Abstract
Background
Alveolar osteitis (dry socket) is a complication of dental extractions more often involving mandibular molar teeth. It is associated with severe pain developing 2 to 3 days postoperatively with or without halitosis, a socket that may be partially or totally devoid of a blood clot, and increased postoperative visits. This is an update of the Cochrane Review first published in 2012.
Objectives
To assess the effects of local interventions used for the prevention and treatment of alveolar osteitis (dry socket) following tooth extraction.
Search methods
An Information Specialist searched four bibliographic databases up to 28 September 2021 and used additional search methods to identify published, unpublished, and ongoing studies.
Selection criteria
We included randomised controlled trials of adults over 18 years of age who were having permanent teeth extracted or who had developed dry socket postextraction. We included studies with any type of local intervention used for the prevention or treatment of dry socket, compared to a different local intervention, placebo or no treatment. We excluded studies reporting on systemic use of antibiotics or the use of surgical techniques because these interventions are evaluated in separate Cochrane Reviews.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We followed Cochrane statistical guidelines and reported dichotomous outcomes as risk ratios (RR) and calculated 95% confidence intervals (CI) using random‐effects models. For some of the split‐mouth studies with sparse data, it was not possible to calculate RR so we calculated the exact odds ratio (OR) instead. We used GRADE to assess the certainty of the body of evidence.
Main results
We included 49 trials with 6771 participants; 39 trials (with 6219 participants) investigated prevention of dry socket and 10 studies (with 552 participants) looked at the treatment of dry socket. 16 studies were at high risk of bias, 30 studies at unclear risk of bias, and 3 studies at low risk of bias.
Chlorhexidine in the prevention of dry socket
When compared to placebo, rinsing with chlorhexidine mouthrinses (0.12% and 0.2% concentrations) both before and 24 hours after extraction(s) substantially reduced the risk of developing dry socket with an OR of 0.38 (95% CI 0.25 to 0.58; P < 0.00001; 6 trials, 1547 participants; moderate‐certainty evidence). The prevalence of dry socket varies from 1% to 5% in routine dental extractions to upwards of 30% in surgically extracted third molars. The number of patients needed to be treated (NNT) with chlorhexidine rinse to prevent one patient having dry socket was 162 (95% CI 155 to 240), 33 (95% CI 27 to 49), and 7 (95% CI 5 to 10) for control prevalence of dry socket 0.01, 0.05, and 0.30 respectively.
Compared to placebo, placing chlorhexidine gel intrasocket after extractions reduced the odds of developing a dry socket by 58% with an OR of 0.44 (95% CI 0.27 to 0.71; P = 0.0008; 7 trials, 753 participants; moderate‐certainty evidence). The NNT with chlorhexidine gel (0.2%) to prevent one patient developing dry socket was 180 (95% CI 137 to 347), 37 (95% CI 28 to 72), and 7 (95% CI 5 to 15) for control prevalence of dry socket of 0.01, 0.05, and 0.30 respectively.
Compared to chlorhexidine rinse (0.12%), placing chlorhexidine gel (0.2%) intrasocket after extractions was not superior in reducing the risk of dry socket (RR 0.74, 95% CI 0.46 to 1.20; P = 0.22; 2 trials, 383 participants; low‐certainty evidence).
The present review found some evidence for the association of minor adverse reactions with use of 0.12%, 0.2% chlorhexidine mouthrinses (alteration in taste, staining of teeth, stomatitis) though most studies were not designed explicitly to detect the presence of hypersensitivity reactions to mouthwash as part of the study protocol. No adverse events were reported in relation to the use of 0.2% chlorhexidine gel placed directly into a socket.
Platelet rich plasma in the prevention of dry socket
Compared to placebo, placing platelet rich plasma after extractions was not superior in reducing the risk of having a dry socket (RR 0.51, 95% CI 0.19 to 1.33; P = 0.17; 2 studies, 127 participants; very low‐certainty evidence).
A further 21 intrasocket interventions to prevent dry socket were each evaluated in single studies, and there is insufficient evidence to determine their effects.
Zinc oxide eugenol versus Alvogyl in the treatment of dry socket
Two studies, with 80 participants, showed that Alvogyl (old formulation) is more effective than zinc oxide eugenol at reducing pain at day 7 (mean difference (MD) ‐1.40, 95% CI ‐1.75 to ‐1.04; P < 0.00001; 2 studies, 80 participants; very low‐certainty evidence)
A further nine interventions for the treatment of dry socket were evaluated in single studies, providing insufficient evidence to determine their effects.
Authors' conclusions
Tooth extractions are generally undertaken by dentists for a variety of reasons, however, all but five studies included in the present review included participants undergoing extraction of third molars, most of which were undertaken by oral surgeons. There is moderate‐certainty evidence that rinsing with chlorhexidine (0.12% and 0.2%) or placing chlorhexidine gel (0.2%) in the sockets of extracted teeth, probably results in a reduction in dry socket. There was insufficient evidence to determine the effects of the other 21 preventative interventions each evaluated in single studies. There was limited evidence of very low certainty that Alvogyl (old formulation) may reduce pain at day 7 in patients with dry socket when compared to zinc oxide eugenol.
Keywords: Adolescent, Adult, Humans, Anti-Bacterial Agents, Anti-Bacterial Agents/therapeutic use, Chlorhexidine, Chlorhexidine/therapeutic use, Dry Socket, Dry Socket/etiology, Dry Socket/prevention & control, Eugenol, Mouthwashes, Mouthwashes/therapeutic use, Pain, Pain/drug therapy, Zinc Oxide
Plain language summary
What treatments can be used to prevent and treat alveolar osteitis (dry socket)?
Key messages
‐ Rinsing with chlorhexidine mouthwash before a dental extraction or beginning 24 hours after may help to prevent a dry socket. ‐ Placing a chlorhexidine gel directly into the socket immediately after tooth extraction may help to prevent a dry socket. ‐ Chlorhexidine rinses cause some minor adverse (unwanted) effects; chlorhexidine intrasocket gels do not appear to cause adverse effects. ‐ Alvogyl reduces the pain of a dry socket when compared to zinc oxide eugenol, but the evidence for this is very uncertain. ‐ Alvogyl does not appear to cause unwanted effects. ‐ We need future studies to strengthen the evidence and investigate the best ways to prevent and treat dry socket in all teeth.
What is dry socket?
Dry socket is a painful condition that sometimes arises after a tooth has been extracted and is more likely to occur following extraction of wisdom teeth in the lower jaw.
What causes dry socket?
It is thought to be linked to the loss of some or all of the blood clot that forms at the bottom of a tooth socket after a tooth is taken out.
How can we prevent dry socket?
An option for prevention of dry socket is to reduce debris and the bacterial load in the mouth, though dry socket is not bacterial in origin. People with poor oral hygiene (food debris and plaque) are at greater risk of dry socket. Improved oral hygiene and rinsing before a dental extraction or beginning 24 hours after may reduce the likelihood of dry socket.
How can we treat dry socket ?
Options for treating dry socket largely focus on reducing pain locally around the tooth extraction site by placing an obtundent (a soothing medicated dressing).
What did we want to find out ?
We wanted to find out if antiseptic rinses, gels, or healing patches could help to prevent dry socket. We also wanted to find out if placing a medicated dressing could treat a dry socket and whether any unwanted side effects were produced.
What did we do?
We searched for studies that compared antiseptic rinses or intrasocket gels with a placebo (dummy) rinse or nothing and a placebo (dummy) intrasocket gel or nothing.
To find the best way to treat dry socket we searched for studies that compared different soothing agents with a placebo (dummy) with other soothing agents or with nothing.
We compared the results of the studies and summarised the findings. We made an assessment of our confidence in the evidence based on the design of the study and the number of patients recruited.
What did we find?
We identified 49 trials; 39 trials (6219 participants) investigated prevention of dry socket and 10 trials (552 participants) investigated the treatment of dry socket.
We found that:
‐ rinsing both before and after tooth extraction (commencing 24 hours after extraction) with chlorhexidine gluconate rinse (at 0.12% and 0.2% strength) probably results in a reduction in dry socket; ‐ placing chlorhexidine gel (0.2% strength) in the socket of an extracted tooth probably results in a reduction in dry socket; ‐ chlorhexidine rinses and gels are equally effective at reducing dry socket, but the evidence for this comparison is very uncertain; ‐ chlorhexidine rinses produced some minor unwanted effects; chlorhexidine intrasocket gels appeared to produce no unwanted effects; ‐ there was a small amount of evidence of very low certainty from two studies that Alvogyl (old formulation) may reduce pain at day 7 in patients with dry socket when compared to zinc oxide eugenol. This evidence relates to the old formulation of Alvogyl which is no longer available. It should be noted that the formulation of Alvogyl has changed, it is now called Alveogyl.
Tooth extractions are generally undertaken by dentists for a variety of reasons, however, all but five studies included in the present review included participants undergoing extraction of third molars, most of which were undertaken by oral surgeons.
What are the limitations of the evidence?
The main limitation of the evidence are that studies:
‐ reported mostly on tooth extractions involving lower wisdom teeth; ‐ were undertaken in ways that introduced errors in the conduct of the study leading to errors in the results; and ‐ produced imprecise results when they were combined.
Due to these errors we have some confidence in the results relating to chlorhexidine rinses and gels but further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
How up to date is this evidence?
The search for existing studies was completed by 28 September 2021.
Summary of findings
Summary of findings 1. Chlorhexidine rinse versus placebo/no treatment for the prevention of dry socket.
| Chlorhexidine rinse versus placebo/no treatment for the prevention of dry socket | ||||||
|
Patient or population: patients having a tooth extraction Setting: primary or secondary care/home use Intervention: chlorhexidine rinse Comparison: placebo/no treatment control | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment controlb | Chlorhexidine rinse | |||||
|
Presence of dry socket
(agreed diagnostic criteria.
Follow‐up: median 7 days) |
Study population |
OR 0.38 (0.25 to 0.58) |
1547 (6 studies) |
⊕⊕⊕⊝
moderatea |
‐ | |
| Low | ||||||
| 10 per 1000 | 4 per 1000 (3 to 6) | |||||
| Moderate | ||||||
| 50 per 1000 | 20 per 1000 (13 to 30) | |||||
| High | ||||||
| 300 per 1000 |
140 per 1000 (97 to 199) |
|||||
| Adverse events | 3 trials reported no adverse events. 1 study reported adverse events (alterations in taste, bad taste, staining) in 24/62 in chlorhexidine group versus 0/40 in placebo group, Peto OR = 8.42 (95% CI 3.31 to 21.39). A further study reported adverse events (bad taste, stomach upset) in 4/40 in chlorhexidine group versus 0/40 in placebo group, Peto OR = 20.87 (95% CI 8.38 to 52.02). Another study reported many adverse events in both experimental and placebo groups but only attributes 1 (stomatitis) to use of chlorhexidine | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded once as 3 trials were assessed as being at high risk of bias and 1 at unclear risk of bias. bThe assumed risk values were set to reflect prevalence rates for routine dental extractions (1% and 5%) and for extraction of mandibular third molars (30%).
Summary of findings 2. Chlorhexidine gel versus placebo/no treatment for the prevention of dry socket.
| Chlorhexidine gel versus placebo/no treatment for the prevention of dry socket | ||||||
|
Patient or population: patients having a tooth extraction Setting: primary or secondary care/home use Intervention: chlorhexidine gel Comparison: placebo/no treatment control | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed riskb | Corresponding risk | |||||
| Placebo/no treatment control | Chlorhexidine gel | |||||
|
Presence of dry socket
(agreed diagnostic criteria.
Follow‐up: median 7 days) |
Study population |
OR 0.44
(0.27 to 0.71) |
753
(7 studies) |
⊕⊕⊕⊝
moderatea |
‐ |
|
| Low | ||||||
| 10 per 1000 | 5 per 1000 (3 to 7) | |||||
| Moderate | ||||||
| 50 per 1000 | 23 per 1000 (14 to 36) | |||||
| High | ||||||
|
300 per 1000 |
159 per 1000 (104 to 234) |
|||||
| Adverse events | No adverse events were reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded once as 2 trials were assessed as being at low risk of bias, 2 at unclear risk, and the other 3 at high risk of bias. bThe assumed risk values were set to reflect prevalence rates for routine dental extractions (1% and 5%) and for extraction of mandibular third molars (30%).
Summary of findings 3. Chlorhexidine gel versus chlorhexidine rinse for the prevention of dry socket.
| Chlorhexidine gel versus chlorhexidine rinse for the prevention of dry socket | ||||||
|
Patient or population: patients having a tooth extraction Setting: primary or secondary care/home use Intervention: chlorhexidine gel Comparison: chlorhexidine rinse | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed riskb | Corresponding risk | |||||
| Chlorhexidine rinse | Chlorhexidine gel | |||||
|
Presence of dry socket
(agreed diagnostic criteria.
Follow‐up: median 7 days) |
Study population |
RR 0.74
(0.46 to 1.20) |
383
(2 studies) |
⊕⊕⊝⊝ lowa | ‐ |
|
| Low | ||||||
| 10 per 1000 | 8 per 1000 (5 to 12) | |||||
| Moderate | ||||||
| 50 per 1000 | 37 per 1000 (23 to 60) | |||||
| High | ||||||
|
300 per 1000 |
222 per 1000 (138 to 360) |
|||||
| Adverse events | No adverse events were reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice: once for 2 studies at high risk of bias and once for imprecision of estimate due to the CI including the possibility of a benefit for both treatment groups. bThe assumed risk values were set to reflect prevalence rates for routine dental extractions (1% and 5%) and for extraction of mandibular third molars (30%).
Summary of findings 4. Platelet rich plasma versus placebo/no treatment for the prevention of dry socket.
| Platelet rich plasma versus placebo/no treatment for the prevention of dry socket | ||||||
|
Patient or population: patients having a tooth extraction Setting: primary or secondary care Intervention: platelet rich plasma Comparison: placebo/no treatment control | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed riskb | Corresponding risk | |||||
| Placebo/no treatment control | Platelet rich plasma | |||||
|
Presence of dry socket
(agreed diagnostic criteria.
Follow‐up: median 7 days) |
Study population |
RR 0.51 (0.19 to 1.33) |
127 (2 studies) |
⊕⊝⊝⊝
very lowa |
‐ |
|
| Low | ||||||
| 10 per 1000 | 6 per 1000 (2 to 14) | |||||
| Moderate | ||||||
| 50 per 1000 | 26 per 1000 (10 to 67) | |||||
| High | ||||||
| 300 per 1000 |
153 per 1000 (57 to 399) |
|||||
| Adverse events | No adverse events were reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 3 times: once for 2 studies at high risk of bias, once for low event rate, and once for the CI including the possibility of benefit for both treatment groups. bThe assumed risk values were set to reflect prevalence rates for routine dental extractions (1% and 5%) and for extraction of mandibular third molars (30%).
Summary of findings 5. Zinc oxide eugenol versus Alvogyl for the treatment of dry socket.
| Zinc oxide eugenol versus Alvogyl for the treatment of dry socket | ||||||
|
Population: adults with dry socket
Setting: primary and secondary care
Intervention: zinc oxide eugenol Comparison: Alvogyl | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Alvogyl | Zinc oxide eugenol | |||||
| Healing | 2 studies used different healing outcomes and we were unable to combine the data | |||||
|
Pain
VAS (0‐10) Follow‐up: 7 days |
The median score in the Alvogyl group was 1.7 | The mean score in the zinc oxide eugenol group was 1.04 higher to 1.75 higher | ‐ | 80
(2 studies) |
⊕⊝⊝⊝ very lowa | This result was inconsistent with the 5‐day data |
| Swelling | No studies reported this outcome | |||||
| Limitation of chewing or swallowing | No studies reported this outcome | |||||
| Fever | No studies reported this outcome | |||||
| Adverse effects | 1 study (Supe 2018) reported 2 patients (8%) in the Alvogyl group and 9 patients (36%) in the zinc oxide eugenol group had delayed healing, classed as non‐healed sockets after 10 days | |||||
| *The basis for the assumed risk is median score. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 3 times as 2 studies at unclear risk of bias, heterogeneity, and differences in the results for day 5 versus day 7.
Background
Teeth are routinely extracted in general dental practice because they are affected by tooth decay or periodontal disease. In spite of generalised overall improvements in oral health, it is estimated that European dentists in general dental practice extract up to seven teeth per week (McCaul 2001). Alveolar osteitis (dry socket) is a complication that may follow tooth extraction, and is known to cause severe pain and repeated dental visits (Noroozi 2009; Veale 2015). The most widely used definition of alveolar osteitis was proposed by Blum, who defined it as postoperative pain in and around an extraction site, which increases in severity between 1 and 3 days after the extraction, accompanied by a partially or totally disintegrated blood clot within the alveolar socket, with or without halitosis (Blum 2002)
Description of the condition
Aetiology and incidence
There is variation in the reported incidence of dry socket; for routine extractions the incidence has been reported to range from 0.5% to 7% (Halabi 2018; Vezeau 2000). For surgical removal of impacted third molars the incidence has been reported to be anywhere from 1% to 37.5% (Caso 2005; Fridrich 1990; Haraji 2013; Oyri 2019; Vezeau 2000). Many studies have discussed risk factors for dry socket, but the exact underlying pathogenesis is not fully understood. There is an acceptance of Birn’s proposed theory of increased localised fibrinolysis leading to clot breakdown within the socket and consequently leading to dry socket (Birn 1973; Blum 2002). But the factors causing fibrinolysis are more ambiguous (Kolokythas 2010). Birn proposed that prolonged trauma or infection of the socket increases localised inflammation within the bone which triggers local release of plasminogen activators (Birn 1973). Nitzan showed in in vitro studies that certain bacteria, in particular Treponema denticola, demonstrate plasmin like fibrinolytic activity and can independently increase fibrinolysis (Nitzan 1983) though this has not been proven clinically. Birn's theory is given weight by many studies which have shown a clear link between prolonged or difficult extractions and an increased incidence of dry socket (Noroozi 2009). Surgical extractions, particularly of third molars, show a significant increase in incidence of dry socket over non‐surgical extractions (Chow 2020). A correlation has also been noted between operator inexperience and an increased incidence of dry socket (Christensen 2012; Larsen 1991). Additionally, bacteria seem to have a potential role to play with the incidence of dry socket shown to increase in patients with poor oral hygiene (Peñarrocha 2001) and pre‐existing pericoronitis (Rud 1970). While differences in the microbial profiles between sockets with dry sockets and normal healing sockets have been shown (Aguilar‐Durán 2019; Shen 2019) the importance and relevance of this is unclear.
Female gender and taking the oral contraceptive pill have been shown to increase incidence of alveolar osteitis, possibly due to the effect of oestrogen on the fibrinolytic system (Cohen 1995; Garcia 2003; Øyri 2020). The relationship between smoking and development of alveolar osteitis is also supported by the literature (Bortoluzzi 2012; Sweet 1978). Disruption of the blood clot through the use of straws is often mentioned, but there is no clear evidence to support this (Bloomer 2012).
Symptoms and diagnosis
Two of the key challenges when conducting this review were the multitude of terminologies used for dry socket and the classification of signs and symptoms that were accepted as determining the presence of dry socket. Dry socket was frequently conflated with an infected socket in some studies. A continuous throbbing pain that radiates to the ear, temple, and neck is the most common symptom of dry socket (Swanson 1989). Classically, this starts 1 to 3 days postextraction and may be accompanied by other signs and symptoms (e.g. foul taste, bad breath, localised swelling, and lymph‐node involvement) (Blum 2002; Chow 2020; Noroozi 2009; Vezeau 2000). The symptoms can persist for up to 10 days after extraction and may include pain so severe that it is not relieved by even the strongest of analgesic medications (Vezeau 2000).
Clinical history and examination are the principal methods of reaching a diagnosis. The clinical picture is of an extraction socket that is visually devoid of a blood clot exposing the bone within the socket, though there may be a greyish debris. But other causative factors for severe postoperative pain should be excluded such as infected retained roots or a surgical site infection (Blum 2002; Kolokythas 2010).
Prevention
There have been a number of theories as to the aetiology of dry socket and a range of preventative agents have been advocated according to the prevailing theory of causation at the time including: plaque control, antiseptic rinses, preoperative systemic antibiotics, and direct placement of medicaments into the socket (Caso 2005; Goldman 1973; Hall 1971; Hedstrom 2007; Kolokythas 2010; Noroozi 2009; Vezeau 2000). Several studies have reported that preoperative and postoperative antiseptic chlorhexidine rinses can be effective in reducing the incidence of dry socket (Berwick 1990; Halabi 2018; Hermesch 1998; Karabit 2019; Larsen 1991; Tjernberg 1979). Other studies have reported on the use of intrasocket antibiotic medicaments (Mitchell 1984; Reekie 2006; Torres‐Lagares 2006a; Trieger 1991; van Eeden 2006), low‐level laser therapy (Shafaee 2020), and intrasocket antifibrinolytic agents (Gersel‐Pedersen 1979; Ritzau 1977). Two Norwegian studies (Akota 1998; Oyri 2019) report significant reductions in the incidence of dry socket when using tetracycline drains postoperatively. Anecdotally, use of such drains are standard practice following removal of wisdom teeth in Norway. The latter studies have not been included in the present review as they do not meet the criteria for an intrasocket intervention and are reported in a separate Cochrane Review (Bailey 2020). Studies have also looked at the use of honey (Abu‐Mostafa 2019), herbal mouthwash (Divya 2019), and gaseous ozone (Ahmedi 2016). More recently the use of autologous platelet concentrates to improve postoperative healing has been advocated (Del Fabbro 2019) in particular platelet rich fibrin (PRF) (Unsal 2018). PRF is a second generation platelet concentrate, which is extracted from plasma through centrifugation of autologous blood, producing a fibrin matrix which contains concentrations of platelets, leucocytes, and growth factors (Unsal 2018). Two systematic reviews have concluded that PRF can reduce the incidence of alveolar osteitis (Xiang 2019; Zhu 2020). Studies have also reported on the use of flap design to minimise trauma and risk of dry socket (Bello 2011; Haraji 2010; Kirk 2007).
The prophylactic use of systemic antibiotics is not generally advocated and there is a consensus that the latter should be reserved for individual patients reporting a history of multiple incidents of dry socket or for the immunocompromised patient (Epstein 2000; Fazakerley 1991; Lodi 2021).
Treatment
Forty‐five per cent of patients with dry socket require multiple postoperative visits, which could have significant consequences for the individual patient as well as societal costs including time off work (Nusair 2007; Vezeau 2000). One study found that patients with dry socket required up to four visits for management of their symptoms (Oyri 2019). Treatment options tend to focus on symptomatic relief, which may include the removal of debris from the socket by irrigation with saline or sterile local anaesthetic, and the use of analgesic medication (Blum 2002). Alternative options include the placement of intrasocket medicaments including antibacterials, topical anaesthetics and obtundents, or combinations of all three (Blum 2002). These intrasocket medications include zinc oxide and eugenol impregnated cotton pellets (Bloomer 2000; Chaurasia 2017), Alvogyl (eugenol, iodoform, and butamben) (Kaya 2011; Supe 2018), Alveogyl (eugenol only), Dentalone, bismuth subnitrate and iodoform paste (BIPP) on ribbon gauze, and metronidazole and lidocaine ointment (Silva 2006). Intrasocket dressings appear to provide an obtundent effect, but there has also been some suggestion that they may cause foreign body reactions and delay healing (Syrjänen 1979). Some studies have also reported the use of lasers for the treatment of dry socket (Jovanović 2011; Kaya 2011). More recently several studies have looked at the use of autologous platelet concentrates, such as plasma rich in growth factors (PRGF) (King 2018) and platelet rich fibrin (Yuce 2019) to promote healing.
It should be noted that Alvogyl (Septodont) has been reformulated and rebranded as Alveogyl (Septodont). The newer version, Alveogyl, no longer contains iodoform (antimicrobial) or butamben (anaesthetic) (Kalsi 2020). It is difficult to establish the exact date when this product was reformulated, but it appears to have been prior to 2014. It is possible that some researchers using this product may have been unaware of the ingredient change as the packaging is almost identical (Kalsi 2020). In view of this, we only included studies in the analysis where the formulation of the product as either Alvogyl or Alveogyl could be confirmed with the authors and matched the timeframe for when these products were in use.
Description of the intervention
Prevention
There are two main approaches taken for prevention of dry socket. One is perioperative rinsing with a variety of mouthwashes from tap water to chlorhexidine and the other involves placement of intrasocket medicaments or autologous platelet concentrates.
Treatment
There are also two approaches taken for treatment of dry socket. One is placement of obtundent or medicated dressings and the other is the use of autologous platelet concentrates.
How the intervention might work
Prevention
There are two main approaches proposed to prevent development of dry socket. The first is to improve oral hygiene and reduce plaque, food debris, and bacterial load around extraction sockets by the use of antiseptic mouthrinses. Most studies focus on chlorhexidine which acts against a broad spectrum of aerobic and anaerobic bacteria, and has been shown to have an immediate bactericidal effect and prolonged bacteriostatic action (Larsen 1991). Chlorhexidine may also be applied to the socket in the form of a bioadhesive gel. Betadine, herbal mouthwashes, honey, and ozone gas have also been variously recommended for their antibacterial properties. Rinsing with tap water has also been suggested.
The second approach is to promote socket healing. Autologous platelet concentrates release growth factors which are thought to improve the healing process. Various types of different tissue patches have also been suggested to support socket healing.
Treatment
Alveolar osteitis is generally managed rather than treated, with pain reduction as the primary aim, though promoting healing and reducing time taken for the patient to return to normal function such as eating and chewing is also important. There are a number of different intrasocket dressings such as zinc oxide eugenol (ZOE), Alvogyl/Alveogyl and Neocone. ZOE and Alvogyl/Alveogyl both contain eugenol which has an obtundent effect. Alvogyl also contains butamben, an anaesthetic and Neocone contains tetracaine. Some intrasocket dressings contain antimicrobial agents such as iodoform (Alvogyl) and polymyxin B (Neocone). The new formulation Alveogyl no longer includes iodoform or butamben (Kalsi 2020). These dressings may also act as a physical barrier against the entry of food debris into the socket.
Autologous platelet concentrates, such as platelet rich growth factor (PRGF), release growth factors that may promote epithelisation of extraction sockets and therefore improve bone coverage. They may also suppress inflammation and have an antimicrobial effect (King 2018). PRGF has been shown to reduce pain scores possibly as a consequence of improved healing, or possibly due to its anti‐inflammatory effects (King 2018).
Why it is important to do this review
Dry socket is a complication of dental extractions that is associated with severe pain and can result in an increase in postoperative visits. Prevention of dry socket as well as the effective management of its sequelae can help in reducing postoperative morbidity for the individual as well as societal costs, for example, lost time from work and healthcare costs. A systematic review of the current best evidence for the effects of the available interventions could help to inform clinical decision‐making for the prevention and management of dry socket.
The review question has previously been identified as clinically important by the public and key stakeholders (Worthington 2015), and is relevant as dental extractions remain an important part of dental practice (McCaul 2001).
This systematic review will summarise the evidence of local interventions for the prevention and management of dry socket. Another Cochrane Review has summarised the evidence of the effects of systemic antibiotics prescribed to prevent infectious complications following tooth extraction and includes dry socket as one of the primary outcomes (Lodi 2021). A further Cochrane Review has evaluated the evidence for surgical techniques (such as surgical drains, wound irrigation and different flap designs) for the removal of mandibular wisdom teeth, which also includes dry socket as a primary outcome (Bailey 2020). In order to avoid duplication, this review evaluates other 'local' interventions for the prevention and treatment of dry socket. This version is an update of the Cochrane Review first published in 2012 (Daly 2012).
Objectives
To assess the effects of local interventions used for the prevention and treatment of alveolar osteitis (dry socket) following tooth extraction.
Methods
Criteria for considering studies for this review
Types of studies
We only considered randomised controlled trials (RCTs) for inclusion in this review. RCTs looking at prevention could have employed a split‐mouth or cross‐over design. All studies included in this review utilised and reported explicit and validated criteria that were used in the diagnosis of dry socket. The diagnosis of dry socket was based on the Blum 2002 criteria (case definition) i.e. a continuous throbbing pain starting 1 to 3 days postextraction, a socket that may be partially or totally devoid of blood clot and which may be accompanied by other signs and symptoms such as foul taste, bad breath, localised swelling, and lymph‐node involvement.
Types of participants
We considered studies that included adults over the age of 18 years who had undergone an extraction (routine or more complex surgical) of one or more permanent teeth under local anaesthesia with or without sedation or under general anaesthesia. We included studies that included participants who were smokers. We excluded participants who were immunocompromised, had any co‐morbidities or medical conditions that might influence the healing of oral tissues.
Types of interventions
We considered studies that included any type of local intervention used for the prevention and treatment of dry socket compared to a different local intervention, placebo or no treatment. We included studies that permitted the use of concomitant pain medication provided it was made available equally to both groups. We excluded studies that examined the effectiveness of local interventions to prevent dry socket and then subsequently to treat dry sockets.
We excluded studies which reported upon the use of systemic antibiotics because these are covered in a separate Cochrane Review (Lodi 2021). Likewise, we excluded studies evaluating the use of different surgical procedures (including drains and lavage volume) to manage dry socket because these interventions are part of another Cochrane Review (Bailey 2020).
Types of outcome measures
We considered studies that included outcome measures that were reported according to clinically important time points i.e. at the end of the intervention and during a follow‐up period of up to 2 weeks.
Adverse effects
Any specific adverse effects related to any clinically diagnosed reactions to any of the active interventions were noted by the review team and reported as an additional table.
Primary outcomes
For prevention of dry socket.
Proportion of participants presenting with a dry socket within 1 week post‐treatment.
For treatment of established dry socket.
Time to heal dry socket and socket healing indices.
Pain: its severity and duration from time of administration of intervention to relief of pain assessed using any patient‐reported validated pain scale.
Swelling: assessed using photography or digital morphometry.
Limitation of chewing or swallowing and time to resumption of normal feeding.
Fever.
Secondary outcomes
Secondary outcomes assessed were for the treatment of dry sockets.
Quality of life as assessed by a validated questionnaire.
Patient satisfaction assessed by any validated measure.
Costs.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials. There were no language, publication year, or publication status restrictions:
Cochrane Oral Health’s Trials Register (searched 28 September 2021) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 8) in the Cochrane Library (searched 28 September 2021) (Appendix 2);
MEDLINE Ovid (1946 to 28 September 2021) (Appendix 3);
Embase Ovid (1980 to 28 September 2021) (Appendix 4).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategies designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Version 6.1 (Lefebvre 2021)).
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 28 September 2021) (Appendix 5);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 28 September 2021) (Appendix 6).
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We checked that none of the included studies in this review were retracted due to error or fraud.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Data collection and analysis was carried out by two review authors working independently. Any disagreements were resolved by discussion.
Selection of studies
Two review authors independently assessed the abstracts of retrieved studies. The search was designed to be sensitive and include controlled clinical trials, these were filtered out early in the selection process if they were not randomised. We obtained full copies of any studies deemed to be relevant or potentially relevant i.e. those appearing to meet the inclusion criteria, or for which there was insufficient information in the title and abstract to make a clear decision. Two review authors then assessed full‐text papers independently and any disagreements on the eligibility of included studies were resolved through discussion and consensus. If necessary, a third review author was consulted. We excluded any studies that did not match the inclusion criteria at this stage or at subsequent stages, and noted the reasons for exclusion in the section.
Data extraction and management
Two review authors collected study details and outcome data independently and in duplicate using a predetermined form designed for this purpose. These were entered into RevMan (RevMan Web 2022) (characteristics of included studies, forest plots, and additional tables). Discrepancies in data were discussed and only included if there was an independently reached consensus. If necessary a third review author was consulted to resolve inconsistencies.
We extracted the following details.
Trial methods: method of allocation; masking of participants, operators, and outcomes; exclusion of participants after randomisation and proportion of losses at follow‐up and number analysed.
Participants: country of origin; sample size and sample size calculation; age; gender; inclusion and exclusion criteria.
Intervention and control: type and procedural information including dose; mode of local use; time of administration relative to extraction details of any other concomitant medication.
Outcomes: primary and secondary outcomes; methods of assessment and completeness of reporting as outlined in the Types of outcome measures section of this review.
This information was used to help assess the clinical diversity and generalisability of any included trials.
If stated, we recorded the sources of funding of any of the included studies.
Assessment of risk of bias in included studies
Studies identified for inclusion in this review were assessed independently by two review authors who graded them using the Cochrane risk of bias tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The independent evaluations were compared and discussed, and any disagreements were resolved. For cross‐over trials we referred to Higgins 2021 (Table 23.2.a) for guidance on managing assessment of risk of bias.
We assessed each trial for the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessors (performance bias).
Completeness of outcome data.
Risk of selective outcome reporting.
Risk of other bias
For each domain a description of what occurred as reported in the journal article was reviewed and a judgement made on the risk of bias: high, unclear, or low risk of bias. The judgement was determined using guidance as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
The assessments for each included study are reported in the corresponding section of the risk of bias tables in RevMan (RevMan Web 2022).
Overall risk of bias
Low risk of bias: all domains are judged to be at low risk of bias.
Unclear risk of bias: one or more domains judged to be at unclear risk of bias.
High risk of bias: one or more domains judged to be at high risk of bias.
The overall risk of bias assessment was undertaken without blinding of review authors to the study authors' names or organisations or the journal type. The independent evaluations were compared and discussed, and any disagreements were resolved.
The results of the risk of bias assessment are presented graphically both by domain and by study.
Measures of treatment effect
The primary measure of intervention effect for the prevention of dry socket was the reduction in incidence of dry socket between the control and intervention groups i.e. proportion of participants presenting with a dry socket within 1 week post‐treatment. For the treatment of dry socket the primary measure of intervention effect was the reduction in the time to heal of the socket and reduction in the incidence of pain, swelling, functional limitation (chewing, swallowing, and time to resumption of normal feeding), and fever.
Secondary measures of intervention effect for the prevention and treatment of dry socket were the continuous outcomes: quality of life, patient satisfaction, and costs between the intervention group and the control.
For dichotomous data, we calculated the risk ratio (RR), together with the 95% confidence interval (CI). For split‐mouth/cross‐over studies, we calculated odds ratios (OR) using the Becker‐Balagtas method (BB OR) outlined in Curtin 2002. We chose the Becker‐Balagtas method because we intended to pool data from split‐mouth studies and parallel‐group studies in the same meta‐analyses, and the Becker‐Balagtas method facilitates this data synthesis (as outlined by Stedman 2011). The split‐mouth studies included in the review did not present the paired data by tooth pairs, only as marginals (as parallel‐group studies, not as cross‐classification), so we chose the conservative intraclass correlation coefficient (ICC) of 0.5 for the split‐mouth studies and 0 for the parallel‐group studies. For continuous outcomes, we used the mean differences (MD) and 95% CIs to summarise the data for each group where the mean difference and standard deviations were calculable from the data presented.
Unit of analysis issues
In parallel‐group studies, we chose the individual to be the unit of analysis.
In split‐mouth studies, we chose the tooth pair within an individual to be the unit of analysis.
In cross‐over and split‐mouth studies, we planned to choose the tooth pair within an individual to be the unit of analysis.
Dealing with missing data
Where possible we calculated missing data from tables and graphs. We contacted authors of included studies to obtain missing trial details and data from the reports.
Assessment of heterogeneity
If a sufficient number of studies had been included in any meta‐analyses, we would have assessed clinical heterogeneity by examining the characteristics of studies and the similarity between types of participants, interventions, and outcomes as specified in the criteria for included studies.
We assessed the significance of discrepancies in estimates of treatment effects from various studies using Cochran's test for heterogeneity and the I2 statistic. The I2 statistic describes the percentage of variability in effect estimates that is due to heterogeneity rather than to sampling error. A value greater than 50% may represent substantial heterogeneity (Higgins 2003). The Cochrane Handbook for Systematic Reviews of Interventions also gives a rough guide to heterogeneity measured by I2 as follows: 0% to 40% may not be important, 30% to 60% represents moderate heterogeneity, 50% to 90% may be classified as substantial heterogeneity, and 75% to 100% represents considerable heterogeneity (Higgins 2021).
In the event that there were insufficient clinically homogeneous trials for any specific intervention or insufficient study data that could be pooled, a narrative synthesis was presented.
Assessment of reporting biases
If sufficient trials had been identified for inclusion in this review, we would have assessed publication bias according to the recommendations on testing for funnel plot asymmetry (as described in Section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), and if asymmetry was identified, other possible causes would have been assessed.
Data synthesis
Two review authors analysed the data and reported them as specified in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). Analysis was conducted at the same level as the allocation. The data for effects related to prevention were analysed and presented separately to those which considered treatment only.
The outcomes specified for this review necessitated repeated observations on the participants over a comparatively short period of time during and after the interventions. Therefore, depending on sufficient data being available, we grouped the outcomes and analysed them according to clinically important time points; at the end of the intervention and during the follow‐up period.
We undertook pooling of data to provide estimates of the efficacy of the interventions if included studies were clinically and statistically homogeneous. We used RRs to pool the dichotomous outcomes where possible. If split‐mouth studies were included in the meta‐analysis, then ORs were calculated as described by Stedman 2011 (Stedman 2011).
In general, for the synthesis of any quantitative data, we used the random‐effects model unless there were fewer than four studies, where we used the fixed‐effect model.
We calculated number needed to treat (NNT) for the pooled estimates using control prevalence rates for dry socket. Dry socket is a complication associated with 0.5% to 7% of routine extraction of teeth affected by periodontal disease and dental decay (Field 1988; Halabi 2018; Vezeau 2000), however, the prevalence of dry socket postextraction of mandibular molars, especially impacted wisdom teeth is much higher (1% to 37.5%) (Caso 2005; Fridrich 1990; Haraji 2013; Oyri 2019; Vezeau 2000). For the assumed risk of dry socket in the control group for the summary of findings table, we set prevalence rates to reflect prevalence rates for routine dental extractions (1% and 5%) and for extraction of mandibular third molars (30%).
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were planned if sufficient studies were identified: complexity of the extraction (surgical removal or simple extraction), and different types of teeth (third molars, molars, premolars). We also planned to undertake a subgroup analysis for chlorhexidine dose. There were insufficient studies to undertake subgroup analyses, however the data are presented for chlorhexidine dose.
Sensitivity analysis
If a sufficient number of studies with similar characteristics had been included in the review, we would have undertaken sensitivity analyses to assess the robustness of the results by excluding studies at high risk of bias.
Summary of findings and assessment of the certainty of the evidence
We constructed summary of findings (SoF) tables for comparisons including more than one trial. Separate SoF tables were undertaken for the prevention and treatment of dry socket, with the primary outcome, the proportion of participants presenting with a dry socket within 1 week post‐treatment for prevention, and the following outcomes for treatment: time to heal dry socket, pain, swelling, limitation of chewing or swallowing, and fever. Adverse events were included as an outcome for all comparisons.
We followed GRADE methods to provide overall grading of the certainty of the evidence, with reference to overall risk of bias of included studies at each outcome, directness of the evidence, consistency of the results, precision of estimates, and risk of publication bias, as described in Section 14.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). The certainty of the evidence was assessed as high, moderate, low, or very low.
Results
Description of studies
Results of the search
The search identified 1371 references through database searching and a further 11 identified through other sources. Figure 1 presents the PRISMA flow diagram for the review. After examination of the titles and abstracts, duplicates were removed and the remaining references eliminated when they did not match the inclusion criteria. Full‐text copies of the remaining 181 studies were obtained and these were then subjected to further evaluation. A number of studies were translated: three were in German (Birke 1970; Neugebauer 2004; Neuner 1969), two were in Russian (Butylin 1977; Zorina 2019), and one each was in Japanese (Anonymous 1966), Serbian (Jovanovic 2011), French (Turcotte 1997), and Polish (Banach 1973). There were seven in Chinese (Bai 2011; Feng 2009; Hu 2005; Huang 2011; Sun 2007; Wen 2004; Xue 2013). The bibliographical references were examined for all potentially eligible studies and six potentially relevant additional citations were identified (Al‐Hamed 2017; Babar 2012; Baslarli 2015; Delilbasi 2002; Haraji 2012b; Kirk 2007).
1.
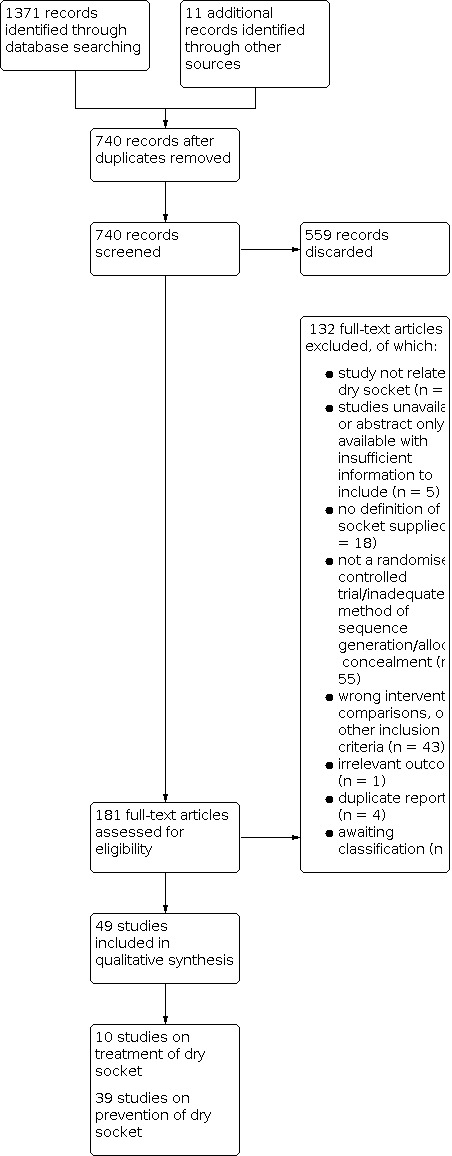
Study flow diagram.
We included 49 studies in this review. See Characteristics of included studies. The majority (39 studies) evaluated interventions for the prevention of dry sockets (Abu‐Mostafa 2015; Abu‐Mostafa 2019; Ahmedi 2016; Alissa 2010; Bai 2011; Babar 2012; Cho 2018; Delilbasi 2002; Divya 2019; Feng 2009; Freudenthal 2015; Gersel‐Pedersen 1979; Ghaeminia 2017; Halabi 2018; Haraji 2013; Hasheminia 2018; Hermesch 1998; Hita‐Iglesias 2008; Hu 2005; Huang 2011; Karabit 2019; Kjellman 1973; Larsen 1991; Metin 2006; Ragno 1991; Reekie 2006; Ritzau 1977; Rodriguez‐Perez 2013; Rubio‐Palau 2015; Shad 2018; Shi 2003; Sun 2007; Torres‐Lagares 2006a; Torres‐Lagares 2006b; Trieger 1991; Tuk 2019; Unsal 2018; van Eeden 2006; Xue 2013). All the prevention trials comprehensively addressed the single (primary) outcome of whether a dry socket occurred or not.
The remaining 10 studies examined treatment strategies for dry sockets occurring after dental extraction (Burgoyne 2010; Chaurasia 2017; Faizel 2015; Kaya 2011; Keshini 2020; King 2018; Lenka 2019; Mitchell 1984; Supe 2018; Yuce 2019). The 10 treatment trials mostly looked at duration and severity of pain, and the time to heal dry sockets.
The search also retrieved 22 reviews: 14 with meta‐analysis (Barona‐Dorado 2014; Canellas 2020; Canellas 2019; Canellas 2017; Canullo 2020; Caso 2005; Del Fabbro 2019; Hedstrom 2007; Rodríguez Sánchez 2017; Shafaee 2020; Xiang 2019; Xu 2019; Yengopal 2012; Zhu 2020) and the remaining seven were narrative reviews (Chow 2020; Kolokythas 2010; Neuner 1969; Noroozi 2009; Turcotte 1997; Veale 2015; Vezeau 2000). All reviews were examined for potentially eligible studies.
Included studies
Forty‐nine studies were considered eligible for inclusion (Figure 1). There were 39 studies on prevention and 10 on the treatment of dry socket. Seven of the prevention studies were designed as split‐mouth studies (Ahmedi 2016; Gersel‐Pedersen 1979; Haraji 2013; Karabit 2019; Trieger 1991; Unsal 2018; van Eeden 2006), one was designed as a cross‐over study (Tuk 2019), and the remainder were of parallel‐group design. Trials in the treatment review will always be of parallel‐group design as there would be insufficient participants with more than one dry socket to undertake this type of study design.
Characteristics of the trial setting and investigators
Thirty‐three of the studies had been conducted in college/university/hospital oral surgery or dentistry departments: Abu‐Mostafa 2015 (Saudi Arabia); Abu‐Mostafa 2019 (Saudi Arabia); Ahmedi 2016 (Kosovo); Alissa 2010 (UK); Bai 2011 (China); Burgoyne 2010 (USA); Chaurasia 2017 (Nepal); Divya 2019 (India); Faizel 2015 (India); Feng 2009 (China); Freudenthal 2015 (Sweden); Gersel‐Pedersen 1979 (Denmark); Hita‐Iglesias 2008 (Spain); Huang 2011 (China); Hu 2005 (China); Karabit 2019 (Syria); Kaya 2011 (Turkey); King 2018 (UK); Kjellman 1973 (Sweden); Metin 2006 (Turkey); Mitchell 1984 (UK); Ritzau 1977 (Denmark); Rodriguez‐Perez 2013 (Spain); Rubio‐Palau 2015 (Spain); Shad 2018 (Pakistan); Sun 2007 (China); Supe 2018 (India); Torres‐Lagares 2006a (Spain); Torres‐Lagares 2006b (Spain); Tuk 2019 (Netherlands); Unsal 2018 (Turkey); Xue 2013 (China); and Yuce 2019 (Turkey). Military clinics were the settings for four of the studies (Babar 2012 (Pakistan); Hermesch 1998; Ragno 1991; van Eeden 2006). Two studies were conducted in private maxillofacial clinics (Haraji 2013 (Tehran) and Hasheminia 2018 (Iran)). Four multicentre studies were conducted, one was in three dental practices in the UK (Reekie 2006), one was conducted in three private dental practices in Australia (Cho 2018), one was conducted in two public dental clinics in Chile (Halabi 2018), and one was conducted in three oral and maxillofacial surgery departments of a university hospital, a hospital, and a private clinic in the Netherlands (Ghaeminia 2017). The settings of the remaining six studies were not stated (Delilbasi 2002; Keshini 2020; Larsen 1991; Lenka 2019; Shi 2003; Trieger 1991).
The skill level and number of operators providing care where stated varied. Single operators provided care in 12 of the included studies (Ahmedi 2016; Bai 2011; Babar 2012; Gersel‐Pedersen 1979; Hu 2005; Karabit 2019; Ragno 1991; Shad 2018; Sun 2007; Tuk 2019; Unsal 2018; Xue 2013). Either oral and maxillofacial surgeons, general dentists, general dentistry residents or senior house officers provided care for participants in 10 studies (Cho 2018; Freudenthal 2015; Ghaeminia 2017; Halabi 2018; Hermesch 1998; Kaya 2011; King 2018; Metin 2006; Rodriguez‐Perez 2013; Yuce 2019). Abu‐Mostafa 2015 and Abu‐Mostafa 2019 stated that dental interns or dental students under supervision of surgery instructors provided the care. Larsen 1991 stated that "multiple surgeons with varying levels of experience" with "formal training in third molar removal" were responsible for provision of care. Reekie 2006 stated that four general dental practitioners were providers of care. The providers of care for 23 of the included studies were not stated (Alissa 2010; Burgoyne 2010; Chaurasia 2017; Delilbasi 2002; Divya 2019; Faizel 2015; Feng 2009; Haraji 2013; Hasheminia 2018; Hita‐Iglesias 2008; Huang 2011; Keshini 2020; Kjellman 1973; Lenka 2019; Mitchell 1984; Ritzau 1977; Rubio‐Palau 2015; Shi 2003; Supe 2018; Torres‐Lagares 2006a; Torres‐Lagares 2006b; Trieger 1991; van Eeden 2006).
Characteristics of the participants
Most prevention studies (29 out of 39) involved sockets of mandibular third molar teeth in adults. 21 studies reported on mandibular third molars which were extracted under local anaesthesia with/without intravenous sedation (Ahmedi 2016; Bai 2011; Babar 2012; Cho 2018; Feng 2009; Freudenthal 2015; Gersel‐Pedersen 1979; Ghaeminia 2017; Hermesch 1998; Hita‐Iglesias 2008; Hu 2005; Huang 2011; Karabit 2019; Kjellman 1973; Larsen 1991; Rodriguez‐Perez 2013; Sun 2007; Torres‐Lagares 2006a; Torres‐Lagares 2006b; Tuk 2019; Xue 2013). Three studies involved third molar teeth where the participants' ages were specified (Divya 2019; Hasheminia 2018; Shad 2018). A further three studies involved third molar teeth where the participants' ages were not specified (Ragno 1991; Shi 2003; Trieger 1991). Five studies investigated prevention of dry socket after mandibular third molar extraction, however, participants below the age of 18 years were enrolled in: Haraji 2013 (age 13 to 71 years); Metin 2006 (age 17 to 46 years); Ritzau 1977 (age 17 to 61 years); Unsal 2018 (age 15 to 43 years); and van Eeden 2006 (age 16 to 32 years). Two further studies investigated prevention of dry socket after mandibular third molar extraction, however, participant age was not stated (Delilbasi 2002; Rubio‐Palau 2015).
Abu‐Mostafa 2019 investigated prevention of dry socket after extraction of a single molar tooth in patients (age 17 to 69 years). Abu‐Mostafa 2015 also reported on prevention of dry socket following a single molar extraction. Halabi 2018 looked at patients aged 18 years and older having routine dental extractions. Reekie 2006 investigated prevention of dry socket after non‐surgical extraction of one or more molar/premolar teeth under local anaesthetic in adult participants (age 18 to 90 years), while Alissa 2010 also reported on teeth other than third molars.
In the treatment studies, Kaya 2011 reported on treatment of dry socket after mandibular third molar extraction, Yuce 2019 reported on third molars, Burgoyne 2010 reported on premolars and molars, Chaurasia 2017 reported on molars and mandibular canines, King 2018 reported on incisors, premolars and molars, Supe 2018 reported on all teeth, and no specific tooth was identified in Faizel 2015; Lenka 2019; and Mitchell 1984. Burgoyne 2010 investigated the treatment of diagnosed dry socket in 17‐ to 58‐year olds, Kaya 2011 investigated the treatment of diagnosed dry sockets in adults over 18 years of age, Supe 2018 investigated the treatment of dry socket in people aged 18 to 51 years, Yuce 2019 investigated the treatment of dry socket in people aged 18 to 40 years. Chaurasia 2017; Faizel 2015; and King 2018 investigated the treatment of dry sockets in males and females of unstated age, and Lenka 2019 and Mitchell 1984 investigated the treatment of diagnosed dry socket, however, age and gender were unspecified. Keshini 2020 reports on 30 participants presenting with dry socket, but the teeth were not identified.
The number of participants in the prevention studies ranged from 19 to 744 with a median of 100. The number of participants in the treatment studies ranged from 30 to 117 with a median of 47.
Characteristics of the interventions
This section is divided into two main parts: the characteristics of the interventions for the 39 studies reporting on the prevention of dry socket and the characteristics of the 10 trials reporting on the treatment of dry socket.
Prevention
Interventions in this section (39 studies) have been divided into two broad categories: rinses and intrasocket interventions.
Chlorhexidine (rinses or intrasocket gels)
Chlorhexidine rinse (pre and postoperatively) versus placebo or saline (postoperatively) (Delilbasi 2002; Halabi 2018; Hermesch 1998; Karabit 2019; Larsen 1991; Ragno 1991).
Chlorhexidine gel (placed in socket at time of surgery) versus placebo/no treatment (Babar 2012; Freudenthal 2015; Haraji 2013; Rubio‐Palau 2015; Shad 2018; Torres‐Lagares 2006a; Torres‐Lagares 2006b).
Chlorhexidine rinse with monoject (postoperatively) versus chlorhexidine rinse (postoperatively) (Cho 2018).
Chlorhexidine rinse (preoperatively) versus chlorhexidine rinse (postoperatively) (Metin 2006).
Chlorhexidine gel versus chlorhexidine rinse (both postoperatively) (Abu‐Mostafa 2015; Hita‐Iglesias 2008).
0.2% chlorhexidine gel versus 1% chlorhexidine gel (Rodriguez‐Perez 2013).
Other (rinses or intrasocket interventions)
Two studies looked at platelet rich plasma and two studies at acellular dermal matrix patches. The remainder of the following interventions were evaluated by one trial only.
Acellular dermal matrix patch versus no treatment (Bai 2011; Sun 2007).
Apernyl versus placebo (Kjellman 1973).
Artemisia desertorum Spreng (Shahaosan or Yunnan) versus placebo control (Shi 2003).
Bovine fibroblastic growth factor versus control (Xue 2013).
Clindamycin phosphate antibiotic solution patch versus saline patch (Trieger 1991).
Gaseous ozone versus control (Ahmedi 2016).
Glucocorticosteroid antibiotic agent versus normal saline (van Eeden 2006).
Herbal mouthwash versus chlorhexidine rinse (Divya 2019).
Heal‐all tissue patch (2 x 2.5 cm) versus no treatment (Huang 2011).
Honey versus chlorhexidine rinse (Abu‐Mostafa 2019).
Iodine (1%) (pre and intraoperatively) versus control (Hasheminia 2018).
Iodine tampon versus monoject irrigation with saline (Tuk 2019).
Irrigation with tap water in monoject versus control (Ghaeminia 2017).
Metronidazole gel versus placebo gel (Reekie 2006).
Oral tissue patch versus control (Hu 2005).
P‐hydroxybenzoic acid versus placebo (Ritzau 1977).
Platelet rich fibrin versus control (Unsal 2018).
Platelet rich plasma versus control (Alissa 2010; Feng 2009).
Tranexamic acid versus placebo (Gersel‐Pedersen 1979).
Treatment
Ten studies reported on the treatment of dry socket. Four studies compared management of dry socket with zinc oxide eugenol and Alvogyl/Alveogyl. It should be noted that Alvogyl (Septodont) has been reformulated and rebranded as Alveogyl (Septodont 2021). Alvogyl is described explicitly as containing iodoform 15.8 gm (antimicrobial) and butamben 25.7 gm (anaesthetic) and eugenol 13.7 gm by Faizel 2015; Kaya 2011; and Supe 2018. The manufacturers have since removed iodoform and butamben leaving eugenol (obtundent) as the only active ingredient. The new product is branded as Alveogyl. It is difficult to clarify exactly when this reformulation occurred but it seems to have been prior to 2014 (Kalsi 2020).
All the studies included in this review (except for Chaurasia 2017) state that they have used Alvogyl and clearly list the ingredients of this product with the exception of Lenka 2019 and King 2018. All included studies were published after 2014, but they may have been using existing stock of Alvogyl or possibly the trial subjects were recruited prior to or during 2014 (Faizel 2015; Kaya 2011; Supe 2018). One study refers in the text to "Alveogyl" but lists the ingredients of Alvogyl (Chaurasia 2017). It is possible that some clinicians may have been unaware of the change in formulation as the packaging of Alveogyl is almost identical to Alvogyl (Kalsi 2020). We have contacted the authors of all the included studies using these products to clarify but we have only received a response from the authors of King 2018.
Zinc oxide eugenol versus Alvogyl (Faizel 2015; Lenka 2019; Supe 2018).
Zinc oxide eugenol versus Alveogyl (Chaurasia 2017).
Neocone versus Alvogyl versus zinc oxide eugenol (Faizel 2015).
Advanced platelet rich fibrin versus saline (Yuce 2019).
Alvogyl versus no treatment (Kaya 2011).
Alvogyl versus plasma rich in growth factors (King 2018).
Alvogyl versus plasma rich fibrin (Keshini 2020).
Alvogyl versus SaliCept (Kaya 2011).
Metronidazole versus placebo (Mitchell 1984).
SaliCept versus no treatment (Kaya 2011).
Topical anaesthetic gel (prilocaine‐lidocaine) versus eugenol (Burgoyne 2010).
Characteristics of the outcome measures
Prevention
The primary (and only) outcome measure for prevention was the presence/absence of a dry socket. This was clearly reported in all 39 studies for prevention. Minor adverse events were reported in eight of the prevention studies (Delilbasi 2002; Gersel‐Pedersen 1979; Hermesch 1998; Kjellman 1973; Metin 2006; Ragno 1991; Ritzau 1977; van Eeden 2006).
Treatment
Ten trials investigated the treatment of dry socket (Burgoyne 2010; Chaurasia 2017; Faizel 2015; Kaya 2011; Keshini 2020; King 2018; Lenka 2019; Mitchell 1984; Supe 2018; Yuce 2019) with the following: topical anaesthetic gel (prilocaine‐lidocaine), Alvogyl/Alveogyl, SaliCept, metronidazole, zinc oxide eugenol, Neocone, plasma rich in growth factors, platelet rich fibrin, and advanced platelet rich fibrin.
Three trials compared the use of Alvogyl and zinc oxide eugenol for the management of dry socket (Faizel 2015; Lenka 2019; Supe 2018). Supe 2018 and Faizel 2015 reported on experience of pain at day 5, 7, and 10 while Lenka reported pain at day 7 only (Lenka 2019). One trial (Chaurasia 2017) compared Alveogyl and zinc oxide eugenol, but data could not be included in the meta‐analysis as the authors appeared to have been using the newer formulation Alveogyl. One trial (Keshini 2020) compared Alvogyl versus platelet rich fibrin examining pain visual analogue scale (VAS) scores on day 1, 3, and 10. Although published in 2020 they report a formulation for Alvogyl postdating formula change.
A number of different outcome measures were used in the different studies. In all the studies pain was one of the main outcome measures, but the method of measuring pain varied. One study (Faizel 2015) measured time (in minutes and days) to achieve initial pain relief and complete resolution of pain following placement of a medicament. One study (Mitchell 1984) looked at duration of treatment. Seven studies (Burgoyne 2010; Chaurasia 2017; Keshini 2020; King 2018; Lenka 2019; Supe 2018; Yuce 2019) measured pain on subsequent days, following placement of a medicament using a VAS scale. The VAS scores were taken on different days in different studies.
Four studies (Faizel 2015; Keshini 2020; King 2018; Supe 2018) used different healing outcomes, including changes to signs of dry socket such as empty sockets, exposed sockets, number of socket walls that were exposed, redness around sockets, and also inflammation and healing scores.
The data from Kaya 2011 were unusable as medians and error bars for pain were presented in graphs. There was no evidence of a difference in pain at 48 hours in Burgoyne 2010 (Additional Table 6). Kaya 2011 (page 1574) stated in the text: "The differences in the changes in the clinical signs and symptoms between the control group and all 3 treatment groups were statistically significant (P < 0.05) on the third day after treatment" and "Regardless of the treatment the VAS scores changed during the follow‐up period (P < 0.001); however the intensity of the pain decreased more rapidly in all the treatment groups than for the control group (P < 0.05)."
1. Results for treatment of dry socket (single studies).
| Comparison | Data | Effect (95% CI) | P value |
| Anaesthetic gel versus eugenol (Burgoyne 2010) Pain at 48 hours (VAS 0 to 10) |
Anaesthetic gel n = 15, mean = 2.49, SD = 2.51 Placebo n = 20, mean = 2.69, SD = 2.46 |
Pain at 48 hours VAS (0 to 10) MD ‐0.20 (‐1.87 to 1.47) |
0.81 |
| Neocone versus Alvogyl (Faizel 2015) Mean time for initial pain relief (minutes) Mean time for complete resolution of pain (days) |
Initial pain relief (minutes) Neocone n = 39, 17.23 minutes, SD = 1.6 Alvogyl n = 39, 7.358 minutes, SD = 1.79 Complete resolution of pain (days) Neocone n = 39, 4.85 days, SD = 0.63 Alvogyl n = 39, 6.47 days, SD = 0.45 |
Initial pain relief (minutes) 9.87 (9.12 to 10.63) Complete resolution of pain (days) ‐1.62 (‐1.86 to ‐1.38) |
< 0.00001 < 0.00001 |
| Neocone versus zinc oxide eugenol (Faizel 2015)
Mean time for initial pain relief (minutes) Mean time for complete resolution of pain (days) |
Initial pain relief (minutes)
Neocone
n = 39, 17.23 minutes, SD = 1.6
Zinc oxide eugenol
n = 39, 25 minutes, SD = 3.08 Complete resolution of pain (days) Neocone n = 39, 4.85 days, SD = 0.63 Zinc oxide eugenol n = 39, 8.64 days, SD = 0.47 |
Initial pain relief (minutes) ‐7.77 (‐8.86 to ‐6.68) Complete resolution of pain (days) ‐3.79 (‐4.04 to ‐3.54) |
< 0.00001 < 0.00001 |
| Alvogyl versus zinc oxide eugenol (Faizel 2015) Mean time for initial pain relief (minutes) Mean time for complete resolution of pain (days) |
Initial pain relief (minutes) Alvogyl n = 39, 7.358 minutes, SD = 1.79 Zinc oxide eugenol n = 39, 25 minutes, SD = 3.08 Complete resolution of pain (days) Alvogyl n = 39, 6.47 days, SD = 0.45 Zinc oxide eugenol n = 39, 8.64 days, SD = 0.47 |
Initial pain relief (minutes) ‐17.64 (‐18.76 to ‐16.52) Complete resolution of pain (days) ‐2.17 (‐2.37 to ‐1.97) |
< 0.00001 < 0.00001 |
| Plasma rich in growth factors (PRGF) versus Alvogyl (King 2018) VAS pain score (cm) |
1st review appointment day 3 (+/‐ 1 day)
PRGF
n = 22, 4.0 +/‐ 2.7
Alvogyl
n = 22, 4.3 +/‐ 2.9 2nd review appointment day 7 (+/‐ 1 day) PRGF n = 22, 2.0 +/‐ 2.0 Alvogyl n = 22, 2.4 +/‐ 2.6 |
VAS pain score (cm) 1st review MD ‐0.30 (‐1.96 to 1.36) 2nd review MD ‐0.40 (‐1.77 to 0.97) |
0.72 0.57 |
| Plasma rich in growth factors (PRGF) versus Alvogyl (King 2018) Exposed bone (present versus absent) (percentage of patients) | 1st review (day 3 +/‐ 1) PRGF n = 22, 9.1% Alvogyl n = 22, 9.1% 2nd review (day 7 +/‐ 1) PRGF n = 22, 0% Alvogyl n = 22, 22.7% |
‐ | 1st review Not specified 2nd review < 0.05 |
| Metronidazole versus placebo (Mitchell 1984) Duration of treatment (days) | Metronidazole n = 26, mean = 5.35, SD = 3.52 Placebo n = 29, mean = 8.52, SD = 8.52 |
Duration of treatment (days) MD ‐3.17 (‐1.04 to ‐5.30) |
0.004 |
| Advanced platelet rich fibrin (A‐PRF) versus control (Yuce 2019)
Mean VAS pain scores (cm) Post‐operatively day 1 |
A‐PRF
n = 20, 5.2 +/‐ 1.06 Control n = 20, 7.25 +/‐ 1.02 |
VAS pain score (cm) MD ‐2.05 (‐2.69 to ‐1.41) |
< 0.00001 |
| Advanced platelet rich fibrin (A‐PRF) versus control (Yuce 2019) Mean VAS pain scores (cm) Post‐operatively day 3 | A‐PRF
n = 20, 2.25 +/‐ 0.64 Control n = 20, 7.05 +/‐ 1.23 |
VAS pain score (cm) MD ‐4.80 (‐5.41 to ‐4.19) |
< 0.00001 |
| Advanced platelet rich fibrin (A‐PRF) versus control (Yuce 2019)
Mean VAS pain scores (cm) Post‐operatively day 5 |
A‐PRF n = 20, 0.8 +/‐ 0.62 Control n = 20, 5.9 +/‐ 0.91 | VAS pain score (cm) MD ‐5.10 (‐5.58 to ‐4.62) |
< 0.00001 |
| Advanced platelet rich fibrin (A‐PRF) versus control (Yuce 2019)
Mean VAS pain scores (cm) Post‐operatively day 7 |
A‐PRF n = 20, 0.45 +/‐ 0.51 Control n = 20, 4.05 +/‐ 0.76 | VAS pain score (cm) MD ‐3.60 (‐4.00 to ‐3.20) | < 0.00001 |
CI = confidence interval; MD = mean difference; n = number; SD = standard deviation; VAS = visual analogue scale.
Mitchell 1984 provided raw data on the duration of treatment and there was a statistically significant reduction in duration of treatment in the metronidazole group.
Excluded studies
One hundred and thirty‐two studies were excluded from this review. The reasons for their exclusion are summarised below.
Not a randomised controlled trial/inadequate method of sequence generation (55 studies): Annibali 2012; Anonymous 1966; Banach 1973; Bloomer 2000; Bloomer 2012; Birke 1970; Brignardello 2012; Butylin 1977; Cebi 2020; Christensen 2012; Cooper 2012; Field 1988; Fotos 1992; Garibaldi 1995; Goldman 1973; Goldsmith 2012; Goyal 2012; Hall 1971; Haraji 2012b; Johnson 1988; Jovanovic 2011; Julius 1982; Kamal 2020a; Kamal 2020b; Keskitalo 1973; Krekmanov 1986; Kudiyirickal 2012; Lao 2012; Liu 2011; Long 2012; Malkawi 2011; MacGregor 1975; Mishra 2012; Mitchell 1986a; Neugebauer 2004; Neuner 1969; Prataap 2017; Qi 2012; Rastogi 2018; Reeshma 2021; Ritzau 1978; Sanchis 2004; Sharma 2017; Sorensen 1987; Swanson 1989; Sweet 1985; Syrjanen 1981a; Tek 2014; Torres Lagares 2006; Tjernberg 1979; Tong 2012; Vedtofte 1974; Wen 2004; Yue 2012; Zanetta‐Barbosa 1994.
Dry socket not defined (18 studies): Anand 2015; Arakeri 2011; Arenaz‐Bua 2010; Asutay 2017; Daniels 2011; Dubovina 2016; Guazzo 2018; Haupt 2015; Hooley 1995; Kaplan 2020; Kirk 2007; MacGregor 1973; Majid 2010; Mehlisch 2010a; Mehlisch 2010b; Nordenram 1973; Sarkar 2019; Zuniga 2011.
Irrelevant outcomes (1 study): Betts 1995.
Wrong interventions, comparisons or other inclusion criteria (43 studies): Afat 2018; Ahmed 2020; Akota 1998; Al‐Hamed 2017; Altman 2011; Al‐Sukhun 2011; Baqain 2012; Baslarli 2015; Bello 2011; Berwick 1990; Bezerra 2011; Butler 1977; Bystedt 1980; Daugela 2018; Dutta 2016; Eshghpour 2014; Eshghpour 2018; Farooq 2019; Gonzalez‐Serrano 2021; Haraji 2010; Hill 2006; Jadhao 2018; Jesudasan 2015; Karthik 2021; Kilinc 2017; Kim 2020; Krekmanov 1981; Lopez‐Cedrun 2011; Mitchell 1986b; Olusanya 2011; Osunde 2014; Osunde 2015; Osunde 2017; Oyri 2019; Ozveri 2020; Rani 2016; Saez‐Alcaide 2020; Schatz 1987; Seethamsetty 2019; Syrjanen 1981b; Torres‐Lagares 2010; Wang 2013; Yuan 2006.
Study not related to dry socket (5 studies): Jolley 1972; Krishnan 2020; Moberly 2007; Scopp 1967; Vu 2021.
Study unavailable or abstract available with insufficient information (5 studies): study unobtainable: Nentwig 1985; Schlund 1968. Abstracts available with insufficient information: Njokanma 2019; Olson 1987; Pichler 2001.
Study awaiting classification (1 study): Zorina 2019.
Duplicate reporting (4 studies): Haraji 2012a; Haraji 2014; Haraji 2015; Paul 2019.
Further information about the reasons for exclusion of these studies is available in the Characteristics of excluded studies tables.
Risk of bias in included studies
Risk of bias assessment was undertaken for all included studies. For the prevention trials, the risk of bias assessment was undertaken for the primary outcome (whether or not the patient had a dry socket).
Allocation
Sequence generation
Random sequence generation was assessed at low risk of bias in 24 studies (49%) (Abu‐Mostafa 2015; Abu‐Mostafa 2019; Alissa 2010; Burgoyne 2010; Chaurasia 2017; Cho 2018; Faizel 2015; Freudenthal 2015; Gersel‐Pedersen 1979; Ghaeminia 2017; Haraji 2013; Hita‐Iglesias 2008; Karabit 2019; King 2018; Larsen 1991; Mitchell 1984; Reekie 2006; Rodriguez‐Perez 2013; Rubio‐Palau 2015; Sun 2007; Torres‐Lagares 2006a; Torres‐Lagares 2006b; Trieger 1991; van Eeden 2006), and unclear in the remainder (25 studies; 51%).
Allocation concealment
Allocation concealment was considered to be low risk of bias in 16 trials (33%) (Alissa 2010; Cho 2018; Freudenthal 2015; Gersel‐Pedersen 1979; Ghaeminia 2017; Halabi 2018; Hermesch 1998; Hita‐Iglesias 2008; King 2018; Mitchell 1984; Ragno 1991; Reekie 2006; Ritzau 1977; Torres‐Lagares 2006a; Torres‐Lagares 2006b; Tuk 2019) and for the remainder of the studies it was deemed as either unclear (29 trials; 32%) or at high risk of bias (4 trials; 8%) (Abu‐Mostafa 2015; Abu‐Mostafa 2019; Haraji 2013; Karabit 2019).
Blinding
Blinding in relation to outcomes
As both the participants and outcome assessors' assessment form part of the diagnosis of dry socket, the blinding assessment was planned to include both (i.e. both groups must be blinded for this category to be assessed as being at low risk of bias). In studies where the operator was not blinded to group allocation, but the participant was blinded, it was difficult to assess the impact on performance bias. If this was unlikely to have resulted in a deviation from the intervention, it was likely to have a small impact on study outcome.
Blinding was considered to be at low risk of bias for both performance and detection bias in 8 trials (17%) (Freudenthal 2015; Haraji 2013; Hermesch 1998; Mitchell 1984; Reekie 2006; Ritzau 1977; Torres‐Lagares 2006b; van Eeden 2006). Blinding (performance bias) was judged as being unclear in 33 trials (67%), at high risk of bias in 2 trials (4%), and at low risk of bias for 14 trials (29%). Blinding (detection bias) was judged as being unclear in 30 trials (61%), at high risk of bias in 3 trials (6%) and at low risk of bias in 16 trials (33%).
Incomplete outcome data
We assumed that dropouts in prevention of dry socket studies probably do not have dry socket or they would be returning for treatment. 35 (72%) of the trials were considered to be at low risk of bias with respect to incomplete outcome data (Ahmedi 2016; Bai 2011; Babar 2012; Delilbasi 2002; Divya 2019; Feng 2009; Freudenthal 2015; Gersel‐Pedersen 1979; Ghaeminia 2017; Halabi 2018; Haraji 2013; Hasheminia 2018; Hermesch 1998; Hita‐Iglesias 2008; Hu 2005; Huang 2011; Karabit 2019; Kaya 2011; Keshini 2020; King 2018; Lenka 2019; Metin 2006; Mitchell 1984; Ragno 1991; Ritzau 1977; Rodriguez‐Perez 2013; Rubio‐Palau 2015; Shad 2018; Sun 2007; Torres‐Lagares 2006a; Torres‐Lagares 2006b; Tuk 2019; van Eeden 2006; Xue 2013; Yuce 2019). 10 trials (20%) were considered to be at unclear risk with respect to incomplete outcomes (Abu‐Mostafa 2019; Burgoyne 2010; Chaurasia 2017; Cho 2018; Faizel 2015; Kjellman 1973; Shi 2003; Supe 2018; Trieger 1991; Unsal 2018), and 4 studies (6%) were considered at high risk (Abu‐Mostafa 2015; Alissa 2010; Larsen 1991; Reekie 2006). 31 studies analysed the same number of participants as were enrolled (Abu‐Mostafa 2019; Ahmedi 2016; Bai 2011; Babar 2012; Burgoyne 2010; Delilbasi 2002; Divya 2019; Feng 2009; Freudenthal 2015; Gersel‐Pedersen 1979; Ghaeminia 2017; Halabi 2018; Haraji 2013; Hasheminia 2018; Hu 2005; Huang 2011; Karabit 2019; Kaya 2011; King 2018; Kjellman 1973; Lenka 2019; Metin 2006; Ragno 1991; Ritzau 1977; Sun 2007; Supe 2018; Torres‐Lagares 2006a; Tuk 2019; van Eeden 2006; Xue 2013; Yuce 2019). Where reported, the range in the number of dropouts was from 3 (Hita‐Iglesias 2008) to 11 (Larsen 1991). One study only assessed participants who returned with pain, so it was assumed that the other participants who did not return did not have dry socket (Reekie 2006).
Selective reporting
Only the reporting of dry socket was considered for this item for the prevention trials. Most trials reported this well and were considered at low risk of bias. Six trials were unclear in their reporting (Abu‐Mostafa 2015; Faizel 2015; Reekie 2006; Shi 2003; Supe 2018; Trieger 1991) and three were considered to be at high risk of reporting bias (Ahmedi 2016; Hermesch 1998; Ragno 1991). Hermesch 1998 only reported dry socket for extracted mandibular third molars although non‐mandibular third molars were also extracted concurrently. Ragno 1991 did not report any data from the questionnaire completed by participants on day 7.
Other potential sources of bias
Thirty‐five of the trials (71%) were considered to be at low risk of bias from other sources. 6 trials (12%) were deemed unclear in this respect (Faizel 2015; Keshini 2020; King 2018; Reekie 2006; Shi 2003; Trieger 1991). The reporting in Trieger 1991 in general was very poor and it was not possible to make a clear judgement in many domains. 8 of the trials (16%) in this review were deemed to be at a high risk of bias from other sources. Of the high risk trials, investigators in Hermesch 1998 randomised at an individual participant level but subsequently analysed participants at an extraction site level; similarly Larsen 1991 randomised individuals, however, subsequent analyses were at tooth level.
Overall assessment of bias
All domains had to be assessed as being at low risk of bias for a study to be considered low risk of bias. If any domain was assessed as being at high risk of bias, the study was assessed as high risk of bias, the remainder were assessed as unclear. Figure 2 presents the review authors' judgements about each risk of bias item presented as percentages across all included studies and Figure 3 presents review authors' judgements about each risk of bias item for each included study.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Three studies were assessed as being at low risk of bias overall (Freudenthal 2015; Mitchell 1984; Torres‐Lagares 2006b), 30 were deemed unclear (Burgoyne 2010; Chaurasia 2017; Cho 2018; Delilbasi 2002; Divya 2019; Faizel 2015; Feng 2009; Gersel‐Pedersen 1979; Ghaeminia 2017; Halabi 2018; Hasheminia 2018; Hu 2005; Kaya 2011; Keshini 2020; King 2018; Kjellman 1973; Lenka 2019; Ritzau 1977; Rodriguez‐Perez 2013; Rubio‐Palau 2015; Shad 2018; Shi 2003; Sun 2007; Supe 2018; Trieger 1991; Tuk 2019; Unsal 2018; van Eeden 2006; Xue 2013; Yuce 2019), and the 16 remaining studies were deemed as being at high risk of bias overall (Abu‐Mostafa 2015; Abu‐Mostafa 2019; Ahmedi 2016; Alissa 2010; Babar 2012; Bai 2011; Haraji 2013; Hermesch 1998; Hita‐Iglesias 2008; Huang 2011; Karabit 2019; Larsen 1991; Metin 2006; Ragno 1991; Reekie 2006; Torres‐Lagares 2006a).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Prevention
Forest plots and summary of findings tables have only been included when there was more than one study for a specific comparison.
Comparison 1: chlorhexidine rinse versus placebo
Primary outcome: presence of dry socket
Six trials involving 1547 participants compared rinsing with chlorhexidine at 0.12% concentration (Halabi 2018; Hermesch 1998; Karabit 2019; Larsen 1991; Ragno 1991) and 0.2% concentration (Delilbasi 2002), both pre and postextraction, to rinsing with a placebo for the prevention of dry socket. One trial (Karabit 2019) was of a split‐mouth design, so generic inverse variance was used to calculate the odds ratio (OR) as described in Stedman 2011. The overall pooled estimate of the six trials showed a benefit in rinsing with chlorhexidine to prevent dry socket with an overall OR of 0.38 (95% confidence interval (Cl) 0.25 to 0.58; 6 studies, 1547 participants; moderate‐certainty evidence). There was moderate heterogeneity (I2 = 48%). As there is only a single study using 0.2% chlorhexidine, the comparison between subgroups is inappropriate. The number of patients needing to use chlorhexidine rinse to prevent one patient developing dry socket (number needed to treat (NNT)) was 162 (95% CI 155 to 240), 33 (95% CI 27 to 49), and 7 (95% CI 5 to 10) for control prevalence of dry socket 0.01, 0.05, and 0.30 respectively (Analysis 1.1).
1.1. Analysis.

Comparison 1: Prevention of dry socket, Outcome 1: Chlorhexidine rinse versus placebo
Adverse events
Additional Table 7 presents reports of any adverse events observed. Three trials, with 1137 participants, reported no adverse events (Halabi 2018; Karabit 2019; Larsen 1991). Two studies reported alteration in taste and bad taste (Delilbasi 2002; Ragno 1991). Delilbasi 2002 found 15% (18/118) of participants using chlorhexidine reported alterations in taste. Ragno 1991 reported that 7.5% (3/40) of participants using chlorhexidine complained of a bad taste from the rinse. One study also reported that 10% of participants (10/118) had staining of their teeth/dentures (Delilbasi 2002). One study (Hermesch 1998) reported many adverse events in both the experimental group and the control group, with 16.2% of the subjects in the chlorhexidine group and 23% of the placebo group reporting a range of adverse events, including paraesthesia, headaches, gingivitis, pain, and pharyngitis (see Additional Table 7 for full details). The study authors can only attribute a single case of stomatitis to the use of chlorhexidine (Hermesch 1998).
2. Adverse events reported in included studies for prevention of dry socket.
| Author/study | Intervention | Adverse events |
| Abu‐Mostafa 2015 | 0.2% chlorhexidine gel versus 0.12% chlorhexidine rinse. 0.12% chlorhexidine rinse group rinsed twice daily for 1 week. Chlorhexidine gel group had intrasocket application of 0.2% chlorhexidine gel immediately postoperatively and also on day 3. All patients given ibuprofen 600 mg every 8 hours for 3 days | No adverse events reported |
| Abu‐Mostafa 2019 | Honey versus 0.2% chlorhexidine rinse. Patients in the honey group had Manuka honey applied topically to the socket immediately postoperatively and also on day 3. Patients in the chlorhexidine group rinsed with 0.2% chlorhexidine twice daily for 7 days. All patients instructed to take 400 mg ibuprofen every 8 hours on 1st and 2nd day postoperatively | No adverse events reported |
| Ahmedi 2016 | Gaseous ozone. In the experimental group intra‐alveolar O3 was used to irrigate the socket for 12 seconds prior to suturing. All patients given 400 mg ibuprofen | No adverse events reported |
| Alissa 2010 | Platelet rich plasma. Patients all given Co‐codamol to take postoperatively if needed | No adverse events reported |
| Bai 2011 | Acellular dermal matrix | No adverse events reported |
| Babar 2012 | 0.2% chlorhexidine gel. In experimental group 0.2% chlorhexidine gel was placed into the socket immediately postoperatively. All patients received 400 mg ibuprofen | No adverse events reported |
| Cho 2018 | Irrigation of the surgical site with 0.2% chlorhexidine versus 0.2% chlorhexidine mouthrinse. The irrigation group used 0.2% chlorhexidine in a monoject syringe to irrigate the socket twice daily for 7 days. The control group used 0.2% chlorhexidine as a mouthrinse twice daily for 7 days. All patients prescribed paracetamol/codeine 500/15 mg 1 to 2 tablets every 4 to 6 hours and 200 mg ibuprofen 1 to 2 tablets every 4 to 6 hours | No adverse events reported |
| Delilbasi 2002 | 0.2% chlorhexidine rinse. Paracetamol for postoperative pain relief. Patients rinsed with 15 ml of chlorhexidine solution "just before tooth removal" (page 302). Intraoperatively the surgical site was irrigated with 15 ml of chlorhexidine solution diluted with 15 ml of saline. The day after surgery patients began rinsing with 15 ml chlorhexidine, twice daily for 7 days | Adverse events reported for Group 1 n = 62 Allergy: n = 0 Staining of teeth: n = 4 Mucosal irritation: n = 0 Alteration in taste: n = 12 Gastrointestinal complaints: n = 0 Bad taste: n = 8 No adverse reactions: n = 38 (From Table IV, page 303) |
| Divya 2019 | Herbal mouthwash versus 0.2% chlorhexidine mouthrinse. Each group rinsed with their allotted mouthrinse twice daily for 7 days | No adverse events reported |
| Feng 2009 | Oral tissue patch versus platelet rich plasma versus oral tissue patch + platelet rich plasma versus control group | No adverse events reported |
| Freudenthal 2015 | 0.2% chlorhexidine gel versus placebo gel. Patients had the allocated gel placed in the socket prior to suturing. All patients prescribed paracetamol and paracetamol/codeine | No adverse events reported |
| Gersel‐Pedersen 1979 | Trans‐4‐amino‐methyl‐cyclohexane acid (AMCA) cones versus lactose cones. All had 0.2% chlorhexidine 3 times a day | Foreign body reaction to the vehicle delivery system in the cones |
| Ghaeminia 2017 | Tap water. Experimental group was instructed to irrigate extraction socket with tap water in a monoject syringe 4 times daily. All patients 1 g paracetamol 4 times daily and ibuprofen 600 mg 3 times daily | No adverse events |
| Halabi 2018 | 0.12% chlorhexidine mouthrinse. Experimental group rinsed with 0.12% chlorhexidine mouthrinse twice daily for 7 days. The control group rinsed with sterile water | No adverse events reported |
| Haraji 2013 | 0.2% chlorhexidine gel versus dry dressing. Experimental group had a gelatin sponge saturated in 0.2% chlorhexidine gel packed into the socket prior to suturing | No adverse events reported |
| Hasheminia 2018 | Povidone iodine 1% mouthwash. Experimental group had gauze soaked in 1% iodine placed over teeth preoperatively and also 1% iodine was used intraoperatively. All patients 400 mg ibuprofen every 6 hours for 3 days | No adverse events reported |
| Hermesch 1998 | 0.12% chlorhexidine rinse. Participants in the experimental group rinsed for 30 seconds twice per day with 15 ml chlorhexidine 7 days preoperatively. On day of surgery supervised rinse before anaesthesia and surgery. Participants suspended rinsing for the remainder of the day and recommenced the next day | Adverse events: Group 1 chlorhexidine n = 136 and Group 2 placebo n = 135 Paraesthesia reported: chlorhexidine 9, placebo 5 Infection: chlorhexidine 4, placebo 3 Trismus: chlorhexidine 0, placebo 5 Gingivitis: chlorhexidine 1, placebo 3 Glossitis: chlorhexidine 2, placebo 2 Abnormal healing: chlorhexidine 1, placebo 3 Nausea: chlorhexidine 0, placebo 4 Sinusitis: chlorhexidine 1, placebo 3 Headache: chlorhexidine 1, placebo 2 Dysphagia: chlorhexidine 0, placebo 2 Oedema (head and neck): chlorhexidine 1, placebo 1 Haemorrhage (prolonged): chlorhexidine 1, placebo 1 Pain: chlorhexidine 1, placebo 1 Pharyngitis: chlorhexidine 1, placebo 1 Rash: chlorhexidine 1, placebo 1 There were single site observations of each of the following: asthenia, bronchitis, cyst, depression, contact dermatitis, dyspepsia, ecchymosis, fever, herpes simplex, hypalgesia, back pain, rhinitis, stomatitis, and tenosynovitis. All but 2 cases of paraesthesia had resolved by end of study, 1 in each treatment group (From Table VII, page 384) |
|
Hita‐Iglesias 2008 |
Chlorhexidine 0.2% gel versus 0.12% chlorhexidine mouthrinse. Participants in the 0.2% chlorhexidine gel group had the gel placed in the socket during surgery and then they were required to apply the gel to the socket twice a day (morning and night‐time) for 7 days beginning on the same day as the surgery. Patients in the rinse group rinsed twice a day (morning and night‐time) for 7 days beginning on the same day as the surgery | No adverse events reported |
| Hu 2005 | Oral tissue patch | No adverse events reported |
| Huang 2011 | Intradermal matrix | No adverse events reported |
| Karabit 2019 | 0.12% chlorhexidine mouthrinse versus aqua distillate with mint flavour. Patients rinsed with their allocated mouthrinse on twice daily on the day before the extraction and for 7 days postoperatively | No adverse events reported |
| Kjellman 1973 | Apernyl as alveolar | Pain and burning sensation (page 200) |
| )Larsen 1991 |
0.12% chlorhexidine rinse versus placebo. All patients received 8 mg dexamethasone (glucocorticoid) IV prior to surgery. Participants were required to rinse twice per day for 30 seconds using 15 ml of the solution for 7 days prior to the surgery. On the day of the surgery they rinsed with the solution immediately prior to surgery (using 15 ml) and postoperatively patients were instructed to begin rinsing the day following surgery | No adverse reactions (page 954) |
|
Metin 2006 |
0.2% chlorhexidine rinse 7 days preoperatively and 7 days postoperatively (group I) versus 7 days postoperatively only (group II) |
Altered taste and numbness (page 3). Numbness in the tongue reported in group I and group II 45.6% and 13.2%. However, disturbance of taste sensation was seen in 56.5% of the patients in group I and in 11.3% of the patients in group II |
|
Ragno 1991 |
0.12% chlorhexidine rinse. Participants rinsed immediately before surgery, the surgical was irrigated intraoperatively and starting the day after the surgery participants were asked to rinse with solution twice daily (15 ml) for 7 days postoperatively |
There were no allergic reactions to the chlorhexidine rinse. 3 participants reported bad taste, 1 reported stomach upset (page 526) but no staining noted. 1 person in the control had a severe surgical reaction which in the author's opinion was not attributable to the medication |
|
Reekie 2006 |
0.25 ml of 25% metronidazole gel intrasocket intervention |
1 participant with nausea and vomiting, 2 complained of a bitter taste |
| Ritzau 1977 | PEPH (p‐hydroxybenzoic acid) | Haematoma and rash |
| Rodriguez‐Perez 2013 | 1% chlorhexidine gel versus 0.2% chlorhexidine gel. All patients had 0.2% chlorhexidine gel placed into the socket prior to suturing. The patients also applied either 0.2% or 1% chlorhexidine gel to the wound twice daily for 7 days postoperatively. All patients 600 mg ibuprofen every 8 hours and 1 g paracetamol every 12 hours | No adverse events reported |
| Rubio‐Palau 2015 | 0.2% chlorhexidine gel versus placebo gel. Experimental group had 0.2% chlorhexidine gel placed in extraction socket prior to suturing. Placebo gel was placed in socket in control group. All patients given diclofenac 50 mg every 8 hours alternated with metamizole 575 mg every 8 hours and omprazole 20 mg per day | 30 (18%) patients gastrointestinal discomfort 10 (6.2%) patients dizziness 2 (1.2%) patients skin rash Study authors attribute these adverse events to the use of the analgesic metamizole (dipyrone) No adverse events reported in relation to chlorhexidine gel |
| Shad 2018 | 0.2% chlorhexidine gel versus placebo gel. Either 0.2% chlorhexidine gel or placebo gel was placed in socket prior to suturing | No adverse events reported |
| Shi 2003 | Artemisia desertorum Spreng (Shahaosan, Yunnan) | No adverse events reported |
| Sun 2007 | Acellular dermal matrix | No adverse events reported |
|
Torres‐Lagares 2006a |
0.2% chlorhexidine gel intrasocket versus placebo. Participants in the 0.2% chlorhexidine gel group had the gel placed in the socket during surgery. Note only 1 application of bioadhesive gel during surgery | No adverse events reported |
|
Torres‐Lagares 2006b |
0.2% chlorhexidine gel intrasocket versus placebo. Participants in the 0.2% chlorhexidine gel group had the gel placed in the socket during surgery. Note only 1 application of bioadhesive gel during surgery | No adverse events reported |
| Trieger 1991 | Gelfoam clindamycin | No adverse events reported |
| Tuk 2019 | 1 x 2 cam iodine tampon placed in surgical site in experimental group. All patients used 0.12% chlorhexidine mouthrinse twice daily for 7 days postoperatively | No adverse events reported |
| Unsal 2018 | Platelet rich fibrin | No adverse events reported |
|
van Eeden 2006 |
Gelfoam covomycin. 0.2% chlorhexidine advised postoperatively every 6 hours for 5 days | Some events may be attributable to intervention or could be normal sequelae of operation |
| Xue 2013 | Recombinant bovine fibroblast growth factor gel | No adverse events reported |
n = number.
Secondary outcomes
Quality of life
No studies reported this outcome.
Patient satisfaction
No studies reported this outcome.
Costs
No studies reported this outcome.
Comparison 2: chlorhexidine gel versus placebo/no treatment
Primary outcome: presence of dry socket
Seven trials compared placing chlorhexidine gel in the extraction socket versus placebo dressing or no treatment (Babar 2012; Freudenthal 2015; Haraji 2013; Rubio‐Palau 2015; Shad 2018; Torres‐Lagares 2006a; Torres‐Lagares 2006b). One trial (Haraji 2013) was of a split‐mouth design, so generic inverse variance was used to calculate the OR as described in Stedman 2011. The meta‐analysis showed the use of chlorhexidine gel in extraction sockets reduced the odds of dry socket (OR 0.44, 95% CI 0.27 to 0.71; P = 0.0008; 7 studies, 753 participants; moderate‐certainty evidence). There was moderate heterogeneity within the data (I2 = 44%). The number of patients needing to have chlorhexidine gel placed in an extraction socket to prevent one dry socket (NNT) was 180 (95% CI 137 to 347), 37 (95% CI 28 to 72), and 7 (95% CI 5 to 15) for control prevalences of dry socket of 0.01, 0.05, and 0.30 respectively (Analysis 1.2).
1.2. Analysis.

Comparison 1: Prevention of dry socket, Outcome 2: Chlorhexidine gel versus placebo/no treatment
Adverse events
Six trials, with 673 participants reported no adverse events from the use of chlorhexidine gel. One study reported that 30 (18%) patients experienced gastrointestinal discomfort, 10 (6.2%) patients dizziness, and 2 (1.2%) patients had a skin rash (Rubio‐Palau 2015). The study authors attribute these adverse events to the use of the analgesic metamizole (dipyrone) and they report no adverse events in relation to the use of chlorhexidine gel.
Secondary outcomes
Quality of life
No studies reported this outcome.
Patient satisfaction
No studies reported this outcome.
Costs
No studies reported this outcome.
Comparison 3: chlorhexidine gel versus chlorhexidine rinse
Primary outcome: presence of dry socket
Two studies, with 383 participants, compared postoperative rinsing with chlorhexidine (0.12%) with application of chlorhexidine gel (0.2%) both intraoperatively and also postoperatively (Abu‐Mostafa 2015; Hita‐Iglesias 2008). The meta‐analysis shows no benefit in using chlorhexidine gel over chlorhexidine rinse. The risk ratio (RR) was 0.74 (95% CI 0.46 to 1.20; P = 0.22; 2 studies, 383 participants; low‐certainty evidence), with substantial heterogeneity (Chi2 = 2.39, degrees of freedom (df) = 1; P = 0.12; I2 = 58%) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Prevention of dry socket, Outcome 3: Chlorhexidine gel versus chlorhexidine rinse
Adverse events
No adverse events were reported in these two trials (Abu‐Mostafa 2015; Hita‐Iglesias 2008).
Secondary outcomes
Quality of life
No studies reported this outcome.
Patient satisfaction
No studies reported this outcome.
Costs
No studies reported this outcome.
Comparison 4: platelet rich plasma versus placebo
Primary outcome: presence of dry socket
Two studies compared placement of platelet rich plasma in extraction sockets following extractions compared with leaving the sockets empty (Alissa 2010; Feng 2009). Platelet rich plasma was not superior (or inferior) to placebo (RR 0.51, 95% CI 0.19 to 1.33; P = 0.17; 2 studies, 127 participants; very low‐certainty evidence). There was no evidence of any heterogeneity within this sample (I2 = 0%) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Prevention of dry socket, Outcome 4: Platelet rich plasma versus placebo
Adverse events
No adverse events were reported in these two trials.
Secondary outcomes
Quality of life
No studies reported this outcome.
Patient satisfaction
No studies reported this outcome.
Costs
No studies reported this outcome.
Comparisons for prevention of dry socket, only including a single study
The remaining 21 interventions to prevent dry socket were each evaluated in single studies as described below, and there is insufficient evidence to determine their effects. The results from these studies are shown in Additional Table 8.
3. Results for prevention of dry socket (single studies).
| Comparison | Data | RR/OR (95% CI) | P value |
| Honey versus 0.2% chlorhexidine rinse (Abu‐Mostafa 2019) | Honey 7/57 0.2% chlorhexidine rinse 4/43 |
RR 1.32 (0.41 to 4.22) | 0.64 |
| Gaseous ozone versus placebo (Ahmedi 2016) | Gaseous O3 1/30 Placebo 5/30 |
RR 0.20 (0.02 to 1.61) | 0.13 |
| Acellular dermal matrix patch versus no treatment (Bai 2011) | Patch 1/200 No patch 15/200 |
RR 0.07 (0.01 to 0.50) | 0.008 |
| Irrigation of the surgical site with 0.2% chlorhexidine in a monoject versus 0.2% chlorhexidine mouthrinse (Cho 2018) | Monoject with chlorhexidine 4/47 Chlorhexidine rinse 16/48 |
RR 0.26 (0.09 to 0.71) | 0.009 |
| Herbal mouthrinse versus 0.2% chlorhexidine mouthrinse (Divya 2019) | Herbal 1/25 Chlorhexidine 2/25 |
RR 0.50 (0.05 to 5.17) | 0.56 |
| Tranexamic acid versus placebo (Gersel‐Pedersen 1979) | Split‐mouth AMCA Y, placebo Y = 3 AMCA Y, placebo N = 6 AMCA N, placebo Y = 3 AMCA N, placebo N = 108 |
RR 0.67 (0.30 to 1.48) |
0.51 |
| Irrigating with monoject syringe filled with tap water versus no rinsing (Ghaeminia 2017) | Monoject with tap water 9/158 No rinsing 28/178 |
RR 0.36 (0.18 to 0.74) | 0.006 |
| Povidone iodine 1% versus control (Hasheminia 2018) | Iodine 5/97 Control 13/92 |
RR 0.36 (0.14 to 0.98) | 0.05 |
| Oral tissue patch versus placebo (Hu 2005) | Tissue patch 1/100 Placebo 8/100 |
RR 0.13 (0.02 to 0.98) | 0.05 |
| Heal‐all tissue patch versus no treatment (Huang 2011) | Patch 0/40 No patch 6/40 |
RR 0.08 (0.00 to 1.32) | 0.08 |
| Apernyl versus placebo (Kjellman 1973) | Apernyl 1/50 Placebo 4/50 |
RR 0.25 (0.03 to 2.16) | 0.21 |
| Rinsing with chlorhexidine both pre and postextraction versus rinsing just postextraction (Metin 2006) | Pre & post 3/46 Post 6/53 |
RR 0.58 (0.15 to 2.17) | 0.42 |
| 1% chlorhexidine gel versus 0.2% chlorhexidine gel (Rodriguez‐Perez 2013) | 1% chlorhexidine gel 3/42 0.2% chlorhexidine gel 6/46 |
RR 0.55 (0.15 to 2.05) | 0.37 |
| Metronidazole gel (postoperatively) versus placebo gel (Reekie 2006) | Metronidazole 8/152 Placebo 15/150 |
RR 0.53 (0.23 to 1.20) | 0.13 |
| P‐hydroxybenzoic acid versus placebo (Ritzau 1977) | P‐hydro 0/24 Placebo 5/21 |
RR 0.08 (0.00 to 1.37) | 0.08 |
| Chinese herbs Shahaosan versus placebo (Shi 2003) | Shahaosan 1/92 Placebo 8/96 |
RR 0.13 (0.02 to 1.02) | 0.05 |
| Chinese herbs Yunnan versus placebo (Shi 2003) | Yunnan 2/86 Placebo 8/96 |
RR 0.28 (0.06 to 1.28) | 0.10 |
| Chinese herbs Shahaosan versus Yunnan (Shi 2003) | Shahaosan 1/92 Yunnan 2/86 |
RR 0.47 (0.04 to 5.06) | 0.53 |
| Acellular dermal matrix versus control (Sun 2007) | Dermal matrix 2/150 Control 13/150 |
RR 0.15 (0.04 to 0.67) | 0.01 |
| Iodine tampon versus rinsing with saline in a syringe (Tuk 2019) | Iodine tampon 0/54 Monoject with saline 0/54 |
No events | ‐ |
| Clindamycin phosphate antibiotic solution patch versus saline patch (Trieger 1991) | Split‐mouth Clindamycin only = 0 Solution only = 7 Both = 0 Neither = 79 Total N = 86 |
OR (exact) 0 (0 to 0.69) | 0.016 |
| Platelet rich fibrin versus control (Unsal 2018) | Platelet rich fibrin 4/50 Control 9/50 |
RR 0.44 (0.15 to 1.35) | 0.15 |
| Glucocorticosteroid antibiotic agent (postoperatively) versus normal saline (van Eeden 2006) | Split‐mouth Glucocorticosteroid only = 0 Normal saline only = 3 Both = 0 Neither = 16 Total N = 19 |
OR (exact) 0 (0 to 2.42) |
0.25 |
| Bovine fibroblastic growth factor versus control (Xue 2013) | Growth factor 1/80 Control 10/80 |
RR 0.10 (0.01 to 0.76) | 0.03 |
CI = confidence interval; N = number; RR/OR = risk ratio/odds ratio.
Acellular dermal matrix patch versus no treatment (Bai 2011; Sun 2007).
Apernyl versus placebo (Kjellman 1973).
Artemisia desertorum Spreng (Shahaosan or Yunnan) versus placebo control (Shi 2003).
Bovine fibroblastic growth factor versus control (Xue 2013).
Chlorhexidine gel 0.2% versus chlorhexidine gel 1% (Rodriguez‐Perez 2013).
Chlorhexidine 0.2% rinse both pre and postoperatively versus postoperatively only (Metin 2006).
Chlorhexidine 0.2% in a monoject syringe versus plain rinsing with chlorhexidine 0.2% (Cho 2018).
Clindamycin phosphate antibiotic solution patch versus saline patch (Trieger 1991).
Gaseous ozone versus control (Ahmedi 2016).
Glucocorticosteroid antibiotic agent versus normal saline (van Eeden 2006).
Herbal mouthwash versus chlorhexidine rinse (Divya 2019).
Heal‐all tissue patch (2 x 2.5 cm) versus no treatment (Huang 2011).
Honey versus chlorhexidine rinse (Abu‐Mostafa 2019).
Iodine (1%) (pre and intraoperatively) versus control (Hasheminia 2018).
Iodine tampon versus monoject irrigation with saline (Tuk 2019).
Irrigation with tap water in monoject versus control (Ghaeminia 2017).
Metronidazole gel versus placebo gel (Reekie 2006).
Oral tissue patch versus control (Hu 2005).
P‐hydroxybenzoic acid versus placebo (Ritzau 1977).
Platelet rich fibrin versus control (Unsal 2018).
Tranexamic acid versus placebo (Gersel‐Pedersen 1979).
Adverse events
Additional Table 7 summarises the adverse events for all included studies.
Treatment
Comparison 5: zinc oxide eugenol versus Alvogyl/Alveogyl
Primary outcomes
Healing
Two studies (Faizel 2015; Supe 2018) looked at healing outcomes but used different outcome measures so they were not comparable. The results were consistent with sockets treated with Alvogyl appearing to heal faster than sockets treated with zinc oxide eugenol, but the variation in the outcome measures used did not allow a meta‐analysis to be carried out.
Supe 2018 reported 2/25 patients (8%) in the Alvogyl group and 9/25 patients (36%) in the zinc oxide eugenol group had delayed healing, classed as non‐healed sockets after 10 days indicating reduced adverse events for Alvogyl (RR 4.50, 95% CI 1.08 to 18.77; P = 0.04).
Pain ‐ VAS
Two studies (Lenka 2019; Supe 2018), with 80 participants, compared Alvogyl and zinc oxide eugenol for treatment of dry socket with visual analogue scores (VAS) (0 to 10) on day 7. The meta‐analysis shows that Alvogyl is more effective than zinc oxide eugenol at reducing pain at day 7 (mean difference (MD) ‐1.40, 95% CI ‐1.75 to ‐1.04; P < 0.00001; 2 studies, 80 participants; very low‐certainty evidence). The pooled data showed considerable heterogeneity (df = 1 (P = 0.05); I2 = 75%) (Analysis 2.1). These studies also provided VAS scores for other days which gave inconsistent results.
2.1. Analysis.

Comparison 2: Treatment of dry socket, Outcome 1: Alvogyl versus zinc oxide eugenol day 7 VAS score (0‐10)
Swelling
No studies reported this outcome.
Limitation of chewing or swallowing
No studies reported this outcome.
Fever
No studies reported this outcome.
Adverse events
No adverse events were reported.
Secondary outcomes
Quality of life
No studies reported this outcome.
Patient satisfaction
No studies reported this outcome.
Costs
No studies reported this outcome.
Comparisons for treatment of dry socket, only including a single study
The following comparisons to treat dry socket were evaluated in single studies as described below, and therefore there is insufficient evidence to determine their effects. The results from these studies are shown in Additional Table 6. Kaya 2011 has four arms. The data reported in Kaya 2011 cannot be used as medians are presented with error bars. The treatment comparisons were.
Topical anaesthetic gel (prilocaine‐lidocaine) versus eugenol (Burgoyne 2010).
Neocone versus zinc oxide eugenol versus Alvogyl (Faizel 2015).
Alvogyl versus no treatment (Kaya 2011).
SaliCept versus no treatment (Kaya 2011).
Alvogyl versus SaliCept (Kaya 2011).
Alvogyl versus plasma rich in growth factors (PRGF) (King 2018).
Alvogyl versus platelet rich fibrin (Keshini 2020).
Metronidazole versus placebo (Mitchell 1984).
Advanced platelet rich fibrin versus placebo (Yuce 2019).
Adverse events
No adverse events were reported for any of these studies.
Discussion
Summary of main results
There were 49 studies included in this review involving 6771 participants, which reported on 35 different interventions. Of the 49 included studies, 39 looked at prevention and 10 looked at treatment of dry socket. The quality of the trials was mixed.
There was moderate‐certainty evidence from six trials, with 1547 participants, comparing chlorhexidine rinse with placebo that rinsing with chlorhexidine perioperatively reduces the risk of developing dry socket. In all the included trials the participants rinsed with chlorhexidine for 7 days postoperatively, starting 24 hours after the extraction. In five out of the six studies the participants also rinsed preoperatively. As the 0.2% chlorhexidine subgroup included only one study, comparisons between subgroups are inappropriate. The number of patients needing to be treated to prevent one patient having dry socket (NNT) varied considerably depending on the prevalence of dry socket. For a 30% prevalence, as might be anticipated following surgical removal of a mandibular third molar, the NNT was 7 (95% confidence interval (CI) 5 to 10) but if the control prevalence is 5%, in line with commonly predicted risk following routine extractions, then the NNT increased to 33 (95% CI 27 to 49) (Table 1). This suggests that rinsing with chlorhexidine may have more of an impact following surgical removal of impacted wisdom teeth.
There was also moderate‐certainty evidence from seven studies, with 753 participants, that placing 0.2% chlorhexidine gel in a socket following extraction is an effective preventative therapy and will reduce the risk of a dry socket. In all these studies the chlorhexidine gel was only applied once, at the end of surgery, prior to suturing. Again the NNT varied considerably from 7 (95% CI 5 to 15) for control prevalence of 30% to 37 (95% CI 28 to 72) for control prevalence of 5% (Table 2).
There is low‐certainty evidence from two studies, with 383 participants, that rinsing with chlorhexidine mouthrinse and placement of 0.2% chlorhexidine gel are equally effective at reducing the risk of developing a dry socket (Table 3).
Placement of platelet rich plasma in extraction sockets was not superior in reducing the risk of having a dry socket (risk ratio (RR) 0.51, 95% CI 0.19 to 1.33; P = 0.17; 2 studies, 127 participants; very low‐certainty of evidence) (Table 4).
The studies reporting on the treatment of dry socket presented a number of different outcomes which made comparison of the results difficult. Pain was an outcome in all the treatment studies but it was measured in different ways and at different time points. There was very low‐certainty evidence from two studies involving 80 participants that placing Alvogyl in a dry socket was more effective than zinc oxide eugenol at reducing pain at day 7, but inconsistent results were found for other time periods (Table 5). It appears that these studies were all using the old formulation of Alvogyl, which contained iodoform, butamben, and eugenol. It should be noted that the newer formulation, branded as Alveogyl, contains eugenol only so these results may not be comparable.
The remaining interventions for the prevention of dry socket and the interventions for the treatment of dry socket were only looked at in single studies and therefore there is insufficient evidence to determine their effects.
Overall completeness and applicability of evidence
A comprehensive search strategy was employed and it is likely that the majority of published trials are included in this review. It is uncertain how many unpublished trials there are as some of the interventions identified were developed by industry and findings from these trials were not always reported in the past.
Most studies included in this review reported on prevention of dry socket after surgical removal of third molars. Only five studies reported on the prevention of dry socket after routine dental extractions (Abu‐Mostafa 2015; Abu‐Mostafa 2019; Alissa 2010; Halabi 2018, Reekie 2006). Two of the included studies were conducted in general dental practice settings (Cho 2018; Reekie 2006) but the majority involved extractions undertaken by experienced oral surgeons in hospital or minor oral surgery clinics. However, most extractions in dentistry are routine extractions undertaken in primary dental care, by general dental practitioners on teeth other than lower third molars. Most extractions do not involve surgical removal of the tooth. It is important that future well‐designed trials of interventions to treat and prevent dry socket are conducted in primary dental care settings. Such studies should recruit patients who are having a range of tooth type extractions including molar and premolar teeth. It is also important that power calculations inform the sample size of the proposed study to ensure trials are large enough to detect clinically important effects of interventions including hypersensitivity reactions and adverse events.
Seven of the trials in this review employed a split‐mouth design, and one trial (Tuk 2019) employed a cross‐over design. Split‐mouth designs are appropriate when the disease is stable and uniformly distributed and the effects of the intervention are short‐lived or reversible (Antczak‐Bouckoms 1990). There is evidence from one trial that development of dry socket increases the risk of developing dry socket in the future (Reekie 2006), therefore this could compromise the use of cross‐over studies, however, split‐mouth studies appear to be appropriate for looking at the prevention of dry socket. It is also important that participants and observers are blinded to the intervention allocation if possible.
There was considerable variation in the design of trials, inclusion and exclusion criteria, definition of dry socket, presurgical regimens, intraoperative procedures, and postoperative medications. The generalisability of the trials was compromised in some trials by excluding smokers and women on oral contraceptive therapy. In many trials, the existence of pericoronitis and infection was a cause for exclusion. These exclusion criteria have the potential for studies to include patient groups where the risk factors for dry socket are reduced so dry socket is less likely to happen.This effect was balanced by the predominance of studies that largely reported on extraction of third molars where the prevalence of dry socket is higher compared to extraction of other teeth. Also, two studies included participants considered at high risk to alveolar osteitis (smokers and those experiencing alveolar osteitis previously) (Halabi 2018; Karabit 2019).
Dry socket is a common consequence of tooth extraction and it is important that this review presents the latest evidence for prevention and treatment. A range of interventions reported in this review reflect prevailing theories of dry socket causation at the time the study was undertaken.
Quality of the evidence
The criteria for assessing overall risk of bias were strict (all domains assessed had to be at low risk of bias for the trial to be deemed at low risk of bias) and only 3 studies (6%) were assessed as being at low risk of bias. Of the remainder, 30 (61%) were assessed as being at unclear risk of bias, and 16 (33%) were assessed at high risk of bias. The quality of reporting could be improved by authors reporting their studies in line with CONSORT and, where possible, undertaking double‐blind trials with adequate outcome assessment. Some studies had to be excluded as they did not have an adequate definition of dry socket and some appeared to conflate infection with dry socket. There was also variation in the secondary outcomes reported in the trials and many did not match the ones included in the protocol for the present review. This was a particular issue with outcomes related the treatment of dry socket specifically in relation to healing and pain. There was also generally inadequate reporting of adverse events.
Twenty‐four studies, with 3609 participants, using chlorhexidine in either mouthrinse or gel form were included in this review. Four studies reported some adverse events associated with the use of chlorhexidine mouthrinse. No studies reported adverse events associated with the use of chlorhexidine gel. Two of the studies reporting adverse events were using 0.12% chlorhexidine mouthrinse (Hermesch 1998; Ragno 1991) and two studies were using 0.2% chlorhexidine mouthrinse (Delilbasi 2002; Metin 2006). Adverse events included: staining of teeth, altered taste and bad taste, gastrointestinal upsets, numbness and paraesthesia. Altered taste and bad taste were the most commonly reported adverse events (Delilbasi 2002; Metin 2006; Ragno 1991). Rubio‐Palau 2015 reported a number of adverse reactions including a skin rash in two patients but the authors attributed these to the use of the analgesic metamizole (dipyrone). None of the included studies reported an allergic reaction to chlorhexidine‐based products, though it should be noted that most of the studies had allergy to chlorhexidine as exclusion criteria.
A Cochrane Review looking at chlorhexidine for reduction of gingivitis (James 2017) found that, apart from staining and calculus formation, the most commonly reported adverse events in the chlorhexidine rinse arms of the included studies were taste disturbance/alteration (reported in 11 studies), effects on the oral mucosa including mucosal irritation, soreness, mild desquamation, mucosal ulceration/erosions and oral mucosal lesions (reported in 13 studies), and a general burning sensation or a burning tongue or both (reported in nine studies). They include no reports of allergic reaction to chlorhexidine (James 2017). There is some evidence from in vitro studies that chlorhexidine may cause a dose‐dependent inhibition of gingival fibroblast proliferation and collagen production, which could potentially negatively affect wound healing (Mariotti 1999), though there is a lack of evidence from clinical studies to support this. The studies included in this review, which looked at chlorhexidine use, did not report on socket healing.
While serious adverse effects and events are rare, it is recommended that all members of the dental team prescribing chlorhexidine mouthwashes and gels for the management of dry socket are aware of the potential for side effects, are competent to manage a medical emergency associated with anaphylaxis, and warn their patients of the potential for adverse events.
Potential biases in the review process
Reporting inconsistency and missing data meant that some studies, particularly the older ones, could not be entered into the meta‐analysis. Also, there were too few studies in some interventions (e.g. platelet rich plasma (only two studies reported)). Both issues could have introduced bias. Additionally, we had too few studies for both prevention and treatment studies to assess publication bias.
Agreements and disagreements with other studies or reviews
Four systematic reviews concluded that rinsing perioperatively with chlorhexidine mouthrinse was effective in preventing dry socket after extractions (Canullo 2020; Caso 2005; Hedstrom 2007; Rodríguez Sánchez 2017). Three of these reviews restricted inclusion to studies reporting on surgical removal of mandibular third molars (Caso 2005; Hedstrom 2007; Rodríguez Sánchez 2017). The findings from the present review are mostly consistent with the findings reported in these reviews. Contrary to our results, Canullo 2020 carried out a subgroup analysis and found that 0.2% chlorhexidine mouthrinse did not significantly reduce the risk of dry socket. Hedstrom 2007 concluded that while it could not be determined that perioperative rinsing with 0.12% chlorhexidine prevented dry socket in all extractions, there was evidence that perioperative rinsing with chlorhexidine could prevent dry sockets in lower third molar extractions.
Canullo 2020; Caso 2005; and Hedstrom 2007 included trials were systemic antibiotics were prescribed, provided both the experimental and control groups received the same regimen. As in this review, Rodríguez Sánchez 2017 excluded any trials prescribing systemic antibiotics. The NNT from the latter's review was eight. Yengopal 2012 included a different group of studies to the present review. In contrast to our review, Yengopal 2012 concluded that chlorhexidine rinse had not been conclusively shown to be significantly better than placebo for reducing the incidence of alveolar osteitis (dry socket) after tooth extraction.
Two systematic reviews looking at surgical removal of mandibular third molars (Canellas 2020; Rodríguez Sánchez 2017) and one systematic review included all tooth types extractions (Canullo 2020) with the latter concluding that 0.2% chlorhexidine intrasocket gel significantly reduced the risk of developing a dry socket after extractions. Two of the reviews (Canullo 2020; Rodríguez Sánchez 2017) concluded, in line with our results, that chlorhexidine gel was as effective as chlorhexidine mouthrinse in reducing the risk of dry socket.
Two systematic reviews looking at placement of platelet rich plasma to aid healing of extraction sockets concluding that there was insufficient data to produce a meta‐analysis with regard to dry socket (Barona‐Dorado 2014; Franchini 2019).
Four systematic reviews (Canellas 2020; Canellas 2017; Xiang 2019; Zhu 2020) concluded that placement of platelet rich fibrin in extraction sockets significantly reduced the risk of dry socket, this is in contrast to our findings. The inclusion criteria for these four reviews differed from the present review as they included trials where systemic antibiotics had been prescribed. Two systematic reviews looked at plasma rich in growth factor with one review, Del Fabbro 2019, concluding that plasma rich in growth factor reduces the risk of dry socket, and one review (Xu 2019) concluding that there was insufficient data for a meta‐analysis.
Authors' conclusions
Implications for practice.
There is moderate‐certainty evidence that rinsing perioperatively with 0.12% and 0.2% chlorhexidine gluconate, probably results in a reduction in dry socket. There is moderate‐certainty evidence that chlorhexidine gel placed in the socket postextraction probably results in a reduction in dry socket. The decision to recommend rinsing with chlorhexidine should be balanced against reported adverse effects of tooth staining, taste alteration, and nausea. Adverse events attributable to the rinses were rare, but patients need to be aware and informed of the potential for adverse events associated with the use of chlorhexidine. There was limited evidence of very low certainty that Alvogyl (old formulation) may reduce pain at day 7 in patients with dry socket when compared to zinc oxide eugenol.
Implications for research.
More well‐designed trials in general dental practice settings with teeth other than third molars and including non‐surgical extractions are needed. Further studies comparing rinsing with chlorhexidine with intrasocket chlorhexidine gel to prevent dry socket would help to determine whether one intervention is better than another. Clinicians and researchers in this area need to decide collectively what outcomes should be measured in both prevention and treatment studies. All studies should specifically enquire into and carefully present data on any adverse events. More research is required into the effectiveness of treatment of dry sockets.
What's new
| Date | Event | Description |
|---|---|---|
| 4 May 2022 | New citation required and conclusions have changed | 28 new studies included in this update bringing the total to 49 included studies |
| 28 September 2021 | New search has been performed | Search updated |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 12, 2012
Acknowledgements
The review authors would like to acknowledge the assistance they have received from members of Cochrane Oral Health and for the helpful comments on this review from the peer reviewers: Dr Thomas James Lamont (Dundee Dental School and Hospital, University of Dundee), Philip Riley (School of Dentistry, The University of Manchester), Lasse A Skoglund (Barts and London School of Medicine and Dentistry), and Dr Louis W McArdle (Senior Specialist Clinical Teacher/Consultant Oral Surgeon, King's College London, GSTT NHS foundation Trust). The authors would like to thank Marina Gallagher and Natalie John of King's College London Dental Institute who obtained many of the references for the 2012 review. Thanks to the Cochrane team Anne Littlewood, Janet Lear, and Luisa M Fernandez Mauleffinch. The authors are grateful to the following: Professor Vasiliy Vlassov, Professor Ken Yaegaki, Dr Nian Fang, Ms Mona Nasser, Professor May Wong, Leia Yulei Qiu, Liliya Ziganshina, Sepideh Banava, and Dr Yuan Chi who kindly translated several papers into the English language which enabled assessment of their eligibility for this review. The authors would also like to thank Zbys Fedorowizc, Professor Tim Newton, and Mona Nasser for their contribution to protocol development, and Professor Tim Newton for his contribution to the 2012 review. Thanks to Zbys Fedorowizc for his contribution to information retrieval and screening of search results in earlier drafts of the 2012 review. Our thanks also to Dr Torres‐Lagares who confirmed that the three included studies that he was involved in each included completely different participants, and to Professor Nicola West for confirming details in relation to King 2018. We would like to thank Dr Huda Jawad for her very helpful assistance in understanding the change of formulation from Alvogyl to Alveogyl.
Cochrane Oral Health supported the authors in the development of this review update. The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision) and Editor (provided feedback on submitted draft to prepare for peer review): Anne‐Marie Glenny, Co‐ordinating Editor, Cochrane Oral Health, The University of Manchester, UK.
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, conducted editorial policy checks) and Copy Editor (copy edited final draft according to Cochrane style manual): Luisa M Fernandez Mauleffinch, Managing Editor, Cochrane Oral Health, The University of Manchester, UK.
Information Specialist (checked accuracy of search sections of the review): Anne Littlewood, Information Specialist, Cochrane Oral Health, The University of Manchester, UK.
Appendices
Appendix 1. Cochrane Oral Health’s Trials Register search strategy
Cochrane Oral Health’s Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see oralhealth.cochrane.org/trials.
#1 (("dry socket*" or "alveolar osteitis" or "alveolar ostitis" or alveolagia or "alveolar periostitis" or "alveolitis sicca dolorosa" or dry socket* or "septic socket*" or "necrotic socket osteomylitis" or "fibrinolytic alveolitis")) AND (INREGISTER) #2 ((infect* and socket*)) AND (INREGISTER) #3 (#1 or #2) AND (INREGISTER) #4 (extract* or remov* or surg*) AND (INREGISTER) #5 (#3 and #4) AND (INREGISTER)
Searches to January 2012 were conducted using the following strategy on the ProCite version of the register:
(("dry socket*" or "alveolar osteitis" or "alveolar ostitis" or alveolagia or "alveolar periostitis" or "alveolitis sicca dolorosa" or dry‐socket* or "septic socket*" or "necrotic socket osteomylitis" or "fibrinolytic alveolitis" or (infect* and socket*)) AND (extract* or remov* or surg*))
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Dry Socket this term only #2 ("alveolar osteitis" in All Text or "alveolar ostitis" in All Text) #3 ((septic in All Text near/4 socket* in All Text) or "necrotic socket osteomylitis" in All Text or "fibrinolytic alveolitis" in All Text) #4 (alveolalgia in All Text or "alveolar periostitis" in All Text or "alveolitis sicca dolorosa" in All Text) #5 ("dry socket*" in All Text or dry‐socket* in All Text or (infect* in All Text near/5 socket* in All Text)) #6 (#1 or #2 or #3 or #4 or #5) #7 MeSH descriptor Tooth Extraction explode all trees #8 ("dental extraction*" in All Text or (tooth in All Text near/3 extract* in All Text) or (teeth in All Text near/3 extract* in All Text) or (teeth in All Text near/3 remov* in All Text) or (tooth in All Text near/3 remov* in All Text)) #9 ((dental in All Text or oral in All Text) and (surgery in All Text or surgical in All Text)) #10 (#7 or #8 or #9) #11 (#6 and #10)
Appendix 3. MEDLINE Ovid search strategy
Dry socket/
("alveolar osteitis" or "alveolar ostitis").mp.
(alveolalgia or "alveolar periostitis" or "alveolitis sicca dolorosa").mp.
((septic adj4 socket*) or "necrotic socket osteomylitis" or "fibrinolytic alveolitis").mp.
("dry socket$" or dry‐socket$ or (infect$ adj5 socket$)).mp.
or/1‐5
exp Tooth Extraction/
("dental extraction$" or (tooth adj3 extract$) or (teeth adj3 extract$) or (teeth adj3 remov$) or (tooth adj3 remov$)).mp.
((dental or oral) and (surgery or surgical)).mp.
or/7‐9
6 and 10
The above subject search was linked with the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials in MEDLINE (as described in Lefebvre 2021, box 3.b).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. Dry socket/ 2. ("alveolar osteitis" or "alveolar ostitis").mp. 3. (alveolalgia or "alveolar periostitis" or "alveolitis sicca dolorosa").mp. 4. ((septic adj4 socket*) or "necrotic socket osteomylitis" or "fibrinolytic alveolitis").mp. 5. ("dry socket$" or dry‐socket$ or (infect$ adj5 socket$)).mp. 6. or/1‐5 7. exp Tooth Extraction/ 8. ("dental extraction$" or (tooth adj3 extract$) or (teeth adj3 extract$) or (teeth adj3 remov$) or (tooth adj3 remov$)).mp. 9. ((dental or oral) and (surgery or surgical)).mp. 10. or/7‐9 11. 6 and 10
The above subject search was linked with the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials in Embase (as described in Lefebvre 2021, box 3.e).
Randomized controlled trial/
Controlled clinical study/
random$.ti,ab.
randomization/
intermethod comparison/
placebo.ti,ab.
(compare or compared or comparison).ti.
((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
(open adj label).ti,ab.
((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
double blind procedure/
parallel group$1.ti,ab.
(crossover or cross over).ti,ab.
((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
(assigned or allocated).ti,ab.
(controlled adj7 (study or design or trial)).ti,ab.
(volunteer or volunteers).ti,ab.
human experiment/
trial.ti.
or/1‐19
random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
(((case adj control$) and random$) not randomi?ed controlled).ti,ab.
(Systematic review not (trial or study)).ti.
(nonrandom$ not random$).ti,ab.
"Random field$".ti,ab.
(random cluster adj3 sampl$).ti,ab.
(review.ab. and review.pt.) not trial.ti.
"we searched".ab. and (review.ti. or review.pt.)
"update review".ab.
(databases adj4 searched).ab.
(rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
Animal experiment/ not (human experiment/ or human/)
or/21‐33
20 not 34
Appendix 5. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
“dry socket”
Appendix 6. World Health Organization International Clinical Trials Registry Platform search strategy
“dry socket” OR “alveolar osteitis”
Data and analyses
Comparison 1. Prevention of dry socket.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Chlorhexidine rinse versus placebo | 6 | 1600 | Odds Ratio (IV, Random, 95% CI) | 0.38 [0.25, 0.58] |
| 1.1.1 0.2% chlorhexidine rinse | 1 | 121 | Odds Ratio (IV, Random, 95% CI) | 0.85 [0.36, 2.01] |
| 1.1.2 0.12% chlorhexidine rinse | 5 | 1479 | Odds Ratio (IV, Random, 95% CI) | 0.34 [0.23, 0.49] |
| 1.2 Chlorhexidine gel versus placebo/no treatment | 7 | 833 | Odds Ratio (IV, Random, 95% CI) | 0.44 [0.27, 0.71] |
| 1.3 Chlorhexidine gel versus chlorhexidine rinse | 2 | 383 | Risk Ratio (IV, Fixed, 95% CI) | 0.74 [0.46, 1.20] |
| 1.4 Platelet rich plasma versus placebo | 2 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.19, 1.33] |
Comparison 2. Treatment of dry socket.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Alvogyl versus zinc oxide eugenol day 7 VAS score (0‐10) | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐1.75, ‐1.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abu‐Mostafa 2015.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled, split‐mouth trial Sample size calculation: not stated Sample size: 311 recruited but 10 excluded, therefore 301 reported Setting: not stated Number of centres: 1 Operators: dental interns or dental students under supervision of surgery instructors in the Colleges Clinics Prevention or treatment of dry socket: prevention Type of teeth: "single extraction was done of upper or lower molar tooth" Recruitment period: November 2012 to April 2013 Funding source: Alhokail Orthodontic Center, Dammam City, Saudi Arabia provided 0.12% CHX mouthwash and 0.2% CHX gel for use in this study Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 65 females, 236 males Mean age: not stated Age range: not stated Number randomised: Intervention Group: 160. Control Group: 151 Number analysed: Intervention Group: 160. Control Group: 141 Lost to follow‐up/dropouts: 10 excluded (reasons: "…because they did not use CHX rinse twice daily for a week") |
|
| Interventions |
All patients: postoperative instructions were given for all patients in addition to the prescription of ibuprofen (Brufen, Hamol Limited, Nottingham, England) 600 mg every 8 hours for 3 days Control group: all patients received a bottle of 0.12% CHX mouthwash (Peridex, Oral Rinse, 3M, ESPE, USA) to start using it on the 2nd day of extraction twice daily for 7 days Intervention group: after performing tooth extraction, CHX 0.2% bioadhesive gel (Elugel, 40 mL gel tube, Pierre Fabre Oral Care, Boulogne, Paris, France) was applied into the extraction socket At the 3rd postoperative day CHX 0.2% bioadhesive gel was applied again into the extraction socket |
|
| Outcomes |
Primary outcome measures: presence of a dry socket Diagnosis: "Acute alveolar osteitis, (dry socket) was diagnosed if the patient presented between the 2nd and 4th days with pain or tenderness in the socket with probing, empty socket and food debris with or without halitosis" Secondary outcome measures: none reported Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Patients were divided randomly into two parallel groups by asking them to choice one card out of two in which the group number was written on its back" Comment: there is an element of randomisation in the patient's choice |
| Allocation concealment (selection bias) | High risk |
Quote: "Patients were divided randomly into two parallel groups by asking them to choice one card out of two in which the group number was written on its back" Comment: allocation is not concealed with the group number being written on the back, could easily be seen by patient or researcher |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It would not be possible to blind participants or personnel as this is a study comparing the professional application of a gel versus patient utilised mouthwash. Participants knowing the intervention would probably not impact outcomes being assessed but this is unlikely to have led to deviations from the intended intervention. Operators knowing the interventions are unlikely to have led to deviations from the intended intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no mention of outcome assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "Three hundred‐eleven patients participated in the study; ten of whom were eventually excluded because they did not use CHX rinse twice daily for a week" Comment: there are no data available for 10 patients who were enrolled in the trial |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement |
| Other bias | High risk |
Quotes: "Extractions were performed by dental interns or dental students under supervision of surgery instructors in the Colleges Clinics" "Simple extractions were done by elevators and forceps, while root separations were done using a surgical handpiece" Comment: a variety in operator experience may introduce bias especially if this is not equally distributed between the groups. Also the rates of AO were higher (as to be expected from the literature) in surgical extractions. It is unclear whether an attempt was made to ensure the type of extraction was equally represented in both groups. Table 3 page 85 shows 19 of the 29 AO observed in the study were related to extractions via root separation |
Abu‐Mostafa 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not reported Sample size: 100 Setting: university clinics Number of centres: 1 Operators: dental interns Prevention or treatment of dry socket: prevention Type of teeth: single molar tooth Recruitment period: October 2016 to August 2017 Funding source: not reported Declarations of interest: not reported |
|
| Participants |
Inclusion criteria: patients with upper or lower molar teeth indicated for extraction Exclusion criteria: patients with uncontrolled systemic diseases, epinephrine contraindications, pregnant women, breastfeeding women, and women who were using oral contraceptives. Patients with allergy to CHX, honey, lidocaine, and ibuprofen were also excluded. Other exclusion criteria included smoking, presence of acute infection, cystic lesions, traumatic extraction with fractured alveolar bone, extraction requiring bone reduction or root separation, and extractions that lasted more than 30 minutes Gender: 48 males, 52 females Mean age: 38.13 years Age range: 17 to 69 years Number randomised: 100 Number analysed: 100 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: regular postoperative care and verbal instructions, instructed to take 400 mg ibuprofen every 8 hours on 1st and 2nd day of the tooth extraction Intervention group 1: 0.2% CHX mouthwash twice daily for 7 days Intervention group 2: Manuka honey topically to extraction socket immediately and on 3rd postoperative day |
|
| Outcomes |
Primary outcome measures: pain, assessment of socket for food debris and postextraction halitosis Diagnosis: VAS, clinical examination of socket, fetid odour from patients mouth during speech Secondary outcome measures: none reported Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Random allocation of the patients into the two parallel groups was done by asking them to choose 1 of the 2 colored cards. The green card indicated Group 1 while the blue indicated Group 2" Comment: there is a random element to this |
| Allocation concealment (selection bias) | High risk |
Quote: "Random allocation of the patients into the two parallel groups was done by asking them to choose 1 of the 2 colored cards" Comment: allocation is not concealed with colour of card denoting group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It would not be possible to blind participants or personnel as this is a study comparing the professional application of a honey intrasocket versus patient rinsing with CHX. Participants knowing the intervention would probably not impact outcomes being assessed, but this is unlikely to have led to deviations from the intended intervention. Operators knowing the interventions are unlikely to have led to deviations from the intended intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no mention of outcome assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants enrolled are accounted for but groups noticeably different in size. No details provided |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk |
Quotes: "Extractions were performed by dental interns under close supervision of surgery instructors in the University Clinics" "Additionally, the patients were instructed to take 400 mg of ibuprofen every 8 hours on the 1st and 2nd day of the tooth extraction" Comment: a variety in operator experience may introduce bias especially if this is not equally distributed between the groups Adherence with pain relief regimen was not reported/recorded and could have impacted subjected reporting of pain on VAS |
Ahmedi 2016.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled, split‐mouth trial Sample size calculation: not stated Sample size: 30 Setting: Department of Oral Surgery in the University Dental Clinical Centre of Kosovo (UDCCK) Number of centres: 1 Operators: 1 oral surgeon Prevention or treatment of dry socket: prevention Type of teeth: bilateral impacted lower 3rd molars in similar position Recruitment period: January 2014 to June 2014 Funding source: none stated Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: not reported Mean age: 27.87 ± 4.11years Age range: 18 to 30 years Number randomised: 30 Number analysed: 30 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All: postoperative instructions and prescribed ibuprofen 400 mg provided Control group: 5 mL of 0.9% saline solution was used to irrigate the socket Intervention group: ozone gas was used to irrigate the socket "We used Prozone equipment to supply the O3 gas, which enabled us to introduce the gas into the socket using plastic attachments for 12 s calibrating the therapeutic dose. This procedure was performed using a surgical suction unit to avoid respiratory aspiration and related complication. Suturing was performed with 3/0 absorbable suture (Ethicon, Somerville, NJ, USA)" |
|
| Outcomes |
Primary outcome measures: dry socket Diagnosis: "Absence of a blood clot, bone exposure, or necrotic blood clot" Secondary outcome measures: none reported Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Randomisation was used to determine which side would comprise the control group (gr1) and which would comprise the experimental (O3) group (gr2)" Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The patients were not aware of these designations" and its unlikely during the procedure (due to anesthesia) that they would have known which intervention they received. All the surgeries were performed by the same oral surgeon who would have known the intervention, but this lack of masking is unlikely to have led to a deviation in the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 1 surgeon did start and end assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants reported on |
| Selective reporting (reporting bias) | High risk | All outcomes were not reported i.e. indication for extraction and difficulty of extraction as per objectives 2 and 3 of the study |
| Other bias | High risk | The indication and difficulty for extraction was not reported, and could have been different in each quadrant i.e. a patient may have had caries/infection in 1 tooth which was not present in the other. These could have influenced the primary outcome of the study (AO) |
Alissa 2010.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled, parallel‐group (pilot) study Sample size calculation: a priori sample size calculation suggested needed 34 in each group Sample size: 23, suggesting 60% power Conducted in: University Dental Hospital of Manchester, Manchester, UK. Patients recruited from consultation clinic Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: molars, premolars, canines and incisors, 17 (60.9%) extracted for dental caries and 5 (21.7%) endodontic failure. Extracted under intravenous sedation Recruitment period: January 2005 to April 2008 Funding source: not stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Age group: mean = 30.5 Intervention group: number randomised 12; analysed 12 Control group: number randomised 11; analysed 7 Number evaluated: 19 |
|
| Interventions |
Comparison: platelet rich plasma (PRP) placed in extraction sockets versus control All patients were treated under intravenous sedation and blood drawn to manufacture the PRP produce before the surgical procedure and before intravenous sedation administered. All patients had a mucoperiosteal flap raised with 2 releasing incisions. Extraction with forceps to minimise trauma, elevators used as appropriate. All sockets carefully curetted to remove granulation tissue and/or periapical infections PRP group (n = 12): platelet rich plasma placed in extraction sockets after extraction and curettage Control group (n = 11): nothing placed in control sockets Co‐interventions and concomitant medication: none stated. Co‐codamol analgesic tablets (codeine phosphate 30 mg and paracetamol 500 mg) |
|
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "A computer‐generated randomization schedule was created by a statistician" page 127 Comment: sequence generation was randomised |
| Allocation concealment (selection bias) | Low risk |
Quote: "The randomization codes were enclosed in sealed, opaque and sequentially numbered envelopes. The patients allocation to either group was revealed by the investigator just before venous cannulation on the day of the patient’s appointment for the extraction" Comment: efforts to conceal allocation done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was probably not possible to blind personnel and participants to group allocation. Strict study criteria required that participants would be blinded to group allocation as they will be involved in reporting of subjective patients symptoms. Operators knowing the interventions are unlikely to have led to deviations from the intended intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | While the criteria for dry socket are clear, it is unclear who and how they made the judgement Insufficient information to permit judgement of 'high' or 'low' risk, therefore unclear risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In relation to AO, 2 patients from the control group did not attend any of the scheduled appointments following tooth extraction, and given the low event rate it may have introduced a bias. There was no loss to follow‐up in the intervention group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. The report appears to be free of selective reporting |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Babar 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not reported Sample size: 100 Setting: Department of Oral and Maxillofacial Surgery, Armed Forces Institute of Dentistry, Pakistan Number of centres: 1 Operators: 1 surgeon Prevention or treatment of dry socket: prevention Type of teeth: mandibular 3rd molars Recruitment period: 1 January to 31 December 2007 Funding source: not reported Declarations of interest: not reported |
|
| Participants |
Inclusion criteria: requiring removal impacted mandibular 3rd molar Exclusion criteria: acute pericoronitis, taking antibiotics, history of smoking, pregnancy, any other bone pathology, taking immunosuppressants Gender: 65 males, 35 females Mean age: 29 Age range: 18 to 40 years Number randomised: 100 Number analysed: 100 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: 400 mg ibuprofen for postoperative pain Intervention group 1: none Intervention group 2: 0.2% CHX gel placed into alveolus |
|
| Outcomes |
Primary outcome measures: acute AO diagnosis Diagnosis: AO determined by presence of pain, blood clot disintegration, halitosis Secondary outcome measures: none reported Adverse outcomes: no adverse outcomes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "divided randomly into two groups" page 92 Comment: a random sequence generation was obtained, method was not described |
| Allocation concealment (selection bias) | Unclear risk | There is no reporting of methods/process to conceal allocation just the statement "100 patients.... were examined clinically and radiologically and then divided randomly into two equal groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It would not be possible to blind participants or personnel as this is a study comparing the professional application of a 0.2% CHX intrasocket versus no intervention, but this lack of masking is unlikely to have led to a deviation in the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no mention of outcome assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants enrolled are accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk |
Quote: "Extractions were performed by dental interns under close supervision of surgery instructors in the University Clinics" Comment: a variety in operator experience may introduce bias especially if this is not equally distributed between the groups |
Bai 2011.
| Study characteristics | ||
| Methods |
Study design: parallel RCT Sample size calculation: not clear that this was undertaken Study size: 400 participants Conducted in: Stomatological Center of Chinese PLA, the 306 Hospital of Chinese PLA, Beijing, China Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular 3rd molar, local anaesthesia Recruitment period: March 2009 to June 2010 Providers of care: 1 surgeon Funding source: no funding |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Age group: mean 27.9 Group 1: number randomised: 200; number analysed: 200 Group 2: number randomised: 200; number analysed: 200 Number evaluated: 200 |
|
| Interventions |
Comparison: acellular dermis matrix versus control Group 1 (n = 200): acellular dermis matrix (1×1 cm) embedded into the extraction sockets Group 2 (n = 200): nothing embedded into the extraction sockets Co‐interventions: extraction of the teeth (the detailed information was not reported) Concomitant medication: not reported |
|
| Outcomes |
When measured: 1 week after the extraction Primary outcome measures:
Secondary outcome measures: none reported Adverse outcomes: none reported |
|
| Notes | Author contact failed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The participants were randomized into intervention group (n = 200) and control group (n = 200)" Comment: the detailed methods of randomisation were not clearly reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient reporting to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient reporting to make a judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient reporting to make a judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None of the participants were reported to have dropped out |
| Selective reporting (reporting bias) | Low risk | All the outcomes in relation to dry socket were reported clearly |
| Other bias | High risk | Although the authors reported the gender and the age, the difficulties of the extraction and the detailed information of the tooth impaction were not reported |
Burgoyne 2010.
| Study characteristics | ||
| Methods |
Study design: randomised parallel‐group controlled trial Study size: 35 Sample size calculation: not stated Prevention or treatment of dry socket: treatment Type of teeth: mandibular 3rd molars, mandibular and maxillary molars and premolars Conducted in: Department of Oral and Maxillofacial Surgery, School of Dentistry, Virginia Commonwealth University (Richmond, VA), USA Number of centres: 1 Recruitment period: not stated Funding source: not stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Age: gauze strip group: mean = 33, range 19 to 53 years; topical anaesthetic gel: mean = 27, range 17 to 58 years Number randomised: 35 Number analysed: 35 |
|
| Interventions |
Comparison: topical anaesthetic gel versus eugenol gauze strip Group 1 (n = 15): 2.5% prilocaine, 2.5% lidocaine (Oraqix; Dentsply Pharmaceutical, York, PA) thermosetting gel syringed into socket Group 2 (n = 20): eugenol on plain gauze into socket Co‐interventions:
|
|
| Outcomes | Self‐assessment 5, 10, 15 minutes after 1st treatment, and then hourly whilst awake over 48 hours. Assessed at 48 hours and if still in pain re‐treated Primary outcome measures:
Secondary outcome measures: none assessed Adverse effects: none reported |
|
| Notes | The pain outcomes sought were to be assessed during waking hours but it was unclear why the investigators considered pain assessments not recorded while the patients slept as "missing data." Quote: "Two analyses were performed, one treating the missing data as ignorable and the other inputting pain scores of 0 during sleep." We used the one which ignored the missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "control or experimental group by use of a randomization table" page 145 Comment: randomisation method described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded to allocation and to what they received. Personnel were not blinded to interventions. Strict study criteria required that personnel would be blinded to group allocation as they will be involved in reporting of patients symptoms, operator not masked is unlikely to have led to deviation in intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear who made outcome assessment, if it was the operator given the low numbers in the trial s/he could remember group allocation, but insufficient information to form a judgement of high risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcomes assessments not carried out during periods of sleep were considered as "missing data" |
| Selective reporting (reporting bias) | Low risk | Although the study protocol was unavailable, the report appears to be free of selective reporting |
| Other bias | Low risk | Appears to be free of other bias |
Chaurasia 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not stated Sample size: 88 patients Setting: Department of Oral and Maxillofacial Surgery of Dhulikhel Hospital, Nepal Number of centres: 1 Operators: not stated Prevention or treatment of dry socket: treatment Type of teeth: mandibular and maxillary 2nd and 3rd molars, mandibular canines Recruitment period: not stated Funding source: none stated Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 36 females, 52 males Mean age: not stated Age range: not stated Number randomised: 88 Number analysed: not clear Lost to follow‐up/dropouts: not stated |
|
| Interventions |
All: in both groups irrigation with normal saline was done to remove debris or infected clot Group A: placement of zinc oxide eugenol paste mixed with cotton pellet (manufacturer‐ Septodent India) Group B: placement of Alveogyl TM (manufacturer‐ Septodent India) The medication was changed every day until the pain subsided |
|
| Outcomes |
Primary outcome measures:
Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The dry socket patients were randomly divided into two groups... They were randomly assigned using randomization table to group A and group B" |
| Allocation concealment (selection bias) | Unclear risk | This was not mentioned, and there is insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not mentioned, and there is insufficient information to make a decision |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | his was not mentioned, and there is insufficient information to make a decision |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether all patients were followed up at all time points |
| Selective reporting (reporting bias) | Low risk | Pain was the main outcome measure and this appears to have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Cho 2018.
| Study characteristics | ||
| Methods |
Study design: single‐blind, RCT Sample size calculation: not stated Sample size: 100 patients (120 teeth as 10 bilateral in each group) Setting: 3 private dental clinics in South East Queensland, Australia Number of centres: 3 Operators: 3 surgeons (all with at least 5 years experience of dentoalveolar surgery) did the operations Prevention or treatment of dry socket: prevention Type of teeth: mandibular 3rd molars Recruitment period: 2014 to 2016 Funding source: none stated Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 44 females, 56 males Mean age: control group: 35 years; intervention group: 36 years Age range: control group: 18 to 72 years; intervention group: 19 to 76 years Number randomised: 100 Number analysed: 95 Lost to follow‐up/dropouts: 5 (unable to contact) |
|
| Interventions | All patients were given a bottle of CHX mouthwash (Savacol® alcohol‐free antiseptic mouth and throat rinse, Colgate‐Palmolive) with verbal and written instructions on how to use it. Patients were prescribed oral analgesia with paracetamol/codeine 500/15 mg 1 or 2 tablets every 4 to 6 hours as needed, and ibuprofen 200 mg 1 or 2 tablets every 4 to 6 hours as needed, both with a maximum of 8 tablets/day Control group: "On the day after the surgery, start mouthwash irrigation twice a day" – rinse only Intervention group: "On the day after the surgery, start mouthwash irrigation twice a day" – use of a plastic syringe (Monoject 412, Covidien) (to irrigate the socket using the CHX) |
|
| Outcomes |
Primary outcome measures: pain Diagnosis: VAS used Secondary outcome measures:
Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a sealed envelope method, we randomly allocated the patients in a 1:1 ratio into the irrigation (intervention) or rinse (control) group (n = 50 in each). This was done postoperatively to minimise operator bias" |
| Allocation concealment (selection bias) | Low risk | Quote: "Using a sealed envelope method, we randomly allocated the patients in a 1:1 ratio into the irrigation (intervention) or rinse (control) group (n = 50 in each)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Randomisation "…was done postoperatively to minimise operator bias" Comment: given the nature of the interventions it is not possible to blind the patient. Insufficient data to make a judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The telephone call 48 hours after the operation included a pain score using a visual analogue scale that had been given out after the operation. The interviewer was blinded to the intervention used. During their appointment at the clinic on the seventh postoperative day (acceptable range 5–10 days), the independent observer reviewed the postoperative course, examined the extraction site" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 patients dropped out and there appears to have been a per‐protocol analysis |
| Selective reporting (reporting bias) | Low risk | All outcome measures appear to have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Delilbasi 2002.
| Study characteristics | ||
| Methods |
Study design: randomised, placebo‐controlled, parallel‐group study Sample size: 177 Power calculation: not reported Conducted in: not stated (authors based in Turkey and Japan) Number of centres: not stated Prevention or treatment of dry socket: prevention Type of teeth: mandibular 3rd molars Recruitment period: not stated Funding source: not stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Age: Group 1: 24.1 years; Group 2: 24.7 years; Group 3: 24.2 years Number randomised: 177 Number analysed: 177 |
|
| Interventions |
Group 1 (n = 62): rinse with 15 mL of CHX solution (Klorhex; Drogsan) for 30 seconds just before tooth removal. Intraoperatively, 15 mL of CHX diluted with 15 mL of sterile saline was used as irrigation. The soft tissue was closed with 3/0 silk suture for transalveolar procedures. The day after surgery, the patients began home use of the CHX solution (15 mL for 30 seconds) twice daily for 7 days Group 2 (n = 56): similarly to Group 1. However, in addition to CHX solution, the patients in Group 2 were prescribed Augmentin (500 mg amoxicillin trihydrate, 125 mg clavulanic acid; SmithKline Beecham) twice daily for 5 days postoperatively Group 3 (n = 59): similarly to Group 1, except for the substitution of sterile saline solution (0.09 % NaCl) for CHX Co‐interventions: all 3 groups were instructed to use only 500 mg paracetamol (Minoset; Roche) for postoperative pain relief |
|
| Outcomes | Assessment days 3 and 7 postoperatively Primary outcome measures: diagnosis of AO Secondary outcome measures: none Adverse outcomes: adverse events reported for Group 1 n = 62. Allergy: n = 0. Staining of teeth: n = 4. Mucosal irritation: n = 0. Alteration in taste: n = 12. Gastrointestinal complaints: n = 0. Bad taste: n = 8. No adverse reactions: n = 38. From Table IV page 303 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "...were randomly allocated into 3 groups" Comment: no information relating to randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned and no information to suggest this was done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants in antibiotic group would not have been blinded to intervention (though this arm is not included in the present reviews meta‐analysis). Personnel were blinded to group allocation. Interventions were in identical bottles, a 3rd operator separate to clinicians undertaking the surgery and doing the assessment. Study criteria required both participant and personnel to be masked to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned and no information to suggest this was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | The report appears to be free of selective reporting |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Divya 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not reported Sample size: 50 Setting: Oral and Maxillofacial Surgery, Chennai, India Number of centres: 1 Operators: not reported Prevention or treatment of dry socket: prevention Type of teeth: impacted 3rd molars Recruitment period: not reported Funding source: none received Declarations of interest: reported as none |
|
| Participants |
Inclusion criteria: patients needing surgical removal of impacted 3rd molar teeth Exclusion criteria: patients with any history of systemic illness or allergy to the components of the mouthwash were excluded from the study Gender: 298 males, 22 females Mean age: 23.5 Age range: 18 to 36 years Number randomised: 50 Number analysed: not reported Lost to follow‐up/dropouts: not reported |
|
| Interventions |
All patients: none Intervention group 1: 0.2% CHX twice daily Intervention group 2: herbal mouthrinse (hioria) |
|
| Outcomes |
Primary outcome measures: pain, dry socket, wound healing Diagnosis: VAS, persistent pain postextraction, bone exposure with loss of blood clot and halitosis Secondary outcome measures: none Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were randomly assigned to Group A (control) and Group B (test), with 25 patients in each group)" Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants reported on |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias observed |
Faizel 2015.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled, parallel‐group trial Sample size calculation: not stated Sample size: 117 sockets Setting: Department of Oral and Maxillofacial Surgery, Peoples College of Dental Sciences and Research Centre, Bhopal, India Number of centres: 1 Operators: not stated Prevention or treatment of dry socket: treatment Type of teeth: any tooth Recruitment period: 1 January 2012 to 28 February 2013 Funding source: none stated Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 69 females, 48 males Mean age: 34.6 years Age range: not stated Number randomised: 117. Intervention group 1: 39. Intervention group 2: 39. Control group: 39 Number analysed: 39 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: "The socket was irrigated with warm sterile saline solution. Curettage was avoided. Loose debris was removed, taking care to avoid" Control group: "A few fibers of Alvogyl were placed with the help of a sterile instrument deep into the socket ensuring that the denuded bone was completely covered followed by the placement of sterile gauze. The gauze was removed after 5 min" Intervention group 1: "A gauze piece soaked with freshly prepared ZOE paste was placed in the extraction socket under aseptic precautions" Intervention group 2: "A single pellet of Neocone was placed inside the socket followed by the placement of a sterile gauze piece to cover the socket. The gauze was removed after 5 min" |
|
| Outcomes |
Primary outcome measures: dry socket Diagnosis: "…clinically established on the basis of the following features by a blinded assessor: 1. Pain in and around the extraction socket with or without radiation that increases in severity for some period from 1 and 3 days after extraction. 2. Partial or total clot loss in the interior of the alveolus with or without halitosis" Secondary outcome measures: "Any other associated findings such as halitosis, lymphadenopathy, etc." Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned using randomisation table to one of the three groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a judgement in relation to participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quotes: "The dressings were evaluated by a blinded assessor at every follow‐up visit and were changed in case of persistence of pain" "Clinical examinations for the signs of healing of dry socket were performed on 1st, 3rd, 5th, 7th, and 10th day by a blinded assessor" Comment: outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data not presented clearly enough to make decision |
| Selective reporting (reporting bias) | Unclear risk | Data not presented clearly enough to make decision |
| Other bias | Unclear risk | No other bias noted |
Feng 2009.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not reported Sample size: 200 patients (200 teeth) Setting: outpatients Number of centres: 1 Operators: not reported Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular molar Recruitment period: June 2007 to May 2008 Funding source: none stated Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 116 males and 84 females Mean age: not reported Age range: 20 to 65 years Number randomised: 200 Number analysed: 200 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: the patient to return to the clinic 1 week after extraction, and may return to the clinic at any time if discomfort occurs. Record the occurrence of postoperative bleeding, swelling and dry socket. If not returned in time, contact for inquiry by phone Control group: blank control group. Under routine local anaesthesia, the impacted teeth were removed and the alveolar bone was reduced Intervention group 1: oral tissue patch alone. Under conventional local anaesthesia, the impacted teeth were extracted, and the oral tissue patches were cut into small pieces according to the shape of the alveolar fossa, and then placed in the root fossa of the human teeth and fitted to the bone wall Intervention group 2: platelet rich plasma (PRP) alone. Under routine local anaesthesia, the impacted teeth were extracted, and the prepared PRP was directly injected into the alveolar socket Intervention group 3: oral tissue patch combined with PRP group. Under routine local anaesthesia, the impacted teeth were extracted, the patch was trimmed according to the shape of the alveolar fossa, and the PRP was placed on the patch and then placed in the alveolar fossa to make the patch fit with the wall of the alveolar fossa |
|
| Outcomes | Note: authors did not specify which was the primary outcome Outcome measures:
Diagnosis criteria:
Diagnostic criteria for postoperative bleeding after tooth extraction: local bleeding on the day after tooth extraction surgery or significant local bleeding 24 hours after surgery At 1 week and 3 months after surgery, the location of the surgical area was radiographs and analysed by Digora. The bone density of the root and canine alveolar fossa was determined, and the growth of alveolar bone in the operative area was compared All the above tests were performed by another doctor who was not the surgeon Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned "randomly assigned to 4 groups, 50 in each group" with no further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported, no details about who the assessors were |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up |
| Selective reporting (reporting bias) | Low risk | The incidence of dry socket was reported fully in Table 2 |
| Other bias | Low risk | No other bias noted |
Freudenthal 2015.
| Study characteristics | ||
| Methods |
Study design: double‐blind, RCT Sample size calculation: "A power calculation showed that 100 patients were required to obtain a power of 80% and a significance level of 5% if the hypothesis was a difference of 20% between groups according to AO frequency (5% and 25%, respectively)" Sample size: 100 molars (95 patients) Setting: Oral and Maxillofacial Surgery Clinic at the South Hospital in Stockholm, Sweden Number of centres: 1 Operators: 3 senior house officers Prevention or treatment of dry socket: prevention Type of teeth: mandibular 3rd molars Recruitment period: February 2011 to March 2012 Funding source: none stated Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: females (105), males (95) Mean age: 33 ± 10.03 years Age range: 19 to 65 years Number randomised: 100 teeth Number analysed: 95 teeth Lost to follow‐up/dropouts: 5 teeth (reasons: "Five teeth had to be excluded from the study owing to medical complications during surgery or the immediate postoperative period. In 2 cases, the surgery could not be completed owing to the patients’ anxiety. In 1 case, it was discovered immediately after surgery that the patient had started corticoid medication. In another 2 cases, the surgery was complicated by a postoperative purulent infection disabling a correct AO diagnosis") |
|
| Interventions |
All patients: "All patients were given Alvedon (paracetamol, [Famar A.V.E, Anthoussa Plant, Greece]) 1 g before the surgical procedure… The socket was rinsed with saline" "Postoperatively, the patients were prescribed Alvedon (paracetamol 1 g) and Citodon (paracetamol 500 mg and codeine 30 mg, [Orion, Espoo, Finland]). They were informed to start using only Alvedon and to add Citodon when needed" Control group: a placebo gel (APL dentalgel, APL, Stockholm, Sweden) containing 0.2% NaF was applied Intervention group: Cervitec gel (Ivoclar Vivadent AG, Schaan, Lichtenstein) 10 mL, containing 0.2% CHX and 0.2% NaF, was placed into the alveolus |
|
| Outcomes |
Primary outcome measures: dry socket Diagnosis: "postoperative pain that increased in severity with time and was accompanied by a partially or totally disintegrated blood cloth within the alveolar socket with or without halitosis" Secondary outcome measures: the intake of analgesic tablets (type and frequency) 1 to 7 days after surgery Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The code list (active gel or placebo) was developed in advance through a randomizing process" |
| Allocation concealment (selection bias) | Low risk | Quote: "The code list was locked away and was not unlocked until the study was completed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the surgical team nor the patients knew whether active gel or placebo was administered" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Measurements were registered by one of the surgeons not taking part in the in the actual surgical procedure" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All eligible participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gersel‐Pedersen 1979.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled, double‐blind, split‐mouth trial Sample size: 120 Sample size calculation: not reported Prevention or treatment of dry socket: prevention Type of teeth: bilaterally impacted mandibular 3rd molars – under local anaesthetic Conducted in: Department of Oral Surgery, Royal Dental College, Copenhagen, Denmark Number of centres: 1 Recruitment period: not stated Funding source: no financial support received |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Mean age: 23.2 years, range 14 to 58 years Number randomised: 120 Number analysed: 120 |
|
| Interventions |
Intervention (n = 120): 4 AMCA (tranexamic acid 40.0 mg) cones Control (n = 120): 4 placebo (lactose (n = 30) or sorbitol (n = 90) 40 mg) Co‐interventions: the surgical areas were irrigated with 50 mL sterile saline and closure of the wounds was accomplished with 2 silk sutures "Oral hygiene was secured by mouthrinses with chlorhexidine gluconate 0.2%, 3 times a day. The pains were alleviated with tablets containing 500 mg acetylsalicylic acid and 10 mg codeine phosphate." Frequency and quantity not reported |
|
| Outcomes | Assessment carried out ''...4, 5 or 6 days (mean = 5 days) after the operation...'' Primary outcome measures: presence of alveolitis sicca dolorosa Secondary outcome measures: none stated Adverse effects: foreign body reaction to the vehicle delivery system in the cones |
|
| Notes | 2 control groups, 2 teeth per patient | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An equal distribution of AMCA/Tplacebo between right and left side was ensured by randomization corresponding to 120 consecutive numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "The code was unknown to the author during the study and first broken when all the patients had been treated" Comment: allocation concealment reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned and no information to suggest this was done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned and no information to suggest this was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in analysis |
| Selective reporting (reporting bias) | Low risk | Although the study protocol was unavailable, the report appears to be free of selective reporting |
| Other bias | Low risk | Appears to be free of other bias |
Ghaeminia 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: "calculation of the sample size was based on a randomized clinical trial investigating the usefulness of cone beam CT (CBCT) in patients with an increased risk for inferior alveolar nerve injury following the removal of mandibular third molars..." Sample size: not stated Setting: the study was performed in 3 participating departments of oral and maxillofacial surgery of Radboud University Medical Centre Nijmegen (RUN), Rijnstate Hospital Arnhem (RHA), and a private clinic in Nijmegen (PCN), the Netherlands Number of centres: 3 Operators: senior surgeons and residents Prevention or treatment of dry socket: prevention Type of teeth: mandibular 3rd molars Recruitment period: 2013 to 2014 stated in paper referenced CBCT Funding source: none stated Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 220 females, 112 males ‐ from table 3 only adds up to 332, but 33 randomised Mean age: not stated Age range: not stated Number randomised: 333 teeth (280 patients) Number analysed: intention‐to‐treat = 333, but also performed a per‐protocol (PP) analysis = 249 Lost to follow‐up/dropouts: 27 lost to follow‐up. A further 57 excluded in the PP analysis due to failure in following the protocol |
|
| Interventions |
Control group: standard postoperative care instructions, without the use of a Monoject® syringe. The standard postoperative instructions were biting on a gauze for 30 minutes, no rinsing and spitting for the first 24 hours, and starting the regular toothbrushing the day after surgery. Paracetamol (4 times a day 1000 mg) in combination with ibuprofen (3 times a day 600 mg) were prescribed postoperatively Intervention group: Monoject® syringe group. After surgery, a curved tip Monoject® syringe (12 cm3) was provided to the patient. In addition to the standard postoperative care instructions, the participants received instructions with regard to the use of Monoject® syringe (by bringing the tip at the distal side of the 2nd molar in or above the tooth socket and irrigate 4 times a day with plain tap water). To avoid early removal of the blood clot, patients were instructed to start irrigating the wound 48 hours after surgery until the 1st postoperative visit 7 days after surgery |
|
| Outcomes |
Primary outcome measures:
Diagnosis: "Surgical wound infection was defined as the presence of a local abscess, onset of facial or cervical abscess/cellulitis, and other signs suggesting an infection (redness, swelling, purulent discharge, fever). The diagnosis of AO was based on the Blum criteria: postoperative pain in and around the extractions site, which increased in severity at any time between 1 and 3 days after the extraction, accompanied by a partially or totally disintegrated blood clot within the alveolar socket with or without halitosis" Secondary outcome measures:
Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a surgical assistant assigned the patients randomly through a computer random generator after logging in the secured website" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation concealment was guaranteed through the Web‐based central concealment" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One blinded investigator per center assessed the primary and secondary outcome measures" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were dropouts, an intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | All outcome measures appear to have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Halabi 2018.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, parallel‐group clinical trial Sample size calculation: the sample size was estimated using data published previously be Halabi et al (2012), expecting an incidence reduction of 2‐3rds. The PA expected incidence of disease (AO) in the placebo group was 6.14%, while the PB expected incidence of disease (AO) in the CHX group was 2.05%. The power of the study was set at 80% (β = 0.20), with a = 0.05 as the significance level Sample size: 822 patients assessed for eligibility, 744 met inclusion criteria and completed follow‐up Setting: public community dental clinics Number of centres: 2 Operators: dental surgery team from the clinics’ emergency department Prevention or treatment of dry socket: prevention Type of teeth: any maxillary or mandibular tooth Recruitment period: April 2013 to December 2015 Funding source: self‐funded by the authors Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 381 females, 363 males Mean age: 43.43 years Age range: not stated Number randomised: intervention group: 372; control group: 372 Number analysed: intervention group: 372; control group: 372 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
Control group: (n = 372) rinse with 15 mL placebo (sterile water in similar bottle) for 30 seconds, twice a day for 7 days, starting 24 hours after extraction Intervention group: (n = 372) rinse with 15 mL CHX 0.12% (Oralgene mouthwash 0.12%, Maver, Chile) for 30 seconds, twice a day for 7 days, starting 24 hours after extraction |
|
| Outcomes |
Primary outcome measures: presence of a dry socket Diagnosis:
Secondary outcome measures: none reported Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "To guarantee that in both groups (treatment and placebo) the risk of alveolar osteitis was similar and comparable, the assignment was performed by randomization, stratified by risk factors" Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "We stored black envelopes in a box containing a paper with the letter C for chlorhexidine or P for placebo (half of each). The envelopes were chosen for each patient after the extraction and transported to another room (without opening them)..." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures appear to have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Haraji 2013.
| Study characteristics | ||
| Methods |
Study design: randomised, split‐mouth study Sample size calculation: "A pilot study of 45 individuals was undertaken. The proportions of dry socket in the control and experimental sides were 35.6% and 13.3% respectively. On the basis of this pilot study, a sample size of 76 patients (76 x 2 sockets) was needed to obtain a 0.9 power (α = 0.5, β = 0.1). Thus it was prospectively determined as 160 extraction sites in 80 patients to gain test powers ≥ 0.9" Sample size: 80 patients (160 teeth) Setting: a private maxillofacial clinic in Tehran, Iran Number of centres: 1 Operators: not stated Prevention or treatment of dry socket: prevention Type of teeth: mandibular 3rd molars Recruitment period: 2010 to 2011 Funding source: none stated Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 41 females, 39 males Mean age: 21.6 ± 2.5 years Age range: 17 to 31 years Number randomised: 80 Number analysed: 80 Lost to follow‐up/dropouts: 4 (failure to attend follow‐up) – unclear whether this is from the 200 screened or 80 included |
|
| Interventions |
Control group: gelatin sponge with colloidal silver (Gelatamp, Rocko) Intervention group: gelatin sponge with colloidal silver (Gelatamp, Rocko) impregnated with 0.2% CHX bioadhesive gel (1,6‐bis|N‐p‐chlorphenyl‐biguanidol hexane digluconate|, Kimia) |
|
| Outcomes |
Primary outcome measures: dry socket Diagnosis: "Diagnosis of dry socket was regarded as positive when the patient experienced postoperative pain that intensified sometime between the first and third days, with total or partial loss of the blood clot" Secondary outcome measures: pain. "..recorded on a visual analogue scale (VAS). The end points were considered 'no pain' and 'intolerable pain'. The results on the VAS were later converted to 10 ordered ranks (0‐9)" Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via a coin toss used to allocate to groups |
| Allocation concealment (selection bias) | High risk | Allocation based on a coin toss by person performing intervention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both the surgeon and patients were blinded to the assignment orders" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjects were evaluated in the first and third post‐operative days. Clinical assessments were performed by the blinded maxillofacial surgeon…" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All eligible participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hasheminia 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not reported Sample size: 189 patients Setting: private dental clinic Number of centres: 1 Operators: not reported Prevention or treatment of dry socket: prevention Type of teeth: impacted 3rd molars Recruitment period: not reported Funding source: not reported Declarations of interest: none reported |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 80 men, 109 women Mean age: 31.3 years (intervention group), 30.4 years (control group) Age range: 22 to 46 years Number randomised: 189 Number analysed: 189 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: 400 mg ibuprofen given 1 hour before the procedure. All patients received 400 mg ibuprofen 6 hourly for 3 days Control group: (n = 92) no intervention Intervention group: (n = 97) gauzes soaked in 1% povidone iodine oral antiseptic solution (Betadine, Purdue Pharma LP, CT, USA) were placed on the 3rd molars and adjacent teeth for 2 to 5 minutes. During the procedure povidone iodine 1% mouthwash was also used |
|
| Outcomes |
Primary outcome measures: presence of dry socket Diagnosis: criteria used not detailed Secondary outcome measures: none Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The patients were grouped into the experimental and control groups using random odd and even number" Comment: method of randomisation is not clearly reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures appear to have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hermesch 1998.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled, parallel‐group study Sample size: 279 Sample size calculation: not reported Conducted in: a military dental clinic (authors from San Antonio, Texas USA) Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular 3rd molar ‐ under local anaesthesia with optional intravenous sedation. However, other 3rd molars extracted concurrently Recruitment period: not stated Funding source: not reported |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Number randomised: 279; 0.12% CHX n = 140; placebo n = 139 Number analysed: 271; 0.12% CHX n = 136; placebo n = 135 Lost to follow‐up/dropouts: 8, reasons for exclusion provided |
|
| Interventions |
Comparison: Group 1 (n = 140): 0.12% CHX gluconate rinse in 11.6% alcohol Group 2 (n = 139): placebo rinse containing 11.6% alcohol Co‐interventions:
|
|
| Outcomes | 3 to 4 days postoperatively telephone contact. Day 7 ‐ clinical evaluation Primary outcome measures:
Secondary outcome measures: not evaluated Adverse effects:
|
|
| Notes | NB: number above = patients, extractions: CHX 239, placebo 240. Randomisation at individual participant level but analysis of data at extraction site level | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each subject was then randomized to a treatment group within these strata" Comment: method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Each bottle was labelled with a unique subject number, which allowed for blinding of treatment assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "Each bottle was labelled with a unique subject number, which allowed for blinding of treatment assignment" Comment: both the placebo and active interventions were identical in appearance and therefore participants were blinded to group allocation, however it is not clear how bottle labelling ensured blinding of personnel. Strict study criteria required that participants and personnel would be blinded to group allocation. Lack of masking of operator is unlikely to have led to deviation in intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All diagnoses were made by 1 of 2 attending clinical examiners involved in this study, without knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants not included in final analysis reported (n = 8) and reasons for exclusion provided. Equal numbers in both intervention groups |
| Selective reporting (reporting bias) | High risk | 271 participants, 973 third molars extracted, 51.7% (239 CHX, 240 placebo) were mandibular molars, no data were reported for the non‐mandibular molars |
| Other bias | High risk | Randomisation at individual participant level but analysis of data at extraction site level |
Hita‐Iglesias 2008.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel‐group, controlled trial Sample size: 73 Sample size calculation: not reported Conducted in: Faculty of Odontology at the University of Seville and the Oral and Maxillofacial Surgery service of the Virgen de Rocio University Hospitals, Seville, Spain Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: mandibular 3rd molars, under local anaesthesia. Difficulty index 4‐7 Koerner scale Recruitment period: from June to November 2005 Funding source: Laboratorios Lacer Barcelona España, donated the CHX samples used in this study |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Age: 18 to 60 years, mean 29 years Gel group 1: randomised 41; analysed 39 Mouthrinse group 2: randomised 32; analysed 31 Number randomised: 73 Number analysed: 70 Lost to follow‐up/dropouts: 3 |
|
| Interventions |
Comparisons: CHX bioadhesive gel versus mouthrinse Group 1 (n = 41): 0.2% CHX bioadhesive gel, placed in wound after extraction then self‐applied to the wound twice a day (day 1 to 7) beginning same day as intervention Group 2 (n = 32): 0.12% CHX mouthrinse twice a day (day 1 to 7) beginning same day as intervention Co‐interventions: ibuprofen 600 mg 3 times a day, paracetamol 500 mg with codeine 14.05 mg 3 times a day |
|
| Outcomes | Subjects evaluated on the 3rd and 7th day postoperatively Primary outcome measures:
Secondary outcome measures: not reported Adverse effects: Quote: "tolerance to the treatment defined as the frequency that patients developed one or more adverse effects .. assessed on a verbal score 1 (max tolerance) to 5 (max intolerance) during the 3rd and 7th postoperative day" page 443 Comment: "tolerance" was not defined in the report nor the type of "adverse effects" which might constitute maximum intolerance, and the relevant data reported in Table 1 were not readily interpretable |
|
| Notes | November 2012: communication from Dr Torres‐Lagares confirmed that Hita‐Iglesias 2008; Torres‐Lagares 2006a and Torres‐Lagares 2006b were separate studies each involving a different group of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomly classified into two groups by means of a simple allocation system using a computer program" Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote: "Having carried out the procedure the envelope corresponding to the patient code was opened" Comment: allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could not be blinded to group allocation, nor could operators. Strict study criteria required that participants and personnel would be blinded to group allocation as they will be involved in reporting of patients symptoms |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All clinical assessment was carried out by a single blind investigator previously trained on diagnostic criteria" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropouts/losses to follow‐up were reported. Similar numbers in both groups, 2 in gel group, 1 in rinse group |
| Selective reporting (reporting bias) | Low risk | Although the study protocol was unavailable, the report appears to be free of selective reporting |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Hu 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not reported Sample size: 200 patients (200 teeth) Setting: outpatient Number of centres: 1 Operators: 1 Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular molar Recruitment period: May to September 2004 Funding source: none stated Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 97 males and 103 females Mean age: 30 Age range: 18 to 64 years Number randomised: 200 Number analysed: 200 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: postoperative medical advice: spit out all the tampons after 30 minutes; not to gargle on that day; return visit at any time if there is anything unusual, otherwise, a week later. Patients who smoked were asked not to smoke for 3 days after surgery Control group: blank control group (no description) Intervention group 1: oral tissue patch The impacted tooth was removed by the same surgeon under routine local block anaesthesia. After the reduction of the alveolar bone, the oral tissue patch was trimmed into the shape of the alveolar fossa and implanted into the alveolar fossa. (If the alveolar interval is too high, in order to avoid the falling off of the implanted oral tissue patch, the oral tissue patch should be trimmed into several parts according to the number of tooth roots pulled out and placed into the root fossa respectively, and fitted to the bone wall of the alveolar fossa. After the patch is covered with blood, a cotton strip should be placed on the wound). All processes are performed by the same person |
|
| Outcomes | Note: authors did not specify which was the primary outcome Outcome measures:
Diagnosis criteria:
Diagnostic criteria for postoperative bleeding after tooth extraction: local bleeding on the day after tooth extraction surgery or significant local bleeding 24 hours after surgery Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "200 random numbers were read from any column or any row in the random number table, and then divided each number by 2. Patients were assigned to the two groups depended on the remainder being odd or even" Comment: randomisation method not clear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The extractions were done by 1 operator who was not involved in the assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up |
| Selective reporting (reporting bias) | Low risk | The incidence of dry socket was reported fully in Table 1 |
| Other bias | Low risk | No other bias noted |
Huang 2011.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Sample size: 80 Sample size calculation: not reported Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular 3rd molar, local anaesthesia with lidocaine Conducted in: Department of Stomatology, the First Affiliated Hospital of Sun Yat‐sen University, Guangzhou, China Number of centres: 1 Recruitment period: May 2008 to July 2010 Providers of care: not reported Funding source: scientific plan of Cantoon Province |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Number randomised: 80 Number evaluated: 80 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
Group 1 (n = 40): acellular dermis matrix (2 × 2.5 cm) embedded into the extraction sockets Group 2 (n = 40): nothing embedded into the extraction sockets Co‐interventions: extraction of the teeth via flap elevation, bone removing and tooth splitting |
|
| Outcomes |
When measured: 1 week after the extraction Primary outcome measures: presence of postoperative alveolitis Secondary outcome measures: none Adverse outcomes: none reported |
|
| Notes | Author contact failed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The participants were randomized into two groups with 40 in each group" Comment: the detailed methods of randomisation were not clearly reported |
| Allocation concealment (selection bias) | Unclear risk | It is not clear from the report how allocation concealment was achieved |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to group allocation (placebo received no intrasocket intervention), personnel were not blinded to group allocation. Strict study criteria required that participants and personnel would be blinded to group allocation as they will be involved in reporting of patients symptoms |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear whether assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None of the participants dropped out |
| Selective reporting (reporting bias) | Low risk | All the outcomes were reported clearly |
| Other bias | High risk | Authors stated that the gender and the age in each group were similar at baseline, but the difficulties of the extraction and the detailed information of the tooth impaction were not reported |
Karabit 2019.
| Study characteristics | ||
| Methods |
Study design: randomised, split‐mouth study Sample size calculation: not reported Sample size: 53 Setting: Department of Oral Maxillofacial Surgery, Syria Number of centres: 1 Operators: 1 surgeon Prevention or treatment of dry socket: prevention Type of teeth: bilateral mesially impacted mandibular molars Recruitment period: 1 August 2016 to 5 January 2017 Funding source: reported as none Declarations of interest: not reported |
|
| Participants |
Inclusion criteria: smoker Exclusion criteria: patient on antibiotic prophylaxis, known to be allergic to CHX, with systemic disorders, epinephrine contraindications or breastfeeding women and who are on oral contraceptives Gender: 31 males, 22 females Mean age: not reported Age range: 18 to 26 years Number randomised: 53 Number analysed: 53 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: none Intervention group 1: 0.12% CHX gluconate Intervention group 2: mint flavoured aqua distillate |
|
| Outcomes |
Primary outcome measures: pain Diagnosis: VAS Secondary outcome measures: presence of clot, exposed alveolar bone Adverse outcomes: visual examination |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Surgical site was chosen randomly by asking the patients to choose one card out of two in which site was written on its back (left or right)." Group allocation patients "divided randomly into two parallel group and we asked the patients to choose one card out of two in which the bottle number was written on its back" |
| Allocation concealment (selection bias) | High risk |
Quote: "Surgical site was chosen randomly by asking the patients to choose one card out of two in which site was written on its back (left or right). ....we asked the patients to choose one card out of two in which the bottle number was written on its back" Comment: unlikely to be concealed to operator |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants appeared blinded
Quote: (page 30) "Group A: the patients received a bottle of 0.12% CHXG mouthwash with mint flavor to start using it on the day before and the day after the extraction twice daily for 7 days. Group B: the patients received a bottle of Aqua distillate with mint flavor to start using as directed in bottle A" Operators appear blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who performed clinical assessments and whether blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All eligible participants reported |
| Selective reporting (reporting bias) | Low risk | All planned outcome observations reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kaya 2011.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel‐group, controlled trial Study size: 104 Sample size calculation: not reported Conducted in: Department of Oral and Maxillofacial Surgery, Atatürk University Faculty of Dentistry, Turkey Number of centres: 1 Prevention or treatment of dry socket: treatment Type of teeth: mandibular permanent 1st molar Recruitment period: 18 months, January 2008 to July 2009 Funding source: not reported |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Number randomised: 104 Number evaluated: 104 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
Group 1 (n = 26): curettage and irrigation alone Group 2 (n = 26): curettage and irrigation followed by Alvogyl applied directly to the socket Group 3 (n = 26): curettage and irrigation followed by SaliCept patch applied directly to the socket Group 4 (n = 26): curettage and irrigation followed by low‐level laser therapy irradiation Co‐interventions: patients were allowed 500 mg of acetaminophen as a rescue medication, as required, and were instructed to record how many times daily the medication was used. Additional follow‐up visits were organised through the department, as necessary |
|
| Outcomes |
Primary outcome measures:
Secondary outcome measures: not stated Adverse outcomes: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The patients were randomly assigned…" Comment: method not clear |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned and no information to suggest this was done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned and no information to suggest this was done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned and no information to suggest this was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in analysis |
| Selective reporting (reporting bias) | Low risk | The report appears to be free of selective reporting |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Keshini 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not reported Sample size: 30 Setting: not reported Number of centres: not reported Operators: not reported Prevention or treatment of dry socket: treatment Type of teeth: not reported Recruitment period: not reported Funding source: not detailed Declarations of interest: none |
|
| Participants |
Inclusion criteria: patients reported with postextraction pain, who fit criteria for dry socket Exclusion criteria: patients under 14 and over 60 years of age Gender: not given Mean age: not given Age range: not given Number randomised: 30 Number analysed: 30 Lost to follow‐up/dropouts: none reported |
|
| Interventions |
All patients: gentle irrigation with warm saline and debridement Intervention group 1:. n = 15, socket packed with Alvogyl and sutured with 3‐0 mersilk Intervention group 2: n = 15, platelet rich fibrin membrane placed in socket and sutured with 3‐0 mersilk |
|
| Outcomes |
Primary outcome measures: pain and wound healing Diagnosis: VAS score, wound healing assessed by number of socket walls exposed Secondary outcome measures: none Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: (page 321) "A total of thirty patients were included in the study ....were randomly divided into two Groups (Group A and Group B), with 15 patients in each group using simple randomization" Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk |
Quote: (page 321) "A total of thirty patients were included in the study ....were randomly divided into two Groups (Group A and Group B), with 15 patients in each group using simple randomization" Comment: not mentioned and no information to suggest this was done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned and no information to suggest this was done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who operator is who performed assessments and whether operator was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None of the participants dropped out |
| Selective reporting (reporting bias) | Low risk | All outcome measures appear to have been reported |
| Other bias | Unclear risk | Sparse reporting of details in relation to participants, setting, teeth extracted, operators and comparison between the 2 groups |
King 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: "The data obtained by Kaya et al were used as reference to calculate the statistical power in the present study. It was assumed that a sample size of 40 sockets (20 per each group), with a standard deviation of 2 and a decrease in pain of 6 points in the VAS, would give a statistical power of 86.9%. Therefore, the aim was to recruit 20 sockets per group with the expectation that obtaining data from 17 per group would give a statistical power of 80% with the P value set at 0.05" Sample size: 38 patients (44 sockets) Setting: UK dental hospital Number of centres: 1 Operators: all initial treatment was conducted by the lead clinician, a qualified dentist Prevention or treatment of dry socket: treatment Type of teeth: incisors, premolars and molars Recruitment period: from the beginning of December 2014 to the end of February 2015 Funding source: "the PRGF consumables used in this study were supplied by the BTI Biotechnology Institute (San Antonio, Spain)" Declarations of interest: "the PRGF consumables used in this study were supplied by the BTI Biotechnology Institute (San Antonio, Spain)" |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 18 females, 20 males Mean age: 40.7 ± 17.3 years Age range: not stated Number randomised: 44 Number analysed: 44 Lost to follow‐up/dropouts: 1 patient did not attend the 1st review appointment and another patient did not attend the 2nd review appointment. The data from the review appointments when the patients did attend were included in the statistical analysis |
|
| Interventions |
Control group: the socket was irrigated with sterile saline and packed with Alvogyl approximately 0.20 g to fill the defect according to the manufacturer’s instructions Intervention group: plasma rich in growth factors (PRGF) 4.9 mL tubes of venous blood containing 3.8% sodium citrate were collected. The blood was centrifuged at 580g for 8 minutes at room temperature (PRGF Model System Centrifuge IV, BTI Biotechnology Institute). The 2 plasma fractions, F1 and F2, were separated using a PRGF Plasma Transfer Device supplied with the PRGF‐Endoret kit. Care was taken not to include leukocytes (the buffy coat) when extracting F2. F1 was poured into a flat glass dish and placed in a PlasmaTherm H Oven heated to 37°C for 15 minutes. The F1 clot underwent retraction to produce the F1 membrane and the liquid supernatant. Then, F2 was activated to induce fibrin clot formation. Before placement of the fibrin clot, PRGF liquid supernatant was used to irrigate the socket. Then, the F1 membrane was applied to cover the surgical area and the socket margins were sutured |
|
| Outcomes |
Outcome measures:
Adverse outcomes: no adverse events reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated, block randomization schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization numbers were assigned by study staff at the study site in ascending numerical order as patients were determined to be fully eligible to participate in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Given the nature of the interventions it would not have been possible to blind the operator and patients. Lack of masking of participant and operator is unlikely to have led to deviation in intervention. Strict study criteria required that participants and personnel would be blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "A second clinician, a qualified dentist, who was blinded to the original treatment, conducted the review appointments" Comment: pre‐treatment assessments were reported to have been undertaken by the treating clinician before randomisation and treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "One patient did not attend the first review appointment and another patient did not attend the second review appointment. The data from the review appointments when the patients did attend was included in the statistical analysis" Comment: this suggest an intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | All outcome measures appear to have been reported |
| Other bias | Unclear risk | Analgesia use was not reported and could have had an impact on pain reported by participants |
Kjellman 1973.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel‐group, controlled trial Sample size: 100 Sample size calculation: not reported Conducted in: Department of Oral Surgery at Södersjukhuset Stockholm, Sweden Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular 3rd molars, degree of difficulty unspecified, under local anaesthesia Recruitment period: not stated Funding source: not stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Number randomised: 100 Number evaluated: 100 |
|
| Interventions |
Group 1 (n = 50): Apernyl cone, consisting of acetylsalicylic acid and p‐oxybenzoic acid (preservative) inserted immediately after extraction Group 2 (n = 50): placebo without acetylsalicylic acid inserted immediately after extraction Co‐interventions: concomitant pain medication unreported |
|
| Outcomes | Assessments performed on days 1, 2 and 4 postoperatively Primary outcome measures: the occurrence of postoperative periosteitis "dry socket" Secondary outcome measures: not evaluated Adverse effects: pain and burning sensation |
|
| Notes | Inadequately reported, sparse trial details and incomplete and largely unusable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "...with the help of a randomising procedure..." Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned and no information to suggest this was done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned and no information to suggest this was done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Very limited data, reported as graph plots. Insufficient information to make a clear judgement |
| Selective reporting (reporting bias) | Low risk | Although the protocol was unavailable all of the outcomes specified in the methods section appear to have been reported |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Larsen 1991.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel‐group, controlled trial Sample size: 150 Sample size calculation: none reported Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular 3rd molars Conducted in: not stated Number of centres: not stated Recruitment period: not stated Funding source: Procter and Gamble Co, Cincinnati, USA |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Number randomised: 150 Number analysed: 139 Lost to follow‐up/dropouts: 11 |
|
| Interventions |
Group 1 (n = 72): 0.12% CHX gluconate 15 mL for 30 seconds a day for 1 week prior to surgery and 1 week after Group 2 (n = 67): placebo rinse (identical appearance) Concomitant medication:
|
|
| Outcomes | Assessment 1 week postoperatively or earlier if pain Primary outcome measures: presence of dry socket Secondary outcome measures: none stated Adverse effects: none stated |
|
| Notes | Sponsored by Procter and Gamble Co, Cincinatti, USA Randomisation was by patient but analysis by sites which will narrow the confidence intervals slightly |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "using a computer software routine supplied by the sponsor ..." " Within strata subjects were randomly assigned into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were unaware of group allocation, personnel were unaware of group allocation. Active and placebo were identical in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who made the assessment of outcomes, while operators were blinded to allocation and appearance of placebo was identical, unlikely that they would have known group allocation when making assessment, however insufficient information to make a judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data available for dropouts (11): unreported from which groups, when and the reasons |
| Selective reporting (reporting bias) | Low risk | Although the study protocol was unavailable, the report appears to be free of selective reporting of the outcomes |
| Other bias | High risk | Participants were stratified then randomised but subsequent analyses were at tooth level. Although we know how many sites were available for analysis, we only know the baseline number of patients |
Lenka 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not stated Sample size: 30 Setting: not stated Number of centres: 1 Operators: not stated Prevention or treatment of dry socket: treatment Type of teeth: not stated Recruitment period: not stated Funding source: self‐funded Declarations of interest: stated as none |
|
| Participants |
Inclusion criteria: pain around the extraction socket after 1 day, foul smell, total or partial loss of clot Exclusion criteria: not stated Gender: not stated Mean age: not stated Age range: not stated Number randomised: 30 Number analysed: not stated Lost to follow‐up/dropouts: not stated |
|
| Interventions |
All patients: local anaesthesia, saline irrigation Intervention group 1:. zinc oxide eugenol paste/gauze pieces dressing Intervention group 2: Alvogyl |
|
| Outcomes |
Primary outcome measures: pain Diagnosis: VAS Secondary outcome measures: none Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The patient selected for the study was those who have severe pain after one day of extraction and proper examination the diagnosis was dry socket are equally and randomly to control and test groups" Comment: no information to make a decision |
| Allocation concealment (selection bias) | Unclear risk | No information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information to make a decision |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to make a decision |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures appear to have been reported |
| Other bias | Low risk | No other bias observed |
Metin 2006.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel‐group, controlled, double‐blind, single‐centre trial Sample size: 99 Sample size calculation: not reported Conducted in: Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Ondokuz, Mayis, University of Samsun, Turkey Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular 3rd molars, under local anaesthesia Recruitment period: not stated Funding source: not reported |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Number randomised: 99 Number analysed: 99 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
Comparison: pre and postoperative CHX rinse versus postoperative only Group 1 (n = 46): rinsed with CHX 0.2% 15 mL for 30 seconds twice per day for both 1 week prior to and 1 week after surgery Group 2 (n = 53): rinsed with CHX 0.2% 15 mL for 30 seconds twice per day for 1 week after surgery Co‐interventions: after removal of the teeth, the surgical sites were rinsed with 10 mL of sterile saline solution, and the soft tissue was closed and sutured with 3‐0 silk suture |
|
| Outcomes | On the 7th day (or on preceding days if pain was present), the extraction sites were evaluated Primary outcome measures: incidence of AO Secondary outcome measures: not evaluated Adverse effects: altered taste and numbness page 3. Numbness in the tongue reported in Group 1 and Group 2 45.6% and 13.2%. However, disturbance of taste sensation was seen in 56.5% of the patients in Group 1 and in 11.3% of the patients in Group 2 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were randomly assigned into two groups" Comment: no information provided on how |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants may have known their group allocation, it is not clear if personnel knew group allocation. A strict study criteria require that participants and personnel are blinded to group allocation but unclear reporting and unclear impact on performance bias. Lack of masking of patient and operator is unlikely to have led to deviation in intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "The same examiners made all the diagnoses" Comment: It would appear that operators made the assessment of the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in the outcome evaluation |
| Selective reporting (reporting bias) | Low risk | Although the protocol was unavailable, all of the outcomes specified in the methods section appear to have been reported |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Mitchell 1984.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel‐group, placebo‐controlled, double‐blind trial Sample: 64 Conducted in: Oral Surgery Department, Newcastle Dental Hospital, UK Number of centres: 1 Prevention or treatment of dry socket: treatment Type of teeth: no tooth specified Recruitment period: not stated Funding source: not reported |
|
| Participants |
Inclusion criteria: a diagnosed dry socket Exclusion criteria: none stated Number randomised: 64, 1 lost to follow‐up but group allocation not stated Group 1 (metronidazole): randomised not reported; 6 lost to follow‐up; analysed 26 (18%) Group 2 (Orabase placebo): randomised 32; 3 lost to follow‐up; analysed 29 (10%) Number analysed: 55 |
|
| Interventions |
Comparison: metronidazole (10%) versus Orabase alone Group 1 (n = 26): metronidazole (10%) in carboxymethylcellulose gelatin (Orabase) paste Group 2 (n = 29): Orabase paste alone Dressing syringed into the sockets Co‐interventions: warm saline irrigation at presentation |
|
| Outcomes | Review at 2 days post‐intervention initially and then re‐application dressing where necessary and review until cure Primary outcome measures: absence of pain Secondary outcome measures:
Adverse effects: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly allocated to the test or placebo group and the operator was unaware of the code breaker" |
| Allocation concealment (selection bias) | Low risk |
Quote: "The patients were randomly allocated to the test or placebo group and the operator was unaware of the code breaker" Comment: probable allocation concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The test and control pastes were packaged in identical 2 mL syringes. Participants and personnel are blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Operator unaware of group allocation and code only broken after study was complete |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout reported but not clear from which group, all other dropouts fully reported. Unlikely to have had large impact on data analysis |
| Selective reporting (reporting bias) | Low risk | Although the study protocol was unavailable, the report appears to be free of selective reporting of the outcomes |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Ragno 1991.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled, double‐blind, parallel‐group study Sample size calculation: not reported Conducted in: Department of Oral and Maxillofacial Surgery, Walter Reed Army Medical Centre, Washington DC, USA Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: mandibular 3rd molars, under intravenous sedation and local anaesthesia Operator: resident surgeon for 8 years Recruitment period: July 1987 to April 1989 Funding source: not stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria: not stated Mean age: not reported Intervention Group: number randomised 40; analysed 40 Control Group: number randomised 40; analysed 40 Number randomised: 40 patients, but sites not (80) Number analysed: 40 patients, but sites not (80) |
|
| Interventions |
Comparisons: on the day of surgery patients first rinsed with 15 mL of designated solution, teeth were then extracted, surgical sites irrigated with 15 mL of designated solution and soft tissue closed and sutured. The day after surgery patients began home use of solutions Group 1 (n = 40; 80 surgical sites): 0.12% CHX rinse, 15 mL twice daily for 7 days postoperatively Group 2 (n = 40; 80 surgical sites): placebo rinse, 15 mL twice daily for 7 days postoperatively Co‐interventions: none stated Concomitant interventions: none stated |
|
| Outcomes | Postoperative examination on days 3 and 7 Primary outcome measures: AO Secondary outcome measures: postoperative questionnaire day 7, though not reported but presume it relates to adverse events associated with mouthrinse Adverse effects: there were no allergic reactions to the CHX rinse. 3 participants reported bad taste, 1 reported stomach upset (page 526) but no staining noted. 1 person in the control had a severe surgical reaction which in the author's opinion was not attributable to the medication |
|
| Notes | On the day of the procedure patients first rinsed with 15 mL of their assigned solution for 30 seconds, after the procedure the sites were rinsed with 15 mL of the same solution | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: ''The patients within each group were randomly assigned" Comment: no mention of process |
| Allocation concealment (selection bias) | Low risk |
Quotes: "The pharmacist manufactured the placebo and maintained the code for patient assignments"...."Decoding of the patient assignments revealed..." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants masked to group intervention. Not clear that personnel were blinded to group allocation. It is unlikely that lack of masking of operator would have led to deviation in intervention. Strict study criteria required that participants and personnel would be blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear who assessor was, not clear blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Postoperative questionnaire completed day 7, no data reported |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Reekie 2006.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel‐group, double‐blind, placebo‐controlled study Sample size: 302 Sample size calculation: undertaken Conducted in: 3 dental practices in the UK Number of centres: 3 Prevention or treatment of dry socket: prevention Type of teeth: routine non‐surgical extraction of 1 or more molar or premolar tooth under local anaesthetic Recruitment period: 2000 to 2003 Funding source: a grant from the Oral and Dental Research Trust |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Number randomised: 302 Number analysed: 27 Only patients calling back with pain were examined and assessed. It was assumed all others did not have dry socket |
|
| Interventions |
Comparison: metronidazole 25% gel versus placebo gel Group 1 (n = 152): 0.25 ml of 25% metronidazole gel (62.5 mg) Group 2 (n = 150): placebo gel (KY jelly) Gel syringed into socket immediately after dental extraction Mean age: 49.5 years, SD 14.77 (range 19 to 93) Co‐interventions: not stated |
|
| Outcomes | Assessment made only on participants with severe pain and who phoned the surgery. Only patients with the classical signs of dry socket were reported Primary outcome measures: presence of dry socket Secondary outcome measures: none assessed or reported Adverse effects: not selected as an outcome but were reported (see Notes) |
|
| Notes | Adverse events: reported: 1 participant with nausea and vomiting, 2 complained of a bitter taste | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Blinding and randomisation was achieved by the manufacturer ....The syringes were allocated a code number derived from a random number sequence" Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote: "Blinding and randomisation was achieved by the manufacturer ..The code constructed by the manufacturer and not broken until the end of the study" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded to group allocation. Placebo and intervention gel had identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Code was not broken until after the study, assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only patients with pain and who phoned the surgery were "offered an appointment the same day to see a dentist" Comment: incomplete outcome data and judged as potentially at high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Not enough data to make a decision |
| Other bias | Unclear risk |
Quote: "Where more than one extraction was needed only one was randomly chosen to be included in the study" Comment: unclear if this would constitute an element of selection bias |
Ritzau 1977.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel‐group, double‐blind, placebo‐controlled study Sample size: 45 Sample size calculation: not reported Conducted in: Royal Dental College in Aarhus, Denmark Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular 3rd molars Recruitment period: February to May 1974 Funding source: not stated |
|
| Participants |
Inclusion criteria: partially and totally impacted mandibular 3rd molars Exclusion criteria: none stated Mean age: 27 (range 17 to 61 years) Number randomised: 45 Number analysed: 45 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
Comparison: propylic ester of p‐hydroxybenzoic acid versus placebo
Group 1 (n = 24): propylic ester of p‐hydroxybenzoic acid inserted in each socket Group 2 (n = 21): placebo tablet gel inserted in each socket Co‐interventions: postoperative analgesic tablets containing 10 mg of codeine phosphate, 500 mg of acetyle acid, and 70 mg of magnesium oxide were prescribed, and the number of tablets consumed was recorded Experimental substance or placebo inserted into postoperative socket immediately after removal of impacted 3rd molar |
|
| Outcomes | Assessment day 7 postoperatively. Patients also assessed if they returned in pain at any point ‐ the day of return was noted Primary outcome measures: occurrence of alveolitis sicca dolorosa Secondary outcome measures: pain Adverse outcomes: haematoma and rash |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "PEPH or placebo were inserted at random selection...." Comment: method not specified |
| Allocation concealment (selection bias) | Low risk |
Quote: '"...The code was unknown to the investigators" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and personnel were blinded to group allocation. Intervention and placebo were identical in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients and personnel were blinded to group allocation. Intervention and placebo were identical in appearance, code for group allocation not broken until after the study completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The report appears to be free of selective reporting of the outcomes |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Rodriguez‐Perez 2013.
| Study characteristics | ||
| Methods |
Study design: RCT with 2 parallel groups Sample size calculation: not reported Sample size: 88 patients Setting: School of Dentistry at the University of Granada, and Oral and Maxillofacial Surgery Service of Virgen de las Nieves Hospital of Granada, Spain Number of centres: 2 Operators: 2 surgeons Prevention or treatment of dry socket: prevention Type of teeth: mandibular 3rd molars Recruitment period: January 2009 to January 2011 Funding source: not reported Declarations of interest: none reported |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 46 female, 42 male Mean age: 26 years Age range: 18 to 44 years Number randomised: 88 Number analysed: 88 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: bioadhesive 0.2% CHX gel applied to the socket postextraction. All patients took 1 g paracetamol every 12 hours and 600 mg ibuprofen every 8 hours Intervention group 1: (n = 46) apply 0.2% CHX gel to the socket twice daily for 7 days Intervention group 2: (n = 42) apply 1% CHX gel to the socket twice daily for 7 days |
|
| Outcomes |
Primary outcome measures: presence of dry socket Diagnosis: Blum’s criteria for AO Secondary outcome measures: Oral Health Related Quality of Life Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly classified into two groups, using a simple allocation using a computer program" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All eligible participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes specified were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rubio‐Palau 2015.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, clinical trial Sample size calculation: "The sample size was calculated with 80 patients treated with chlorhexidine gel and 80 with placebo, with a significance level of 5% and a statistical power of 80% to detect as significant a difference corresponding to an incidence of 11% in the chlorhexidine group and 30% in the placebo group" Sample size: 160 patients Setting: Minor Outpatient Surgery Unit of Hospital Vall d’Hebron, Spain Number of centres: 1 Operators: not reported Prevention or treatment of dry socket: prevention Type of teeth: mandibular 3rd molars Recruitment period: April 2008 to November 2010 Funding source: CHX and placebo gels provided by Laboratorios Lacer (C/ Sardenya 350, Barcelona, Spain) Declarations of interest: none reported |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 86 females, 74 males Mean age: 25.04 years Age range: not reported Number randomised: 160 Number analysed: 160 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: diclofenac 50 mg every 8 hours alternated with metamizole 575 mg every 8 hours and omeprazole 20 mg per day Control group: (n = 80) a single dose of bioadhesive placebo was placed in the socket postextraction Intervention group: (n = 80) a single dose of 0.2% CHX bioadhesive gel was placed in the socket postextraction |
|
| Outcomes |
Primary outcome measures: presence of dry socket Diagnosis: uncontrolled pain between 1 and 3 days postextraction with 1 or more of the following: partial or total disintegration of the clot, detritus, empty socket/exposed bone +/‐ halitosis Secondary outcome measures: none Adverse outcomes: 30 patients reported gastrointestinal discomfort, dizziness in 10 patients, skin rash in 2 patients (these patients were in both groups ‐ they report gastrointestinal upset was due to metamizole) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization of patients was performed by the Department of Statistics by a random list grouping the total of 160 patients in groups of 4 so that the distribution of the two groups (chlorhexidine and placebo) were homogeneous throughout the sample" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "neither the surgeon nor the patient knew the substance" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All eligible participants reported |
| Selective reporting (reporting bias) | Low risk | All specified outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Shad 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not reported Sample size: 180 patients Setting: Dentistry Department of Ayub Medical College, Abbottabad, Pakistan Number of centres: 1 Operators: experienced surgeon with 5 years experience Prevention or treatment of dry socket: prevention Type of teeth: impacted 3rd molars Recruitment period: January 2015 to July 2017 Funding source: not reported Declarations of interest: none reported |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: male 83, female 97 Mean age: 27.47 years Age range: 21 to 35 years Number randomised: 180 patients Number analysed: 180 patients Lost to follow‐up/dropouts: 0 |
|
| Interventions |
Control group: 0.2% bioadhesive CHX gel placed in socket after extraction Intervention group: placebo gel placed in socket after extraction |
|
| Outcomes |
Primary outcome measures: presence of dry socket Diagnosis: postoperative pain which increased in intensity 2 to 3 days after extraction and there was partial/total disintegration of clot resulting in an empty socket and denuded bone with or without halitosis Secondary outcome measures: none Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a decision |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make a decision. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for in analysis |
| Selective reporting (reporting bias) | Low risk | All outcome measures appear to have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Shi 2003.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel‐group, controlled trial Sample size: 274 Sample size calculation: not reported Prevention or treatment of dry socket: prevention Type of teeth: impacted wisdom teeth Conducted in: not stated (appears to be 4th Medical University, Xi, Shaanxi China) Number of centres: not stated Recruitment period: November to April 2001 Funding source: not stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Number randomised: 274 Number analysed: not fully reported |
|
| Interventions |
Group 1 (n = 92): Shahaosan Group 2 (n = 86): Yunnan white drug Group 3 (n = 96): blank control Co‐interventions: none stated |
|
| Outcomes | Assessments 5 to 7 days postextraction. Quote: "incidence and intensity of dry socket in each group were observed and evaluated by scores" Primary outcome measures: incidence of dry socket Secondary outcome measures: self‐assessed POSSE (Postoperative Symptom Severity Scale) global assessment to include pain and "influence of daily life." Time and frequency of assessment unreported Adverse effects: no report of any assessment |
|
| Notes | 3 different colour capsules, unclear if both A and B are active interventions. Unclear if this was systemic or topical and method and timing of administration not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "patients with extraction of impacted tooth were randomly divided into 3 groups" Comment: method of sequence generation not clear |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported, unable to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Very limited data reported, unable to make a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Very limited data reported, unable to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Very limited data reported, unable to make a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Very limited data reported, unable to make a clear judgement |
| Other bias | Unclear risk | Very limited data reported, unable to make a clear judgement |
Sun 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not reported Sample size: 300 patients Setting: outpatient Number of centres: 1 Operators: 1 Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular 3rd molar Recruitment period: March 2004 to December 2005 Funding source: none stated Declarations of interest: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria: not reported Gender: 140 males, 160 females Mean age: 30 years Age range: 18 to 49 years Number randomised: 300 Number analysed: 300 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: the patient to return to the clinic 1 week after extraction, and may return to the clinic at any time if discomfort occurs. Inquiring the patients about postoperative bleeding and swelling in detail, the patients with suspected dry socket must be confirmed after examination by 2 attending doctors, and the patients who failed to return in time should be followed up by telephone Control group: blank control group (no description) Intervention group 1: oral tissue patch. Under conventional local anaesthesia, the impacted teeth were pulled out, the oral tissue patch was placed into the alveolar fossa, and the wound was sutured in counterposition. A yarn ball was placed on top to bite tightly, and the yarn ball was spat out after 0.5 hours. All operations are performed by the same doctor |
|
| Outcomes | Note: authors did not specify which was the primary outcome Outcome measures:
Diagnosis criteria:
Diagnostic criteria for postoperative bleeding after tooth extraction: local bleeding on the day after tooth extraction surgery or significant local bleeding 24 hours after surgery. Diagnostic criteria of swelling after tooth extraction: local swelling on the day after tooth extraction or cheek swelling after 24 hours Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The patients were randomly divided into two groups by using the randomised table, 150 people in each group (with no further details being reported) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported, no details about who the assessors were |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up |
| Selective reporting (reporting bias) | Low risk | The outcome (incidence of dry socket) was reported fully in Table 2 |
| Other bias | Low risk | No other bias reported |
Supe 2018.
| Study characteristics | ||
| Methods |
Study design: single‐blinded, prospective study Sample size calculation: not reported Sample size: 50 patients Setting: department of oral and maxillofacial surgery Number of centres: not stated Operators: not reported Prevention or treatment of dry socket: treatment Type of teeth: all teeth Recruitment period: September 2013 to June 2015 Funding source: none Declarations of interest: none |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 29 females, 21 males Mean age: 32.32 years Age range: 18 to 51 years Number randomised: 50 Number analysed: 50 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: all patients received diclomol 50 mg Intervention group 1: patients received Alvogel (combination of iodoform and butylparaminobenzoate) paste as an intrasocket medication Intervention group 2: patients received zinc oxide eugenol as an intrasocket medication |
|
| Outcomes |
Primary outcome measures: pain (VAS scale) Diagnosis: not clearly stated Secondary outcome measures: time required for complete pain relief, healing (measured by amount of socket covered by initial granulation tissue) Adverse outcomes: 2 patients in group 1 (Alvogyl), and 9 patients in group 2 (zinc oxide eugenol) showed delayed healing |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a decision |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make a decision. All patients are accounted for in terms of healing scores at day 14 (final follow‐up). It should be noted that pain scores have only been reported for 4/5 days |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a decision |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Torres‐Lagares 2006a.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled "...prospective, parallel, single‐blind clinical trial" Sample size: 30 Sample size calculation: not reported Conducted in: Faculty of Odontology of the University of Seville, Spain Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: 1 or 2 impacted mandibular 3rd molars, under local anaesthesia Recruitment period: September to December 2001 Funding source: not stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Number randomised: 30 Number analysed: 30 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
Group 1 (n = 17): 10 mL of 0.2% CHX bioadhesive gel applied intra‐alveolar postextraction Group 2 (n = 13): no intrasocket medication Co‐interventions: ''All the patients took, as postoperative treatment, 14.05 mg codeine phosphate and 500 mg of paracetamol on demand...'' |
|
| Outcomes | Unclear if assessed on days 3 and 7 postoperatively Primary outcome measures: development of AO Secondary outcome measures: none stated Adverse effects: none stated |
|
| Notes | November 2012: communication from Dr Torres‐Lagares confirmed that Hita‐Iglesias 2008; Torres‐Lagares 2006a and Torres‐Lagares 2006b were separate studies each involving a different group of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The aforementioned allocation into one group or another was carried out by computer before the start of the study" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: ''...an envelope was opened, in which it indicated whether the patient should receive the bio‐adhesive gel or not" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients did not know allocation but operators would have. It is unlikely that lack of masking of operator would lead to deviation in intervention. Strict study criteria required that participants and personnel would be blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Operators detecting presence of dry socket could have remembered group allocation potential for high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the protocol and analysed |
| Selective reporting (reporting bias) | Low risk | The report appears to be free of selective reporting of the outcomes |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Torres‐Lagares 2006b.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled, parallel‐group, double‐blind study Sample size: 103 Sample size calculation: not reported Conducted in: Faculty of Dentistry of the University of Seville, Spain Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular 3rd molars ‐ under local anaesthesia Recruitment period: January to June 2003 Funding source: Laboratorios Lacer, Barcelona, Spain, donated the medication used in this study |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Number randomised: 103 Number analysed: 94 |
|
| Interventions |
Comparisons: 0.2% CHX bioadhesive gel versus no application Group 1 (n = 49): 10 mL of 0.2% CHX bioadhesive gel applied intra‐alveolar postextraction Group 2 (n = 45): placebo gel ‐ excipient containing Co‐interventions: all the patients took, as postoperative treatment, 14.05 mg codeine phosphate and 500 mg of paracetamol on demand |
|
| Outcomes |
Primary outcome measures: development of AO Secondary outcome measures: none stated Adverse effects: none stated |
|
| Notes | No clear statement of when follow‐up was done November 2012: communication from Dr Torres‐Lagares confirmed that Hita‐Iglesias 2008; Torres‐Lagares 2006a and Torres‐Lagares 2006b were separate studies each involving a different group of participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''The random assignment was carried out by means of a random number list" |
| Allocation concealment (selection bias) | Low risk | Quote: ''The allocation of patients into one group or the other was carried out by computer before the start of the study. The gel was identifiable by a patient inclusion code number" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the patient nor the operator knew group allocation, the code list was kept in a sealed enveloped and was not opened until after the study. Placebo did not contain active ingredient" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Operator did not know group allocation. The code list was kept in a sealed enveloped and was not opened until after the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of patients not included in final check‐up visit stated, not included in analysis: 5 patients (1 in the control group and 4 in the test group) did not have their final check‐up visit |
| Selective reporting (reporting bias) | Low risk | The report appears to be free of selective reporting of the outcomes |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Trieger 1991.
| Study characteristics | ||
| Methods |
Study design: randomised, split‐mouth study Prevention or treatment of dry socket: prevention Type of teeth: mandibular bony 3rd molar impactions Conducted in: setting unreported Number of centres: not stated Recruitment period: not stated Funding source: "This study was made possible with a grant from Upjohn, Kalamazoo, Michigan" |
|
| Participants |
Inclusion criteria: bilateral mandibular 3rd molar bony impactions Exclusion criteria: no antibiotics 2 weeks prior Number randomised: 172 sites in 86 patients Number analysed: unclear |
|
| Interventions |
Group 1 (n = 86): gelfoam square saturated with 1 mL clindamycin phosphate solution (150 mg/mL)
Group 2 (n = 86): gelfoam square saturated with saline placebo Co‐interventions: concomitant analgesic medication allowed but no details reported |
|
| Outcomes | Not clear when assessments were made and by whom Primary outcome measures: dry socket Secondary outcome measures: not reported Adverse events: unreported and unclear if assessed |
|
| Notes | We assume there were no dropouts but study poorly reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...according to a randomized distribution" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement if adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Very limited data reported, unable to make a clear judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Very limited data reported, unable to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Very limited data reported, unable to make a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Very limited data reported, unable to make a clear judgement |
| Other bias | Unclear risk | Very limited data reported, unable to make a clear judgement |
Tuk 2019.
| Study characteristics | ||
| Methods |
Study design: cross‐over RCT Sample size calculation: yes, using a paired‐samples t test, an a of 5%, a power of 90%, 2‐tailed testing, and an effect size of 0.5 (a moderate effect size was used because the effect should be clinically relevant) results in a total sample size of 44. Using a paired‐samples t test, an a of 5%, a power of 90%, 2‐tailed testing, and an effect size of 0.5 (a moderate effect size was used because the effect should be clinically relevant) results in a total sample size of 44 Sample size: 54 patients Setting: Department of Oral and Maxillofacial Surgery, the Netherlands Number of centres: 1 Operators: 1 Prevention or treatment of dry socket: prevention Type of teeth: bilateral symmetrically, horizontally impacted mandibular 3rd molars Recruitment period: 1 January 2016 to 31 July 2017 Funding source: not reported Declarations of interest: reported as none |
|
| Participants |
Inclusion criteria: native Dutch speakers with bilateral horizontally impacted mandibular 3rd molars, with a Gregory‐Pell grade 3B impaction, who were planning to undergo bilateral mandibular 3rd molar surgery, age of 18 years or older; American Society of Anesthesiologists status 1; patients with no systemic diseases or medical conditions, no discernible active pathology associated with the 3rd molars, and no acute pericoronitis; and patients without periodontal disease Exclusion criteria: smoking, allergy to ibuprofen, presence of systemic disease, history of a recent and/or symptomatic peptic ulcer, antiplatelet or anticoagulant therapy, pregnancy or lactation, recent (< 15 days) local infection before surgery, previous radiation therapy to the maxillofacial region, local pathology (e.g. cyst or tumour) associated with the 3rd molars, or lack of consent to undergo the procedure or participate in the study Gender: 25 male, 29 female Mean age: 25.1 years Age range: not reported Number randomised: 54 Number analysed: 54 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: surgical site closure, ibuprofen 600 mg 3 times a day, 0.12% CHX mouthwash Intervention group 1: 1 x 2 cm iodine tampon Intervention group 2: saline rinsing using a monoject syringe 4 times daily 48 hours postoperatively |
|
| Outcomes |
Primary outcome measures: oral health related quality of life, pain, swelling, limited mouth opening, postoperative infection and AO Diagnosis: OHIP‐14, VAS, questionnaire, the presence of purulent discharge in the extraction socket and/or excessive swelling with fluctuation, with or without pain; presence of a local abscess; or onset of facial or cervical cellulitis plus other signs suggesting infection, such as pain, increased heat, erythema, and/or fever. A diagnosis of AO was established if there was new onset or increasing pain more than 36 hours after removal of the mandibular 3rd molar in combination with an absence of the hematic clot in the socket, resulting in exposed bone. A putrid smell may be present in combination with intense neuralgia‐type pain. Gentle probing or irrigation of the wound aggravated the pain. All elements needed to be present for the diagnosis of AO Secondary outcome measures: self‐care: pain medication, ice cooling Adverse outcomes: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a surgical assistant allocated the patient to either trial condition using a computer random generator... After the allocation to a trial condition, another surgical assistant assigned a side to either the experimental treatment or the control treatment, again using a computer random generator" |
| Allocation concealment (selection bias) | Low risk |
Quote: "The concealment of allocation was guaranteed through a sealed envelope" Comment: suitable washout period of 8 weeks |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants likely to be aware of procedure. Operator would be aware of procedure. Unlikely that lack of masking would have caused deviation in intervention. Strict study criteria required that participants and personnel would be blinded to group allocation, but it is likely that they were aware as experimental was very different from control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One independent assessor evaluated the presence of wound infection or AO. Infection was defined by any of the following" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures appear to have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias. There was a suitable washout period to eliminate carry out |
Unsal 2018.
| Study characteristics | ||
| Methods |
Study design: split‐mouth randomised study Sample size calculation: not given Sample size: 50 patients Setting: Department of Oral and Maxillofacial Surgery, Ankara University, Turkey Number of centres: 1 Operators: 1 surgeon Prevention or treatment of dry socket: prevention Type of teeth: partially erupted lower 3rd molars Recruitment period: February 2013 to February 2014 Funding source: none Declarations of interest: none |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Gender: 17 males, 33 females Mean age: 23.96 years Age range: 15 to 43 years Number randomised: 50 Number analysed: 50 Lost to follow‐up/dropouts: none reported |
|
| Interventions |
All patients: rinsed with 0.2% CHX 3 times daily postoperatively Control group: no intervention Intervention group: platelet rich fibrin inserted into socket |
|
| Outcomes |
Primary outcome measures: presence of AO Diagnosis: patients experiencing “severe” level of pain for at least 4 days postoperatively, with the occurrence of situations outside of the normal healthy healing tissue Secondary outcome measures: periodontal probing depths on the distal surface of the mandibular 2nd molar at 3 months Adverse outcomes: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a decision |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All postoperative evaluations were performed by the surgeon blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make a decision |
| Selective reporting (reporting bias) | Low risk | All outcome measures appear to have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
van Eeden 2006.
| Study characteristics | ||
| Methods |
Study design: randomised controlled, split‐mouth trial Sample size: 19 Sample size calculation: not reported Setting: Military Hospital in South Africa Number of centres: 1 Prevention or treatment of dry socket: prevention Type of teeth: bilateral impacted mandibular 3rd molars of similar difficulty assessed clinically and radiologically. Carried out under general anaesthesia Recruitment period: not stated Funding source: not stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Mean age: 21.4 years (16 to 32 range) Number randomised: 19 Number analysed: 19 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
Comparison: covomycin D versus inert gel foam carrier Group 1 (n = 19): 1 mm covomycin D (2.0 mg chloramphenicol, 5.0 mg neomycin sulphate and 0.5 mg dexamethasone) delivered within an inert gel foam carrier Group 2 (n = 19): inert gel foam carrier ‐ 1 mL normal saline Co‐interventions:
|
|
| Outcomes | Pain scores were recorded at 6‐hour intervals from the day of surgery until day 6. The patients were examined clinically on day 6 Primary outcome measures: development of AO, diagnostic criteria Secondary outcome measures: none stated Adverse effects: some of the events may be attributable to intervention or could be normal sequelae of operation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...preselected on a random basis by the flip of a coin" |
| Allocation concealment (selection bias) | Unclear risk | No mention of this. Therefore, insufficient information to make a clear judgement if adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to group allocation. Personnel were blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "..patients were examined clinically by an independent surgeon blinded to the site of intrasocket medication" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for at day 6 |
| Selective reporting (reporting bias) | Low risk | The report appears to be free of selective reporting of the outcomes |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Xue 2013.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size: not stated Sample size calculation: 160 Setting: outpatient clinic Number of centres: 1 Operators: 1, associate chief physician with 10 years experience Prevention or treatment of dry socket: prevention Type of teeth: impacted mandibular molar Recruitment period: June 2011 to June 2012 Funding source: no direct or indirect financial or benefit sponsorship from any manufacturer, relevant employer or other economic organisation: none stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria: patients have systemic diseases such as severe heart disease, high blood pressure, diabetes, and malnutrition Gender: 72 males, 88 females Mean age: 28 years Age range: 20 to 40 years Number randomised: 160 Number analysed: 160 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: preoperative preparation: detailed inquiries were made about the patient's chief complaint, history of present disease, and past history, and tooth extraction contraindication was excluded. Good communication with patients, so that patients have an understanding of the necessity of the operation and the possible complications during and after the operation, so that patients can relax, avoid tension and fear, and can cooperate well during the operation. The situation of the impacted mandibular teeth was examined and the curved oral pantogram was taken to understand the resistance of the impacted teeth and the relationship between the impacted teeth and the adjacent teeth and the mandibular inferior dental nerve. Before operation, 75% ethanol was used to disinfect the perioral and facial skin, and then the face was covered with a sterile towel. The tooth extraction operative area was disinfected with 1% iodine tincture. Routine preparation scalpel, gingival separator, turbine, split drill, tooth brace, tooth forceps, needle holder, suture, aspirator. Impacted teeth were extracted: the experimental group was treated with 2% lidocaine and 1% adrenaline hydrochloride at 1:200000 to block the inferior alveolar nerve, lingual nerve and buccal nerve, and then infiltrated with anaesthesia at the medial and distal buccal angles of the impacted teeth to enhance the anaesthetic effect and reduce the intraoperative and postoperative bleeding. Buccal and distal incisions were made with a number 11 sharp knife blade, and the buccal and distal bone surfaces were exposed after flaps were turned over. According to the bone coverage, buccal, distal bone and accretion were removed with a fast turbine, and the impacted crowns were ground vertically, then the crowns and roots were removed. The tooth extraction sockets were carefully examined and the residual bone or tooth fragments were removed. Reposition was performed in patients with displacement of bone plates, and immediate trimming was performed in those with sharp bone processes. The doctors' advice after tooth extraction was the same in both groups. Patients were required not to gargle or brush their teeth within 24 hours after tooth extraction surgery, not to accept any antibiotics 1 week after the surgery, not to smoke, and to return to the doctor in time with abnormal conditions, such as bleeding Control group: the control group did not intervene after pulling out the cotton ball for 30 minutes Intervention group 1: recombinant bovine basic fibroblast growth factor gel intervention: after the extraction of the affected teeth, 0.1 mL recombinant bovine basic fibroblast growth factor gel was dropped into the bottom of the tooth extraction socket with a 1 mL injector immediately after tooth extraction was extracted, and the cotton ball was bit for 30 minutes after the blood filling of the tooth extraction fossus |
|
| Outcomes | Note: authors did not specify which was the primary outcome Outcome measures:
Diagnostic criteria for dry socket syndrome: diagnosis mainly depends on clinical manifestations:
Adverse outcomes: no local or systemic reactions, such as hypersensitivity or tissue hyperplasia, occurred in the experimental group after the application of recombinant bovine basic fibroblast growth factor gel |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 160 random numbers were read from any column or any row in the random number table, and then divided each number by 2. Patients were assigned to the two groups depended on the remainder being odd or even. Insufficient clarity to understand the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported, no details about who the assessors were |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up |
| Selective reporting (reporting bias) | Low risk | All outcomes specified were reported, the incidence of dry socket was reported in Table 2 |
| Other bias | Low risk | No other bias observed |
Yuce 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Sample size calculation: not reported Sample size: 40 Setting: university oral and maxillofacial surgery department Number of centres: 1 Operators: 1 surgeon for initial treatment; 3 surgeons for assessments Prevention or treatment of dry socket: treatment Type of teeth: 3rd molar Recruitment period: not reported Funding source: no funding received Declarations of interest: no conflicts of interest |
|
| Participants |
Inclusion criteria: between 18 and 40 years of age; no systemic disease; positively diagnosed and untreated AO in 3 days after extraction of mandibular 3rd molars of class A and 1 according to the Pell–Gregory classification Exclusion criteria: the use of medications that may interfere with the healing process; smoking; pregnancy or lactation; were menstruating; presence of any conditions such as inflammation, periodontitis, gingivitis and dental abscess in the area of the teeth; undergoing antibiotic or anti‐inflammatory drug therapies in the 7 days before extraction; using oral contraceptives and radiation therapy or chemotherapy in the 12 months before extraction and would require raising a flap and/or removing bone to remove the tooth Gender: 55% female, 45% male Mean age: 31.2 years Age range: not reported Number randomised: 40 Number analysed: 40 Lost to follow‐up/dropouts: 0 |
|
| Interventions |
All patients: extraction sockets were curetted to remove debris and gently cleaned by irrigation with saline only, diclofenac 50 mg every 12 hours as needed Intervention group 1: saline irrigation postoperatively on day 1, 3, 5 and 7 Intervention group 2: extraction sockets were packed with A‐PRF + (advanced platelet rich fibrin) and stitched; suture removed at day 7 |
|
| Outcomes |
Primary outcome measures: pain, soft tissue healing, bone density Diagnosis: VAS; index of Landry, Turnbull and Howley, OPT i‐Dixel 2.1.8.2 software by the average gray level values Secondary outcome measures: none reported Adverse outcomes: reported as none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a decision |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a decision |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Given the nature of the interventions it would not have been possible to blind the operator and patients. Lack of masking unlikely to have led to deviation in the intervention. Strict study criteria required that participants and personnel would be blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No sample sizes numbers presented in results tables or in text; flow chart would suggest no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | The report appears to be free of selective reporting bias |
| Other bias | Low risk | No other observed bias |
AO = alveolar osteitis; CHX = chlorhexidine; NaCl = sodium chloride; RCT = randomised controlled trial; SD = standard deviation; VAS = visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afat 2018 | Antibiotics prescribed postoperatively. All patients received amoxicillin 1 g twice daily for 1 week |
| Ahmed 2020 | Details of intervention unclear |
| Akota 1998 | The intervention used was an impregnated gauze drain which was specifically excluded from this review |
| Al‐Hamed 2017 | Antibiotics prescribed postoperatively. All patients received amoxicillin 500 mg 4 times daily for 5 days |
| Al‐Sukhun 2011 | 3 analgesics taken systemically to assess their ability to control pain after extraction. They were not local measures |
| Altman 2011 | Not dry socket |
| Anand 2015 | Dry socket not clearly defined; page 26: “for the purpose of this investigation, the diagnosis of dry socket was based on clinical symptoms rather than the appearance of dry socket” |
| Annibali 2012 | Not an RCT. Consensus statement on management of third molars |
| Anonymous 1966 | Not an RCT. Translated from the Japanese by Professor Ken Yaegaki, Nippon Dental University Department of Oral Health, Tokyo, Japan |
| Arakeri 2011 | Dry socket not defined |
| Arenaz‐Bua 2010 | Dry socket not defined, general comments on sequelae |
| Asutay 2017 | Unclear criteria for diagnosis of dry socket; page 1533: “if patients experienced persistent and progressive pain, it considered to dry socket” |
| Banach 1973 | Not an RCT (translated from Polish) |
| Baqain 2012 | The intervention was surgical (flap design) which was specifically excluded from the review |
| Baslarli 2015 | Antibiotics prescribed postoperatively; all patients received amoxicillin 1000 mg twice for 5 days |
| Bello 2011 | The intervention was wound closure techniques excluded from this review |
| Berwick 1990 | Dropouts substituted making trial results invalid |
| Betts 1995 | Evaluated the efficacy of lidocaine jelly for the alleviation of pain experienced during the instrumentation of extraction sites diagnosed with alveolar osteitis. Assessment of pain related to instrumentation only |
| Bezerra 2011 | Systemic amoxicillin antibiotic, not local intervention |
| Birke 1970 | Not an RCT (translated from German) |
| Bloomer 2000 | Quote: "In a consecutive manner, 1 of the 2 lower third molar sockets was packed on each patient. Sockets were packed in a series of 25 on 1 side then changed to a series of 25 on the opposite side throughout the series, totaling 100 patients" Comment: CCT quasi‐randomised |
| Bloomer 2012 | Not an RCT/inadequate method of sequence generation |
| Brignardello 2012 | Study about techniques, not dry socket |
| Butler 1977 | The interventions were lavage techniques which are excluded from this review |
| Butylin 1977 | Cohort study (translated from Russian) |
| Bystedt 1980 | Interventions were systemic antibiotics |
| Cebi 2020 | Randomisation unclear; page 681: "patients were assigned to groups A,B,C." Diagnostic criteria for dry socket also unclear |
| Christensen 2012 | Not an RCT. Expert opinion/commentary |
| Cooper 2012 | Pain study, not dry socket |
| Daniels 2011 | Dry socket not defined. Pain management study |
| Daugela 2018 | Antibiotics prescribed; all patients received clindamycin 600 mg 1 hour before and 6 hours after surgery |
| Dubovina 2016 | Criteria for diagnosis and follow‐up of dry socket unclear |
| Dutta 2016 | Antibiotics prescribed; page 47: "Each patient received identical postoperative antibiotics" |
| Eshghpour 2014 | Antibiotics prescribed postoperatively; all patients received amoxicillin 500 mg 3 times daily for 7 days |
| Eshghpour 2018 | Antibiotics prescribed postoperatively; all patients received amoxicillin 500 mg 3 times daily for 7 days |
| Farooq 2019 | It does not appear from the text that a clinician diagnosed the dry socket and while they mention Blum’s criteria they have conflated this with abscess in an “alveolitics” score they have developed where main items are not shown |
| Field 1988 | Quote: "The trial was 'open' and on arrival extraction cases were consecutively allocated to one of three groups" Comment: CCT quasi‐randomised |
| Fotos 1992 | Quote: "70 randomly selected healthy patients" page 383, "each subject was treated at one extraction site with a saline solution whereas the other site received CHX" page 384 Comment: no evidence of randomisation of participants to intervention |
| Garibaldi 1995 | Quote: "Patients were assigned in sequential (A, B, C, A, B, C, etc) order" Comment: CCT quasi‐randomised |
| Goldman 1973 | CCT, non‐randomised study |
| Goldsmith 2012 | Intervention involved surgical techniques which are excluded from the review |
| Gonzalez‐Serrano 2021 | Antibiotics administered as part of intervention; amoxicillin 750 mg 3 times a day for 7 days |
| Goyal 2012 | Not dry socket, instrument evaluation |
| Guazzo 2018 | No criteria given for “alveolitis” |
| Hall 1971 | Not an RCT. Expert opinion/commentary |
| Haraji 2010 | Interventions were envelope versus modified triangular flap designs, which are excluded from this review |
| Haraji 2012a | Duplicate reporting |
| Haraji 2012b | Criteria for diagnosis of dry socket is unclear – they use a scale that includes the presence of pus |
| Haraji 2014 | Duplicate reporting: "The authors used some information collected during a double‐blinded randomised control trial reported previously." Some of these data previously reported in Haraji 2013 |
| Haraji 2015 | Duplicate reporting |
| Haupt 2015 | No definition or criteria given for diagnosis of dry socket |
| Hill 2006 | Pain study, not dry socket |
| Hooley 1995 | No clinical diagnosis of dry socket made, only used pain |
| Jadhao 2018 | Antibiotics were prescribed; all patients received co‐amoxiclav 625 mg twice a day for 7 days |
| Jesudasan 2015 | Antibiotics prescribed postoperatively; all patients received metronidazole 400 mg 3 times daily for 3 days |
| Johnson 1988 | CCT, non‐randomised study |
| Jolley 1972 | Quote: "The purpose of this was to determine the effectiveness of the gel in controlling pain from ill‐fitting dentures after extractions and in other situations" page 72 Comment: not dry socket |
| Jovanovic 2011 | Not an RCT (after contact with author) |
| Julius 1982 | Not an RCT. Split‐mouth where all left‐hand sides received intervention and all right‐hand sides received the control |
| Kamal 2020a | Not an RCT. Quote page 2: "selected patients were divided into two groups based on their choice to be treated with either conventional treatment (group I) or CGF treatment (group II)" |
| Kamal 2020b | Not an RCT. Quote page 613: "Sixty patients with one dry socket each, at University Dental Hospital Sharjah, were divided into three treatment groups based on their choice" |
| Kaplan 2020 | Presence of dry socket not one of the main outcome measures. No criteria given for diagnosis of dry socket |
| Karthik 2021 | Antibiotics administered as part of intervention |
| Keskitalo 1973 | Quote: "Alternate patients were treated with Apernyl cones" Comment: CCT quasi‐randomised |
| Kilinc 2017 | Antibiotics prescribed; all patients given amoxicillin + clavulanic acid 2 g/day for 5 days |
| Kim 2020 | Presence of dry socket is not an outcome measure |
| Kirk 2007 | Definition of dry socket not provided |
| Krekmanov 1981 | Only systemic interventions |
| Krekmanov 1986 | Unsure if randomised |
| Krishnan 2020 | Presence of dry socket is not an outcome measure |
| Kudiyirickal 2012 | Not an RCT, not dry socket, looks at oral facial infections |
| Lao 2012 | Not dry socket |
| Liu 2011 | Not dry socket |
| Long 2012 | The intervention was a surgical technique which is excluded from this review |
| Lopez‐Cedrun 2011 | Systemic antibiotics, not a local intervention |
| MacGregor 1973 | Outcomes specified were pain and swelling, no clinical diagnosis of dry socket |
| MacGregor 1975 | Quote: "Successive patients were entered in to the trials... arrangements were made that there was an equal distribution of experiments and controls" Comment: no evidence of randomisation |
| Majid 2010 | No definition of dry socket in report. Study refers to generalised sequelae |
| Malkawi 2011 | Cross‐sectional study, not an RCT |
| Mehlisch 2010a | No definition of dry socket. Study refers to pain management |
| Mehlisch 2010b | No definition of dry socket. Study refers to pain management |
| Mishra 2012 | Not dry socket, dental pain |
| Mitchell 1986a | Quote: "They were allocated into groups according to a predetermined protocol" Comment: inadequate method of sequence generation, quasi‐randomised CCT |
| Mitchell 1986b | Systemic intervention, not a local intervention |
| Moberly 2007 | Wrong outcome; this study is comparing efficacy of different analgesics |
| Nentwig 1985 | Study unobtainable |
| Neugebauer 2004 | Participants "randomised into two groups." Randomisation at participant level but allocation of intervention 'split‐mouth' at extraction socket level Comment: open allocation. CCT (German translation) |
| Neuner 1969 | Study to treat pain and there is no mention that it is an RCT (German translation) |
| Njokanma 2019 | Only abstract available with insufficient information |
| Nordenram 1973 | Outcomes reported were "postoperative complications": pain/swelling/infection but with no independent assessments of classical 'dry socket' |
| Olson 1987 | Abstract only, insufficient information for inclusion |
| Olusanya 2011 | Systemic antibiotics, not a local intervention |
| Osunde 2014 | Antibiotics prescribed postoperatively; all patients received amoxicillin 500 mg 8 hourly and metronidazole 200 mg 8 hourly for 5 days |
| Osunde 2015 | Antibiotics prescribed; quote: “all patients received the same antibiotics” |
| Osunde 2017 | Antibiotics prescribed; all patients received amoxicillin 500 mg 8 hourly and metronidazole 200 mg 8 hourly for 5 days |
| Oyri 2019 | The intervention used was an impregnated gauze drain which was specifically excluded from this review |
| Ozveri 2020 | Antibiotics prescribed; all patients received amoxicillin + clavulanic acid 625 mg every 12 hours for 7 days |
| Paul 2019 | Retracted by the editorial board of the journal due to “significant double publication” |
| Pichler 2001 | Abstract only, insufficient information for inclusion |
| Prataap 2017 | Not an RCT |
| Qi 2012 | Not dry socket, pain management study |
| Rani 2016 | Postoperative antibiotics prescribed |
| Rastogi 2018 | Not an RCT |
| Reeshma 2021 | Not an RCT |
| Ritzau 1978 | Unclear if RCT |
| Saez‐Alcaide 2020 | Antibiotics prescribed; all patients were prescribed amoxicillin 750 mg every 8 hours for 7 days |
| Sanchis 2004 | Quote: "We divided the cases into a group of 100 patients who underwent extraction...." Comment: non‐randomised controlled (no treatment) study |
| Sarkar 2019 | Criteria for diagnosis of dry socket not provided |
| Schatz 1987 | Age range of a group of participants outside the inclusion criteria and no subgroup data reported. Open allocation sequence |
| Schlund 1968 | Study unobtainable |
| Scopp 1967 | Wrong outcome; this study evaluates the clinical efficacy of different analgesics |
| Seethamsetty 2019 | Antibiotics prescribed; all patients were given amoxicillin |
| Sharma 2017 | Not an RCT |
| Sorensen 1987 | Quote: "Patients were randomly selected and divided so that approximately half would receive...." Comment: non‐RCT |
| Swanson 1989 | Quote: "One hundred impacted lower third molars were to be operated. They were to be included in the study in the random order in which they came to the surgery by routine booking methods" Comment: CCT quasi‐randomised |
| Sweet 1985 | Quote: "The rinses were chosen by a random‐selection technique" Comment: CCT quasi‐randomised |
| Syrjanen 1981a | Controlled study, non‐RCT |
| Syrjanen 1981b | Does not measure dry socket as an outcome |
| Tek 2014 | Consecutive rather than randomisation of site |
| Tjernberg 1979 | Quote: "Patients referred to the department for the surgical removal of a partially erupted, lower third molar were randomly distributed into two pools" Comment: CCT quasi‐randomised |
| Tong 2012 | Not dry socket |
| Torres Lagares 2006 | Not an RCT/inadequate method of sequence generation |
| Torres‐Lagares 2010 | Patients in the study have bleeding disorders |
| Vedtofte 1974 | Quote: "The cones were packed in randomly numbered packets and the code was unknown to the investigators" Comment: inadequate method of randomisation |
| Vu 2021 | Dry socket not a primary outcome measure |
| Wang 2013 | Antibiotics prescribed; all patients in the study received amoxicillin 500 mg 3 times daily and tinidazole 1 g once daily for 5 days |
| Wen 2004 | Quote: "The method of random‐digit dialling was adopted to divide groups" Translated from the Chinese by Dr Nian Fang. "Patients were divided into 2 groups Group A and Group B, with complete randomization according to the visit order....." Comment: suggestive of quasi‐randomisation Inconsistencies between the English abstract and the translated version of the Chinese paper and lack of clarity in the methodology as reported did not provide any degree of confidence that adequate measures had been taken to satisfactorily randomised the participants or to conceal the allocation sequence in this study |
| Yuan 2006 | Antibiotics prescribed; all patients received cephalosporin 4 g intravenous and metronidazole 250 ml infusion for 3 days |
| Yue 2012 | No dry socket, pain management study |
| Zanetta‐Barbosa 1994 | Following email communication with the principal investigator, "the first patient was decided by a coin toss and the following was always allocated by alternation in the other group" Comment: CCT quasi‐randomised |
| Zuniga 2011 | Dry socket not defined. Pain management study |
CCT= controlled clinical trial; RCT = randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Zorina 2019.
| Methods | No details |
| Participants | No details |
| Interventions | No details |
| Outcomes | No details |
| Notes |
Differences between protocol and review
The original protocol was only for the treatment of dry socket. We have expanded the scope of the review to incorporate the prevention of dry socket.
We added the primary outcome for treatment of dry socket: time to heal.
We reworded 'type of participant' to clarify the inclusion of any extracted teeth.
We changed analyses from fixed‐effect to random‐effects as requested by peer reviewer.
We have added undertaking a subgroup analysis for chlorhexidine dose in the methods.
Contributions of authors
Conceiving the idea: Blánaid Daly (BD).
Writing the protocol: BD, Mohammad O Sharif (MOS), Tim Newton (TN), and Kate Jones (KJ).
Organising retrieval of papers: BD, MOS, Anna Beattie (AB).
Writing to authors of papers for additional information: BD, MOS, AB.
Providing additional data about papers: BD, AB.
Data collection for the review: BD, MOS, AB.
Screening search results: BD, AB, MOS, Helen Worthington (HW).
Screening retrieved papers against inclusion criteria: BD, AB, MOS, HW.
Appraising quality of papers: BD, AB, MOS, KJ.
Extracting data from papers: BD, MOS, AB, HW, KJ.
Obtaining and screening data on unpublished studies: AB, MOS, HW.
Entering data into Review Manager: BD, AB, HW, KJ, MOS.
Analysis of data: BD, AB, HW.
Writing the review: BD, AB, KJ, HW, MOS.
Sources of support
Internal sources
-
The University of Manchester, UK
Cochrane Oral Health is supported by The University of Manchester and the NIHR Manchester Biomedical Research Centre.
-
Manchester Academic Health Sciences Centre (MAHSC), UK
Cochrane Oral Health is supported by the MAHSC and the NIHR Manchester Biomedical Research Centre.
External sources
-
Cochrane Oral Health Global Alliance, UK
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships-alliances). Contributors in recent years have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Swiss Society of Endodontology, Switzerland.
-
National Institute for Health and Care Research (NIHR), UK
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, the NHS, or the Department of Health and Social Care.
Declarations of interest
There are no financial conflicts of interest and the review authors declare that they do not have any associations with any parties who may have vested interests in the results of this review. Helen V Worthington is an Editor with Cochrane Oral Health, and was previously Co‐ordinating Editor. She was not involved in conducting the editorial process for the review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abu‐Mostafa 2015 {published data only}
- Abu-Mostafa N, Alqahtani A, Abu-Hasna M, Alhokail A, Alsadsani A. A randomized clinical trial compared the effect of intra-alveolar 0.2% chlorohexidine bio-adhesive gel versus 0.12% chlorohexidine rinse in reducing alveolar osteitis following molar teeth extractions. Medicina Oral, Patologia Oral y Cirugia Bucal 2015;20(1):e82-7. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abu‐Mostafa 2019 {published data only}
- Abu-Mostafa N, Al-Daghamin S, Al-Anazi A, Al-Jumaah N, Alnesafi A. The influence of intra-alveolar application of honey versus chlorhexidine rinse on the incidence of alveolar osteitis following molar teeth extraction. A randomized clinical parallel trial. Journal of Clinical and Experimental Dentistry 2019;11(10):e871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmedi 2016 {published data only}
- Ahmedi J, Ahmedi E, Sejfija O, Agani Z, Hamiti V. Efficiency of gaseous ozone in reducing the development of dry socket following surgical third molar extraction. European Journal of Dentistry 2016;10(3):381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alissa 2010 {published data only}
- Alissa R, Esposito M, Horner K, Oliver R. The influence of platelet-rich plasma on the healing of extraction sockets: an explorative randomised clinical trial. European Journal of Oral Implantology 2010;3(2):121-34. [PubMed] [Google Scholar]
Babar 2012 {published data only}
- Babar A, Ibrahim MW, Baig NJ, Shah I, Amin E. Efficacy of intra-alveolar chlorhexidine gel in reducing frequency of alveolar osteitis in mandibular third molar surgery. Journal of the College of Physicians and Surgeons Pakistan 2012;22(2):91-4. [PubMed] [Google Scholar]
Bai 2011 {published data only}
- Bai Z-C, Shi S-G, Li Li-L, Niu Z-Y, Zhang Y-R. Application of accellular dermal matrix embedded in socket after wisdom tooth extraction. Journal of Clinical Rehabilitative Tissue Engineering Research 2011;15(34):6457-60. [Google Scholar]
Burgoyne 2010 {published data only}
- Burgoyne CC, Giglio JA, Reese SE, Sima AP, Laskin DM. The efficacy of a topical anesthetic gel in the relief of pain associated with localized alveolar osteitis. Journal of Oral and Maxillofacial Surgery 2010;68:144-8. [DOI] [PubMed] [Google Scholar]
Chaurasia 2017 {published data only}
- Chaurasia NK, Upadhyaya C, Dixit S. Comparative study to determine the efficacy of zinc oxide eugenol and alveogyl in treatment of dry socket. Kathmandu University Medical Journal (KUMJ) 2017;15:203-6. [PubMed] [Google Scholar]
Cho 2018 {published data only}
- Cho H, David MC, Lynham AJ, Hsu E. Effectiveness of irrigation with chlorhexidine after removal of mandibular third molars: a randomised controlled trial. British Journal of Oral & Maxillofacial Surgery 2018;56:54-9. [DOI] [PubMed] [Google Scholar]
Delilbasi 2002 {published data only}
- Delilbasi C, Saracoglu U, Keskin A. Effects of 0.2% chlorhexidine gluconate and amoxicillin plus clavulanic acid on the prevention of alveolar osteitis following mandibular third molar extractions. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radioloy, and Endodontics 2002;94(3):301-4. [DOI] [PubMed] [Google Scholar]
Divya 2019 {published data only}
- Divya R, Senthilnathan KP, Santhosh Kumar Mp, Senthil Murugan PS. Effectiveness of herbal and chlorhexidine mouth rinses in the prevention of post-operative complications during third molar surgery. Drug Invention Today 2019;11(10):2644-7. [Google Scholar]
Faizel 2015 {published data only}
- Faizel S, Thomas S, Yuvaraj V, Prabhu S, Tripathi G. Comparison between neocone, alvogyl and zinc oxide eugenol packing for the treatment of dry socket: a double blind randomised control trial. Journal of Maxillofacial and Oral Surgery 2015;14(2):312-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2009 {published data only}
- Feng P, Hu J, She X. Clinical investigation of using oral tissue patch and platelet rich plasma (PRP) after tooth extraction. Journal of Oral Science Research 2009;25:302-4. [Google Scholar]
Freudenthal 2015 {published data only}
- Freudenthal N, Sternudd M, Jansson L, Wannfors K. A double-blind randomized study evaluating the effect of intra-alveolar chlorhexidine gel on alveolar osteitis after removal of mandibular third molars. Journal of Oral and Maxillofacial Surgery 2015;73(4):600-5. [DOI] [PubMed] [Google Scholar]
Gersel‐Pedersen 1979 {published data only}
- Gersel-Pedersen N. Tranexamic acid in alveolar sockets in the prevention of alveolitis sicca dolorosa. International Journal of Oral Surgery 1979;8(6):421-9. [DOI] [PubMed] [Google Scholar]
Ghaeminia 2017 {published data only}
- Ghaeminia H, Hoppenreijs TJ, Xi T, Fennis JP, Maal TJ, Berge SJ, et al. Postoperative socket irrigation with drinking tap water reduces the risk of inflammatory complications following surgical removal of third molars: a multicenter randomized trial. Clinical Oral Investigations 2017;21:71-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halabi 2018 {published data only}
- Halabi D, Escobar J, Alvarado C, Martinez N, Munoz C. Chlorhexidine for prevention of alveolar osteitis: a randomised clinical trial. Journal of Applied Oral Science 2018;26:e20170245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haraji 2013 {published data only}
- Haraji A, Rakhshan V, Khamverdi N, Alishahi HK. Effects of intra-alveolar placement of 0.2% chlorhexidine bioadhesive gel on dry socket incidence and postsurgical pain: a double-blind split-mouth randomized controlled clinical trial. Journal of Orofacial Pain 2013;27(3):256-62. [DOI] [PubMed] [Google Scholar]
Hasheminia 2018 {published data only}
- Hasheminia D, Moaddabi A, Moradi S, Soltani P, Moannaei M, Issazadeh M. The efficacy of 1% Betadine mouthwash on the incidence of dry socket after mandibular third molar surgery. Journal of Clinical and Experimental Dentistry 2018;10:e445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hermesch 1998 {published data only}
- Hermesch C, Hilton T, Baker R, Biesbrock A, Hamlin J, McClanahan S, et al. Prophylactic use of Peridex® reduces the incidence of alveolar osteitis following third molar extraction. Journal of Dental Research 1997;76(1 Suppl IADR Abstracts):243 (Abs No 1835). [Google Scholar]
- Hermesch CB, Hilton TJ, Biesbrock AR, Baker RA, Cain-Hamlin J, McClanahan SF, et al. Perioperative use of 0.12% chlorhexidine gluconate for the prevention of alveolar osteitis: efficacy and risk factor analysis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 1998;85(4):381-7. [DOI] [PubMed] [Google Scholar]
Hita‐Iglesias 2008 {published data only}
- Hita-Iglesias P, Torres-Lagares D, Flores-Ruiz R, Magallanes-Abad N, Basallote-Gonzalez M, Gutierrez-Perez JL. Effectiveness of chlorhexidine gel versus chlorhexidine rinse in reducing alveolar osteitis in mandibular third molar surgery. Journal of Oral and Maxillofacial Surgery 2008;66(3):441-5. [DOI] [PubMed] [Google Scholar]
Hu 2005 {published data only}
- Hu K, Kong L, Peng L. Prevention of postexodontic complications by oral tissue patch embedded in socket after tooth extraction: a clinical randomised and controlled study. Journal of Practical Stomatology 2005;21(2):214-6. [Google Scholar]
Huang 2011 {published data only}
- Huang D-Y, Nie E-M, Guo J-B, Ji L. Application of HEAL-ALL patch on the prevention of dry socket syndrome after tooth extraction. Journal of Clinical Rehabilitative Tissue Engineering Research 2011;15(29):5409-12. [Google Scholar]
Karabit 2019 {published data only}
- Karabit ZZ, Al Kattan FZ. The effect of chlorhexidine gluconate 0.12% rinse in reducing dry socket following teeth extraction in smoker patients: randomized clinical trial (triple blind). New Armenian Medical Journal 2019;13(3):28-33. [Google Scholar]
Kaya 2011 {published data only}
- Kaya GŞ, Yapici G, Savaş Z, Güngörmüş M. Comparison of alvogyl, SaliCept patch, and low-level laser therapy in the management of alveolar osteitis. Journal of Oral and Maxillofacial Surgery 2011;69(6):1571-7. [DOI] [PubMed] [Google Scholar]
Keshini 2020 {published data only}
- Keshini MP, Shetty SK, Sundar S, Chandan SN, Manjula S. Assessment of healing using alvogyl and platelet rich fibrin in patients with dry socket - an evaluative study. Annals of Maxillofacial Surgery 2020;10(2):320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2018 {published data only}
- King EM, Cerajewska TL, Locke M, Claydon NCA, Davies M, West NX. The efficacy of plasma rich in growth factors for the treatment of alveolar osteitis: a randomized controlled trial. Journal of Oral and Maxillofacial Surgery 2018;76:1150-9. [DOI] [PubMed] [Google Scholar]
Kjellman 1973 {published data only}
- Kjellman O. Apernyl as alveolar inlay in connection with the removal of impacted third molars of the lower jaw. A clinical double blind investigation of 100 patients. Svensk Tandlakare Tidskrift 1973;6(2):197-200. [PubMed] [Google Scholar]
Larsen 1991 {published data only}
- Larsen PE. The effect of a chlorhexidine rinse on the incidence of alveolar osteitis following the surgical removal of impacted mandibular third molars. Journal of Oral and Maxillofacial Surgery 1991;49(9):932-7. [DOI] [PubMed] [Google Scholar]
Lenka 2019 {published data only}
- Lenka S, Rathor K, Satyarup D, Varu R, Dalai R. Comparison between alvogyland zinc oxide eugenol packing for the treatment of dry socket: a clinical study. Indian Journal of Public Health Research and Development 2019;10(11):845-8. [Google Scholar]
Metin 2006 {published data only}
- Metin M, Tek M, Sener I. Comparison of two chlorhexidine rinse protocols on the incidence of alveolar osteitis following the surgical removal of impacted third molars. Journal of Contemporary Dental Practice 2006;7(2):79-86. [PubMed] [Google Scholar]
Mitchell 1984 {published data only}
- Mitchell L. Topical metronidazole in the treatment of 'dry socket'. British Dental Journal 1984;156(4):132-4. [DOI] [PubMed] [Google Scholar]
Ragno 1991 {published data only}
- Ragno JR, Szkutnik AJ. Evaluation of 0.12% chlorhexidine rinse on the prevention of alveolar osteitis. Oral Surgery, Oral Medicine, and Oral Pathology 1991;72(5):524-6. [DOI] [PubMed] [Google Scholar]
Reekie 2006 {published data only}
- Reekie D, Downes P, Devlin CV, Nixon GM, Devlin H. The prevention of 'dry socket' with topical metronidazole in general dental practice. British Dental Journal 2006;200(4):210-3. [DOI] [PubMed] [Google Scholar]
Ritzau 1977 {published data only}
- Ritzau M, Swangsilpa K. The prophylactic use of propylic ester of p-hydrobenzoic acid on alveolitis sicca dolorosa. A preliminary report. Oral Surgery, Oral Medicine, and Oral Pathology 1977;43(1):32-7. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Perez 2013 {published data only}
- Rodriguez-Perez M, Bravo-Perez M, Sanchez-Lopez JD, Munoz-Soto E, Romero-Olid MN, Baca-Garcia P. Effectiveness of 1% versus 0.2% chlorhexidine gels in reducing alveolar osteitis from mandibular third molar surgery: a randomized, double-blind clinical trial. Medicina Oral, Patologia Oral y Cirugia Bucal 2013;18(4):e693-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubio‐Palau 2015 {published data only}
- Rubio-Palau J, Garcia-Linares J, Hueto-Madrid JA, Gonzalez-Lagunas J, Raspall-Martin G, Mareque-Bueno J. Effect of intra-alveolar placement of 0.2% chlorhexidine bioadhesive gel on the incidence of alveolar osteitis following the extraction of mandibular third molars. A double-blind randomized clinical trial. Medicina Oral, Patologia Oral y Cirugia Bucal 2015;20(1):e117-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shad 2018 {published data only}
- Shad S, Hussain SM, Tahir MW, Rahat Geelani SR, Khan SM, Abbasi MM. Role of 0.2% bio-adhesive chlorhexidine gel in reducing incidence of alveolar osteitis. Journal of Ayub Medical College, Abbottabad 2018;30:524-8. [PubMed] [Google Scholar]
Shi 2003 {published data only}
- Shi L, Hu K, Peng L. Effect evaluation of artemisia desertorum spreng. Chinese Journal of Clinical Rehabilitation 2003;7(1):166-7. [Google Scholar]
Sun 2007 {published data only}
- Sun H-P, Feng L, Weng R. Clinical study of prevention of postoperatvie complications by acellular dermal matrix embedded in socket after tooth extraction. Journal of Clinical Rehabilitive Tissue Engineering Research 2007;15(3):102-4. [Google Scholar]
Supe 2018 {published data only}
- Supe NB, Choudhary SH, Yamyar SM, Patil KS, Choudhary AK, Kadam VD. Efficacy of Alvogyl (combination of iodoform + butylparaminobenzoate) and zinc oxide eugenol for dry socket. Annals of Maxillofacial Surgery 2018;8:193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torres‐Lagares 2006a {published data only}
- Torres-Lagares D, Infante-Cossio P, Gutierrez-Perez JL, Romero-Ruiz MM, Garcia-Calderon M, Serrera-Figallo MA. Intra-alveolar chlorhexidine gel for the prevention of dry socket in mandibular third molar surgery. A pilot study. Medicina Oral, Patologia Oral y Cirugia Bucal 2006;11(2):179-84. [PubMed] [Google Scholar]
Torres‐Lagares 2006b {published data only}
- Torres-Lagares D, Gutierrez-Perez JL, Infante-Cossio P, Garcia-Calderon M, Romero-Ruiz MM, Serrera-Figallo MA. Randomized, double-blind study on effectiveness of intra-alveolar chlorhexidine gel in reducing the incidence of alveolar osteitis in mandibular third molar surgery. International Journal of Oral and Maxillofacial Surgery 2006;35(4):348-51. [DOI] [PubMed] [Google Scholar]
Trieger 1991 {published data only}
- Trieger N, Schlagel GD. Preventing dry socket. A simple procedure that works. Journal of the American Dental Association 1991;122(2):67-8. [DOI] [PubMed] [Google Scholar]
Tuk 2019 {published data only}
- Tuk JG, Lindeboom JA, Sana F, van Wijk AJ, Milstein D. Alveolar iodine tampon packing reduces postoperative morbidity after third molar surgery. Journal of Oral and Maxillofacial Surgery 2019;77(12):2401-11. [DOI] [PubMed] [Google Scholar]
Unsal 2018 {published data only}
- Unsal H, H Erbasar GN. Evaluation of the effect of platelet-rich fibrin on the alveolar osteitis incidence and periodontal probing depth after extracting partially erupted mandibular third molars extraction. Nigerian Journal of Clinical Practice 2018;21(2):201-5. [DOI] [PubMed] [Google Scholar]
van Eeden 2006 {published data only}
- Eeden SP, Butow K. Post-operative sequelae of lower third molar removal: a literature review and pilot study on the effect of Covomycin D. SADJ Journal of the South African Dental Association 2006;61(4):154-9. [PubMed] [Google Scholar]
Xue 2013 {published data only}
- Xue L-F, Xu Y-X, Yue J, Wang S-Y, Xiao W-L, Zhang C-Y. Recombinant bovine basic fibroblast growth factor gel prevents dry socket syndrome after tooth extraction. Chinese Journal of Tissue Engineering Research 2013;17(34):6097-102. [Google Scholar]
Yuce 2019 {published data only}
- Yuce E, Komerik N. Potential effects of advanced platelet rich fibrin as a wound-healing accelerator in the management of alveolar osteitis: a randomized clinical trial. Nigerian Journal of Clinical Practice 2019;22(9):1189-95. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Afat 2018 {published data only}
- Afat IM, Akdogan ET, Gonul O. Effects of leukocyte- and platelet-rich fibrin alone and combined with hyaluronic acid on early soft tissue healing after surgical extraction of impacted mandibular third molars: a prospective clinical study. Journal of Cranio-maxillo-facial Surgery 2018;47:280-6. [DOI] [PubMed] [Google Scholar]
Ahmed 2020 {published data only}
- Ahmed T, Rehman A, Qureshi N, Khan M, Azeem S, Alam I. Effectiveness of pre-procedural antimicrobial mouthwash rinse with a simple water rinse on the dry socket after third molar extractions. Medical Forum Monthly 2020;31(12):44-8. [Google Scholar]
Akota 1998 {published data only}
- Akota I, Alvsaker B, Bjornland T. The effect of locally applied gauze drain impregnated with chlortetracycline ointment in mandibular third-molar surgery. Acta Odontologica Scandinavica 1998;56(1):25-9. [DOI] [PubMed] [Google Scholar]
Al‐Hamed 2017 {published data only}
- Al-Hamed F, Tawfik M, Abdelfadil E. Clinical effects of platelet-rich-fibrin (PRF) following surgical extraction of lower third molar. Saudi Journal for Dental Research 2017;8:19-25. [Google Scholar]
Al‐Sukhun 2011 {published data only}
- Al-Sukhun J, Penttila H. The cyclooxygenase-2 inhibitor celecoxib and alveolar osteitis. Journal of the Irish Dental Association 2011;57(1):50-3. [PubMed] [Google Scholar]
Altman 2011 {published data only}
- Altman RD, Daniels S, Manvelian G. Acute pain relief by a proprietary, nano-formulated lower-dose oral indomethacin. Arthritis and Rheumatism 2011;63(S10):1. [Google Scholar]
Anand 2015 {published data only}
- Anand KP, Patro S, Mohapatra A, Mishra S. The efficacy of tranexamic acid in the reduction of incidence of dry socket: an institutional double blind study. Journal of Clinical and Diagnostic Research 2015;9(9):ZC25-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Annibali 2012 {published data only}
- Annibali S, De Biase A, Pippi R, Sfasciotti GL. A consensus conference on management of the lower third molar. Italian Society of Odontostomatological Surgery. Minerva Stomatologica 2012;60(10):509-27. [PubMed] [Google Scholar]
Anonymous 1966 {published data only}
- Anonymous. Drug therapy for dry socket. Nihon Shika Ishikai Zasshi [Journal of the Japan Dental Association] 1966;19(8):653-4. [PubMed] [Google Scholar]
Arakeri 2011 {published data only}
- Arakeri G, Brennan PA. Povidone-iodine: an anti-oedematous agent? International Journal of Oral and Maxillofacial Surgery 2011;40(2):173-6. [DOI] [PubMed] [Google Scholar]
Arenaz‐Bua 2010 {published data only}
- Arenaz-Búa J, Luaces-Rey R, Sironvalle-Soliva S, Otero-Rico A, Charro-Huerga E, Patiño-Seijas B, et al. A comparative study of platelet-rich plasma, hydroxyapatite, demineralized bone matrix and autologous bone to promote bone regeneration after mandibular impacted third molar extraction. Medicina Oral, Patologia Oral y Cirugia Bucal 2010;15(3):e483-9. [DOI] [PubMed] [Google Scholar]
Asutay 2017 {published data only}
- Asutay F, Yolcu U, Gecor O, Acar AH, Ozturk SA, Malkoc S. An evaluation of effects of platelet-rich-fibrin on postoperative morbidities after lower third molar surgery. Nigerian Journal of Clinical Practice 2017;20:1531-6. [DOI] [PubMed] [Google Scholar]
Banach 1973 {published data only}
- Banach J, Malinowski J, Spychalska M, Zielinska B. Clinical evaluation of Apernyl preparation. Czasopismo Stomatologiczne [Journal of Stomatology] 1973;26(8):861-4. [PubMed] [Google Scholar]
Baqain 2012 {published data only}
- Baqain ZH, Al-Shafii A, Hamdan AA, Sawair FA. Flap design and mandibular third molar surgery: a split mouth randomized clinical study. International Journal of Oral and Maxillofacial Surgery 2012;41(8):1020-4. [DOI] [PubMed] [Google Scholar]
Baslarli 2015 {published data only}
- Baslarli O, Tumer C, Ugur O, Vatankulu B. Evaluation of osteoblastic activity in extraction sockets treated with platelet-rich fibrin. Medicina Oral, Patologia Oral y Cirugia Bucal 2015;20(1):e111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bello 2011 {published data only}
- Bello SA, Olaitan AA, Ladeinde AL. A randomized comparison of the effect of partial and total wound closure techniques on postoperative morbidity after mandibular third molar surgery. Journal of Oral and Maxillofacial Surgery 2011;69(6):e24-30. [DOI] [PubMed] [Google Scholar]
Berwick 1990 {published data only}
- Berwick JE, Lessin ME. Effects of a chlorhexidine gluconate oral rinse on the incidence of alveolar osteitis in mandibular third molar surgery. Journal of Oral and Maxillofacial Surgery 1990;48(5):444-8. [DOI] [PubMed] [Google Scholar]
Betts 1995 {published data only}
- Betts NJ, Makowski G, Shen YH, Hersh EV. Evaluation of topical viscous 2% lidocaine jelly as an adjunct during the management of alveolar osteitis. Journal of Oral and Maxillofacial Surgery 1995;53(10):1140-4. [DOI] [PubMed] [Google Scholar]
Bezerra 2011 {published data only}
- Bezerra TP, Studart-Soares EC, Scaparo HC, Pita-Neto IC, Batista SH, Fonteles CS. Prophylaxis versus placebo treatment for infective and inflammatory complications of surgical third molar removal: a split-mouth, double-blind, controlled, clinical trial with amoxicillin (500 mg). Journal of Oral and Maxillofacial Surgery 2011;69(11):e333-9. [DOI] [PubMed] [Google Scholar]
Birke 1970 {published data only}
- Birke WP, Furtig W. Drug therapy of post extraction pain. Deutsche Stomatologie 1970;20(5):370-90. [PubMed] [Google Scholar]
Bloomer 2000 {published data only}
- Bloomer CR. Alveolar osteitis prevention by immediate placement of medicated packing. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2000;90(3):282-4. [DOI] [PubMed] [Google Scholar]
Bloomer 2012 {published data only}
- Bloomer CR. Straws do not cause dry sockets when third molars are extracted. Texas Dental Journal 2012;129(1):25-32. [PubMed] [Google Scholar]
Brignardello 2012 {published data only}
- Brignardello-Petersen R, Carrasco-Labra A, Araya I, Yanine N, Beyene J, Shah PS. Is adjuvant laser therapy effective for preventing pain, swelling, and trismus after surgical removal of impacted mandibular third molars? A systematic review and meta-analysis. Journal of Oral and Maxillofacial Surgery 2012;70(8):1789-801. [DOI] [PubMed] [Google Scholar]
Butler 1977 {published data only}
- Butler DP, Sweet JB. Effect of lavage on the incidence of localized osteitis in mandibular third molar extraction sites. Oral Surgery, Oral Medicine, and Oral Pathology 1977;44(1):14-20. [DOI] [PubMed] [Google Scholar]
Butylin 1977 {published data only}
- Butylin AM. Effect of PC powder on the healing of the socket after tooth extraction. Stomatologiia 1977;56(2):95. [PubMed] [Google Scholar]
Bystedt 1980 {published data only}
- Bystedt H, Nord CE, Nordenram A. Effect of azidocillin, erythromycin, clindamycin and doxycycline on postoperative complications after surgical removal of impacted mandibular third molars. International Journal of Oral Surgery 1980;9(3):157-65. [DOI] [PubMed] [Google Scholar]
Cebi 2020 {published data only}
- Cebi AT. Evaluation of the effects of intra-alveolar irrigation with clindamycin, rifampicin and sterile saline in alveolar osteitis treatment. Journal of Stomatology, Oral and Maxillofacial Surgery 2020;121(6):680-3. [DOI] [PubMed] [Google Scholar]
Christensen 2012 {published data only}
- Christensen JA, Matzen LH, Wenzel A. Should removal of lower third molars be included in the pre-graduate curriculum for dental students? An evaluation of post-operative complications after student operations. Acta Odontologica Scandinavica 2012;70(1):42-8. [DOI] [PubMed] [Google Scholar]
Cooper 2012 {published data only}
- Cooper SA, Voelker M. Evaluation of onset of pain relief from micronized aspirin in a dental pain model. Inflammopharmacology 2012;20(4):233-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daniels 2011 {published data only}
- Daniels SE, Bandy DP, Christensen SE, Boice J, Losada MC, Liu H, et al. Evaluation of the dose range of etoricoxib in an acute pain setting using the postoperative dental pain model. Clinical Journal of Pain 2011;27(1):1-8. [DOI] [PubMed] [Google Scholar]
Daugela 2018 {published data only}
- Daugela P, Grimuta V, Sakavicius D, Jonaitis J, Juodzbalys G. Influence of leukocyte- and platelet-rich fibrin (L-PRF) on the outcomes of impacted mandibular third molar removal surgery: a split-mouth randomized clinical trial. Quintessence International 2018;49:377-88. [DOI] [PubMed] [Google Scholar]
Dubovina 2016 {published data only}
- Dubovina D, Mihailovic B, Bukumiric Z, Vlahovic Z, Miladinovic M, Mikovic N, et al. The use of hyaluronic and aminocaproic acid in the treatment of alveolar osteitis. Vojnosanitetski Pregled 2016;73(11):1010-5. [DOI] [PubMed] [Google Scholar]
Dutta 2016 {published data only}
- Dutta S, Passi D, Singh P, Sharma S, Singh M, Srivastava D. A randomized comparative prospective study of platelet-rich plasma, platelet-rich fibrin, and hydroxyapatite as a graft material for mandibular third molar extraction socket healing. National Journal of Maxillofacial Surgery 2016;7(1):45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eshghpour 2014 {published data only}
- Eshghpour M, Dastmalchi P, Nekooei AH, Nejat A. Effect of platelet-rich fibrin on frequency of alveolar osteitis following mandibular third molar surgery: a double-blinded randomized clinical trial. Journal of Oral and Maxillofacial Surgery 2014;72(8):1463-7. [DOI] [PubMed] [Google Scholar]
Eshghpour 2018 {published data only}
- Eshghpour M, Danaeifar N, Kermani H, Nejat A H. Does intra-alveolar application of chlorhexidine gel in combination with platelet-rich fibrin have an advantage over application of platelet-rich fibrin in decreasing alveolar osteitis after mandibular third molar surgery? A double-blinded randomized clinical trial. Journal of Oral and Maxillofacial Surgery 2018;76:939.e1-7. [DOI] [PubMed] [Google Scholar]
Farooq 2019 {published data only}
- Farooq M, Kayani S, Hashim M, Hassan F, Toosy W, Raza Naqvi S. Effectiveness of 1% versus 0.2% chlorhexidine gels in reducing alveolar osteitis from mandibular third molar surgery: a randomized, double-blind clinical trial. Medical Forum Monthly 2019;30(10):2-7. [Google Scholar]
Field 1988 {published data only}
- Field EA, Nind D, Varga E, Martin MV. The effect of chlorhexidine irrigation on the incidence of dry socket: a pilot study. British Journal of Oral and Maxillofacial Surgery 1988;26(5):395-401. [DOI] [PubMed] [Google Scholar]
Fotos 1992 {published data only}
- Fotos PG, Koorbusch GF, Sarasin DS, Kist RJ. Evaluation of intra-alveolar chlorhexidine dressings after removal of impacted mandibular third molars. Oral Surgery, Oral Medicine, and Oral Pathology 1992;73(3):383-8. [DOI] [PubMed] [Google Scholar]
Garibaldi 1995 {published data only}
- Garibaldi JA, Greenlaw J, Choi J, Fotovatjah M. Treatment of post-operative pain. Journal of the California Dental Association 1995;23(4):71-2, 74. [PubMed] [Google Scholar]
Goldman 1973 {published data only}
- Goldman DR, Kilgore DS, Panzer JD, Atkinson WH. Prevention of dry socket by local application of lincomycin in Gelfoam. Oral Surgery, Oral Medicine, and Oral Pathology 1973;35(4):472-4. [DOI] [PubMed] [Google Scholar]
Goldsmith 2012 {published data only}
- Goldsmith SM, De Silva RK, Tong DC, Love RM. Influence of a pedicle flap design on acute postoperative sequelae after lower third molar removal. International Journal of Oral and Maxillofacial Surgery 2012;41(3):371-5. [DOI] [PubMed] [Google Scholar]
Gonzalez‐Serrano 2021 {published data only}
- Gonzalez-Serrano J, Lopez-Pintor R, Cecilia-Murga R, Torres J, Hernandez G, Lopez-Quiles J. Application of propolis extract, nanovitamin C and nanovitamin E to prevent alveolar osteitis after impacted lower third molar surgery. A randomized, double-blind, split-mouth, pilot study. Medicina Oral, Patologia Oral y Cirugia Bucal 2021;26(2):e118-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goyal 2012 {published data only}
- Goyal M, Marya K, Jhamb A, Chawla S, Sonoo PR, Singh V, et al. Comparative evaluation of surgical outcome after removal of impacted mandibular third molars using a Piezotome or a conventional handpiece: a prospective study. British Journal of Oral and Maxillofacial Surgery 2012;50(6):556-61. [DOI] [PubMed] [Google Scholar]
Guazzo 2018 {published data only}
- Guazzo R, Perissinotto E, Mazzoleni S, Ricci S, Peñarrocha-Oltra D, Sivolella S. Effect on wound healing of a topical gel containing amino acid and sodium hyaluronate applied to the alveolar socket after mandibular third molar extraction: a double-blind randomized controlled trial. Quintessence International 2018;49(10):831-40. [DOI] [PubMed] [Google Scholar]
Hall 1971 {published data only}
- Hall HD, Bildman BS, Hand CD. Prevention of dry socket with local application of tetracycline. Journal of Oral Surgery 1971;29(1):35-7. [PubMed] [Google Scholar]
Haraji 2010 {published data only}
- Haraji A, Motamedi MH, Rezvani F. Can flap design influence the incidence of alveolar osteitis following removal of impacted mandibular third molars? General Dentistry 2010;58(5):e187-9. [PubMed] [Google Scholar]
Haraji 2012a {published data only}
- Haraji A, Khamverdi N, Khanzadealishahi H. The effect of 0.2% chlorhexidine gel in prevention of pain and dry socket following mandibular third molar surgery. Journal of Research in Dental Sciences 2012;9(2):63-7. [Google Scholar]
Haraji 2012b {published data only}
- Haraji A, Lassemi E, Motamedi M, Alavi M, Abidnejad S. Effect of plasma rich in growth factors on alveolar osteitis. National Journal of Maxillofacial Surgery 2012;3(1):38-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haraji 2014 {published data only}
- Haraji A, Rakhshan V. Single-dose intra-alveolar chlorhexidine gel application, easier surgeries, and younger ages are associated with reduced dry socket risk. Journal of Oral and Maxillofacial Surgery 2014;72(2):259-65. [DOI] [PubMed] [Google Scholar]
Haraji 2015 {published data only}
- Haraji A, Rakhshan V. Chlorhexidine gel and less difficult surgeries might reduce post-operative pain, controlling for dry socket, infection and analgesic consumption: a split-mouth controlled randomised clinical trial. Journal of Oral Rehabilitation 2015;42(3):209-19. [DOI] [PubMed] [Google Scholar]
Haupt 2015 {published data only}
- Haupt A, Haupt D, Miloro M, Kolokythas A. Influence of Immediate Post-Extraction Irrigation With Chlorhexidine Rinse on Postoperative Pain and Incidence of Alveolar Osteitis of Impacted Mandibular Third Molars. Journal of Oral and Maxillofacial Surgery 2015;73(9):e52-3. [Google Scholar]
Hill 2006 {published data only}
- Michael Hill C, Sindet-Pederson S, Seymour RA, Hawkesford JE 2nd, Coulthard P, Lamey PJ, et al. Analgesic efficacy of the cyclooxygenase-inhibiting nitric oxide donor AZD3582 in postoperative dental pain: comparison with naproxen and rofecoxib in two randomized, double-blind, placebo-controlled studies. Clinical Therapeutics 2006;28(9):1279-95. [DOI] [PubMed] [Google Scholar]
Hooley 1995 {published data only}
- Hooley JR, Golden DP. The effect of polylactic acid granules on the incidence of alveolar osteitis after mandibular third molar surgery. A prospective randomized study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 1995;80(3):279-83. [DOI] [PubMed] [Google Scholar]
Jadhao 2018 {published data only}
- Jadhao VA, Rao A, Hande P, Mahajani M, Raktade P, Gedam R, et al. The efficiency of three irrigating solutions after surgical removal of impacted mandibular third molars: a cross-sectional study. Journal of Contemporary Dental Practice 2018;19(9):1147-51. [PubMed] [Google Scholar]
Jesudasan 2015 {published data only}
- Jesudasan JS, Wahab PUA, Sekhar MRM. Effectiveness of 0.2% chlorhexidine gel and a eugenol-based paste on postoperative alveolar osteitis in patients having third molars extracted: a randomised controlled clinical trial. British Journal of Oral & Maxillofacial Surgery 2015;53(9):826-30. [DOI] [PubMed] [Google Scholar]
Johnson 1988 {published data only}
- Johnson WS, Blanton EE. An evaluation of 9-aminoacridine/Gelfoam to reduce dry socket formation. Oral Surgery, Oral Medicine, and Oral Pathology 1988;66(2):167-70. [DOI] [PubMed] [Google Scholar]
Jolley 1972 {published data only}
- Jolley HM, Torneck CD, Siegel I. A topical choline salicylate gel for control of pain and inflammation in oral conditions - a controlled study. Journal of the Canadian Dental Association 1972;38(2):72-4. [PubMed] [Google Scholar]
Jovanovic 2011 {published data only}
- Jovanović G, Urić N, Krunić N, Tijanić M, Stojanović S. Assessment of the effectiveness of low level laser in the treatment of alveolar osteitis [Procena efikasnosti primene lasera male snage u terapiji alveolarnogosteitisa]. Vojnosanitetski Pregled 2011;68(6):506-10. [DOI] [PubMed] [Google Scholar]
Julius 1982 {published data only}
- Julius LL, Hungerford RW, Nelson WJ, McKercher TC, Zellhoefer RW. Prevention of dry socket with local application of Terra-Cortril in gelfoam. Journal of Oral and Maxillofacial Surgery 1982;40(5):285-6. [DOI] [PubMed] [Google Scholar]
Kamal 2020a {published data only}
- Kamal A, Salman B, Razak N, Qabbani A, Samsudin R. The efficacy of concentrated growth factor in the healing of alveolar osteitis: a clinical study. International Journal of Dentistry 2020;2020:9038629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamal 2020b {published data only}
- Kamal A, Salman B, Razak N, Samsudin A. A comparative clinical study between concentrated growth factor and low-level laser therapy in the management of dry socket. European Journal of Dentistry 2020;14(4):613-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 2020 {published data only}
- Kaplan V, Hasanoglu Erbasar GN, Cigerim L, Altay Turgut H, Cerit A. Effect of St John's wort oil and olive oil on the postoperative complications after third molar surgery: randomized, double-blind clinical trial. Clinical Oral Investigations 2020;25(4):2429-38. [PMID: 10.1007/s00784-020-03639-0. Epub 2020 Oct 15.] [DOI] [PubMed] [Google Scholar]
Karthik 2021 {published data only}
- Karthik KP, Balamurugan RB. Evaluation and comparison of anti-inflammatory properties of ibuprofen using two drug delivery systems after third molar surgery: using chitosan microspheres as a carrier for local drug delivery in to the third molar socket and through the oral route. British Journal of Oral & Maxillofacial Surgery 2021;59(2):191-6. [DOI] [PubMed] [Google Scholar]
Keskitalo 1973 {published data only}
- Keskitalo E, Persson G. A clinical trial of Apernyl cones and tamponade with Ward's Wondr Pak in the treatment of dry socket. Svensk Tandlakare Tidskrift [Swedish Dental Journal] 1973;66(5):475-9. [PubMed] [Google Scholar]
Kilinc 2017 {published data only}
- Kilinc A, Ataol M. How effective is collagen resorbable membrane placement after partially impacted mandibular third molar surgery on postoperative morbidity? A prospective randomized comparative study. BMC Oral Health 2017;17:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2020 {published data only}
- Kim JW, Seong TW, Cho S, Kim SJ. Randomized controlled trial on the effectiveness of absorbable collagen sponge after extraction of impacted mandibular third molar: split-mouth design. BMC Oral Health 2020;20(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirk 2007 {published data only}
- Kirk DG, Liston PN, Tong DC, Love RM. Influence of two different flap designs on incidence of pain, swelling, trismus, and alveolar osteitis in the week following third molar surgery. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2007;104:e1-e6. [DOI] [PubMed] [Google Scholar]
Krekmanov 1981 {published data only}
- Krekmanov L. Alveolitis after operative removal of third molars in the mandible. International Journal of Oral Surgery 1981;10(3):173-9. [DOI] [PubMed] [Google Scholar]
Krekmanov 1986 {published data only}
- Krekmanov L, Nordenram A. Postoperative complications after surgical removal of mandibular third molars. Effects of penicillin V and chlorhexidine. International Journal of Oral and Maxillofacial Surgery 1986;15(1):25-9. [DOI] [PubMed] [Google Scholar]
Krishnan 2020 {published data only}
- Krishnan S, Periasamy S, Arun M. Evaluating the efficacy of Abgel soaked in manuka honey following extraction of infected mandibular molars. International Journal of Pharmaceutical Research 2020;12(1):1789-97. [Google Scholar]
Kudiyirickal 2012 {published data only}
- Kudiyirickal M, Hollinshead F. Clinical profile of orofacial infections: an experience from two primary care dental practices. Medicina Oral, Patologia Oral y Cirugia Bucal 2012;17(4):e533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lao 2012 {published data only}
- Lao L, Huang Y, Feng C, Berman BM, Tan MT. Evaluating traditional Chinese medicine using modern clinical trial design and statistical methodology: application to a randomized controlled acupuncture trial. Statistics in Medicine 2012;31(7):619-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2011 {published data only}
- Liu W, Dutta S, Hosmane BS. Exposure-survival analysis to quantify the proportion of subjects with meaningful pain relief in dental pain. Journal of Clinical Pharmacology 2011;51(51):1366-7. [Google Scholar]
Long 2012 {published data only}
- Long H, Zhou Y, Liao L, Pyakurel U, Wang Y, Lai W. Coronectomy vs total removal for third molar extraction: a systematic review. Journal of Dental Research 2012;91(7):659-65. [DOI] [PubMed] [Google Scholar]
Lopez‐Cedrun 2011 {published data only}
- López-Cedrún JL, Pijoan JI, Fernández S, Santamaria J, Hernandez G. Efficacy of amoxicillin treatment in preventing postoperative complications in patients undergoing third molar surgery: a prospective, randomized, double-blind controlled study. Journal of Oral and Maxillofacial Surgery 2011;69(6):e5-14. [DOI] [PubMed] [Google Scholar]
MacGregor 1973 {published data only}
- MacGregor AJ, Hutchinson D. The effect of Nivemycin on pain and swelling following lower third molar removal. British Journal of Oral Surgery 1973;10(3):321-5. [DOI] [PubMed] [Google Scholar]
MacGregor 1975 {published data only}
- MacGregor AJ, Hutchinson D. The effect of sulfonamides on pain and swelling following removal of ectopic third molars. International Journal of Oral Surgery 1975;4(5):184-90. [DOI] [PubMed] [Google Scholar]
Majid 2010 {published data only}
- Majid OW, Mahmood KW. Effect of submucosal and intramuscular dexamethasone on postoperative sequelae after third molar surgery: comparative study. Journal of Oral and Maxillofacial Surgery 2011;49(8):647-52. [DOI] [PubMed] [Google Scholar]
Malkawi 2011 {published data only}
- Malkawi Z, Al-Omiri MK, Khraisat A. Risk indicators of postoperative complications following surgical extraction of lower third molars. Medical Principles and Practice 2011;20(4):321-5. [DOI] [PubMed] [Google Scholar]
Mehlisch 2010a {published data only}
- Mehlisch DR, Aspley S, Daniels SE, Southerden KA, Christensen KS. A single-tablet fixed-dose combination of racemic ibuprofen/paracetamol in the management of moderate to severe postoperative dental pain in adult and adolescent patients: a multicenter, two-stage, randomized, double-blind, parallel-group, placebo-controlled, factorial study. Clinical Therapeutics 2010;32(6):1033-49. [DOI] [PubMed] [Google Scholar]
Mehlisch 2010b {published data only}
- Mehlisch DR, Aspley S, Daniels SE, Bandy DP. Comparison of the analgesic efficacy of concurrent ibuprofen and paracetamol with ibuprofen or paracetamol alone in the management of moderate to severe acute postoperative dental pain in adolescents and adults: a randomized, double-blind, placebo-controlled, parallel-group, single-dose, two-center, modified factorial study. Clinical Therapeutics 2010;32(5):882-95. [DOI] [PubMed] [Google Scholar]
Mishra 2012 {published data only}
- Mishra H, Khan FA. A double-blind, placebo-controlled randomized comparison of pre and postoperative administration of ketorolac and tramadol for dental extraction pain. Journal of Anaesthesiology, Clinical Pharmacology 2012;28(2):221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 1986a {published data only}
- Mitchell R. Treatment of fibrinolytic alveolitis by a collagen paste (Formula K). A preliminary report. International Journal of Oral and Maxillofacial Surgery 1986;15(2):127-33. [DOI] [PubMed] [Google Scholar]
Mitchell 1986b {published data only}
- Mitchell DA. A controlled clinical trial of prophylactic tinidazole for chemoprophylaxis in third molar surgery. British Dental Journal 1986;160(8):284-6. [DOI] [PubMed] [Google Scholar]
Moberly 2007 {published data only}
- Moberly JB, Xu J, Desjardins PJ, Daniels SE, Bandy DP, Lawson JE, et al. A randomized, double-blind, celecoxib- and placebo-controlled study of the effectiveness of CS-706 in acute postoperative dental pain. Clinical Therapeutics 2009;29(1):399-412. [DOI] [PubMed] [Google Scholar]
Nentwig 1985 {published data only}
- Nentwig GH, Kniha H. Treatment of postsurgical bone infections. Fortschritte der Kiefer- und Gesichts-Chirurgie 1985;30:35-7. [PubMed] [Google Scholar]
Neugebauer 2004 {published data only}
- Neugebauer J, Jozsa M, Kubler A. Antimicrobial photodynamic therapy for prevention of alveolar ostitis and post-extraction pain. Mund-, Kiefer- und Gesichtschirurgie 2004;8(6):350-5. [DOI] [PubMed] [Google Scholar]
Neuner 1969 {published data only}
- Neuner O. Experiences with Apernyl in the treatment of and prevention of pain after extraction. Schweizerische Monatsschrift fur Zahnheilkunde 1969;79(5):630-5. [PubMed] [Google Scholar]
Njokanma 2019 {published data only}
- Njokanma A, Fatusi O, Ogundipe O, Salawu L. The effect of platelet rich fibrin on surgical outcome of mandibular third molar surgery. International Journal of Oral and Maxillofacial Surgery 2019;48(Suppl 1):66. [Google Scholar]
Nordenram 1973 {published data only}
- Nordenram A, Sydnes G, Ödegaard J. Neomycin-bacitracin cones in impacted third molar sockets. International Journal of Oral Surgery 1973;2(6):279-83. [DOI] [PubMed] [Google Scholar]
Olson 1987 {published data only}
- Olson RAJ, Fridrich KL. Alveolitis sicca dolorosa following impacted mandibular 3rd molar surgical removal. Journal of Dental Research 1987;66(1 Suppl):174 (Abs No 542). [Google Scholar]
Olusanya 2011 {published data only}
- Olusanya AA, Arotiba JT, Fasola OA, Akadiri AO. Prophylaxis versus pre-emptive antibiotics in third molar surgery: a randomised control study. Nigerian Postgraduate Medical Journal 2011;18(2):105-10. [PubMed] [Google Scholar]
Osunde 2014 {published data only}
- Osunde OD, Adebola RA, Adeoye JB, Bassey GO. Comparative study of the effect of warm saline mouth rinse on complications after dental extractions. International Journal of Oral and Maxillofacial Surgery 2014;43(5):649-53. [DOI] [PubMed] [Google Scholar]
Osunde 2015 {published data only}
- Osunde OD, Bassey GO. Role of warm saline mouth rinse in prevention of alveolar osteitis: a randomized controlled trial. Nigerian Journal of Medicine 2015;24(1):28-31. [PubMed] [Google Scholar]
Osunde 2017 {published data only}
- Osunde OD, Anyanechi CE, Bassey GO. Prevention of alveolar osteitis after third molar surgery: comparative study of the effect of warm saline and chlorhexidine mouth rinses. Nigerian Journal of Clinical Practice 2017;20:470-3. [DOI] [PubMed] [Google Scholar]
Oyri 2019 {published data only}
- Oyri H, Jonsdottir O, Jensen JL, Bjornland T. The use of a tetracycline drain reduces alveolar osteitis: a randomized prospective trial of third molar surgery under local anesthetics and without the use of systemic antibiotics. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 2019;128(3):205-12. [DOI] [PubMed] [Google Scholar]
Ozveri 2020 {published data only}
- Ozveri Koyuncu B, Isik G, Ozden Yuce M, Gunbay S, Gunbay T. Effect of concentrated growth factors on frequency of alveolar osteitis following partially-erupted mandibular third molar surgery: a randomized controlled clinical study. BMC Oral Health 2020;20(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2019 {published data only}
- Paul S, Choudhury R, Kumari N, Rastogi S, Sharma A, Singh V, et al. Is treatment with platelet-rich fibrin better than zinc oxide eugenol in cases of established dry socket for controlling pain, reducing inflammation, and improving wound healing? Journal of the Korean Association of Oral and Maxillofacial Surgeons 2019;45(2):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Pichler 2001 {published data only}
- Pichler JR, Beirne OR. Chlorhexidine rinsing and dry socket after surgically extracting third molars. Journal of Dental Research 2001;80(Special Issue (AADR Abstracts)):222 (Abs No 1492). [Google Scholar]
Prataap 2017 {published data only}
- Prataap N, Sunil PM, Sudeep CB, Ninan VS, Tom A, Arjun MR. Platelet-rich plasma and incidence of alveolar osteitis in high-risk patients undergoing extractions of mandibular molars: a case-control study. Journal of Pharmacy and Bioallied Sciences 2017;9(Supp 1):S173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qi 2012 {published data only}
- Qi D, May L, Zimmerman B, Peng P, Atillasoy E, Shelburne S, et al. A randomized, double-blind, placebo-controlled, efficacy and safety study of acetaminophen 1000 mg and acetaminophen 650 mg in postoperative dental pain. Journal of Pain 2012;13(4 Suppl 1):S74. [Google Scholar]
Rani 2016 {published data only}
- Rani A, Mohanty S, Sharma P, Dabas J. Comparative evaluation of Er:Cr:YSGG, diode laser and Alvogyl in the management of alveolar osteitis: a prospective randomized clinical study. Journal of Maxillofacial and Oral Surgery 2016;15(3):349-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rastogi 2018 {published data only}
- Rastogi S, Choudhury R, Kumar A, Manjunath S, Sood A, Upadhyay H. Versatility of platelet rich fibrin in the management of alveolar osteitis - A clinical and prospective study. Journal of Oral Biology and Craniofacial Research 2018;8(3):188-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeshma 2021 {published data only}
- Reeshma S, Dain CP. Comparison of platelet-rich fibrin with zinc oxide eugenol in the relief of pain in alveolar osteitis. Health Science Reports 2021;4(3):e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ritzau 1978 {published data only}
- Ritzau M, Therkildsen P. Antifibrinolytic prevention of alveolitis sicca dolorosa. International Journal of Oral Surgery 1978;7(6):534-40. [DOI] [PubMed] [Google Scholar]
Saez‐Alcaide 2020 {published data only}
- Saez-Alcaide LM, Molinero-Mourelle P, Gonzalez-Serrano J, Rubio-Alonso L, Bornstein MM, Lopez-Quiles J. Efficacy of a topical gel containing chitosan, chlorhexidine, allantoin and dexpanthenol for pain and inflammation control after third molar surgery: a randomized and placebo-controlled clinical trial. Medicina Oral, Patologia Oral y Cirugia Bucal 2020;25(5):e644-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchis 2004 {published data only}
- Sanchis JM, Sáez U, Penarrocha M, Gay C. Tetracycline compound placement to prevent dry socket: a postoperative study of 200 impacted mandibular third molars. Journal of Oral and Maxillofacial Surgery 2004;62(5):587-91. [DOI] [PubMed] [Google Scholar]
Sarkar 2019 {published data only}
- Sarkar S, Prashanth NT, Shobha ES, Rangan V, Nikhila G. Efficacy of platelet rich fibrin versus chitosan as a hemostatic agent following dental extraction in patients on antiplatelet therapy. Journal of Oral Biology and Craniofacial Research 2019;9(4):336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schatz 1987 {published data only}
- Schatz JP, Fiore-Donno G, Henning G. Fibrinolytic alveolitis and its prevention. International Journal of Oral and Maxillofacial Surgery 1987;16(2):175-83. [DOI] [PubMed] [Google Scholar]
Schlund 1968 {published data only}
- Schlund A. Experiences with "Terracortril Gel" in oral surgery. DDZ - das Deutsche Zahnarzteblatt 1968;22(2):82-4. [PubMed] [Google Scholar]
Scopp 1967 {published data only}
- Scopp IW, Morgan FH, Gillette WB, Fredrics HJ, Peskin MJ, Kleinman D. Dialog. A double-blind clinical study of dialog, darvon and a placebo in the management of postoperative dental pain. Journal of Oral Therapeutics and Pharmacology 1967;4(2):123-7. [PubMed] [Google Scholar]
Seethamsetty 2019 {published data only}
- Seethamsetty S, Sarepally G, Sanober A, Qureshi Y, Fatima U, Arif SM. A comparative evaluation of the effectiveness of chitosan-based dressing and conventional method of hemostasis in patients on oral antithrombotic therapy without therapy interruption. Journal of Pharmacy & Bioallied Sciences 2019;11(5):S18-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2017 {published data only}
- Sharma A, Aggarwal N, Rastogi S, Choudhury R, Tripathi S. Effectiveness of platelet-rich fibrin in the management of pain and delayed wound healing associated with established alveolar osteitis (dry socket). European Journal of Dentistry 2017;11(4):508-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorensen 1987 {published data only}
- Sorensen DC, Preisch JW. The effect of tetracycline on the incidence of postextraction alveolar osteitis. Journal of Oral and Maxillofacial Surgery 1987;45(12):1029-33. [DOI] [PubMed] [Google Scholar]
Swanson 1989 {published data only}
- Swanson AE. A double-blind study on the effectiveness of tetracycline in reducing the incidence of fibrinolytic alveolitis. Journal of Oral and Maxillofacial Surgery 1989;47(2):165-7. [DOI] [PubMed] [Google Scholar]
Sweet 1985 {published data only}
- Sweet JB, Macynski AA. Effect of antimicrobial mouth rinses on the incidence of localized alveolitis and infection following mandibular third molar oral surgery. Oral Surgery, Oral Medicine, and Oral Pathology 1985;59(1):24-6. [DOI] [PubMed] [Google Scholar]
Syrjanen 1981a {published data only}
- Syrjanen SM, Syrjanen KJ. A new combination of drugs intended to be used as a preventive measure for the postextraction complications. A preliminary report. International Journal of Oral Surgery 1981;10(1):17-22. [DOI] [PubMed] [Google Scholar]
- Syrjanen SM, Syrjanen KJ. The effects on extraction wound healing of a new drug combination introduced for use in the prevention of post-extraction complications. A preliminary report. British Journal of Oral Surgery 1981;19(1):57-66. [DOI] [PubMed] [Google Scholar]
Syrjanen 1981b {published data only}
- Syrjanen SM, Syrjanen KJ. Prevention and treatment of post-extraction complications. A comparison of the effect of Alvogyl and a new drug combination on wound healing. Proceedings of the Finnish Dental Society 1981;77(6):305-11. [PubMed] [Google Scholar]
Tek 2014 {published data only}
- Tek M, Akkas I, Toptas O, Ozan F, Sener I, Bereket C. Effects of the topical hemostatic agent Ankaferd Blood Stopper on the incidence of alveolar osteitis after surgical removal of an impacted mandibular third molar. Nigerian Journal of Clinical Practice 2014;17(1):75-80. [DOI] [PubMed] [Google Scholar]
Tjernberg 1979 {published data only}
- Tjernberg A. Influence of oral hygiene measures on the development of alveolitis sicca dolorosa after surgical removal of mandibular third molars. International Journal of Oral Surgery 1979;8(6):430-4. [DOI] [PubMed] [Google Scholar]
Tong 2012 {published data only}
- Tong SE, Daniels SE, Black P, Chang S, Protter A, Desjardins PJ. Novel p38alpha mitogen-activated protein kinase inhibitor shows analgesic efficacy in acute postsurgical dental pain. Journal of Clinical Pharmacology 2012;52(5):717-28. [DOI] [PubMed] [Google Scholar]
Torres Lagares 2006 {published data only}
- Torres Lagares D, Infante Cossío P, Gutiérrez Pérez JL, Romero Ruiz MM, Serrera Figallo MA. Gel de Clorhexidina intra-alveolar en la prevención de la alveolitis tras la extracción de terceros molares inferiores: estudio piloto. Medicina Oral, Patologia Oral y Cirugia Bucal 2006;11(2):113-8. [PubMed] [Google Scholar]
Torres‐Lagares 2010 {published data only}
- Torres-Lagares D, Gutierrez-Perez JL, Hita-Iglesias P, Magallanes-Abad N, Flores-Ruiz R, Basallote-Garcia M, et al. Randomized, double-blind study of effectiveness of intra-alveolar application of chlorhexidine gel in reducing incidence of alveolar osteitis and bleeding complications in mandibular third molar surgery in patients with bleeding disorders. Journal of Oral and Maxillofacial Surgery 2010;68(6):1322-6. [DOI] [PubMed] [Google Scholar]
Vedtofte 1974 {published data only}
- Vedtofte P, Ritzau M, Donatsky O. Use of propylic ester of p-hydroxy-benzoic acid after removal of impacted mandibular third molars. International Journal of Oral Surgery 1974;3(6):394-9. [DOI] [PubMed] [Google Scholar]
Vu 2021 {published data only}
- Vu N, Chuenchompoonut V, Jansisyanont P, Sangvanich P, Pham T, Thunyakitpisal P. Acemannan-induced tooth socket healing: a 12-month randomized controlled trial. Journal of Dental Sciences 2021;16(2):643-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang YZ, Guan QL, Li YX, Guo JL, Jiang L, Jia MY, et al. Use of "gelatamp" colloidal silver gelatin sponge to prevent dry socket after extracting mandibular impacted teeth. Shanghai Kou Qiang Yi Xue [Shanghai Journal of Stomatology] 2013;22(1):108-10. [PubMed] [Google Scholar]
Wen 2004 {published data only}
- Wen N, Han L, Lu HX, Mao TQ, Wang ZY, Liu HC. Protection of basic fibroblast growth factor on dry socket after mandibular impacted tooth extraction. Zhongguo Linchuang Kangfu 2004;8(5):858-9. [Google Scholar]
Yuan 2006 {published data only}
- Yuan KF, Lai QG, Li DR, Xia YF, Cui YX. Application of low intensity ultrasound in the extraction of totally impacted mandibular third molars. Shanghai Kou Qiang Yi Xue [Shanghai Journal of Stomatology] 2006;15(5):497-9. [PubMed] [Google Scholar]
Yue 2012 {published data only}
- Yue Y, Christensen S, Buchanan W, Reed K, Collaku A, Otto J. Two randomized, double-blind, placebo-controlled efficacy studies assessing the efficacy and speed of onset of pain relief of Panadol Advance in post-surgical dental pain. Journal of Pain 2012;13(4 Suppl 1):S85. [Google Scholar]
Zanetta‐Barbosa 1994 {published data only}
- Zanetta-Barbosa D, Candelas L, Rocha F, Marquez IM. A clinical study on the efficacy of two treatments for dry socket [Estudo clínico da eficácia de dois tipos de tratamento para alveolite]. Revista do Centro de Ciencias Biomedicas da Universidade Federal de Uberlandia 1994;10(1):35-46. [Google Scholar]
Zuniga 2011 {published data only}
- Zuniga JR, Noveck RJ, Schmidt WK, Boesing SE, Hersh EV. Onset of action of diclofenac potassium liquid-filled capsules in dental surgery patients. Current Medical Research and Opinion 2011;27(9):1733-9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Zorina 2019 {published data only}
- Zorina O, Petrukhina N, Boriskina O. Alveolar osteitis treatment using Holisal gel. Stomatologiia 2019;98(6):58-64. [DOI] [PubMed] [Google Scholar]
Additional references
Aguilar‐Durán 2019
- Aguilar-Durán L, Figueiredo R, Seminago R, Roig FJ, Llorens C, Valmaseda-Castellón E. A metagenomic study of patients with alveolar osteitis after tooth extraction. A preliminary case-control study. Clinical Oral Investigations 2019;23(11):4163–72. [DOI] [PubMed] [Google Scholar]
Antczak‐Bouckoms 1990
- Antczak-Bouckoms AA, Tulloch JF, Berkey CS. Split-mouth and cross-over designs in dental research. Journal of Clinical Periodontology 1990;17(7 Pt 1):446-53. [DOI] [PubMed] [Google Scholar]
Bailey 2020
- Bailey E, Kashbour W, Shah N, Worthington HV, Renton TF, Coulthard P. Surgical techniques for the removal of mandibular wisdom teeth. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD004345. [DOI: 10.1002/14651858.CD004345.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barona‐Dorado 2014
- Barona-Dorado C, Gonzalez-Regueiro I, Martin-Ares M, Arias-Irimia O, Martinez-Gonzalez JM. Efficacy of platelet-rich plasma applied to post-extraction retained lower third molar alveoli. A systematic review. Medicina Oral, Patologia Oral y Cirugia Bucal 2014;19(2):e142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bello 2011
- Bello SA, Olaitan AA, Ladeinde AL. A randomized comparison of the effect of partial and total wound closure techniques on postoperative morbidity after mandibular third molar surgery. Journal of Oral and Maxillofacial Surgery 2011;69(6):e24-30. [DOI] [PubMed] [Google Scholar]
Berwick 1990
- Berwick JE, Lessin ME. Effects of a chlorhexidine gluconate oral rinse on the incidence of alveolar osteitis in mandibular third molar surgery. Journal of Oral and Maxillofacial Surgery 1990;48(5):444-8. [DOI] [PubMed] [Google Scholar]
Birn 1973
- Birn H. Etiology and pathogenesis of fibrinolytic alveolitis ('dry socket'). International Journal of Oral Surgery 1973;2(5):211-63. [Google Scholar]
Bloomer 2000
- Bloomer CR. Alveolar osteitis prevention by immediate placement of medicated packing. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2000;90(3):282-4. [DOI] [PubMed] [Google Scholar]
Bloomer 2012
- Bloomer CR. Straws do not cause dry sockets when third molars are extracted. Texas Dental Journal 2012;129(1):25-32. [PubMed] [Google Scholar]
Blum 2002
- Blum IR. Contemporary views on dry socket (alveolar osteitis): a clinical appraisal of standardization, aetiopathogenesis and management: a critical review. International Journal of Oral and Maxillofacial Surgery 2002;31(3):309-17. [DOI] [PubMed] [Google Scholar]
Bortoluzzi 2012
- Bortoluzzi MC, Capella DL, Barbieri T, Marchetti S, Dresch CP, Tirello C. Does smoking increase the incidence of postoperative complications in simple exodontia? International Dental Journal 2012;62(2):106–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Canellas 2017
- Canellas JVDS, Ritto FG, Medeiros PJD. Evaluation of postoperative complications after mandibular third molar surgery with the use of platelet-rich fibrin: a systematic review and meta-analysis.. International Journal of Oral and Maxillofacial Surgery 2017;46(9):1138-46. [DOI] [PubMed] [Google Scholar]
Canellas 2019
- Canellas J, Medeiros P, Figueredo C, Fischer R, Ritto F. Platelet-rich fibrin in oral surgical procedures: a systematic review and meta-analysis. International Journal of Oral and Maxillofacial Surgery 2019;48:395-414. [DOI] [PubMed] [Google Scholar]
Canellas 2020
- Canellas J, Fraga S, Santoro M, Netto J, Tinoco E. Intrasocket interventions to prevent alveolar osteitis after mandibular third molar surgery: a systematic review and network meta-analysis. Journal of Cranio-Maxillofacial Surgery 2020;48(9):902-13. [DOI] [PubMed] [Google Scholar]
Canullo 2020
- Canullo L, Laino L, Longo F, Filetici P, D'Onofrio I, Troiano G. Does chlorhexidine prevent complications in extractive, periodontal, and implant surgery? A systematic review and meta-analysis with trial sequential analysis. International Journal of Oral & Maxillofacial Implants 2020;35(6):1149-58. [DOI] [PubMed] [Google Scholar]
Caso 2005
- Caso A, Hung LK, Beirne OR. Prevention of alveolar osteitis with chlorhexidine: a meta-analytic review. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2005;99(2):155-9. [DOI] [PubMed] [Google Scholar]
Chow 2020
- Chow O, Wang R, Ku D, Huang W. Alveolar osteitis: a review of current concepts. Journal of Oral and Maxillofacial Surgery 2020;78:1288-96. [DOI] [PubMed] [Google Scholar]
Christensen 2012
- Christensen J, Matzen LH, Wenzel A. Should removal of lower third molars be included in the pre-graduate curriculum for dental students? An evaluation of post-operative complications after student operations. Acta Odontologica Scandinavica 2012;70(1):42–8. [DOI] [PubMed] [Google Scholar]
Cohen 1995
- Cohen M, Simecek J. Effects of gender-related factors on the incidence of localized alveolar osteitis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 1995;79(4):416-22. [DOI] [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta-analysis combining parallel and cross-over clinical trials. II: binary outcomes. Statistics in Medicine 2001;21(15):2145-59. [DOI] [PubMed] [Google Scholar]
Del Fabbro 2019
- Del Fabbro M, Panda S, Taschieri S. Adjunctive use of plasma rich in growth factors for improving alveolar socket healing: a systematic review. Journal of Evidence-based Dental Practice 2019;19(2):166-76. [DOI] [PubMed] [Google Scholar]
Epstein 2000
- Epstein JB, Chong S, Le ND. A survey of antibiotic use in dentistry. Journal of the American Dental Association 2000;131(11):1600-9. [DOI] [PubMed] [Google Scholar]
Fazakerley 1991
- Fazakerley M, Field EA. Dry socket: a painful post-extraction complication (a review). Dental Update 1991;18(1):31-4. [PubMed] [Google Scholar]
Field 1988
- Field EA, Nind D, Varga E, Martin MV. The effect of chlorhexidine irrigation on the incidence of dry socket: a pilot study. British Journal of Oral and Maxillofacial Surgery 1988;26(5):395-401. [DOI] [PubMed] [Google Scholar]
Franchini 2019
- Franchini M, Cruciani M, Mengoli C, Masiello F, Marano G, D'Aloja E, et al. The use of platelet-rich plasma in oral surgery: a systematic review and meta-analysis. Blood Transfusion 2019;17(5):357-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fridrich 1990
- Fridrich KL, Olson RAJ. Alveolar osteitis following surgical removal of mandibular third molars. Anesthesia Progress 1990;37(1):32-41. [PMC free article] [PubMed] [Google Scholar]
Garcia 2003
- Garcia AG, Grana PM, Sampedro FG, Diago MP, Rey JM. Does oral contraceptive use affect the incidence of complications after extraction of a mandibular third molar? British Dental Journal 2003;194(8):453-5. [DOI] [PubMed] [Google Scholar]
Goldman 1973
- Goldman DR, Kilgore DS, Panzer JD, Atkinson WH. Prevention of dry socket by local application of lincomycin in Gelfoam. Oral Surgery, Oral Medicine, and Oral Pathology 1973;35(4):472-4. [DOI] [PubMed] [Google Scholar]
Hall 1971
- Hall HD, Bildman BS, Hand CD. Prevention of dry socket with local application of tetracycline. Journal of Oral Surgery 1971;29(1):35-7. [PubMed] [Google Scholar]
Hedstrom 2007
- Hedstrom L, Sjogren P. Effect estimates and methodological quality of randomised controlled trials about prevention of alveolar osteitis following tooth extraction: a systematic review. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2007;103(1):8-15. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Higgins 2021
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
James 2017
- James P, Worthington HV, Parnell C, Harding M, Lamont T, Cheung A, et al. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD008676. [DOI: 10.1002/14651858.CD008676.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jovanović 2011
- Jovanović G, Urić N, Krunić N, Tijanić M, Stojanović S. Assessment of the effectiveness of low level laser in the treatment of alveolar osteitis [Slovenian] [Procena efikasnosti primene lasera male snage u terapiji alveolarnogosteitisa]. Vojnosanitetski Pregled 2011;68(6):506-10. [DOI] [PubMed] [Google Scholar]
Kalsi 2020
- Kalsi H, Major R, Jawad H. Alvogyl or alveogyl? British Dental Journal 2020;229:211. [DOI] [PubMed] [Google Scholar]
Kirk 2007
- Kirk DG, Liston PN, Tong DC, Love RM. Influence of two different flap designs on incidence of pain, swelling, trismus, and alveolar osteitis in the week following third molar surgery. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2007;104:e1-e6. [DOI] [PubMed] [Google Scholar]
Koerner 1994
- Koerner KR. The removal of impacted third molars. Principles and procedures. Dental Clinics of North America 1994;38(2):255-78. [PubMed] [Google Scholar]
Kolokythas 2010
- Kolokythas A, Olech E, Miloro M. Alveolar osteitis: a comprehensive review of concepts and controversies. International Journal of Dentistry 2010;2010:249073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsen 1991
- Larsen PE. The effect of a chlorhexidine rinse on the incidence of alveolar osteitis following surgical removal of impacted third molars. Journal of Oral and Maxillofacial Surgery 1991;49:932-7. [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Lodi 2021
- Lodi G, Azzi L, Varoni EM, Pentenero M, Del Fabbro M, Carrassi A, et al. Antibiotics to prevent complications following tooth extractions. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD003811. [DOI: 10.1002/14651858.CD003811.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mariotti 1999
- Mariotti AJ, Rumpf DA. Chlorhexidine-induced changes to human gingival fibroblast collagen and non-collagen protein production. Journal of Periodontology 1999;70: 1443-8. [DOI] [PubMed] [Google Scholar]
McCaul 2001
- McCaul LK, Jenkins WM, Kay EJ. The reasons for the extraction of various tooth types in Scotland: a 15-year follow-up. British Dental Journal 2001;190(12):658-62. [DOI] [PubMed] [Google Scholar]
Nitzan 1983
- Nitzan D. On the genesis of “dry socket”. Journal of Oral and Maxillofacial Surgery 1983;41(11):706-10. [DOI] [PubMed] [Google Scholar]
Noroozi 2009
- Noroozi AR, Philbert RF. Modern concepts in understanding and management of the "dry socket" syndrome: comprehensive review of the literature. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2009;107(1):30-5. [DOI] [PubMed] [Google Scholar]
Nusair 2007
- Nusair YM, Younis MH. Prevalence, clinical picture, and risk factors of dry socket in a Jordanian dental teaching center. Journal of Contemporary Dental Practice 2007;8(3):53-63. [PubMed] [Google Scholar]
Peñarrocha 2001
- Peñarrocha M, Sanchis JM, Sáez U, Gay C, Bagán JV. Oral hygiene and postoperative pain after mandibular third molar surgery. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2001;92(3):260–4. [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.8.1. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rodríguez Sánchez 2017
- Rodríguez Sánchez F, Rodríguez Andrés C, Arteagoitia Calvo I. Does chlorhexidine prevent alveolar osteitis after third molar extractions? Systematic review and meta-analysis. Journal of Oral and Maxillofacial Surgery 2017;75(5):901-14. [DOI] [PubMed] [Google Scholar]
Rud 1970
- Rud J. Removal of impacted lower third molars with acute pericoronitis and necrotising gingivitis. British Journal of Oral Surgery 1970;7(3):153-60. [DOI] [PubMed] [Google Scholar]
Septodont 2021
- Septodont. Alveogyl. www.septodont.ie/sites/ie/files/2019-03/packInsert-Alveogyl_0.pdf (accessed 4 May 2021).
Shafaee 2020
- Shafaee H, Bardideh E, Nazari M, Asadi R, Shahidi B, Rangrazi A. The effects of photobiomodulation therapy for treatment of alveolar osteitis (dry socket): systematic review and meta-analysis. Photodiagnosis and Photodynamic Therapy 2020;32:102000. [DOI] [PubMed] [Google Scholar]
Shen 2019
- Shen LH, Xiao E, Wang EB, Zheng H, Zhang Y. High-throughput sequencing analysis of microbial profiles in the dry socket. Journal of Oral and Maxillofacial Surgery 2019;77(8):1548–56. [DOI] [PubMed] [Google Scholar]
Silva 2006
- Silva LJ, Poi WR, Panzarini SR, Rodrigues TS, Simonato LE. Clinical evaluation of an ointment with 10% metronidazole and 2% lidocaine in the treatment of alveolitis. Minerva Stomatologica 2006;55(7-8):431-6. [PubMed] [Google Scholar]
Stedman 2011
- Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2011;40(6):1732-4. [DOI] [PubMed] [Google Scholar]
Swanson 1989
- Swanson AE. A double-blind study on the effectiveness of tetracycline in reducing the incidence of fibrinolytic alveolitis. Journal of Oral and Maxillofacial Surgery 1989;47(2):165-7. [DOI] [PubMed] [Google Scholar]
Sweet 1978
- Sweet JB, Butler DP. Predisposing and operative factors: effect on the incidence of localized osteitis in mandibular third-molar surgery. Oral Surgery, Oral Medicine, and Oral Pathology 1978;46(2):206-15. [DOI] [PubMed] [Google Scholar]
Syrjänen 1979
- Syrjänen SM, Syrjänen KJ. Influence of Alvogyl on the healing of extraction wound in man. International Journal of Oral Surgery 1979;8(1):22-30. [DOI] [PubMed] [Google Scholar]
Tjernberg 1979
- Tjernberg A. Influence of oral hygiene measures on the development of alveolitis sicca dolorosa after surgical removal of mandibular third molars. International Journal of Oral Surgery 1979;8(6):430-4. [DOI] [PubMed] [Google Scholar]
Turcotte 1997
- Turcotte JY. [Alveolitis--current opinion] [French]. Journal (Canadian Dental Association) 1997;63(3):206-10. [PubMed] [Google Scholar]
Veale 2015
- Veale B. Alveolar osteitis: a critical review of the aetiology and management. Oral Surgery 2015;8:68-77. [Google Scholar]
Vezeau 2000
- Vezeau PJ. Dental extraction wound management: medicating postextraction sockets. Journal of Oral and Maxillofacial Surgery 2000;58(5):531-7. [DOI] [PubMed] [Google Scholar]
Worthington 2015
- Worthington H , Clarkson J, Weldon J. Priority oral health research identification for clinical decision-making. Evidence-based Dentistry 2015;16(3):69-71. [DOI] [PubMed] [Google Scholar]
Xiang 2019
- Xiang X, Shi P, Zhang P, Shen J, Kang J. Impact of platelet-rich fibrin on mandibular third molar surgery recovery: a systematic review and meta-analysis. BMC Oral Health 2019;19(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2019
- Xu R, Xia R. Efficacy of plasma rich in growth factor used for dry socket management: a systematic review. Medicina Oral, Patologia Oral y Cirugia Bucal 2019;24(6):e704-e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yengopal 2012
- Yengopal V, Mickenautsch S. Chlorhexidine for the prevention of alveolar osteitis. International Journal of Oral and Maxillofacial Surgery 2012;41(10):1253–64. [DOI] [PubMed] [Google Scholar]
Zhu 2020
- Zhu J, Zhang S, Yuan X, He T, Liu H, Wang J, et al. Effect of platelet-rich fibrin on the control of alveolar osteitis, pain, trismus, soft tissue healing, and swelling following mandibular third molar surgery: an updated systematic review and meta-analysis. International Journal of Oral and Maxillofacial Surgery 2020;50(3):398-406. [DOI: 10.1016/j.ijom.2020.08.014] [DOI] [PubMed] [Google Scholar]
Øyri 2020
- Øyri H, Jensen JL, Barkvoll P, Jonsdottir OH, Reseland J, Bjørnland T. Incidence of alveolar osteitis after mandibular third molar surgery. Can inflammatory cytokines be identified locally? Acta Odontologica Scandinavica 2020;79(3):205-11. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Daly 2008
- Daly B, Newton T, Nasser M, Jones K, Fedorowicz Z. Intrasocket interventions for the treatment of dry socket. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD006968. [DOI: 10.1002/14651858.CD006968] [DOI] [Google Scholar]
Daly 2012
- Daly B, Sharif MO, Newton T, Jones K, Worthington HV. Local interventions for the management of alveolar osteitis (dry socket). Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD006968. [DOI: 10.1002/14651858.CD006968.pub2] [DOI] [PubMed] [Google Scholar]


