Abstract
Background and objectives
During the COVID-19 pandemic, B cell depleting therapies pose a clinical concern for patients with neuroimmune conditions, as patients may not mount a sufficient immune response to SARS-CoV-2 infection and vaccinations. Studies to-date have reported conflicting results on the degree of antibody production post-SARS-CoV-2 infection and vaccinations in B cell depleted patients, focusing primarily on short-term immune profiling. Our objective was to follow longitudinal immune responses in COVID-19 B cell depleted patients with neuroimmune disorders post-COVID-19 and SARS-CoV-2-vaccination.
Methods
CD20 B cell depleted autoimmune patients and age/sex-matched controls positive for SARS-CoV-2 were recruited at Dell Medical School, UT Austin between 2020 and 2021, followed prospectively for 12 months and evaluated at multiple time points for spike S1 receptor binding domain (RBD) antibody titers, B and T cell composition, and frequency of T cells specific for SARS-CoV-2 antigens.
Results
Immune responses post-SARS-CoV-2 infection and vaccination were evaluated in a cohort of COVID-19 B cell depleted neuroimmune patients (n = 5), COVID-19 non-B cell depleted autoimmune patients (n = 15), COVID-19 immunocompetent patients (n = 117), and healthy controls (n = 6) for a total of 259 samples in 137 participants. 4/5 B cell-depleted patients developed detectable anti-spike RBD antibodies, which were boosted by vaccination in 2 patients. While spike RBD antibodies were associated with presence of CD20+ B cells, very few B cells were required. In contrast, patients whose B cell compartment primarily consisted of CD19+CD20– Bcells during acute COVID-19 disease or vaccination did not seroconvert. Interestingly, circulating Bcells in B cell depleted patients were significantly CD38high with co-expression of CD24 and CD27, indicating that B cell depletion may impact B cell activation patterns. Additionally, all B cell depleted patients mounted a sustained T cell response to SARS-CoV-2 antigens, regardless of seroconversion. Specifically, all patients developed naïve, central memory, effector memory, and effector memory RA+ T cells, suggesting intact T cell memory conversion in B cell depleted patients compared to controls.
Discussion
We present the longest COVID-19 immune profiling analysis to date in B cell depleted patients, demonstrating that both humoral and cellular immune responses can be generated and sustained up to 12 months post SARS-CoV-2 infection and vaccination. Notably, failure to establish humoral immunity did not result in severe disease. We also highlight specific T and B cell signatures that could be used as clinical biomarkers to advise patients on timing of SARS-CoV-2 vaccinations.
Keywords: COVID-19 CD20 B cell depletion therapy Multiple Sclerosis Autoimmune
Graphical abstract

1. Introduction
B cells are mediators of the adaptive immune response, differentiate into plasma cells and plasma blasts, and are thus critical for the generation of antibodies and immune memory in response to viral pathogens and vaccine antigens (LeBien and Tedder, 2008). B cell depleting therapies that target CD19+ and CD20+ B cells have emerged over the last decade as a cornerstone treatment approach for rheumatological (Bryl, 2021), neurological (Lee et al., 2021), and oncological diseases (Pierpont et al., 2018). During the coronavirus disease 2019 (COVID-19) pandemic, a major clinical question facing patients on these therapies is whether they can generate a meaningful immune response to the SARS-CoV-2 infection and vaccination compared to the general population.
The main adverse effects of B cell depleting therapies are hypogammaglobulinemia and suboptimal memory responses to infections and vaccinations due to depletion of naïve B cells and impaired generation of new plasma cells and antibodies (Sacco and Abraham, 2018). Given the widespread use of B cell depleting therapies, it is important to understand what impact these therapies have on immunological memory to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) acute infection and vaccination.
Most studies evaluating immune responses in B cell depleted patients have focused either on vaccinated patients or patients exposed to SARS-CoV-2 with limited patient follow up from timing of the initial disease or vaccine exposure. Several studies have demonstrated attenuated or absent antibody levels in patients on B cell depleting therapies post COVID-19 or post SARS-CoV-2 vaccinations (Conte, 2022; Gaitzsch et al., 2021; Lucchini et al., 2020; Thornton and Harel, 2020; Zabalza et al., 2021). However, other studies have found meaningful humoral responses to SARS-CoV-2 infection and vaccinations (Habek et al., 2022; Moser et al., 2022). Thus, it is currently unresolved whether patients on B cell depleting therapies can mount a predictable humoral response post exposure to SARS-CoV-2 infection and vaccinations and which factors may account for the observed heterogeneity in antibody titer responses. Additionally, as B cells can contribute to establishing T cell mediated immune responses (Petersone et al., 2018), it is important to assess whether Bcell depletion influences T cell responses in B cell depleted patients. Some studies have documented preserved T cell immune responses in B cell depleted patients post SARS-CoV-2 infection and vaccination (Apostolidis et al., 2021), though the longevity of these T cell immune responses is not known. It is also unclear what the optimal time window is between B cell therapy administration and booster COVID-19 vaccinations.
We hypothesized that the time since the last B cell treatment and presence of more CD19+ CD20+ B cells that contain naïve B cells would be the driving factor for generation of an antibody response to SARS-CoV-2 infection or vaccination. To test this hypothesis, we recruited a cohort of patients on B cell depleting therapies who had contracted COVID-19 and followed them prospectively for 12 months. Patients were evaluated at multiple time points for Spike Subunit S1 Receptor Binding Domain (RBD) antibody titers, circulating B and T cell frequencies and SARS-CoV-2 spike, nucleocapsid, membrane and envelope protein T cell antigen responses. To our knowledge, we present the longest follow up of B cell depleted patients who had experienced SARS-CoV-2 infection and vaccinations with detailed immunological phenotyping.
2. Methods
2.1. Standard protocol approvals, registrations, and patient consents
Patients positive for COVID-19 by PCR or antigen test on anti-CD20 B cell depleting therapies (COVID-19 B cell depleted) and control subjects (COVID-19 Controls and Healthy Controls, confirmed to be PCR or antigen negative immediately prior to enrollment and tested seronegative for SARS-CoV-2 antibodies) were enrolled between June 2020 and January 2021 at the Dell Medical School Neuroimmunology Center at University of Texas at Austin (UT Austin) and followed over the course of 12 months. Blood samples were collected at early (D8-13), mid (D16-34), and late (D52-225) timepoints after symptom onset, or in the case of asymptomatic patients, days from first SARS-CoV-2 PCR positive test result. Four out of the five B cell depleted patients received two intramuscular doses of the Pfizer/BioNTech or Moderna/Spikevax mRNA vaccines over the duration of the study (Table 1 ).
Table 1.
B cell depleted patient demographics.
| Patient | Sex | Age | Acute COVID-19 Symptoms | COVID-19 Severity | SARS-CoV-2 Vaccination | Autoimmune Disorder | Comorbidities | B Cell Depleting Therapy | Other Medications |
|---|---|---|---|---|---|---|---|---|---|
| B1 | Female | 34 | Fever, Cough, Fatigue, Anorexia, Myalgia, Nasal Congestion, Headache, Nausea, Anosmia, Ageusia, Neck Pain, Night Sweats | Mild | N/A | Multiple Sclerosis | N/A | Rituximab | Naltrexone, Vitamin B12 |
| B2 | Female | 33 | Asymptomatic | Mild | Pfizer | Multiple Sclerosis | N/A | Ocrevus | Topiramate |
| B3 | Female | 25 | Fever, Cough, Anorevia, Sore Throat, Nasal Congestion, Headache, Anosmia | Mild | Pfizer | Multiple Sclerosis | N/A | Ocrevus | Nitrofurantoin |
| B4 | Female | 40 | Fever, Cough, Fatigue, Anorexia, Shortness of breath, Myalgia, Sore Throat, Nasal Congestion, Headache, Diarrhea, Nausea, Vomiting, Anosmia, Ageusia, Reduced Mobility, Muscle Weakness | Mild | Pfizer | Multiple Sclerosis | Hypertension | Ocrevus | Amlodipine, Ferrous Gluconate, Norethindrone Acetate |
| B5 | Female | 44 | Fever, Cough, Fatigue, Myalgia, Sore Throat, Headache, Diarrhea, Nausea, Vomiting, Abdominal Pain, Rash, Chills, Sweats | Hospitalized | Moderna | Lupus, Sjogrens Syndrome | Diabetes, H pylori Gastritis, Fatty Liver | Rituximab | Azithromycin, Enoxaparin, Ceftriazone, Vancomycin, Hydroxychloroquine, Metformin |
Patient data were anonymized for analysis. The study was approved by the UT Austin Institutional Review Board (IRB ID: 2020-04-0117) and was in compliance with the October 2013 Declaration of Helsinki principles. All subjects gave written informed consent in adherence to local and national regulations.
2.2. Antibody testing
The immunoassays for the detection of SARS-CoV-2 S1 RBD IgG antibodies were performed by Babson Diagnostics, Inc. using the Siemens Healthineers Atellica IM sCOVG assay following manufacturer's instruction (Healthineers, 2022). The relative light units (RLU) detected by the analyzer directly correlated with the amount of antibody present in the sample. The validated measuring range for sCOVG at the Babson Diagnostics laboratory is 0.00 to 107.00 Index where an index value ≥1.00 indicated a reactive (Ab positive) result. Samples with a result <1.00 were reported as non-reactive (Ab negative). Reactive samples with an index value >107.00 were reprocessed by performing an additional 1:20 pre-dilution of the sample to provide numeric results up to 2140.00 Index.
2.3. PBMC isolation and cryopreservation
Venous blood was collected in BD heparin tubes (BD 367874) before being pooled into one 50 mL conical tube and diluted 1:1 with PBS + 3% FBS and mixed by inversion. The blood mixture was then layered over 15 mL of Lymphoprep (STEMCELL Technologies 1858) inside a 50 mL SepMate tube (STEMCELL Technologies 85,450), after which the manufacturer's protocol was followed. Once isolated, PBMCs were resuspended in sterile filtered freezing media (45% RPMI-1640, 45% FBS and 10% DMSO) at a minimum of 5 × 106 cells/mL/tube and transferred to cryogenic storage tubes. The tubes were stored at -80°C for 24 h in Mr. Frosty freezing containers with isopropyl alcohol, before being transferred to -150°C storage.
2.4. Unstimulated PBMC flow cytometry
PBMCs were thawed for 4 min at 37 °C and quickly transferred to 9 mL of cold FACS wash buffer (FWB1), comprised of 1x PBS with 2% FBS and 5 mM EDTA. The suspended samples were then spun at 1500 rpm at 4 °C for 8 min. The supernatant was then decanted, and cells were resuspended in 10 mL of cold FWB1, with trypan blue used for counting on a hemocytometer. The suspended samples were then spun at 1500 rpm at 4°C for 8 min, decanted and resuspended at a concentration of 1×107 cells/ml. 3–5 million cells were isolated for Panel A (Supp Table 1), and 1-3 million cells were isolated for Panel B (Supp Table 2). Samples were spun at 1500 rpm at 4°C for 5 min and then decanted for staining. Panel A's B cell binding spike tetramers were formed from biotinylated SARS-CoV-2 spike protein and fluorophore-conjugated streptavidin per the manufacturer's instructions with 5 µL per test for PE and 10 µL per test for BUV395 conjugated tetramers. For Panel B, the CCR7 antibody stain was added to each sample and incubated at 37 °C for 15 min before the rest of the cocktail was added and incubated for an additional 30 min at 4 °C. For Panel A, the antibody cocktail was added to each sample and incubated for 30 min at 4 °C. After incubation, 1 mL cold FWB1 was added to each sample spun at 1500 rpm @ 4°C for 5 min then decanted, with this step being repeated a second time. Cells were briefly vortexed before addition of 100 µL of Cytofix (BD 554655) followed by a 20 min incubation in the dark at 4 °C. After incubation, 1 mL of cold FWB1 was added to each sample then centrifuged at 1800 rpm @ 4C for 5 min, decanted, and resuspended in 250 µL of FWB1. The samples were then run on a Cytek Aurora (Ultraviolet, Violet, Blue, Yellow-green, and Red). Gating schemes for Panel A are shown in Supp Fig. 1 and gating schemes for Panel B are shown in Supp Fig. 2.
2.5. Stimulation assay
PBMCs were thawed at 37 °C and quickly transferred to 9 mL of cold T cell media (Supp Table 3). Cells were centrifuged at 1500 RPM for 7 min at 4 °C. The supernatant was then decanted and the remaining pellet was resuspended in 10 mL of cold media, with Trypan Blue used for counting on a hemocytometer. The cells were then centrifuged at 1500 RPM for 7 min at 4°C, decanted, and resuspended at 1 × 107 cells/mL in media. The cells were then incubated with anti-CD40 antibody (0.5 ng/mL) for 15 min at 37°C/5% CO2. Meanwhile, 1 mg/mL stocks for each stimulation peptide pool (cytomegalovirus (CMV), SARS-CoV-2 spike (S), SARS-CoV-2 membrane (M), SARS-CoV-2 nucleocapsid (N), SARS-CoV-2 envelope (E); kindly provided by Dr. Alessandro Sette and Daniella Weiskopf) were diluted to 2 ug/mL (2X concentration)in medium. 100 µL of each 2X stimulus solution was added to a designated well for each patient sample in a 96-well round-bottom plate. 100 µL of PBMCs treated with CD40 antibody (1.0 × 106 cells/100 µL) was added to the appropriate stimulus solution for a final stimulus peptide concentration of 1 µg/mL. The plate was then incubated for 24 h at 37°C/5% CO2
2.6. Stimulated PBMC flow cytometry
Cells from the stimulation assays were then centrifuged at 1500 RPM for 5 min at 4 °C, decanted, and vortexed. 100 µL of the master mix (Panel C, Supp Table 4) was added to each sample, vortexed, and incubated for 30 min at 4 °C in the dark. After incubation, cells were washed twice with 1 mL cold FWB2, comprised of PBS with 1% fetal bovine serum, and spun at 1500 rpm @ 4 °C for 5 min. The cell as were briefly vortexed before addition of 100 µL of Cytofix (BD 554655) followed by a 20 min incubation in the dark at 4 °C. After incubation, 1 mL of cold FWB2 was added to each sample then centrifuged at 1800 rpm @ 4°C for 5 min, decanted, and resuspended in 250 µL of FWB2. A Cytek Aurora (Ultraviolet, Violet, Blue, Yellow-green, and Red) was used to acquire flow cytometry data. To avoid bias by a single marker of activation, multiple activation markers were used. Gating schemes for Panel C are shown in Supp Fig. 3. The stimulation index was calculated by dividing the frequency of the indicated T cell subset that is positive for the indicated activation markers in the antigen-stimulated versus unstimulated conditions.
2.7. Data analysis
All flow data was analyzed using FlowJo (v10.8.1) with gating strategies shown in Supp Figs. 1–3. Graphing and analyses were conducted using R 4.1.0. For pairwise comparisons, the Wilcoxon rank-sum test was used with Benjamini/Hockberg p-value corrections. The packages scales, lubridate, along with the tidyverse were used for analysis (Grolemund and Wickham, 2011; Hadley Wickham, 2022; Wickham et al., 2019).
2.8. Data availability
Anonymized data used within this article will be made available by request from any qualified investigator.
3. Results
3.1. Study cohort
We enrolled a total of 5 patients on anti-CD20 B cell depleting therapies (rituximab, n = 2 or ocrelizumab, n = 3) who were confirmed by SARS-CoV-2 RT-PCR (Table 1). The control cohort for RBD antibody trajectory analysis consisted of age- and sex-matched patients including 5 healthy non-B cell depleted controls (Healthy Controls, HC), and 132 COVID-19 controls (CC) made up of 15 autoimmune non-B cell depleted COVID-19+ controls, and 117 non-autoimmune non-B cell depleted COVID-19+ controls. Our total cohort consisted of 259 samples from 137 participants.
3.2. SARS-CoV-2 spike antibody plasma titers
We measured SARS-CoV-2 RBD-reactive IgG antibody titers in plasma using Siemens Healthineers Atellica IM sCOVG. Four out of the five B cell depleted patients in our cohort had detectable RBD IgG antibodies at one or more timepoints post-infection (patients B2-B5), as shown in Figs. 1 and 5. B cell depleted patients on average had a lower initial antibody response to SARS CoV-2 (Fig. 1C), though two patients reached antibody titers comparable to COVID-19 controls within the first 30 days post symptom onset.
Fig. 1.
Longitudinal SARS-CoV-2 spike RBD antibody titers in COVID-19 B cell depleted patients. Spike RBD antibody titers over (A) 12 months and (B) first 40 days post symptom onset for COVID-19. (C) Comparison of peak spike RBD antibody titer detected per patient in samples from the first 30 days. The cut-off for a positive antibody response is 1 a.u. (dotted line). For pairwise comparisons the Wilcoxon rank-sum test with Benjamini-Hochberg corrections was used. For asymptomatic patients, days from their first SARS-CoV-2 PCR positive test result were used instead of days from symptom onset.
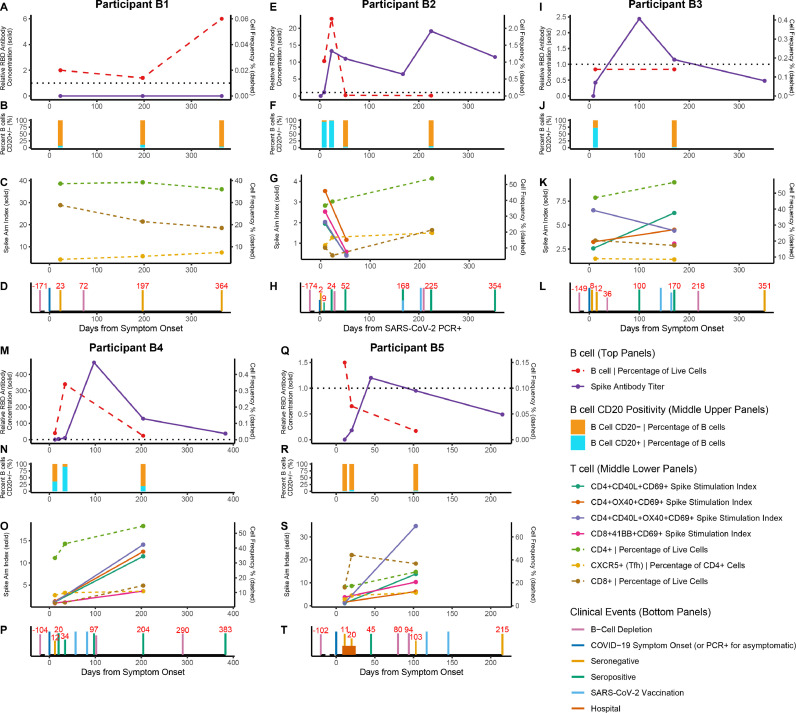
Fig. 5.
B cell depleted COVID-19 immune trajectories over one year. Humoral and cellular response trajectories and clinical event timeline over a course of 12 months in B cell depleted patients. (A, E, I, M, Q) B cell frequency of all PBMCs via flow cytometry (dashed lines, right axis), and spike RBD IgG antibody titer (solid lines, left axis). The black dotted line is at 1 a.u. indicating the positivity threshold for the spike RBD IgG antibody assay. (B, F, J, N, R) Percent of B cells positive for CD20 at each patient visit. (C, G, K, O, S) T cell dynamics across visits with stimulation indices of CD4+ and CD8+ T cells (solid lines, left axis) and frequency of CD4+, CD8+, and Tfh T cells in unstimulated PBMCs (dashed lines, right axis). (D, H, L, P, T) Patient timeline of clinical events including B cell depleting therapy administration, symptom onset, seroconversion, vaccination, and hospitalization. In asymptomatic patient B2, days from their first SARS-CoV-2 PCR positive test result was used instead of days from symptom onset.
Over the course of 12 months post SARS-Co-V-2 infection, the rate of decline of RBD IgG antibodies in B cell depleted patients remained similar to the COVID-19 controls, even after anti-CD20 treatment (Figs. 1 and 5). Antibody titers increased in response to vaccination in two out of four B cell depleted vaccinated patients (Fig. 5). At 12 months, 2 of the 5 B cell depleted patients had sustained positive antibody titers. These results demonstrate that patients on B cell depleting therapies can effectively seroconvert in response to SARS-CoV-2 acute infection and/or vaccinations.
3.3. Peripheral Blood Mononuclear Cell (PBMC) flow cytometry results
In evaluating B cells by flow cytometry, all B cell depleted patients had detectable B cells at the early (day 8–13), mid (day 16–34), or late (day 52–225) timepoints from COVID-19 symptom onset. Across all timepoints combined, B cell depleted patients had a significantly lower frequency of B cells compared to COVID-19 controls and healthy controls (CC p = 7.5e-5, HC p = 3.2e-3) (Fig. 2A ). Due to CD20 B cell depletion, a larger percentage of circulating B cells were CD20–(CC = 7.5e-5, HC = 4.7e-4) across all timepoints combined, although the frequency of this population among live cells was not significantly different (CC p = 0.90, HC p = 0.98) (Fig. 2B and C). Interestingly, a larger proportion of CD20+ B cells, which comprises most B cell subsets, including naïve and transitional B cells, compared to CD19+CD20– plasmablasts/plasma cells, correlated with a positive antibody response to SARS-CoV-2 RBD protein (Fig. 5). Notably, two patients who either did not seroconvert or weakly seroconverted primarily had CD19+CD20– B cells (Fig. 5, Participants B1 and B5) compared to COVID-19 controls who had both CD20+ and CD20– B cells.
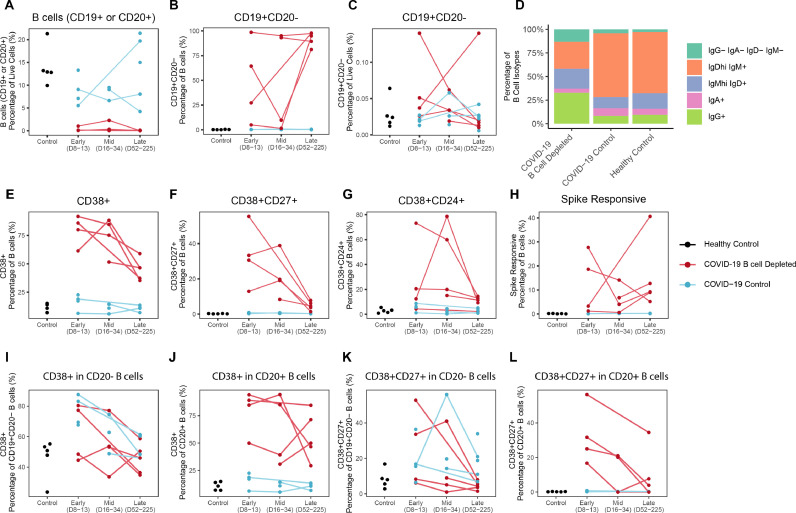
Fig. 2.
Longitudinal characterization of circulating B cells in COVID-19 B cell depleted patients, COVID-19 and healthy controls. Timepoints were grouped into early (days 8-13), mid (days 16-34), and late (days 52-225) in days from symptom onset, or for asymptomatic patient, days from first SARS-CoV-2 PCR positive test. (A) Frequency of B cells in live PBMCs. (B) Frequency of CD19+ CD20- cells in B cells and (C) live PBMCs. (D) Average Isotype frequency of B cells per group. Frequency of (E) CD38+, (F) CD27+CD38+, (G) CD24+CD38+, and (H) spike responsive B cells (CD19+ and/or CD20+). Frequency of (I) CD38+ in CD20- B cells, (J) CD38+ in CD20+ B cells, (K) CD38+CD27+ in CD20- B cells, and (L) CD38+CD27+ in CD20+ B cells.
Across all timepoints combined, B cell depleted patients demonstrated fewer IgDhigh IgM+ B cells (CC p = 8.1e-6, HC p = 1.9e-3) with a trend toward a higher percentage of IgG+ B cells compared to COVID-19 controls (CC p = 2.4e-2, HC p = 0.15) (Fig. 2D). Additionally, circulating B cells displayed significantly elevated expression of CD38 in B cell depleted patients (CC p = 1.2e-6, HC p = 4.7e-4) (Fig. 2E), a marker associated with antibody secretion and activation. The circulating CD38+ B cells differed in CD24+ and CD27+ expression between individuals (Fig. 2F and G), with a higher frequency of both CD38+CD27+ (CC p = 7.3e-5, HC p = 4.7e-4) and CD38+CD24+ (CC p = 4.3e-4, HC p = 5.6e-3) cells across all timepoints in B cell depleted patients (Fig. 2F and G). The percentage of B cells responding to spike was significantly higher in the B cell depleted patients likely due to having an overall lower number of diverse naïve non-Spike responding B cells in circulation as a result of depletion (Fig. 2H, CC p = 7.5e-5, HC p = 4.7e-4).
Interestingly, when evaluating CD38 expression between the CD20– and CD20+ B cell fractions, the CD20+ fraction in the B cell depleted patients had an increased percentage of CD38+ compared to COVID-19 controls across all timepoints (CD20– B cells: CC p = 0.15, HC p = 0.77; CD20+ B cells: CC = 7.4e-5, HC = 3.2e-3) (Fig. 2I and J). This relationship is also true when looking at CD38 and CD27 co-expression, particularly for the early timepoint (CD20– B cells: CC p = 1.00, HC p = 0.66; CD20+ B cells: CC p = 0.057, HC p = 0.048) although the effect weakens with all timepoints combined (CD20– B cells: CC p = 0.077, HC p = 0.85; CD20+ B cells: CC p = 0.30, HC p = 0.30) (Fig. 2K and L).
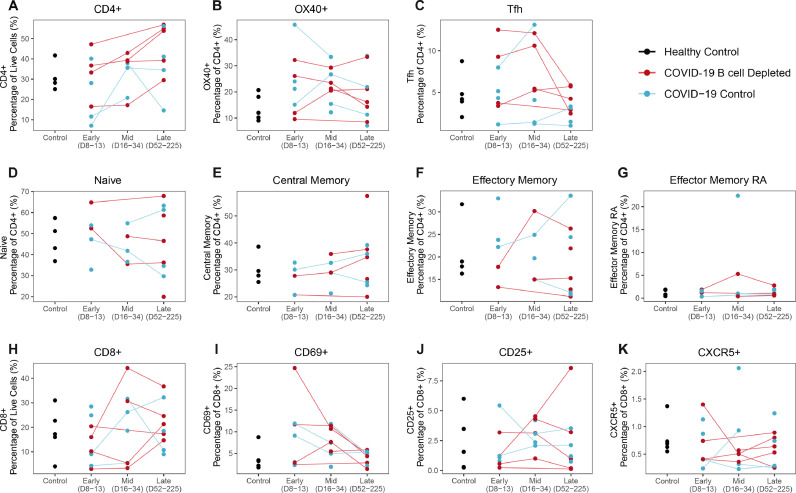
The frequency of CD4+ T cells was slightly elevated in B cell depleted patients (Fig. 3A ), although this difference was not statistically significant. B cell depleted patients had comparable CD8+ T cell frequencies to COVID-19 matched controls across all timepoints (Fig. 3H). CD4+ subsets, including OX40+, Tfh, naïve, central memory (TCM), effector memory (TEM), effector memory CD45RA (TEMRA), and CD8+ T cell subsets, including CD8+ CD69+, CD8+ CD25+, CD8+ CXCR5+ were comparable to the COVID-19 matched controls (Fig. 3B–K). Altogether, immunophenotyping of the T cell compartment did not reveal any statistically significant differences in T cell subsets between B cell depleted patients and controls.
Fig. 3.
Longitudinal characterization of circulating T cells in COVID-19 B cell depleted patients, COVID-19 and healthy controls. Timepoints were grouped into early (days 8-13), mid (days 16-34), and late (days 52-225) in days from symptom onset, or for asymptomatic patients, days from first SARS-CoV-2 PCR positive test. Trajectory plots of CD4+ and CD8+ T cell subset frequencies. (A) Frequency of CD4+ T cells and CD4+ subtypes including (B) OX40+ CD4+ T cells, (C) Tfh CD4+ T cells, (D) Naïve CD4+ T cells, (E) TCM, (F) TEM, (G) TEMRA. (H) Frequency of CD8+ T cells and frequency of subtypes in CD8+ cells including (I) CD69+ CD8+ T cells, (J) CD25+ CD8+ T cells, and (K) CXCR5+ CD8+ T cells.
3.4. T cell responses to SARS-CoV-2 antigens
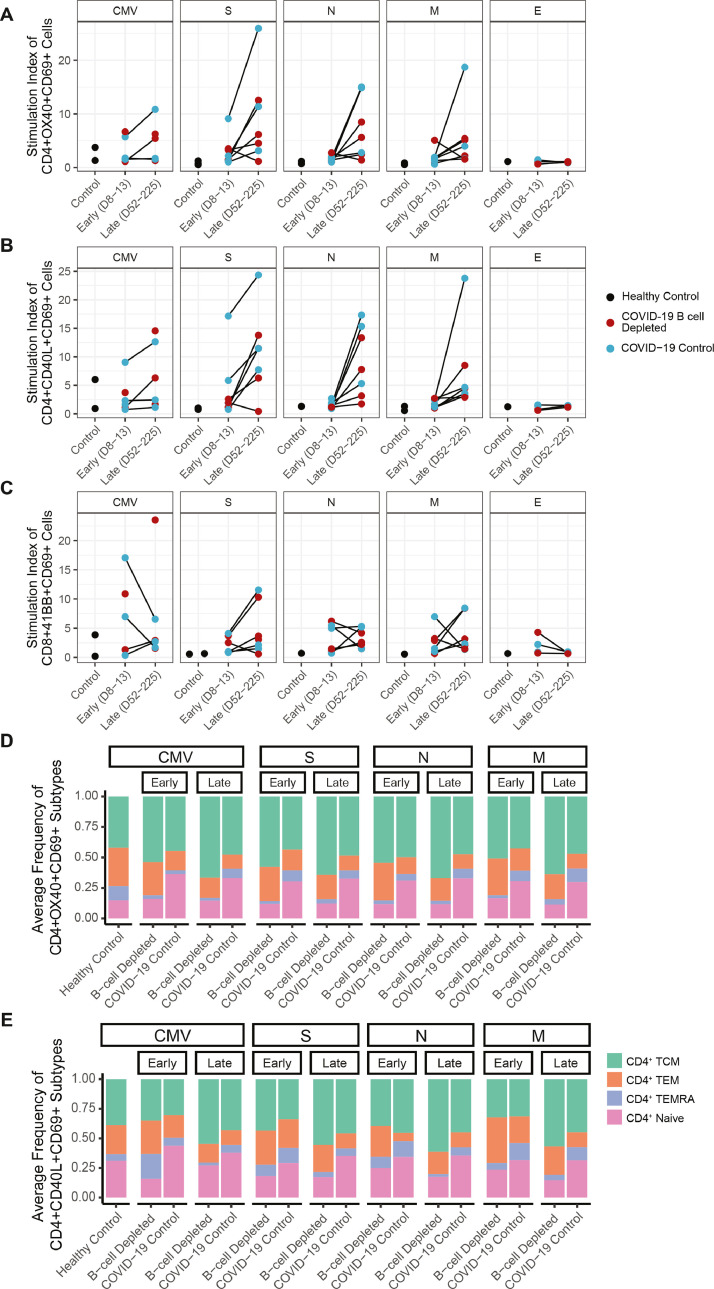
All evaluated patients with COVID-19, regardless of B cell depletion status or seroconversion, mounted an activated T cell response to SARS-CoV-2 antigens, as assessed by in vitro T cell stimulation assays with peptide pools evaluating the SARS-CoV-2 spike (S), nucleocapsid (N), and membrane (M) proteins. After stimulation for 24 h, activated CD4+ T cells were quantified by flow cytometry, based on increased expression of CD40L, CD69 and OX40 (Fig. 4 ). Activation in response to stimulation was primarily observed for CD4+ T cells (Fig. 4A-B), with less robust activation of CD8+ T cells assessed by 41-BB and CD69 (Fig. 4C). Both COVID-19 controls and B cell depleted groups followed a similar pattern of mild reactivity at the early (8–13 days post symptoms onset) timepoint and higher reactivity at the late timepoint (52–225 days post COVID-19 symptom onset), consistent with prior studies (Dan et al., 2021). Envelope protein stimulation showed no reactivity at either timepoint, consistent with other studies (Grifoni et al., 2020) (Fig. 4A–C).
Fig. 4.
COVID-19 T cell stimulation in B cell depleted patients. Stimulation Index to cytomegalovirus (CMV), spike (S), nucleocapsid (N), membrane (M), and envelope (E) megapools for (A) OX40+ CD69+ CD4+ T cells, (B) CD40L+ CD69+ CD4+ T cells, (C) 41BB+CD69+ CD8+ T cells. Average percentages of Naïve, TCM, TEM, and TEMRA for CMV, PMA, S, N, and M megapool stimulation in (D) OX40+ CD69+ CD4+ T cells and (E) CD40L+ CD69+ CD4+ T cells. Timepoints were grouped into early (days 8-13) and late (days 52-225) in days from symptom onset, or for asymptomatic patients, days from first SARS-CoV-2 PCR positive test.
All patients on B cell depleted therapies developed detectable SARS-CoV-2 reactive T cells that differentiated into TEM, TCM, TEMRA subsets (Fig. 4D,E). Interestingly, there was a lower frequency of naïve T cells across all timepoints in B cell depleted patients in response to cytomegalovirus (CMV), spike, nucleocapsid, and membrane protein stimulation compared to COVID-19 controls (Fig. 4D,E).
3.5. Patient immune trajectory timelines
In Fig. 5 , data from RBD antibody testing, PBMC flow cytometry, and SARS-CoV-2 T cell stimulation were compiled for B cell depleted patients demonstrating individualized trajectories of immune responses to both acute SARS-CoV-2 infection and vaccination. All numbered events were scaled to post symptom onset of COVID-19 (day 0, PSO).
Participant B1 did not develop a measurable RBD antibody response and had very low detectable CD20+ B cells. This patient's COVID-19 symptom onset occurred 190 days (6.2 months) post B cell depletion and the patient was not vaccinated over the course of 364 days of follow up PSO.
Participant B2 developed COVID-19 180 days (5.9 months) post B cell depletion. The patient mounted a detectable RBD antibody titer that increased in response to vaccination. This patient had detectable live B cells at the time of acute COVID-19 symptoms, with a majority of CD20+ B cells. Of note, this patient was seronegative on day 2 and seroconverted by day 9 post SARS-CoV-2 PCR, and antibodies persisted even after B cell depletion therapy administration on day 30 post SARS-CoV-2 PCR.
Participant B3 developed COVID-19 149 days (4.9 months) post B cell depletion and displayed a detectable RBD antibody titer in response to COVID-19 infection, which peaked by day 100 PSO, with the patient found to be seronegative by day 351 PSO. The patient was seronegative at days 8 and 12 PSO but was positive by day 100 PSO, despite receiving B cell depletion therapy on day 36 PSO and no vaccination events occurring prior to day 100 PSO. The patient did not have a higher RBD antibody response post vaccinations administered on day 143 and 164 PSO.
Participant B4 developed COVID-19 104 days (3.4 months) post B cell depletion, and remained seronegative on days 12, 20, and 34 PSO. By day 57 PSO, the patient was vaccinated and by day 100 PSO, there was a high titer of antibodies detected, which declined but sustained as positive until at least day 383 PSO. Spike stimulated T cell response was observed at both day 12 and day 204 PSO.
Participant B5 developed COVID-19 107 days (3.5 months) post B cell depletion and remained seronegative on days 9 and 20 PSO. By day 45 PSO, the patient developed a detectable RBD antibody response, which declined such that the patient was seronegative at days 101 and 213 PSO. The patient received B cell depletion therapy on day 80 and 94 PSO, and was vaccinated on day 117 and 145 PSO, but did not develop an increase in spike antibody titer post vaccination. Spike specific T cell response was seen on day 11 PSO and persisted to at least day 103 PSO.
4. Discussion
The goal of this study was to evaluate longitudinal humoral and cellular immune responses in patients on B cell depleting therapies in response to SARS CoV-2 viral infection and vaccination in comparison to non-B cell depleted COVID-19 patients and healthy controls. To our knowledge, we present the longest follow up course of comprehensive immunological trajectories in the B cell depleted patient population post COVID-19 infection and vaccinations.
In our cohort, 80% of patients (4/5, B2-B5) on B cell depleting therapies developed detectable spike RBD antibodies in response to native SARS-CoV-2 infection and/or vaccination. Two of the four patients (B2 and B4) who seroconverted demonstrated sustained RBD antibody titers for up to 12 months post initial SARS-CoV-2 exposure. Patient B1 who failed to develop SARS-CoV-2 antibodies, only had detectable CD19+CD20– B cells at the time of acute COVID-19 disease and was hypogammaglobulenimic. In comparison, all of the patients who developed a RBD antibody response had detectable CD20+ B cells during the acute SARS-CoV-2 infection, though very few CD20+ B cells were necessary for the response. This finding is in line with other recent publications demonstrating that timing from the last B cell depletion and threshold of B cell presence impact generation of antibodies (Apostolidis et al., 2021; König et al., 2021; Mrak et al., 2021; Rimar et al., 2021; Sabatino et al., 2022; Sormani et al., 2021; Tolf et al., 2022). Our results extend prior COVID-19 investigations in B cell depleted patients suggesting that it is the presence of CD20+ B cells in acute disease that is predictive of seroconversion and that CD19+CD20– B cells alone may not be sufficient, consistent with the fact that CD19+CD20– B cells comprise plasmablasts and plasma cells.
Additionally, compared to our control cohort, B cell depleted patients had lower peak spike RBD titer at the sampled timepoints in the first 30 days. This finding suggests that while spike RBD titer can be established in B cell depleted patients, it may not be comparable to the titer in non-immunosuppressed individuals, as demonstrated by patients B3 and B5, and may potentially not be fully protective. Of note, SARS-CoV-2 RBD antibody titer does not reflect the total spike antibody fraction, which is often higher than S1 RBD antibody titer, though the RBD titer better reflects the neutralizing antibody titer (Yuan et al., 2021). Interestingly, patient B5 who developed a very low S1 RBD antibody response to both SARS-CoV-2 infection and vaccination had a diagnosis of lupus and Sjogren's and was on other immunosuppressant therapies in addition to B cell depletion with rituximab. Additionally, this patient had been receiving 1000 mg rituximab twice every 6 months for 5 years, a double dose compared to typical B cell depletion regimens in MS patients (B1-B4), suggesting that higher dose of immunosuppression may influence seroconversion post SARS-CoV-2 infection. Studies to-date have provided disparate results on whether patients on B cell depleting therapies can develop antibodies in response to SARS-CoV-2 infection and/or vaccination. In patients with MS, some studies support variable generation of antibodies on B cell depleting therapies (Moser et al., 2022; Apostolidis et al., 2021; König et al., 2021) while studies in patients with lupus, vasculitis, and lymphoma largely do not demonstrate antibody production (Gaitzsch et al., 2021; Marty et al., 2022; Simon et al., 2022). In context of these studies, the observation of our patient with lupus developing very low RBD antibody titers raises the question of whether the type of autoimmune disorder, higher dosing of B cell therapy, or concomitant immunosuppression with multiple drugs may influence the humoral response to SARS-CoV-2 infection and/or vaccination.
CD20+ B cell repopulation was variable in timing in different patients in our cohort, though CD19+CD20– B cells were found in all B cell depleted patients throughout the evaluated time course at comparable levels to both healthy and COVID-19 controls. Intriguingly, circulating B cells were significantly higher for CD38 and either CD24 or CD27 and trended towards being disproportionately IgG+ with significantly less IgDhigh IgM+ B cells. These B cell markers have previously been associated with transitional (CD27– CD24high CD38high), mature naïve (CD27low CD38high), antigen-experienced (CD27high CD38high), and memory B cells (CD27var CD38−), marginal zone B cells (CD27+IgMhigh IgD+) (Bautista et al., 2020) as well as plasmablasts (CD20− CD27high CD38high) (Palanichamy et al., 2009).
Another interesting observation in our cohort was that patients who developed antibodies in response to SARS-CoV-2 infection and/or vaccination, sustained RBD-specific antibody titers, even after receiving their next dose(s) of B cell depletion, albeit at lower levels than controls. This finding is in line with known downregulation of CD20 on plasma cells and plasmablasts, suggesting that the SARS-CoV-2-specific B cells have differentiated into the latter cell types and are no longer susceptible to CD20 depletion. This finding generalizes to other vaccinations for B cell depleted patients requiring two-three step vaccines (e.g. hepatitis and herpes zoster), suggesting that administration of the second or third step vaccinations could be successfully timed in close proximity following B cell depletion as long as the first vaccination triggered plasmablast (CD19+CD20– B cell) generation.
In terms of T cell immune responses, we observed an increased frequency of SARS-CoV-2 (S, N, and M) reactive T cells in all patients post SARS-CoV-2 infection regardless of B cell depletion or vaccination status. Stimulation with SARS-CoV-2 peptide pools demonstrated that T cells had vigorous antigen recall responses similar to patients who are not on B cell depletion therapies. All patients had responding naïve, TCM, TEM, and TEMRA CD4 T cells, with no deficit in memory subsets in depleted patients, suggesting intact T cell memory conversion. These T cell immune responses were sustained over time for at least 3-10 months post COVID-19. Thus, our data are in line with other studies demonstrating that T cell immunity is not impaired in B cell depleted patients (Apostolidis et al., 2021; Gadani et al., 2021; Habek et al., 2022). Further, our data demonstrate that patients with MS on B cell depleting therapies who do not develop antibodies or have very low titers may still have a mild COVID-19 disease course, suggesting that other arms of the immune system, such as T cells and possibly natural killer cells, are capable of controlling the infection in the absence of a strong antibody response. These findings support the idea that antibody titers are not the only important metric of a successful immune response (Sette and Crotty, 2021).
Ultimately, the question is the relative extent to which antibodies versus cellular immunity are critical to modulating the COVID-19 disease course and preventing future COVID-19 re-infections. In prior studies, lymphoma patients on B cell therapies have long and severe clinical courses (Gaitzsch et al., 2021; Kos et al., 2020). Conversely, patients with MS on B cell depleting therapies typically have a mild COVID-19 disease course (Safavi et al., 2020; Schiavetti et al., 2022; Sormani et al., 2022; Wurm et al., 2020) unless other co-morbidities are present. To better understand which aspects of the immune system contribute to disease severity and re-infections in immunocompromised patients, future studies should compare humoral, cellular, and innate immune responses in large longitudinal cohorts.
Our study has limitations, including a small sample size of B cell depleted patients and a higher number of MS patients compared to patients with other rheumatological and oncological conditions. However, our study focuses for the first time on a longitudinal cohort of B cell depleted patients from a personalized medicine standpoint by performing deep immunological assessment of both the humoral and cellular compartments in patients over the course of 12 months. The results of our study expand the knowledge on immune trajectories post SARS-CoV-2 infection and vaccination and demonstrate that patients on B cell depleting therapies are capable of mounting an effective adaptive immune response in association with a mild clinical disease course.
5. Conclusion
In conclusion, we found that B cell depleted patients contracting COVID-19 are able to develop spike RBD antibody responses, although antibody titers were generally lower and not sustained across patients. A minimal number of CD20+ B cells was necessary to establish spike RBD antibody titer. Interestingly, the majority of circulating B cells post-CD20 depletion were CD38high, suggesting an activated state of B cells. Similar to other studies, we found that T cell responses to SARS-CoV-2 antigens were preserved even if patients did not seroconvert. In summary, our study presents the longest follow up of B cell depleted patients, highlighting specific T-and B cell signatures that can be used in the clinical setting to advise patients on timing of SARS-CoV-2 vaccination.
Study funding
This work was supported by R01AI104870-S1 (L.I.R.E, E.M., T.A.T), Austin Public Health grant 4700 NI210000003 (E.M. and L.I.R.E), NIAAA T32 Training Grant AA007471, funds from Babson Diagnostics (E.M.), and institutional Dell Medical School Startup funding (E.M.). Servers contributed by Advanced Micro Devices (AMD to L.I.R.E.) were used for data analysis.
CRediT authorship contribution statement
Sam A. Bazzi: Writing – original draft, Writing – review & editing, Funding acquisition, Conceptualization, Formal analysis. Cole Maguire: Writing – original draft, Writing – review & editing, Funding acquisition, Conceptualization, Formal analysis. Nisha Holay: Formal analysis. Janelle Geltman: Writing – original draft, Writing – review & editing, Funding acquisition. Kerin Hurley: Writing – original draft, Writing – review & editing, Funding acquisition. Chris DiPasquale: Writing – original draft, Writing – review & editing, Funding acquisition. Melissa Abigania: Funding acquisition. Eric Olson: Writing – original draft, Writing – review & editing, Funding acquisition. Lauren I.R. Ehrlich: Writing – original draft, Writing – review & editing, Funding acquisition, Conceptualization, Formal analysis, Funding acquisition. Todd A. Triplett: Writing – original draft, Writing – review & editing, Funding acquisition, Conceptualization, Formal analysis, Funding acquisition. Esther Melamed: Writing – original draft, Writing – review & editing, Funding acquisition, Conceptualization, Formal analysis, Funding acquisition.
Declaration of Competing Interest
Sam Bazzi: Nothing to disclose Cole Maguire: Nothing to disclose Kerin Hurley: Nothing to disclose Janelle Geltman: Nothing to disclose Lauren Ehrlich: Nothing to disclose Todd Triplett: Funding from OnKure Esther Melamed: has received research funding from Babson Diagnostics, honorarium from Multiple Sclerosis Association of America and has served on advisory boards of Genentech, Horizon, Teva and Viela Bio.
Acknowledgments
We are grateful to Jacob Rogers, Maisey Schuler, Blaine Caslin, Richard Salinas for technical support; Dennis Wylie for statistical guidance; Hilary Selden for assistance with ordering supplies; Erica Brown for phlebotomy assistance; Dr. Ethan Meltzer, Dr. Leorah Freeman, Ashlea Lucas, Sarah Walter, and Hilaire Ridlon for referring patients to this study. We thank Dr. Alessandro Sette and Daniella Weiskopf for providing SARS-CoV-2 peptide pools. We appreciate Dell Medical School Neurology Departments administrative support, by Bethaney Watson, Tran de la Torre, and Karen Rascon.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2022.104195.
Appendix. Supplementary materials
References
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., E Markowitz C., Mexhitaj I., Jacobs D., Babb A., Betts M.R., Prak E.T.L., Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D., Vásquez C., Ayala-Ramírez P., Téllez-Sosa J., Godoy-Lozano E., Martínez-Barnetche J., Franco M., Angel J. Differential expression of IgM and IgD discriminates two subpopulations of human circulating IgM+IgD+CD27+ B cells that differ phenotypically, functionally, and genetically. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryl E. B cells as target for immunotherapy in rheumatic diseases–current status. Immunol. Lett. 2021;236:12–19. doi: 10.1016/j.imlet.2021.05.006. [DOI] [PubMed] [Google Scholar]
- Conte W.L. B cell depleters attenuate the humoral response to SARS-CoV-2 vaccines in multiple sclerosis patients: a case-control study. Mult. Scler. Relat. Disord. 2022;57 doi: 10.1016/j.msard.2021.103413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani S.P., Reyes-Mantilla M., Jank L., Harris S., Douglas M., Smith M.D., Calabresi P.A., Mowry E.M., Fitzgerald K.C., Bhargava P. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitzsch E., Passerini V., Khatamzas E., Strobl C.D., Muenchhoff M., Scherer C., Osterman A., Heide M., Reischer A., Subklewe M. COVID-19 in patients receiving CD20-depleting immunochemotherapy for B cell lymphoma. Hemasphere. 2021;5(7) doi: 10.1097/HS9.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitzsch E., Passerini V., Khatamzas E., Strobl C.D., Muenchhoff M., Scherer C., Osterman A., Heide M., Reischer A., Subklewe M., Leutbecher A., Tast B., Ruhle A., Weiglein T., Stecher S.S., Stemmler H.J., Dreyling M., Girl P., Georgi E., Wolfel R., Mateyka L., D'Ippolito E., Schober K., Busch D.H., Kager J., Spinner C.D., Treiber M., Rasch S., Lahmer T., Iakoubov R., Schneider J., Protzer U., Winter C., Ruland J., Quante M., Keppler O.T., von Bergwelt-Baildon M., Hellmuth J., Weigert O. COVID-19 in patients receiving CD20-depleting Immunochemotherapy for B cell Lymphoma. Hemasphere. 2021;5(7):e603. doi: 10.1097/HS9.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., Marrama D., de Silva A.M., Frazier A., Carlin A.F., Greenbaum J.A., Peters B., Krammer F., Smith D.M., Crotty S., Sette A. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolemund G., Wickham H. Dates and times made easy with lubridate. J Stat Softw. 2011;40(3):1–25. [Google Scholar]
- Habek M., Zeljko C., Savic Mlakar A., Bendelja K., Rogic D., Adamec I., Barun B., Gabelic T., Krbot Skoric M. Humoral and cellular immunity in convalescent and vaccinated COVID-19 people with multiple sclerosis: effects of disease modifying therapies. Mult. Scler. Relat. Disord. 2022;59 doi: 10.1016/j.msard.2022.103682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley Wickham, D.S., Scales: scale functions for visualization. (Version 1.1.1) (2022).
- Healthineers, S., SARS-CoV-2 IgG (sCOVG): assay for the detection of IgG antibodies to SARS-CoV-2 (2022).
- König M., Lorentzen Å.R., Torgauten H.M., Schikora-Rustad S., Vaage E.B., Mygland Å., Wergeland S., Aarseth J., Aaberge I.A.S., Torkildsen Ø. Humoral immunity to SARS-CoV-2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J. Neurol. Neurosurg. Psychiatry. 2021 doi: 10.1136/jnnp-2021-327612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos I., Balensiefer B., Roth S., Ahlgrimm M., Sester M., Schmidt T., Thurner L., Bewarder M., Bals R., Lammert F., Stilgenbauer S., Kaddu-Mulindwa D. Prolonged course of COVID-19-associated pneumonia in a B cell depleted patient after rituximab. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBien T.W., Tedder T.F. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.S., Rojas O.L., Gommerman J.L. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat. Rev. Drug Discovery. 2021;20(3):179–199. doi: 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini M., Bianco A., Del Giacomo P., De Fino C., Nociti V., Mirabella M. Is serological response to SARS-CoV-2 preserved in MS patients on ocrelizumab treatment? A case report. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102323. 102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty P.K., Van Keulen V.P., Erskine C.L., Shah M., Hummel A., Stachowitz M., Fatis S., Granger D., Block M.S., Duarte-García A., Warrington K.J., Theel E.S., Zhou X., Zeng H., Specks U., Escalante P., Peikert T. Antigen specific humoral and cellular immunity following SARS-CoV-2 vaccination in ANCA-associated vasculitis patients receiving B cell depleting therapy. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.834981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T., Otto F., O’Sullivan C., Hitzl W., Pilz G., Harrer A., Trinka E., Wipfler P. Recall response to COVID-19 antigen is preserved in people with multiple sclerosis on anti-CD20 medications - a pilot study. Mult. Scler. Relat. Disord. 2022;59 doi: 10.1016/j.msard.2022.103560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T., Otto F., O’Sullivan C., Hitzl W., Pilz G., Harrer A., Trinka E., Wipfler P. Recall response to COVID-19 antigen is preserved in people with multiple sclerosis on anti-CD20 medications – a pilot study. Mult. Scler. Relat. Disord. 2022;59 doi: 10.1016/j.msard.2022.103560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak D., Tobudic S., Koblischke M., Graninger M., Radner H., Sieghart D., Hofer P., Perkmann T., Haslacher H., Thalhammer R., Winkler S., Bluml S., Stiasny K., Aberle J.H., Smolen J.S., Heinz L.X., Aletaha D., Bonelli M. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T cell-mediated immunity. Ann. Rheum. Dis. 2021;80(10):1345–1350. doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- Palanichamy A., Barnard J., Zheng B., Owen T., Quach T., Wei C., Looney R.J., Sanz I., Anolik J.H. Novel human transitional B cell populations revealed by B cell depletion therapy. J. Immunol. 2009;182(10):5982–5993. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersone L., Edner N.M., Ovcinnikovs V., Heuts F., Ross E.M., Ntavli E., Wang C.J., Walker L.S.K. T Cell/B Cell collaboration and autoimmunity: an intimate relationship. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont T.M., Limper C.B., Richards K.L. Past, present, and future of rituximab—the world's first oncology monoclonal antibody therapy. Front. Oncol. 2018;8:163. doi: 10.3389/fonc.2018.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimar D., Slobodin G., Paz A., Henig I., Zuckerman T. SARS-COV-2 vaccination after stem cell transplantation for scleroderma. Ann. Rheum. Dis. 2021;80(10):1354–1355. doi: 10.1136/annrheumdis-2021-220677. [DOI] [PubMed] [Google Scholar]
- Sabatino Jr J.J., Mittl K., Rowles W.M., McPolin K., Rajan J.V., Laurie M.T., Zamecnik C.R., Dandekar R., Alvarenga B.D., Loudermilk R.P. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine–induced antibody and T cell immunity and function. JCI Insight. 2022;7(4) doi: 10.1172/jci.insight.156978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco K.A., Abraham R.S. Consequences of B cell-depleting therapy: hypogammaglobulinemia and impaired B cell reconstitution. Immunotherapy. 2018;10(8):713–728. doi: 10.2217/imt-2017-0178. [DOI] [PubMed] [Google Scholar]
- Safavi F., Nourbakhsh B., Azimi A.R. B cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult. Scler. Relat. Disord. 2020;43 doi: 10.1016/j.msard.2020.102195. 102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavetti I., Cordioli C., Stromillo M.L., Teresa Ferrò M., Laroni A., Cocco E., Cola G., Pasquali L., Rilla M.T., Signoriello E., Iodice R., Di Sapio A., Lanzillo R., Caleri F., Annovazzi P., Conte A., Liberatore G., Ruscica F., Docimo R., Bonavita S., Ulivelli M., Cavalla P., Patti F., Ferraro D., Clerico M., Immovilli P., Di Filippo M., Salvetti M., Sormani M.P. Breakthrough SARS-CoV-2 infections in MS patients on disease-modifying therapies. Mult. Scler. 2022 doi: 10.1177/13524585221102918. [DOI] [PubMed] [Google Scholar]
- Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Tascilar K., Schmidt K., Manger B., Weckwerth L., Sokolova M., Bucci L., Fagni F., Manger K., Schuch F. Humoral and cellular immune responses to SARS–CoV-2 infection and vaccination in autoimmune disease patients with B cell depletion. Arthritis Rheumatol. 2022;74(1):33–37. doi: 10.1002/art.41914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M., Musc-19 Study G. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Inglese M., Carmisciano L., Laroni A., Lapucci C., Visconti V., Serrati C., Gandoglia I., Tassinari T. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. EBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J.R., Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in Two MS patients treated with ocrelizumab. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolf A., Wiberg A., Müller M., Nazir F.H., Pavlovic I., Laurén I., Mangsbo S., Burman J. Factors associated with serological response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with rituximab. JAMA Netw. Open. 2022;5(5) doi: 10.1001/jamanetworkopen.2022.11497. e2211497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H., Averick M., Bryan J., Chang W., McGowan L., François R., Grolemund G., Hayes A., Henry L., Hester J., Kuhn M., Pedersen T., Miller E., Bache S., Müller K., Ooms J., Robinson D., Seidel D., Spinu V., Takahashi K., Vaughan D., Wilke C., Woo K., Yutani H. Welcome to the Tidyverse. J. Open Source Softw. 2019;4(43) [Google Scholar]
- Wurm H., Attfield K., Iversen A.K., Gold R., Fugger L., Haghikia A. Recovery from COVID-19 in a B cell-depleted multiple sclerosis patient. Mult. Scler. 2020;26(10):1261–1264. doi: 10.1177/1352458520943791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Liu H., Wu N.C., Wilson I.A. Recognition of the SARS-CoV-2 receptor binding domain by neutralizing antibodies. Biochem. Biophys. Res. Commun. 2021;538:192–203. doi: 10.1016/j.bbrc.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalza A., Cárdenas-Robledo S., Tagliani P., Arrambide G., Otero-Romero S., Carbonell-Mirabent P., Rodriguez-Barranco M., Rodríguez-Acevedo B., Restrepo Vera J.L., Resina-Salles M. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2021;28(10):3384–3395. doi: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data used within this article will be made available by request from any qualified investigator.