Abstract
Background
SARS-CoV-2 has evolved, leading to the emergence of new Variants Of Concern (VOCs) with significant impact on transmissibility. Although the transmission process is complex, higher nasopharyngeal viral load (NP-VL) can be considered as a proxy for greater transmissibility.
Objectives
The aim of this analysis was to compare NP-VL across a set of representative VOCs observed in mildly symptomatic patients.
Study design
Observational single-center comparative analysis of patients with early mild-to-moderate COVID-19, enrolled within the early treatment access program of Lazzaro Spallanzani Institute (March 2021-March 2022). NP-VL before drug administration was estimated through RT-PCR, based on cycle threshold values (CTs); VOCs were identified by Sanger sequencing. VOCs’ average treatment effect (ATE) was estimated on the CTs fitted in the log2 scale, controlling for potential confounders.
Results
A total of 707 patients were included. VOCs were: 10% Alpha, 3% Gamma, 34% Delta, 34% BA.1, 19% BA.2. Mean CTs for BA.1 and BA.2 were lower than Delta and BA.1, respectively. After adjusting for calendar time, age, immunodeficiency and vaccination, CTs for Gamma were lower than those seen for Alpha and higher than Delta, for Delta were similar to BA.1, for BA.2 were lower than Delta and BA.1.
Conclusions
Our analysis shows higher NP-VL of BA.2 compared to previously circulating VOCs, even after controlling for factors potentially contributing to the amount of nasopharyngeal viral RNA, included vaccination, supporting the increased transmissibility of BA.2. Further studies are necessary to clarify this mechanism and to provide guidance for public health measures.
Keywords: SARS-CoV-2, Variants of concern (VOCs), Nasopharyngeal viral load, Cycle threshold (CT) values, Increased transmissibility, Omicron BA.2
1. Background
The ongoing evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to the emergence of new viral variants, named Variants of Concern (VOCs) in case of significant impact on transmissibility, disease severity, immunity and/or susceptibility to available therapies and vaccines, likely to contribute to an increased risk for global public health [1,2]. As of November 2021, the time of first appearance in South Africa, the SARS-CoV-2 Omicron (B.1.1.529) variant, its sub-lineages and descendent lineages, have become the predominant VOCs circulating worldwide. Focusing on BA.2, it replaced BA.1 as the major circulating strain in a few weeks, due to its growth advantage, related to mechanisms of enhanced immunologic evasiveness or inherent increased transmissibility [3], [4], [5], [6], [7], [8].
Although the transmission process is complex [9], higher viral load (VL) in nasopharyngeal swabs (NPS) can be considered one of the main factors influencing the infectiousness of patients affected by Coronavirus disease 2019 (COVID-19) [9], [10], [11], [12]. Higher VLs have been described around the time of symptoms’ onset, in case of severe disease, in patients with advanced age and for certain VOCs [10,[13], [14], [15], [16]], while the effect of vaccination on viral shedding and transmission remains controversial [3,5,17]. At present, the rapid spread of Omicron BA.1 has been mainly attributed to immune escape mechanisms as several studies have shown reduced vaccine protection against infection but no evidence for higher VL [4,5,[18], [19], [20], [21]]; for BA.2 similar evidence is limited [3,4,6,22].
2. Objectives
We hereby aimed to compare VL measured on NPS samples of a cohort of mildly symptomatic individuals, receiving early treatment in the outpatients setting, who were infected with a set of representative VOCs, including Omicron sub-lineages BA.1 and BA.2. Moreover, we also compared a number of participants’ clinical, demographic and laboratory characteristics, according to the identified VOC.
3. Study design
3.1. Study procedures and ethics
This comparative analysis uses the data of a prospective observational study on the effectiveness of early treatment with monoclonal antibodies or antiviral agents for outpatients with mild-to-moderate COVID-19. The study was approved by the Scientific Committee of the Italian Medicines Agency (AIFA) and by the Ethical Committee of the National Institute for Infectious Diseases “Lazzaro Spallanzani” in Rome, Italy, as National Review Board for COVID-19 pandemic in Italy (approval number 380/2021). All consecutive patients presenting at our Institute to be evaluated for early treatment, from March 23rd, 2021 to March 15th, 2022, were enrolled after having signed the informed consent. For this analysis, we considered the first scheduled visit for each participant, which included medical examination, vital signs’ recording, self-reported symptoms’ evaluation, laboratory testing and drug administration. All the data were anonymously collected into an electronic database.
Semi-quantitative estimation of the VL in NPS was assessed by Real-Time Polymerase Chain Reaction (RT-PCR), based on cycle threshold (CT) values. According to the laboratory workflow and testing availability during the COVID-19 pandemic, DiaSorin Simplexa® COVID-19 Direct platform (DiaSorin, Saluggia, Italy) was used during the first period, including Alpha, Gamma and the most part of Delta variant infections, while Abbott Alinity m RealTime System (Abbott Laboratories, Wiesbaden, Germany), more recently, including all the Omicron and the remaining part of Delta variant detections. The results of the Delta analyses were splitted accordingly, in order to compare only results obtained using the same platform. Identification of SARS-CoV-2 variants was conducted by Sanger sequencing of the Spike coding gene.
Data on vaccination were extracted from the regional register (Anagrafe Vaccinale Regione Lazio) and, if not available, we collected self-reported vaccination status from clinical charts. Considering the minimum interval of at least 120 days between the completion of the primary vaccine schedule and the booster dose, participants were defined: 1.unvaccinated, if they did not receive any vaccine dose, or they received only the first dose of a 2-dose series less than 14 days earlier; 2.partially vaccinated, if they received only the first dose of a 2-dose series more than 14 days earlier, or if they completed the vaccine schedule less than 14 days earlier; 3.recent fully vaccinated or boosted, if they completed the vaccine schedule between 14 and 120 days earlier, or if they received the booster dose; 4.waned fully vaccinated or unboosted, if they completed the vaccine schedule more than 120 days earlier, without having received the booster dose.
Symptoms’ severity was assessed by a self-reported questionnaire. The total symptom score (range, 0-39) was achieved by rating 13 symptoms (cough, sore throat, nasal congestion, rhinorrhea, shortness of breath, body aches and pain, fatigue, feeling feverish, chills, headache, nausea/ lack of appetite, vomiting, diarrhea) from none/absent to mild, moderate or severe (scored from 0 to 3) and combining them to provide an overall score.
All procedures contributing to the work described comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
3.2. Statistical analysis
Descriptive characteristics were provided using medians and interquartile ranges (IQR) for continuous variables, and frequencies and percentages for categorical variables, and were compared by VOCs using Mann-Whitney (continuous variables) or Chi-square (categorical variables) test.
For the crude mean comparisons and regression analyses, CT values were fitted in the log2 scale; we adopted the log transformation because the distribution of CT values in the raw scale was positively skewed and significantly deviating from the normal distribution.
In the unadjusted analysis, means of log2 CT values were compared by VOCs, through Kruskal-Wallis test with P-values adjusted using the Dunn's multiple comparison method. We emulated a number of distinct 2-arms parallel trials in which the specific VOC was the exposure/intervention of interest and log2 CT the primary outcome. The average causal effect of VOCs was estimated using marginal models in which, to control for the effects of the confounding variables, we modelled both the exposure (through inverse probability weighting) and the outcome (via regression) or both (doubly robust). When there was a discrepancy between results, we have used the Doubly Robust results as they are valid against a mis-classification of one of the two models. According to our assumptions regarding the underlying causal links between measured factors (Fig. 1 ), we identified the following key time-fixed confounders: age, vaccination status, calendar time of study entry and immunodeficiency, all measured at the time of drug administration. In addition, in order to explore the association between vaccination and log2 CT, we compared mean values by vaccination categories and evaluated the strength of the association by means of ANOVA (Fisher and Holm-Šídák tests). Finally, because our results are valid only under the strong assumption of no unmeasured confounding being present, we also calculated an e-value using the online calculator devised by VanderWeele and Ding [23].
Fig. 1.
Key assumptions regarding the underlying causal link between measured factors. According to our assumptions, age, vaccination status, calendar time of study entry and immunodeficiency at time of drug administration were identified as main confounders of our comparison of interest. VOC, variant of concern; CT, cycle threshold values.
Even though it was a homogeneous cohort of mildly symptomatic individuals, in the subset of participants for whom the symptom score could be calculated, we investigated whether the difference in CT values by VOC might vary by severity of symptoms. Using the median value of the scores, we classified participants in two groups (asymptomatic or mild symptomatic versus more symptomatic) and we tested effect measure modification by symptoms’ severity from formally fitting an interaction term in the linear regression models.
A statistically significant difference in the variables tested was indicated as P value <.05 (two-sided). Statistical analysis was performed using SAS version 9.4 (Carey North Carolina, USA).
4. Results
A total of 707 patients were included and identified VOCs were distributed as follows: 67 (10%) Alpha (B.1.1.7), 24 (3%) Gamma (P.1), 240 (34%) Delta (B.1.617.2), 242 (34%) BA.1, 134 (19%) BA.2. Main characteristics of the study population and comparisons by VOCs, are reported in Table 1 . Briefly, the median (IQR) age was 65 (54,75) years, 331 (47%) were female and the median distance from symptoms’ onset was 4 (3,5) days. Several characteristics differed across the subgroups. In particular, compared to Omicron sub-lineages, patients identified with Alpha, Gamma and Delta showed more alterations in inflammatory parameters, in terms of lower lymphocytes cell count and higher ferritin and, when compared to BA.1, C-reactive protein levels (P < .001 for all); moreover, in case of earlier circulating VOCs, we observed lower peripheral oxygen saturation (P < .001), and a higher proportion of patients with fever (P = .029). No evidence for a difference in mean symptom score was observed (P = .504).
Table 1.
Main characteristics of the study population and comparison by Variant of Concern.
| Characteristics | Variant of Concern | ||||||
|---|---|---|---|---|---|---|---|
| Alpha | Gamma | Delta | Omicron BA.1 | Omicron BA.2 | PValue* | Total | |
| N= 67 | N= 24 | N= 240 | N= 242 | N= 134 | N= 707 | ||
| Gender, n (%) | |||||||
| Female | 29 (43.3%) | 12 (50.0%) | 112 (46.7%) | 116 (47.9%) | 62 (46.3%) | .966 | 331 (46.8%) |
| Age, years | |||||||
| Median (IQR) | 64 (56, 72) | 73 (55, 80) | 62 (52, 73) | 65 (51, 77) | 70 (61, 78) | <.001 | 65 (54, 75) |
| Older than 65, n (%) | 30 (44.8%) | 14 (58.3%) | 98 (40.8%) | 120 (49.6%) | 87 (64.9%) | <.001 | 349 (49.4%) |
| Days from symptoms onset | |||||||
| Median (IQR) | 4 (3, 6) | 4 (4, 6) | 5 (3, 6) | 4 (3, 5) | 3 (2, 4) | <.001 | 4 (3, 5) |
| Comorbidities/risk factors, n (%) | |||||||
| Diabetes | 24 (35.8%) | 9 (37.5%) | 48 (20.0%) | 54 (22.3%) | 16 (11.9%) | <.001 | 151 (21.4%) |
| Obesity (BMI>30) | 36 (53.7%) | 14 (58.3%) | 127 (52.9%) | 141 (58.3%) | 75 (56.0%) | .814 | 393 (55.6%) |
| Cardiovascular disease | 11 (16.4%) | 3 (12.5%) | 33 (13.8%) | 41 (17.1%) | 28 (20.9%) | .472 | 116 (16.5%) |
| Chronic respiratory disease | 2 (3.0%) | 0 (0.0%) | 5 (2.1%) | 12 (5.0%) | 8 (6.0%) | .232 | 27 (3.8%) |
| Renal impairment | 4 (6.0%) | 0 (0.0%) | 6 (2.5%) | 5 (2.1%) | 2 (1.5%) | .307 | 17 (2.4%) |
| Hepatic Disease | 11 (16.4%) | 8 (33.3%) | 34 (14.2%) | 46 (19.0%) | 29 (21.6%) | .105 | 128 (18.1%) |
| Cancer | 7 (10.4%) | 3 (12.5%) | 35 (14.6%) | 41 (17.1%) | 26 (19.4%) | .485 | 112 (15.9%) |
| Primary/secondary immunodeficiency | 7 (10.4%) | 6 (25.0%) | 23 (9.6%) | 39 (16.1%) | 24 (17.9%) | .048 | 99 (14.0%) |
| Neurologic disease | 3 (4.5%) | 1 (4.2%) | 8 (3.3%) | 13 (5.4%) | 3 (2.2%) | .617 | 28 (4.0%) |
| Vital signs at baseline | |||||||
| SpO2, median (IQR) | 97 (95, 98) | 96 (95, 97) | 97 (96, 98) | 98 (97, 99) | 98 (97, 99) | <.001 | 98 (97, 98) |
| Fever (>37.5°C), n (%) | 6 (9.0%) | 1 (4.2%) | 16 (6.8%) | 4 (1.7%) | 4 (3.1%) | .029 | 31 (4.5%) |
| Laboratory values, median (IQR) | |||||||
| Ferritin, ng/ml | 207.0 (97,360) | 215.5 (140.5,398.5) | 163.0 (72,272) | 89.50 (48,176.5) | 106.0 (56,181) | <.001 | 129.0 (60,235.5) |
| C-reactive protein, mg/dl | 1.40 (0.50,2.98) | 2.12 (0.87,5.43) | 1.22 (0.51,3.03) | 0.76 (0.30,1.50) | 1.51 (0.44,2.56) | <.001 | 1.08 (0.40,2.51) |
| Lymphocytes, cells/uL | 1260 (890,1540) | 1020 (650,1340) | 1140 (840, 1510) | 1495 (1090,2040) | 1350 (950,1650) | <.001 | 1280 (910,1730) |
| Baseline SARS-CoV-2 Serology, n (%) | .030 | ||||||
| Anti-N positive | 0 (0.0%) | 0 (0.0%) | 6 (2.5%) | 7 (2.9%) | 0 (0.0%) | 13 (1.8%) | |
| Anti-S Positive | 22 (32.8%) | 6 (25.0%) | 161 (67.1%) | 195 (80.6%) | 118 (88.1%) | 502 (71.0%) | |
| Negative | 0 (0.0%) | 0 (0.0%) | 47 (19.6%) | 37 (15.3%) | 14 (10.4%) | 98 (13.9%) | |
| Unknown | 45 (67.2%) | 18 (75.0%) | 26 (10.8%) | 3 (1.24%) | 2 (1.5%) | 94 (13.3%) | |
| Vaccination, n (%) | <.001 | ||||||
| Unvaccinated | 8 (11.9%) | 3 (12.5%) | 42 (17.5%) | 20 (8.3%) | 8 (6.0%) | 81 (11.5%) | |
| Partially vaccinated | 20 (29.9%) | 6 (25.0%) | 24 (10%) | 7 (2.9%) | 1 (0.7%) | 58 (8.2%) | |
| Recent fully/boosted vaccinated | 39 (58.2%) | 15 (62.5%) | 79 (32.9%) | 149 (61.6%) | 82 (61.2%) | 364 (51.5%) | |
| Waned fully/unboosted vaccinated | 0 (0.0%) | 0 (0.0%) | 91 (37.9%) | 65 (26.8%) | 43 (32.1%) | 199 (28.1%) | |
| Unknown | 0 (0.0%) | 0 (0.0%) | 4 (1.7%) | 1 (0.4%) | 0 (0.0%) | 5 (0.7%) | |
| Vaccine type, n (%) | .009 | ||||||
| BNT162b2 | 43 (72.9%) | 15 (71.4%) | 127 (66.5%) | 159 (73.3%) | 89 (71.2%) | 433 (70.6%) | |
| mRNA-1273 | 14 (23.7%) | 6 (28.6%) | 27 (14.1%) | 35 (16.1%) | 22 (17.6%) | 104 (17.0%) | |
| ChAdOx1 | 2 (3.4%) | 0 (0.0%) | 27 (14.1%) | 23 (10.6%) | 14 (11.2%) | 66 (10.8%) | |
| Ad26.COV2.S | 0 (0.0%) | 0 (0.0%) | 10 (5.2%) | 0 (0.0%) | 0 (0.0%) | 10 (1.6%) | |
| Other/unknown | 8 (11.9%) | 3 (12.5%) | 49 (20.4%) | 25 (10.3%) | 9 (6.7%) | 94 (13.3%) | |
| Baseline CT | |||||||
| Mean ± SD | 19.64 ± 4.19 | 17.51 ± 3.67 | 19.39 ± 4.41 | 19.01 ± 4.00 | 16.06 ± 3.03 | <.001 | 18.59 ± 4.18 |
| Less than 25, n (%) | 60 (89.6%) | 24 (100.0%) | 213 (88.8%) | 220 (90.9%) | 133 (99.3%) | .003 | 650 (91.9%) |
| MASS score, median (IQR) | 2 (1, 4) | 2 (1, 5) | 2 (0, 3) | 3 (1, 5) | 3 (2, 5) | <.001 | 3 (1, 4) |
| Baseline symptom score, median (IQR) | 10 (6, 15) | 12 (9, 15) | 9 (5, 15) | 10 (6, 16) | 10 (5, 15) | .501 | 10 (5, 15) |
Abbreviations: n, number of participants in group; IQR, interquartile range; BMI, body mass index; SpO2, peripheral oxygen saturation; CT, cycle threshold values; SD, standard deviation; MASS, Monoclonal Antibodies Screening Score.
Chi-square or Mann-Whitney test, as appropriate.
In unadjusted analysis, mean log2 CT values were lower for BA.1 than Delta P = .031), and for BA.2 than Delta and BA.1 (P < .001); no differences were observed by comparing Alpha, Gamma and Delta VOCs (Fig. 2 ). Assuming a linear model with no interactions, including calendar time of study entry, age, immunodeficiency and vaccination as covariates, we found several significant differences in the average potential log2 CT outcome values indicating a gradient in increased VL by more recent circulating VOCs: BA.2 showed lower values than both BA.1 [Average Treatment Effect - ATE (95% Confidence Interval - CI) 0.16 (0.09;0.24), P < .001] and Delta [ATE -0.30 (95%CI -0.53;-0.07), P < .001] which, in turn, showed lower values than Alpha [ATE 0.28 (95%CI -0.10;0.66), P < .001] and especially when compared to Gamma [ATE 1.07 (95%CI 0.00;2.14), P < .001]. Interestingly, the difference between Alpha and Delta was marked in the subset of participants who were more symptomatic (symptoms score >11) with an IPW ATE of 0.16 (95%CI 0.02;0.29), while tended to go in the opposite direction in those with no or mild symptoms (score ≤11): -0.08 (95%CI -0.19;0.03), interaction P = .006). We did not detect any other significant interactions between VOC and symptoms’ severity.
Fig. 2.
Dot-plots showing means and Standard Deviation (SD) of nasopharyngeal viral RNA levels detected in (a) patients with Alpha, Gamma and Delta (DiaSorin assay) variants infection; in (b) patients with Delta (Abbott Alinity assay), Omicron BA.1 and Omicron BA.2 variants infection. Viral RNA levels are expressed as log2 Cycle Threshold (CT) values. Means in log2 CT values and SD are shown. Statistical analysis of the comparisons between variants of concern (VOCs) was performed by Kruskal-Wallis test and specific contrasts PValue corrected by Dunn's method for multiple comparisons. Horizontal dashed line represents the limit of detection (CT=40.0), CT values ≥40 are considered negative. n, number of participants in group.
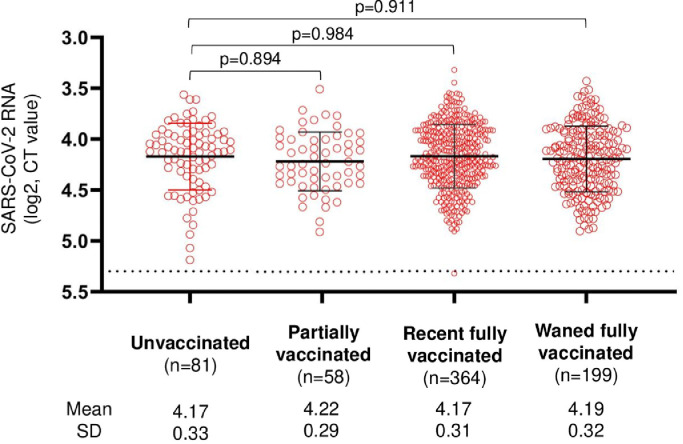
Gamma showed lower values when compared with Alpha [ATE 0.22 (95%CI 0.06;0.38), P = .002]. Under our model specifications, we did not find any evidence for a difference, when we compared log2 CT values for BA.1 versus Delta VOCs (Table 2 a, b) and the main confounder explaining this finding was the calendar time of study entry (data not shown). In order to evaluate the robustness of our results against unmeasured confounding, we calculated an e-value for standardised mean differences. The smallest ATE with a significant P value was of 0.16 log2 CT (standard error 0.035). On the basis of this value, the calculator provides an e-value of 1.58 for the point estimate and 1.39 for the lower limit of the 95%CI. Finally, as reported in the unadjusted analysis in Fig. 3 , there was no evidence for a difference in mean log2 CT values by vaccination status: 0.02 (95%CI -0.08;0.12, P = .688) for partially vaccinated participants, 0.04 (95%CI -0.03;0.11, P = .324) for fully vaccinated ones and 0.06 (95%CI -0.23;0.14, P = .163) in case of unboosted vaccination.
Table 2.
Potential Outcomes and ATE from fitting linear regression models, considering nasopharyngeal viral load detected in (a) patients with Alpha versus Gamma versus Delta (DiaSorin assay) variants infection; in (b) patients with Delta (Abbott Alinity assay) versus Omicron BA.1 versus Omicron BA.2 variants infection. Viral RNA levels are expressed as log2 CT values.
| a) | Potential CT values and ATE from fitting a linear regression model (log2 scale) | |||
|---|---|---|---|---|
| Mean (log2) in VOC 1 i(95% CI) | Mean (log2)iin VOC 2 i(95% CI) | ATE* (95% CI) | P Value | |
| Alpha vs Gamma | ||||
| IPWs | 4.26 (4.19, 4.33) | 4.06 (3.93, 4.18) | 0.20 (0.07, 0.34) | .004 |
| Double Robust | 4.26 (4.19, 4.33) | 4.04 (3.90, 4.18) | 0.22 (0.06, 0.38) | .002 |
| Regression adjustment | 4.26 (4.19, 4.33) | 4.04 (3.91, 4.16) | 0.22 (0.08, 0.36) | .003 |
| Alpha vs Delta | ||||
| IPWs | 4.26 (4.19, 4.34) | 4.22 (4.17, 4.27) | 0.04 (-0.04, 0.13) | .301 |
| Double Robust | 4.43 (4.06, 4.80) | 4.15 (4.09, 4.22) | 0.28 (-0.10, 0.66) | <.001 |
| Regression adjustment | 4.43 (4.11, 4.74) | 4.15 (4.09, 4.22) | 0.28 (-0.04, 0.60) | .091 |
| Gamma vs Delta | ||||
| IPWs | 4.10 (3.97, 4.22) | 4.22 (4.17, 4.27) | -0.12 (-0.26, 0.01) | .072 |
| Double Robust | 5.26 (4.19, 6.33) | 4.19 (4.14, 4.24) | 1.07 (0.00, 2.14) | <.001 |
| Regression adjustment | 5.21 (4.37, 6.04) | 4.19 (4.14, 4.24) | 1.02 (0.18, 1.85) | .017 |
| b) | Potential CT values and ATE from fitting a linear regression model (log2 scale) | |||
| Mean (log2)iin VOC 1 i(95% CI) | Mean (log2)iin VOC 2 i(95% CI) | ATE* (95% CI) | P Value | |
| Omicron BA1 vs Delta | ||||
| IPWs | 4.25 (4.21, 4.29) | 4.31 (4.23, 4.39) | -0.06 (-0.15, 0.03) | .177 |
| Double Robust | 4.25 (4.21, 4.30) | 4.31 (4.09, 4.53) | -0.05 (-0.28, 0.17) | .360 |
| Regression adjustment | 4.25 (4.21, 4.29) | 4.37 (3.91, 4.82) | -0.12 (-0.57, 0.34) | .614 |
| Omicron BA2 vs Delta | ||||
| IPWs | 3.99 (3.94, 4.04) | 4.36 (4.27, 4.46) | -0.37 (-0.48, -0.27) | <.001 |
| Double Robust | 3.99 (3.94, 4.05) | 4.29 (4.07, 4.52) | -0.30 (-0.53, -0.07) | <.001 |
| Regression adjustment | 4.02 (3.93, 4.12) | 4.38 (3.52, 5.23) | -0.35 (-1.22, 0.51) | .419 |
| Omicron BA1 vs Omicron BA2 | ||||
| IPWs | 4.21 (4.16, 4.26) | 4.04 (3.98, 4.10) | 0.17 (0.09, 0.25) | <.001 |
| Double Robust | 4.20 (4.15, 4.26) | 4.04 (3.98, 4.10) | 0.16 (0.09, 0.24) | <.001 |
| Regression adjustment | 4.20 (4.15, 4.25) | 4.06 (3.92, 4.20) | 0.14 (-0.01, 0.29) | .064 |
Abbreviations: ATE, Average Treatment Effect; CT, cycle threshold; VOC, Variant of Concern; CI, Confidence Interval; vs, versus; IPWs, Inverse Probability Weightings.
weighted for calendar time of study entry, age, immunodeficiency and vaccination (none, partial cycle, booster/recent full cycle, unboosted/waned full cycle).
Fig. 3.
Dot-plots showing means and Standard Deviation (SD) of nasopharyngeal viral RNA levels, stratified by vaccination categories (unvaccinated, partially vaccinated, recent fully vaccinated or boosted, waned fully vaccinated or unboosted). Viral RNA levels are expressed as log2 Cycle Threshold (CT) values. Means in log2 CT values and SD are shown. Statistical analysis of the comparison between stratification groups was performed by means of analysis of variance (ANOVA, with global Fisher F test and specific contrasts PValues corrected by Holm-Šídák's method for multiple comparisons). Horizontal dashed line represents the limit of detection (CT=40.0), CT values ≥40 are considered negative. n, number of participants in group.
5. Discussion
In our analysis, we observed a difference in nasopharyngeal VL according to the VOC considered, after controlling for potential confounders, including vaccination, with the exception of the comparison between Delta and Omicron BA.1 variants for which we found less statistical evidence for a difference.
Our results are in line with those of several other studies which explored the association between VOCs and VL. Increased VL and greater transmissibility have been reported with the Alpha and Delta variants compared to ancestral strain [15,16], or with Delta towards the wild-type virus or Alpha and Gamma [3,9,16,24]. Studies on the virologic characteristics of BA.1 variant, reported that VL in diagnostic specimens did not differ from those of previous VOCs [21], or were even lower, despite its great contagiousness, probably related to immune evasiveness mechanisms [4,5,[18], [19], [20]]. Likewise, our data showed similar VL for Delta compared to BA.1. In contrast, for BA.2 we observed higher VL when compared to both BA.1 and Delta variants. To date, few studies exist on infections caused by Omicron BA.2 in comparison with previous VOCs, and on the impact of infection- and vaccine-derived antibodies on infectiousness. In vitro [4,6] and in vivo [3,6,8] data, have confirmed the selective advantage of BA.2 over BA.1, suggesting that it is mainly due to an intrinsic greater transmissibility, probably related to its high VL [3,6]. The mutation profiles of BA.1 and BA.2 are substantially different and it is reasonable to assume that the virologic properties of BA.2, as immune resistance and pathogenicity, are different from those of BA.1. The largest study conducted thus far is from Denmark, comparing Omicron BA.1 and BA.2 transmission among household contacts; the authors observed that both unvaccinated, fully vaccinated and booster-vaccinated individuals had a higher susceptibility for BA.2 compared to BA.1, indicating an inherent increased transmissibility of BA.2 [3]. Our findings of higher VL in BA.2 samples, independently from vaccination, are consistent with these results. Of note, in the unadjusted analysis exploration with vaccination status as the only predictor in the model, vaccination did not seem to have an effect on CT values and, as a consequence, it was a weak confounder for the association between VOCs and SARS-CoV-2 RNA.
Interestingly, our attempt to study the association between VOC and CT values according to symptoms’ severity showed differences only for Alpha and Delta variants, suggesting that viral load for Alpha was lower than Delta particularly in more symptomatic participants. Our study population, enrolled in the earliest stages of the disease, reported an overall low symptom score and, probably, it is not the ideal setting for performing this kind of analysis and we cannot rule out selection bias due to missing data for the symptom score. Nonetheless, our findings shed light on the need to more deeply evaluate this aspect in order to examine the role of other potential SARS-CoV-2 viral load determinants, possibly in a population with a more heterogeneous symptom set.
Finally, among patients harbouring Omicron variants, we observed symptom scores similar to those seen for other VOCs but a better clinical and laboratory profile, when assessing peripheral oxygen saturation, fever and inflammatory parameters. Even though this analysis is limited to the early phase of the illness, our findings are somewhat in line with those of previous reports suggesting that BA.2 leads to an equally mild course of COVID-19 as BA.1, compared to previous VOCs [8,[25], [26], [27]].
A strength of our analysis is the homogeneity of our study population which included mildly symptomatic individuals enrolled at an early stage of their disease, with a precise assessment of the timing of symptoms’ onset and with risk factors for progression to severe COVID-19 given by the eligibility criteria for early treatment; moreover, to our knowledge is one of the largest studies to date with a high prevalence of Omicron sub-lineage BA.2, in the outpatient setting.
However, several limitations need to be mentioned. Firstly, we used as primary endpoint the CT values, determined by the amount of viral RNA in the sample, as a surrogate measure for VL; albeit higher VL, as those observed in our population enrolled around the time of symptoms’ onset [13,14], have been correlated with positive viral culture [28], [29], [30], this condition alone is not sufficient for our measure to be a consistent surrogate of viral culture in order to rule out a surrogate paradox [31]. Second, as in all analyses of observational data, we cannot rule out that results can be explained by unmeasured confounding and some of the findings rely on a correctly specified model for the outcome and propensity score model. However, we have performed a sensitivity analysis and established that, in order to be able to attenuate our smallest detected association to the null, this unmeasured confounder(s) would need to decrease CT values by at least 1.4 log2. Of note, none of the identified measured confounders showed such a big effect on the outcome (data not shown). In addition, doubly robust results are valid despite the mis-classification of one of the two models. Furthermore, it is established that viral shedding may contribute to the degree of virus transmissibility, as shown in other studies [9,15]. Unfortunately, all our patients were treated shortly after the analysed measure of CT and were isolated post-diagnosis, which prevented us from conducting a comparison of viral shedding by VOC. As a statistical limitation, although we calculated robust standard errors, there might be an inflation of type I error due to multiple contrasts when we performed the marginal models. Finally, methods for RT-PCR tests and vaccine access have varied over the 12 months of enrolment period; we have mitigated this potential limitation, by restricting the analysis to CT values measured using the same platform, and controlling in the model for vaccination status.
In conclusion, although the process of human-to-human transmission is complex and several factors, such as varying recommended protection measures, the overall incidence, the context of contacts (household or community), the increase in immunity against SARS-CoV-2 in the population, and the intrinsic characteristics of viral variants, can play a role, VL is considered as a key element of transmission [9], [10], [11], [12]; our data show higher levels of VL for Omicron sub-lineage BA.2 versus other VOCs and therefore corroborate epidemiologic data suggesting greater infectiousness of BA.2 compared to other variants and support the hypothesis of an increased inherent transmissibility rather than an enhanced immunologic escape. In a context of continuous viral evolution and new emerging VOCs (e.g. Omicron sub-lineages BA.4 and BA.5), with still uncertain impact on transmissibility and disease severity, it is essential to strengthen surveillance and sequencing capacities. It is also crucial to better understand mechanisms of increased transmissibility, through more focused studies, in order to shape public health and social decision. Promotion of vaccination, encouragement of mask-wearing and physical distancing remain effective measures to reduce population-level transmission.
Funding
The study was performed in the framework of the SARS-CoV-2 surveillance and response program implemented by the Lazio Region Health Authority. This study was supported by funds to the National institute for Infectious Diseases “Lazzaro Spallanzani”, IRCCS, Rome (Italy), from Italian Ministry of Health (Programme CCM 2020; Ricerca Corrente - Linea 1 on emerging and re-emerging infections) and from the European Commission - Horizon 2020 (CoNVat, Grant agreement ID 101003544; KRONO, Grant agreement ID 101005075). Alessandro Cozzi-Lepri work is supported by EuCARE project funded bythe EU under the HORIZON Europe programme, Grant agreement n. 6944397.
CRediT authorship contribution statement
Ilaria Mastrorosa: Conceptualization, Investigation, Resources, Writing – original draft, Project administration. Alessandro Cozzi-Lepri: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft. Francesca Colavita: Conceptualization, Investigation, Resources, Writing – review & editing, Project administration. Eleonora Lalle: Conceptualization, Investigation, Resources. Valentina Mazzotta: Conceptualization, Investigation, Resources, Project administration. Claudia Cimaglia: Data curation. Jessica Paulicelli: Data curation. Giulia Matusali: Investigation, Resources. Lavinia Fabeni: Investigation, Resources. Fabrizio Carletti: Investigation, Resources. Silvia Rosati: Investigation, Resources. Serena Vita: Investigation, Resources. Giuseppina Giannico: Data curation. Pierluca Piselli: Data curation. Elisa Biliotti: Investigation, Resources. Samir Al Moghazi: Investigation, Resources. Silvia Mosti: Investigation, Resources. Enrico Girardi: Supervision, Funding acquisition. Emanuele Nicastri: Supervision, Funding acquisition. Anna Rosa Garbuglia: Supervision, Funding acquisition. Fabrizio Maggi: Supervision, Funding acquisition. Francesco Vaia: Supervision, Funding acquisition. Andrea Antinori: Conceptualization, Project administration, Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest for the present study.
Acknowledgments
The authors gratefully acknowledge the nurse staff, all the patients, and all members of the INMI COVID-19 Outpatient Treatment Study Group:
Samir Al-Moghazi, Chiara Agrati, Andrea Antinori, Tommaso Ascoli-Bartoli, Francesco Baldini, Bartolini Barbara, Rita Bellagamba, Giulia Berno, Nazario Bevilacqua, Elisa Biliotti, Giulia Bonfiglio, Licia Bordi, Elena Boschiavo, Marta Camici, Emanuela Caraffa, Rita Casetti, Carlotta Cerva, Stefania Cicalini, Claudia Cimaglia, Francesca Colavita, Angela Corpolongo, Rita Corso, Alessandro Cozzi Lepri, Gianpiero D'Offizi, Federico De Zottis, Silvia Di Bari, Virginia Di Bari, Francesca Di Bella, Davide Roberto Donno, Angela D'Urso, Lavinia Fabeni, Massimo Francalancia, Marisa Fusto, Roberta Gagliardini, Paola Gallì, Francesca Gavaruzzi, Letizia Giancola, Giuseppina Giannico, Emanuela Giombini, Enrico Girardi, Giulia Gramigna, Elisabetta Grilli, Susanna Grisetti, Cesare Ernesto Maria Gruber, Giuseppina Iannicelli, Daniela Inzeo, Eleonora Lalle, Alessandra Lamonaca, Simone Lanini, Fabio Leotta, Raffaella Libertone, Laura Loiacono, Gaetano Maffongelli, Alessandra Marani, Andrea Mariano, Ilaria Mastrorosa, Giulia Matusali, Valentina Mazzotta, Silvia Meschi, Eugenia Milozzi, Annalisa Mondi, Vanessa Mondillo, Emanuele Nicastri, Giovanna Onnelli, Sandrine Ottou, Claudia Palazzolo, Fabrizio Palmieri, Sara Pantanella, Jessica Paulicelli, Carmela Pinnetti, Pierluca Piselli, Maria Maddalena Plazzi, Alessandra Oliva, Alessia Rianda, Silvia Rosati, Martina Rueca, Alessandra Sacchi, Giuseppe Sberna, Laura Scorzolini, Fabrizio Taglietti, Giuseppina Tarabù, Francesca Trotti, Francesco Vaia, Alessandra Vergori, Serena Vita, Pietro Vittozzi.
References
- 1.World Health Organization (WHO). “Tracking SARS-CoV-2 variants (last updated on 11 August 2022)”. Available at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants. Accessed 15 September 2022.
- 2.Campbell F., Archer B., Laurenson-Schafer H., et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyngse F.P., Kirkeby C.T., Denwood M., et al. Transmission of SARS-CoV-2 omicron VOC subvariants BA.1 and BA.2: evidence from danish households. medRxiv [Preprint]. Posted online 30 January 2022. Available from: 10.1101/2022.01.28.22270044.
- 4.Yu J., Collier A.Y., Rowe M., Mardas F., et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 Variants. N. Engl. J. Med. 2022;386:1579–1580. doi: 10.1056/NEJMc2201849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puhach O., Adea K., Hulo N., et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat. Med. 2022 doi: 10.1038/s41591-022-01816-0. [published online ahead of print, 2022 Apr 8] Available from: [DOI] [PubMed] [Google Scholar]
- 6.Yamasoba D., Kimura I., Nasser H., et al. Virological characteristics of SARS-CoV-2 BA.2 variant. bioRxiv [Preprint]. Posted online 15 February 2022. Available from: 10.1101/2022.02.14.480335.
- 7.UK Health Security Agency SARS-CoV-2 variants of concern and variants under investigation in England. Tech. Brief. 2022;35 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050999/Technical-Briefing-35-28January2022.pdf UKHSA; last updated on 28 JanuaryAvailable at: Accessed 15 September 2022. [Google Scholar]
- 8.Fonager J., Bennedbæk M., Bager P., et al. Molecular epidemiology of the SARS-CoV-2 variant Omicron BA.2 sub-lineage in Denmark, 29 November 2021 to 2 January 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.10.2200181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies N.G., Abbott S., Barnard R.C., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyngse F.P., Mølbak K., Skov R.L., et al. Increased transmissibility of SARS-CoV-2 lineage B.1.1.7 by age and viral load. Nat. Commun. 2021;12:7251. doi: 10.1038/s41467-021-27202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marc A., Kerioui M., Blanquart F., et al. Quantifying the relationship between SARS-CoV-2 viral load and infectiousness. Elife. 2021;10:e69302. doi: 10.7554/eLife.69302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marks M., Millat-Martinez P., Ouchi D., et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect. Dis. 2021;21:629–636. doi: 10.1016/S1473-3099(20)30985-3. Erratum in: Lancet Infect Dis. 2021; 21: e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stankiewicz Karita H.C., Dong T.Q., Johnston C., et al. Trajectory of viral RNA load among persons with incident SARS-CoV-2 G614 infection (Wuhan Strain) in association with COVID-19 symptom onset and severity. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Néant N., Lingas G., Le Hingrat Q., et al. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2017962118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong S.W.X., Chiew C.J., Ang L.W., et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta) Clin. Infect. Dis. 2021:ciab721. doi: 10.1093/cid/ciab721. [published online ahead of print, 2021 Aug 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo C.H., Morris C.P., Sachithanandham J., et al. Infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta variant is associated with higher recovery of infectious virus compared to the Alpha variant in both unvaccinated and vaccinated individuals. Clin. Infect. Dis. 2022;75(1):e715–e725. doi: 10.1093/cid/ciab986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singanayagam A., Hakki S., Dunning J., et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022;22:183–195. doi: 10.1016/S1473-3099(21)00648-4. Erratum in: Lancet Infect Dis. 2021; 21: e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyngse F.P., Mortensen L.H., Denwood M.J., et al. SARS-CoV-2 Omicron VOC transmission in Danish households. medRxiv [Preprint]. Posted online 27 December 2021. Available from: 10.1101/2021.12.27.21268278.
- 19.Eggink D., Andeweg S.P., Vennema H., et al. Increased risk of infection with SARS-CoV-2 Omicron BA.1 compared with Delta in vaccinated and previously infected individuals. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.4.2101196. the Netherlands, 22 November 2021 to 19 January 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sentis C., Billaud G., Bal A., et al. SARS-CoV-2 Omicron variant, Lineage BA.1, is associated with lower viral load in nasopharyngeal samples compared to Delta variant. Viruses. 2022;14:919. doi: 10.3390/v14050919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matic N., Lowe C.F., Ritchie G., Young M., Lawson T., Romney M.G. Omicron (B.1.1.529) SARS-CoV-2 viral load among nasopharyngeal and oral samples compared to other Variants of Concern and impact on diagnostic testing strategy. Clin. Microbiol. Infect. 2022 doi: 10.1016/j.cmi.2022.04.022. [published online ahead of print, 2022 May 11] S1198-743X(22)00234-8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsebom F.C.M., Andrews N., Stowe J., et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00309-7. [published online ahead of print, 2022 May 24] S1473-3099(22)00309-7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann. Intern. Med.. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 24.King K.L., Wilson S., Napolitano J.M., Sell K.J., Rennert L., Parkinson C.L., Dean D. SARS-CoV-2 variants of concern Alpha and Delta show increased viral load in saliva. PLoS One. 2022;17 doi: 10.1371/journal.pone.0267750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veneti L., Bøås H., Bråthen Kristoffersen A., et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA.1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.4.2200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolter N., Jassat W., Walaza S., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyberg T., Ferguson N.M., Nash S.G., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Kampen J.J.A., van de Vijver D., Fraaij P.L.A., et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat. Commun. 2021;12(1):267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Scola B., Le Bideau M., Andreani J., et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jefferson T., Spencer E.A., Brassey J., Heneghan C. Viral cultures for coronavirus disease 2019 infectivity assessment: a systematic review. Clin. Infect. Dis. 2021;73:e3884–e3899. doi: 10.1093/cid/ciaa1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Echt D.S., Liebson P.R., Mitchell L.B., et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]