Abstract
Triple-negative breast cancer (TNBC) is the most aggressive breast cancer subtype. It disproportionately affects BRCA mutation carriers and young women, especially African American (AA) women. Chemoresistant TNBC is a heterogeneous and molecularly unstable disease that challenges our ability to apply personalized therapies. With the approval of immune checkpoint blockade (ICB) for TNBC, the addition of pembrolizumab to systemic chemotherapy has become standard of care (SOC) in neoadjuvant systemic therapy (NST) for high-risk early-stage TNBC. Pembrolizumab plus chemotherapy significantly increased the pathologic complete response (pCR) and improved event-free survival in TNBC. However, clinical uncertainties remain because similarly treated TNBC partial responders with comparable tumor responses to neoadjuvant therapy often experience disparate clinical outcomes. Current methods fall short in accurately predicting which high-risk patients will develop chemo-resistance and tumor relapse. Therefore, novel treatment strategies and innovative new research initiatives are needed. We propose that the EGFR-K-RAS-SIAH pathway activation is a major tumor driver in chemoresistant TNBC. Persistent high expression of SIAH in residual tumors following NACT/NST reflects that the EGFR/K-RAS pathway remains activated (ON), indicating an ineffective response to treatment. These chemoresistant tumor clones persist in expressing SIAH (SIAHHigh/ON) and are linked to early tumor relapse and poorer prognosis. Conversely, the loss of SIAH expression (SIAHLow/OFF) in residual tumors post-NACT/NST reflects EGFR/K-RAS pathway inactivation (OFF), indicating effective therapy and chemo-sensitive tumor cells. SIAHLow/OFF signal is linked to tumor remission and better prognosis post-NACT/NST. Therefore, SIAH is well-positioned to become a novel tumor-specific, therapy-responsive, and prognostic biomarker. Potentially, this new biomarker (SIAHHigh/ON) could be used to quantify therapy response, predict chemo-resistance, and identify those patients at the highest risk for tumor relapse and poor survival in TNBC.
Keywords: Triple-negative breast cancer (TNBC), chemo-resistance, seven in absentia (SINA) and human homologs of SINA (SIAH) E3 ligase, ubiquitin-mediated proteolysis, EGFR/K-RAS/SIAH pathway activation in TNBC, neoadjuvant chemotherapy prognosis, patient risk stratification, detection of chemo-resistance, precision quantification of therapy efficacy, and treatment optimization
INTRODUCTION
Triple-negative breast cancer
Triple-negative breast cancer (TNBC) represents 15%-20% of all breast cancers diagnosed in the United States, and it is characterized by the absence of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2)[1-5]. https://seer.cancer.gov/statfacts/html/breast.html. TNBC is the most aggressive subtype of breast cancer, and it disproportionately affects BRCA1 mutation carriers and young women, especially those with Western African ancestry[6-13]. This molecular subtype is nearly twice as common in African American women (AA) than in Caucasian women[6,8,9,14-17]. TNBC is a genetically diverse, highly heterogeneous, and molecularly unstable disease, which challenges our ability to tailor effective individualized treatments for patients[10,18]. TNBC has unique and aggressive tumor biology, and it constitutes a major health threat with the worst prognosis and the highest mortality of all breast cancer subtypes[10,19,20]. Looking more closely, one in three patients with high-risk early-stage TNBC will develop tumor relapse, which typically occurs within the first three years of initial diagnosis; only a third of women with locoregional TNBC will survive their disease; and of those with metastatic TNBC, less than 1 in 9 will survive their disease[5,10,21-23]. TNBC has a 5-year overall survival (OS) of 78.5%, and the 5-year survival rates for localized, regional, and metastatic diseases are 91.2%, 65.4%, and 12.2%, respectively, which is the worst among the major molecular subtypes in breast cancer (https://seer.cancer.gov/explorer/application.html?site=623&data_type=4&graph_type=5&compareBy=stage&chk_stage_101=101&chk_stage_104=104&chk_stage_105=105&chk_stage_106=106&series=9&hdn_sex=3&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&advopt_display=2). Thus, the dismal prognosis, chemo-resistance, and high mortality of regional and metastatic TNBC highlights a critical unmet need for the development of improved therapies and the discovery of reliable prognostic biomarkers such as SIAH, which can single out the highest risk patients at the first-line neoadjuvant setting, identify chemoresistant tumors that are difficult to treat and prone to develop early relapse, optimize effective treatment sequences, and select the best combinational strategies for better clinical outcomes and prolonged survival.
Standard treatment regimens in TNBC
Standard chemotherapy remains the backbone of systemic therapy in TNBC[20,24-28]. https://www.cancer.org/cancer/breast-cancer/treatment/treatment-of-triple-negative.html. Neoadjuvant chemotherapy (NACT) was the previous standard of care (SOC) to treat high-risk and locally advanced TNBC prior to July 26, 2021[10,29]. The addition of immune checkpoint blockade (ICB) to chemotherapy is now the current SOC for neoadjuvant systemic therapy (NST) to treat high-risk early-stage TNBC. Immuno-oncology (IO) therapy is an exciting scientific breakthrough in the treatment of TNBC[30-32]. Immunotherapy that targets programmed death receptor-1 (PD-1) has shown great promise in treating a subset of TNBC patients in combination with chemotherapy[33,34]. Pembrolizumab plus chemotherapy significantly improved the pCR rates in high-risk early-stage TNBC in the neoadjuvant setting[35,36]. As shown in the KEYNOTE-522 trial, neoadjuvant pembrolizumab plus chemotherapy led to an improved pCR rate (65%) in high-risk early-stage TNBC[35]. Notably, the TNBC pIR patients with residual disease at the time of surgery seemed to benefit the most from the addition of IO-therapy in the neoadjuvant and adjuvant settings[37]. Surprisingly, the 3-year event-free survival (EFS) benefit associated with pembrolizumab was independent of PD-L1 expression in high-risk early-stage TNBC[35]. Based on the I-SPY 2 trial, the pCR rate doubled to 60% when pembrolizumab was added to standard chemotherapy to treat stage II/III TNBC patients with T2/N1 or higher stage tumors[30,36]. In contrast, as reported in KEYNOTE-355, KEYNOTE-119, Impassion130, and Impassion131, PD-(L)1-targeted immunotherapies plus chemotherapy have shown only modest survival benefit in PD-L1-positive TNBC in advanced and metastatic settings[33,34,38]. At the same time, unfortunately, the grade 3 or 4 treatment-related adverse events were significantly increased in response to the new IO-regimens[39].
Unmet needs in TNBC
As more and more TNBC patients are treated with pembrolizumab plus chemotherapy in both neoadjuvant and/or metastatic settings, ICB resistance is evidently emerging, and serious adverse side effects were reported in a subset of TNBC patients[35,38]. Despite the benefit of this newly FDA-approved IO-therapy, 30%-44% of high-risk early-stage TNBC patients and 60%-70% of PD-L1-positive metastatic TNBC patients who receive the IO-therapy did not show any objective improvement[35,38]. Without a proper guide, pembrolizumab plus chemotherapy is often administered “blindly” in the neoadjuvant setting following the newly FDA-approved standard IO-regimens to treat high-risk early-stage TNBC. How to maximize the current SOC chemo- and IO-therapy in combination while limiting chemo- and IO-resistance, and minimizing the side effects of immunotherapy is a difficult problem and an unmet need for a large number of TNBC patients.
Pathology following completion of NACT/NST, with or without immunotherapy (pembrolizumab), produces a binary response: pathologic complete response (pCR) or pathologic incomplete response (pIR)[40,41]. pCR is a reliable prognostic marker that correlates with tumor remission and long-term survival, whereas pIR is associated with an increased risk of early tumor relapse and poor prognosis[36,42-47]. Incomplete responders can be further classified by the residual cancer burden (RCB classes I-III); the higher the RCB classification, the higher the likelihood of tumor relapse and mortality[21,40,41,48-51]. TNBC patients with high-risk and high-grade residual disease are now commonly treated with additional adjuvant chemotherapy, including capecitabine, which may be combined with immunotherapy (pembrolizumab) post-operatively[35,36,38,52-54]. Clinical uncertainties remain, because although many TNBC patients with the identical clinical and pathological tumor stages by the American Joint Committee on Cancer (AJCC) TNM classification, and similar residual cancer burden (RCB) after a non-pCR (pIR) diagnosis post-NACT/NST, will often experience disparate clinical outcomes and survival[55,56]. Current methods to stratify these high-risk patients fall short in predicting the risk of tumor recurrence and forecasting survival. There is no reliable prognostic biomarker that can be used to predict with certainty and molecular precision which RCB (I-II-III) tumors will stay in remission and which ones will relapse rapidly[3]. Few therapeutic agents, alone or in combination, are effective at eradicating chemoresistant and metastatic TNBC[57-60]. Therefore, the development of a new tumor-specific biomarker that can be used to stratify high-risk TNBC patients in the first-line neoadjuvant setting, quantifying treatment efficacy in real time in the clinical setting is essential. Additionally, utilizing this same biomarker to detect the emergence of chemoresistant tumor clones at a single tumor cell resolution, forecast risk for early tumor relapse, and predict patient survival would equip us with a new therapy-responsive prognostic biomarker to quantify, guide, and treat TNBC more effectively[3].
Chemo-resistance in TNBC
Chemo-resistance is a vexing problem and a major life-threatening feature of TNBC[25,57,61]. Activation of multiple signaling pathways, context-dependent compensatory pathway cross-talk, synergy, antagonism, and signaling network “rewiring” are all implicated in the development of chemoresistant phenotypes in TNBC. These include Wnt/β-catenin, Notch, Hedgehog, NFκB, PI3K/mTOR, Hippo/YAP, JAK/Stat, TGFβ, hypoxia, p53 loss of function and BRCA mutations, altered metabolism, and increased transporter and efflux pump activity[25,57,62-66]. Single-cell sequencing has revealed that plasticity, heterogeneity, rapid molecular evolution of innate and acquired chemo-resistance, cellular senescence, and dynamic remodeling of epithelial-mesenchymal transition (TME)/mesenchymal-epithelial transition (MET) states of tumor-initiating cells or cancer stem cells in TNBC contribute to cancer recurrences[49,62,67-71]. These aforementioned topics have been reviewed extensively in the TNBC literature. Here, our discussion will focus on persistent activation of the EGFR/K-RAS/MAPK/SIAH pathway, which drives chemo-resistance, early tumor relapse, and high mortality in TNBC[3,72].
Persistent EGFR/K-RAS/MAPK/SIAH pathway activation drives TNBC malignancy
Genomic landscape studies indicate that EGFR/K-RAS/MAPK pathway activation is a major impetus driving TNBC malignancy, early tumor relapse, local invasion, and metastatic spread[73,74]. Aberrant EGFR/K-RAS/MAPK/SIAH pathway activation is highly prevalent in chemoresistant, recurrent, locally advanced, and metastatic TNBC[3,75-78]. Heightened EGFR/K-RAS/MAPK activation has multiple deleterious effects on the tumor/tumor microenvironment (TME), which is associated with decreased tumor-infiltrating lymphocytes (TIL) detection in TNBC and is correlated with increased metastases and poor prognosis in breast cancer[73,74,78]. With the new FDA-approved chemo- and immunotherapy combination to treat high-risk early-stage TNBC, it is important to maximize the therapeutic benefit and identify chemoresistant tumor cells as early as possible, but also minimize the adverse toxicities and immune side-effects of these IO-combination therapies in the neoadjuvant and adjuvant settings.
SIAH is the most conserved downstream signaling gatekeeper in the EGFR/K-RAS/MAPK signaling pathway
Due to the extraordinary conservation of EGFR/RAS/MAPK/SIAH signaling pathway across metazoan species, the molecular insights and core principles gleaned from Drosophila EGFR/RAS/SINA studies have shed light on the evolutionarily conserved principles and fundamental aspects of mammalian EGFR/K-RAS/MAPK/SIAH signaling pathway, and guided anti-EGFR/K-RAS/MAPK/SIAH drug development in human cancer[77,79-85]. As a RING-domain E3 ubiquitin ligase, the human homologs of SINA (SIAH) or Drosophila Seven-In-Absentia (SINA) are the most downstream gatekeeper and the most evolutionarily conserved signaling component in the EGFR/K-RAS/MEK/MAPK pathway identified thus far [Figure 1A, 1E and 1F][72,77,82-88]. Due to its conserved signaling gatekeeper function as a major network bottleneck, SIAHON/OFF expression is well-positioned to serve as a direct readout of tumor-driving EGFR/K-RAS/MEK/MAPK pathway activation (ON)/inactivation (OFF) in TNBC [Figure 1B][76,77,85,89]. SIAHLow/OFF in TNBC post-NACT correlates with tumor remission, effective therapy, and good prognosis [Figure 1C], whereas SIAHHigh/ON in TNBC post-NACT correlates with early relapse, ineffective therapy, and poor survival [Figure 1D][76]. Therefore, SIAH is likely to be an excellent prognostic biomarker to stratify incomplete responders in the first-line neoadjuvant setting[3,76,89]. SIAH may be used to identify chemoresistant tumor cells as early as possible in the neoadjuvant setting as a therapy-responsive biomarker in order to identify the difficult-to-treat cancers at the highest-risk for early relapse and treatment-resistance. Furthermore, we propose that SIAH can be used to augment residual cancer burden (RCB I-III) classification in quantifying the efficacy of SOC treatment regimens, detect chemoresistant tumor clones, forecast early tumor relapse, and predict patient survival. Having this accuracy in real time allows the clinician to pivot with precision to select more effective drugs and optimize the sequence of combination therapies for treatment-refractory TNBC [Figure 1][3,76,77,85].
Figure 1.
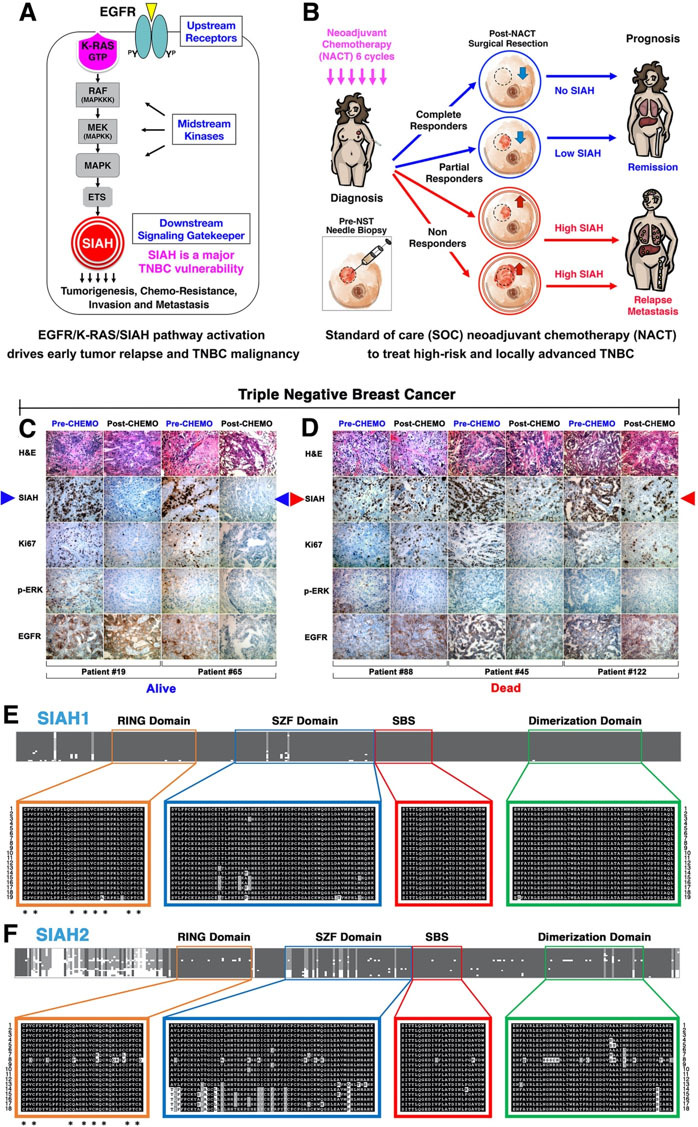
SIAH is the most conserved downstream signaling gatekeeper in the EGFR/K-RAS/SIAH pathway, whose persistent activation is driving TNBC malignancy, tumor relapse, and metastasis. (A) SIAH is the most evolutionarily conserved and the most downstream signaling module identified in the EGFR/K-RAS signaling pathway thus far. (B) Loss of SIAH expression (SIAHLow/OFF) after effective NACT is correlated with EGFR/K-RAS pathway inactivation and tumor regression/remission, whereas persistent SIAH expression (SIAHHigh/ON) after ineffective NACT is correlated with EGFR/K-RAS pathway activation and tumor progression/early relapse. Persistent high SIAH expression (SIAHHigh/ON) in high-risk residual tumors post NACT is correlated with EGFR/K-RAS pathway activation, chemo-resistance, and early tumor relapse. (C-D) TNBC tumors were stained with H&E, SIAH, Ki67, phospho-ERK, and EGFR. SIAH outperforms Ki67. SIAH is prognostic and Ki67 is not prognostic in NACT-treated high-risk and locally advanced breast cancer. We found that SIAHLow/OFF post-NACT correlates with tumor remission and prolonged survival (Alive at 5 years) (C). We found that persistent SIAHHigh/ON expression in residual tumors post-NACT is associated with tumor relapse and poor survival (Dead before3-5 years) (D). (E) SIAH1 and (F) SIAH2 are extraordinarily conserved across metazoan species. Conclusion: We found that SIAHON/OFF expression is a binary code that reflects tumor-driving EGFR/K-RAS/SIAH pathway activationON/inactivationOFF in TNBC primary and residual tumors As such, SIAH is strategically well positioned to become a new TNBC target, and a new tumor-specific, therapy-responsive, and prognostic biomarker to risk-stratify pIR patients, detect the emergence of treatment-refractory tumors, quantify NACT/NST efficacy, augment RCB classifications, forecast early relapse, and predict patient survival in real time in the clinic. SIAH: Human homologs of Drosophila Seven In Absentia (SINA); NACT: neoadjuvant chemotherapy; RCB: residual cancer burden; TNBC: triple-negative breast cancer.
The SIAHON/OFF binary code reflects this major tumor-driving EGFR/K-RAS/MAPK pathway activation (ON) and inactivation (OFF) in TNBC
Supported by strong evidence in developmental, evolutionary, and cancer biology, we hypothesize that persistent EGFR–K-RAS–SIAH pathway activation is a major tumor-driving force in TNBC, and that SIAH is a new tumor-specific, therapy-responsive, and prognostic biomarker for patient risk stratification, therapy quantification, and treatment optimization[76,77,82-85,89]. We propose that the persistent high expression of SIAH (SIAHHigh/ON) post-NACT/NST reflects tumor-driving EGFR/K-RAS/MAPK pathway activation (ON), resulting in tumor progression, immuno-suppression, and chemo-resistance, versus the loss of SIAH expression (SIAHLow/OFF) post-NACT/NST reflects this tumor-driving pathway is inactivated (OFF), resulting in tumor regression, immune responsiveness, and chemo-sensitivity[3,76]. This new tumor-specific, therapy-responsive, and prognostic SIAHON/OFF binary code can potentially be used to identify those TNBC pIR patients at the highest risk for early tumor relapse, detect multidrug-resistant residual tumor clones in real time, combine and guide precise therapies in the first-line setting.
Clinical utility of SIAH as a tumor-specific, therapy-responsive, and prognostic biomarker for risk stratification, early relapse, and survival prediction in TNBC
We generated an anti-SIAH monoclonal antibody for tumor IHC/IF/FACS staining[82,83]. Our pilot study of 57 NACT-treated TNBC patients with residual disease showed that SIAHLow/OFF correlates with tumor remission and good prognosis [Figure 1C]. For those with SIAHHigh/ON in their residual tumors, it predicts chemoresistant tumor cells/clones, early tumor relapse, and poor prognosis post-NACT/NST [Figure 1D][76]. The prognostic impact of SIAH expression seemed to be far superior to that of Ki67 and phospho-ERK in NACT/NST-treated breast cancer[76]. SIAH could be used to risk stratify incomplete responders, identify chemo- and IO-resistance, quantify therapy efficacy, and predict relapse and survival [Figure 2A]. SIAHLow/OFF in TNBC residual tumors reflects that the EGFR/K-RAS/MAPK/SIAH pathway is OFF, and indicates chemo-sensitivity, effective NACT/NST, and good prognosis after surgery [Figure 2B]. SIAHHigh/ON in TNBC residual tumors reflects that the EGFR/K-RAS/MAPK/SIAH pathway is ON, which indicates chemo-resistance, ineffective NACT/NST, and/or the need for additional adjuvant therapies to prevent progressive disease and early tumor relapse [Figure 2C]. Our studies have demonstrated that SIAH offers a tumor-specific, therapy-responsive, and prognostic biomarker in TNBC with high molecular precision and full dynamic range (0%-100%)[76,89]. Therefore, SIAHON/OFF expression can be used to identify chemoresistant tumor clones, differentiate partial responders, forecast tumor relapse, and predict patient survival in NACT-treated breast cancer[76,89]. Moreover, we have shown that SIAHON/OFF expression is a direct readout of EGFR/K-RAS/MAPK pathway activation (ON)/inactivation (OFF)[3,76,77,84,85]. Hence, studying the “ON/OFF” of the major tumor-driving EGFR/K-RAS/MAPK/SIAH pathway may represent an opportunity to risk-stratify TNBC patients before and after NACT/NST (IO-therapy). As a binary biomarker with high tumor specificity and detection sensitivity as well as a full dynamic range (0%-100%), we propose to validate that SIAH is a powerful new prognostic biomarker that can be used to risk-stratify patients, detection of chemo-resistance, quantify NACT/NST efficacy, forecast early tumor relapse, and predict patient survival in TNBC[72,76,77,82,83].
Figure 2.
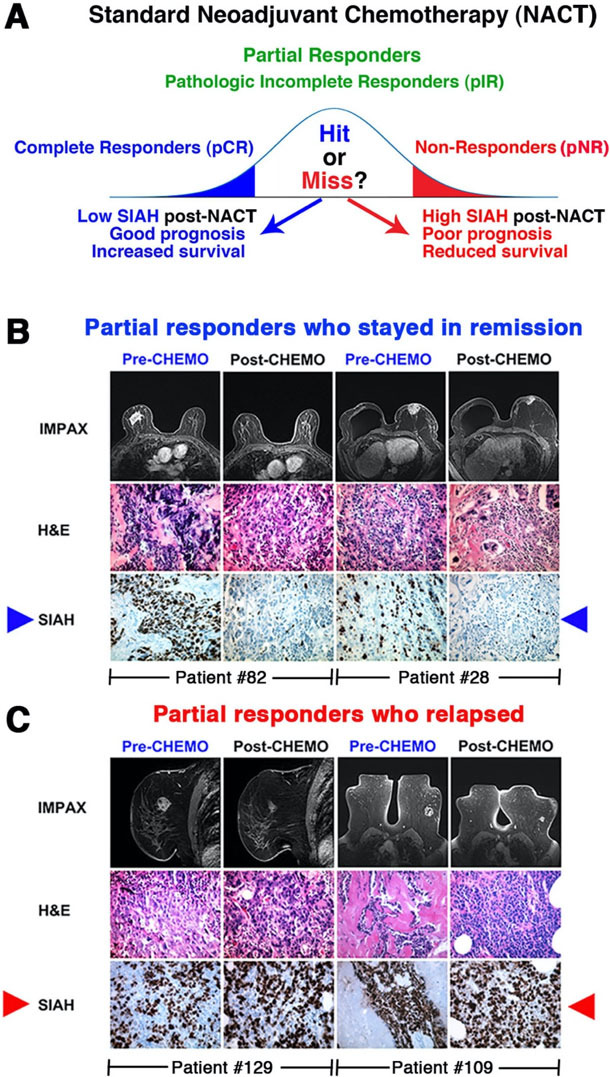
SIAH marks proliferating tumor cells at a single-cell resolution, and SIAH is a therapy-responsive and prognostic biomarker that can be used to risk-stratify incomplete responders in NACT/NST-treated high-risk and locally advanced breast cancer. IMPAX stands for the IMPAX digital mammography, a powerful diagnostic platform for breast imaging at the Sentara Breast Centers. (A) pCR is a good prognostic marker associated with long-term survival post-NACT/NST. However, for the pIR patients with residual disease, additional tools are needed to distinguish which patients are at high risk for early tumor relapse and thus who may need additional adjuvant chemotherapies. (B) The pIR patients with no or low SIAH expression in residual tumors post-NACT/NST stayed in remission and have prolonged survival (examples: patients #82 and #28). (C) In contrast, the pIR patients with persistent high SIAH expression in residual disease post-NACT/NST developed early relapse and succumbed to their chemoresistant and metastatic diseases (examples: patients #109 and #129). Conclusion: We propose that persistent high SIAH expression in residual tumors is associated with early tumor relapse and poor prognosis, while no or low SIAH expression in residual tumors is associated with tumor remission and good prognosis post-NACT/NST. SIAH: Human homologs of Drosophila Seven In Absentia (SINA); Pir: pathologic incomplete response; NACT: neoadjuvant chemotherapy; NST: neoadjuvant systemic therapy; PCR: athologic complete response.
CONCLUSION
Focusing on EGFR/K-RAS/MAPK/SIAH pathway activation as a major tumor driver in TNBC to risk-stratify patients and detect chemo-resistance may represent a significant step forward. In our pilot study, we found that all TNBC primary tumors are highly proliferative, heavily decorated with SIAH, and the median SIAH expression in untreated TNBC tumors was 70% in both lymph node (LN) positive and LN-negative subsets[76]. In contrast, 30% of patients with residual disease displayed persistent high SIAH expression and had high relapse rates and poor outcomes[76]. As a tumor-specific, therapy-responsive, and prognostic biomarker in TNBC, SIAH has a full dynamic range, high sensitivity, high specificity, and molecular precision and could be used to risk-stratify patients and detect chemoresistant tumor cells in the first-line neoadjuvant settings. Ultimately, we hope to translate our findings (SIAH as a new prognostic biomarker) for clinical use to facilitate early detection of ineffective therapy in the first-line neoadjuvant setting, detect chemoresistant tumor cells at a single tumor cell resolution, augment RCB I-II-III classifications with high-precision, accurately calculate the risk of early tumor relapse and predict long-term survival in TNBC. If successful, we can use this new, dynamic, therapy-responsive, interactive, and tumor-specific biomarker, SIAH, to address the unmet need of identifying chemoresistant TNBC, and risk-stratify pIR patients by tumor relapse and poor prognosis in the first-line neoadjuvant setting. By leveraging the tumor-driving EGFR/K-RAS/SIAH pathway activation (ON)/inactivation (OFF) in TNBC, we hope to differentiate TNBC pIR patients by correlating SIAHHigh (high-risk) versus SIAHLow (low-risk) expression in residual tumors post-NACT/NST. This precision biomarker may also be used to detect treatment disparity amongst incomplete responders, forecast early tumor relapse, and predict survival. The next steps should include the successful executions of several independent large-scale multicenter biomarker validation studies leading to FDA approval of SIAH as a new tumor-specific, therapy-responsive, and prognostic biomarker for TNBC risk stratification, detection of chemo-resistance, therapy quantification in real time, treatment optimization in the clinic. Importantly, SIAH is a strategically positioned cancer target for us to develop a new anti-TNBC targeted therapy to eradicate multidrug-resistant, undruggable, and incurable TNBC malignancy in the future.
DECLARATIONS
Acknowledgements
Correspondence should be addressed to A.H.T. The authors thank Mrs. Jennie Capps, Mrs. Linda Church, and Mrs. Cheryl McLeskey at the Chesapeake Bay Wine Classic Foundation (CBWCF); Dr. Judith Salerno, Mrs. Sharon Laderberg, and Mrs. Miki Donovan at the Susan G. Komen Foundation for their staunch support, fundraising efforts, and kind encouragements. The authors thank Elizabeth A. Harden, M.D., and the medical and scientific advisory board of the Dorothy G. Hoefer Foundation for Breast Cancer for identifying and financially supporting this project at its inception. The authors thank the Sentara-EVMS-VOA-VCU top leadership for their support. The authors thank our colleagues at the Institutional Review Boards (IRB) for supporting our clinical research endeavors at Sentara-EVMS-VOA. The authors dedicate this concept paper and clinical review to our brave TNBC patients, their loving families, our dedicated surgeons, oncologists, pathologists, radiologists, and the outstanding Sentara-EVMS-VOA-VCU Massey Cancer Center breast cancer teams who are fighting multidrug-resistant, recurrent, and metastatic diseases in Virginia.
Authors’ contributions
Formulated the original idea, novel concept, central hypothesis, data collection, experimental execution, designed the figures and wrote the early drafts: Tang AH
Provided the seed money and visionary leadership to support this study since its conception: Hoefer RA
Advised, guided, contributed, and supported this original idea, novel concept, and large-scale biomarker validation studies: Hoefer RA, Guye ML, Bear HD
All authors have met the four criteria of the authorship requirements as listed by the ICMJE.
All authors have made important contribution, improved the intellectual content, and added their scientific and clinical expertise to strengthen, augment, and support this work.
All authors have read, edited, and approved the finalized manuscript for publication.
All authors have contributed to writing, editing, revising, proofreading, and rewriting of this manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by DOD-BCRP Level II Breakthrough Award (BC180907 to A.H.T.), National Institutes of Health National Cancer Institute (R01 CA140550 to A.H.T.), the Center for Innovative Technology (CIT) - Commonwealth Research Commercialization Fund (CRCF) (MF14S-009-LS to A.H.T.), and Dorothy G. Hoefer Foundation (Breast Cancer Grant to A.H.T.). As the corresponding author and the principal investigator, AHT has the full responsibility in making the decision to submit this review article for publication with the consultation, support and agreements of all the co-authors. None of authors have been paid to write this article by a pharmaceutical company or other federal, state and local funding agencies and foundations. The statement declaring that the funding agency and supporting source had no involvement in making any publication decisions in here.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Active IRB approval: The Institutional Review Boards (IRB) approval is in place to conduct this study.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
References
- 1.Mahtani R, Kittaneh M, Kalinsky K, et al. Breast Cancer Therapy Expert Group (BCTEG) Advances in therapeutic approaches for triple-negative breast cancer. Clin Breast Cancer. 2021;21:383–90. doi: 10.1016/j.clbc.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8–16. doi: 10.1097/PPO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 3.Gupta GK, Collier AL, Lee D, et al. Perspectives on triple-negative breast cancer: current treatment strategies, unmet needs, and potential targets for future therapies. Cancers (Basel) 2020;12:2392. doi: 10.3390/cancers12092392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo SP, Wu QS, Chen H, et al. Validation of the prognostic significance of the prognostic stage group according to the eighth edition of american cancer joint committee on cancer staging system in triple-negative breast cancer: an analysis from surveillance, epidemiology, and end results 18 database. J Surg Res. 2020;247:211–9. doi: 10.1016/j.jss.2019.09.072. [DOI] [PubMed] [Google Scholar]
- 5.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 6.Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/black people 2022. CA Cancer J Clin. 2022;72:202–29. doi: 10.3322/caac.21718. [DOI] [PubMed] [Google Scholar]
- 7.Nwagu GC, Bhattarai S, Swahn M, Ahmed S, Aneja R. Prevalence and mortality of triple-negative breast cancer in west africa: biologic and sociocultural factors. JCO Glob Oncol. 2021;7:1129–40. doi: 10.1200/GO.21.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69:211–33. doi: 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 9.Newman LA, Jenkins B, Chen Y, et al. Hereditary susceptibility for triple negative breast cancer associated with western sub-saharan african ancestry: results from an international surgical breast cancer collaborative. Ann Surg. 2019;270:484–92. doi: 10.1097/SLA.0000000000003459. [DOI] [PubMed] [Google Scholar]
- 10.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–90. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23 Suppl 6:vi7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 12.Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2011;125:627–36. doi: 10.1007/s10549-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–92. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 14.Scott LC, Mobley LR, Kuo TM, Il'yasova D. Update on triple-negative breast cancer disparities for the United States: a population-based study from the United States Cancer Statistics database, 2010 through 2014. Cancer. 2019;125:3412–7. doi: 10.1002/cncr.32207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas A, Rhoads A, Pinkerton E, et al. Incidence and survival among young women with stage I-III breast cancer: SEER 2000-2015. JNCI Cancer Spectr. 2019;3:pkz040. doi: 10.1093/jncics/pkz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the united states. Cancer Epidemiol Biomarkers Prev. 2018;27:619–26. doi: 10.1158/1055-9965.EPI-17-0627. [DOI] [PubMed] [Google Scholar]
- 17.Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in african american women: a review. JAMA Surg. 2017;152:485–93. doi: 10.1001/jamasurg.2017.0005. [DOI] [PubMed] [Google Scholar]
- 18.Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12:381–94. doi: 10.1038/nrclinonc.2015.73. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder MC, Rastogi P, Geyer CE Jr, Miller LD, Thomas A. Early and locally advanced metaplastic breast cancer: presentation and survival by receptor status in surveillance, epidemiology, and end results (SEER) 2010-2014. Oncologist. 2018;23:481–8. doi: 10.1634/theoncologist.2017-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreopoulou E, Schweber SJ, Sparano JA, McDaid HM. Therapies for triple negative breast cancer. Expert Opin Pharmacother. 2015;16:983–98. doi: 10.1517/14656566.2015.1032246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy WR, Tricarico C, Gabani P, et al. Predictors of distant metastases in triple-negative breast cancer without pathologic complete response after neoadjuvant chemotherapy. J Natl Compr Canc Netw. 2020;18:288–96. doi: 10.6004/jnccn.2019.7366. [DOI] [PubMed] [Google Scholar]
- 22.Savard MF, Khan O, Hunt KK, Verma S. Redrawing the lines: the next generation of treatment in metastatic breast cancer. Am Soc Clin Oncol Educ Book. 2019;39:e8–e21. doi: 10.1200/EDBK_237419. [DOI] [PubMed] [Google Scholar]
- 23.Gabani P, Merfeld E, Srivastava AJ, et al. Predictors of locoregional recurrence after failure to achieve pathologic complete response to neoadjuvant chemotherapy in triple-negative breast cancer. J Natl Compr Canc Netw. 2019;17:348–56. doi: 10.6004/jnccn.2018.7103. [DOI] [PubMed] [Google Scholar]
- 24.Gradishar WJ, Moran MS, Abraham J, et al. NCCN Guidelines Insights: Breast Cancer, Version 4.2021. J Natl Compr Canc Netw. 2021;19:484–93. doi: 10.6004/jnccn.2021.0023. [DOI] [PubMed] [Google Scholar]
- 25.Nedeljković M, Damjanović A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells. 2019;8:957. doi: 10.3390/cells8090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciriello G, Gatza ML, Beck AH, et al. TCGA Research Network. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–19. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isakoff SJ, Mayer EL, He L, et al. TBCRC009: A multicenter phase ii clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902–9. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreopoulou E, Kelly CM, McDaid HM. Therapeutic advances and new directions for triple-negative breast cancer. Breast Care (Basel) 2017;12:21–8. doi: 10.1159/000455821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savas P, Loi S. Expanding the role for immunotherapy in triple-negative breast cancer. Cancer Cell. 2020;37:623–4. doi: 10.1016/j.ccell.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Killock D. Chemotherapy as a TONIC to invigorate PD-1 inhibition in TNBC. Nat Rev Clin Oncol. 2019;16:464. doi: 10.1038/s41571-019-0232-2. [DOI] [PubMed] [Google Scholar]
- 32.Sidaway P. Setting dictates efficacy of pembrolizumab in TNBC. Nat Rev Clin Oncol. 2019;16:66. doi: 10.1038/s41571-018-0157-1. [DOI] [PubMed] [Google Scholar]
- 33.Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 34.Adams S, Diéras V, Barrios CH, et al. Patient-reported outcomes from the phase III IMpassion130 trial of atezolizumab plus nab-paclitaxel in metastatic triple-negative breast cancer. Ann Oncol. 2020;31:582–9. doi: 10.1016/j.annonc.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Schmid P, Cortes J, Pusztai L, et al. KEYNOTE-522 Investigators. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–21. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 36.Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6:676–84. doi: 10.1001/jamaoncol.2019.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid P, Cortes J, Dent R, et al. KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556–67. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 38.Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. The Lancet. 2020;396:1817–28. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 40.Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35:1049–60. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 42.Weiss A, Campbell J, Ballman KV, et al. Factors associated with nodal pathologic complete response among breast cancer patients treated with neoadjuvant chemotherapy: results of CALGB 40601 (HER2+) and 40603 (Triple-Negative) (Alliance) Ann Surg Oncol. 2021;28:5960–71. doi: 10.1245/s10434-021-09897-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeVasseur N, Sun J, Gondara L, et al. Impact of pathologic complete response on survival after neoadjuvant chemotherapy in early-stage breast cancer: a population-based analysis. J Cancer Res Clin Oncol. 2020;146:529–36. doi: 10.1007/s00432-019-03083-y. [DOI] [PubMed] [Google Scholar]
- 44.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet. 2014;384:164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 45.Wu K, Yang Q, Liu Y, Wu A, Yang Z. Meta-analysis on the association between pathologic complete response and triple-negative breast cancer after neoadjuvant chemotherapy. World J Surg Oncol. 2014;12:95. doi: 10.1186/1477-7819-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biswas T, Efird JT, Prasad S, Jindal C, Walker PR. The survival benefit of neoadjuvant chemotherapy and pCR among patients with advanced stage triple negative breast cancer. Oncotarget. 2017;8:112712–9. doi: 10.18632/oncotarget.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–9. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Symmans WF, Yau C, Chen YY, et al. Assessment of residual cancer burden and event-free survival in neoadjuvant treatment for high-risk breast cancer: an analysis of data from the I-SPY2 randomized clinical trial. JAMA Oncol. 2021;7:1654–63. doi: 10.1001/jamaoncol.2021.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Echeverria GV, Ge Z, Seth S, et al. Resistance to neoadjuvant chemotherapy in triple-negative breast cancer mediated by a reversible drug-tolerant state. Sci Transl Med. 2019;11:eaav0936. doi: 10.1126/scitranslmed.aav0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carbognin L, Furlanetto J, Vicentini C, et al. Neoadjuvant strategies for triple negative breast cancer: ‘state-of-the-art’ and future perspectives. Anticancer Agents Med Chem. 2015;15:15–25. doi: 10.2174/1871520614666141019191616. [DOI] [PubMed] [Google Scholar]
- 51.Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer. 2015;121:2544–52. doi: 10.1002/cncr.29348. [DOI] [PubMed] [Google Scholar]
- 52.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–59. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 53.Gradishar WJ, Anderson BO, Abraham J, et al. Breast Cancer, Version 3.2020, NCCN clinical practice guidelines in Oncology. J Natl Compr Canc Netw. 2020;18:452–78. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Zhou Y, Mao F, et al. Adjuvant addition of capecitabine to early-stage triple-negative breast cancer patients receiving standard chemotherapy: a meta-analysis. Breast Cancer Res Treat. 2020;179:533–42. doi: 10.1007/s10549-019-05513-4. [DOI] [PubMed] [Google Scholar]
- 55.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–51. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 56.Carey LA, Winer EP. I-SPY 2--toward more rapid progress in breast cancer treatment. N Engl J Med. 2016;375:83–4. doi: 10.1056/nejme1603691. [DOI] [PubMed] [Google Scholar]
- 57.Zong Y, Pegram M. Research advances and new challenges in overcoming triple-negative breast cancer. Cancer Drug Resist. 2021;4:517–42. doi: 10.20517/cdr.2021.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Reilly D, Sendi MA, Kelly CM. Overview of recent advances in metastatic triple negative breast cancer. World J Clin Oncol. 2021;12:164–82. doi: 10.5306/wjco.v12.i3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konner M. Progress in the treatment of breast cancer. N Engl J Med. 2020;382:e4. doi: 10.1056/NEJMc1915045. [DOI] [PubMed] [Google Scholar]
- 60.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 61.Cao J, Zhang M, Wang B, Zhang L, Zhou F, Fang M. Chemoresistance and metastasis in breast cancer molecular mechanisms and novel clinical strategies. Front Oncol. 2021;11:658552. doi: 10.3389/fonc.2021.658552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marra A, Curigliano G. Adjuvant and neoadjuvant treatment of triple-negative breast cancer with chemotherapy. Cancer J. 2021;27:41–9. doi: 10.1097/PPO.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 63.Kumar S, Nandi A, Singh S, et al. Dll1+ quiescent tumor stem cells drive chemoresistance in breast cancer through NF-κB survival pathway. Nat Commun. 2021;12:432. doi: 10.1038/s41467-020-20664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu SZ, Roden DL, Wang C, et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020;39:e104063. doi: 10.15252/embj.2019104063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cazet AS, Hui MN, Elsworth BL, et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat Commun. 2018;9:2897. doi: 10.1038/s41467-018-05220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kabraji S, Solé X, Huang Y, et al. AKT1low quiescent cancer cells persist after neoadjuvant chemotherapy in triple negative breast cancer. Breast Cancer Res. 2017;19:88. doi: 10.1186/s13058-017-0877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Chen H, Mo H, et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell. 2021;39:1578–1593.e8. doi: 10.1016/j.ccell.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 68.Chakrabarty A, Chakraborty S, Bhattacharya R, Chowdhury G. Senescence-induced chemoresistance in triple negative breast cancer and evolution-based treatment strategies. Front Oncol. 2021;11:674354. doi: 10.3389/fonc.2021.674354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kvokačková B, Remšík J, Jolly MK, Souček K. Phenotypic heterogeneity of triple-negative breast cancer mediated by epithelial-mesenchymal plasticity. Cancers (Basel) 2021;13:2188. doi: 10.3390/cancers13092188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marra A, Trapani D, Viale G, Criscitiello C, Curigliano G. Practical classification of triple-negative breast cancer: intratumoral heterogeneity, mechanisms of drug resistance, and novel therapies. NPJ Breast Cancer. 2020;6:54. doi: 10.1038/s41523-020-00197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim C, Gao R, Sei E, et al. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell. 2018;173:879–893.e13. doi: 10.1016/j.cell.2018.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta G, Lee CD, Guye ML, et al. Unmet clinical need: developing prognostic biomarkers and precision medicine to forecast early tumor relapse, detect chemo-resistance and improve overall survival in high-risk breast cancer. Ann Breast Cancer Ther. 2020;4:48–57. doi: 10.36959/739/525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dias Carvalho P, Guimarães CF, Cardoso AP, et al. KRAS oncogenic signaling extends beyond cancer cells to orchestrate the microenvironment. Cancer Res. 2018;78:7–14. doi: 10.1158/0008-5472.CAN-17-2084. [DOI] [PubMed] [Google Scholar]
- 74.Loi S, Dushyanthen S, Beavis PA, et al. RAS/MAPK Activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res. 2016;22:1499–509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang W, Wang X, Zhang C, Xue L, Yang L. Expression and clinical significance of MAPK and EGFR in triple-negative breast cancer. Oncol Lett. 2020;19:1842–8. doi: 10.3892/ol.2020.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Reesema LLS, Zheleva V, Winston JS, et al. SIAH and EGFR, two RAS pathway biomarkers, are highly prognostic in locally advanced and metastatic breast cancer. EBioMedicine. 2016;11:183–98. doi: 10.1016/j.ebiom.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Sciver RE, Njogu MM, Isbell AJ, et al. Blocking SIAH proteolysis, an important K-RAS vulnerability, to control and eradicate K-RAS-driven metastatic cancer. In: Azmi AS, editor Conquering RAS : from biology to cancer therapy. Amsterdam ; Boston: Elsevier/AP, Academic Press is an imprint of Elsevier. 2016. [DOI] [Google Scholar]
- 78.Wright KL, Adams JR, Liu JC, et al. Ras signaling is a key determinant for metastatic dissemination and poor survival of luminal breast cancer patients. Cancer Res. 2015;75:4960–72. doi: 10.1158/0008-5472.CAN-14-2992. [DOI] [PubMed] [Google Scholar]
- 79.Simon MA, Carthew RW, Fortini ME, Gaul U, Mardon G, Rubin GM. Signal transduction pathway initiated by activation of the sevenless tyrosine kinase receptor. Cold Spring Harb Symp Quant Biol. 1992;57:375–80. doi: 10.1101/sqb.1992.057.01.042. [DOI] [PubMed] [Google Scholar]
- 80.Zipursky SL, Rubin GM. Determination of neuronal cell fate: lessons from the R7 neuron of Drosophila. Annu Rev Neurosci. 1994;17:373–97. doi: 10.1146/annurev.ne.17.030194.002105. [DOI] [PubMed] [Google Scholar]
- 81.Zipursky SL, Rubin GM. Determination of neuronal cell fate: lessons from the R7 neuron of Drosophila. Annu Rev Neurosci. 1994;17:373–97. doi: 10.1146/annurev.ne.17.030194.002105. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt RL, Park CH, Ahmed AU, et al. Inhibition of RAS-mediated transformation and tumorigenesis by targeting the downstream E3 ubiquitin ligase seven in absentia homologue. Cancer Res. 2007;67:11798–810. doi: 10.1158/0008-5472.CAN-06-4471. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed AU, Schmidt RL, Park CH, et al. Effect of disrupting seven-in-absentia homolog 2 function on lung cancer cell growth. J Natl Cancer Inst. 2008;100:1606–29. doi: 10.1093/jnci/djn365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pepper IJ, Van Sciver RE, Tang AH. Phylogenetic analysis of the SINA/SIAH ubiquitin E3 ligase family in Metazoa. BMC Evol Biol. 2017;17:182. doi: 10.1186/s12862-017-1024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Sciver RE, Lee MP, Lee CD, et al. A new strategy to control and eradicate “undruggable” oncogenic k-ras-driven pancreatic cancer: molecular insights and core principles learned from developmental and evolutionary biology. Cancers (Basel) 2018;10:142. doi: 10.3390/cancers10050142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang AH, Neufeld TP, Kwan E, Rubin GM. PHYL acts to down-regulate TTK88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell. 1997;90:459–67. doi: 10.1016/s0092-8674(00)80506-1. [DOI] [PubMed] [Google Scholar]
- 87.Hu G, Chung YL, Glover T, Valentine V, Look AT, Fearon ER. Characterization of human homologs of the Drosophila seven in absentia (sina) gene. Genomics. 1997;46:103–11. doi: 10.1006/geno.1997.4997. [DOI] [PubMed] [Google Scholar]
- 88.Carthew RW, Rubin GM. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990;63:561–77. doi: 10.1016/0092-8674(90)90452-k. [DOI] [PubMed] [Google Scholar]
- 89.Siewertsz van Reesema LL, Lee MP, Zheleva V et al. RAS pathway biomarkers for breast cancer prognosis. Clin Lab Int. 2016;40:18–23. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


