remained. In the next step, titles and abstracts were screened regarding their relevancy to the systematic review topic. After the exclusion of 228 irrelevant studies, we identified 36 potentially eligible studies. We checked the full text of these studies, and finally, 11 original clinical trials met the criteria to be included in the meta-analysis [51–61]. No further studies that were appropriate for inclusion were identified via hand searching and checking references. Figure 1 illustrates the process of study selection according to the PRISMA guideline. The agreement between the two independent reviewers for study selection was great for both titles/abstracts (kappa = 1.00; percentage agreement = 99.92%) and full text (kappa = 1.00; percentage agreement = 100%).
Fig. 1.
Study selection process according to the Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guideline
Study characteristics
Table 1 shows the characteristics of the studies included in the meta-analysis. Levels of IL-6 were measured in nine studies [51–59], of which five studies had control groups [51, 54, 55, 58, 59]. Regarding TNF-α levels, six studies assessed its levels [52, 53, 55, 56, 60, 61], of which three investigated controls as well [55, 60, 61].
Table 1.
Characteristics of the included studies
| Studies | Patients | Controls | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Exercise Protocol | Type of Exercise | Source | Assessed Cytokines | N | Mean Age (years) | Male, % | Mean Disease Duration | Mean EDSS score | Type of Controls | N | Mean age (years) | Male, % |
| Berkowitz et al., 2019 | Israel | A session of training for 2 h | Aerobic exercises | Serum | IL-4, IL-6, IL-10, IL-17A, IFN-ɣ, TNF-α | 14 | 33.80 | 0 | - | - | Healthy people who exercised | 9 | 28.3 | 0 |
| Devasahayam et al., 2020 | Canada | Training for ten weeks (3x/week) | Aerobic exercises | Serum | IL-6, BDNF | 7 | - | - | - | - | - | - | - | - |
| Devasahayam et al., 2021 | Canada | A session of graded exercise test (GXT) | Aerobic exercises | Serum | IL-6, BDNF | 14 | 54.07 | 28% | 16.57 | - | Healthy people who exercised | 8 | 50.71 | 37% |
| Donia et al., 2019 | Canada | A session of training for 1 h | Aerobic exercises | Serum | IL-6, TNF-α, IFN-ɣ, IL-1RA | 13 | 57.20 | 23% | - | - | - | - | - | - |
| Faramarzi et al., 2020 | Iran | Training for 12 weeks (3x/week) | Combined aerobic and resistance exercises | Plasma | IL-6, IFN-ɣ | 46 | - | 0% | - | - | MS patients who didn't exercise | 43 | - | 0% |
| Kierkegaard et al., 2016 | Sweden | Training for 12 weeks (2x/week) | Resistance exercises | Serum | IL-1RA, IL-4, IL-5, IL-6, IL-7, IL-8, IL-12p70, IL-13, IL-17 | 17 | - | - | - | - | - | - | - | - |
| Kordi et al., 2014 | Iran | Training for 8 weeks (4x/week) | Combined aerobic and resistance exercises | Serum | IL-10, TNF-α | 27 | 33.68 | - | - | 1.78 | MS patients who didn't exercise | 8 | 33.63 | - |
| Mokhtarzade et al., 2021 | Iran | Training for 6 months (5x/week) | Combined aerobic and resistance exercises | Serum | IL-10, TNF-α | 21 | 35.06 | 28% | 4.35 | 2.14 | MS patients who didn't exercise | 21 | 36.38 | 23% |
| Raisi et al., 2018 | Iran | Training for 12 weeks (3x/week) | Combined aerobic and resistance exercises | Serum | IL-6 | 48 | - | 0% | - | - | MS patients who did only stretching trainings | 48 | - | 0% |
| Schulz et al., 2004 | Germany | A session of training for 30 min | Aerobic exercises | Plasma | IL-6, sIL-6R, BDNF, NGF | 15 | 39 | 26% | 11.40 | 2.30 | MS patients who didn't exercise | 13 | 40 | 38% |
| White et al., 2006 | United States | Training for 8 weeks (2x/week) | Resistance exercises | Serum | IL-2, IL-4, IL-6, IL-10, IFN-ɣ, TNF-α | 10 | 47 | 0% | - | 3.80 | - | - | - | - |
Quality assessment
The median total PEDro score was 5 (IQR = 1; mean ± SD = 5.5 ± 0.8; range: 4 to 7) out of 10, indicating that the included studies were of good quality overall (Table 2). All studies passed the following criteria: (1) Between-group statistical comparison and (2) point estimates and variability. Moreover, neither the participants nor the raters were blinded in any of the included investigations.
Table 2.
Quality assessment of the included studies, based on the PEDro scale
| Berkowitz et al., 2019 | Devasahayam et al., 2020 | Devasahayam et al., 2021 | Donia et al., 2019 | Faramarzi et al., 2020 | Kierkegaard et al., 2016 | Kordi et al., 2014 | Mokhtarzade et al., 2021 | Raisi et al., 2018 | Schulz et al., 2004 | White et al., 2006 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eligibility criteria | * | * | * | * | * | * | * | * | |||
| Random allocation | * | * | * | * | * | ||||||
| Concealed allocation | * | * | * | * | * | ||||||
| Baseline comparability | * | * | * | * | * | * | * | * | * | ||
| Masked participants | |||||||||||
| Masked therapists | |||||||||||
| Masked assessors | |||||||||||
| Adequate follow-up | * | * | * | * | * | * | * | * | |||
| Intention to treat analysis | * | * | * | ||||||||
| Between-group statistical comparison | * | * | * | * | * | * | * | * | * | * | * |
| Point estimates and variability | * | * | * | * | * | * | * | * | * | * | * |
| Total score | 5 | 4 | 5 | 6 | 6 | 5 | 5 | 7 | 6 | 6 | 5 |
Meta-analysis
All statistical indices for within- and between-group meta-analysis are reported in Table 3. Overall, for pre–post-comparisons within individuals with MS, IL-6 did neither change after acute nor regular exercise. For TNF-α, overall concentrations decreased from pre- to post-intervention, though no change in TNF-α concentrations was observed for acute exercise when considered separately. Interestingly, regular exercise resulted in significant differences from pre- to post-intervention. Compared to controls, in individuals with MS, neither IL-6 nor TNF-α concentrations decreased or increased after acute and regular exercise.
Table 3.
Results of within- and between-group meta-analyses.
| Comparison | Subgroup | No. studies | No. Cases | No. Controls | Meta-analysis | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect size | 95% Confidence interval (%) | p | Eggers | I2 (%) | Q | p | ||||||
| Pre–Post | ||||||||||||
| IL-6 | Overall | 9 | 184 | NA | −0.0925 | −0.2976; 0.1125 | 0.3765 | 0.45 | 0.0% | 3.69 | 0.8843 | |
| Acute Exercise | 4 | 56 | −0.0724 | −0.4459; 0.3010 | 0.7037 | 0.8997 | 0.0% | 2.98 | NA | |||
| Regular Exercise | 5 | 128 | −0.1012 | −0.3465; 0.1442 | 0.4189 | 0.0% | 0.69 | |||||
| TNF-α | Overall | 6 | 102 | NA | −0.5162 | −0.9195; -0.1129 | 0.0121 | 0.27 | 48.6% | 9.73 | 0.0833 | |
| Acute Exercise | 2 | 27 | −0.2190 | −0.7551; 0.3171 | 0.4233 | 0.2591 | 0.0% | 0.28 | NA | |||
| Regular Exercise | 4 | 75 | −0.6705 | −1.2429; -0.0982 | 0.0217 | 63.7% | 8.26 | |||||
| MS-Control | ||||||||||||
| IL-6 | Overall | 5 | 137 | 121 | −0.0888 | −0.3351; 0.1575 | 0.4798 | 0.18 | 0.0% | 2.66 | 0.6162 | |
| Acute Exercise | 3 | 43 | 30 | 0.1023 | −0.3673; 0.5719 | 0.6694 | 0.3579 | 0.0% | 0.61 | NA | ||
| Regular Exercise | 2 | 94 | 91 | −0.1623 | −0.4750; 0.1503 | 0.3088 | 14.4% | 1.17 | ||||
| TNF-α | Overall | 3 | 62 | 38 | −0.2364 | −0.6551; 0.1824 | 0.2686 | 0.23 | 0.0% | 0.60 | 0.7410 | |
| Acute Exercise | 1 | 14 | 9 | 0.0108 | −0.8266; 0.8482 | NA | 0.5041 | NA | NA | NA | ||
| Regular Exercise | 2 | 48 | 29 | −0.3188 | −0.8023; 0.1648 | 0.1963 | 0.0% | 0.15 | ||||
Within-group analysis: individuals with multiple sclerosis: pre- vs. post-comparisons for acute and regular exercise. Between-group analysis: individuals with vs. without multiple sclerosis; comparisons for acute and regular exercising
MS = multiple sclerosis; IL-6 = interleukin-6; TNF-α = Tumor Necrosis Factor-alpha. NA = Not Applicable
Significant p-values are in Bold
IL-6
Comparison of pre- and post-intervention IL-6 concentration
In nine studies, levels of IL-6 were assessed (N = 184) before and after the exercise program. The combined mean ± SD of the age of participants was 46.59 ± 12.91 years, as reported by six studies (n = 73) [51, 52, 55–57, 59]. The cumulative number of female and male participants was 172 and 12, respectively, reported by eight studies [51, 52, 54–59]. Three studies reported the EDSS score of patients [51, 52, 57] (n = 32), and the combined EDSS mean ± SD was 3.72 ± 1.79. The combined mean ± SD of the disease duration was 14.84 ± 7.92 years (n = 39), reported by three studies [51, 57, 59].
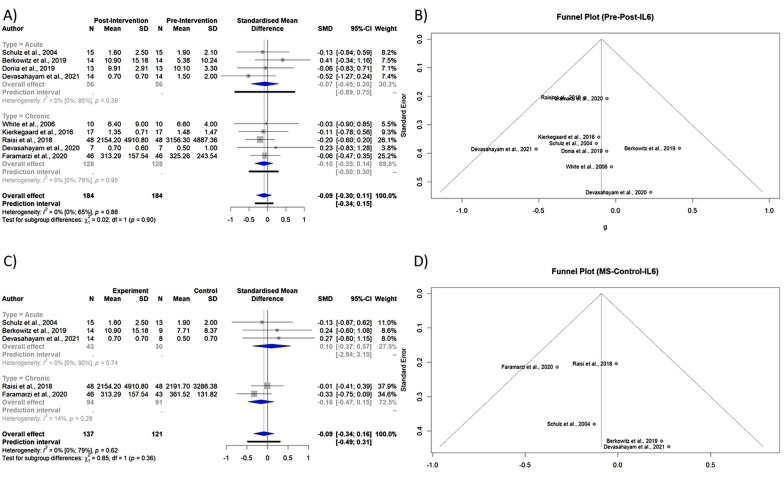
Meta-analysis showed that IL-6 levels were lower after exercise but did not reach statistical significance (Table 3, Fig. 2A). A drapery plot is used to represent the meta-analysis findings based on the study's p-value functions (p-value on the y-axis and the effect size on the x-axis), which are depicted in the Additional file 1 for all four meta-analyses.
Fig. 2.
A Forest plot of subgroup meta-analysis of pre- and post-intervention levels of IL-6 B Funnel plot of meta-analysis of pre- and post-intervention levels of IL-6 C Forest plot of subgroup meta-analysis of PwMS vs. Control levels of IL-6 D Funnel plot of meta-analysis of PwMS vs. Control levels of IL-6
The Egger's test (p-value = 0.45) and funnel plot (Fig. 2B) exhibited no evidence of publication bias. The Eggers' test does not indicate the presence of substantial funnel plot asymmetry. The heterogeneity between studies was not statistically significant (p-value = 0.8843).
Comparison of post-intervention IL-6 levels between PwMS and controls
In five studies, levels of IL-6 were compared between PwMS (N = 137) and controls (N = 121). The combined mean ± SD of participants' age was 42.21 ± 11.92 years reported by three studies (n = 43) [51, 55, 59]. The cumulative number of female and male participants was 177 and 8, respectively [51, 54, 55, 58, 59]. Just one study reported the EDSS score of the patients [59]. The combined mean ± SD of disease duration was 13.89 ± 7.19 years [51, 59].
No statistically significant difference was observed comparing post-intervention levels of IL-6 between PwMS and controls (Table 3, Fig. 2C).
The Egger's test (p-value = 0.18) and funnel plot (Fig. 2D) disclosed no evidence of publication bias. The Eggers' test does not indicate the presence of substantial funnel plot asymmetry. The heterogeneity between studies was not statistically significant (p-value = 0.6162).
TNF-α
Comparison of pre- and post-intervention TNF-α concentration
In six studies, levels of TNF-α were determined before and after the exercise program in PwMS (N = 102). The combined mean ± SD of the age of participants was 32.2 ± 12.08 years, as reported by five studies (n = 85) [52, 55, 56, 60, 61]. The cumulative number of female and male participants was 74 and 9, respectively [52, 55, 56, 61]. Three studies reported the EDSS score of the patients (n = 58) [52, 60, 61], and the combined EDSS mean ± SD was 2.25 ± 1.16. One study reported the disease duration time of the participants [61].
TNF-α levels were lower after exercise intervention, but not achieving statistical significance (SMD −0.5161, 95% CI [−1.0488; 0.0166], p-value = 0.0551, test of heterogeneity: I2 = 48.6%, p-value = 0.0836 (Table 3, Fig. 3A).
Fig. 3.
A Forest plot of subgroup meta-analysis of pre- and post-intervention levels of TNF-α B Funnel plot of meta-analysis of pre- and post-intervention levels of TNF-α C and D Result of sensitivity analysis
The Egger's test (p-value = 0.27) and funnel plot (Fig. 3B) showed no evidence of publication bias. The Eggers' test does not indicate the presence of substantial funnel plot asymmetry. The heterogeneity between studies was statistically significant (p-value = 0.0836).
Sensitivity analysis (leave-one-out analysis) showed that the effect size remained significant after omitting each study, and the heterogeneity did significantly reduce (Fig. 3C and D).
Comparison of post-intervention TNF-α levels between PwMS and controls
In three studies, the levels of TNF-α were compared between PwMS (N = 62) and controls (N = 48). The combined mean ± SD of the age of participants was 34.17 ± 7.9 years for 62 reported patients of three studies [55, 60, 61]. The cumulative number of female and male participants was 29 and 6, respectively, reported by two studies [55, 61]. Two studies reported the EDSS score of the patients [60, 61], and the combined EDSS mean ± SD was 1.93 ± 0.94 for 48 reported patients. One study reported the disease duration time of the participants [61].
No statistically significant difference was observed comparing post-intervention levels of TNF-α between PwMS and controls (Table 3, Fig. 4A).
Fig. 4.

A Forest plot of subgroup meta-analysis of PwMS vs. Control levels of TNF-α B Funnel plot of meta-analysis of PwMS vs. Control levels of TNF-α
The Egger's test (p-value = 0.23) and funnel plot (Fig. 4B) demonstrated no evidence of publication bias. The Eggers' test does not indicate the presence of substantial funnel plot asymmetry. The heterogeneity between studies was not statistically significant (p-value = 0.7410).
Discussion
To the best of our knowledge, the current review is the first meta-analysis evaluating the acute and long-term impacts of exercise on serum IL-6 and TNF-α in PwMS and compared to healthy controls. Results can be summarized in five points: First, in PwMS, acute and regular exercise had no impact on IL-6 levels. Second, for TNF-α, overall concentrations decreased from pre- to post-intervention; however, third, no change in TNF-α concentrations was observed for acute exercise when considered separately. Fourth, regular exercise resulted in significant differences from pre- to post-intervention. Lastly, compared to controls, in PwMS, IL-6 and TNF-α concentrations did not change after acute and regular exercise. Given these, the present results significantly add to the current literature: Both acute and regular exercise does either not impact or favorably impact IL-6 and TNF-α levels in PwMS; as such, both acute and regular exercises do not further deteriorate the immune system; this is disturbed in PwMS.
Blood and CSF cytokine alterations have been reported in MS patients, but the results are not consistent given the heterogeneity of studies. Most studies involved a small number of MS patients, not controlling for the phase of the disease (e.g., remission vs. relapse/acute, relapsing–remitting vs. progressive) [62–67]. According to a recent meta-analysis of these studies, TNF-α was significantly higher in PwMS compared to healthy controls (p-value < 0.001), but differences in IL-6 blood concentrations in PwMS and healthy controls were not (p-value = 0.064) [68]. Similarly, CSF levels of TNF-α was significantly higher in PwMS, unlike IL-6 [68].
Exercise has been associated with changes in the peripheral levels of cytokines. For example, Ostrowskie et al. demonstrated that IL-6 plasma concentration of athletes increases significantly after 2.5 h of treadmill running, while TNF-α remains unchanged [69]. Kouvelioti et al. observed significant elevations of IL-1β, IL-6, and TNF-α serum levels after high-intensity interval running and cycling [70]. Townsend et al. assessed the circulating levels of TNF-α after heavy resistance exercise in men. They reported that TNF-α elevates immediately after resistance exercise but decreases at 24 and 48 h after that [71]. Different exercise protocols, including type, duration, and intensity alongside distinct measurement methods, and targeted population might explain differences among these studies [70] [72, 73]. Age and gender are other factors contributing to different post-exercise cytokine changes [74].
Several studies have also investigated exercise-induced cytokine alterations in neurodegenerative diseases [75–77]. For example, plasma levels of IL-6, but not TNF-α, increased after a 16-week duration of moderate-to-high-intensity aerobic physical exercise in Alzheimer’s disease patients [78]. Conversely, TNF-α levels decreased [79] or remained unaltered in PD patients after 8-week course of aerobic exercise [80].
In contrast to these studies in healthy subjects and people with neurodegenerative disorders, PwMS did not change their TNF-α and IL-6 blood levels after physical activity. The impaired aerobic capacity in PwMS could explain the observed differences between healthy people and PwMS regarding cytokine changes. The decreased oxygen transportation/mitochondrial phosphorylation in MS is associated with disease severity and results in different cytokine alterations [81].
Given that systemic IL-6 regulation alterations may be significant in the establishment of central nervous system lesions [82], reductions in this cytokine may have substantial clinical outcomes in people with MS. Previous research indicates that excessively high IL-6 concentrations in the peripheral may cause excess inflammation, which may aggravate disease activity in MS [83]. Moreover, increased IL-6 may interfere with microbial pathogen clearance [83] and contribute in T-cell activation, thereby contributing to MS disease processes [83, 84]. Furthermore, plasma IL-6 levels may be a marker of skeletal muscle-controlled metabolic regulation. IL-6 has both paracrine and endocrine effects. IL-6 may influence the release of more IL-6 from local skeletal muscle [85], or it may circulate and influence hepatic glucose release [86]. Also, when glucose reliance reduces, resting basal IL-6 concentrations have been reported to decrease with training [87]. As a result, reductions in IL-6 may represent a training response and may reflect metabolic alterations. More investigations that clearly determine the impact of variations in IL-6 in MS patients would be beneficial.
Recent studies demonstrate that TNF-α may be neuroprotective by increasing the proliferation of oligodendrocytes and stimulating remyelination [82, 88, 89], despite the fact that it has been connected to MS-related inflammatory demyelination [90–92]. In fact, intravenous anti-TNF-α medication proved ineffective in MS patients and may have worsened their symptoms [88, 93]. As a result, resolving TNF-α's paradoxical involvement in disease activity is challenging. One reason might be the occurrence of two distinct signaling pathways mediated by two distinct TNF-α receptors (p55 and p75) [88, 89]. Exercise may cause activation of the "good" inflammatory TNF-α-p75 receptor pathway, which stimulates cell growth and proliferation [88]. All of these data suggested that TNF-α plays a critical part in the disease progression of MS and that blocking its effects may lower the severity of MS symptoms. TNF-α inhibitors are being employed as an effective treatment option in a variety of autoimmune and inflammatory conditions [94]. Remarkably, none of the previous trials and investigations supported the use of anti-TNF drugs in MS.
As previously explained, PwMS hesitated to do exercises for a long time because they feared disease exacerbation [95]. Our study revealed that IL-6 and TNF-alpha levels insignificantly decrease after the acute phases of acute physical exercises. As the relapse phase of RRMS patients is associated with higher IL-6 and TNF-alpha levels than healthy controls [96], this might imply the anti-inflammatory effect of physical activity and its protection from disease exacerbation in patients. However, neurologists should take caution when recommending PwMS to exercise, as aggressive training could result in excess heat and injuries. These factors are associated with disease exacerbation in these people [97, 98]. If prescribed cautiously, physical activity can lead to a better quality of life in PwMS [99].
The limitations of the current meta-analysis reflect the limitations of the available studies investigating the effect of exercise on PwMS. Most studies enrolled small number of patients and involved short-term interventions (less than 26 weeks), with different types of exercise. Moreover, most recruited patients had low levels of disability (EDSS scores of < 4) and relapsing–remitting MS, not presenting medical comorbidities, decreasing the potential generalizability of the findings.
Further investigations, including a more significant number of patients with diverse forms of MS, are necessary to confirm these findings. These approaches can improve the overall quality and scope of the evidence on MS rehabilitation research.
Conclusion
Among adult individuals with MS, both acute and regular exercise did not deteriorate but positively impacted two cytokines, namely, IL-6 and TNF-α levels in PwMS. Given this, the present systematic review and meta-analysis support the benefit of acute and regular exercise among PwMS. According to the results of our study, on a molecular basis, exercise does not result in a disturbed and inflammatory immune system in PwMS, and even lead to a reduction in TNF-α. These findings are in contrary to the belief of some neurologists and patients.
Supplementary Information
Acknowledgements
The authors sincerely thank the authors of the included articles in this review due to sharing the relevant data.
Abbreviations
- PwMS
People with multiple sclerosis
- MS
Multiple sclerosis
- CNS
Central nervous system
- TNF-α
Tumor necrosis factor-alpha
- IL-6
Interleukin-6
- ELISA
Enzyme-linked immunosorbent assay
- EDSS
Expanded disability status scale
- SMD
Standardized mean difference
- IQR
Interquartile range
- SD
Standard deviation
Author contributions
PS and NR contributed to conceptualization; HS performed data curation; PS was involved in formal analysis, methodology, and visualization; funding acquisition is not applicable; PS and HS investigated this study; PS and NR contributed to project administration; NR did supervision; AT, SB, DS, and NR performed validation; PS, HS, NK, and FR were involved in writing—original draft; PS, AT, SB, DS, and NR contributed to writing—review & editing. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All of the data will be available for secondary analysis in necessary cases from the corresponding author through the email address.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
“We declare this research has not been published before nor under review for publication in any journal. None of the paper's contents have been previously published. All authors have read and approved the manuscript. The authors declare no conflict of interests. No funding was received for performing this study. We also declare that all co-authors have read and approved the submission.”
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Parnian Shobeiri and Homa Seyedmirzaei contributed equally
References
- 1.McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325(8):765–779. doi: 10.1001/jama.2020.26858. [DOI] [PubMed] [Google Scholar]
- 2.Majdinasab N, Motl RW, Mokhtarzade M, Zimmer P, Ranjbar R, Keytsman C, et al. Acute responses of cytokines and adipokines to aerobic exercise in relapsing vs. remitting women with multiple sclerosis. Complement Ther Clin Pract. 2018;31:295–301. doi: 10.1016/j.ctcp.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Deckx N, Wens I, Nuyts AH, Hens N, De Winter BY, Koppen G, et al. 12 weeks of combined endurance and resistance training reduces innate markers of inflammation in a randomized controlled clinical trial in patients with multiple sclerosis. Mediators Inflamm. 2016 doi: 10.1155/2016/6789276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White LJ, Dressendorfer RH. Exercise and multiple sclerosis. Sports Med. 2004;34(15):1077–1100. doi: 10.2165/00007256-200434150-00005. [DOI] [PubMed] [Google Scholar]
- 5.Petajan JH, White AT. Recommendations for physical activity in patients with multiple sclerosis. Sports Med. 1999;27(3):179–191. doi: 10.2165/00007256-199927030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt S, Jöstingmeyer P. Depression, fatigue and disability are independently associated with quality of life in patients with multiple sclerosis: results of a cross-sectional study. Mult Scler Relat Disord. 2019;35:262–269. doi: 10.1016/j.msard.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Edwards T, Pilutti LA. The effect of exercise training in adults with multiple sclerosis with severe mobility disability: a systematic review and future research directions. Mult Scler Relat Disord. 2017;16:31–39. doi: 10.1016/j.msard.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Ensari I, Adamson BC, Motl RW. Longitudinal association between depressive symptoms and walking impairment in people with relapsing-remitting multiple sclerosis. J Health Psychol. 2016;21(11):2732–2741. doi: 10.1177/1359105315584837. [DOI] [PubMed] [Google Scholar]
- 9.Coote S, Uszynski M, Herring MP, Hayes S, Scarrott C, Newell J, et al. Effect of exercising at minimum recommendations of the multiple sclerosis exercise guideline combined with structured education or attention control education—secondary results of the step it up randomised controlled trial. BMC Neurol. 2017;17(1):119. doi: 10.1186/s12883-017-0898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razazian N, Kazeminia M, Moayedi H, Daneshkhah A, Shohaimi S, Mohammadi M, et al. The impact of physical exercise on the fatigue symptoms in patients with multiple sclerosis: a systematic review and meta-analysis. BMC Neurol. 2020;20(1):93. doi: 10.1186/s12883-020-01654-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD009956.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadeghi Bahmani D, Kesselring J, Papadimitriou M, Bansi J, Pühse U, Gerber M, et al. In patients with multiple sclerosis, both objective and subjective sleep, depression, fatigue, and paresthesia improved after 3 weeks of regular exercise. Front Psychiatry. 2019;10:265. doi: 10.3389/fpsyt.2019.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadeghi Bahmani D, Gonzenbach R, Motl RW, Bansi J, Rothen O, Niedermoser D, et al. Better objective sleep was associated with better subjective sleep and physical activity; Results from an exploratory study under naturalistic conditions among persons with multiple sclerosis. Int J Environ Res Public Health. 2020;17(10):3522. doi: 10.3390/ijerph17103522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadeghi Bahmani D, Gonzenbach R, Kesselring J, Bansi J, Motl RW, Cordier D, et al. Among persons with multiple sclerosis (MS), objective sleep, psychological functioning, and higher physical activity scores remained stable over 2 years-results from a small study under naturalistic conditions. Front Psychiatry. 2020;11:586244. doi: 10.3389/fpsyt.2020.586244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briken S, Rosenkranz SC, Keminer O, Patra S, Ketels G, Heesen C, et al. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J Neuroimmunol. 2016;299:53–58. doi: 10.1016/j.jneuroim.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Mouzaki A, Rodi M, Dimisianos N, Emmanuil A, Kalavrizioti D, Lagoudaki R, et al. Immune parameters that distinguish multiple sclerosis patients from patients with other neurological disorders at presentation. PLoS ONE. 2015;10(8):e0135434. doi: 10.1371/journal.pone.0135434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaskow BJ, Baecher-Allan C. Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(4):a029025. doi: 10.1101/cshperspect.a029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oreja-Guevara C, Ramos-Cejudo J, Aroeira LS, Chamorro B, Diez-Tejedor E. TH1/TH2 Cytokine profile in relapsing-remitting multiple sclerosis patients treated with Glatiramer acetate or Natalizumab. BMC Neurol. 2012;12:95. doi: 10.1186/1471-2377-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpe E, Battistini L, Borsellino G. Advances in T helper 17 cell biology: pathogenic role and potential therapy in multiple sclerosis. Mediators Inflamm. 2015;2015:475158. doi: 10.1155/2015/475158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunkl M, Frascolla S, Amormino C, Volpe E, Tuosto L. T helper cells: the modulators of inflammation in multiple sclerosis. Cells. 2020;9(2):482. doi: 10.3390/cells9020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy A, Pahan K. Myelin basic protein-primed t helper 2 cells suppress microglial activation via AlphaVBeta3 integrin: implications for multiple sclerosis. J Clin Cell Immunol. 2013;7:158. doi: 10.4172/2155-9899.1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellano V, Patel DI, White LJ. Cytokine responses to acute and chronic exercise in multiple sclerosis. J Appl Physiol. 2008;104(6):1697–1702. doi: 10.1152/japplphysiol.00954.2007. [DOI] [PubMed] [Google Scholar]
- 23.Kjolhede T, Dalgas U, Gade AB, Bjerre M, Stenager E, Petersen T, et al. Acute and chronic cytokine responses to resistance exercise and training in people with multiple sclerosis. Scand J Med Sci Sports. 2016;26(7):824–834. doi: 10.1111/sms.12504. [DOI] [PubMed] [Google Scholar]
- 24.Popko K, Gorska E, Stelmaszczyk-Emmel A, Plywaczewski R, Stoklosa A, Gorecka D, et al. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res. 2010;15(Suppl 2):120–2. doi: 10.1186/2047-783X-15-S2-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauptmann J, Johann L, Marini F, Kitic M, Colombo E, Mufazalov IA, et al. Interleukin-1 promotes autoimmune neuroinflammation by suppressing endothelial heme oxygenase-1 at the blood–brain barrier. Acta Neuropathol. 2020;140(4):549–567. doi: 10.1007/s00401-020-02187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palle P, Monaghan KL, Milne SM, Wan ECK. Cytokine signaling in multiple sclerosis and its therapeutic applications. Med Sci (Basel) 2017;5(4):23. doi: 10.3390/medsci5040023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105(26):9041–9046. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stampanoni Bassi M, Iezzi E, Mori F, Simonelli I, Gilio L, Buttari F, et al. Interleukin-6 disrupts synaptic plasticity and impairs tissue damage compensation in multiple sclerosis. Neurorehabil Neural Repair. 2019;33(10):825–835. doi: 10.1177/1545968319868713. [DOI] [PubMed] [Google Scholar]
- 29.Mee-Inta O, Zhao Z-W, Kuo Y-M. Physical exercise inhibits inflammation and microglial activation. Cells. 2019;8(7):691. doi: 10.3390/cells8070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steensberg A. The role of IL-6 in exercise-induced immune changes and metabolism. Exerc Immunol Rev. 2003;9:40–47. [PubMed] [Google Scholar]
- 31.Rezaee S, Kahrizi S, Nabavi SM, Hedayati M. Vegf and tnf-α responses to acute and chronic aerobic exercise in the patients with multiple sclerosis. Asian J Sports Med. 2020;11(3):1–6. doi: 10.5812/asjsm.98312. [DOI] [Google Scholar]
- 32.Peake JM, Della Gatta P, Suzuki K, Nieman DC. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev. 2015;21:8–25. [PubMed] [Google Scholar]
- 33.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 34.Zuo Q, Qu F, Li N, Wang S, Liu J, Xu C, et al. Eccentric exercise results in a prolonged increase in interleukin-6 and tumor necrosis factor-α levels in rat skeletal muscle. J Muscle Res Cell Motil. 2019;40(3–4):379–387. doi: 10.1007/s10974-019-09554-6. [DOI] [PubMed] [Google Scholar]
- 35.Gligoroska JP, Manchevska S. The effect of physical activity on cognition–physiological mechanisms. Materia Sociomedica. 2012;24(3):198. doi: 10.5455/msm.2012.24.198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101(4):1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 37.Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults. Sports Med. 2002;32(12):741–760. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- 38.Prakash RS, Snook EM, Motl RW, Kramer AF. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res. 2010;1341:41–51. doi: 10.1016/j.brainres.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klaren RE, Hubbard EA, Motl RW, Pilutti LA, Wetter NC, Sutton BP. Objectively measured physical activity is associated with brain volumetric measurements in multiple sclerosis. Behav Neurol. 2015 doi: 10.1016/j.brainres.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kjølhede T, Siemonsen S, Wenzel D, Stellmann J-P, Ringgaard S, Pedersen BG, et al. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Mult Scler J. 2018;24(10):1356–1365. doi: 10.1177/1352458517722645. [DOI] [PubMed] [Google Scholar]
- 41.Velikonja O, Čurić K, Ožura A, Jazbec SŠ. Influence of sports climbing and yoga on spasticity, cognitive function, mood and fatigue in patients with multiple sclerosis. Clin Neurol Neurosurg. 2010;112(7):597–601. doi: 10.1016/j.clineuro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Beier M, Bombardier CH, Hartoonian N, Motl RW, Kraft GH. Improved physical fitness correlates with improved cognition in multiple sclerosis. Arch Phys Med Rehabil. 2014;95(7):1328–1334. doi: 10.1016/j.apmr.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Briken S, Gold S, Patra S, Vettorazzi E, Harbs D, Tallner A, et al. Effects of exercise on fitness and cognition in progressive MS: a randomized, controlled pilot trial. Mult Scler J. 2014;20(3):382–390. doi: 10.1177/1352458513507358. [DOI] [PubMed] [Google Scholar]
- 44.Sandroff BM, Dlugonski D, Pilutti LA, Pula JH, Benedict RH, Motl RW. Physical activity is associated with cognitive processing speed in persons with multiple sclerosis. Mult Scler Relat Disord. 2014;3(1):123–128. doi: 10.1016/j.msard.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Sandroff BM, Balto JM, Klaren RE, Sommer SK, DeLuca J, Motl RW. Systematically developed pilot randomized controlled trial of exercise and cognition in persons with multiple sclerosis. Neurocase. 2016;22(5):443–450. doi: 10.1080/13554794.2016.1237658. [DOI] [PubMed] [Google Scholar]
- 46.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tooth LR, Ottenbacher KJ. The κ statistic in rehabilitation research: an examination. Arch Phys Med Rehabil. 2004;85(8):1371–1376. doi: 10.1016/j.apmr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 49.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 50.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):1–13. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schulz KH, Gold SM, Witte J, Bartsch K, Lang UE, Hellweg R, et al. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. J Neurol Sci. 2004;225(1–2):11–18. doi: 10.1016/j.jns.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 52.White LJ, Castellano V, McCoy SC. Cytokine responses to resistance training in people with multiple sclerosis. J Sports Sci. 2006;24(8):911–914. doi: 10.1080/02640410500357036. [DOI] [PubMed] [Google Scholar]
- 53.Kierkegaard M, Lundberg IE, Olsson T, Johansson S, Ygberg S, Opava C, et al. High-intensity resistance training in multiple sclerosis—an exploratory study of effects on immune markers in blood and cerebrospinal fluid, and on mood, fatigue, health-related quality of life, muscle strength, walking and cognition. J Neurol Sci. 2016;362:251–257. doi: 10.1016/j.jns.2016.01.063. [DOI] [PubMed] [Google Scholar]
- 54.Raisi Z, Faramarzi M, Banitalebi E, Samieyan M. The effect of 12 weeks combined (Strength, endurance, pilates, PNF) exercise training on fibrin d-dimer (FDD) and interleukin-6 levels in female multiple sclerosis patients with different levels of disability. J Zanjan Univ Med Sci Health Serv. 2018;26(116):35–47. [Google Scholar]
- 55.Berkowitz S, Achiron A, Gurevich M, Sonis P, Kalron A. Acute effects of aerobic intensities on the cytokine response in women with mild multiple sclerosis. MulT Scler Relat Disord. 2019;31:82–86. doi: 10.1016/j.msard.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 56.Donia SA, Allison DJ, Gammage KL, Ditor DS. The effects of acute aerobic exercise on mood and inflammation in individuals with multiple sclerosis and incomplete spinal cord injury. NeuroRehabilitation. 2019;45(1):117–124. doi: 10.3233/NRE-192773. [DOI] [PubMed] [Google Scholar]
- 57.Devasahayam AJ, Chaves AR, Lasisi WO, Curtis ME, Wadden KP, Kelly LP, et al. Vigorous cool room treadmill training to improve walking ability in people with multiple sclerosis who use ambulatory assistive devices: a feasibility study. BMC Neurol. 2020;20(1):33. doi: 10.1186/s12883-020-1611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faramarzi M, Banitalebi E, Raisi Z, Samieyan M, Saberi Z, Ghahfarrokhi MM, et al. Effect of combined exercise training on pentraxins and pro- inflammatory cytokines in people with multiple sclerosis as a function of disability status. Cytokine. 2020;134:155196. doi: 10.1016/j.cyto.2020.155196. [DOI] [PubMed] [Google Scholar]
- 59.Devasahayam AJ, Kelly LP, Williams JB, Moore CS, Michelle P. Fitness shifts the balance of BDNF and IL-6 from inflammation to repair among people with progressive multiple sclerosis. Biomolecules. 2021;11(4):504. doi: 10.3390/biom11040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kordi MR, Anooshe L, Khodadade S, Maghsodi N, Sanglachi B, Hemmatinafar M. Comparing the effect of three methods of combined training on serum levels of Ghrelin, pro and anti-inflammatory cytokines in multiple sclerosis (MS) patients. J Zanjan Univ Med Sci Health Serv. 2014;22(91):39–51. [Google Scholar]
- 61.Mokhtarzade M, Molanouri Shamsi M, Abolhasani M, Bakhshi B, Sahraian MA, Quinn LS, et al. Home-based exercise training influences gut bacterial levels in multiple sclerosis. Complement Ther Clin Pract. 2021;45:101463. doi: 10.1016/j.ctcp.2021.101463. [DOI] [PubMed] [Google Scholar]
- 62.Kraus J, Kuehne B, Tofighi J, Frielinghaus P, Stolz E, Blaes F, et al. Serum cytokine levels do not correlate with disease activity and severity assessed by brain MRI in multiple sclerosis. Acta Neurol Scand. 2002;105(4):300–308. doi: 10.1034/j.1600-0404.2002.1o199.x. [DOI] [PubMed] [Google Scholar]
- 63.Obradović D, Kataranovski M, Dinčić E, Obradović S, Čolić M. Tumor necrosis factor-alfa and interleukin-4 in cerbrospinal fluid and plasma in different clinical forms of multiple sclerosis. Vojnosanit Pregl. 2012;69(2):151–156. doi: 10.2298/VSP1202151O. [DOI] [PubMed] [Google Scholar]
- 64.Okuda Y, Sakoda S, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T. IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glycoprotein 35–55 induced experimental autoimmune encephalomyelitis. J Neuroimmunol. 1999;101(2):188–196. doi: 10.1016/S0165-5728(99)00139-3. [DOI] [PubMed] [Google Scholar]
- 65.Olsson T. Cytokine-producing cells in experimental autoimmune encephalomyelitis and multiple sclerosis. Neurology. 1995;45(6 Suppl 6):S11–S15. doi: 10.1212/WNL.45.6_Suppl_6.S11. [DOI] [PubMed] [Google Scholar]
- 66.Rieckmann P, Albrecht M, Kitze B, Weber T, Tumani H, Broocks A, et al. Cytokine mRNA levels in mononuclear blood cells from patients with multiple sclerosis. Neurology. 1994;44(8):1523. doi: 10.1212/WNL.44.8.1523. [DOI] [PubMed] [Google Scholar]
- 67.Trenova AG, Manova MG, Kostadinova II, Murdjeva MA, Hristova DR, Vasileva TV, et al. Clinical and laboratory study of pro-inflammatory and antiinflammatory cytokines in women with multiple sclerosis. Folia Med. 2011;2013:03–22. doi: 10.2478/v10153-010-0034-x. [DOI] [PubMed] [Google Scholar]
- 68.Bai Z, Chen D, Wang L, Zhao Y, Liu T, Yu Y, et al. Cerebrospinal fluid and blood cytokines as biomarkers for multiple sclerosis: a systematic review and meta-analysis of 226 studies with 13,526 multiple sclerosis patients. Front Neurosci. 2019;13:1026. doi: 10.3389/fnins.2019.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998;513:889–94. doi: 10.1111/j.1469-7793.1998.889ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kouvelioti R, Kurgan N, Falk B, Ward WE, Josse AR, Klentrou P. Cytokine and sclerostin response to high-intensity interval running versus cycling. Med Sci Sports Exerc. 2019;51(12):2458–2464. doi: 10.1249/MSS.0000000000002076. [DOI] [PubMed] [Google Scholar]
- 71.Townsend JR, Hoffman JR, Fragala MS, Jajtner AR, Gonzalez AM, Wells AJ, et al. TNF-α and TNFR1 responses to recovery therapies following acute resistance exercise. Front Physiol. 2015;6:48. doi: 10.3389/fphys.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998;508:949–53. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernecker C, Scherr J, Schinner S, Braun S, Scherbaum WA, Halle M. Evidence for an exercise induced increase of TNF-α and IL-6 in marathon runners. Scand J Med Sci Sports. 2013;23(2):207–214. doi: 10.1111/j.1600-0838.2011.01372.x. [DOI] [PubMed] [Google Scholar]
- 74.Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA. Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-alpha levels after prolonged running. Am J Physiol Cell Physiol. 2001;280(4):C769–C774. doi: 10.1152/ajpcell.2001.280.4.C769. [DOI] [PubMed] [Google Scholar]
- 75.Rocha NP, Ribeiro FM, Furr-Stimming E, Teixeira AL. Neuroimmunology of Huntington's disease: revisiting evidence from human studies. Mediators Inflamm. 2016;2016:8653132. doi: 10.1155/2016/8653132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su F, Bai F, Zhang Z. Inflammatory cytokines and Alzheimer's disease: a review from the perspective of genetic polymorphisms. Neurosci Bull. 2016;32(5):469–480. doi: 10.1007/s12264-016-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson's disease. J Neural Transm Suppl. 2000;58:143–151. [PubMed] [Google Scholar]
- 78.Jensen CS, Bahl JM, Østergaard LB, Høgh P, Wermuth L, Heslegrave A, et al. Exercise as a potential modulator of inflammation in patients with Alzheimer's disease measured in cerebrospinal fluid and plasma. Exp Gerontol. 2019;121:91–98. doi: 10.1016/j.exger.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Zoladz JA, Majerczak J, Zeligowska E, Mencel J, Jaskolski A, Jaskolska A, et al. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson's disease patients. J Physiol Pharmacol. 2014;65(3):441–448. [PubMed] [Google Scholar]
- 80.Soke F, Kocer B, Fidan I, Keskinoglu P, Guclu-Gunduz A. Effects of task-oriented training combined with aerobic training on serum BDNF, GDNF, IGF-1, VEGF, TNF-α, and IL-1β levels in people with Parkinson's disease: a randomized controlled study. Exp Gerontol. 2021;150:111384. doi: 10.1016/j.exger.2021.111384. [DOI] [PubMed] [Google Scholar]
- 81.Langeskov-Christensen M, Heine M, Kwakkel G, Dalgas U. Aerobic capacity in persons with multiple sclerosis: a systematic review and meta-analysis. Sports Med. 2015;45(6):905–923. doi: 10.1007/s40279-015-0307-x. [DOI] [PubMed] [Google Scholar]
- 82.Sharief M. Cytokines in multiple sclerosis: pro-inflammation or pro-remyelination? Mult Scler J. 1998;4(3):169–173. doi: 10.1177/135245859800400315. [DOI] [PubMed] [Google Scholar]
- 83.Özenci V, Kouwenhoven M, Link H. Cytokines in multiple sclerosis: methodological aspects and pathogenic implications. Mult Scler J. 2002;8(5):396–404. doi: 10.1191/1352458502ms837rr. [DOI] [PubMed] [Google Scholar]
- 84.Ozenci V, Kouwenhoven M, Huang Y, Xiao B, Kivisakk P, Fredrikson S, et al. Multiple sclerosis: levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-b-1b treatment. Scand J Immunol. 1999;49(5):554. doi: 10.1046/j.1365-3083.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 85.Pedersen BK, Fischer CP. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr Opin Clin Nutr Metab Care. 2007;10(3):265–271. doi: 10.1097/MCO.0b013e3280ebb5b3. [DOI] [PubMed] [Google Scholar]
- 86.Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53(7):1643–1648. doi: 10.2337/diabetes.53.7.1643. [DOI] [PubMed] [Google Scholar]
- 87.Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance. Exerc immunol rev. 2006;12(6–33):41. [PubMed] [Google Scholar]
- 88.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP-Y. TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nature Neurosci. 2001;4(11):1116–22. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 89.Arnett HA, Wang Y, Matsushima GK, Suzuki K, Ting JP-Y. Functional genomic analysis of remyelination reveals importance of inflammation in oligodendrocyte regeneration. J Neurosci. 2003;23(30):9824–32. doi: 10.1523/JNEUROSCI.23-30-09824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burks JS, Johnson KP. Multiple sclerosis: diagnosis, medical management, and rehabilitation. New York: Demos Medical; 2000. [Google Scholar]
- 91.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966(1):290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 92.Huang Y-M, Xiao B-G, Özenci V, Kouwenhoven M, Teleshova N, Fredrikson S, et al. Multiple sclerosis is associated with high levels of circulating dendritic cells secreting pro-inflammatory cytokines. J Neuroimmunol. 1999;99(1):82–90. doi: 10.1016/S0165-5728(99)00106-X. [DOI] [PubMed] [Google Scholar]
- 93.Van Oosten B, Barkhof F, Truyen L, Boringa J, Bertelsmann F, Von Blomberg B, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47(6):1531–1534. doi: 10.1212/WNL.47.6.1531. [DOI] [PubMed] [Google Scholar]
- 94.Kemanetzoglou E, Andreadou E. CNS demyelination with TNF-α blockers. Curr Neurol Neurosci Rep. 2017;17(4):1–15. doi: 10.1007/s11910-017-0742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horton S, MacDonald DJ, Erickson K, Dionigi RA. A qualitative investigation of exercising with MS and the impact on the spousal relationship. Eur Rev Aging Phys Act. 2015;12:3. doi: 10.1186/s11556-015-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peiravian F, Rajaian H, Samiei A, Gholijani N, Gharesi-Fard B, Mokaram P, et al. Altered serum cytokine profiles in relapse phase of relapsing-remitting multiple sclerosis. Iran J Immunol. 2016;13(3):186–196. [PubMed] [Google Scholar]
- 97.Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731. doi: 10.1136/bmj.38041.724421.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chacko G, Patel S, Galor A, Kumar N. Heat exposure and multiple sclerosis-a regional and temporal analysis. Int J Environ Res Public Health. 2021;18(11):5962. doi: 10.3390/ijerph18115962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Motl RW, McAuley E, Snook EM, Gliottoni RC. Physical activity and quality of life in multiple sclerosis: intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med. 2009;14(1):111–124. doi: 10.1080/13548500802241902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data will be available for secondary analysis in necessary cases from the corresponding author through the email address.