Abstract
Background
Hypertrophic and keloid scars are common skin conditions resulting from abnormal wound healing. They can cause itching, pain and have a negative physical and psychological impact on patients’ lives. Different approaches are used aiming to improve these scars, including intralesional corticosteroids, surgery and more recently, laser therapy. Since laser therapy is expensive and may have adverse effects, it is critical to evaluate the potential benefits and harms of this therapy for treating hypertrophic and keloid scars.
Objectives
To assess the effects of laser therapy for treating hypertrophic and keloid scars.
Search methods
In March 2021 we searched the Cochrane Wounds Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL EBSCO Plus and LILACS. To identify additional studies, we also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses, and health technology reports. There were no restrictions with respect to language, date of publication, or study setting.
Selection criteria
We included randomised controlled trials (RCTs) for treating hypertrophic or keloid scars (or both), comparing laser therapy with placebo, no intervention or another intervention.
Data collection and analysis
Two review authors independently selected studies, extracted the data, assessed the risk of bias of included studies and carried out GRADE assessments to assess the certainty of evidence. A third review author arbitrated if there were disagreements.
Main results
We included 15 RCTs, involving 604 participants (children and adults) with study sample sizes ranging from 10 to 120 participants (mean 40.27). Where studies randomised different parts of the same scar, each scar segment was the unit of analysis (906 scar segments). The length of participant follow‐up varied from 12 weeks to 12 months. All included trials had a high risk of bias for at least one domain: all studies were deemed at high risk of bias due to lack of blinding of participants and personnel. The variability of intervention types, controls, follow‐up periods and limitations with report data meant we pooled data for one comparison (and only two outcomes within this). Several review secondary outcomes ‐ cosmesis, tolerance, preference for different modes of treatment, adherence, and change in quality of life ‐ were not reported in any of the included studies.
Laser versus no treatment:
We found low‐certainty evidence suggesting there may be more hypertrophic and keloid scar improvement (that is scars are less severe) in 585‐nm pulsed‐dye laser (PDL) ‐treated scars compared with no treatment (risk ratio (RR) 1.96; 95% confidence interval (CI): 1.11 to 3.45; two studies, 60 scar segments).
It is unclear whether non‐ablative fractional laser (NAFL) impacts on hypertrophic scar severity when compared with no treatment (very low‐certainty evidence).
It is unclear whether fractional carbon dioxide (CO2) laser impacts on hypertrophic and keloid scar severity compared with no treatment (very low‐certainty evidence).
Eight studies reported treatment‐related adverse effects but did not provide enough data for further analyses.
Laser versus other treatments:
We are uncertain whether treatment with 585‐nm PDL impacts on hypertrophic and keloid scar severity compared with intralesional corticosteroid triamcinolone acetonide (TAC), intralesional Fluorouracil (5‐FU) or combined use of TAC plus 5‐FU (very low‐certainty evidence). It is also uncertain whether erbium laser impacts on hypertrophic scar severity when compared with TAC (very low‐certainty evidence).
Other comparisons included 585‐nm PDL versus silicone gel sheeting, fractional CO2 laser versus TAC and fractional CO2 laser versus verapamil. However, the authors did not report enough data regarding the severity of scars to compare the interventions.
As only very low‐certainty evidence is available on treatment‐related adverse effects, including pain, charring (skin burning so that the surface becomes blackened), telangiectasia (a condition in which tiny blood vessels cause thread‐like red lines on the skin), skin atrophy (skin thinning), purpuric discolorations, hypopigmentation (skin colour becomes lighter), and erosion (loss of part of the top layer of skin, leaving a denuded surface) secondary to blistering, we are not able to draw conclusions as to how these treatments compare.
Laser plus other treatment versus other treatment:
It is unclear whether 585‐nm PDL plus TAC plus 5‐FU leads to a higher percentage of good to excellent improvement in hypertrophic and keloid scar severity compared with TAC plus 5‐FU, as the certainty of evidence has been assessed as very low.
Due to very low‐certainty evidence, it is also uncertain whether CO2 laser plus TAC impacts on keloid scar severity compared with cryosurgery plus TAC.
The evidence is also very uncertain about the effect of neodymium‐doped yttrium aluminium garnet (Nd:YAG) laser plus intralesional corticosteroid diprospan plus 5‐FU on scar severity compared with diprospan plus 5‐FU and about the effect of helium‐neon (He‐Ne) laser plus decamethyltetrasiloxane, polydimethylsiloxane and cyclopentasiloxane cream on scar severity compared with decamethyltetrasiloxane, polydimethylsiloxane and cyclopentasiloxane cream.
Only very low‐certainty evidence is available on treatment‐related adverse effects, including pain, atrophy, erythema, telangiectasia, hypopigmentation, regrowth, hyperpigmentation (skin colour becomes darker), and depigmentation (loss of colour from the skin). Therefore, we are not able to draw conclusions as to how these treatments compare.
Authors' conclusions
There is insufficient evidence to support or refute the effectiveness of laser therapy for treating hypertrophic and keloid scars. The available information is also insufficient to perform a more accurate analysis on treatment‐related adverse effects related to laser therapy. Due to the heterogeneity of the studies, conflicting results, study design issues and small sample sizes, further high‐quality trials, with validated scales and core outcome sets should be developed. These trials should take into consideration the consumers' opinion and values, the need for long‐term follow‐up and the necessity of reporting the rate of recurrence of scars to determine whether lasers may achieve superior results when compared with other therapies for treating hypertrophic and keloid scars.
Plain language summary
Laser therapy for hypertrophic and keloid scars
What was studied in the review?
Hypertrophic and keloid scars are raised and bumpy scars that form when a wound does not heal correctly. These scars can be discoloured or reddened and can also cause pain and itching. A range of treatments are available, including silicone gels and steroids.
Laser therapy may be an alternative treatment for these types of scars. During laser therapy, areas of skin are targeted by a powerful beam of light which can break down damaged tissue. Different types of laser therapy are available depending on the patient's skin type and the nature of the scar. Laser therapy is expensive and has potentially harmful side effects, so it is important to establish whether it is safe and effective.
What is the aim of this review?
The aim of this review was to investigate whether laser therapy is an effective treatment for people with hypertrophic and keloid scars. To answer this question, researchers from Cochrane collected and analysed all relevant studies to answer this question and found 15 randomised controlled trials.
What are the main results of the review?
We included 15 studies dating from 1999 to 2019, involving 604 participants (children and adults of both sexes). The study sizes were small (10 to 120 participants), with the length of participant follow‐up varying from 12 weeks to 12 months. The studies analysed the change in the severity of scars assessed by health professionals or participants.
In the studies, different kinds of laser devices were compared with no treatment and with other treatment methods. Laser therapy combined with another treatment was also compared with this treatment alone.
We cannot be sure whether laser therapy alone or combined with other treatments improves hypertrophic or keloid scars severity when compared with no treatment or other treatments, as the certainty of all available evidence is low or very low. This is due to the small number of studies, different comparisons, conflicting results, small number of participants, and lack of available data.
Some side effects of laser treatment such as damage to the skin or underlying blood vessels, redness, and numbness were reported. However, the certainty of the evidence is too low to be sure how common these side effects are.
Key messages
Taken together, the results of these studies do not allow us to be sure if using any kind of laser therapy is more or less effective than other available treatments for hypertrophic and keloid scars. As the studies provided only very low‐certainty evidence regarding possible side effects, we are not very confident in the results of the currently available studies, and we cannot be sure whether any type of laser therapy leads to more harm than benefits compared with no treatment or other treatments.
How up to date is this review?
We searched for studies published up to 23 March 2021.
Summary of findings
Background
Description of the condition
Hypertrophic and keloid scars (usually referred to as keloids) represent common skin conditions (Bouzari 2007) which result from abnormal wound healing (Seifert 2009). They can affect any part of the skin's surface that has suffered traumatic or infectious injury (Köse 2008), however, those areas of the skin where there is increased stretching tension (e.g. trunk, upper arm/shoulder (deltoid region), and knees) are more susceptible to the appearance of keloid and hypertrophic scars. They present as raised scars with a smooth surface, firm when palpitated, and their colour can vary from pink‐purple to pale (hypopigmented) or dark (hyperpigmented). They can be associated with symptoms such as itching (pruritus) and pain (Asilian 2006). In some cases, due to the physical and psychological impacts caused by these scars, patients with keloid and hypertrophic scars may report impairment in their quality of life (Bock 2006).
The anatomical location of the initial skin lesion, a history of trauma or infection associated with the initial injury, a burn injury, sutures under tension, adolescence, pregnancy, and family history are considered to be risk factors for the appearance of hypertrophic scars and keloids (Alster 2003; Seifert 2009). During the normal healing process, several cells and chemical substances work together to promote tissue repair. In this process the production and subsequent degradation of collagen usually act in equilibrium, resulting in a scar healing normally. This balance is altered in keloids and hypertrophic scars, where there is higher production of collagen and lower levels of collagen degradation (Cho 2010). The collagen accumulates in the lesion, resulting in excessive scar tissue. Despite their similarities, keloids and hypertrophic scars have some clinical, pathological, and evolutive differences (Seifert 2009).
Both genetic predisposition and skin injury play major roles in the development of keloid and hypertrophic scars (Alster 2003). While keloids may occur at any age, they usually occur in individuals between 10 and 30 years of age (Berman 1996). They affect between 4.5% and 16% of black and Hispanic populations, with an incidence of up to 16% in black Africans (Alster 2003). They occur less frequently in populations with lighter skin. The incidence of hypertrophic scars is probably higher than that of keloid scars (Köse 2008), ranging from 5% to 37% in white people (Li‐Tsang 2005), but precise data are lacking. The prevalence of hypertrophic scars ranges from 15% to 63% in white people (Li‐Tsang 2005). Both keloid and hypertrophic scars tend to recur after treatment (Cassuto 2010).
Clinically, keloids appear as raised scars, exceeding the boundaries of the original injury (Mutalik 2005). They can arise within a few months of the initial injury and often show gradual and undefined growth. They can be of different sizes and patterns and are frequently associated with itching and burning. Keloid scars do not regress spontaneously and usually affect the chest, shoulders, back, posterior neck and ear lobes, but rarely the palms and soles (Seifert 2009). In contrast, hypertrophic scars do not exceed the limits of the original skin injury, and are rarely wider than 1 cm. They usually occur around four weeks after the original injury, grow strongly for a few months, and then tend to regress spontaneously within one year (Seifert 2009).
Due to the recurrent aspect of the lesions, their slowly progressive nature, and the lack of a gold standard therapy, the treatment of hypertrophic scars and keloids represents a significant challenge (Cassuto 2010). Frequently, the treatment of both types of scars is performed by associating two or more techniques (Bouzari 2007, Gupta 2011), looking for a synergistic and or complementary action, or both at different levels of the healing process, with variable results reported in the literature. The main therapies used in the treatment of hypertrophic and keloid scars include medicinal treatments, compressive treatment, surgical treatments, treatment with radiation, and treatment with light sources.
Among the medicinal treatments, corticosteroids are considered as first‐line drugs in the treatment of hypertrophic and keloid scars, and are most often used in intralesional applications (Gupta 2011). Other medicinal treatments are the use of intralesional bleomycin (Alster 2003) and intralesional 5‐ Fluorouracil (Mutalik 2005). Silicone is used in its varied forms, including gel, cream, spray or flexible gel sheeting (Alster 2003). Regarding surgical treatments, cryosurgery can be useful as it causes tissue ischaemia (which is a reduction in blood flow) and necrosis of scar tissue (Alster 2003; Berman 1996). Surgeries to remove scar volume, by removal of its core, or of the entire scar can also be performed (Gupta 2011). Other treatments include light sources such as Intense Pulsed Light (IPL), which aims to promote vascular ischaemia, interfering with collagen production (Erol 2008), and laser therapy. Different laser devices are used, aiming to improve the appearance of hypertrophic and keloid scars by direct destruction of the tissue (fractional or conventional ablative lasers), with consequent reduction in scar volume; coagulation of scar tissue (non‐ablative lasers, fractioned or not), with consequent remodeling of the local collagen and scar improvement; or even by destruction of the scar microvascularisation, leading to ischaemia and consequent reduction in scar volume (Alster 2003; Berman 1996).
Description of the intervention
Different laser systems have been used in the treatment of hypertrophic and keloid scars over the last 30 years (Bouzari 2007; Gupta 2011). The mechanism of lasers follows the principle of 'selective photothermolysis' (Anderson 1983). According to this principle, a light with a specific wavelength emitted by a laser device acts on a specific target, which responds to this wavelength. This target is called "chromophore".
Different skin structures respond to different wavelengths, so it is necessary to find a laser device that emits a laser beam with a wavelength equal or similar to that of the structure that needs to be reached. This laser beam will 'search' for the structure within the skin that has the same or similar wavelength (the "chromophore"), and then destroy it selectively ("selective photothermolysis").
The patient's skin type (skin type I: pale white skin, blue or green eyes, blond or red hair; type II: fair skin, blue eyes; type III: darker white skin; type IV: light brown skin; type V: brown skin; type VI: dark brown or black skin), the energy released by the device in a certain area (also called fluence), the spot size of the laser light and the speed with which this beam reaches the target, are all factors that influence the results obtained with laser therapy.
A laser can be classified as ablative or non‐ablative according to its effect on tissue. Ablative lasers remove part or all of the tissues on which they are applied. They act on the water present in these tissues, causing them to vaporise. Examples of ablative laser devices include carbon dioxide (CO2), argon, and 2940 nm erbium‐doped yttrium aluminium garnet (Er:YAG) laser. Non‐ablative lasers act on different structures, such as the melanin pigment or intracellular haemoglobin, leading to necrosis (death) of these structures, without, however, removing them. An example of a non‐ablative laser device is the pulsed‐dye laser (PDL) (Manuskiatti 2007).
How the intervention might work
The exact mechanism of action by which laser therapy could improve hypertrophic and keloid scars severity is still unknown. Laser devices such as CO2 (Gupta 2011; Kantor 1985), argon, and 2940 nm Er:YAG (Mutalik 2005) can cause scar removal through the interaction of the laser energy with the water present in the treated skin, leading to reduction in the lesion volume. Non‐ablative lasers (e.g. 585 nm PDL,1064 nm Q‐switched neodymium‐doped yttrium aluminium garnet (Nd:YAG) laser with low fluence, 532 nm frequency‐doubled Nd:YAG, and 1064 nm Nd:YAG) can cause destruction (coagulation/necrosis) of the capillaries, through the absorption of laser light energy by blood inside the veins (i.e. intravascular haemoglobin; Asilian 2006). The destruction of blood vessels decreases blood flow (hypoperfusion) to the treated area, with a consequent decrease in local tissue oxygenation (hypoxia). Changes occur in the tissues as a result of the hypoxia, with the production of new collagen, heating of collagen fibres, dissociation of disulphide bonds (which keep collagen fibres together), and collagen fibre realignment (Cho 2010; Mutalik 2005). In this way, laser therapy reorganises collagen deposition in the hypertrophic and keloid scars and improves their clinical aspect and symptoms (Karsai 2007). The number of laser treatment sessions and the intervals between each session may vary according to the scar and laser device used. Every patient is individually examined and treated by his/her doctor, according to the characteristics of the scar in question.
Why it is important to do this review
Hypertrophic and keloid scars result from abnormal wound healing and have cosmetic implications. They occur in women and men of all ethnicities, often between the ages of 10 and 30 years, and can have a negative impact on a person's physical functioning and quality of life (Bock 2006). There are many different treatments for hypertrophic and keloid scars, but there is no gold standard therapy. Laser therapy is an option currently used in the treatment and prevention of keloids and hypertrophic scars, which underlines the need for a systematic review of the most reliable studies.
Objectives
To assess the effects of laser therapy for treating hypertrophic and keloid scars.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials (RCTs) comparing laser therapy with no intervention or another intervention for treating hypertrophic or keloid scars (or both). We did not include cluster‐randomised and cross‐over studies.
Types of participants
People with hypertrophic or keloid scars (or both), who had been diagnosed by a health professional, with no restrictions regarding age, sex, or ethnicity.
Types of interventions
We considered trials in which laser therapy was used to treat hypertrophic or keloid scars (or both), with any kind of laser device, using any fluency, course duration, number of sessions, and follow‐up time, compared either with no intervention or any other type of therapy.
Types of outcome measures
Primary outcomes
Severity of keloid or hypertrophic scars (or both) measured by health professional and/or participant using a specific scale (as defined by the authors).
Incidence and severity of treatment‐related adverse effects.
Secondary outcomes
Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain (non‐treatment related).
Cosmesis/aesthetic scar appearance (defined by patient opinion and/or health professional observations).
Patient tolerance (measured by reported side effects and adverse reactions).
Preference for different modes of treatment measured by patient choice after receiving at least two different types of treatment.
Adherence (measured by health professional and/or patient report).
Patient's quality of life measured by a validated scale (36‐Item Short Form Health Survey (SF‐36), EuroQol (EQ‐5D58); (Ching 2003).
Recurrence of the condition.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
Cochrane Wounds Specialised Register (searched 23 March 2021);
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library via the Cochrane Register of Studies (searched 23 March 2021);
MEDLINE Ovid including In‐Process & Other Non‐Indexed Citations (1946 to 23 March 2021);
Embase Ovid(1974 to 23 March 2021);
CINAHL Plus EBSCO (Cumulative Index to Nursing and Allied Health Literature (1937 to 23 March 2021);
LILACS (Latin American and Caribbean Health Science Information database) via VHL (Virtual Health Library; 1982 to 23 March 2021).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, MEDLINE Ovid, Embase Ovid and CINAHL Plus EBSCO can be found in Appendix 1. In MEDLINE Ovid, we combined the subject‐specific strategy with the sensitivity‐ and precision‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (2008 revision) (Lefebvre 2021). We combined the Embase Ovid search with the Ovid Embase filter developed by Cochrane UK (Lefebvre 2021). We combined the CINAHL Plus EBSCO search with the trial filter developed by Glanville 2019. There were no restrictions with respect to language, date of publication, or study setting.
We also searched the following clinical trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 23 March 2021);
World Health Organization International Clinical Trials Registry Platform (www.who.int/clinical-trials-registry-platform; searched 23 March 2021).
Searching other resources
We aimed to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses, and health technology assessment reports. We contacted specialists in the field, authors of the included studies, and laser device manufacturers for any possible unpublished data. We did not perform a separate search for adverse effects of interventions used, we only considered treatment‐related adverse effects described in included studies.
Data collection and analysis
Data collection and analysis were carried out according to methods stated in the published protocol (Leszczynski 2015), based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Selection of studies
After merging the search results and removing duplicate records, we examined titles and abstracts to select the relevant reports. At least two review authors (RL, ACPNP or CAPS) independently screened the studies identified by the literature search. We retrieved and examined the full text of selected studies for compliance with eligibility criteria and in the case of any disagreements (at this or at any other stage as listed below), a third review author (EMKS) was consulted. Reasons for exclusion of studies after full text retrieval are recorded in the 'Characteristics of excluded studies' table. We included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart to document the screening process, Figure 1 (Moher 2009).
1.
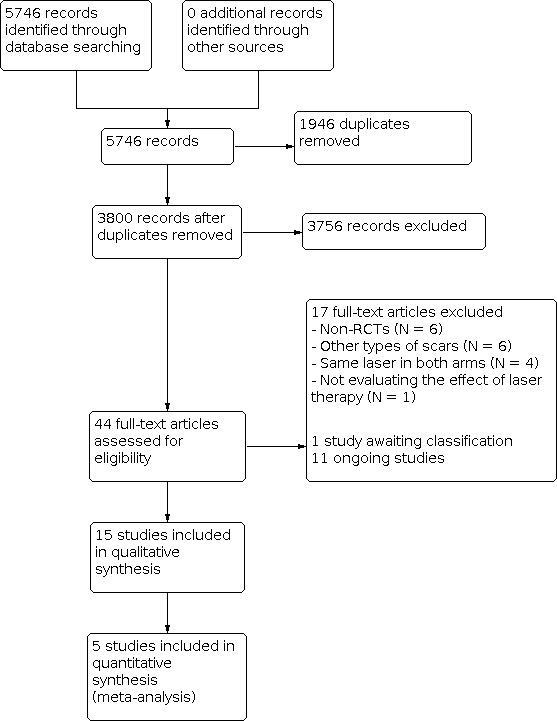
Study flow diagram.
Data extraction and management
At least two review authors (RL, ACPNP or CAPS) extracted data independently and collected data on a data extraction form. We resolved discrepancies in the results by discussion. We collected the following information.
Study features:
publication details (e.g. year, country, authors);
study design;
population data (e.g. age, ethnicity, baseline aspects of keloid and hypertrophic scars, such as severity, duration, symptoms, history of prior trauma or infection at the scar sites, and history concerning treatments and responses);
details of interventions (e.g. number of laser treatment sessions, regimen, fluence, scheme, adjunctive therapies, increasing or decreasing fluence, who delivered the intervention, the location of the intervention);
size of the scars;
cause of the scars;
treatment duration;
number of participants randomised into each treatment group;
number of participants in each group who were cured or failed treatment;
numbers of participants lost to follow‐up;
duration of follow‐up;
source of funding for the trial;
care setting.
Outcomes and results:
types of outcome measures;
timing of outcomes;
results;
treatment‐related adverse effects.
Follow‐up data were extracted on the basis that long‐term follow‐up occurs 12 months or more from the beginning of the treatment; intermediate‐term follow‐up occurs between three months and 12 months from the beginning of the treatment; and short‐term follow‐up occurs less than three months from the beginning of the treatment. These criteria were established by the authors of this review, since there is no consensus about the follow‐up in the literature.
Where studies had multiple publications, the main study report was used as the reference, with additional details being supplemented from secondary papers.
Assessment of risk of bias in included studies
At least two review authors (RL, ACPNP or CAPS) independently assessed each included study using the Cochrane tool for assessing risk of bias (Higgins 2021). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other issues (see Appendix 2 for details of the criteria on which the judgements were based). We completed a risk of bias table for each eligible study. We discussed any disagreements among all review authors to achieve a consensus. We presented assessment of risk of bias using a risk of bias summary figure (see Figure 2), which presents all the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader can give the results of each study.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
The measures of treatment effect were calculated whenever data were available. We calculated risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous variables. We also calculated the mean difference (MD) and 95% CIs for continuous outcomes.
Unit of analysis issues
When multiple scars of the body received the same intervention, with a separate outcome judgement made for each body part, and the number of body parts was used as the denominator in the analysis, we planned to analyse the data similarly to the situation in cluster‐randomised trials (Higgins 2021). We anticipated a possible unit‐of analysis issue if individual participants with multiple scars were randomised, the allocated treatment was used on multiple scars per participant (or perhaps only for some participants), and then data were presented and analysed by scar not person. This is a type of clustered data, such that the participant is the 'cluster', and presents a unit of analysis error which inflates precision. If there had been studies that contained some or all clustered data, we would have reported this alongside information on whether data had been (incorrectly) treated as independent. We would have recorded this as part of the risk of bias assessment. We would not have undertaken further calculation to adjust for clustering. However, no studies of this type were included.
Dealing with missing data
Where there were missing or unavailable data, we contacted the study authors to request additional information. Because the study authors failed to respond, we reported dropout rates in the 'Characteristics of included studies' tables of the review, and used intention‐to‐treat analysis. All participants were analysed according to their randomisation allocation. Although we planned to perform sensitivity analysis, excluding participants with missing data, to assess the strength of the results (Higgins 2021), due to the lack of information in the included RCTs, this was not possible.
Assessment of heterogeneity
We considered clinical and methodological heterogeneity and quantified heterogeneity among the pooled estimates using the I2 measure calculated by Review Manager software (RevMan 2020). This illustrates the percentage of the variability in the effect estimates resulting from heterogeneity rather than sampling error. Whilst the I2 measure was used to quantify heterogeneity, a holistic assessment of heterogeneity more broadly taking into account study, intervention, population and outcome features was used to guide synthesis decisions.
Assessment of reporting biases
We planned to assess reporting biases or small study effects by drawing a funnel plot (trial effect versus trial size), assuming a sufficient number of studies (more than 10 for each outcome) were included in the review. As we did not have this number of studies, a funnel plot was not created.
Data synthesis
We considered clinical and methodological heterogeneity. We planned to pool data when studies appeared appropriately similar in terms of type of scar, intervention type, duration of follow‐up, and outcome type. Within comparisons, we pooled paired data separately (e.g. from split‐scar designs) from data from parallel‐group trials where both study types were available. (Lesaffre 2009). Where data synthesis was inappropriate, we provide a narrative overview.
We presented data using forest plots where possible. For dichotomous outcomes we presented the summary estimate as an RR with 95% CI. Where continuous outcomes were measured in the same way across studies, we present pooled mean differences (MD) with 95% CI. We planned to pool standardised mean difference (SMD) estimates where studies measured the same outcome using different methods; however, no outcomes were measured using different methods. We included studies where multiple treatment groups relevant to the review were compared with only one control group, and present the data separately for these comparisons.
Subgroup analysis and investigation of heterogeneity
We intended to perform subgroup analyses to consider the following:
types of laser devices;
different fluences and schemes;
duration of treatment.
If we had found substantial heterogeneity and sufficient data, we planned to investigate the possible causes by exploring the impact of the condition of the individuals and the interventions (e.g. participant characteristics such as skin type, and addition of adjuvant therapies) using subgroup analyses. However, these analyses were not possible because of insufficient data.
Sensitivity analysis
If there had been an adequate number of studies, we would have performed sensitivity analyses based on separation of studies according to risk of bias. This would have been performed by excluding the studies most susceptible to bias on the basis of our risk of bias assessment, namely those with inadequate randomisation sequence generation, allocation concealment, high levels of post‐randomisation losses or exclusions, and unclear or unblinded outcome assessment.
Summary of findings and assessment of the certainty of the evidence
We present the main results of the review in summary of findings tables. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2021a). The summary of findings tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. We evaluated the certainty of evidence considering within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2021b). We present the following outcomes in the summary of findings tables:
severity of keloid or hypertrophic scars;
incidence and severity of treatment‐related adverse effects.
Results
Description of studies
Results of the search
We searched the databases previously described (up to 23 March 2021) and this search retrieved 5746 citations. After assessing the abstracts for inclusion criteria, 44 citations were assessed in full. Of these, 17 studies were excluded with justification, 11 studies are ongoing, one study is awaiting classification and 15 randomised controlled trials (RCTs) were included in this systematic review, Figure 1.
Included studies
We included 15 RCTs in the review (Alsharnoubi 2018; Asilian 2006; Azzam 2016; Behera 2016; Blome‐Eberwein 2016; Chen 2017; Daoud 2019; Khattab 2019; Lin 2011; Manuskiatti 2001; Manuskiatti 2002; Omranifard 2007; Srivastava 2019; Verhaeghe 2013; Wittenberg 1999), with a total of 604 participants. The included studies evaluated different types of lasers with various types of control interventions, including no intervention and different injectable drugs (see Characteristics of included studies).
Care setting
Six studies were conducted in the USA (Blome‐Eberwein 2016; Daoud 2019; Lin 2011; Manuskiatti 2001; Manuskiatti 2002; Wittenberg 1999), two in Iran (Asilian 2006; Omranifard 2007), one in Belgium (Verhaeghe 2013), three in Egypt (Alsharnoubi 2018; Azzam 2016; Khattab 2019), two in India (Behera 2016; Srivastava 2019), and one in China (Chen 2017).
Four studies were conducted in private clinics (Asilian 2006; Manuskiatti 2001; Manuskiatti 2002; Omranifard 2007), six in hospitals (Alsharnoubi 2018; Behera 2016; Chen 2017; Lin 2011; Srivastava 2019; Verhaeghe 2013), two in outpatient clinics of University departments or University Hospitals (Azzam 2016; Khattab 2019), one in an outpatient burn centre (Blome‐Eberwein 2016), and two studies did not specify care settings (Daoud 2019; Wittenberg 1999).
Design
All of the 15 included studies were single‐centre RCTs, published between 1999 and 2019. The length of participant follow‐up of the studies ranged from 12 weeks to 12 months.
Five studies (Alsharnoubi 2018; Behera 2016; Blome‐Eberwein 2016; Khattab 2019; Verhaeghe 2013) compared two trial arms. Six studies (Asilian 2006; Chen 2017; Daoud 2019; Omranifard 2007; Wittenberg 1999; Srivastava 2019) assessed three trial arms each. Three studies (Azzam 2016; Lin 2011; Manuskiatti 2001) evaluated four trial arms each, and one study (Manuskiatti 2002) assessed five trial arms.
Among the included studies, six were parallel group (Asilian 2006; Behera 2016; Chen 2017; Khattab 2019; Omranifard 2007; Srivastava 2019), and nine were split‐scar design (Alsharnoubi 2018; Azzam 2016; Blome‐Eberwein 2016; Daoud 2019; Lin 2011; Manuskiatti 2001; Manuskiatti 2002; Verhaeghe 2013; Wittenberg 1999).
Sample sizes
The number of participants included in each RCT ranged from 10 (Lin 2011; Manuskiatti 2001; Manuskiatti 2002) to 120 (Omranifard 2007), with a total of 604 participants included across the review and a mean of 40.27 participants per trial. A total of 906 scar segments (unit of analysis) were assessed.
Participants
Participants included children and adults (age range from 2 to 81 years) of both sexes, skin types I‐VI, presenting hypertrophic and/or keloid scars. Almost all studies specified inclusion and exclusion criteria, except three (Blome‐Eberwein 2016; Daoud 2019 Manuskiatti 2002), which reported only inclusion criteria. Some of the exclusion criteria were pregnancy, lactation, scars with previous treatment, and participants with a history of isotretinoin use within the six months prior to laser treatment. Three trials only included participants with hypertrophic scars (Lin 2011; Verhaeghe 2013; Wittenberg 1999).
Interventions
In this review, we included trials comparing laser therapy for treating hypertrophic and/or keloid scars with other therapies (including no treatment). Different kinds of laser devices were evaluated, as well as different laser treatment regimens. The number of laser sessions varied from one (Behera 2016) to 24 (Alsharnoubi 2018), with a mean of 6.13 sessions, and the frequency of administration varied from twice a week (Alsharnoubi 2018) to one session every eight weeks (Wittenberg 1999). Different kinds of laser treatments were compared with no treatment (Table 4), with other treatments (Table 5) or were combined with other kinds of laser treatments and compared with other treatments (Table 6). Only two authors reported who had performed the laser sessions (in Blome‐Eberwein 2016 and Verhaeghe 2013 where laser sessions were performed by health professionals).
1. Laser versus no treatment outcome details.
| Comparison | Outcome | Trials (participants) | Definition of outcome in trial/measurement details | Data reported |
| 585‐nm PDL versus no treatment | Scar severity | 2 RCTs (Manuskiatti 2001; 10 participants; 40 segments and Manuskiatti 2002, 10 participants; 20 segments) | Manuskiatti 2001 and Manuskiatti 2002: categories of 25% increments of improvement in scar severity after 32 weeks (graded by the patient) | Number of participants with an improvement of 50% or higher in the scar severity Manuskiatti 2001: Laser: 23/30 Control: 4/30 Manuskiatti 2002: Laser: 8/10 Control: 4/10 |
| 585‐nm PDL versus no treatment | Incidence and severity of treatment‐related adverse effects | 3 RCTs (Manuskiatti 2001; 10 participants; 40 segments, Manuskiatti 2002, 10 participants; 20 segments, and Wittenberg 1999; 20 participants; 40 segments) | Manuskiatti 2001: immediate treatment reactions, including purpuric discolorations and erosion secondary to blistering, and adverse sequelae during 32 weeks. Manuskiatti 2002: immediate treatment reactions included mild to moderate pain during treatment, burning sensation, spots of purpura, erosion secondary to blistering. Treatment‐related adverse sequelae including hypopigmentation, telangiectasia, and skin atrophy during 32 weeks. Wittenberg 1999: no definition was provided. Duration: 40 weeks. |
Manuskiatti 2001 and 2002: in all participants treated with the PDL the area became purpuric and a small number of participants with skin phototype VI reported erosions.
Manuskiatti 2002: mild to moderate
discomfort or pain related to treatment was in 90% (9/10) of the participants during laser pulsing. Wittenberg 1999: one (1/20) participant dropped out due to pain during laser treatment. |
| 585‐nm PDL versus no treatment | Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain | 3 RCTs (Manuskiatti 2001; 10 participants; 30 segments and Manuskiatti 2002: 10 participants; 20 segments; Wittenberg 1999; 20 participants; 40 segments) | Manuskiatti 2001 and 2002: scar height evaluated using a dial calliper, erythema using a hand‐held colorimeter after 32 weeks. Wittenberg 1999: blood flow (erythema), elasticity and volume, burning, pruritus, and pain not related to treatment after 40 weeks. |
Manuskiatti 2001, 2002: scar height improvement in laser treated areas when compared with control (P < 0.05, and P = 0.005, respectively) (data presented only in graphs) Wittenberg 1999: No statistically significant differences between groups were detected for erythema (P = 0.26), elasticity (P = 0.76), volume (P = 0.13), burning (P = 0.75), pruritus (P = 0.99), and pain not related to treatment (P = 0.41) (results presented only in graphs). |
| NAFL versus no treatment | Scar severity | 2 RCTS (Verhaeghe 2013; 22 participants, 44 segments; Lin 2011; 20 participants; 20 scars) | Verhaeghe 2013:
HPGA and PGA using a VAS ranging from 0 to 100 mm (0 = normal skin and 100 = worst possible scar) ‐ smallest clinically important minimum relevant difference was 20 POSAS: containing vascularisation, pigmentation, thickness, relief, pliability, and surface area (observer part) and pain not related to treatment, itching, colour, stiffness, thickness, and relief (patient part) (range 6 to 60) after 3 months. Lin 2011: categories of 25% increments of improvement in scar severity (graded by the participants and 2 blinded observers) after 3 months. |
Verhaeghe 2013 (36 segments analysed): Number of participants who got better according to HPGA and PGA. HPGA: Laser: 10/18 Control: 5/18 PGA: Laser: 10/18 Control: 1/18 POSAS: Patient part (P = .047) Observer part: not significant (details not provided) Lin 2011: HDTA laser: not significant LDTA: P = 0.001 (data presented only in graphs) |
| NAFL versus no treatment | Incidence and severity of treatment‐related adverse effects | 2 RCTS (Verhaeghe 2013; 22 participants, 44 segments; Lin 2011; 20 participants; 20 scars) | Verhaeghe 2013: treatment‐related adverse effects included erythema, edema, burning sensation, crusts, purpura, vesicles, hyperpigmentation. Duration: 3 months Lin 2011: side effects, including worsening (erythema, pigmentation, or texture), discolouration, exfoliation, swelling, scabbing, and pain related to treatment, rated on a quartile scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe) during the 3 month‐period. |
Verhaege 2013: Percentage of participants reporting treatment‐related adverse effects 4 days after treatment (out of 18 analysed): NAFL treatment: Erythema: 70% Edema: 20% Burning sensation: 18% Crusts: 15% Purpura: 7% purpura Vesicles: 3% After 3 months: Hyperpigmentation: 1 participant *In a small group of participants, the treated part improved less than the untreated part. Median pain (related to treatment) score (IQR) on a VAS (0 = no pain and 100 = worst possible pain) was: Session 1: 37.0 (26.0–53.5) Session 2: 41.0 (26.7–60.7) Session 3: 53.0 (22.5–71.0) Session 4: 48.0 (23.5–78.5) Lin 2011: Number of participants reporting scar worsening HDTA: 3/10 Higher risk of erythema, exfoliation, and pain related to treatment with HDTA compared with LDTA (P = 0.05, P = 0.02, P = 0.01, respectively) (data on side effects were presented only in graphs). |
| NAFL versus no treatment | Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain | 2 RCTS (Verhaeghe 2013; 22 participants, 44 segments; Lin 2011; 20 participants; 20 scars) | Verhaeghe 2013: redness, pigmentation, and skin texture after 3 months Lin 2011: erythema, pigmentation, texture after 3 months |
Verhaeghe 2013: No significant difference in redness, pigmentation, and skin texture (details not provided) Lin 2011: Mean + SD Erythema: HDTA: 1.33 + 1.26 Control: 0.89 + 0.95 LDTA: 1.06 + 1.26 Control: 0.94 + 1.20 Pigmentation: HDTA: 1.06 + 0.95 Control: 0.94 + 0.82 LDTA: 0.79 + 0.76 Control: 0.71 + 0.76 Texture: HDTA: 2 + 0.76 Control: 1.78 + 0.89 LDTA: 2.19 + 0.95 Control: 1.63 + 0.95 |
| Fractional CO2 Laser versus no treatment | Scar severity | 3 RCTS (Azzam 2016: 30 participants; 60 segments, Blome‐Eberwein 2016: 36 participants; 80 scars, Daoud 2019: 23 participants; 46 segments) | Azzam 2016: patient satisfaction, as follows: excellent = more than 75 %, good = 50 to 75%, moderate = 25 to 50 % and poor = less than 25 % improvement after 3 months Azzam 2016 and Blome‐Eberwein 2016 used the VSS, including pliability, height, colour, and vascularity Blome‐Eberwein 2016 and Daoud 2019: POSAS including: vascularisation, pigmentation, thickness, relief, pliability, and surface area (observer), itching, colour, and pain not related to treatment stiffness, thickness, and relief (patient) (range 6 to 60) after up to 6 months |
Azzam 2016: Number of participants that reported each satisfaction Keloid scar participants Excellent: 6/12 Good: 3/12 Moderate: 3/12 Hypertrophic scar patients Excellent: 2/7 Good: 1/7 Moderate: 2/7 Poor: 2/7 Azzam 2016: VSS (Mean + SD) Keloid scar patients Laser: 5.7 + 2.2 Control: 7.6 + 1.0 Hypertrophic scar patients Laser: 4.6 + 2.5 Control: 7.6 + 2.9 Blome‐Erbewein 2016 VSS (Mean + SD) Hypertrophic scar patients Laser: 6.5 + 2.39 Control: 6.41 + 2.31 Blome‐Eberwein 2016 POSAS Pre‐treatment Laser: 32.64 ± 12.41 Control: 29.91 ± 13.03 Post‐treatment Laser: 28.51 ± 12.85 Control: 24.38 ± 11.41 Daoud 2019 POSAS Significant improvements in all categories except for colour (P < 0.001) (results presented in graphs) |
| Fractional CO2 Laser versus no treatment | Incidence and severity of treatment‐related adverse effects | 1 RCT (Daoud 2019 23 participants; 46 scar segments) | No definition provided | The authors mention that no treatment‐related adverse effects were reported |
| Fractional CO2 Laser versus no treatment | Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain | 2 RCTS (Azzam 2016: 30 participants; 60 segments;,Blome‐Eberwein 2016: 36 participants; 80 scars) | Azzam 2016: pruritus and pain not related to treatment after 3 months Blome‐Eberwein 2016 Scar pliability and height evaluated with suction cup and ultrasound, colour; erythema and pigmentation assessed with Dermaspectrometer), sensation, pruritus, and pain not related to treatment evaluated with POSAS after up to 6 months |
Azzam: number of participants complaining of pruritus and pain Pruritus: 16 Pain: 5 (details not provided) Blome‐Eberwein 2016: pliability, height, recoil Mean and SD – before and after treatment Scar height Pre‐intervention Laser: 3.15 ± 0.37 Control: 2.658 ± 0.344 Post‐treatment: Laser: 2.34 ± 0.313 Control: 2.46 ± 0.342 |
HDTA: high‐density treatment arm; HPGA: Health Professional Global Assessment; IQR: interquartile range; LDTA: low‐density treatment arm; PGA: patient global assessment; POSAS: Patient and Observer Scar Assessment Scale; (In POSAS, highest values indicate worse scar or sensation); SD: standard deviation; VAS: visual analogue scale; VSS: Vancouver Scar Scale.
2. Laser versus other treatments outcome details.
| Comparison | Trials (participants) | Outcome | Definition of outcome in trial/measurement details | Data reported |
| 585‐nm PDL versus TAC | 2 RCTs (Omranifard 2007: 80 participants; 80 scars and Manuskiatti 2002: 10 participants; 20 segments) | Scar severity | Omranifard 2007: Patient Satisfaction assessed by VBS, including pigmentation, vascularity, pliability and height (photographs were taken). Self‐assessment: number of participants who considered their scars better or much better after up to 12 months Manuskiatti 2002: categories of 25% increments of improvement in scar severity after 32 weeks (graded by the participant) | Omranifard 2007: VBS (Mean + SD) Post‐treatment PDL: 4.2 + 1.6 TAC: 6.7 + 1.6 Self‐assessment PDL: 70% TAC: 30% Manuskiatti 2002: Number of participants with a 50% improvement or higher PDL: 8/10 TAC: 10/10 |
| 585‐nm PDL versus TAC | 2 RCTs (Omranifard 2007: 80 participants; 80 scars and Manuskiatti 2002: 10 participants; 20 segments) | Incidence and severity of treatment‐related adverse effects | Omranifard 2007: complications, such as textural or discolouration (hypo‐ or hyperpigmentation) during up to 12 months Manuskiatti 2002: Immediate treat reactions included mild to moderate pain during treatment, burning sensation, spots of purpura, erosion secondary to blistering. Treatment‐related adverse sequelae including hypopigmentation, telangiectasia, and skin atrophy during 32 weeks. | Omranifard 2007: no complication was observed Manuskiatti 2002: Sequelae: PDL: 0/10 TAC: 5/10 (Hypopigmentation 2, Telangiectasia 2, skin atrophy 1) Mild to moderate pain related to treatment: PDL: 9/10 Control: 10/10 (further details not provided) |
| 585‐nm PDL versus TAC | 2 RCTs (Omranifard 2007: 80 participants; 80 scars and Manuskiatti 2002: 10 participants; 20 segments) | Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain | Omranifard 2007: vascularity, using a transparent tool for blanching the scar. Scar height scores: calliper by measuring the maximum vertical elevation of the scar above the normal skin after up to 12 months Manuskiatti 2002: scar height evaluated using a dial calliper, erythema using a hand‐held colorimeter after 32 weeks. | Omranifard 2007: Vascularity (Mean) Pre‐treatment PDL: 2.3 TAC: 2.3 Post‐treatment PDL: 1.1 TAC: 1.95 Height (Mean) Pre‐treatment PDL: 2.16 TAC: 2.18 Post‐treatment PDL: 1.32 TAC: 1.93 Manuskiatti 2002: No significant difference between groups in height (results presented only in graphs) |
| 585‐nm PDL versus 5‐FU | 1 RCT (Manuskiatti 2002: 10 participants; 20 segments) | Scar severity | Manuskiatti 2002: categories of 25% increments of improvement in scar severity after 32 weeks (graded by the participant). | Manuskiatti 2002: Number of participants with a 50% improvement or higher PDL: 8/10 5‐FU: 10/10 |
| 585‐nm PDL versus 5‐FU | 1 RCT (Manuskiatti 2002: 10 participants; 20 segments) | Incidence and severity of treatment‐related adverse effects | Manuskiatti 2002: immediate treatment reactions included mild to moderate pain during treatment and spots of purpura during 32 weeks. | Manuskiatti 2002:
Mild to moderate pain during the injection:
PDL: 9/10
5‐FU: 10/10
Purpura: PDL: 10/10 5‐FU: 2/10 No permanent sequelae were reported in the areas submitted to laser therapy or 5‐FU. |
| 585‐nm PDL versus 5‐FU | 1 RCT (Manuskiatti 2002: 10 participants; 20 segments) | Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain | Manuskiatti2002: scar height evaluated using a dial calliper, erythema using a hand‐held colorimeter after 32 weeks. | No significant difference between groups in height (results presented only in graphs) |
| 585‐nm PDL versus TAC plus 5‐FU | 1 RCT (Manuskiatti 2002: 10 participants; 20 segments) | Scar severity | Manuskiatti 2002: categories of 25% increments of improvement in scar severity after 32 weeks (graded by the participant). | Manuskiatti 2002: Number of participants with a 50% improvement or higher PDL: 8/10 TAC plus 5‐FU: 9/10 |
| 585‐nm PDL versus TAC plus 5‐FU | 1 RCT (Manuskiatti 2002: 10 participants; 20 segments) | Incidence and severity of treatment‐related adverse effects | Manuskiatti 2002: immediate treatment reactions included mild to moderate pain during treatment and spots of purpura during 32 weeks. | Manuskiatti 2002:
Mild to moderate pain during the injection:
PDL: 9/10
5‐FU: 10/10
Purpura: PDL: 10/10 5‐FU: 3/10 No permanent sequelae were reported in the areas submitted to laser therapy or 5‐FU. |
| 585‐nm PDL versus TAC plus 5‐FU | 1 RCT (Manuskiatti 2002: 10 participants; 20 segments) | Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain | Manuskiatti2002: scar height evaluated using a dial calliper, erythema using a hand‐held colorimeter after 32 weeks. | Mild to moderate pain not related to treatment PDL: 9/10 TAC plus 5‐FU: 10/10 No significant difference between groups in height (results presented only in graphs). |
| 585‐nm PDL versus Silicone Gel Sheeting | 1 RCT (Wittenberg 1999; 20 participants; 40 segments) | Incidence and severity of treatment‐related adverse effects | Wittenberg 1999: No definition is provided | Wittenberg 1999: one participant dropped out due to pain during laser treatment. One participant did not use SGS because of skin irritation. |
| Erbium laser versus TAC | 1 RCT (Omranifard 2007: 80 participants; 80 scars) | Scar severity | Omranifard 2007: Patient Satisfaction assessed by VBS, including pigmentation, vascularity, pliability and height. (photographs were taken) Self‐assessment: improvement of the scars severity after up to 12 months. | Omranifard 2007: VBS (Mean + SD) Post‐treatment Erbium: 4.6 + 1.9 TAC: 6.7 + 1.6 Self‐assessment (number of participants who considered their scars better or much better) Erbium: 65% TAC: 30% |
| Erbium laser versus TAC | 1 RCT (Omranifard 2007: 80 participants; 80 scars) | Incidence and severity of treatment‐related adverse effects | Omranifard 2007: complications, such as textural or discoloration (hypo‐ or hyperpigmentation) during up to 12 months. | Omranifard 2007: no complication was observed |
| Erbium laser versus TAC | 1 RCT (Omranifard 2007: 80 participants; 80 scars) | Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain | Omranifard 2007: vascularity, using a transparent tool for blanching the scar. Scar height scores: calliper by measuring the maximum vertical elevation of the scar above the normal skin after up to 12 months. | Omranifard 2007: Vascularity (Mean) pre‐treatment Erbium: 2.4 TAC: 2.3 Post‐treatment Erbium: 1.15 TAC: 1.95 Height (Mean) Pre‐treatment Erbium: 2.18 TAC: 2.18 Post‐treatment Erbium: 1.39 TAC: 1.93 |
| Fractional CO2 Laser versus TAC | 1 RCT (Srivastava 2019; 40 participants; 40 scars) | Scar severity | Srivastava 2019: VSS | Srivastava 2019 Results of full scale were not reported |
| Fractional CO2 Laser versus TAC | 1 RCT (Srivastava 2019; 40 participants; 40 scars) | Incidence and severity of treatment‐related adverse effects | Srivastava 2019: pain at injection site, telangiectasia, skin atrophy and charring were evaluated and reported (when they occurred) during 24 weeks. | Srivastava 2019: Number of participants reporting treatment‐related adverse effects Pain at injection site FCO2: 2/20 TAC: 8/20 Telangiectasia FCO2: 0/20 TAC: 2/20 Skin atrophy FCO2: 0/20 TAC: 1/20 Charring FCO2: 3/20 TAC: 0/20 |
| Fractional CO2 Laser versus TAC | 1 RCT (Srivastava 2019; 40 participants; 40 scars) | Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain | Srivastava 2019: height measured with callipers; vascularity by visual inspection; pliability by palpation; pigmentation after blanching (using a piece of clear plastic sheet till scar flattening occurred) and comparing it with the surrounding skin, pruritus, and pain not related to treatment after 24 weeks. | Srivastava 2019: Height (Mean + SD) Pre‐treatment FCO2: 1.95 + 0.76 TAC: 1.75 + 0.64 Post‐treatment FCO2: 0.25 + 0.44 TAC: 0 + 0 Vascularity Pre‐treatment FCO2: 2.05 + 0.69 TAC: 1.65 + 0.49 Post‐treatment FCO2: 0.45 + 0.51 TAC: 0 + 0 Pliability Pre‐treatment FCO2: 1.85 + 0.67 TAC: 1.9 + 0.64 Post‐treatment FCO2: 0.9 + 0.31 TAC: 0 + 0 Pigmentation Pre‐treatment FCO2: 1.60 + 0.50 TAC: 1.7 + 0.47 Post‐treatment FCO2: 0.8 + 0.41 TAC: 0.8 + 0.41 |
| Fractional CO2 Laser versus Verapamil | 1 RCT (Srivastava 2019; 40 participants 40 scars) | Scar severity | Srivastava 2019: VSS | Srivastava 2019: Results of full scale were not reported |
| Fractional CO2 Laser versus Verapamil | 1 RCT (Srivastava 2019; 40 participants 40 scars) | Incidence and severity of treatment‐related adverse effects | Srivastava 2019: pain at injection site, telangiectasia, skin atrophy and charring were evaluated and reported (when they occurred) during 24 weeks. | Srivastava 2019: Number of participants reporting treatment‐related adverse effects Pain at injection site FCO2: 2/20 Verapamil: 0/20 Telangiectasia FCO2: 0/20 Verapamil: 0/20 Skin atrophy FCO2: 0/20 Verapamil: 0/20 Charring FCO2: 3/20 Verapamil: 0/20 |
| Fractional CO2 Laser versus Verapamil | 1 RCT (Srivastava 2019; 40 participants 40 scars) | Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain | Srivastava 2019: height measured with callipers; vascularity by visual inspection; pliability by palpation; pigmentation after blanching (using a piece of clear plastic sheet till scar flattening occurred) and comparing it with the surrounding skin, pruritus, and pain not related to treatment after 24 weeks. | Srivastava 2019: Height (Mean + SD) Pre‐treatment FCO2: 1.95 + 0.76 Verapamil: 2.05 + 0.6 Post‐treatment FCO2: 0.25 + 0.44 Verapamil: 0.05 + 0.22 Vascularity Pre‐treatment FCO2: 2.05 + 0.69 Verapamil: 1.95 + 0.69 Post‐treatment FCO2: 0.45 + 0.51 Verapamil: 0.1 + 0.31 Pliability Pre‐treatment FCO2: 1.85 + 0.67 Verapamil: 2.1 + 0.64 Post‐treatment FCO2: 0.9 + 0.31 Verapamil: 0 + 0 Pigmentation Pre‐treatment FCO2: 1.60 + 0.50 Verapamil: 1.65 + 0.49 Post‐treatment FCO2: 0.8 + 0.41 Verapamil: 0.55 + 0.51 |
5‐FU: Fluorouracil; PDL: Pulsed‐Dye Laser; TAC: Triamcinolone acetonide;VSS: Vancouver Scar scale.
3. Laser plus other treatments versus other treatments outcome details.
| Comparison | N. of Trials | Outcome | Definition of outcome in trial/measurement details | Data reported |
| 585‐nm PDL plus TAC plus 5 FU versus TAC plus 5‐FU | 1 RCT (Asilian 2006; 43 participants; 43 scars) | Scar severity | Asilian 2006: patient and Observer: no improvement; poor = up to 25% improvement; fair = 26% to 50% improvement; good = 51% to 75% improvement; (for the observer assessment photographs were taken) after 12 weeks | Asilian 2006: Number of participants and observers reporting good to excellent improvement OA 585‐nm PDL plus TAC plus 5 FU: 14/20 TAC plus 5‐FU: 8/20 PSA 585‐nm PDL plus TAC plus 5 FU: 15/20 TAC plus 5‐FU: 11/20 |
| 585‐nm PDL plus TAC plus 5 FU versus TAC plus 5‐FU | 1 RCT (Asilian 2006; 43 participants; 43 scars) | Incidence and severity of treatment‐related adverse effects | Asilian 2006: treatment‐related adverse effects including the presence of purpuric areas by observer interviews during 12 weeks | Asilian 2006: In the TAC plus 5‐FU plus PDL group the lesions became purpuric which lasted from 7 to 10 days |
| 585‐nm PDL plus TAC plus 5 FU versus TAC plus 5‐FU | 1 RCT (Asilian 2006; 43 participants; 43 scars) | Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain | Asilian 2006: scar length and width using a dial calliper, height using a calliper, erythema, pliability, and pruritus: graded by the observer on a 5‐point scale: 0 = no; 1 = mild; 2 = moderate; 3 = severe; and 4 = very severe. | Asilian 2006: Erythema Pre‐treatment PDL plus TAC plus 5‐FU: 3.2 TAC plus 5‐FU: 3.3 Post‐treatment TAC plus 5‐FU plus PDL: 1.2 TAC plus 5‐FU: 1.9 Reduction in height (average lesion) TAC plus 5‐FU: 77% PDL plus TAC plus 5‐FU: 79% |
| CO2 Laser plus TAC versus Cryosurgery plus TAC | 1 RCT (Behera 2016: 60 participants; 101 scars) | Scar severity | Behera 2016: patient and observer on a 5‐point scale as, poor = up to 25% improvement; fair = 26% to 50% improvement; good = 51% to 75% improvement; and excellent = 76% to 100% improvement (for the observer assessment photographs were taken) VSS after up to 12 months | Behera 2016: Number (%) of participants with an improvement of 50% or higher in the scar: PSA: CO2 Laser plus TAC: 27 (75%); Cryosurgery plus TAC: 21 (77.78%) OA: CO2 Laser plus TAC: 22 (61.12%); Cryosurgery plus TAC: 23 (85.18%) VSS: CO2 Laser plus TAC: 19 (52.78%); Cryosurgery plus TAC: 17 (62.96%) |
| CO2 Laser plus TAC versus Cryosurgery plus TAC | 1 RCT (Behera 2016: 60 participants; 101 scars) | Recurrence | Behera 2016: no definition was provided (after up to 12 months) | Behera 2016: CO2 Laser plus TAC: 6 (16.66%) Cryosurgery plus TAC: 0 (0%) |
| CO2 Laser plus TAC versus Cryosurgery plus TAC | 1 RCT (Behera 2016: 60 participants; 101 scars) | Incidence and severity of treatment‐related adverse effects | Behera 2016: no definition was provided (during up to 12 months) | Behera 2016: Number (%) of participants with atrophy CO2 Laser plus TAC: 17 (47.23%); Cryosurgery plus TAC: 15 (55.56%) |
| CO2 Laser plus TAC versus Cryosurgery plus TAC | 1 RCT (Behera 2016: 60 participants; 101 scars) | Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain | Behera 2016: height: using a dial calliper, volume, pruritus, and pain not related to treatment after up to 12 months | Behera 2016:
Height (mean percentage reduction + SD): CO2 Laser plus TAC: 49.31 + 50.42; Cryosurgery plus TAC: 65.46 + 35.63 Volume (Mean percentage reduction + SD): CO2 Laser plus TAC: 54.81 + 47.96; Cryosurgery plus TAC: 72.69 + 37.75 |
| Nd:YAG laser plus Intralesional corticosteroid plus 5‐FU versus Intralesional corticosteroid plus 5‐FU | 1 RCT (Chen 2017: 46 participants; 46 scars) | Scar severity | Chen 2017: patient and observer on a 5‐point scale as, poor = up to 25% improvement; fair = 26% to 50% improvement; good = 51% to 75% improvement; and excellent = 76% to 100% improvement (for the observer assessment photographs were taken) after 3 months. | Chen 2017: OA: Number (%) of participants with an improvement of 51% or higher in the scar: Nd:YAG laser plus Intralesional corticosteroid plus 5‐FU: 16 (69.57%); Intralesional corticosteroid plus 5‐FU: 11 (47.83%) PSA: Nd:YAG laser plus Intralesional corticosteroid plus 5‐FU: 18 (78.26%); Intralesional corticosteroid plus 5‐FU: 13 (56.52%%) |
| Nd:YAG laser plus Intralesional corticosteroid plus 5‐FU versus Intralesional corticosteroid plus 5‐FU | 1 RCT (Chen 46 participants; 46 scars) | Incidence and severity of treatment‐related adverse effects | Chen 2017: no definition was provided (during 3 months) | Chen 2017: Almost all injections were painful. Nd:YAG plus Intralesional corticosteroid plus 5‑FU: the site treated by Nd:YAG became purpuric, which lasted for 7 to 10 days. No adverse textural or pigmentary alterations, and no ulcers or erosions were observed in either groups. No further information was provided regarding adverse events in the trial. |
| Nd:YAG laser plus Intralesional corticosteroid plus 5‐FU versus Intralesional corticosteroid plus 5‐FU | 1 RCT (Chen 46 participants; 46 scars) | Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain | Chen 2017: erythema, pliability, and pruritus were graded by the observer on a 5‐point scale: 0 = no erythema; 1 = mild erythema; 2 = moderate erythema; 3 = severe erythema; and 4 = very severe erythema after 3 months. | Chen 2017: Erythema (P < 0.05), pliability (P < 0.05), and pruritus (P < 0.05) were significantly lower in the laser group (data provided only in graphs). |
| He‐Ne laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream versus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream | 1 RCT (Alsharnoubi 2018: 15 participants; 30 segments) | Scar severity | Alsharnoubi 2018: VSS, including skin thickness, pigmentation, and vascularity after 3 months. | Alsharnoubi 2018: VSS: median values Pre‐treatment: 9 (value of the whole scar) Post treatment He‐Ne laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 4; Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 6; Height score: Pre‐treatment: 2 (value of the whole scar) Post treatment He‐Ne laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 1; Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 2 |
| He‐Ne laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream versus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream | 1 RCT (Alsharnoubi 2018: 15 participants; 30 scar segments) | Incidence and severity of treatment‐related adverse effects | Definition was not provided | No treatment‐related adverse effects were reported. |
| He‐Ne laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream versus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream | 1 RCT (Alsharnoubi 2018: 15 participants; 30 scar segments) | Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain | Alsharnoubi 2018: scar thickness using an ultrasound imaging system, perfusion (erythema) using a Laser Doppler perfusion imager. | Alsharnoubi 2018: Skin thickness (mean ± SD) Pre‐treatment: 0.52 ± 0.17 mm (whole scar) Post treatment He‐Ne laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 0.34 ± 0.09 mm; Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 0.43 ± 0.13 mm Perfusion Pre‐treatment: 1.27 ± 0.54 V (whole scar) Post treatment He‐Ne laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 0.8 ± 0.23 V; Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 0.77 ± 0.24 V Pigmentation Pre‐treatment: 2 (whole scar) Post Treatment He‐Ne laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 0; Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 2; Vascularity Pre‐treatment: 2 (whole scar) Post Treatment He‐Ne laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 1 Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream: 1 (data presented in graphs) |
| PDL plus intralesional verapamil versus intralesional verapamil | 1 RCT (Khattab 2019; 40 participants; 50 keloid scars) | Scar severity | Khattab 2019: VSS, keloid height measured with callipers; pliability by palpation; vascularity by visual inspection, and pigmentation scored after blanching and comparing it with the surrounding skin after 24 weeks. | Khattab 2019: Height (Mean + SD) PDL plus verapamil: 0.21 ± 0.56; Verapamil: 3.10±1.85 Pliabilty: PDL plus verapamil: 0.20±0.41; Verapamil: 2.07±0.26 Vascularity PDL plus verapamil: 0.03 ± 0.70; Verapamil: 0.87±0.74 Pigmentation PDL plus verapamil: 0.13 ± 0.35; Verapamil: 0.27±0.70 |
| PDL plus intralesional verapamil versus intralesional verapamil | 1 RCT (Khattab 2019; 40 participants; 50 keloid scars) | Incidence and severity of treatment‐related adverse effects | Khattab 2019: treatment‐related adverse effects were regrowth, pain, hyperpigmentation, depigmentation, and purpura during 24 weeks. | Khattab 2019: Treatment‐related adverse events: Regrowth PDL plus verapamil: 3 (12%) Verapamil: 2 (8%) Pain PDL plus verapamil: 1 (4%); Verapamil: 0 (0%) Hyperpigmentation PDL plus verapamil: 2 (8%) Verapamil: 0 (0%) Depigmentation PDL plus verapamil: 1 (4%) Verapamil: 1 (4%) Purpura PDL plus verapamil: 7 (28%) Verapamil: 0 (0%) Total PDL plus verapamil: 14 (56%) Verapamil: 3 (12%) |
| PDL plus intralesional verapamil versus intralesional verapamil | 1 RCT (Khattab 2019; 40 participants; 50 keloid scars) | Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain | Khattab 2019: height, vascularity, pliability, and pigmentation were assessed at 24 weeks. | Khattab 2019:
Height
PDL plus verapamil: 0.21 + 0.56;
Verapamil: 3.1 + 1.85
Vascularity
PDL plus verapamil: 0.03 + 0.07; Verapamil: 0.87 + 0.74 Pliability PDL plus verapamil: 0.2 + 0.41; Verapamil: 2.07 + 0.26 Pigmentation PDL plus verapamil: 0.13 + 0.35; Verapamil: 0.27 + 0.7 |
5‐FU: Fluorouracil; NdYAG: neodymium‐doped yttrium aluminium garnet; PDL: Pulsed‐Dye Laser; SD: standard deviation; TAC: Triamcinolone acetonide;VSS: Vancouver Scar scale.
Outcomes
The primary outcome of severity of keloid or hypertrophic scars (or both), assessed using a range of specific scales across studies, for both participant and health professional‐reported data, and incidence and severity of treatment‐related adverse effects were reported in 12 of the studies evaluated (Alsharnoubi 2018; Asilian 2006; Behera 2016; Chen 2017; Daoud 2019; Khattab 2019; Lin 2011; Manuskiatti 2001; Manuskiatti 2002; Omranifard 2007; Srivastava 2019; Verhaeghe 2013). Azzam 2016 ,and Blome‐Eberwein 2016 did not report treatment‐related adverse effects. Wittenberg 1999 only reported treatment‐related adverse effects.
Secondary outcomes were reported as follows.
Alsharnoubi 2018: skin thickness, pigmentation, and vascularity
Asilian 2006: colour, height, length, width, pliability, and pruritus
Azzam 2016: pruritus and pain
Behera 2016: height, volume, pruritus and pain
Blome‐Eberwein 2016: colour, height, pliability, sensation, pruritus and pain
Chen 2017: erythema, pliability, and pruritus
Daoud 2019: colour, matte versus shiny, contour, distortion, and texture
Khattab 2019: overall appearance, dyschromia (discolourisation), the degree of hypertrophy, and texture
Lin 2011: size, colour, and texture
Manuskiatti 2001: colour, height, pliability, texture, pruritus and pain
Manuskiatti 2002: colour, height, and pliability
Omranifard 2007: colour, height, and patient tolerance
Srivastava 2019: height, pigmentation, vascularity, and pliability
Verhaeghe 2013: pain, itching, colour, stiffness, thickness, relief, vascularity, pigmentation, pliability, and surface area
Wittenberg 1999: size, pliability, colour, pruritus and pain.
Callipers, colorimeters, ultrasound, cutometer, spectrometer, molds, and forms were used to evaluate these parameters.
Excluded studies
Of the 44 full‐text articles evaluated, 17 were excluded and the reasons for their exclusion are described in Characteristics of excluded studies.
One study is awaiting classification (Maari 2017) and 11 are ongoing studies see Characteristics of ongoing studies.
Risk of bias in included studies
We evaluated and presented the risk of bias of each RCT as part of the Characteristics of included studies tables. Figure 2 presents the judgments of the review authors on the methodological appraisal as percentages across all included studies. Figure 3 presents the judgements of the review authors on the methodological appraisal of each risk of bias item of the included studies. All studies were assessed as being at high risk of bias for at least one domain.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven studies described their sequence generation methods (Azzam 2016; Behera 2016; Blome‐Eberwein 2016; Lin 2011; Srivastava 2019; Verhaeghe 2013; Wittenberg 1999) and were rated as low risk of bias. Four RCTs (Azzam 2016; Behera 2016; Blome‐Eberwein 2016; Verhaeghe 2013) also provided an adequate description of allocation concealment and were rated as low risk of bias: meaning they were considered as low risk for bias arising from the randomisation process.
Blinding
Since we were comparing a procedure (laser therapy) with other therapies and blinding is difficult from a practical perspective, participants and interventionists were not blinded in any of the RCTs evaluated and they were considered as high risk for performance bias. Twelve trials (Alsharnoubi 2018; Asilian 2006; Azzam 2016; Behera 2016; Blome‐Eberwein 2016; Chen 2017; Daoud 2019; Lin 2011; Omranifard 2007; Srivastava 2019; Verhaeghe 2013; Wittenberg 1999) had blinded observers, so were rated as low risk for detection bias for non‐participant reported outcomes. The remaining three RCTs Khattab 2019; Manuskiatti 2001; Manuskiatti 2002) did not mention the blinding of the evaluators and were rated as unclear risk of bias.
Incomplete outcome data
In four trials (Manuskiatti 2001; Manuskiatti 2002; Omranifard 2007; Srivastava 2019), all participants completed the study, and these trials were considered as low risk of bias. Two RCTs (Verhaeghe 2013; Wittenberg 1999) reported dropouts, but explained the reasons, and were also rated as low risk of bias. Two trials (Alsharnoubi 2018; Khattab 2019) did not provide enough information to judge if there were losses, and these trials were rated as unclear risk of bias. Two trials (Blome‐Eberwein 2016; Lin 2011) had substantial losses and although the reasons for the losses were explained, the authors performed as‐treated analyses. Thus, these trials were rated as high risk of bias. Five trials (Asilian 2006; Azzam 2016; Behera 2016; Chen 2017; Daoud 2019) had losses and did not provide enough information about the reasons for dropouts, so were considered at high risk of bias.
Selective reporting
Four trials (Alsharnoubi 2018; Asilian 2006; Azzam 2016; Omranifard 2007) did not describe the data for all parameters analysed, and their protocols were not available, so they were considered as high risk of bias. Eight RCTs (Blome‐Eberwein 2016; Chen 2017; Khattab 2019; Lin 2011; Manuskiatti 2001; Manuskiatti 2002; Verhaeghe 2013; Wittenberg 1999) reported all parameters specified in the methods section or in their protocols, so were rated as low risk of bias. Three trials (Behera 2016; Daoud 2019; Srivastava 2019) were considered as unclear risk of bias as the authors described the findings for all parameters analysed, however, they did not explain what kind of tool they used to analyse these parameters and no trial protocol was available.
Other potential sources of bias
All split‐scar trials performed paired analyses as required so we did not consider them to be at risk of bias in terms of analytical approach. There were no other potential sources of bias noted in any of the included studies, and all of them were rated as low risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Laser therapy compared with no treatment for treating hypertrophic and keloid scars.
| Laser therapycompared with no treatment for treating hypertrophic and keloid scars | ||||||
| Patient or population: patients with hypertrophic and keloid scars Setting: outpatient Intervention: laser therapy (various types ‐ 585‐nm Pulsed‐Dye Laser (PDL), Non‐Ablative Fractional Laser (NAFL), Fractional CO2) Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | № of scar segment (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with Laser therapy | |||||
| Scar severity ‐ 585‐nm Pulsed‐Dye Laser (PDL) versus no treatment ‐ patient self‐assessment of scar improvement of 50% or higher ‐ hypertrophic and keloid scars ‐ follow‐up: 32 weeks | Study population | RR 1.96 (1.11 to 3.45) | 60 (2 studies) | ⊕⊕⊝⊝1,2 Low | There may be more hypertrophic and keloid scar improvement (that is scars are less severe) in 585‐nm PDL‐treated scars compared with no treatment after 32 weeks. |
|
| 400 per 1000 | 784 per 1000 | |||||
| Incidence and severity of treatment‐related adverse effects ‐ 585‐nm PDL versus no treatment ‐ mild to moderate discomfort or pain related to treatment ‐ hypertrophic and keloid scars ‐follow‐up: 32 weeks | Two split‐scar trials (n = 60) reported this outcome. In these studies, participants reported mild to moderate discomfort or pain in 10 out of 30 (10/30) (33%) PDL treated areas versus 0 out of 30 (0/30) (0%) no treatment areas (RR 8.62; 1.10 to 67.39). | ⊕⊝⊝⊝2,3 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in 585‐nm PDL‐treated hypertrophic and keloid scars compared with no treatment after 32 weeks. | |||
| Incidence and severity of treatment‐related adverse effects ‐ 585‐nm PDL versus no treatment ‐ purpura ‐ hypertrophic and keloid scars ‐ follow‐up: 32 weeks | Two split‐scar trials (n = 60) reported this outcome. In these studies, purpura was observed in 40 out of 40 (40/40) (100%) PDL treated areas versus 0 out of 20 (0/20) (0%) no treatment areas (RR 21.32; 3.14 to 144.86). | ⊕⊝⊝⊝2,3 Very low | ||||
| Scar severity ‐ Non‐Ablative Fractional Laser (NAFL) versus no treatment ‐ health professional global assessment measured on a visual analogue scale (VAS) ranging from 0 to 100 mm (0 = as normal skin and 100 = worst possible scar) ‐ hypertrophic scars ‐ follow‐up: 3 months | Study population | RR 2.00 (0.85 to 4.69) |
36 (1 study) |
⊕⊝⊝⊝3,4 Very low | It is uncertain whether there is any difference in the scar severity in NAFL‐treated hypertrophic scars compared with no treatment after 3 months. | |
| 278 per 1000 | 556 per 1000 |
|||||
| Scar severity ‐ NAFL versus no treatment ‐ patient global assessment measured on a VAS ranging from 0 to 100 mm (0 = as normal skin and 100 = worst possible scar) ‐ hypertrophic scars ‐ follow‐up: 3 months | One split‐scar trial (n = 36) reported this outcome. In this study, the authors reported an improvement in scar severity in NAFL treated hypertrophic scars compared with no treatment on the patient global assessment at 1 month (reported P = 0.006) and 3 months (reported P = 0.02). | ⊕⊝⊝⊝5,6 Very low | ||||
| Scar severity ‐ NAFL versus no treatment ‐ Patient and Observer Scar Assessment Scale (POSAS) (higher scores = worse scar appearance) ‐ hypertrophic scars ‐ follow‐up: up to 3 months | One split‐scar trial (n = 36) reported this outcome. In this study, the authors reported an improvement in scar severity in NAFL treated hypertrophic scars compared with no treatment on the participant part of the scale at 1 month and 3 months. The size of the difference was not reported and no data for the observer part of the scale was presented. | ⊕⊝⊝⊝5,6 Very low | ||||
| Incidence and severity of treatment‐related adverse effects ‐ NAFL versus no treatment ‐ scar worsening ‐ hypertrophic scars ‐ follow‐up: 3 months | One split‐scar trial (n = 20) reported this outcome. In this study, 3 out of 10 (3/10) (30%) NAFL treated areas versus 0 out of 10 (0/10) (0%) no treatment areas were considered by the patients to have worsened (RR 7.00; 0.41 to 120.16). | ⊕⊝⊝⊝3,7 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in NAFL‐treated hypertrophic scars compared with no treatment after 3 months. | |||
| Incidence and severity of treatment‐related adverse effects ‐ NAFL versus no treatment ‐ hyperpigmentation ‐ hypertrophic scars ‐ follow‐up: 3 months | One split‐scar trial (n = 36) reported this outcome. In this study, hyperpigmentation was observed in 1 out of 18 (1/18) (6%) NAFL treated areas versus 0 out of 18 (0/18) (0%) no treatment areas (RR 3.00; 0.13 to 69.09). | ⊕⊝⊝⊝3,5 Very low | ||||
| Scar severity ‐ Fractional Carbon Dioxide (CO2) Laser versus no treatment ‐ Vancouver Burn Scar (VBS) scale (higher scores = worse scar appearance) ‐ hypertrophic scars ‐ follow‐up: up to 3 months | Study population | NA | 104 (2 studies) |
⊕⊝⊝⊝6,8 Very low | It is uncertain whether there is any difference in the scar severity in Fractional CO2‐treated hypertrophic and keloid scars compared with no treatment after up to 3 months, and in Fractional CO2‐treated hypertrophic scars compared with no treatment after at least 1 month. | |
| Baseline mean in the no treatment group was 7.6 | MD 1.30 lower (4.32 lower to 1.71 higher) | |||||
| Scar severity ‐ Fractional CO2 Laser versus no treatment ‐ VBS (higher scores = worse scar appearance) ‐ keloid scars ‐ follow‐up: 3 months | Study population | NA | 24 (1 study) |
⊕⊝⊝⊝6,9 Very low | ||
| Baseline mean in the no treatment group was 7.6 | MD 1.90 lower (3.02 lower to 0.78 lower) | |||||
| Scar severity ‐ Fractional CO2 Laser versus no treatment ‐ POSAS scale (higher scores = worse scar appearance) ‐ hypertrophic scars ‐ follow‐up: at least 1 month | Study population | NA | 80 (1 study) |
⊕⊝⊝⊝6,10 Very low | ||
| Baseline mean in the no treatment group was 29.9 | MD 4.13 higher (1.24 lower to 9.50 higher) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). ƚThe assumed risk in the comparison group is based on the event rate observed in the control arms of included trials. Where no events occurred, the risk was not calculated. VAS: visual analogue scale ‐ ranging from 0 to 100 mm (0 = normal skin and 100 = worst possible scar); Patient self‐assessment ‐ based on a 4‐point scale (1 = 0 to 25% improvement, 2 = 25 to 50% improvement, 3 = 50 to 75% improvement, and 4 = 75% or greater improvement); POSAS: Patient and Observer Scar Assessment Scale ‐ the lowest score (6) reflects normal skin, and the highest score (60) reflects the worst imaginable scar; VBS: Vancouver Burn Scar Assessment Scale ‐ severity of scar was determined by numeric value from a minimum of 0 to 13 as the most severe form. CI: Confidence Interval; CO2: carbon dioxide;LDTA: (Low‐Density Treatment Arm of NAFL), HDTA: (High‐Density Treatment Arm of NAFL); MD: Mean Difference; NAFL: Non‐Ablative Fractional Laser ;PDL: Pulsed‐Dye Laser; RR: Risk Ratio; VBS: Vancouver Burn Scar Assessment Scale. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded 1 level for serious imprecision due to small number of events.
2 Downgraded 1 level for serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome), and unclear sequence generation and allocation concealment).
3 Downgraded 2 levels for very serious imprecision due to small number of events and large confidence interval.
4 Downgraded 1 level for serious risk of bias (lack of blinding of participants).
5 Downgraded 1 level for serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome)).
6 Downgraded 2 levels for very serious imprecision due to small sample size and large confidence interval.
7 Downgraded 1 level for serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome), incomplete outcome data, and unclear allocation concealment).
8 Downgraded 1 level for serious risk of bias (selective reporting in one study, and lack of blinding of participants and incomplete outcome data in 2 studies).
9 Downgraded 1 level for serious risk of bias (lack of blinding of participants, incomplete outcome data and selective reporting).
10 Downgraded 1 level for serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome) and incomplete outcome data).
Summary of findings 2. Laser therapy compared with other treatments for treating hypertrophic and keloid scars.
| Laser therapy compared with other treatments for treating hypertrophic and keloid scars | ||||||
| Patient or population: participants with hypertrophic and keloid scars Setting: outpatient Intervention: laser therapy (various types ‐ 585‐nm Pulsed‐Dye Laser (PDL), Erbium Laser, Fractional Carbon Dioxide (CO2) Laser) Comparison: other treatments (various types ‐ triamcinolone acetonide (TAC), 5‐Fluorouracil (5‐FU), verapamil) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of scar segments (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other treatments | Risk with Laser therapy | |||||
| Scar severity ‐ 585‐nm Pulsed‐Dye Laser (PDL) versus Triamcinolone acetonide (TAC) ‐ Vancouver Burn Scar (VBS) (Higher score = worse scar appearance) ‐ hypertrophic scars ‐ follow‐up: up to 12 months | Study population | NA | 80 (1 study) | ⊕⊝⊝⊝1,2 Very low | It is uncertain whether there is any difference in the scar severity in PDL‐treated hypertrophic and keloid scars compared with TAC after up to 12 months. | |
| Baseline mean in the other treatment group was 6.7 | MD 2.5 lower (3.2 lower to 1.8 lower) | |||||
| Scar severity ‐ 585‐nm PDL versus TAC ‐ patient self‐assessment of scar improvement of 50% or higher, or patients considering the scar better or much better ‐ hypertrophic and keloid scars ‐ follow‐up: up to 12 months | One split‐scar trial (n = 20) and one parallel trial (n = 80) reported this outcome. In the split scar trial, 8 out of 10 (8/10) (80%) PDL treated areas versus 10 out of 10 (10/10) (100%) TAC treated areas were considered to have improved 50% or more by participants (RR 0.81; 0.57 to 1.14), and in the parallel trial, 28 out of 40 participants (28/40) (70%) treated with PDL and 12 out of 40 participants (12/40) (30%) treated with TAC considered their scars better or much better (RR 2.33; 1.39 to 3.91). | ⊕⊝⊝⊝3,4 Very low | ||||
| Incidence and severity of treatment‐related adverse effects ‐ 585‐nm PDL versus TAC: sequelae ‐ hypertrophic and keloid scars ‐ follow‐up: 32 weeks | Study population | RR 0.09 (0.01 to 1.45) | 20 (1 study) | ⊕⊝⊝⊝3,5 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in PDL‐treated hypertrophic and keloid scars compared with TAC after 32 weeks. |
|
| 500 per 1000 | 45 per 1000 | |||||
| Incidence and severity of treatment‐related adverse effects ‐ 585‐nm PDL versus TAC ‐ mild to moderate pain related to treatment ‐ hypertrophic and keloid scars ‐ follow‐up: 32 weeks | One split‐scar trial (n = 20) reported this outcome. In this study, participants reported to have felt mild to moderate pain during the intervention in 10 out of 10 (10/10) (100%) TAC treated areas versus in 9 out of 10 (9/10) (90%) PDL treated areas (RR 0.90; 0.69 to 1.18). | ⊕⊝⊝⊝3,5 Very low | ||||
| Scar severity ‐ 585‐nm PDL versus 5‐FU ‐ patient self‐assessment of scar improvement of 50% or higher ‐ hypertrophic and keloid scars ‐ follow‐up: 32 weeks | One split‐scar trial (n = 20) reported this outcome. In this study, 8 out of 10 (8/10) (80%) PDL treated areas versus 10 out of 10 (10/10) (100%) 5‐FU treated areas were reported by the participants to have improved 50% or more (RR 0.81; 0.57 to 1.14). | ⊕⊝⊝⊝3,5 Very low | It is uncertain whether there is any difference in the scar severity in PDL‐treated hypertrophic and keloid scars compared with 5‐FU after 32 weeks. | |||
| Incidence and severity of treatment‐related adverse effects ‐ 585‐nm PDL versus 5‐FU ‐ mild to moderate pain related to treatment ‐ hypertrophic and keloid scars ‐ follow‐up: 32 weeks | One split‐scar trial (n = 20) reported this outcome. In this study, participants reported to have felt mild to moderate pain during the intervention in 9 out of 10 (9/10) (90%) PDL treated areas versus in 10 out of 10 (10/10) (100%) 5‐FU treated areas (RR 0.90; 0.69 to 1.18). | ⊕⊝⊝⊝3,5 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in PDL‐treated hypertrophic and keloid scars compared with 5‐FU after 32 weeks. | |||
| Incidence and severity of treatment‐related adverse effects ‐ 585‐nm PDL versus 5‐FU ‐ purpura ‐ hypertrophic and keloid scars ‐ follow‐up: 32 weeks | One split‐scar trial (n = 20) reported this outcome. In this study, purpura was observed in 10 out of 10 (10/10) (100%) PDL treated areas versus in 2 out of 10 (2/10) (20%) 5‐FU treated areas (RR 4.20; 1.40 to 12.58). | ⊕⊝⊝⊝3,5 Very low | ||||
| Scar severity ‐ 585‐nm PDL versus TAC plus 5‐FU ‐ patient self‐assessment of scar improvement of 50% or higher ‐ hypertrophic and keloid scars ‐ follow‐up: 32 weeks | Study population | RR 0.89 (0.61 to 1.29) | 20 (1 study) | ⊕⊝⊝⊝3,5 Very low | It is uncertain whether there is any difference in the scar severity in PDL‐treated hypertrophic and keloid scars compared with TAC plus 5‐FU after 32 weeks. | |
| 900 per 1000 | 801 per 1000 | |||||
| Incidence and severity of treatment‐related adverse effects ‐ 585‐nm PDL versus TAC plus 5‐FU ‐ mild to moderate pain related to treatment ‐ hypertrophic and keloid scars ‐ follow‐up: 32 weeks | One split‐scar trial (n = 20) reported this outcome. In this study, participants reported to have felt mild to moderate pain during the intervention in 9 out of 10 (9/10) (90%) PDL treated areas versus in 10 out of 10 (10/10) (100%) TAC plus 5‐FU treated areas (RR 0.90; 0.69 to 1.18). | ⊕⊝⊝⊝3,5 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in PDL‐treated hypertrophic and keloid scars compared with TAC plus 5‐FU after 32 weeks. | |||
| Incidence and severity of treatment‐related adverse effects ‐ 585‐nm PDL versus TAC plus 5‐FU ‐ purpura ‐ hypertrophic and keloid scars ‐ follow‐up: 32 weeks | One split‐scar trial (n = 20) reported this outcome. In this study, purpura was observed in 10 out of 10 (10/10) (100%) PDL treated areas versus in 3 out of 10 (3/10) (30%) TAC plus 5‐FU treated areas (RR 3.00; 1.25 to 7.19). | ⊕⊝⊝⊝3,5 Very low | ||||
| Incidence and severity of treatment‐related adverse effects ‐ 585‐nm PDL versus silicone gel sheeting ‐ pain during laser treatments ‐ hypertrophic scars ‐ follow‐up: 24 weeks | One split‐scar trial (n = 40) reported this outcome. In this study, participants reported to have felt pain during laser treatment in 1 out of 20 (1/20) (5%) PDL treated areas versus in 0 out of 20 (0/20) (0%) silicone gel sheeting treated areas (RR 3.00; 0.13 to 69.52). | ⊕⊝⊝⊝3,6 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in PDL‐treated hypertrophic scars compared with silicone gel sheeting after 24 weeks. | |||
| Incidence and severity of treatment‐related adverse effects ‐ 585‐nm PDL versus silicone gel sheeting ‐ skin irritation ‐ hypertrophic scars ‐ follow‐up: 24 weeks | One split‐scar trial (n = 40) reported this outcome. In this study, participants reported to have felt skin irritation related to laser treatment in 0 out of 20 (0/20) (0%) PDL treated areas versus in 1 out of 20 (1/20) (5%) silicone gel sheeting treated areas (RR 0.33; 0.01 to 7.72). | ⊕⊝⊝⊝3,6 Very low | ||||
| Scar severity ‐ erbium laser versus TAC ‐ VBS (Higher scores = worse scar appearance) ‐ hypertrophic scars ‐ follow‐up: up to 12 months | Baseline mean in the other treatment group was 6.7 | MD 2.10 lower (2.87 lower to 1.33 lower) | NA | 80 (1 study) | ⊕⊝⊝⊝1,2 Very low | It is uncertain whether there is any difference in the scar severity in erbium‐treated hypertrophic scars compared with TAC after up to 12 months. |
| Scar severity ‐ erbium laser versus TAC ‐ patient self‐assessment ‐ patients considering the scar much better ‐ hypertrophic scars ‐ follow‐up: up to 12 months | Study population | RR 2.17 (1.28 to 3.66) | 80 (1 study) | ⊕⊝⊝⊝3,7 Very low | ||
| 300 per 1000 | 651 per 1000 | |||||
| Incidence and severity of treatment‐related adverse effects ‐ fractional carbon dioxide (CO2) laser versus TAC ‐ pain at injection site ‐ keloid scars ‐ follow‐up: 24 weeks | Study population | RR 0.25 (0.06 to 1.03) |
40 (1 study) | ⊕⊝⊝⊝3,8 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in fractional CO2‐treated keloid scars compared with TAC after 24 weeks. | |
| 400 per 1000 | 100 per 1000 | |||||
| Incidence and severity of treatment‐related adverse effects ‐ fractional CO2 laser versus TAC ‐ telangectasia ‐ keloid scars ‐ follow‐up: 24 weeks | Study population | RR 0.20 (0.01 to 3.92) |
40 (1 study) | ⊕⊝⊝⊝3,8 Very low | ||
| 100 per 1000 | 20 per 1000 | |||||
| Incidence and severity of treatment‐related adverse effects ‐ fractional CO2 laser versus TAC ‐ skin atrophy ‐ keloid scars ‐ follow‐up: 24 weeks | Study population | RR 0.33 (0.01 to 7.72) |
40 (1 study) | ⊕⊝⊝⊝3,8 Very low | ||
| 50 per 1000 | 17 per 1000 | |||||
| Incidence and severity of treatment‐related adverse effects ‐ fractional CO2 laser versus TAC ‐ charring ‐ keloid scars ‐ follow‐up: 24 weeks | One parallel trial (n = 40) reported this outcome. In this study, charring was observed in 3 out of 20 (3/20) (15%) participants treated with fractional CO2 laser and 0 out of 20 (0/20) (0%) participants treated with TAC (RR 7.00; 0.38 to 127.32). | ⊕⊝⊝⊝3,8 Very low | ||||
| Incidence and severity of treatment‐related adverse effects ‐ fractional CO2 laser versus verapamil ‐ pain at injection site ‐ keloid scars ‐ follow‐up: 24 weeks | One parallel trial (n = 40) reported this outcome. In this study, pain at injection site was reported by 2 out of 20 (2/20) (10%) participants treated with fractional CO2 laser group and 0 out of 20 (0/20) (0%) participants treated with verapamil (RR 5.00; 0.26 to 98.00). | ⊕⊝⊝⊝3,8 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in fractional CO2‐treated keloid scars compared with verapamil after 24 weeks. | |||
| Incidence and severity of treatment‐related adverse effects ‐ fractional CO2 laser versus verapamil ‐ charring ‐ keloid scars ‐ follow‐up: 24 weeks | One parallel trial (n = 40) reported this outcome. In this study, charring was observed in 3 out of 20 (3/20) (15%) participants treated with fractional CO2 laser group and 0 out of 20 (0/20) (0%) participants treated with verapamil group (RR 7.0; 0.38 to 127.32) | ⊕⊝⊝⊝3,8 Very low | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). ƚThe assumed risk in the comparison group is based on the event rate observed in the control arms of included trials. Where no events occurred, the risk was not calculated. Vancouver Burn Scar (VBS) Assessment Scale ‐ severity of scar was determined by numeric value from a minimum of 0 to 13 as the most severe form. Patient self‐assessment ‐ based on a 4‐point scale (1 = 0 to 25% improvement, 2 = 25 to 50% improvement, 3 = 50 to 75% improvement, and 4 = 75% or greater improvement). CI: Confidence Interval; CO2: carbon dioxide; MD: Mean Difference; PDL: Pulsed‐Dye Laser;RR: Risk Ratio; TAC: Triamcinolone acetonide;5‐FU: 5‐fluorouracil;VBS: Vancouver Burn Scar Assessment Scale;. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded 2 levels for very serious imprecision due to small sample size and large confidence interval.
2 Downgraded 1 level due to serious risk of bias (lack of blinding of participants, selective reporting, and unclear sequence generation and allocation concealment).
3 Downgraded 2 levels for very serious imprecision due to small number of events and large confidence interval.
4 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome) and unclear sequence generation and allocation concealment in 2 studies, and selective reporting in 1 study).
5 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome) and unclear sequence generation and allocation concealment).
6 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome) and unclear allocation concealment).
7 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome) and selective reporting, and unclear sequence generation and allocation concealment).
8 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome), and unclear allocation concealment and selective reporting).
Summary of findings 3. Laser therapy plus other treatment compared with other treatment for treating hypertrophic and keloid scars.
| Laser therapy plus other treatment compared with other treatment for treating hypertrophic and keloid scars | ||||||
| Patient or population: participants with hypertrophic and keloid scars Setting: outpatient Intervention: laser therapy (various types ‐ 585‐nm Pulsed‐Dye Laser (PDL), erbium laser, carbon dioxide (CO2) laser, Neodymium‐doped yttrium aluminium garnet (Nd:YAG) laser, Helium‐Neon (He‐Ne) laser) plus other treatments (various types ‐ triamcinolone acetonide (TAC), 5‐Fluorouracil (5‐FU), Diprospan, decamethyltetrasiloxane, polydimethylsiloxane and cyclopentasiloxane cream and verapamil) Comparison: other treatments (various types ‐ triamcinolone acetonide (TAC), 5‐Fluorouracil (5‐FU), Diprospan, decamethyltetrasiloxane, polydimethylsiloxane and cyclopentasiloxane cream, cryosurgery and verapamil) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of scar segments (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other treatment | Risk with Laser therapy plus other treatment | |||||
| Scar severity ‐ 585‐nm pulsed‐dye laser (PDL) plus triamcinolone acetonide (TAC) plus 5‐Fluorouracil (5‐FU) versus TAC plus 5‐FU ‐ blinded observer assessment of good to excellent scar improvement ‐ hypertrophic and keloid scars ‐ follow‐up: 12 weeks | Study population | RR 1.75 (0.95 to 3.22) |
40 (1 study) | ⊕⊝⊝⊝1,2 Very low | It is uncertain whether there is any difference in the scar severity in PDL plus TAC plus 5‐FU‐treated hypertrophic and keloid scars compared with TAC plus 5‐FU after 12 weeks. | |
| 400 per 1000 | 700 per 1000 | |||||
| Scar severity ‐ 585‐nm PDL plus TAC plus 5 FU versus TAC plus 5‐FU ‐ patient self‐assessment of good to excellent scar improvement ‐ hypertrophic and keloid scars ‐ follow‐up: 12 weeks | Study population | RR 1.36 (0.85 to 2.18) |
40 (1 study) | ⊕⊝⊝⊝1,3 Very low | ||
| 550 per 1000 | 748 per 1000 | |||||
| Scar severity ‐ carbon dioxide (CO2) laser plus TAC versus cryosurgery plus TAC. Mean percentage reduction ‐ blinded observer assessment ‐ keloid scars ‐ follow‐up: 12 months | Study population | NA | 60 (1 study) |
⊕⊝⊝⊝4,5 Very low | It is uncertain whether there is any difference in the scar severity in CO2 plus TAC‐treated keloid scars compared with cryosurgery plus TAC after 12 months. | |
| Baseline mean in the other treatment group was 74.44 | MD 16.11 lower (34.49 lower to 2.27 higher) | |||||
| Scar severity ‐ CO2 laser plus TAC versus cryosurgery plus TAC Mean percentage reduction ‐ patient self‐assessment score (higher scores = worse scar appearance) ‐ keloid scars ‐ follow‐up: 12 months | Study population | NA | 60 (1 study) |
⊕⊝⊝⊝4,6 Very low | ||
| Baseline mean in the other treatment group was 74.26 | MD 7.59 lower (22.83 lower to 7.65 higher) | |||||
| Incidence of treatment‐related adverse effects: CO2 laser plus TAC versus cryosurgery plus TAC ‐ atrophy ‐ keloid scars ‐ follow‐up: 12 months | Study population | RR 1.13 (0.70 to 1.82) |
60 (1 study) |
⊕⊝⊝⊝4,6 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in CO2 plus TAC‐treated keloid scars compared with cryosurgery plus TAC after 12 months. |
|
| 500 per 1000 | 565 per 1000 | |||||
| Incidence of treatment‐related adverse effects ‐ CO2 laser plus TAC versus cryosurgery plus TAC ‐ erythema ‐ keloid scars ‐ follow‐up: 12 months | Study population | RR 1.50 (0.47 to 4.78) |
60 (1 study) |
⊕⊝⊝⊝4,6 Very low | ||
| 133 per 1000 | 200 per 1000 | |||||
| Incidence of treatment‐related adverse effects ‐ CO2 laser plus TAC versus cryosurgery plus TAC ‐ telangiectasia ‐ keloid scars ‐ follow‐up: 12 months | Study population | RR 0.33 (0.07 to 1.52) |
60 (1 study) |
⊕⊝⊝⊝4,6 Very low | ||
| 200 per 1000 | 66 per 1000 | |||||
| Incidence of treatment‐related adverse effects ‐ CO2 laser plus TAC versus cryosurgery plus TAC ‐ hypopigmentation ‐ keloid scars ‐ follow‐up: 12 months | Study population | RR 0.60 (0.16 to 2.29) |
60 (1 study) |
⊕⊝⊝⊝4,6 Very low | ||
| 167 per 1000 | 100 per 1000 | |||||
| Scar severity ‐ Neodymium‐doped yttrium aluminium garnet (Nd:YAG) laser plus Diprospan plus 5‐FU versus Diprospan plus 5‐FU ‐ blinded observer assessment of good to excellent scar improvement ‐ keloid scars ‐ follow‐up: 3 months | Study population | RR 1.45 (0.88 to 2.41) |
46 (1study) |
⊕⊝⊝⊝1,7 Very low | It is uncertain whether there is any difference in the scar severity in Nd:YAG plus Diprospan plus 5‐FU‐treated keloid scars compared with Diprospan plus 5‐FU after 3 months. | |
| 478 per 1000 | 693 per 1000 | |||||
| Scar severity: Nd:YAG laser plus Diprospan plus 5‐FU versus Diprospan plus 5‐FU ‐ patient self‐assessment of scar improvement of 50% or higher ‐ keloid scars ‐ follow‐up: 3 months | Study population | RR 1.38 (0.91 to 2.10) |
46 (1study) |
⊕⊝⊝⊝1,8 Very low | ||
| 565 per 1000 | 780 per 1000 | |||||
| Incidence and severity of treatment‐related adverse effects: Nd:YAG laser plus Diprospan plus 5‐FU versus Diprospan plus 5‐FU ‐ keloid scars ‐ follow‐up: 3 months | One parallel trial (n = 46) reported this outcome. In this study, almost all injections were reported by participants as being painful, and the sites treated by Nd:YAG laser became purpuric (which lasted for 7 to 10 days). | ⊕⊝⊝⊝1,8 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in Nd:YAG plus Diprospan plus 5‐FU‐treated keloid scars compared with Diprospan plus 5‐FU after 3 months. | |||
| Scar severity ‐ Helium‐Neon (He‐Ne) laser plus decamethyltetrasiloxane, polydimethylsiloxane and cyclopentasiloxane cream versus decamethyltetrasiloxane, polydimethylsiloxane and cyclopentasiloxane cream ‐ Vancouver Scar scale (VSS) (higher scores = worse scar appearance) ‐ hypertrophic scars ‐ follow‐up: 12 weeks | One split‐scar trial (n = 30) reported this outcome. In this study, a significant decrease in the median values of VSS of the intervention area compared with the control area (P = 0.003) | ⊕⊝⊝⊝4,9 Very low | It is uncertain whether there is any difference in the scar severity in He‐Ne plus decamethyltetrasiloxane, polydimethylsiloxane and cyclopentasiloxane cream‐treated hypertrophic scars compared with decamethyltetrasiloxane, polydimethylsiloxane and cyclopentasiloxane cream after 12 weeks. | |||
| Incidence of treatment‐related adverse effects ‐ 595‐nm PDL plus verapamil versus verapamil ‐ regrowth ‐ keloid scars ‐ follow‐up: 24 weeks | Study population | RR 1.50 (0.27 to 8.22) |
50 (1study) |
⊕⊝⊝⊝1,10 Very low | It is uncertain whether there is any difference in the incidence and severity of treatment‐related adverse effects in PDL plus verapamil‐treated keloid scars compared with verapamil after 24 weeks. |
|
| 80 per 1000 | 120 per 1000 | |||||
| Incidence of treatment‐related adverse effects ‐ 595‐nm PDL plus verapamil versus verapamil ‐ pain related to treatment ‐ keloid scars ‐ follow‐up: 24 weeks | One parallel trial (n = 50) reported this outcome. In this study, pain at injection site was reported by 1 out of 25 (1/25) (4%) participants treated with 595‐nm PDL plus verapamil and by 0 out of 25 (0/15) (0%) participants treated with verapamil (RR 3.00; 0.13 to 70.30) | ⊕⊝⊝⊝1,10 Very low | ||||
| Incidence of treatment‐related adverse effects ‐ 595‐nm PDL plus verapamil versus verapamil ‐ hyperpigmentation ‐ keloid scars ‐ follow‐up: 24 weeks | One parallel trial (n = 50) reported this outcome. In this study, hyperpigmentation was observed in 2 out of 25 (2/25) (8%) participants treated with 595‐nm PDL plus verapamil and by 0 out of 25 (0/15) (0%) participants treated with verapamil (RR 5.00; 0.25 to 99.16) | ⊕⊝⊝⊝1,10 Very low | ||||
| Incidence of treatment‐related adverse effects ‐ 595‐nm PDL plus verapamil versus verapamil ‐ depigmentation ‐ keloid scars ‐ follow‐up: 24 weeks | Study population | RR 1.00 (0.07 to 15.12) |
50 (1study) |
⊕⊝⊝⊝1,10 Very low | ||
| 40 per 1000 | 40 per 1000 | |||||
| Incidence of treatment‐related adverse effects ‐ 595‐nm PDL plus verapamil versus verapamil ‐ purpura ‐ keloid scars ‐ follow‐up: 24 weeks | One parallel trial (n = 50) reported this outcome. In this study, hyperpigmentation was observed in 7 out of 25 (7/25) (28%) participants treated with 595‐nm PDL plus verapamil and by 0 out of 25 (0/15) (0%) participants treated with verapamil (RR 15.00; 0.90 to 249.30) | ⊕⊝⊝⊝1,10 Very low | ||||
| Incidence of treatment‐related adverse effects ‐ 595‐nm PDL plus verapamil versus verapamil ‐ total ‐ keloid scars ‐ follow‐up: 24 weeks | Study population | RR 4.67 (1.53 to 14.26) |
50 (1study) |
⊕⊝⊝⊝1,10 Very low | ||
| 120 per 1000 | 560 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). ƚThe assumed risk in the comparison group is based on the event rate observed in the control arms of included trials. Where no events occurred, the risk was not calculated. ‐ Diprospan contains betamethasone disodium phosphate plus betamethasone dipropionate. Patient self‐assessment ‐ based on a 4‐point scale (1 = 0 to 25% improvement, 2 = 25 to 50% improvement, 3 = 50 to 75% improvement, and 4 = 75% or greater improvement); VBS: Vancouver Burn Scar Assessment Scale ‐ severity of scar was determined by numeric value from a minimum of 0 to 13 as the most severe form. CI: Confidence Interval; ; CO:2 carbon dioxide;5‐FU: 5‐fluorouracil; He‐Ne: Helium‐Neon; NdYAG:neodymium‐doped yttrium aluminium garnet; PDL: Pulsed‐Dye Laser; RR: Risk Ratio; TAC: Triamcinolone acetonide. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded 2 levels for very serious imprecision due to small number of events and large confidence interval.
2 Downgraded 1 level due to serious risk of bias (lack of blinding of participants, incomplete outcome data and selective reporting, and unclear sequence generation and allocation concealment).
3 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome), incomplete outcome data and selective reporting, and unclear sequence generation and allocation concealment)
4 Downgraded 2 levels for very serious imprecision due to small sample size and large confidence interval.
5 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and incomplete outcome data, and unclear selective reporting).
6 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome) and incomplete outcome data, and unclear selective reporting).
7 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and incomplete outcome data, and unclear sequence generation and allocation concealment).
8 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome) and incomplete outcome data, and unclear sequence generation and allocation concealment).
9 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and selective reporting, and unclear sequence generation, allocation concealment and incomplete outcome data).
10 Downgraded 1 level due to serious risk of bias (lack of blinding of participants and assessors (patient‐reported outcome), and unclear sequence generation, allocation concealment and incomplete outcome data).
The variability of intervention types, controls, follow‐up periods and limited data reported meant we could only pool data for one comparison (and only two outcomes within this). We report below the results of the effects of interventions, with subgroup analyses, considering the latest time points available in the included studies. The secondary outcomes ‐ cosmesis, tolerance, preference for different modes of treatment, adherence, and change in quality of life ‐ were not reported in any of the included studies.
1. Laser versus no treatment
585‐nm pulsed‐dye laser (PDL) versus no treatment (3 studies)
Scar severity measured by health professional and/or participant using a specific scale
We pooled data from Manuskiatti 2001 and Manuskiatti 2002. There may be more hypertrophic and keloid scar improvement (that is scars are less severe) in 585‐nm PDL‐treated scars compared with no treatment after 32 weeks: risk ratio (RR) 1.96 (95% confidence interval (CI): 1.11 to 3.45, two studies, 60 scar segments (based on unblinded patient self‐assessment of improvement of 50% or higher between the laser treated areas and the control areas) (Analysis 1.1). This is low‐certainty evidence, downgraded once for serious imprecision and once for serious risk of bias.
1.1. Analysis.

Comparison 1: 585‐nm Pulsed‐Dye Laser (PDL) versus no treatment, Outcome 1: Severity of scar: Patient self‐assessment (32 weeks)
Incidence and severity of treatment‐related adverse effects
Three studies reported incidence of adverse effects related to treatment (Wittenberg 1999, Manuskiatti 2001, Manuskiatti 2002) (Table 4). It is uncertain whether the 585‐nm PDL impacts on the incidence of treatment‐related adverse effects. This is very low certainty evidence, downgraded twice for very serious imprecision and once for serious risk of bias (Table 1) (Analysis 1.2).
1.2. Analysis.

Comparison 1: 585‐nm Pulsed‐Dye Laser (PDL) versus no treatment, Outcome 2: Incidence and severity of treatment‐related adverse effects (32 weeks)
Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain
Three studies which were at high risk of bias evaluated these outcomes (Wittenberg 1999, Manuskiatti 2001 and Manuskiatti 2002).
In Wittenberg 1999, the study authors reported there were no significant differences in blood flow (erythema), elasticity and volume between intervention and control groups at the end of the follow up period. Results were presented graphically and not analysed here further. There was also no reported difference between groups in patients who rated scar burning, pruritus, and pain not related to treatment.
In Manuskiatti 2001 and Manuskiatti 2002, the authors reported scar height reduction in laser treated areas when compared with control (P < 0.05, and P = 0.005, respectively). The results were presented graphically, with no data for further analysis.
Nonablative fractional laser (NAFL) versus no treatment (2 studies)
Scar severity measured by health professional and/or participant using a specific scale
From Verhaeghe 2013 it is unclear whether NAFL impacts on hypertrophic scar severity compared with no treatment when measured using a blinded health professional global assessment at three months, as this is very low‐certainty evidence, downgraded twice due to very serious imprecision and once for serious risk of bias) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Non‐ablative Fractional Laser (NAFL) versus no treatment, Outcome 1: Severity of scar: Health Professional Global Assessment (3 months)
In Verhaeghe 2013, the authors described an improvement in the patient global assessment (PGA) at one month (reported P = 0.006) and three months (reported P = 0.02) after the final NAFL treatment with no further data available. The certainty of evidence was assessed as very low, downgraded twice due to very serious imprecision and once for serious risk of bias.
In Verhaeghe 2013 the authors reported an improvement in NAFL treated hypertrophic scars compared with no treatment on the participant part of the Patient and Observer Scar Assessment Scale (POSAS) at one month and three months, however the size of the difference was not reported. No data for the observer part of the scale was presented. This is very low‐certainty evidence, downgraded twice due to very serious imprecision and once for serious risk of bias.
For patient self‐assessments in Lin 2011 we were unable to fully analyse the data, because the results were presented only in graphs and did not allow extraction.
Incidence and severity of treatment‐related adverse effects
It is uncertain whether NAFL treatment leads to a higher incidence of treatment‐related adverse effects. This is very low‐certainty evidence, downgraded twice for very serious imprecision and once for serious risk of bias (Table 1) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Non‐ablative Fractional Laser (NAFL) versus no treatment, Outcome 2: Incidence and severity of treatment‐related adverse effects (3 months)
Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain
In a small study which was at high risk of bias (Lin 2011), two different parameters of NAFL, including a high‐density treatment arm (HDTA) and a low‐density treatment arm (LDTA), were compared with a control intervention for the treatment of hypertrophic scars. In this study, small differences that were imprecise were noted between HDTA treated groups compared with the control group for a range of outcomes: erythema mean difference ((MD) 0.44; 95% CI ‐0.54 to 1.42), pigmentation (MD 0.12; 95% CI ‐0.66 to 0.90]) and texture (MD 0.22; 95% CI ‐0.50 to 0.94). Similarly, small and imprecise differences were also found for LDTA treated groups when compared with the control group: erythema (MD 0.12; 95% CI: ‐0.96 to 1.20), pigmentation (MD 0.08; 95% CI: ‐0.59 to 0.75) and texture (MD 0.56; 95% CI: ‐0.27 to 1.39) (Analysis 2.3). Erythema, pigmentation and texture were assessed based on a four‐point scale (where 0 was no improvement or worsened, and 3 was a marked improvement).
2.3. Analysis.

Comparison 2: Non‐ablative Fractional Laser (NAFL) versus no treatment, Outcome 3: Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain (3 months)
Verhaeghe 2013 reported no improvement in redness and pigmentation of skin scars (skin reflectance measurements) treated with NAFL, when compared with control, one and three months after the final treatment.
Fractional CO2 laser versus no treatment (3 studies)
Azzam 2016 compared ablative fractional CO2 laser for treating hypertrophic and keloid scars with no treatment. Blome‐Eberwein 2016 and Daoud 2019 compared ablative fractional CO2 laser for treating hypertrophic scars with no treatment.
Scar severity measured by health professional and/or participant using a specific scale
It is uncertain whether fractional CO2 impacts on hypertrophic scar severity compared with no treatment as evaluated with the Vancouver Burn Scar (VBS) scale by blinded observers (Azzam 2016; Blome‐Eberwein 2016) or impacts on keloid scar severity (Azzam 2016) after up to six months. The evidence is of very low certainty, as we downgraded twice for very serious imprecision, and once for serious risk of bias (Analysis 3.1).
3.1. Analysis.

Comparison 3: Fractional carbon dioxide (CO2) versus no treatment, Outcome 1: Severity of scar: Vancouver Burn Scar (VBS) scale (up to 6 months)
The evidence is very uncertain about the effect of fractional CO2 compared with no treatment on scar severity based on the POSAS values (Analysis 3.2). The evidence is of very low certainty, as we downgraded twice for very serious imprecision and once for serious risk of bias (Table 1).
3.2. Analysis.

Comparison 3: Fractional carbon dioxide (CO2) versus no treatment, Outcome 2: Severity of scar: Patient and Observer Scar Assessment Scale (POSAS) (at least one month)
Incidence and severity of treatment‐related adverse effects
These data were not assessed in Azzam 2016 and Blome‐Eberwein 2016. In Daoud 2019; there were no treatment‐related adverse effects reported.
Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain
In a study which was at high risk of bias (Blome‐Eberwein 2016), pliability, height (in the suction cup), and "recoil" of hypertrophic scars were reported as not improving significantly in the laser group or in the control group. No, or only small, differences that were imprecise were noted between fractional CO2 laser‐treated groups compared with control group for a range of outcomes: scar height assessed by ultrasound (MD ‐0.12 millimetres (mm); 95% CI ‐0.27 to 0.03); erythema and pigmentation assessed by dermatospectrometry (MD 0.73; 95% CI ‐1.27 to 2.73, and MD ‐1.14; ‐7.16 to 4.88, respectively), and scar‐related pruritus and pain not related to treatment assessed as part of the POSAS (MD 0.41; 95% CI ‐0.66 to 1.48, and MD 0.41; 95% CI ‐0.63 to 1.45, respectively) (Analysis 3.3).
3.3. Analysis.

Comparison 3: Fractional carbon dioxide (CO2) versus no treatment, Outcome 3: Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain (12 to 18 weeks)
Azzam 2016 reported that scar pain not related to treatment decreased in six (54.5%) of the 11 participants (three had keloid and three had hypertrophic scars), and the pruritus disappeared in 11 (68.8%) (seven had keloid and four had hypertrophic scars) of the 16 cases after treatment. Results for this trial were not presented by treatment arm and no further data were available for analysis.
2. Laser versus other treatments
585‐nm PDL versus triamcinolone acetonide (TAC) (2 studies)
Scar severity measured by health professional and/or participant using a specific scale
It is uncertain whether PDL impacts on hypertrophic scar severity compared with TAC when measured using blinded observer assessment (Omranifard 2007) (Analysis 4.1) or on hypertrophic and keloid scar severity compared with TAC when measured using patient self‐assessment (PSA) (Manuskiatti 2002, Omranifard 2007) (Analysis 4.2), as the certainty of evidence was assessed as very low, downgraded twice due to very serious imprecision and once due to serious risk of bias.
4.1. Analysis.

Comparison 4: 585‐nm Pulsed‐Dye Laser (PDL) versus Triamcinolone acetonide (TAC), Outcome 1: Severity of scars: Vancouver Burn Scar (VBS) scale (up to 12 months)
4.2. Analysis.

Comparison 4: 585‐nm Pulsed‐Dye Laser (PDL) versus Triamcinolone acetonide (TAC), Outcome 2: Severity of scars: Patient self‐assessment (up to 12 months)
Incidence and severity of treatment‐related adverse effects
In Omranifard 2007, no complications were reported. In Manuskiatti 2002, it is unclear whether 585‐nm PDL leads to more treatment‐related adverse effects, including sequelae (Analysis 4.3) or treatment‐related pain (Analysis 4.3) than TAC, as the evidence was rated as very low certainty, downgraded twice due to very serious imprecision and once due to serious risk of bias (Table 2).
4.3. Analysis.

Comparison 4: 585‐nm Pulsed‐Dye Laser (PDL) versus Triamcinolone acetonide (TAC), Outcome 3: Incidence and severity of treatment‐related adverse effects (up to 12 months)
Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain
In Manuskiatti 2002, the results were presented in graphs, with no data for further analysis. In Omranifard 2007 no measures of variability were provided and no further analysis was possible (Table 5)
585‐nm PDL versus 5‐Fluorouracil (5‐FU) (1 study)
Scar severity measured by health professional and/or participant using a specific scale
It is uncertain whether PDL impacts on hypertrophic and keloid scar severity measured using patient self‐assessment compared with 5‐FU (Manuskiatti 2002) (Analysis 5.1). The certainty of the evidence is very low, downgraded twice due to very serious imprecision and once due to serious risk of bias.
5.1. Analysis.

Comparison 5: 585‐nm Pulsed‐Dye Laser (PDL) versus 5‐Fluorouracil (5‐FU), Outcome 1: Severity of scars: Patient self‐assessment (32 weeks)
Incidence and severity of treatment‐related adverse effects
It is uncertain whether 585‐nm PDL treatment impacts on the incidence of treatment‐related adverse effects compared with 5‐FU. The certainty of the evidence is very low, downgraded twice due to very serious imprecision and once due to serious risk of bias (Table 2) (Analysis 5.2).
5.2. Analysis.

Comparison 5: 585‐nm Pulsed‐Dye Laser (PDL) versus 5‐Fluorouracil (5‐FU), Outcome 2: Incidence and severity of treatment‐related adverse effects (32 weeks)
Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
The results were presented only graphically and further data extraction and analyses were not possible.
585‐nm PDL versus TAC plus 5‐FU (1 study)
Scar severity measured by health professional and/or participant using a specific scale
It is uncertain whether PDL impacts on hypertrophic and keloid scar severity compared with TAC plus 5‐FU for the participant assessments. The certainty of the evidence is very low downgraded twice due very serious imprecision and once due to serious risk of bias (Analysis 6.1) (Manuskiatti 2002).
6.1. Analysis.

Comparison 6: 585‐nm Pulsed‐Dye Laser (PDL) versus Triamcinolone acetonide (TAC) plus 5‐Fluorouracil (5‐FU), Outcome 1: Severity of scars: Patient self‐assessment (32 weeks)
Incidence and severity of treatment‐related adverse effects
It is uncertain whether 585‐nm PDL treatment impacts on the incidence of treatment‐related adverse effects compared with TAC plus 5‐FU. The certainty of the evidence is very low, downgraded twice due to very serious imprecision and once due to serious risk of bias (Table 2) (Analysis 6.2).
6.2. Analysis.

Comparison 6: 585‐nm Pulsed‐Dye Laser (PDL) versus Triamcinolone acetonide (TAC) plus 5‐Fluorouracil (5‐FU), Outcome 2: Incidence and severity of treatment‐related adverse effects (32 weeks)
Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
Authors reported the changes in height, erythema and pliability between intervention areas only in graphical formats that did not allow data extraction and further analyses.
585‐nm PDL versus silicone gel sheeting (1 study)
Scar severity measured by health professional and/or participant using a specific scale
This outcome was not reported.
Incidence and severity of treatment‐related adverse effects
It is uncertain whether 585‐nm PDL treatment impacts on the incidence of treatment‐related adverse effects compared with silicone gel sheeting. The certainty of the evidence is very low, downgraded twice due to very serious imprecision and once due to serious risk of bias (Table 2) (Analysis 7.1).
7.1. Analysis.

Comparison 7: 585‐nm Pulsed‐Dye Laser (PDL) versus Silicone Gel Sheeting, Outcome 1: Incidence and severity of treatment‐related adverse effects (24 weeks)
Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
In a small study which was at high risk of bias (Wittenberg 1999), the study authors reported that 585‐nm PDL treatment did not improve blood flow, volume and pruritus in hypertrophic scars when compared with silicone gel sheeting. There was also no reported difference between groups in patients who rated scar elasticity, burning, and pain not related to treatment. Results were presented graphically and not analysed further here.
Erbium laser versus TAC (1 study)
Scar severity measured by health professional and/or participant using a specific scale
It is uncertain whether erbium laser impacts on hypertrophic scar severity compared with TAC treatment assessed with VBS score (Analysis 8.1) and obtained through patient self‐assessment measured by the number of patients considering the scar better or much better (Analysis 8.2) (Omranifard 2007). The certainty of this evidence was very low, downgraded twice due to very serious imprecision and once due to serious risk of bias.
8.1. Analysis.

Comparison 8: Erbium laser versus Triamcinolone acetonide (TAC), Outcome 1: Severity of scars: Vancouver Burn Scar (VBS) scale (up to 12 months)
8.2. Analysis.

Comparison 8: Erbium laser versus Triamcinolone acetonide (TAC), Outcome 2: Severity of scars: Patient self‐assessment (up to 12 months)
Incidence and severity of treatment‐related adverse effects
The authors reported no complications such as permanent pigmentation change, ulceration or infection in either treatment group.
Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
These outcomes were not reported.
Fractional CO2 Laser versus TAC (1 study)
Scar severity measured by health professional and/or participant using a specific scale
The authors did not report the means of the full Vancouver Scar Scale (VSS), reporting only separate results of keloid scar height, vascularity, pigmentation, and pliability (Srivastava 2019).
Incidence and severity of treatment‐related adverse effects
It is uncertain whether fractional CO2 laser treatment impacts on treatment‐related adverse effects, including the incidence of pain at injection site, telangiectasia (a condition in which tiny blood vessels cause thread‐like red lines on the skin), skin atrophy, and charring (skin burning so that its surface becomes blackened) compared with TAC (Analysis 9.1). The certainty of evidence was rated as very low. We downgraded the evidence twice for very serious imprecision and once for serious risk of bias (Table 2).
9.1. Analysis.

Comparison 9: Fractional carbon dioxide (CO2) laser versus intralesional Triamcinolone acetonide (TAC), Outcome 1: Incidence of treatment‐related adverse effects (24 weeks)
Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
A small study which was at high risk of bias assessed these outcomes (Srivastava 2019). In this study, height was measured with callipers, vascularity was assessed by visual inspection, pliability was assessed by palpation, and pigmentation was scored after blanching and comparing it with the surrounding skin.
After 12 weeks of treatment, the results of this study suggested no or only a small difference between treatments in the assessment of height (MD 0.90; 95% CI 0.60 to 1.20), vascularity (MD 0.70; 95% CI 0.37 to 1.03), and pliability (MD 0.40; 95% CI 0.13 to 0.67) (Analysis 9.2). Regarding pigmentation, no further analysis was possible based on reported data.
9.2. Analysis.

Comparison 9: Fractional carbon dioxide (CO2) laser versus intralesional Triamcinolone acetonide (TAC), Outcome 2: Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (12 weeks)
After 24 weeks of treatment, it was not possible to analyse whether there were differences between fractional laser and TAC for scar height, vascularity and pliability based on reported data. Data on keloid scar pigmentation suggested no, or only a small difference, between treatments (MD 0.00; 95% CI ‐0.25 to 0.25; one study, 40 participants, 40 scars) (Analysis 9.3).
9.3. Analysis.

Comparison 9: Fractional carbon dioxide (CO2) laser versus intralesional Triamcinolone acetonide (TAC), Outcome 3: Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (24 weeks)
Fractional CO2 laser versus intralesional verapamil (1 study)
Scar severity measured by health professional and/or participant using a specific scale
Although the authors used VSS, they did not report the means of the full scale, reporting only the separate results of keloid scar height, vascularity pigmentation, and pliability (Srivastava 2019).
Incidence and severity of treatment‐related adverse effects
It is uncertain whether fractional CO2 laser treatment impacts on the incidence of treatment‐related adverse effects, including pain at injection site and charring (skin burning so that its surface becomes blackened) compared with intralesional verapamil (Analysis 10.1). No treatment‐related adverse effects occurred in the verapamil group. No events of teleangiectasia or skin atrophy (skin thinning) occurred in either groups. The certainty of evidence is very low, downgraded twice due to very serious imprecision and once due to serious risk of bias (Table 2).
10.1. Analysis.

Comparison 10: Fractional carbon dioxide (CO2) laser versus Intralesional Verapamil, Outcome 1: Incidence of treatment‐related adverse effects (24 weeks)
Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
Only one small study which was at high risk of bias assessed these outcomes (Srivastava 2019). In this study, height was measured with callipers, pliability was assessed by palpation, vascularity was assessed by visual inspection, and pigmentation was scored after blanching and comparing it with the surrounding skin.
After 12 weeks of treatment, the results of this study suggested no or only a small difference between treatments in the assessment of height (MD 0.30; 95% CI ‐0.00 to 0.60), pigmentation (MD ‐0.40; 95% CI ‐0.68 to ‐0.12), vascularity (MD 0.10; 95% CI ‐0.17 to 0.37), and pliability (MD 0.10; 95% CI ‐0.19 to 0.39) (Analysis 10.2).
10.2. Analysis.

Comparison 10: Fractional carbon dioxide (CO2) laser versus Intralesional Verapamil, Outcome 2: Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (12 weeks)
After 24 weeks of treatment, these results also suggested no, or only a small difference between treatments in the assessment of keloid scar height (MD 0.20; 95% CI ‐0.02 to 0.42), scar pigmentation (MD 0.25; 95% CI ‐0.04 to 0.54), and scar vascularity (MD 0.35; 95% CI 0.09 to 0.61) (Analysis 10.3). No quantitative analysis was possible regarding pliability as SD in the verapamil group was 0.
10.3. Analysis.

Comparison 10: Fractional carbon dioxide (CO2) laser versus Intralesional Verapamil, Outcome 3: Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (24 weeks)
3. Laser plus other treatment versus other treatment
585‐nm PDL plus TAC plus 5 FU versus TAC plus 5‐FU (1 study)
Scar severity measured by health professional and/or participant using a specific scale
The evidence is uncertain about the effect of 585‐nm PDL plus TAC plus 5 FU compared with TAC plus 5‐FU on hypertrophic and keloid scar severity assessed by a blinded observer assessment, and by patient self‐assessment at 12 weeks (Analysis 11.1) (Asilian 2006). The certainty of evidence was rated as very low, downgraded twice for imprecision and once for serious risk of bias.
11.1. Analysis.

Comparison 11: 585‐nm Pulsed‐Dye Laser (PDL) plus Triamcinolone acetonide (TAC) plus 5‐Fluorouracil (5‐FU) versus TAC plus 5‐FU, Outcome 1: Severity of scars (12 weeks)
Incidence and severity of treatment‐related adverse effects
At the end of the study, no treatment‐related adverse effects were observed in either group.
Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
A small study which was at high risk of bias, (Asilian 2006), assessed these outcomes. At the end of the study, the study authors reported that the erythema score in the TAC plus 5‐FU plus PDL group was lower than in the TAC plus 5‐FU group (reported P< 0.05) (Table 6). In this study, erythema was graded by the observer on a 5‐point scale, where 0 was no erythema and 4 was very severe erythema. Results were presented graphically and not analysed further here.
CO2 laser plus TAC versus cryosurgery plus TAC (1 study)
Scar severity measured by health professional and/or participant using a specific scale
It is unclear whether CO2 Laser plus TAC impacts on keloid scar severity compared with cryosurgery plus TAC when measured using blinded observer assessment, patient self‐assessment and VBS Assessment Scale, respectively (Analysis 12.1). The certainty of this evidence was rated as very low downgraded twice due to very serious imprecision and once due to serious risk of bias.
12.1. Analysis.

Comparison 12: Carbon dioxide (CO2) Laser plus Triamcinolone acetonide (TAC) versus Cryosurgery plus TAC, Outcome 1: Severity of scars (12 months)
Incidence and severity of treatment‐related adverse effects
It is also uncertain whether there is a difference between groups in the incidence of treatment‐related adverse effects (Analysis 12.2). The certainty of this evidence was rated as very low, downgraded twice due to very serious imprecision and once due to serious risk of bias.
12.2. Analysis.

Comparison 12: Carbon dioxide (CO2) Laser plus Triamcinolone acetonide (TAC) versus Cryosurgery plus TAC, Outcome 2: Incidence and severity of treatment‐related adverse effects (12 months)
Change in scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
A small study which was at high risk of bias, (Behera 2016), assessed this outcomes. After 12 months, the results of this study suggested no or only a small difference in scar height (MD ‐ 16.15 mm, 95% CI: ‐38.24 to 5.94, one study, 60 scar segments) or volume (MD ‐17.88, 95% CI: ‐39.72 to 3.96, one study, 60 segments scars) (Analysis 12.3). The authors reported a difference between groups in pain not related to treatment (P = 0.0010) favouring CO2 laser, but no difference in pruritus score (P = 0.2262). We could not extract or analyse relevant data further.
12.3. Analysis.

Comparison 12: Carbon dioxide (CO2) Laser plus Triamcinolone acetonide (TAC) versus Cryosurgery plus TAC, Outcome 3: Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain(12 months)
Recurrence of the condition
In Behera 2016, at 12 months, there was a recurrence of 6 keloid scars (16.66%), all of which belonged to the CO2 laser group (RR: 13.00; 95% CI: 0.76 to 220.96; one study, 60 scar segments) (Analysis 12.4)
12.4. Analysis.

Comparison 12: Carbon dioxide (CO2) Laser plus Triamcinolone acetonide (TAC) versus Cryosurgery plus TAC, Outcome 4: Recurrence of the condition (12 months)
Neodymium‐doped yttrium aluminium garnet (Nd:YAG) laser plus intralesional corticosteroid (diprospan, including betamethasone disodium phosphate plus betamethasone dipropionate) plus 5‐FU versus intralesional corticosteroid (diprospan, including betamethasone disodium phosphate plus betamethasone dipropionate) plus 5‐FU (1 study)
Scar severity measured by health professional and/or participant using a specific scale
It is uncertain whether Nd:YAG laser plus intralesional corticosteroid diprospan plus 5‐FU impacts on keloid scar severity compared with Intralesional corticosteroid diprospan plus 5‐FU when measured using the number of patients and blinded observers reporting good to excellent improvement (Analysis 13.1) (Chen 2017). This evidence is of very low certainty downgraded twice due to very serious imprecision and once due to serious risk of bias.
13.1. Analysis.

Comparison 13: Neodymium‐doped yttrium aluminum garnet (Nd:YAG) laser plus intralesional corticosteroid diprospan plus 5‐Fluorouracil (5‐FU) versus Intralesional corticosteroid diprospan plus 5‐FU, Outcome 1: Severity of scars (3 months)
Incidence and severity of treatment‐related adverse effects
It is also uncertain whether there is a difference between groups in the incidence of treatment‐related adverse effects. Almost all injections were reported by participants as being painful, and the sites treated by Nd:YAG laser became purpuric (which lasted for 7‐10 days). No treatment‐related adverse textural or pigmentary alterations, and no ulcers or erosions were observed in either groups. The certainty of evidence is very low, downgraded twice due to very serious imprecision and once due to serious risk of bias
Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
A small study which was at high risk of bias, (Chen 2017), assessed these outcomes. During this study, the observer compared images of the lesions, and rated the erythema, pliability and pruritus of the lesions on a 5‑point scale where 0 was no and 4 was very severe. In the third month, erythema (P < 0.05), pliability (P< 0.05), and pruritus (P < 0.05) were reported by the authors to be significantly lower in the Nd:YAG plus intralesional corticosteroid plus 5‐FU laser group than in the intralesional corticosteroid plus 5‐FU group. These data were provided only in graphs and we could not extract further data to analyse.
He‐Ne laser plus decamethyltetrasiloxane, polydimethylsiloxane and cyclopentasiloxane cream versus decamethyltetrasiloxane, polydimethylsiloxane and cyclopentasiloxane cream (1 study)
Scar severity measured by health professional and/or participant using a specific scale
In Alsharnoubi 2018, the authors reported a significant decrease in the median values of VSS of the intervention area compared with the control areas (P = 0.003). Study authors reported these results only in graphical formats that did not allow data extraction and further analyses. This evidence was rated as very low certainty, downgraded twice due to very serious imprecision and once due to serious risk of bias.
Incidence and severity of treatment‐related adverse effects
It is also uncertain whether there is a difference between groups in the incidence of treatment‐related adverse effects. No treatment‐related adverse effects were reported in either groups.
Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
A small study which was at high risk of bias, (Alsharnoubi 2018) assessed these outcomes. The skin thickness after the treatment measured by ultrasonography was slightly smaller in the laser treated hypertrophic scar segments (MD ‐0.09 mm; 95% CI ‐0.17 to ‐0.01; one study; 15 participants; 30 segments) (Analysis 14.1)
14.1. Analysis.

Comparison 14: Helium–neon (He‐Ne) laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream versus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream, Outcome 1: Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (12 weeks)
595 nm PDL plus intralesional verapamil versus intralesional verapamil (1 study)
Scar severity measured by health professional and/or participant using a specific scale
In Khattab 2019, although the authors used the VSS, they did not report the means of the full scale, reporting only the separate results of keloid scar height, vascularity pigmentation, and pliability.
Incidence and severity of treatment‐related adverse effects
It is uncertain whether there is a difference in the incidence of treatment‐related adverse effects (Analysis 15.1). The certainty of evidence was rated as very low, downgraded twice due to very serious imprecision and once due to serious risk of bias (Table 3).
15.1. Analysis.

Comparison 15: 595‐nm Pulsed‐Dye Laser (PDL) plus intralesional verapamil versus intralesional verapamil, Outcome 1: Incidence of treatment‐related adverse effects (24 weeks)
Change in scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain
A small study which was at high risk of bias assessed these outcomes (Khattab 2019). After 12 weeks of treatment, measures of keloid scar height (MD ‐2.61; 95% CI ‐3.68 to ‐1.54; one study; 40 participants; 50 scars) and pliability (MD ‐2.06; 95% CI ‐2.46 to ‐1.66; one study; 40 participants; 50 scars) were lower in 595 nm PDL plus verapamil group. However, the results of this study suggested no or only a small difference between groups for vascularity (MD 0.06; 95% CI ‐0.34 to 0.46) and pigmentation (MD ‐0.27; 95% CI ‐0.62 to 0.08) (Analysis 15.2).
15.2. Analysis.

Comparison 15: 595‐nm Pulsed‐Dye Laser (PDL) plus intralesional verapamil versus intralesional verapamil, Outcome 2: Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (change from baseline) (12 weeks)
After 24 weeks of treatment, laser was superior to verapamil in the assessment of height (MD ‐2.89; 95% CI ‐3.65 to ‐2.13; one study; 40 participants; 50 scars), vascularity (MD ‐0.84; 95% CI ‐1.24 to ‐0.44; one study; 40 participants; 50 scars), and pliability (MD ‐1.87 95%CI ‐2.06 to ‐1.68; one study; 40 participants; 50 scars). No statistically significant difference was found between groups for pigmentation (Analysis 15.3).
15.3. Analysis.

Comparison 15: 595‐nm Pulsed‐Dye Laser (PDL) plus intralesional verapamil versus intralesional verapamil, Outcome 3: Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (change from baseline) (24 weeks)
Discussion
Summary of main results
In this review, we found eight studies (Wittenberg 1999, Manuskiatti 2001, Manuskiatti 2002, Lin 2011, Verhaeghe 2013, Azzam 2016, Blome‐Eberwein 2016, Daoud 2019) that compared laser treatments with no treatment. There is low‐certainty evidence from two randomised controlled trials (RCTs) (Manuskiatti 2001, Manuskiatti 2002) indicating that 585‐nm pulsed‐dye laser (PDL) may reduce keloid and hypertrophic scar severity compared with no treatment, based on unblinded patient self‐assessments of improvement of 50% or more over a 32‐week period of follow‐up. Of note, although these studies suggest that PDL may reduce scar severity compared with no treatment, we have not found any adequate studies showing the minimally clinically important differences in any of the severity rating scales, which makes it difficult for the professionals to transpose these results to clinical practice. The evidence for all other outcomes is uncertain. Adverse effects associated with treatment delivery and secondary outcomes, including erythema, elasticity, volume, and height were assessed by small studies at high risk of bias that either reported imprecise estimates or presented results only graphically, without further data to analyse.
Other types of laser therapy, such as non‐ablative fractional laser (NAFL) (Lin 2011, Verhaeghe 2013) and fractional CO2 (Azzam 2016, Blome‐Eberwein 2016, Daoud 2019), were compared with no treatment. However, results regarding the effectiveness of NAFL on the severity of scars are not consistent when assessed by participants compared with assessment by health professionals. Similarly, the results regarding the fractional CO2 laser are conflicting. While one study on keloid scars showed a possible benefit of using fractional CO2 as compared with no treatment, the pooled results from two RCTs on hypertrophic scars did not show the same results. Because the studies provide inconsistent results, have a small number of patients, exhibit important methodological limitations, and have poorly reported their data, no conclusion can be drawn from these results.
We also found four studies that compared laser treatments with other treatments (Wittenberg 1999, Manuskiatti 2002, Omranifard 2007, Srivastava 2019) and five studies that compared laser combined with other treatments versus other treatments (Asilian 2006, Behera 2016, Chen 2017, Alsharnoubi 2018, Khattab 2019). However, the evidence from these studies is also limited, mainly by the risk of bias and imprecision of effect estimates. As the overall certainty of the available evidence for all these comparisons and all outcomes is very low, it is also unclear whether laser alone or combined with other treatments, in general, results in a difference in the severity of scars when compared with other treatments.
Overall completeness and applicability of evidence
The small number of participants, varying schemes of laser therapy, different types of lasers, and comparisons of different treatment options, made it difficult to compare the results obtained. Currently, several types of lasers are available, such as 585 nm PDL, NAFL, Fractional CO2 Laser, erbium, neodymium‐doped yttrium aluminium garnet (Nd:YAG), and helium‐neon (He‐Ne) laser. The best available evidence is of low‐certainty from two RCTs (Manuskiatti 2001, Manuskiatti 2002) on 585‐nm PDL. The results of these studies suggest 585‐nm PDL may reduce keloid and hypertrophic scars severity compared with no treatment. Of note, PDL 585 nm was one of the first developed types of lasers, and availability of the devices in clinical practice may have influenced the amount of research performed with the types of lasers (Vrijman 2011).
As a result of the lack of robust, homogeneous evidence, most of the data were presented as narrative analyses to permit the individual evaluation of the results obtained with each type of laser. Some outcomes which we originally intended to evaluate, such as cosmesis, patient's preferred treatment, adherence, and quality of life, were not addressed in the included studies and the majority of the trials had short follow‐up periods. To help guide clinical practice, we also planned to perform subgroup analyses on different types of laser devices, fluences, schemes, and duration of treatment. However, current available evidence is insufficient to draw any conclusions or support the routine use of any type of laser to treat hypertrophic and keloid scars in clinical practice.
Quality of the evidence
Fifteen RCTs with a total of 604 participants were included. Nevertheless, several limitations in the included studies should be underscored. Although blinding of participants and personnel may be very difficult from a practical perspective, except for one study (Verhaeghe 2013), all trials included in this review had other important methodological limitations and a high or unclear risk of bias for at least two domains. Of note, only one trial (Behera 2016) reported the scar recurrence rates during the follow‐up period, which is clinically important outcome, and a prevalent condition. This limitation brings into question the long‐term effects of these interventions.
Of note, several of our results were based on split‐scar trials. Although all split‐scar trials used paired analyses and we have not combined the results of split‐scar trials with those from parallel studies, we should interpret these results carefully. We should not rule out the possibility of "spill‐over" of the treatment from one scar segment to the other.
Due to small sample sizes, small numbers of studies, unblinded assessments, and attrition bias, the certainty of the evidence comparing laser treatment with no treatment, laser treatment with other treatments, and laser plus other treatment with other treatment, is low to very low. Furthermore, in some cases, data were also insufficiently reported or reported in ways that did not allow extraction and further analysis. Therefore, none of the available trials were of high‐methodological quality and the true efficacy of laser therapy may not be fully clarified until high‐quality trials are published.
Potential biases in the review process
The most important databases, clinical trials, contacts with specialists and other research sources were included in our study searches in order to identify and collect all relevant RCTs, regardless of language or publication status. Moreover, strict collection and evaluation of data was performed by the review authors in order to avoid errors in the conduct of this systematic review. Some data were only available in incomplete formats in graphs and did not allow for extraction. Although we tried to contact the authors, we did not receive a response. We were also unable to assess publication bias due to the insufficient number of included RCTs in each comparison. Nevertheless, we believe this scientifically‐rigorous systematic review exposes literature gaps and provides critical data upon which to base further trials and protocols.
Agreements and disagreements with other studies or reviews
We did not find a systematic review that evaluated laser therapy for treating both hypertrophic and keloid scars, but we found a systematic review assessing the effects of laser and intense pulsed light (IPL) for the treatment of hypertrophic scars (Vrijman 2011). Differently from this review, Vrijman 2011 evaluated not only RCTs, but also non‐randomised trials, in which only hypertrophic scars were treated with laser or IPL. Our search strategy was also broader than in Vrijman 2011, and included MEDLINE, Embase and CENTRAL, as well as other databases, such as EBSCO CINAHL, LILACS, Clinicaltrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform and European Union (EU) Clinical Trials Register Platform (ICTRP). We excluded trials comparing laser in both arms without control areas and non‐randomised clinical trials, in order to minimise bias. Due to the different parameters used to select the studies and evaluate the data between this systematic review and that published by Vrijman 2011, the findings could not be compared.
Authors' conclusions
Implications for practice.
There is currently limited research evidence about the impact of laser therapy on severity of hypertrophic and keloid scars. Due to the heterogeneity of the studies and conflicting results, we cannot draw any conclusions on whether lasers offer net benefit when compared with traditional therapies for treating hypertrophic and keloid scars. Further high‐quality trials, with long‐term follow‐up, and which report the rate of scar recurrence, are needed to better understand the potential impacts of laser as a treatment for these scar types and to guide future clinical practice.
Implications for research.
A broad range of different laser devices and protocols were used to treat hypertrophic scars and keloids resulting in multiple comparisons, often with limited evidence. As this is a high‐priority clinical decision uncertainty in the field, more randomised controlled trials (RCTs) assessing laser therapy for treating these types of scars are needed. Given the number of comparisons, focusing research on treatments where there is a signal of effectiveness, such as on 585‐nm pulsed‐dye laser (PDL), should be considered.
New studies should be standardised, in order to provide more homogeneous and reliable data for a proper comparison of the results. For example, studies should evaluate the same laser device (e.g. 585‐nm PDL), delivered with the same fluency, in an equal regimen for treating similar scars (including the same scar age, size, and body region) of participants with the same phototype, and keloids and hypertrophic scars should be assessed separately (and their cause described, e.g. surgical wounds and post‐burn scars), as they are physiologically different and could respond distinctly to different treatment modalities. The follow‐up period should be suitably long to allow evaluation of the long‐term effects of laser therapy, recurrence events, or even worsening after treatment. Validated scales and forms (including quality of life forms) should be used. Also, to ensure a "spill‐over" of the treatment from one scar segment to the other is avoided, it may be useful for further trials ‐ if using a split‐scar design ‐ to leave treatment‐free areas (e.g. 2‐3 centimetres (cm)) between the treated scar segments.
Of note, there are no current core outcome sets in this area and they should be developed taking into consideration consumers' opinion and values. The validated scale chosen should be one that evaluates both objective (measured by blinded observers, colorimeters, ultrasound, graduated callipers etc) and subjective (participant opinion about the overall improvement of the scar severity, symptoms, cosmesis, pain during treatment etc) characteristics. The Patient and Observer Scar Assessment Scale (POSAS) scale is an example. In addition, there is a need for clear and complete reporting of outcome data for the interventions being compared and reporting of the rate of recurrence of scars during the follow‐up periods.
History
Protocol first published: Issue 4, 2015
Acknowledgements
The authors are grateful for the advice of peer referees Giovanni Casazza, Janet Gunderson, David Margolis, Emma Maund and Lisa O’Brien who commented on the protocol, and Gill Norman, Mamta Shah, Jacky Edwards and Janet Yarrow who commented on the review. They would also like to thank copy‐editors, Elizabeth Royle and Heather Maxwell and S. Zeinab Mousavi and Ali Yadollahpour for providing translation services.
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Laser therapy EXPLODE ALL AND INREGISTER
2 MESH DESCRIPTOR Phototherapy EXPLODE ALL AND INREGISTER
3 (laser* or ultraviolet or phototherap* or photoradiation therap* or photon therap* or light therap* or heat therap*) AND INREGISTER
4 #1 or #2 or #3 AND INREGISTER
5 MESH DESCRIPTOR Cicatrix EXPLODE ALL AND INREGISTER
6 (keloid* or cicatrix or cicatrices) AND INREGISTER
7 (hypertrophic near3 (scar or scars or scarred or scarring)) AND INREGISTER
8 #5 or #6 or #7 AND INREGISTER
9 #4 AND #8 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) via the Cochrane Register of Studies
1 MESH DESCRIPTOR Laser therapy EXPLODE ALL AND CENTRAL:TARGET
2 MESH DESCRIPTOR Phototherapy EXPLODE ALL AND CENTRAL:TARGET
3 (laser* or ultraviolet or phototherap* or photoradiation therap* or photon therap* or light therap* or heat therap*) AND CENTRAL:TARGET
4 #1 or #2 or #3 AND CENTRAL:TARGET
5 MESH DESCRIPTOR Cicatrix EXPLODE ALL AND CENTRAL:TARGET
6 (keloid* or cicatrix or cicatrices) AND CENTRAL:TARGET
7 (hypertrophic near3 (scar or scars or scarred or scarring)) AND (CENTRAL:TARGET)
8 #5 or #6 or #7 AND CENTRAL:TARGET
9 #4 AND #8 AND CENTRAL:TARGET
Trial Registry specific search ofThe Cochrane Central Register of Controlled Clinical Trials (CENTRAL) via the Cochrane Register of Studies linked to above CENTRAL search
10 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET
11 http*:SO AND CENTRAL:TARGET
12 #10 OR #11 AND CENTRAL:TARGET
13 #9 AND #12
Ovid MEDLINE
1 exp Phototherapy/
2 exp Laser Therapy/
3 (laser* or ultraviolet or phototherap* or photoradiation therap* or photon therap* or light therap* or heat therap*).tw.
4 or/1‐3
5 exp Cicatrix/
6 (keloid* or cicatrix or cicatrices).tw.
7 (hypertrophic adj3 (scar or scars or scarred or scarring)).tw.
8 or/5‐7
9 4 and 8
10 randomized controlled trial.pt.
11 controlled clinical trial.pt.
12 randomi?ed.ab.
13 placebo.ab.
14 clinical trials as topic.sh.
15 randomly.ab.
16 trial.ti.
17 or/10‐16
18 exp animals/ not humans.sh.
19 17 not 18
20 9 and 19
Ovid Embase
1 exp laser/
2 exp phototherapy/
3 (laser* or ultraviolet or phototherap* or photoradiation therap* or photon therap* or light therap* or heat therap*).tw.
4 or/1‐3
5 exp scar/
6 (keloid* or cicatrix or cicatrices).tw.
7 (hypertrophic adj3 (scar or scars or scarred or scarring)).tw.
8 or/5‐7
9 4 and 8
10 Randomized controlled trial/
11 Controlled clinical study/
12 Random$.ti,ab.
13 randomization/
14 intermethod comparison/
15 placebo.ti,ab.
16 (compare or compared or comparison).ti.
17 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
18 (open adj label).ti,ab.
19 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
20 double blind procedure/
21 parallel group$1.ti,ab.
22 (crossover or cross over).ti,ab.
23 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 orintervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
24 (assigned or allocated).ti,ab.
25 (controlled adj7 (study or design or trial)).ti,ab.
26 (volunteer or volunteers).ti,ab.
27 human experiment/
28 trial.ti.
29 or/10‐28
30 (random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
31 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
32 (((case adj control$) and random$) not randomi?ed controlled).ti,ab.
33 (Systematic review not (trial or study)).ti.
34 (nonrandom$ not random$).ti,ab.
35 Random field$.ti,ab.
36 (random cluster adj3 sampl$).ti,ab.
37 (review.ab. and review.pt.) not trial.ti.
38 we searched.ab. and (review.ti. or review.pt.)
39 update review.ab.
40 (databases adj4 searched).ab.
41 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
42 Animal experiment/ not (human experiment/ or human/)
43 or/30‐42
44 29 not 43
45 9 and 44
EBSCO CINAHL Plus
S34 S10 AND S33
S33 S32 NOT S31
S32 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25
S31 S29 NOT S30
S30 MH (human)
S29 S26 OR S27 OR S28
S28 TI (animal model*)
S27 MH (animal studies)
S26 MH animals+
S25 AB (CLUSTER W3 RCT)
S24 MH (crossover design) OR MH (comparative studies)
S23 AB (control W5 group)
S22 PT (randomized controlled trial)
S21 MH (placebos)
S20 MH (sample size) AND AB (assigned OR allocated OR control)
S19 TI (trial)
S18 AB (random*)
S17 TI (randomised OR randomized)
S16 MH cluster sample
S15 MH pretest‐posttest design
S14 MH random assignment
S13 MH single‐blind studies
S12 MH double‐blind studies
S11 MH randomized controlled trials
S10 S5 AND S9
S9 S6 OR S7 OR S8
S8 TI ( (hypertrophic N3 (scar or scars or scarred or scarring)) ) OR AB ( (hypertrophic N3 (scar or scars or scarred or scarring)) )
S7 TI ( keloid* or cicatrix or cicatrices) OR AB ( keloid* or cicatrix or cicatrices)
S6 (MH "Cicatrix+")
S5 S1 OR S2 OR S3 OR S4
S4 TI (laser* or ultraviolet or phototherap* or photoradiation therap* or photon therap* or light therap* or heat therap*) or AB (laser* or ultraviolet or phototherap* or photoradiation therap* or photon therap* or light therap* or heat therap*)
S3 (MH "Phototherapy+")
S2 (MH "Lasers+")
S1 (MH "Laser Therapy+")
LILACS (Latin American and Caribbean Health Science Information database)
# 1 MH:"Terapia a Laser" OR (Terapia por Laser) OR (Laser Therapy) OR (R Vaporização a Laser) OR MH:E02.594$ OR MH:E04.014.520$ OR mh:"FotoTerapia a Laser" OR (Bisturi a Laser) OR (Escalpelo a Laser) OR (Cirurgia a Laser) OR Terapia OR Fototerapia OR Phototherapy OR (Terapia por Fotorradiação) OR (Terapia por Luz) OR MH:E02.774$ OR Laser$ OR (Therapy Photoradiation) OR (Light Therap$) OR (Photoradiation Therapies) OR (Phototherap$) OR (Therapies Light) OR (Therapies Photoradiation)
#2 MH:Cicatrix OR Cicatriz OR Cicatrizes OR Escaras OR MH:A10.165.450.300$ OR MH:C23.550.355.274$ OR MH:G16.762.891.249$ OR MH:Cicatriz Hipertrófica OR (Cicatriz Hipertrófica) OR (Cicatrix, Hypertrophic) OR (Escaras Hipertróficas) OR MH:A10.165.450.300.125$ OR MH:C23.550.355.274.505$ OR MH:Queloide OR Queloide OR Keloid OR MH:A10.165.450.300.425$ OR MH:C17.300.200.425$ OR MH:C23.550.355.274.510
#3 #1 AND #2
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
phototherapy OR laser OR ultraviolet OR photoradiation OR photon OR light OR heat | Cicatrix
phototherapy OR laser OR ultraviolet OR photoradiation OR photon OR light OR heat | Scars, Hypertrophic
phototherapy OR laser OR ultraviolet OR photoradiation OR photon OR light OR heat | Scar Keloid
World Health Organization International Clinical Trials Registry Platform (www.who.int/clinical-trials-registry-platform)
hypertrophic scars AND phototherapy OR hypertrophic scars AND laser OR hypertrophic scars AND ultraviolet OR hypertrophic scars AND photoradiation OR hypertrophic scars AND photon OR hypertrophic scars AND light OR hypertrophic scars AND heat
cicatrix AND phototherapy OR cicatrix AND laser OR cicatrix AND ultraviolet OR cicatrix AND photoradiation OR cicatrix AND photon OR cicatrix AND light OR cicatrix AND heat
keloid scars AND phototherapy OR keloid scars AND laser OR keloid scars AND ultraviolet OR keloid scars AND photoradiation OR keloid scars AND photon OR keloid scars AND light OR keloid scars AND heat
Appendix 2. Criteria for 'Risk of bias' judgements
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random‐number table; using a computer random‐number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed, non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following:
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Either of the following:
Insufficient information provided to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected treatment‐related adverse effects).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. 585‐nm Pulsed‐Dye Laser (PDL) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Severity of scar: Patient self‐assessment (32 weeks) | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.11, 3.45] |
| 1.2 Incidence and severity of treatment‐related adverse effects (32 weeks) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Mild to moderate discomfort or pain related to treatment | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 8.62 [1.10, 67.39] |
| 1.2.2 Purpura | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 21.32 [3.14, 144.86] |
Comparison 2. Non‐ablative Fractional Laser (NAFL) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Severity of scar: Health Professional Global Assessment (3 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Incidence and severity of treatment‐related adverse effects (3 months) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.1 High‐Density Treatment Arm (HDTA) versus control, scar worsening (3 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.2 Non‐ablative fractional Laser (NAFL) versus no treatment, mild hyperpigmentation (3 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain (3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.1 High‐Density Treatment Arm (HDTA) versus control, erythema (3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.2 High‐Density Treatment Arm (HDTA) versus control, pigment (3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.3 High‐Density Treatment Arm (HDTA) versus control, texture (3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.4 Low‐Density Treatment Arm (LDTA) versus control, erythema (3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.5 Low‐Density Treatment Arm (LDTA) versus control, pigment (3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.6 Low‐Density Treatment Arm (LDTA) versus control, texture (3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Fractional carbon dioxide (CO2) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Severity of scar: Vancouver Burn Scar (VBS) scale (up to 6 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 Hypertrophic scar | 2 | 104 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐4.32, 1.71] |
| 3.1.2 Keloid scar | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.02, ‐0.78] |
| 3.2 Severity of scar: Patient and Observer Scar Assessment Scale (POSAS) (at least one month) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 Scar size, colour, height, length, width, pliability, skin surface texture, pruritus and pain (12 to 18 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.1 Height | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.2 Erythema | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.3 Pigmentation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.4 Pain not related to treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.5 Pruritus | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. 585‐nm Pulsed‐Dye Laser (PDL) versus Triamcinolone acetonide (TAC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Severity of scars: Vancouver Burn Scar (VBS) scale (up to 12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 Severity of scars: Patient self‐assessment (up to 12 months) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2.1 Split‐scar trial | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2.2 Parallel trial | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.3 Incidence and severity of treatment‐related adverse effects (up to 12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3.1 Sequelae (32 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3.2 Mild to moderate pain during the intervention (32 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. 585‐nm Pulsed‐Dye Laser (PDL) versus 5‐Fluorouracil (5‐FU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Severity of scars: Patient self‐assessment (32 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2 Incidence and severity of treatment‐related adverse effects (32 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2.1 Mild to moderate discomfort or pain related to treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2.2 Purpura | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. 585‐nm Pulsed‐Dye Laser (PDL) versus Triamcinolone acetonide (TAC) plus 5‐Fluorouracil (5‐FU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Severity of scars: Patient self‐assessment (32 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2 Incidence and severity of treatment‐related adverse effects (32 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.2.1 Mild to moderate discomfort or pain related to treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.2.2 Purpura | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. 585‐nm Pulsed‐Dye Laser (PDL) versus Silicone Gel Sheeting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Incidence and severity of treatment‐related adverse effects (24 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1.1 Pain during treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1.2 Skin irritation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Erbium laser versus Triamcinolone acetonide (TAC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Severity of scars: Vancouver Burn Scar (VBS) scale (up to 12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.2 Severity of scars: Patient self‐assessment (up to 12 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 9. Fractional carbon dioxide (CO2) laser versus intralesional Triamcinolone acetonide (TAC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Incidence of treatment‐related adverse effects (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1.1 Pain at injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1.2 Telangiectasia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1.3 Skin atrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1.4 Charring | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.2 Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.2.1 Height (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.2.2 Vascularity (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.2.3 Pliability (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.3 Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (24 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.3.1 Pigmentation (24 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 10. Fractional carbon dioxide (CO2) laser versus Intralesional Verapamil.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Incidence of treatment‐related adverse effects (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1.1 Charring (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1.2 Pain at injection site (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2 Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.2.1 Height | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.2.2 Pigmentation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.2.3 Vascularity | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.2.4 Pliability | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.3 Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (24 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.3.1 Height | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.3.2 Pigmentation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.3.3 Vascularity | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 11. 585‐nm Pulsed‐Dye Laser (PDL) plus Triamcinolone acetonide (TAC) plus 5‐Fluorouracil (5‐FU) versus TAC plus 5‐FU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Severity of scars (12 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1.1 Scar improvement good to excellent: observer assessment (12 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1.2 Scar improvement good to excellent: Patient self‐assessment (12 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 12. Carbon dioxide (CO2) Laser plus Triamcinolone acetonide (TAC) versus Cryosurgery plus TAC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Severity of scars (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1.1 Mean percentage reduction in Observer Assessment score (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1.2 Mean percentage reduction in Patient Self‐Assessment score (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1.3 Mean percentage reduction in Vancouver Burn Scale (VBS) score (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.2 Incidence and severity of treatment‐related adverse effects (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.2.1 Atrophy (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.2.2 Erythema (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.2.3 Telangiectasia (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.2.4 Hypopigmentation (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.3 Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain(12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.3.1 Mean percentage reduction of scar height (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.3.2 Mean percentage reduction of scar volume (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.4 Recurrence of the condition (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 13. Neodymium‐doped yttrium aluminum garnet (Nd:YAG) laser plus intralesional corticosteroid diprospan plus 5‐Fluorouracil (5‐FU) versus Intralesional corticosteroid diprospan plus 5‐FU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Severity of scars (3 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1.1 Scar improvement good to excellent: Observer assessment (3 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1.2 Scar improvement good to excellent: Patient self‐assessment (3 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 14. Helium–neon (He‐Ne) laser plus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream versus Decamethyltetrasiloxane, Polydimethylsiloxane and Cyclopentasiloxane cream.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1.1 Skin thickness (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 15. 595‐nm Pulsed‐Dye Laser (PDL) plus intralesional verapamil versus intralesional verapamil.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Incidence of treatment‐related adverse effects (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1.1 Regrowth (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1.2 Treatment‐related pain (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1.3 Hyperpigmentation (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1.4 Depigmentation (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1.5 Purpura (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1.6 Total (24 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.2 Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (change from baseline) (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.2.1 Height (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.2.2 Vascularity (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.2.3 Pliability (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.2.4 Pigmentation (12 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.3 Scar size, colour, height, length, width and pliability, skin surface texture, pruritus and pain (change from baseline) (24 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.3.1 Height (24 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.3.2 Vascularity (24 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.3.3 Pliability (24 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.3.4 Pigmentation (24 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alsharnoubi 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled study (single‐blinded, intra‐individual, split‐scar) conducted in Egypt. Scar segment as the unit of randomisation. Duration of the study: not reported Follow‐up time: 12 weeks (intermediate‐term follow‐up) Protocol was published before recruitment of participants: the study was conducted after the approval of the Laser Institute’s Research Ethical Committee. Details of trial registration: not reported Funding sources: none declared |
|
| Participants |
Number of participants assigned: 15 participants (with a total of 30 scar segments treated) Group 1 (He‐Ne laser plus decamethyltetrasiloxane, polydimethylsiloxan and cyclopentasiloxane cream): 15 scar segments Group 2 (decamethyltetrasiloxane, polydimethylsiloxan and cyclopentasiloxane cream): 15 scar segments Loss: not described Number of participants assessed: 15 participants (with a total of 30 segment scars assessed) Group 1: 15 scar segments Group 2: 15 scar segments Inclusion criteria:
Exclusion criteria:
Age (years): 4.73 ± 1.79 (mean /± SD); age range 2 to 10. Gender: ‐Male: 5 (33.3%) ‐Female: 10 (66.7%) Scar location: not specified Consanguinity: ‐Positive: 9 (60%) ‐Negative: 6 (40%) Scars aetiology: ‐Flame burn: 8 (53.3%) ‐Scaled burn: 7 (46.7%) Skin Type: ‐Type I: 0 (0%) ‐Type II: 1 (6.67%) ‐Type III: 5 (33.3%) ‐Type IV: 9 (60%) ‐Type V: 0 (0%) Skin thickness (by ultrasonography): ‐At baseline: 0.52 ± 0.17 mm (mean ± SD value of the whole scar) Skin perfusion (by Laser Doppler perfusion imager ‐ LDPI): ‐At baseline: 1.27 ± 0.54 V (mean ± SD value of the whole scar) Vancouver Scar Scale (VSS): ‐At baseline: 9 (value of the whole scar) High score: ‐At baseline: 2 (value of the whole scar) Pigmentation score: ‐At baseline: 2 (value of the whole scar) Vascularity score: ‐At baseline: 2 (value of the whole scar) |
|
| Interventions | The hypertrophic scars were divided into 2 halves; 1 half, received He‐Ne laser scanning technique plus topical treatment. The other half, the controlled area, received only Scaro cream.
Group 1: a laser device "bravo terza serie HE NE laser (ASA s.r.i)" with a wave length 632.8‐nm (head source with aimed beam) was used perpendicularly to half of hypertrophic scar, during 25 min in each application, with a power density of 119 mW/ cm2 and energy density of 16 J/cm2,using the scanning technique. The distance between the laser probe and the burn was 70 cm length. The treatment interval was twice per week, for 12 weeks. In addition, participants received topical treatment (with Scaro cream Scaro cream ‐ company: Macro; active ingredients: decamethyltetrasiloxane plus polydimethylsiloxane plus cyclopentasiloxane). Group 2: half of hypertrophic scar received only topical treatment (with Scaro cream Scaro cream ‐ company: Macro; active ingredients: decamethyltetrasiloxane plus polydimethylsiloxane plus cyclopentasiloxane). |
|
| Outcomes |
Primary outcomes: Vancouver Scar Scale (VSS): Graded by a blinded observer to evaluate the skin thickness, pigmentation, and vascularity, before the treatment (pre‐test), and 3 months after (post‐test). Secondary Outcomes: Change in scar thickness: an ultrasound imaging system (LOGIQ P6 GE healthcare, Japan) was used to measure the skin thickness before the treatment (pre‐test), and 3 months after (post‐test). Change in scar perfusion (erythema): skin perfusion was also evaluated before the treatment (pre‐test), and 3 months after (post‐test). A Laser Doppler perfusion imager (LDPI): A PIM II laser Doppler perfusion imager used (Lisca AB, Linköping, Sweden) was used. |
|
| Notes | Descriptive statistics and T test were conducted for comparison of the mean age between both groups. T test was conducted for comparison of treatment mean values of scar thickness, and perfusion between both groups. Paired T test was conducted for comparison between treatment mean values of scar thickness, and perfusion before, and after treatment in each group, as well as areas in‐between the study, and control areas in each group. Mann–Whitney U test was conducted for comparison of VSS between both groups. Wilcoxon signed ranks test was conducted for comparison of VSS between treatment before and after in each group. The level of significance for all statistical tests was set at P < 0.05. All statistical measures were performed through the statistical package for social studies (SPSS) version 19 for windows. The authors declare that they have no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was cited but the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and health professionals performing the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each scar was evaluated by a blinded evaluator before the treatment (pre‐test), and 3 months after (post‐test)." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The results are presented and the number of participants included in each analysis is not provided. It was not clear if there were losses during the study. |
| Selective reporting (reporting bias) | High risk | No trial protocol is available. There are no data about the treated and control areas before the study, only values for the whole scar (before the study) and values for treated and control areas at the end of the study, regarding to the parameters evaluated. |
| Other bias | Low risk | No other sources of bias were found. |
Asilian 2006.
| Study characteristics | ||
| Methods |
Study design: randomised clinical trial (single‐blinded, parallel group) conducted in Iran. Participant was the unit of randomisation. Duration of the study: not reported Follow‐up time: 12 weeks (intermediate‐term follow‐up) Protocol was published before recruitment of participants: not reported Details of trial registration: not reported Funding sources: none declared |
|
| Participants |
Number of participants assigned: 69 participants (with a total of 69 scars treated ‐ 1 scar per participant). Group 1(intralesional TAC): 23 individuals Group 2(intralesional TAC plus 5‐FU): 23 individuals Group 3(intralesional TAC plus 5‐FU plus 585‐nm PDL): 23 individuals Loss: 9 participants. Number of participants assessed: 60 participants Group 1: 20 individuals Group 2: 20 individuals Group 3: 20 individuals Inclusion criteria:
Exclusion criteria:
Age (years): Group 1: 23.4 / 8.0 (mean / ± SD) Group 2: 25.3 / 11.9 (mean / ± SD) Group 3: 25.5 / 13 (mean / ± SD) Gender: Group 1: male: 7; 35 (number; %); female: 13; 65 (number; %) Group 2: male: 8; 40 (number; %); female: 12; 60 (number; %) Group 3: male: 9; 45 (number; %); female: 11; 55 (number; %) |
|
| Interventions | In all segment groups only 1 lesion (preferably on the trunk or proximal extremities) per participant was treated Group 1: intralesional triamcinolone acetonide (TAC, 10 mg/mL) injected at weekly intervals for a total of 8 weeks Group 2: intralesional TAC (0.1mL of 40 mg/mL) plus 5‐Fluorouracil (5‐FU) (0.9 mL of 50 mg/mL). This combination was injected weekly for 8 weeks Group 3: intralesional TAC (0.1 mL of 40 mg/mL) plus 0.9 mL of 5‐FU (50mg/mL) combination injected weekly for 8 weeks in addition to 585‐nm flashlamp‐pumped 585‐nm Pulsed‐Dye Laser (PDL) at the 1st, 4th, and 8th weeks. Laser features: Nlite System, EUPhotonics, Swansea, Wales, UK, with a pulse duration of 250 microseconds, at an energy density of 5 to 7.5 J/cm² with a 5 mm spot and a single pass of spots overlapping 10% to 20% without cooling. |
|
| Outcomes |
Primary outcomes: Overall Appearance (by Patient Self‐assessment): Graded by participants at the 4th, 8th, and 12th weeks of the study on a 5‐point scale: no improvement; poor = up to 25% improvement; fair = 26% to 50% improvement; good = 51% to 75% improvement; and excellent = 76% to 100% improvement. Overall Appearance (Observer Assessment): Graded by the observer at the 4th, 8th, and 12th weeks of the study, by comparison of standardised photographs taken at 4‐week intervals. The overall appearance (improvement) scale used was similar to the patient self‐assessment scale. Treatment‐related adverse effects: The evaluation of participants's symptoms and treatment‐related adverse effects was done by observer interviews during the study. Atrophy and telangiectasia were seen in 37% of participants in the TAC group. In the Segment 3 group, the areas treated with the PDL became purpuric, which lasted from 7 to 10 days. On conclusion of the study, neither treatment‐related adverse textural nor pigmentary alterations were observed in either Segment group 2 or 3. No ulcers or erosions were seen. Secondary Outcomes: Change in scar length: a dial calliper was used to determine the greatest length of the lesion (in mm). Change in scar width: a dial calliper was used to determine the greatest width of the lesion (in mm). Change in scar height: a dial calliper was used to determine the greatest height of the lesion (in mm). Change in scar erythema: erythema was graded by the observer on a 5‐point scale: 0 = no erythema; 1 = mild erythema; 2 = moderate erythema; 3 = severe erythema; and 4 = very severe erythema. Change in scar pliability: pliability was graded by the observer on a 5‐point scale: 0 = no induration; 1 = mild induration; 2 = moderate induration; 3 = severe induration; and 4 = very severe induration. Change in scar pruritus: severity of pruritus was graded by the participant on a 5‐point scale: 0 = no pruritus; 1 = mild pruritus; 2 = moderate pruritus; 3 = severe pruritus; and 4 = very severe pruritus. |
|
| Notes | The authors have indicated no significant conflict of interest with commercial supporters. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was cited but the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation process was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and health professionals performing the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessments were carried out on the basis of participant satisfaction, observations, and the measurements of a blinded observer (a dermatology resident) and photographic records at the beginning and the 4th, 8th, and 12th weeks" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Nine of the 69 participants did not complete the study. The reasons were not specified. |
| Selective reporting (reporting bias) | High risk | No trial protocol is available. Among the evaluated parameters (length, width, height, erythema, and pliability) evaluated by the observer, only the final findings of height and erythema were reported. |
| Other bias | Low risk | No other sources of bias were found. |
Azzam 2016.
| Study characteristics | ||
| Methods |
Study design: prospective randomised intra‐individual (split‐scar) controlled trial conducted in Egypt. Scar segment as the unit of randomisation. Duration of the study: not reported Follow‐up time: 3 months (intermediate‐term follow‐up) Protocol was published before recruitment of participants: participants were recruited from the outpatient clinic of Dermatology Department, Kasr Alainy, Cairo University, after approval of the study by the local ethical committee Details of trial registration: not reported Funding sources: no funding sources were used to support this work |
|
| Participants |
Number of participants assigned: 30 participants (with a total of 30 scars treated ‐ divided into 2 halves = 60 segments) Keloid Group: 18 participants (total of 36 segments enrolled) Segment 1A: a total of 18 segments treated Segment 2A: a total of 18 segments not treated (control) Hypertrophic scar Group: 12 participants (total of 24 segments enrolled) Segment 1B: a total of 12 segments treated Segment 2B: a total of 12 segments not treated (control) Loss: 11 Keloid group: 6 participants (3 dropped after the 3rd laser session, 2 did not come for the 3rd month follow‐up, and 1 did not come for the 6‐month follow‐up visit). Hypertrophic scar group: 5 participants (3 dropped after the 2nd and 2 after the 3rd laser session). Number of participants assessed: 19 individuals Keloid Group: 12 individuals (total of 24 segments) Hypertrophic scar Group: 7 individuals (total of 14 segments) Inclusion criteria:
Exclusion criteria:
Age (years): (keloid group) 31.4 ± 11.1 (mean); (hypertrophic scar group) 24.5 ± 9.4 (mean) Gender: (keloid group) male: 11; 61 (number; %); female: 7; 39 (number; %); (hypertrophic scar group) male: 4, 33.3 (number; %); female: 8, 66.7 (number; %) Duration of scars (years): (both groups) 4.62; 0.167‐20 (mean; range) Skin phototypes: (both groups) II‐VI Scar Location: (both groups) head and neck, and upper limbs (most common sites of lesion) |
|
| Interventions |
Keloid group: Segment 1A: 1 half of the keloid scar was treated with a fractional CO2 laser (smart stack, 30 W, stack 4, 1000‐μs dwelling time, and 800‐μm spacing) Segment 2A: 1 half of the scar was not treated, serving as an internal control Hypertrophic scar group: Segment 1B: 1 half of the hypertrophic scar was treated with a fractional CO2 laser (smart stack, 25 W, stack 3, 600‐μs dwelling time, 700‐μm spacing for skin type III and 800 μm for skin type IV) Segment 2B: 1 half of the scar was not treated, serving as an internal control Obs: in both 1A and 1B groups, the equipment used was the fractional CO2 laser, DEKA, SMARTXIDE DOT, Italy. Topical anaesthetic cream (lidocaine 25 % and prilocaine 25 % ‐ Pridocaine®) was applied under occlusion 60 minutes prior to the laser session and wiped off just before. Four laser sessions, 6 weeks apart, were performed. The follow‐up was done at 1 month, 3 months, and 6 months after the last treatment. |
|
| Outcomes |
Primary outcomes: Patient Satisfaction: Assessed at the end of the study, and graded according to the following: excellent (more than 75 % improvement), good (50 to 75 % improvement), moderate (25% to 50 % improvement), and poor (less than 25 % improvement) Vancouver Burn Scar Assessment Scale (VBS): Treatment outcome was evaluated by the Vancouver Burn Scar (VBS) before and 1, 3, and 6 months after the last laser session by a blinded observer. Four components were considered, such as pigmentation, vascularity, pliability and height. Severity of scar was determined by numeric value from a minimum of 0 to 13 as the most severe form on this scale. Digital photographs were taken using a Sony Cyber shot DSC‐H10, Japan. Treatment‐related adverse effects: Were not assessed Secondary Outcomes: Change in scar pruritus: method not described Change in scar pain: method not described |
|
| Notes | Vancouver Burn Scar Assessment Scale (VBS) is cited along this study as Vancouver Scar Score (VSS). Histological and immunohistochemical results: Three biopsies (4 mm skin punch) were obtained from each patient's scar, before and 1, and 3 months after the last laser session, respectively. In addition, a 4th skin biopsy was obtained from normal skin in the same anatomical region before treatment as control. All skin biopsies were immediately fixed in 10 % formol saline and processed into paraffin blocks Paraffin sections of 5‐μm thickness were prepared and subjected to the following stains: hematoxylin and eosin for histological evaluation, Masson’s trichrome stain for collagen fibres, and anti‐matrix metalloproteinase (MMP9) immunohistochemistry. Skin biopsies obtained 3 months after the last laser session were not subjected to MMP‐9 immunohistochemistry. All stained paraffin sections were examined using an Olympus light microscope and photomicrographs were captured using a digital camera. The authors declared no funding sources were used and that no conflicts of interest were present. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by the participants drawing lots between sealed opaque envelopes, containing cards with treatment code of either laser treatment of test site "right" and no treatment of test site "left", and vice versa. |
| Allocation concealment (selection bias) | Low risk | The laser‐treated site was concealed from the evaluator throughout the study and was revealed only to the investigator who treated the participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and health professionals performing the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Scars were photographed and graded clinically by a blinded evaluator. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Eleven participants dropped out of the study (6 participants of keloid group and 5 of hypertrophic scars group) and the reasons were not described by the author. |
| Selective reporting (reporting bias) | High risk | No trial protocol is available. No skin biopsies were obtained from not treated areas of each participant's scar, in order to compare histological and immunohistochemical changes between treated and not treated scar areas. |
| Other bias | Low risk | No other sources of bias were found. |
Behera 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled, hospital‐based, parallel designed, and single (rater) blinded study, conducted in a tertiary care Institute, in South India. Participants as the unit of randomisation but multiple scar per person. Duration of the study: November 2011 to November 2013 Follow‐up time: 12 months (long‐term follow‐up) Protocol was published before recruitment of participants: the study was conducted after obtaining Institute ethics committee clearance and bears trial registration number Details of trial registration: bears trial registration number CTRI/2015/01/005400 Funding sources: none declared |
|
| Participants |
Number of participants assigned: 60 participants (with a total of 101 scars treated). Group 1 (1 CO2 laser session plus 4 intralesional TAC sessions): 30 individuals (with a total of 56 keloid scars treated) Group 2 (1 cryosurgery session plus 4 intralesional TAC sessions): 30 individuals (with a total of 45 keloid scars treated) Loss: 23 participants (13 from group 1, 10 from group 2) Number of participants assessed: 37 participants Group 1: 17 individuals Group 2: 20 individuals Inclusion criteria:
Exclusion criteria:
Age, gender, and duration of keloids in Group 1 and Group 2: the author only described that the participant characteristics were comparable between the 2 groups with respect to age, gender, and duration of the keloid. No further information was available. Scar Location: Group 1: presternal: 26; chest: 10; shoulder: 7; upper limb: 7; breast: 2; lower limb: 2; ear: 1; back: 1 Group 2: chest: 14; presternal: 10; shoulder: 9; upper limb: 5; lower limb: 5; ear: 1; breast: 1 |
|
| Interventions |
Group 1 (1 CO2 laser session plus 4 intralesional TAC sessions): participants were treated with carbon dioxide laser (COLMS, DINONA), in continuous mode, with maximum power up to 10 W, followed by intralesional triamcinolone acetonide (ILTAC) injection. After this, the patients were given ILTA (40 mg/mL) every month for 3 months. Group 2 (1 cryosurgery session plus 4 intralesional TAC sessions): participants were treated with cryotherapy using contact cryoprobe with 2 freeze thaw cycles of 20 seconds (Frigitronics, Nitrous oxide as cryogen) followed by ILTAC injection. After this, the patients were given ILTAC (40 mg/mL) every month for 3 months. |
|
| Outcomes |
Primary outcomes Overall Appearance (by Patient Self‐assessment): Assessed by participants at 3, 6, and 12 months and graded on a 5‐point scale as, no improvement; poor = up to 25% improvement; fair = 26% to 50% improvement; good = 51% to 75% improvement; and excellent = 76% to 100% improvement Overall Appearance (Observer Assessment): Assessed by 2 blinded observers at 3, 6, and 12 months, based on a serial photograph and graded on a 5‐point scale as, no improvement; poor = up to 25% improvement; fair = 26% to 50% improvement; good = 51% to 75% improvement; and excellent = 76% to 100% improvement Vancouver Burn Scar Assessment Scale (VBS): Assessed at 3, 6, and 12 months and graded on a 5‐point scale as, no improvement; poor = up to 25% improvement; fair = 26% to 50% improvement; good = 51% to 75% improvement; and excellent = 76% to 100% improvement Treatment‐related adverse effects: The evaluation of participants's treatment‐related adverse effects was done by a blinded investigator during the study. Secondary Outcomes: Change in scar height: a dial calliper was used to determine the percentage of height reduction of the lesion (in mm) Change in scar volume: reduction of scar volume was evaluated but the method used to assess this parameter was not described Change in scar pain: reduction of scar pain was evaluated but the method used to assess this parameter was not described Change in scar pruritus: reduction of scar pruritus was evaluated but the method used to assess this parameter was not described |
|
| Notes | Vancouver Burn Scar Assessment Scale (VBS) is cited along this study as Vancouver Scar Scale (VSS). Cryosurgery is cited along this study as cryotherapy. The authors have indicated no significant interest with commercial supporters. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by simple randomisation" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation was concealed and noted in a sealed file and not handed over till statistical analysis was completed by investigator" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and health professionals performing the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Results were assessed by a blinded observer. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Twenty‐three of the 60 participants did not complete the study. The reasons were not specified. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was retrospectively registered (CTRI/2015/01/005400). All parameters listed in the methods section to assess changes in the scars were described, however, the method used to assess the lesion's volume was not described. |
| Other bias | Low risk | No other sources of bias were found. |
Blome‐Eberwein 2016.
| Study characteristics | ||
| Methods |
Study design: prospective randomised intra‐individual, single‐blinded, controlled trial conducted in the USA. Scar as the unit of randomisation. Duration of the study: not reported Follow‐up time: 12 to 18 weeks (intermediate‐term follow‐up) Protocol was published before recruitment of participants: participants were recruited from the outpatient burn centre at Lehigh Valley Health Network, Allentown, Pennsylvania Details of trial registration: not reported Funding sources: not reported |
|
| Participants |
Number of participants assigned: 36 participants (80 scars treated) Group 1 (fractional CO2 laser): 48 scar segments enrolled Group 2 (control group): 32 scar segments enrolled Loss: 4 participants (2 dropped out because of pain, 1 moved away, and 1 was unwilling to schedule follow‐up appointment) Number of participants assessed: 32 individuals with a total of 80 scar segments enrolled Inclusion criteria:
Exclusion criteria:
Participants included aged 18 years or older and were quote: "burn survivors and survivors of other skin loss diseases with mature, matched, hypertrophic, blatant scars", according to the authors. |
|
| Interventions |
Group 1: fractional CO2 laser treatments were performed with the Lutronic® e‐CO2 laser (Lutronic, Inc., Fremont, CA) on the treatment scars after the topical anaesthetic cream (lidocaine / tetracaine: 23% / 7%) had been applied for 45 minutes. The topical cream was removed, the fractional laser was performed as following: 40 mJ to 90 mJ, 5 to 8.4% coverage, 1 pass, and 30 W. After each session, the treated area was covered with Aquaphor® ointment (Beiersdorf AG, Hamburg, Germany). Treatments were performed every 4 to 6 weeks for 3 consecutive treatments. Group 2: control group (not treated). |
|
| Outcomes |
Primary outcomes Vancouver Scar Scale (VSS): The VSS considered 4 components (appearance, pigmentation, hypertrophy and vascularity), and was assessed by a blinded evaluator. Patient and Observer Scar Assessment Scale (POSAS): The POSAS is a scar assessment scale that combine participant and observer evaluation. The observer assessed 5 components: appearance, pigmentation, hypertrophy, vascularity and roughness (results from 5 to 50; the highest score reflects the worst scar). The participants evaluated appearance, pain, itching, texture, and pigmentation (results from 6 to 60). Secondary Outcomes: Change in scar pliability: tool used was the Cutometer MPA 580 (Courage & Khazaka, Cologne, Germany). This tool measures the response of skin when being suctioned by defined suction force into a small probe. Change in scar height: tool used was the high‐resolution ultrasound 35 MHz system (Longport Inc., Glen Mills, PA). The thickness was measured in millimetres. Change in scar colour (erythema and pigmentation): tool used was the Dermaspectrometer (Cortex Technology, Cyberderm, Media, PA), which uses narrow‐band reflectance spectrometry to evaluate coloration, and is able to distinguish erythrocytes from melanin. Change in scar sensation: tool used was the Semmes‐Weinstein Aesthesiometer monofilament testing set (North Coast Medical, Campbell, CA), which consists of a series of different diameter and weight filaments, that lightly touches the examined area of skin. Change in scar pain: assessed as part of the POSAS (results from 1‐10) Change in scar pruritus: assessed as part of the POSAS (results from 1‐10) |
|
| Notes | Several participants had multiple scars in the same body area and required only 1 control scar. No declaration of funding sources was available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using SAS/STAT software, Version 9.1 of the SAS System for Windows, copyright© 2004 (SAS Institute Inc., Cary, NC) before the start of study enrolment" |
| Allocation concealment (selection bias) | Low risk | Quote: "To prevent bias, clinicians remained blinded to the treatment assignment for each patient until each scarring site on that given patient was designated by one of the research team assistants" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and health professionals performing the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Results were assessed by a blinded observer. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four patients dropped out. The reasons were described by the authors (quote: "too painful without anaesthesia: 2, moved away: 1, and unwilling to schedule follow‐up appointment: 1"). As‐treated analyses were performed. |
| Selective reporting (reporting bias) | Low risk | No trial protocol is available, but all parameters listed in the methods section to assess changes in the scars were described. |
| Other bias | Low risk | No other sources of bias were found. |
Chen 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled, hospital‐based, parallel designed, and single‐blinded study, conducted in China. Participants as the unit of randomisation. Duration of the study: May 2014 to May 2015 Follow‐up time: 12 weeks (intermediate‐term follow‐up) Protocol was published before recruitment of participants: the study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Nanjing, China) Details of trial registration: not reported Funding sources: supported by grants from the National Natural Science Foundation of China (nos. 81573072 and 81371757) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (no. JX10231801) |
|
| Participants |
Number of participants assigned: 69 participants (with a total of 69 scars treated ‐ 1 scar per participant) Group 1 (intralesional Diprospan ‐ betamethasone disodium phosphate and betamethasone dipropionate): 23 individuals Group 2 (intralesional Diprospan plus 5‐FU): 23 individuals Group 3 (intralesional Diprospan plus 5‐FU plus 1064‐nm Nd:YAG): 23 individuals Loss: 7 participants. Number of participants assessed: 62 participants Group 1: number of individuals not specified Group 2: number of individuals not specified Group 3: number of individuals not specified Inclusion criteria:
Exclusion criteria:
Age (years): Group 1: 27.2 / 6.4 (mean /± SD) Group 2: 26.5 / 7.5 (mean /± SD) Group 3: 26.5 / 9.5 (mean /± SD) Gender: male: 42% female: 58% Scar Location: face and neck: 4.7%; trunk: 67.8%; proximal extremities: 22.5%; distal extremities: 5% Duration of scars (months): Group 1: 29.8 / 25.6 (mean / ± SD) Group 2: 29.2 / 33.6 (mean / ± SD) Group 3: 39.8 / 26.8 (mean / ± SD) Erythema score at baseline: Group 1: 3.5 / 0.8 (mean / ± SD) Group 2: 3.7 / 0.7 (mean / ± SD) Group 3: 3.6 / 0.8 (mean / ± SD) Pruritus score at baseline: Group 1: 3.4 / 0.6 (mean / ± SD) Group 2: 3.3 / 0.5 (mean / ± SD) Group 3: 3.5 / 0.7 (mean / ± SD) Pliability score at baseline: Group 1: 3.0 / 1.1 (mean / ± SD) Group 2: 2.9 / 1.0 (mean / ± SD) Group 3: 2.9 / 0.9 (mean / ± SD) No statistically significant differences were observed between the study groups regarding mean age (P = 0.872), duration (P = 0.557), erythema (P = 0.401), pruritus (P = 0.628) and pliability (P = 0.664) at baseline |
|
| Interventions | In all segment groups only 1 lesion (preferably on the trunk or proximal extremities) per participant was treated Group 1: keloids were treated once a month by intralesional injection of Diprospan (1 ml/ampoule contains 2 mg betamethasone disodium phosphate and 5 mg betamethasone dipropionate) for 3 months. Group 2: keloids received a combination of 0.5 ml 5‐FU (25 mg/ml; Jinyao Pharmaceutical Group, Tianjin, China) and 1 ml Diprospan, injected monthly for 3 months. Group 3: keloids were treated by a 1,064‑nm Nd:YAG (Lumenis One; Lumenis, Santa Clara, CA, USA) with a single 12‐msec pulse at an energy density of 90‐100 J/cm² with a 6 mm spot and a single pass of spots overlapping 5% to 10%, without cooling, for 3 sessions at a 1‐month interval. Each session consisted of 3 passes (without local anaesthesia), unless the participant felt intolerable pain at the 2nd pass. This way, the session was ceased. Intralesional injection of Diprospan plus 5‐FU was similar to that in group 2 and was given immediately after each Nd:YAG treatment for a total of 3 sessions. |
|
| Outcomes |
Primary outcomes Overall Appearance (by Patient Self‐assessment): Assessed by participants 1 month after each session (at the 1st, 2nd, and 3rd months of the study), based on a 5‐point scale as, no improvement; poor improvement = up to 25%; fair improvement = 26% to 50%, good improvement = 51% to 75% improvement; and excellent improvement = 76% to 100%. Overall Appearance (Observer Assessment): Assessed by a blinded observer 1 month after each session (at the 1st, 2nd, and 3rd months of the study), by comparing the standardised photographs taken at the 4‐week intervals. The scales of overall appearance (improvement) were similar to those of the patient self‑assessment. Treatment‐related adverse effects: Treatment‐related adverse effects were reported according to the group in which they occurred. Secondary Outcomes: Change in scar erythema: erythema was graded by the observer on a 5‐point scale: 0 = no erythema; 1 = mild erythema; 2 = moderate erythema; 3 = severe erythema; and 4 = very severe erythema. The percentage of lesion improvement was defined as the percentage of erythema reduction compared with the erythema at baseline. Change in pliability: pliability was graded by the observer on a 5‐point scale: 0 = no induration; 1 = mild induration; 2 = moderate induration; 3 = severe induration; and 4 = very severe induration. The percentage of lesion malacia was defined as the percentage of reduction to the baseline pliability. Change in scar pruritus: severity of pruritus was graded by the participant on a 5‐point scale: 0 = no pruritus; 1 = mild pruritus; 2 = moderate pruritus; 3 = severe pruritus; and 4 = very severe pruritus. The percentage of itch reduction was defined as the percentage of pruritus reduction to the baseline pruritus. |
|
| Notes |
Statistical analysis: The data were analysed using the SPSS 11.5 software package for Windows (SPSS Inc., Chicago, IL, USA). Standard 2‐tailed and paired Student's t‐tests were used to compare the differences between values at time‐points of assessment and at baseline. One‐way analysis of variance followed by post hoc and X² tests were used to compare the difference between baseline and 1, 2 and 3 months. All statistical tests were 2‑tailed. P < 0.05 was considered to indicate a significant difference. This work was supported by grants from the National Natural Science Foundation of China (nos. 81573072 and 81371757) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (no. JX10231801). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was cited but the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation process was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blinded study. Participants and health professionals performing the treatment can't be blinded. Objective assessment of the results was made by a blinded observer. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Results were assessed by a blinded observer. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seven participants did not complete the study. The reasons were not specified. |
| Selective reporting (reporting bias) | Low risk | No trial protocol is available, but all parameters listed in the methods to assess changes in the scars were described. |
| Other bias | Low risk | No other sources of bias were found. |
Daoud 2019.
| Study characteristics | ||
| Methods |
Study design: prospective randomised intra‐individual (split‐scar) controlled trial with 3 treatment arms conducted in the USA. Scar segment as the unit of randomisation. Duration of the study: not reported Follow‐up time: 6 months (intermediate‐term follow‐up) Protocol was published before recruitment of participants: the study was board‐approved but there is no mention about protocol publishing. Details of trial registration: not reported Funding sources: this trial received funding from the Lumenis corporation. |
|
| Participants |
Number of participants assigned: 23 participants (with a total of 69 scar segments enrolled) Group 1 (fractional CO2 laser): 23 segments Group 2 (fractional CO2 laser plus Intense Pulsed Light ‐ IPL) : 23 segments Group 3 (control ‐ no treatment): 23 segments Loss: 4 participants. Number of participants assessed: 19 participants Group 1: 19 segments Group 2: 19 segments Group 3: 19 segments Inclusion criteria:
Exclusion criteria:
Age (years): 18 to 60 Gender: not specified Scar Location: not specified Skin phototypes: I‐IV Duration of scars (months): at least 12 months |
|
| Interventions | Scars were divided into 3 segments of equal area and randomised into either single laser, multi‐laser, or control (no laser treatment). Analgesia was achieved topically with BLT (20% / 8% / 4%). Group 1: submitted to a total of 4 treatment sessions at 6 to 8‐week interval, with single laser CO2 AFL (Lumenis UltraPulse Encore™, Yokneam, Israel, 15 to 20 mJ, 10% Density, 200 to 300 Hz, 120 μm spot). Group 2: submitted to a total of 4 treatment sessions at 6 to 8‐week interval, with IPL (Lumenis M22 IPL, Yokneam, Israel, 15 mm × 35 mm footprint, settings varied based on scar) immediately followed by CO2 AFL (same parameters of group 1). Group 3: no treatment. |
|
| Outcomes |
Primary outcomes Manchester Scar Scale (MSS): The MSS considered 5 categories (colour, matte versus shiny, contour, distortion and texture) and was assessed by a blinded observer. Lower scores indicate a better scar character profile. The average decrease in MSS score was reported and means scar severity (improvement). Patient and Observer Scar Assessment Scale (POSAS): The POSAS is a scar assessment scale that combine participant and blinded observer evaluation of 5 components: appearance, pigmentation, hypertrophy, vascularity and roughness (results from 5 to 50; the highest score reflects the worst scar). Secondary Outcomes: Digital Photography assessment: Digital photographs (pre‐ and post‐treatment) were evaluated by blinded observers, in order to identify which one was taken before and after treatment; they also analysed if there was improvement of the scars (appearance). |
|
| Notes |
Statistical analysis: Data were analysed using a parametric analysis of variance (ANOVA) between the 3 groups (control, CO2 alone, CO2 plus IPL) and the 2 time periods (pre‐treatment and post‐treatment). A 2 x 3 ANOVA with repeated measures was used for statistical comparisons. The trial received funding from the Lumenis corporation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was cited but the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation process was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and health professionals performing the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Results were assessed by a blinded observer. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four lost to follow‐up prior to the third treatment. The reasons were specified (loss of communication). As‐treated analyses were performed. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol is available. All parameters listed in the methods section to assess changes in the scars were described, however, the methods used to assess these parameters were not described. |
| Other bias | Low risk | No other sources of bias were found. |
Khattab 2019.
| Study characteristics | ||
| Methods |
Study design: randomised parallel controlled trial conducted in Egypt. Participants as the unit of randomisation. Duration of the study: March 2018 to March 2019 Follow‐up time: 24 weeks (intermediate‐term follow‐up) Protocol was published before recruitment of participants: not reported Details of trial registration: not reported Funding sources: none declared |
|
| Participants |
Number of participants assigned: 40 participants with a total of 56 keloid scars Group 1 (intralesional Verapamil): total of 20 participants treated (26 keloids treated) Group 2 (PDL plus intralesional Verapamil): total of 20 participants treated (30 keloids treated) Loss: not described Number of participants assessed: 40 (56 scars) Inclusion criteria:
Exclusion criteria:
Age (mean) Group 1: 30.45 Group 2: 32.65 Race: not reported Gender: Group 1: male 11; 55 (number; %); female 09; 45 (number; %) Group 2: male 09; 45 (number; %); female 11; 55 (number; %) Skin phototypes: not reported Duration of scar (months): 6 months to 20 years Scar Location: Group 1: extremity 10; 50; trunk: 2; 10; face: 2; 10. Group 2: extremity 9; 45; trunk: 4; 20; face: 2; 10. Scar Aetiology: Group 1: trauma: 8; 40 (number; %); burn: 6; 30 (number; %); spontaneously: 6; 30 (number; %). Group 2: trauma: 10; 50 (number; %); burn: 6; 20 (number; %); spontaneously: 4; 20 (number; %). Previous Treatment: not reported |
|
| Interventions |
Group 1: 20 participants were only treated with intralesional verapamil 2.5 mg/mL (verapamil hydrochloride ‐ Verahexal Knoll AG, Ludwigshafen, Germany), every 3 weeks, for a maximum of 8 sessions or until complete flattening of the scar. Group 2: 20 participants treated with PDL (4‐15 J/cm2, 7 mm spot, 1.5 msec pulse duration, 595‐nm wavelength, DCD, 30 msec spray: 20 msec delay) every 6 to 8 weeks, and intralesional verapamil. A minimum of 4 sessions was advised to the participants for the purpose of this study. Laser beams of various shapes were used which included square, hexagonal or line for various shapes and areas of keloids, based on suitability and convenience. The single pass was given to all the participants selected. Post‐procedure cooling was done following the laser treatment. Systemic antibiotic, azithromycin 500 mg once a day for 3 days, and topical antibacterial cream containing fusidic acid for a week were prescribed. |
|
| Outcomes | At every session, pretreatment photographs were taken under the identical camera and lighting conditions and measurements were recorded for both the groups. Primary outcomes Participants Satisfaction Scale (PSS): Clinical and photographic assessment to evaluate the improvement in overall appearance, dyschromia, the degree of hypertrophy, and texture using a modified Manchester quartile score (MQS)(14). The following 4‐point scale was utilised: 0 ‐ less than 25% improvement; 1‐ 25% to 50% improvement; 2‐ to 50% to 75% improvement; 3 ‐ more than 75% improvement. For each participant, scores in each category were then averaged to assign an overall score. Vancouver scar scale: Assessed scars on 4 domains: vascularity, pigmentation, pliability, and height. The scores range from 0 to 14. The maximum score is 14, indicating the worst result; a score of 0 indicates normal skin. For VSS, keloid height was measured with callipers; pliability was assessed by palpation; vascularity was assessed by visual inspection, and pigmentation was scored after blanching and comparing it with the surrounding skin. |
|
| Notes |
Statistical analysis:
Data are presented as percentage, mean, and SD. Percentages were compared using the X2‐test, linear correlations, and statistical program at 0.05, 0.01, and 0.001 level of P. The Wilcoxon test was used to test the significant improvement of VSS parameters in each group. The VSS scores were compared between the 2 groups using the Mann‐Whitney U test. A P value < 0.05 was considered to be statistically significant. Statistical analysis was done using SPSS version 19. Financial support and sponsorship: nil. Conflicts of interest: there are no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was cited but the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Not cited or described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and health professionals performing the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no mention about the blinding of the evaluators. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The results are presented and the number of participants included in each analysis is not provided. It was not clear if there were losses during the study. |
| Selective reporting (reporting bias) | Low risk | No trial protocol is available, but all parameters listed in the methods section to assess the scar changes were described. |
| Other bias | Low risk | No other sources of bias were found. |
Lin 2011.
| Study characteristics | ||
| Methods |
Study design: prospective randomised intra‐individual (split‐scar) controlled trial with 2 treatment arms conducted in the USA. Scar segment as the unit of randomisation. Duration of the study: recruiting between October 2008 and March 2009, plus 4.5 months of study duration Follow‐up time: 4.5 months (intermediate‐term follow‐up) Protocol was published before recruitment of participants: participants were recruited according to a protocol approved by the Massachusetts General Hospital's institutional review board Details of trial registration: not reported Funding sources: none declared |
|
| Participants |
Number of participants assigned: 20 participants with (with a total of 20 scars treated ‐ 1 scar per participant) Group A (NAFL‐ Non‐ablative fractional laser / High‐Density Treatment Arm ‐ HDTA): 10 participants (total of 20 segments enrolled) Segment 1A: a total of 10 segments treated Segment 2A: a total of 10 segments not treated (control) Group B (NAFL ‐ Non‐ablative fractional laser / Low‐Density Treatment Arm ‐ LDTA): 10 participants (total of 20 segments enrolled) Segment 1B: a total of 10 segments treated Segment 2B: a total of 10 segments not treated (control) Loss: 3 participants. Number of participants assessed: 17 individuals Group A: 9 individuals (total of 18 segments) Group B: 8 individuals (total of 16 segments) Inclusion criteria:
Exclusion criteria:
Age: (group A) 42; 28 to 67 years (mean; range); (group B) 40; 23 to 66 (mean; range) Gender: female: 18; 90 (number; %); male: 2; 10 (number; %). Duration of scars: (group A) 8.3 years; 0.5 to 29 years (mean; range); (group B) 7.3 years; 1.5 to 23 years (mean; range). Skin phototypes: (group A) I‐VI; (group B) II‐VI Scar Location: (group A) neck, chest, back, abdomen, arm, sacrum; (group B) neck, chest, arm, abdomen, shoulder, suprapubic Scar Length: (group A) 9.58 cm; 1‐23.5 cm (mean; range); (group B) 6.94 cm; 2.2‐17 cm (mean; range) |
|
| Interventions |
Group A (NAFL ‐ HDTA): Segment 1A: half of the hypertrophic scar was treated with a Non‐Ablative Fractional Laser (NAFL) at 1550 nm wavelength (40 mJ and per cent coverage of 26%) Segment 2A: half of the scar was not treated, serving as an internal control Group B (NAFL ‐ LDTA): Segment 1B: half of the hypertrophic scar was treated with a Non‐Ablative Fractional Laser (NAFL) at 1550 nm wavelength (40 mJ and per cent coverage of 14%) Segment 2B: half of the scar was not treated, serving as an internal control Obs: in both 1A and 1B groups, the equipment used was the Fraxel Re:Store laser (Solta Medical, Hayward, CA). For each treatment, 8 passes were used. Each participant received a treatment every 2 weeks (in 1 half of the scar) for a total of 4 treatments. The follow‐up was done at 1 month and 3 months after the last treatment. |
|
| Outcomes |
Primary outcomes Overall Appearance (by Blinded Observers): Two blinded dermatologists analysed scar severity improvement according to overall appearance, assessing standardised photographs taken prior to every treatment and at the 1‐month and 3‐month follow‐up visits. They rated both the control side and the treatment side at 1 month and 3 months, using a quartile scale (0 = no improvement or worsened, 1 = minor improvement, 2 = moderate improvement, and 3 = marked improvement). Overall Appearance (by Patient Self‐assessment): At the end of the study participants evaluated the improvement of treated sides of the scar using a 4‐point scale (1 = 0 to 25% improvement, 2 = 25% to 50% improvement, 3 = 50% to 75% improvement, and 4 = 75% or greater improvement). Treatment‐related adverse effects: Side effects, including erythema, discolouration, exfoliation, pain, swelling, and scabbing, were rated on a quartile scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe). Results were presented only in a figure, for both treatment sides (HDTA and LDTA). Secondary Outcomes: Changes in Pigmentation, Erythema and Texture: 2 blinded dermatologists rated independently both the control side and the treatment side at 1 month and 3 months, using a quartile scale (0 = no improvement or worsened, 1 = minor improvement, 2 = moderate improvement, and 3 = marked improvement). Volume Measurement: obtained by a blinded observer using optical frequency domain imaging (OFDI), a 2nd generation of optical coherence tomography to measure the change in scar depth within the scar mould. Measurements were made prior to treatment, and at the 1‐month and 3‐month follow‐up visits. Two representative scars from each arm were calculated. |
|
| Notes | One participant from HDTA did not complete the study due to perceived worsening of the scar. Two participants from LDTA did not complete the study: 1 moved away before the 3‐month follow‐up, and 1 had to stop the treatments due to an unrelated emergent illness. The article was presented as an abstract at the 2009 ASLMS annual meeting under the support of a USAF travel grant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each scar was divided in half and a treatment side was randomly assigned using a coin flip. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was a single‐blinded trial. Participants and health professionals performing the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers were blinded and rated each half of the scar (control and treatment) independently. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three participants dropped out of the study. The reasons were described by the author. One of them gave up because of scar worsening. As‐treated analyses were performed. |
| Selective reporting (reporting bias) | Low risk | No trial protocol is available, but all parameters listed in the methods section to assess changes in the scars were described. |
| Other bias | Low risk | No other sources of bias were found. |
Manuskiatti 2001.
| Study characteristics | ||
| Methods |
Study design: prospective randomised intra‐individual (split‐scar) controlled trial with 3 treatment arms, conducted in the USA. Scar segment as the unit of randomisation. Duration of the study: not reported Follow‐up time: 32 weeks (intermediate‐term follow‐up) Protocol was published before recruitment of participants: not reported Details of trial registration: not reported Funding sources: none |
|
| Participants |
Number of participants assigned: 10 participants (keloidal or hypertrophic median sternotomy scars of 10 participants were divided into 4 segments, with a total of 40 segments evaluated) Segment 1 (585‐nm PDL 3J/cm²): a total of 10 segments treated Segment 2 (585‐nm PDL 5J/cm²): a total of 10 segments treated Segment 3 (585‐nm PDL 7J/cm²): a total of 10 segments treated Segment 4 (no treatment ‐ control segment): a total of 10 segments not treated Loss: none Number of participants assessed: 10 participants (40 segments) Segment 1: a total of 10 segments treated Segment 2: a total of 10 segments treated Segment 3: a total of 10 segments treated Segment 4: a total of 10 segments not treated Inclusion criteria:
Exclusion criteria:
Age: 59 ± 19 years of age (mean ± SD) Gender: female: 6; 60 (number; %); male: 4; 40 (number; %) Duration of scars: at least 6 months Skin phototypes: Skin type I: 1; 10 (number; %) Skin type II: 5; 50 (number; %) Skin type III: 2; 20 (number; %) Skin type VI: 2; 20 (number; %) Scar Location: median sternotomy scars Scar Length: not specified Scar Cause: post‐surgery |
|
| Interventions | Each scar was divided equally into 4 segments with an area of 1 cm untreated between individual treated segments to avoid the effect of overlapping pulses on the adjacent treated area and to have a well‐defined segment for observation of each treatment modality. Three segments of each scar were randomly treated with a 585‐nm PDL (Photogenica V, Cynosure Inc, Bedford, Mass). Laser spots were overlapped by 10%, and the entire lesion of each segment, including a few millimetres surrounding the lateral aspects of the lesion, was irradiated during each session. The location assignment of each of the 4 segments (superior to inferior) was rotated sequentially with each participant to minimise potential effects of scar location. Segment 1: 1 segment of each participant's scar was treated with a 585‐nm PDL using a 450‐msec pulse and 5‐mm spot size at an energy density of 3 J/cm² for 6 treatment sessions at 4‐week intervals. Segment 2: 1 segment of each participant's scar was treated with a 585‐nm PDL using a 450‐msec pulse and 5‐mm spot size at an energy density of 5 J/cm² for 6 treatment sessions at 4‐week intervals. Segment 3: 1 segment of each participant's scar was treated with a 585‐nm PDL using a 450‐msec pulse and 5‐mm spot size at an energy density of 7 J/cm² for 6 treatment sessions at 4‐week intervals. Segment 4: 1 segment of each participant's scar was not treated (control). |
|
| Outcomes |
Primary outcomes Overall Scar Improvement (Patient Self‐assessment): At the end of the study, participants subjectively evaluated the improvement of their scars' severity compared with a standardised photograph taken before treatment. The results were graded as being completely clear (100% improvement) or placed in a category of 25% increments. Treatment‐related adverse effects: The areas treated with the PDL became purpuric, which lasted from 7 to 10 days. There were erosions secondary to blistering on some areas treated with a fluence of 5 J/cm² and on all areas treated with 7 J/cm² in participants with skin type VI. All erosions healed within 7 days. Neither treatment‐related adverse textural nor pigmentary alterations were observed on conclusion of the study. Secondary Outcomes: Change in scar height: dial calliper (Mitutoyo, Japan) was used to determine the scar height by measuring the maximum vertical elevation of the scar above normal skin. Change in scar erythema: scar erythema was quantified with a hand‐held colorimeter (ChromaMeter CR‐200; Minolta, Ramsey, NJ). A higher erythema value indicates an increased saturation towards redness. The mean of 3 measurements was obtained from each area under study. Change in scar pliability: scar pliability was graded according to a standard scale used to assess functional mobility of the scar related to the contracture and elastic texture of the scar. Change in scar symptoms: all participants (4/4) who had pruritic or irritative sensations reported cessation of the symptoms after one laser session. Change in scar texture: an improvement in scar texture towards that of adjacent normal skin was also observed in all laser‐irradiated segments. |
|
| Notes | The authors have indicated no significant interest with commercial supporters and no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was cited but the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There is no mention about blinding of participants or health professionals. It is likely that the blinding could have been broken, and the outcomes are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no mention about the blinding of the evaluators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Low risk | No trial protocol is available, but all parameters listed in the methods section to assess changes in the scars were described. |
| Other bias | Low risk | No other sources of bias were found. |
Manuskiatti 2002.
| Study characteristics | ||
| Methods |
Study design: prospective, intra‐individual (split‐scar), randomised controlled trial conducted in the USA. Scar segment as the unit of randomisation Duration of the study: not reported Follow‐up time: 32 weeks (intermediate‐term follow‐up) Protocol was published before recruitment of participants: not reported Details of trial registration: not reported Funding sources: none reported |
|
| Participants |
Number of participants assigned: 10 participants (keloidal or hypertrophic median sternotomy scars of 10 participants were divided into 5 segments, with a total of 50 segments evaluated) Segment 1 (585‐nm PDL 5J/cm²): a total of 10 segments treated (1 segment of each participant's scar) Segment 2 (TAC ‐ 20mg/mL): a total of 10 segments treated (1 segment of each participant's scar) Segment 3 (5‐FU ‐ 50mg/mL): a total of 10 segments treated (1 segment of each participant's scar) Segment 4 (TAC ‐ 1mg/mL plus 5‐FU ‐ 45mg/mL): a total of 10 segments treated (1 segment of each participant's scar) Segment 5 (no treatment ‐ control segment): a total of 10 segments not treated (1 segment of each participant's scar) Loss: none Number of participants assessed: 10 (50 segments) Segment 1: a total of 10 segments treated Segment 2: a total of 10 segments treated Segment 3: a total of 10 segments treated Segment 4: a total of 10 segments treated Segment 5: a total of 10 segments not treated Inclusion criteria:
Exclusion criteria:
Age: aged 25 to 74 years (range) Gender: female: 6; 60 (number; %); male: 4; 40 (number; %) Duration of scars (months): 7; 6 to 11.5 (mean; range) Skin phototypes: Skin type I: 1; 10 (number; %) Skin type II: 5; 50 (number; %) Skin type III: 2; 20 (number; %) Skin type VI: 2; 20 (number; %) Scar Location: median sternotomy scars Scar Length: not specified Scar Cause: post‐surgery |
|
| Interventions | Each scar was divided equally into 5 segments, with an untreated area of 1 cm between individual treated segments to avoid the global effect of PDL and intralesional formulas on the adjacent treated segments and to have a well‐defined segment for observation of each treatment modality. Five segments were randomly treated with 4 different regimens. One segment of each scar received no treatment and served as a control. The assignment of modality per segment was sequentially rotated from superior to inferior in each participant to adjust for effects of location. Segment 1: laser irradiation with a 585‐nm PDL (Photogenica V; Cyanosure Inc, Bedford, Mass) at an energy density of 5 J/cm² with a 7 mm spot without cooling for 6 treatment sessions at 4‐week intervals Segment 2: intralesional TAC (Kenalog; Westwood‐Squibb, Buffalo, NY) at a concentration of 20 mg/mL every 4 weeks for a total of 6 treatments Segment 3: intralesional 5‐FU (Roche, Nutley, NJ) at a concentration of 50 mg/mL for a total of 10 treatments (every 2 weeks for the first 8 treatments and every 4 weeks for the last 2 treatments) Segment 4: intralesional TAC (1 mg/mL) mixed with 5‐FU (45 mg/mL) for a total of 10 treatments (every 2 weeks for the first 8 treatments and every 4 weeks for the last 2 treatments) Segment 5: untreated ‐ control |
|
| Outcomes |
Primary outcomes Overall Scar Improvement (Patient Self‐assessment): At the end of the study participants subjectively evaluated the improvement of their scars' severity compared with a standardised photograph taken before treatment. The results were graded as being completely clear (100% improvement) or placed in a category of 25% increments. Treatment‐related adverse effects: Almost all the participants (90%) reported mild to moderate discomfort during PDL treatment sessions. Purpuric discolorations were seen in the laser‐irradiated segments of all participants. Erosion secondary to blistering was observed in some areas treated with laser in 2 participants with skin phototype VI. Mild to moderate pain was reported by 100% of the participants, in the segments treated with TAC, 5‐FU (lasting from 30 minutes to several hours), and TAC plus 5‐FU, during injections. Spots of purpura were seen at the 5‐FU and TAC plus 5‐FU injection sites in 20% to 30% (respectively) of the participants at each follow‐up visit. One participant developed localised superficial tissue slough at the TAC plus 5‐FU injection site after the first treatment visit, but this reaction was not observed after the subsequent treatments. Treatment‐related adverse sequelae, including hypopigmentation (20%), telangiectasia (20%), and skin atrophy (10%), were seen in 50% (5/10) of the segments that received TAC injection alone. Some of these treatment‐related adverse effects were initially noted as early as week 8, and all persisted through the 32‐week follow‐up. No persistent treatment‐related adverse sequelae were observed in segments treated with the other modalities. Secondary Outcomes: Change in scar height: dial calliper (Mitutoyo Corporation, Kawasaki, Japan) was used to determine scar height by measuring the maximum vertical elevation of the scar above normal skin. Measurements were done at weeks 8, 16, 24 and 32. Change in scar erythema: scar erythema was measured using a handheld colorimeter (ChromaMeter CR‐200; Minolta, Ramsey, NJ). A higher erythema value indicates increased saturation toward red. The mean of 3 measurements obtained from each area under study was used. Measurements were done at weeks 8, 16, 24 and 32. Change in scar pliability: scar pliability was graded according to a standard scale used to assess functional mobility of the scar related to the contracture and elastic texture of the scar. Measurements were done at weeks 8, 16, 24 and 32. Change in histological findings: punch biopsy samples were obtained from 2 representative participants at week 32: 2 from the PDL‐ and TAC‐treated segments of one participant and 2 from the TAC plus 5‐FU and control segments of another participant. Each biopsy sample stained with hematoxylin‐eosin was examined for the pattern, arrangement, and characteristics of collagen bundles and fibroblasts and the vascularity features of individually treated segments. |
|
| Notes | No declaration of funding sources was available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was cited but the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There is no mention about blinding of participants or health professionals. It is likely that the blinding could have been broken, and the outcomes are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no mention about the blinding of the evaluators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Low risk | No trial protocol is available, but all parameters listed in the methods section to assess changes in the scars were described. |
| Other bias | Low risk | No other sources of bias were found. |
Omranifard 2007.
| Study characteristics | ||
| Methods |
Study design: prospective single‐blinded randomised parallel controlled trial conducted in Iran. Participants as the unit of randomisation. Duration of the study: not reported Follow‐up time: 12 months (long‐term follow‐up) Protocol was published before recruitment of participants: study protocol was compatible with the guidelines of the 1975 Declaration of Helsinki and was approved by the authors institutional review board. Details of trial registration: not reported Funding sources: none declared |
|
| Participants |
Number of participants assigned: 120 participants (1 scar per participant was treated) Group 1 (PDL): 40 participants Group 2 (erbium laser): 40 participants Group 3 (corticosteroid): 40 participants Loss: none Number of participants assessed: 120 participants Group 1: 40 participants Group 2: 40 participants Group 3: 40 participants Inclusion criteria:
Exclusion criteria:
Age: participants' ages ranged from 10 to 35 years (mean of 27.2 ± 4.8). Participants's age did not present significant differences among the 3 groups. Gender: (segment 1) male 9; 22.5 (number; %); female 31; 77.5 (number; %); (segment 2) male 7; 17.5 (number; %); female 33; 82.5 (number; %) (segment 3) male 8; 20 (number; %); female 32; 80 (number; %). Gender ratio did not present significant differences among the 3 groups. Duration of scar: The mean duration of scars was 8.9 ± 2.6 (mean ± SD) months. Duration of scar did not present significant differences among the 3 groups. Skin phototypes: Fitzpatrick class III Scar location: Hypertrophic scars on the head and neck Scar length: Hypertrophic scars of > 4 cm |
|
| Interventions |
Group 1: treated with N‐lite PDL application of 585‐nm flashlamp‐pumped pulsed dye laser to the hypertrophic scars with max 9 J/cm² fluence, a 5‐mm spot size and a 1.5‐µsec pulse duration. The fluence was decreased to 7.0 to 7.5 J/cm² if participants developed blisters after the first treatment session. Group 2: received Erbium laser application of 2940‐nm and a 0‐1 msec pulse duration and repetition of 30 Hz with max 2 J/cm² (carl busel BLM 10005). Group 3: received corticosteroid injection (triamcinolone acetonide at 5 to 10 mg/ml monthly) and pressure garments. Obs: in all 3 segments of participants, treatment sessions were performed at 4‐week intervals for a maximum period of 12 months, but were terminated sooner if the scar resolved or when the participants were satisfied with the improvement in their scars severity or refused any further treatment. |
|
| Outcomes |
Primary outcomes: Patient Satisfaction: assessed by a simple questionnaire Vancouver Burn Scar Assessment Scale (VBS): evaluated by the Vancouver Burn Scar (VBS) assessment scale. Four components were considered, such as pigmentation, vascularity, pliability and height. Severity of scar was determined by numeric value from a minimum of 0 to 13 as the most severe form on this scale. To minimise the inter‐observer errors, all measurements were done by 3 trained general physicians throughout the study. Digital photographs were taken by 1 photographer with 1 camera in exactly similar settings such as resolution, magnification and angle. Treatment‐related adverse effects: were assessed by a simple questionnaire. Secondary Outcomes: Change in scar mean vascularity scores: for assessment of pigmentation and vascularity, a transparent tool for blanching the scar was used. Change in scar mean height scores: calliper used to determine the scar height by measuring the maximum vertical elevation of the scar above the normal skin. Patient tolerance: all participants tolerated the laser and conventional treatment well. |
|
| Notes | Participants were referred to this study by dermatologists or plastic surgeons. No declaration of funding sources was available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was cited but the method was not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and health professionals performing the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "This study was a single blinded randomised clinical trial ... to minimize the inter‐observer errors, all measurements were done by three trained general physicians throughout the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | High risk | No trial protocol is available. Despite that the authors reported that the treatment outcome was evaluated by the Vancouver Burn Scar (VBS) assessment scale (considering pigmentation, vascularity, pliability and height), they only described 2 characteristics (vascularity and height). Other values and complications, such as textural or discolouration (hypo‐ or hyperpigmentation) and participant satisfaction (assessed by a simple questionnaire) were not described. |
| Other bias | Low risk | No other sources of bias were found. |
Srivastava 2019.
| Study characteristics | ||
| Methods |
Study design: randomised clinical trial (single‐blinded, parallel group) conducted in India. Participants as the unit of randomisation. Duration of the study: May 2017 to April 2018 Follow‐up time: 24 weeks (intermediate‐term follow‐up) Protocol was published before recruitment of participants: not reported. The study protocol was approved by the institutional Screening Committee and Ethics Committee. Informed consent was obtained from all the participants. Details of trial registration: not reported Funding sources: none declared |
|
| Participants |
Number of participants assigned: 60 participants (with a total of 60 scars treated ‐ 1 scar per participant). Group 1(fractional CO2 laser): 20 participants Group 2(intralesional TAC): 20 participants Group 3(intralesional verapamil): 20 participants Loss: none. Number of participants assessed: 60 participants Group 1: 20 individuals Group 2: 20 individuals Group 3: 20 individuals Inclusion criteria:
Exclusion criteria:
Participants Age (years): Group 1: 32.65 / 9.74 (mean / %) Group 2: 30.45 / 9.33 (mean / %) Group 3: 29.45 / 8.24 (mean / %) Gender: Group 1: male: 9; 45 (number; %); female: 11; 55 (number; %) Group 2: male: 11; 55 (number; %); female: 9; 45 (number; %) Group 3: male: 9; 45 (number; %); female: 11; 55 (number; %) Scar Location: Group 1: extremity: 5; 25 (number; %); face: 4; 20 (number; %); trunk: 4; 20 (number; %); presternal: 7; 35 (number; %) Group 2: extremity: 4; 20 (number; %); face: 2; 10 (number; %); trunk: 6; 30 (number; %); presternal: 8; 40 (number; %) Group 3: extremity: 4; 20 (number; %); face: 3; 15 (number; %); trunk: 4: 20 (number; %); presternal: 9; 45 (number; %) Scar Aetiology: Group 1: burn: 6; 30 (number; %); infection: 6; 30 (number; %); surgery: 3; 15 (number; %); trauma: 5; 25 (number; %) Group 2: burn: 5; 25 (number; %); infection: 6; 30 (number; %); surgery: 3; 15 (number; %); trauma: 6; 30 (number; %) Group 3: burn: 4; 20 (number; %); infection: 5; 25 (number; %); surgery: 4; 20 (number; %); trauma: 7; 35 (number; %) |
|
| Interventions |
Group 1: fractional CO2 laser was delivered with a spot density of 25 spots/cm2 and energy of 30–50 millijoules level, every 3 weeks, for 24 weeks. Group 2: intralesional TAC (a maximum of 2 mL of 40 mg/mL) was injected every 3 weeks for 24 weeks or till scar flattening occurred, whichever was earlier. Group 3: intralesional Verapamil (a maximum of 2 mL of 2.5mg/mL) was injected every 3 weeks for 24 weeks or till scar flattening occurred, whichever was earlier. |
|
| Outcomes |
Primary outcomes: Vancouver Scar Scale (VSS): For VSS, keloid height was measured with callipers; pliability was assessed by palpation; vascularity was assessed by visual inspection; and pigmentation was scored after blanching and comparing it with the surrounding skin. Blanching was achieved using a piece of clear plastic sheet till scar flattening occurred (VSS < 2). Treatment‐related adverse effects: Pain at injection site, telangiectasia, skin atrophy and charring were evaluated and reported (when they occurred). Secondary Outcomes: Change in scar pruritus: method not described. Change in scar pain: method not described. Change in scar height: measured with callipers. Change in scar pliability: assessed by palpation. Change in scar vascularity: assessed by visual inspection. Change in scar pigmentation: scored after blanching and comparing it with the surrounding skin. Scars were evaluated at each stage by taking serial photographic records and objective measurements by VSS. Assessment was done by a resident doctor who was not part of the treating team. Mean values of the score were recorded. |
|
| Notes | Comparative analysis among the 3 groups was done using scatter plots to determine the correlation in the treatment groups. Proportions were compared between the groups using chi square test and ANOVA was used for evaluating difference in means of groups. Statistical analysis was carried out with SPSS software for Windows Version 23.0 (Armonk, NY). A P value of < 0.05 was considered to be significant. Funding sources: none declared. The authors declare that no competing financial interests exist. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All the patients eligible for the study were selected from the outpatient clinic and randomised according to the Chit‐in‐Box method". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blinded study. Participants and health professionals performing the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessment was done by a resident doctor who was not part of the treating team". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol is available. All parameters listed to assess changes in the scars were described (although methods used to assess pruritus and pain have not been described). |
| Other bias | Low risk | No other sources of bias were found. |
Verhaeghe 2013.
| Study characteristics | ||
| Methods |
Study design: prospective, split lesion design, single‐blinded, intraindividual randomised controlled trial conducted in Belgium. Scar segment as unit of randomisation. Duration of the study: January 2010 to July 2011 Follow‐up time: 6 months from the beginning of treatment (intermediate‐term follow‐up). Protocol was published before recruitment of participants: the local ethical committee approved the study (registration number B 67020097471) Details of trial registration: registered on clinicaltrials.gov (NCT01056211) Funding sources: this work was supported by a grant of the Klinisch Onderzoeksfonds (Clinical Research Fund) of Ghent University Hospital |
|
| Participants |
Number of participants assigned: 22 participants with a total of 22 scars (1 scar per person) Segment 1 (NAFL‐ Nonablative fractional laser): total of 22 segments treated Segment 2 (control): total of 22 segments not treated Loss: 4 participants Number of participants assessed: 18 participants (total of 36 segments) Segment 1 (NAFL‐ Nonablative fractional laser): 18 segments Segment 2 (control): 18 segment Inclusion criteria:
Exclusion criteria:
Age: 41; 17 to 62 (mean; range) Race: Asian: 2; Caucasian: 16 Gender: male 3; 16.7 (number; %); female 15; 83.3 (number; %) Skin phototypes: Skin type I: 2; 11.1 (number; %) Skin type II: 7; 38.9 (number; %) Skin type III: 6; 33.3 (number; %) Skin type IV: 3; 16.7 (number; %) Duration of scar (months): 38; 1 to 223 (mean; range) Scar Location: lower limb: 2; head and neck: 3; joints: 4; upper limb: 4; trunk: 5 Scar cause: surgery: 15; trauma: 3 Previous Treatment: silicone dressing: 8; local corticosteroids: 5; moisturiser: 10; pressure therapy: 1; pulsed dye laser treatment: 1; none: 1 |
|
| Interventions |
Segment 1: 1 scar area was treated with 1540‐nm non‐ablative fractional laser therapy (Starlux 300 with Lux 1540‐nm fractional hand piece, Palomar Medical Technologies, Burlington, MA). A 10‐mm hand piece (100 microbeams/cm²) was used, with a 15‐ms pulse duration, and energies from 45 to 85 mJ/microbeam (mB) in 3 to 4 passes. The energy level was 45 to 55 mJ/mB during the 1st treatment and was increased 5 to 10 mJ/mB every subsequent session depending on side effects. The maximum delivered energy was 85 mJ/mB. Passes were applied by covering the entire treatment area 3 to 4 successive times. Participants were treated without topical or infiltrative anaesthesia. A single health professional performed all treatments. Segment 2: a scar area, similar to scar area treated with NAFL (in a same anatomic region), received no treatment (control group). |
|
| Outcomes |
Primary outcomes: Severity of scar: Health Professional Global Assessment: measured for the treated and untreated control side on a visual analogue scale (VAS) ranging from 0 to 100 mm (0 = normal skin and 100 = worst possible scar) at baseline and 1 and 3 months after the last treatment. The health professional performing the evaluations was blinded to the treatment. Patient global assessment (PGA): measured for the treated and untreated control side on a VAS ranging from 0 to 100 mm (0 = as normal skin and 100 = worst possible scar) at baseline and 1 and 3 months after the last treatment. Patient and Observer Scar Assessment Scale (POSAS): scar assessment scale validated for the evaluation of burn scars and linear surgical scars. The combination of a participant and an observer scale allows for a more‐complete evaluation of the scar. The observer scale contains 6 parameters: vascularisation, pigmentation, thickness, relief, pliability, and surface area. The participant scale also contains 6 items: pain, itching, colour, stiffness, thickness, and relief. Each of the 6 items has a 10‐step score, with 10 indicating the worst imaginable scar or sensation. The total score of both scales is calculated by adding the scores of each of the 6 items (range 6 to 60). The lowest score (6) reflects normal skin, and the highest score (60) reflects the worst imaginable scar. Treatment‐related adverse effects: participants were interviewed 4 days after the treatment, over the telephone, and the treatment‐related adverse effects were registered on a standard form. Long‐term treatment‐related adverse effects were registered 3 months after the last treatment. Treatment‐related pain (patient self‐assessment): the participant assessed treatment‐related pain after each treatment on a VAS from 0 to 100 mm (0 = no pain and 100 = worst possible pain). Secondary outcomes: Skin reflectance measurements: quantified skin redness and pigmentation before and 1 and 3 months after the last treatment (DSM II Color meter, Cortex Technology, Hadsund, Denmark). The erythema (E) and melanin (M) index was measured 3 times for the treated side, the untreated control side, and the healthy surrounding skin at each time point. An average of the E and M index of the treated and untreated control area was calculated and subtracted from the average of the E and M index of the surrounding normal skin to adjust for fluctuations in the vascular bed and pigmentation (ΔE and ΔM). The location of the measurement was marked on plastic templates at baseline, and measurements were taken at 1‐ and 3‐month follow‐up at the same location. Digital photographs were taken for documentation in JPEG format using a digital camera. |
|
| Notes | The authors have indicated no significant interest with commercial supporters. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization procedure involved computer‐generated randomization lists in which allocation was indicated. The random allocation sequence was created using a digital randomization program." |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocated treatment was concealed from the assessor throughout the study and revealed only to the treating physician" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participant and treating physician were not blinded because this was not feasible from a practical perspective" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The health professional performing the evaluations was blinded to the treatment. Quote: "The physician performing the evaluations was blinded to the treatment". "Blinded on‐site response evaluations were performed 1 and 3 months after the final treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant withdrew during the study for personal, non‐treatment‐related reasons and did not complete allocated treatments. Two participants were lost to follow‐up for personal non‐treatment‐related reasons, and 1 participant was excluded from statistical analysis because the blinded assessor judged that both parts of the scar were not identical at baseline. Eighteen participants were included in the statistical analyses. |
| Selective reporting (reporting bias) | Low risk | Protocol: NCT01056211. All parameters listed to assess changes in the scars were described in the overall result, such as health professional global assessment, patient global assessment (PGA), Patient and Observer Scar Assessment Scale (POSAS) and Skin reflectance measurements. |
| Other bias | Low risk | No other sources of bias were found. |
Wittenberg 1999.
| Study characteristics | ||
| Methods |
Study design: prospective single‐blinded randomised, intra‐individual (split‐scar) controlled trial conducted in the USA. Scar segment as unit of randomisation. Duration of the study: 1 December 1996 to 31 May 1997 Follow‐up time: 40 weeks (intermediate‐term follow‐up) Protocol was published before recruitment of participants: approved by the institutional review board of the Henry Ford Health System, Detroit, Michigan Details of trial registration: not reported Funding sources: funding was provided through the Clarence S. Livingood Fund under the direction of Edward A. Krull, MD, and by a small projects fund, both at Henry Ford Hospital, Detroit, Michigan |
|
| Participants |
Number of participants assigned: 20 participants (each participant's scar was divided in 3 segments ‐ 60 segments) Segment 1 (FLPDL ‐ 585‐nm Flashlamp‐Pumped Pulsed‐dye Laser): a total of 20 segments treated Segment 2 (SGS ‐ silicone gel sheeting): a total of 20 segments treated Segment 3 (control): a total of 20 segments not treated Loss: 1 One participant dropped out of the study and another 1 stopped using SGS (silicone gel sheeting). Number of participants assessed: 19 participants (with a total of 57 segments enrolled). Segment 1: a total of 19 segments treated Segment 2: a total of 18 segments treated Segment 3: a total of 19 segments not treated Inclusion criteria:
Exclusion criteria:
Age: 49; 24 to 81 (mean; range) Gender: male: 5; 25 (number; %) female: 15; 75 (number; %) Duration of scar (months): 32; 4 to 240 (mean; range) Skin phototypes: Skin type II: 10; 50 (number; %) Skin type V: 2; 10 (number; %) Skin type VI: 8; 40 (number; %) Scar location: hip: 1; neck: 1; knee: 1; shoulder: 1; back: 1; thigh: 2; abdomen: 4; chest: 9 Previous treatment: silicone gel sheeting: 2; intralesional corticosteroid: 3; none: 15 |
|
| Interventions |
Segment 1: 1/3 of each participant’s scar was treated with an FLPDL (model SPTL‐1; Candela Laser Corp, Wayland, Mass) at a wavelength of 585‐nm, a pulsed duration of 450 microseconds, fluence per pulse between 6.5 and 8.0 J/cm², and a spot size of 5 mm with a 10% to 20% overlap. The fluence used on each participant was determined by the threshold dose—the lowest dose that produced nonblanchable purpura filling the entire spot size. Participants were given the option of applying a topical anaesthetic agent (2.5% lidocaine and 2.5% prilocaine cream) 2 hours before laser therapy. After laser treatments, the site was iced for 15 to 20 minutes. Participants (n = 19) received 4 laser treatments at 8‐week intervals. Segment 2: participants were instructed to wear SGS (Cica Care; Smith and Nephew, Largo, Fla) on the designated site (corresponding to 1/3 of their scar) for at least 12 continuous hours per day and to wash the SGS and test site with soap and water before and after treatment. The SGS was held in place using a dressing retention sheet (Hypafix tape; Smith and Nephew). Participants (n = 18) were treated for 24 weeks with SGS. Segment 3: 1/3 of the scar was randomised to control and left untreated for the study duration. |
|
| Outcomes |
Primary outcomes Treatment‐related adverse effects: One participant dropped out of the study at week 24 because of pain during laser treatments. One participant did not use SGS because of skin irritation. Secondary outcomes Changes in burning pruritus and pain (not related to treatment): at each visit, participants rated their pain, burning, and pruritus based on a quartile scale from 1 (absent or minimal) to 4 (severe). Change in scar erythema: blood flow (erythema) was evaluated using a laser doppler. Measurements were taken at 0, 8, 16, 24, and 40 weeks. Change in scar elasticity: elasticity was evaluated with the handheld elastometer at 8, 16, 24, and 40 weeks. Change in scar volume: polydimethyl vinyl siloxane material was used to make negative impressions of the scars. Then, by a specific process final scar volumes were obtained. Change in scar histological analysis: in participants who consented, punch biopsy samples were taken from the treated and control sections of each scar and from healthy skin 3 cm from the scars. Each biopsy sample was fixed in formaldehyde, embedded in paraffin, and stained with hematoxylin‐eosin and Giemsa. A masked observer compared the degree of fibrosis in the biopsy samples at weeks 0 and 40. |
|
| Notes | The initial goal for this study was to enrol 30 participants, allowing a minimal difference between any 2 group means of 0.61 SD to be detected with 90% power. These calculations were based on paired t test analysis and assumed 2‐sided testing with α = .05. Twenty participants were enrolled in the study, which reduced the power to detect minimal differences to 74%. Funding was provided through the Clarence S. Livingood Fund under the direction of Edward. A. Krul, MD, and by a small projects fund, both at Henry Ford Hospital, Detroit, Mich. Smith and Nephew, Largo, Fla, for supplying silicone gel sheeting, and Hypafix tape; Heraeus Kulzer Dental, South Bend, Ind, for supplying dental impression material. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Only the patient and the individual performing the treatments knew the treatment assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The measurements were taken by a masked observer at constant sites on the skin during each visit" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant dropped out of the study (because of pain during laser treatments) and another one did not use the silicone gel sheeting until the end of the trial (because of skin irritation). |
| Selective reporting (reporting bias) | Low risk | No trial protocol is available, but all parameters listed in the methods section to assess changes in the scars were described. |
| Other bias | Low risk | No other sources of bias were found. |
AFL: ablative fractional laser;CO2: carbon dioxide; 5‐FU: Fluorouracil; HDTA: high‐density treatment arm; He‐Ne: helium‐neon; LDPI: Laser Doppler perfusion imager; LDTA: low‐density treatment arm MSS:Manchester Scar Scale; NdYAG:neodymium‐doped yttrium aluminium garnet; OFDI:optical frequency domain imaging; PDL: Pulsed‐Dye Laser; POSAS: Patient and Observer Scar Assessment Scale; RCT: randomised controlled trial; SD: standard deviation; SPSS: statistical package for social studies; TAC: triamcinolone acetonide; VBS: Vancouver Burn Scar Assessment Scale; VSS: Vancouver Scar Score.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alster 2003a | Same laser in both arms |
| Blome‐Eberwein 2018 | Same laser in both arms |
| Chan 2004 | Non‐randomised study |
| Christophel 2012 | The study included all types of postsurgical scars |
| El‐Zawahry 2015 | Non‐randomised study |
| Freitas 2013 | The study included all types of scars |
| Gaida 2004 | Study about burn scars, not specifically about hypertrophic scars and / or keloids |
| Ghalambor 2006 | Non‐randomised study |
| Hædersdal 2009 | Study about thermal burn scars, not specifically about hypertrophic scars and keloids; one of the exclusion criteria was participants with tendency to produce hypertrophic scars or keloids. |
| Lueangarun 2020 | Not evaluating the effect of the laser therapy |
| Manuskiatti 2007 | Same laser in both arms, without a control area |
| Ouyang 2018 | Same laser in both arms |
| Sheridan 1997 | Non‐randomised study |
| Stern 1989 | Non‐randomised, not controlled study |
| Tierney 2009 | The study was about scars in general after Mohs surgery |
| Van Drooge 2015 | Different type of scars (atrophic and hypertrophic) |
| Wagner 2011 | Non‐randomised, non‐controlled study |
Characteristics of studies awaiting classification [ordered by study ID]
Maari 2017.
| Methods | Randomised, controlled, within‐patient, single‐blinded, pilot study |
| Participants | Burn hypertrophic scars |
| Interventions | Laser treatment on burn HSc relative to self‐matched control scars in 12 patients. The randomly selected scars were treated with the CORE fractional CO2 laser (Candela/Syneron, Mississauga, ON) with the Fusion Mode at the following settings: 25% fractional coverage, ring energy of 17.9 J/cm2, CORE energy of 311 J/cm2 for 2 passes/treatment session. |
| Outcomes | Scar pliability, thickness, vascularity, pain, itch and patient satisfaction. Each site were measured using the Cutometer, Mexameter, and high‐frequency ultrasound at baseline, immediately prior to the 3rd treatment and 12 weeks after the final treatment. |
| Notes | This study did not show a statistical difference between laser treated scar and control in erythema (P .3), pliability (P .2), or thickness (P.4). Larger studies are needed to further evaluate fractional laser as a treatment modality for burn victims. Reported as abstract. No clear description of the control arm (if active ‐ with or without laser or inactive control). |
HSc hypertrophic scar.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12619001317189.
| Study name | Ablative, fractional CO2 laser and medical needling to enhance burn scarring in children: a pilot randomised trial |
| Methods | Randomised controlled trial |
| Participants | Participants will be aged 16 years or younger at the time of recruitment and have hypertrophic burn scarring of any size at up to 2‐months post‐burn |
| Interventions | Intervention arm 1 ‐ Ablative fractional CO2 laser therapy plus standard care Intervention arm 2 ‐ Medical needling plus standard care |
| Outcomes | Primary outcome: scar thickness measured using high frequency ultrasound. Secondary outcome: adverse effects to the laser intervention may include redness, changes in pigmentation (hyperpigmentation or hypopigmentation), a hypersensitive scar, blistering and irritation around hair follicles, infection, scarring, and swelling and bruising for 7 to 10 days after the procedure. Adverse effects to the medical needling intervention may include swelling, tingling, and redness for a day or more. Caregivers report of their child's overall opinion of scar appearance using the Patient Observer Scar Assessment Scale. Caregivers report of their child's scar stiffness using the Patient Observer Scar Assessment Scale. Children's report of overall scar appearance (if the child is aged 8 years or older) will be measured using the patient report of the Patient Observer Scar Assessment Scale. Generic health‐related quality of life of children will be reported using the Child Health Utility‐9D. Incremental cost per QALY measured using the CHU‐9D, trial intervention resource use, and healthcare resource use (including co‐interventions).[12‐month time horizon] Itch severity: measured using a 0 to 10 numeric rating scale for the last month. Pain severity: measured using a 0 to 10 numeric rating scale for the last month. Satisfaction with scar response to treatment measured using a 0 to 10 numeric rating scale. Data will be gathered from patients aged 8 years and older and all parents/caregivers. Scar and skin microbiome as measured using swabs to extract total RNA and perform 16S rRNA sequencing. Scar pigmentation measured using the DSM‐II ColorMeter L* parameter. Scar pigmentation measured using the DSM‐II Colormeter melanin parameter. Scar vascularity measured using the DSM‐II ColorMeter a* parameter. Scar‐specific health‐related quality of life (including sensory symptoms) measured using the Brisbane Burn Scar Impact Profile. Serum proteins linked to wound and scar outcomes measured using a blood sample taken when children are under a light anaesthetic. Therapists report of scar height using the Patient Observer Scar Assessment Scale. Time to scar maturation in days from the burn injury to scar maturation, which is usually the end of active treatment or time of discontinuation or reduced length of application of scar interventions is recommended by health professionals. As reported by treating health professionals. |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
ACTRN12621000288820.
| Study name | A randomised controlled trial of ablative fractional CO2 laser and medical needling in children with burn scars |
| Methods | Randomised controlled trial: stratified allocation will be used in the study with scar thickness of the treatment site (< 2 mm, greater than or equal to 2 mm) as the unit of stratification. Randomisation will be at the level of the individual in random blocks. |
| Participants | Both males and females aged 18 years or younger at the time of recruitment and have: hypertrophic burn scarring of any size at 6‐months or more post‐burn with functional and/or cosmetic implications determined by patient‐report on the Brisbane Burn Scar Impact Profile |
| Interventions | Intervention arm 1 ‐ Ablative fractional CO2 laser therapy plus standard care: ablative fractional CO2 laser therapy will be delivered by a surgeon to each participant under a general anaesthetic using a Lumenis Ablative Fractional CO2 laser with SCAAR FX setting; ARTG no. 182239. The dosage applied by the laser intervention will be delivered at 1 of 3 depth settings (1 to 2 mm, 60 to 70mj – shallow skin lesion, 2 to 3 mm, 70 to 120mj – medium skin lesion, > 3mm, 120 to 150mj – deep skin lesion), which will be determined by ultrasound measurement of scar thickness pre‐operatively; and with a standard device setting of 1% density, square size 10, 250 Hz, 1 pass with minimal overlapping. The dose applied and areas these doses were applied will be recorded as part of a fidelity checklist. Delivered individually over 1 session at 6 months or more post‐burn. Following the intervention any blood and/or exudate will be cleaned, the site will be covered with a Sorbact dressing immediately post‐intervention for 24 hours and following that QV intensive moisturising cream will be applied as many times a day as needed to prevent dryness of the skin site. The duration of the intervention will vary for each participant and will be determined by the treating surgeon but will usually be for no longer than 1 hour under general anaesthetic. A hospital‐specific fact sheet will be provided to the caregivers of participants with post‐procedural information (e.g. ongoing treatment, aftercare of the treatment site). Standard care (see below) will continue with the exception of brief cessation of standard care interventions post‐operatively. Silicone and pressure garment therapy will be recommenced after re‐epithelialisation post laser intervention where appropriate. Intervention arm 2 ‐ Medical needling plus standard care: medical needling will be delivered mechanically by a surgeon to each patient under a general anaesthetic using 3mm needles attached to a roller (Environ Roll‐CIT 3mm). The dose applied and areas these doses were applied will be recorded as part of a fidelity checklist. Delivered individually in 1 session at 6‐months or more post‐burn. Following the intervention any blood and/or exudate will be cleaned, the site will be covered with a Sorbact dressing immediately post‐intervention for 24 hours and following that QV intensive moisturising cream will be applied as many times a day as needed to prevent dryness of the skin site. The duration of the intervention will vary for each participant and will be determined by the treating surgeon but will usually be for no longer than 1 hour under general anaesthetic. A hospital‐specific fact sheet will be provided to the caregivers of participants with post‐procedural information (e.g. ongoing treatment, aftercare of the treatment site). Standard care (see below) will continue with the exception of brief cessation of standard care interventions post‐operatively. Silicone and pressure garment therapy will be recommenced after re‐epithelialization post needling as appropriate. |
| Outcomes | Scar thickness measured using high frequency ultrasound. Scar pigmentation measured using the DSM‐II ColorMeter L* parameter. Scar pigmentation measured using the DSM‐II Colormeter melanin parameter. Burn‐scar specific, health‐related quality of life (child itch severity). Brisbane Burn Scar Impact Profile itch items. Reported by parent proxies or children aged 8 years or older. Burn‐scar specific, health‐related quality of life (child pain severity). Brisbane Burn Scar Impact Profile pain items. Reported by parent proxies or children aged 8 years or older. Burn‐scar specific, health‐related quality of life (perceived scar tightness). Brisbane Burn Scar Impact Profile scar tightness items. Reported by parent proxies or children aged 8 years or older. Burn‐scar specific, health‐related quality of life (child appearance). Brisbane Burn Scar Impact Profile appearance subscale. Reported by parent proxies or children aged 8 years or older. Burn‐scar specific, health‐related quality of life (child daily living). Brisbane Burn Scar Impact Profile daily living subscale. Reported by parent proxies or children aged 8 years or older. Generic health‐related quality of life: CHU‐9D utility measure. Reported by parent proxies or children aged 8 years or older. School functioning: PEDS‐QL school functioning subscale. Reported by children aged 8 years or older. Days missed from school or daycare: a single question for the number of days missed from school or daycare reported by parent proxies or children aged 8 years or older. Days missed from employment: a single question for number of days missed from employment self‐reported by a caregiver. Satisfaction with scar response to treatment measured using a 0 to 10 numeric rating scale. Data will be gathered from patients aged 8 years and older and all parents/caregivers. Adverse effects to the laser intervention may include redness, changes in pigmentation (hyperpigmentation or hypopigmentation), a hypersensitive scar, blistering and irritation around hair follicles, infection, scarring, swelling and bruising for 7 to 10 days after the procedure. This outcome will be measured by participant/proxy self‐report using a study‐specific questionnaire. Adverse effects to the medical needling intervention may include swelling, tingling, and redness for a day or more. Burn‐scar specific, health‐related quality of life (child friendships and social interaction). Brisbane Burn Scar Impact Profile friendships and social interaction subscale. Reported by parent proxies or children aged 8 years or older. Burn‐scar specific, health‐related quality of life (child mobility). Brisbane Burn Scar Impact Profile mobility subscale. Reported by parent proxies or children aged 8 years or older. Burn scar specific health‐related quality of life (child overall impact) ‐ Brisbane Burn Scar Impact Profile overall impact subscale. Burn scar specific health‐related quality of life (child emotional reactions) ‐ Brisbane Burn Scar Impact Profile emotional reactions subscale. Burn scar specific health‐related quality of life (child physical symptoms) ‐ Brisbane Burn Scar Impact Profile physical symptoms subscale. |
| Starting date | Not reported |
| Contact information | Dr Zephanie Tyack The University of Queensland 62 Graham St. South Brisbane QLD 4101 Australia +61 7 3069 7343 z.tyack@uq.edu.au |
| Notes |
ChiCTR1900027249.
| Study name | The efficacy and safety of low energy carbon dioxide fractional in the treatment of paediatric hypertrophic scar: a perspective, randomised, self‐controlled study |
| Methods | Randomised, self‐controlled study trial |
| Participants | Participants should be/have: 1. Aged 1 to 13 years; 2.Hypertrophic scar was diagnosed by at least 3 physicians; 3. Hypertrophic scar area should be at least 6 cm2; 4. Within 18 months of trauma or burn; 5. Skin type III/IV; 6. No glucocorticoid injection, other laser therapy and other invasive treatments have been given in the past year. |
| Interventions | Treatment group: a total of 3 lasers, each 4 weeks apart; Control group: without laser treatment, the remaining measures the same. |
| Outcomes | Primary outcome: Patient and Observer Scar Assessment Scale (POSAS) Score; Secondary outcome: Visual Analogue Scale. |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
ChiCTR‐ONH‐17012350.
| Study name | A randomised, parallel controlled study: the treatment of hypertrophic scars by combining fractional CO2 laser with narrow‐spectrum intense pulsed light |
| Methods | Randomised, parallel controlled study |
| Participants | Participants aged 18 to 55 years, with hypertrophic scars among original regular margins, with length more than 8 cm, thickness more than 2 mm, more than 6 months with no retreat and persistent growth, redness and pruritus without self‐healing and vanishment, and no infiltration and crab claws. |
| Interventions | Intervention 1: fractional CO2 laser and narrow‐spectrum intense pulsed light Intervention 2: narrow‐spectrum intense pulsed light |
| Outcomes | Scar area, pruritus, pain, colour, rigidity, thickness, smoothness, hyperemia |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
CTRI/2015/01/005400.
| Study name | CTRI/2015/01/005400 |
| Methods | Randomised, parallel group, active controlled trial |
| Participants | Participants with keloids irrespective of gender, site of involvement, duration of lesions to be included in the study if they are: 1. Keloid of size ≤ 5 cm in any dimension. 2. Multiple keloids ≤ 5 in numbers. |
| Interventions | Intervention 1: intralesional steroids with carbon dioxide laser: carbon dioxide laser at baseline followed by intralesional steroid for 3 months at 1‐month interval.
Intervention 2: intralesional steroid with cryotherapy: cryotherapy at baseline followed by intralesional steroid for 3 months at 1‐month interval. |
| Outcomes | Primary outcome: improvement in scar severity. Secondary: recurrence rate following treatment with these two different combination therapies. |
| Starting date | 01 March 2012 |
| Contact information | Biswanath Behera: Department of Dermatology, Jipmer Gorimedu, Pondicherry‐605006 Room n. 303, Harvey House 3, Hostel Complex, Jiipmer, Puducherry,605006 India biswanathbehera61@gmail.com |
| Notes |
CTRI/2019/08/020883.
| Study name | Study and compare the effect of Intralesional Radiofrequency and carbon dioxide laser in keloid and hypertrophic scar |
| Methods | Randomised, parallel group, active controlled trial |
| Participants | Patients with keloid and hypertrophic scar, progressive or stationary lesion, size < 10 cm in any dimension, no previous treatment in last 6 weeks. |
| Interventions | Intervention1: Intralesional Radiofrequency ablation: the device used‐RF generator. Mode ‐ cut/ coagulation Max. power output of 10 W. Intracath no 22 will be used with the insulation and small nick was made at the base of middle and the tip of a needle. The uninsulated part of the intracath tip will be inserted into the keloid, and the pre‐set energy will be applied to the keloid tissue until a maxi temperature of 90°C will be reached. Injection triamcinolone acetonide 20 mg/mlL will be infiltrated around the margins just after surgery and again after a gap of every 4 weeks for 6 months. The same procedure will be repeated every monthly consequently for 3 months, if needed (until excellent response achieved). Intervention2: carbon dioxide laser surface ablation: technique ‐ single or stage‐wise manner by multiple puncture technique. Small full thickness punctures will be created throughout the keloid tissue with a gap of 1 to 3 mm between the 2 punctures by CO2 laser. Single pulse (for firm keloid) or continuous (for hard keloid) mode will be used with 20 W power. Injection triamcinolone acetonide 20 mg/mL will be infiltrated around the margins just after surgery and again after a gap of every 4 weeks for 6 months. The same procedure will be repeated every monthly consequently for 3 months, if needed (until excellent response achieved). |
| Outcomes | Primary outcome: improvement in scar severity: excellent response (90 % ‐ almost complete flattening); good response (71 to 90 % ‐ significant flattening); moderate response (51 to 70 % ‐ adequate flattening); poor response (< 50 % ‐ inadequate flattening); time point: 28 weeks Secondary outcomes: size, height, The Vancouver Scar Scale |
| Starting date | 16 September 2019 |
| Contact information | Dr Hita Mehta: Room No.115 First floor Department of Dermatology New OPD building Sir T General hospital Bhavnagar Bhavnagar GUJARAT 364001 India hitamehta88@gmail.com |
| Notes |
IRCT2016052318210N7.
| Study name | Treatment of burn scars using ablative CO2 fractional laser and topical rapamycin (sirolimus) |
| Methods | Randomised open‐label controlled trial |
| Participants | Patients with burn hypertrophic scar who will come to RasoulAkram hospital, excluding: pregnancy or breastfeeding women, and psychological disorders that could not understand or fill consent inform. |
| Interventions | Intervention 1: CO2 fractional laser: device will set with skin types of participants, anatomic place and scars colour and depth. Laser will be applied in 3 sections monthly. Intervention 2: Rapamycin cream: participants will be treated with Rapamycin 1% cream for 12 weeks. Intervention 3: combination therapy of CO2 fractional laser (on burn scars that will be applied in 3 sections monthly) added with Rapamayci 1% Cream. |
| Outcomes | Primary outcome: contracture and texture. Secondary outcomes: satisfaction, skin colour, atrophy. |
| Starting date | Not reported |
| Contact information | Elham Behrangi: Hazrat‐e Rasool General Hospital Niyayesh St. Satarkhan St. Tehran Iran Tel:+98 (21) 66517341‐9: Elham.Behrangi@gmail.com |
| Notes |
NCT02996097.
| Study name | Fractional laser assisted steroid therapy versus intralesional steroids in the treatment of keloids |
| Methods |
Study design: randomised controlled trial Duration of the study: 16 weeks Details of trial registration: NCT02996097 Funding sources: none declared |
| Participants |
Number of participants assigned: 30 participants/lesions. 2 keloids on each participant will be selected as treatment sites Inclusion criteria:
Exclusion criteria:
Age: > 18 years old Gender: 15 female and 15 male |
| Interventions |
Intervention 1: CO2 ablative laser plus intralesional triamcinolone acetonide: a topical eutectic mixture of local aesthetics (EMLA) cream or tetracaine 7% / lidocaine 23% will be applied to the both treatment sites and after sufficient anaesthesia is attained, 1 keloid will be treated with the fractional CO2 laser using standard protocol as practised in our clinics followed by intralesional triamcinolone acetonide. One lesion would be treated with fractional CO2 ablative laser followed with intralesional triamcinolone acetonide at 4 weeks intervals. Intervention 2: intralesional triamcinolone acetonide alone: a topical EMLA cream or tetracaine 7% / lidocaine 23% will be applied to the both treatment sites and after sufficient anaesthesia is attained, 1 keloid will be treated with the fractional CO2 laser using standard protocol as practised in our clinics followed by intralesional triamcinolone acetonide. The other chosen lesion would be treated with intralesional triamcinolone acetonide alone at 4‐week intervals. |
| Outcomes | Primary outcomes: mean change in Composite Observer Score for Patient and Observer Scar Assessment Scale (POSAS) and mean change in Composite Patient Score for Patient and Observer Scar Assessment Scale (POSAS) |
| Starting date | Not reported |
| Contact information | Dr. Ginette Okoye: ginette.okoye@howard.edu |
| Notes | Although the authors report the results in clinicaltrials.gov, no information on the time points and details of the assessments are available. We have contacted study authors, but did not have any response. |
NCT03240718.
| Study name | Pilot study of the ablative fractional CO2 laser in hypertrophic scars in adult burn patients |
| Methods | Randomised, controlled, within‐patient, single‐blinded pilot study |
| Participants | Participants with Fitzpatrick skin type < IV (37); that have sustained a thermal burn injury, at least 2 independent sites that show clinical evidence of hypertrophic scar and are 2 months or more post‐injury. |
| Interventions | Intervention 1: CORE fractional CO2 laser treatment. Intervention 2: Standard care. |
| Outcomes | Primary outcomes: skin characteristics changes: erythema, elasticity and thickness measures. Secondary outcomes: Satisfaction Questionnaire. |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
NCT04769089.
| Study name | Effects of pulsed‐dye and CO2 laser in treatment of hypertrophic burn scars |
| Methods | Randomised, double‐blinded, controlled trial |
| Participants | Participants between 18 and 65 years old with at least 4 areas of burn scar located on the trunk or extremities measuring 3 cm x 3 cm and not previously treated with laser, with a minimum of 1 month after burn closure |
| Interventions | Intervention 1: Pulse Dye Laser: Pulse Dye laser treatment will be applied to 1 randomised area with the 10 mm hand piece, duration of 0.45 ms, and fluence of 5.25 J/s. Intervention 2: CO2 Laser: CO2 laser treatment will be applied to 1 randomised area with the deep Fx hand piece, 300 Hz, 15 mJ, 15% density. Intervention 3: combination: Pulse Dye laser and CO2 treatment will be applied to 1 randomised area. Pulse Dye will be applied with the 10 mm hand piece, duration of 0.45 ms, and fluence of 5.25 J/s followed by the CO2 laser treatment with the deep Fx hand piece, 300 Hz, 15 mJ, 15% density. Intervention 4: mo treatment: 1 area will be randomised to receive no laser treatment. |
| Outcomes | Primary outcome: effectiveness of laser treatments: 4 to 6 weeks post treatment ‐ using the Patient and Observer Scar Assessment Scale (POSAS), evaluate the effectiveness of Pulse Dye Laser (PDL) and Carbon Dioxide (CO2) laser treatment on symptoms related to Hypertrophic Burn Scar (HBS). Secondary outcome: improvement of symptoms: 4 to 6 weeks post treatment ‐ to longitudinally compare the change in symptoms by treatment modality between treatment sessions by assessment with the Patient and Observer Scar Assessment Scale (POSAS) measured for each participant and 2 observers (to increase score reliability) at each follow‐up visit. |
| Starting date | Not reported |
| Contact information | Not provided |
| Notes |
PACTR202004808998657.
| Study name | Fractional carbon dioxide laser versus fractional microneedle radiofrequency in the treatment of post burn scars: a comparative randomised split scar study |
| Methods | Randomised open‐label split scar study |
| Participants | Patients with post burn hypertrophic scars more than 1 year. Participants should be more than 16 years old, be both males and females. Patents with 2 separate lesions, each of them about 2 to 3 cm OR a single large sized lesion equal to or more than 10 cm. |
| Interventions | Fractional carbon dioxide laser: in each participant, a large lesion will be outlined and divided into 2 parts 2 to 3 cm apart so that 1 will be randomly assigned to fractional CO2 laser and the other 1 will receive fractional microneedle radiofrequency. If the lesion size was small 2 symmetrical lesions were picked. Topical anaesthesia will be applied beforehand. The parameters of fractional CO2 laser that will be used in the treatment 20 watt, 800 to 1000 μs dwell time, according to height 2 to 3 stacks, 500 μm spacing. All patents will receive 4 treatment sessions 6 weeks apart. Fractional microneedle radiofrequency: in each patient, a large lesion will be outlined and divided into 2 parts 2 cm to 3 cm apart so that 1 will be randomly assigned to fractional CO2 laser and the other 1 will receive fractional microneedle radiofrequency. If the lesion size is small 2 symmetrical lesions will be picked. The parameters of fractional microneedle radiofrequency; 7 to 8 v, exposure time: 800 ms. depth: 3 to 3.5 mm (using non‐insulated needles), frequency: 2 Hz. Topical anaesthesia will be applied beforehand. All patents will receive 4 treatment sessions 6 weeks apart. |
| Outcomes | Assessment will be done via : a) clinical evaluation: clinical assessment will be done by calculating patient and observer scar assessment scale before every session and 1 month after the last session. b) Photographic documentation: before every session and 1 month after least session. c) Histopathological and biochemical evaluation. Pre treatment biopsy: one 3 mm a punch biopsy will be taken per lesion to be treated prior to treatment sessions. Post treatment biopsy: one 3 mm punch biopsy will be taken per lesion 1 month after the last session. Biopsies will be fixed in 10% natural buffered formalin, and then embedded in paraffin blocks. Sections will be prepared for routine staining by H & E. Sections will be graded as regards the appearance and pattern of dermal collagen and elastin before and 1 month after the last session. Ten sections will be prepared for histochemical staining of collagen fibbers using Masson trichrome stain and elastic fibres using Orcein stain and will be evaluated by an image analyser. Biochemical evaluation: the tissue level of TGFβ1will be measured by ELISA in biopsies obtained from the scar area. |
| Starting date | Not reported |
| Contact information | Shereen Tawfic: shereemosamat@yahoo.com |
| Notes |
CO2: carbon dioxide;ELISA: enzyme‐linked immunosorbent assay; Pes QoL: Pediatric Quality of Life Inventory; POSAS: Patient and Observer Scar Assessment Scale.
Differences between protocol and review
In the data collection and analysis section, the selection of studies was performed independently by three review authors (RL, ACPNP and CAPS) instead of two (RL and CAPS). Additionally, our primary outcomes were described in our protocol as 'Change in severity of keloid or hypertrophic scars (or both)' and 'Incidence and severity of adverse effects and 'we changed the wording to 'severity of keloid or hypertrophic scars (or both)' and ''Incidence and severity of treatment‐related adverse effects'', respectively, to improve understanding of these outcomes. Furthermore, in the unit of analysis issues section, we intended to deem the individual participant as our unit of analysis (unit to be randomised for interventions to be compared), i.e. the number of observations in an analysis should match the number of individuals randomised. However, as the majority of the studies in this systematic review randomised different parts of the same scar for different interventions (split scar), we used each segment of the scar assessed as a unit of analysis. In the analysis of split scar lesions, when different parts of the scar were randomised to different interventions, we performed the analysis of continuous data as a paired t‐test. Regarding the data extraction, the source of funding for the trial and care setting were added post hoc.
Contributions of authors
Rafael Leszczynski: conceived, designed and co‐ordinated the review; extracted data; analysed or interpreted data; undertook and checked certainty assessment; produced the first draft of the review; contributed to writing or editing the review; performed previous work that was the foundation of the current review; wrote to study author / experts / companies; provided data; performed translations; approved the final review prior to submission; and is a guarantor of the review.
Carolina da Silva: extracted data; analysed or interpreted data; produced the first draft of the review; contributed to writing or editing the review; advised on the review; provided data; performed translations; and approved the final review prior to submission.
Ana Carolina Pereira Nunes Pinto: co‐ordinated the review, selected studies, extracted data; performed statistical analysis; interpreted data; undertook and checked certainty assessment; contributed to writing and editing the review; and checked and approved the final review prior to submission.
Uliana Kuczynski (consumer co‐author): checked and approved the final review prior to submission.
Edina da Silva: designed and co‐ordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; provided data; performed translations; and approved the final review prior to submission.
Contributions of editorial base
Jo Dumville (Joint Co‐ordinating Editor): advised on methodology, interpretation and content, and approved the final review prior to publication.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process, advised on interpretation and content, and edited the review.
Sophie Bishop (Information Specialist): ran the searches and edited the search methods section of the review.
Tom Patterson (Editorial Assistant): edited the reference sections and Plain language summary.
Sources of support
Internal sources
-
Cochrane Centre, Brazil
The Brazilian Cochrane Centre provided institutional support for ACPNP, CAPS, RL, and EMKS
External sources
-
The National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Wounds. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Declarations of interest
Rafael Leszczynski: works as a health professional. Carolina da Silva: works as a health professional. Ana Carolina Pereira Nunes Pinto: none known. Uliana Kuczynski: none known. Edina MK da Silva: none known.
New
References
References to studies included in this review
Alsharnoubi 2018 {published data only}
- Alsharnoubi J, Shoukry KE, Fawzy MW, Mohamed O. Evaluation of scars in children after treatment with low-level laser. Lasers in Medical Science 2018;33(9):1991-5. [DOI] [PubMed] [Google Scholar]
Asilian 2006 {published data only}
- Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatologic Surgery 2006;32(7):907-15. [DOI] [PubMed] [Google Scholar]
Azzam 2016 {published data only}
- Azzam OA, Bassiouny DA, El-Hawary MS, El Maadawi ZM, Sobhi RM, El-Mesidy MS. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers in Medical Science 2016;31(1):9-18. [DOI] [PubMed] [Google Scholar]
Behera 2016 {published data only}
- Behera B, Kumari R, Thappa DM, Malathi M. Therapeutic efficacy of intralesional steroid with carbon dioxide laser versus with cryotherapy in treatment of keloids: a randomized controlled trial. Dermatologic Surgery 2016;42(10):1188-98. [DOI] [PubMed] [Google Scholar]
Blome‐Eberwein 2016 {published data only}
- Blome-Eberwien S, Gogal C, Weiss MJ, Boorse D, Pagella P. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. Journal of Burn Care & Research 2016;37(6):379-87. [DOI] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen X, Liu J, Jameel AA, Valeska M, Zhang J, Xu Y, et al. Combined effects of long-pulsed neodymium-yttrium-aluminum-garnet laser, diprospan and 5-fluorouracil in the treatment of keloid scars. Experimental and Therapeutic Medicine 2017;13:3607-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daoud 2019 {published data only}
- Daoud AA, Gianatasio C, Rudnick A, Michael M, Waibel J. Efficacy of combined Intense Pulsed Light (IPL) with fractional CO2 laser ablation in the treatment of large hypertrophic scars: a prospective, randomized control trial. Lasers in Surgery and Medicine 2019;51(8):1-8. [DOI] [PubMed] [Google Scholar]
Khattab 2019 {published data only}
- Khattab FM, Nasr M, Khashaba SA, Bessar H. Combination of pulsed dye laser and verapamil in comparison with verapamil alone in the treatment of keloid. Journal of Dermatological Treatment 2019;31(2):186-90. [DOI] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin JY, Warger WC, Izikson L, Anderson RR, Tannous Z. A prospective, randomized controlled trial on the efficacy of fractional photothermolysis on scar remodeling. Lasers in Surgery and Medicine 2011;43:265-72. [DOI] [PubMed] [Google Scholar]
Manuskiatti 2001 {published data only}
- Manuskiatti W, Fitzpatrick RE, Goldman MP. Energy density and numbers of treatment affect response of keloidal and hypertrophic sternotomy scars to the 585-nm flashlamp-pumped pulsed-dye laser. Journal of the American Academy of Dermatology 2001;45(4):557-65. [DOI] [PubMed] [Google Scholar]
Manuskiatti 2002 {published data only}
- Manuskiatti W, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars. Comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Archives of Dermatology 2002;138(9):1149-55. [DOI] [PubMed] [Google Scholar]
Omranifard 2007 {published data only}
- Omranifard M, Rasti M. Comparing the effects of conventional method, pulse dye laser and erbium laser for the treatment of hypertrophic scars in Iranian patients. Journal of Research in Medical Sciences 2007;12(6):277-81. [Google Scholar]
Srivastava 2019 {published data only}
- Srivastava S, Kumari H, Singh A. Comparison of fractional CO2 laser, verapamil, and triamcinolone for the treatment of keloid. Advances in Wound Care 2019;8(1):7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verhaeghe 2013 {published data only}
- Verhaeghe E, Ongenae K, Bostoen J, Lambert J. Nonablative fractional laser resurfacing for the treatment of hypertrophic scars: a randomized controlled trial. Dermatologic Surgery 2013;39(3 Pt 1):426-34. [DOI] [PubMed] [Google Scholar]
Wittenberg 1999 {published data only}
- Wittenberg GP, Fabian BG, Bogomilsky JL, Schultz LR, Rudne EJ, Chaffins ML, et al. Prospective, single-blind, randomized, controlled study to assess the efficacy of the 585-nm flashlamp-pumped pulsed-dye laser and silicone gel sheeting in hypertrophic scar treatment. Archives of Dermatology 1999;135(9):1049-55. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alster 2003a {published data only}
- Alster T. Laser scar revision: comparison study of 585-nm pulsed dye laser with and without intralesional corticosteroids. Dermatologic Surgery 2003;29(1):25-9. [DOI] [PubMed] [Google Scholar]
Blome‐Eberwein 2018 {published data only}
- Blome-Eberwein SA, Boorse D, Pagella P, Gogal C, Sobotor M. Does topical steroid after fractional CO2 laser treatment of scars make a clinical difference? Journal of Burn Care & Research 2018;39(1):S42-3. [Google Scholar]
Chan 2004 {published data only}
- Chan HH, Wong DS, Ho WS, Lam LK, Wei W. The use of pulsed dye laser for the prevention and treatment of hypertrophic scars in Chinese persons. Dermatologic Surgery 2004;30(7):987-94. [DOI] [PubMed] [Google Scholar]
Christophel 2012 {published data only}
- Christophel JJ, Elm C, Endrizzi BT, Hilger PA, Zelickson B. A randomized controlled trial of fractional laser therapy and dermabrasion for scar resurfacing. Dermatologic Surgery 2012;38(4):595-602. [DOI] [PubMed] [Google Scholar]
El‐Zawahry 2015 {published data only}
- El-Zawahry BM, Sobhi RM, Bassiouny DA, Tabak SA. Ablative CO2 fractional resurfacing in treatment of thermal burn scars: an open-label controlled clinical and histopathological study. Journal of Cosmetic Dermatology 2015;14:324-31. [DOI] [PubMed] [Google Scholar]
Freitas 2013 {published data only}
- Freitas CP, Melo C, Alexandrino AM, Noites A. Efficacy of low-level laser therapy on scar tissue. Journal of Cosmetic and Laser Therapy 2013;15:171-6. [DOI] [PubMed] [Google Scholar]
Gaida 2004 {published data only}
- Gaida K, Koller R, Isler C, Aytekin O, Al-Awami M, Meissl G, et al. Low level laser therapy - a conservative approach to the burn scar? Burns 2004;30:262-7. [DOI] [PubMed] [Google Scholar]
Ghalambor 2006 {published data only}
- Ghalambor AA, Pipelzadeh MH. Low level CO2 laser therapy in burn scars: which patient benefit most? Pakistan Journal of Medical Sciences 2006;22(2):158-61. [Google Scholar]
Hædersdal 2009 {published data only}
- Hædersdal M, Moreau KE, Beyer DM, Nymann P, Alsbjørn B. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers in Surgery and Medicine 2009;41:189-95. [DOI] [PubMed] [Google Scholar]
Lueangarun 2020 {published data only}
- Lueangarun S, Sukcharoen T, Tempark T. Efficacy of combing fractional carbon dioxide laser and silicone gel in treatment of hypertrophic scars and keloids: a split-lesion, double-blinded, randomized, controlled trials. In: Journal of the Dermatology Nurses' Association. Conference: 24th World Congress of Dermatology; 2019 June 11-15; Milan (Italy). Vol. 12. 2020.
Manuskiatti 2007 {published data only}
- Manuskiatti W, Wanitphakdeedecha R, Fitzpatrick RE. Effect of pulse width of a 595-nm flashlamp-pumped pulsed dye laser on the treatment response of keloidal and hypertrophic sternotomy scars. Dermatologic Surgery 2007;33(2):152-61. [DOI] [PubMed] [Google Scholar]
Ouyang 2018 {published data only}
- Ouyang H, Li G, Lei Y, Gold MH, Tan J. Comparison of the effectiveness of pulsed dye laser vs pulsed dye laser combined with ultrapulse fractional CO2 laser in the treatment of immature red hypertrophic scars. Journal of Cosmetic Dermatology 2018;17:54-60. [DOI] [PubMed] [Google Scholar]
Sheridan 1997 {published data only}
- Sheridan RL, MacMillan K, Donelan M, Choucair R, Grevelink J, Petras L, et al. Tunable dye laser neovessel ablation as an adjunct to the management of hypertrophic scarring in burned children: pilot trial to establish safety. Journal of Burn Care & Rehabilitation 1997;18:317-20. [DOI] [PubMed] [Google Scholar]
Stern 1989 {published data only}
- Stern JC, Lucente FE. Carbon dioxide laser excision of earlobe keloids: a prospective study and critical analysis of existing data. Archives of Otolaryngology - Head and Neck Surgery 1989;115(9):1107-11. [DOI] [PubMed] [Google Scholar]
Tierney 2009 {published data only}
- Tierney E, Mahmoud BH, Srivastava D, Ozog D, Kouba DJ. Treatment of surgical scars with nonablative fractional laser versus pulsed dye laser: a randomized controlled trial. Dermatologic Surgery 2009;35(8):1172-80. [DOI] [PubMed] [Google Scholar]
Van Drooge 2015 {published data only}
- Van Drooge AM, Vrijman C, Van der Veen W, Wolkerstorfer A. A randomized controlled pilot study on ablative fractional CO2 laser for consecutive patients presenting with various scar types. Dermatologic Surgery 2015;41(3):371-7. [DOI] [PubMed] [Google Scholar]
Wagner 2011 {published data only}
- Wagner JA, Paasch U, Bodendorf MO, Simon JC, Grunewald S. Treatment of keloids and hypertrophic scars with the triple-mode Er:YAG laser: a pilot study. Medical Laser Application 2011;26(1):10-5. [Google Scholar]
References to studies awaiting assessment
Maari 2017 {published data only}
- Maari C. Randomized, controlled, within-patient, single-blinded pilot study to evaluate the efficacy of the ablative fractional CO2 laser in the treatment of hypertrophic scars in adult burn patients. Journal of the American Academy of Dermatology 2017;76(6 (Suppl 1)):AB212. [DOI: 10.1016/j.jaad.2017.04.1113] [DOI] [Google Scholar]
References to ongoing studies
ACTRN12619001317189 {published data only}
- ACTRN12619001317189. Ablative, fractional CO2 laser and medical needling to enhance burn scarring in children: a pilot randomised trial. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619001317189 2019.
ACTRN12621000288820 {published data only}
- ACTRN12621000288820. A randomised controlled trial of ablative fractional CO2 laser and medical needling in children with burn scars. anzctr.org.au/ACTRN12621000288820.aspx 2021.
ChiCTR1900027249 {published data only}
- ChiCTR1900027249. The efficacy and safety of low energy carbon dioxide fractional in the treatment of pediatric hypertrophic scar: a perspective, randomized, self-controlled study. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900027249 2019.
ChiCTR‐ONH‐17012350 {published data only}
- ChiCTR-ONH-17012350. A randomized, parallel controlled study: the treatment of hypertrophic scars by combining fractional CO2 laser with narrow-spectrum intense pulsed light. www.chictr.org.cn/showproj.aspx?proj=20887 2017.
CTRI/2015/01/005400 {published data only}
- CTRI/2015/01/005400. Therapeutic efficacy of laser therapy (carbon dioxide laser)with intralesional steroid injection versus cold therapy (cryotherapy) with intralesional steroid injection in the treatment of keloidal scar:a randomized controlled trial. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=7531 2015.
CTRI/2019/08/020883 {published data only}
- CTRI/2019/08/020883. Study and compare the effect of intralesional radiofrequency and carbon dioxide laser in keloid and hypertrophic scar. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=31318 2019.
IRCT2016052318210N7 {published data only}
- IRCT2016052318210N7. Treatment of burn scars using ablative CO2 fractional laser and topical rapamycin (sirolimus). en.irct.ir/trial/16592 2017.
NCT02996097 {published data only}
- NCT02996097. Fractional laser assisted steroid therapy vs intralesional steroids in the treatment of keloids. clinicaltrials.gov/show/NCT02996097 2016.
NCT03240718 {published data only}
- NCT03240718. Pilot study of the ablative fractional CO2 laser in hypertrophic scars in adult burn patients. clinicaltrials.gov/ct2/show/NCT03240718 2017.
NCT04769089 {published data only}
- NCT04769089. Effects of pulsed dye and CO2 laser in treatment of hypertrophic burn scars. clinicaltrials.gov/show/NCT04769089 2021.
PACTR202004808998657 {published data only}
- PACTR202004808998657. Fractional carbon dioxide laser versus fractional microneedle radiofrequency in the treatment of post burn scars: a comparative randomized split scar study. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=8138 2019.
Additional references
Alster 2003
- Alster TS, Tanzi EL. Hypertrophic scars and keloids. Etiology and management. American Journal of Clinical Dermatology 2003;4(4):235-43. [DOI] [PubMed] [Google Scholar]
Anderson 1983
- Anderson RR, Parrish JA. Selective phototermolysis: precise microsurgery by selective absorption of pulsed radiation. Science 1983;220:524-7. [DOI] [PubMed] [Google Scholar]
Berman 1996
- Berman B, Bieley HC. Adjunct therapies to surgical management of keloids. Dermatologic Surgery 1996;22(2):126-30. [DOI] [PubMed] [Google Scholar]
Bock 2006
- Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Archives of Dermatological Research 2006;297(10):433-81. [DOI] [PubMed] [Google Scholar]
Bouzari 2007
- Bouzari N, Davis SC, Nouri K. Laser treatment of keloids and hypertrophic scars. International Journal of Dermatology 2007;46(1):80-8. [DOI] [PubMed] [Google Scholar]
Cassuto 2010
- Cassuto DA, Scrimali L, Siragó P. Treatment of hypertrophic scars and keloids with an LBO laser (532 nm) and silicone gel sheeting. Journal of Cosmetic and Laser Therapy 2010;12(1):32-7. [DOI] [PubMed] [Google Scholar]
Ching 2003
- Ching S, Thoma A, McCabe RE, Antony MM. Measuring outcomes in aesthetic surgery: a comprehensive review of the literature. Plastic and Reconstructive Surgery 2003;111(1):469-80. [DOI] [PubMed] [Google Scholar]
Cho 2010
- Cho SB, Lee JH, Lee SH, Lee SJ, Bang D, Oh SH. Efficacy and safety of 1064-nm Q-switched Nd:YAG laser with low fluence for keloids and hypertrophic scars. Journal of the European Academy of Dermatology and Venereology 2010;24(9):1070-4. [DOI] [PubMed] [Google Scholar]
Erol 2008
- Erol OO, Gurlek A, Agaoglu G, Topcuoglu E, Oz H. Treatment of hypertrophic scars and keloids using intense pulsed light (IPL). Aesthetic Plastic Surgery 2008;32:902-9. [DOI] [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
Gupta 2011
- Gupta S, Sharma VK. Standard guidelines of care: keloids and hypertrophic scars. Indian Journal of Dermatology, Venereology, and Leprology 2011;77(1):94-100. [DOI] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Kantor 1985
- Kantor GR, Wheeland RG, Bailin PL, Walker NP, Ratz JL. Treatment of earlobe keloids with carbon dioxide laser excision: a report of 16 cases. Journal of Dermatologic Surgery and Oncology 1985;11(11):1063-7. [DOI] [PubMed] [Google Scholar]
Karsai 2007
- Karsai S, Roos S, Hammes S, Raulin C. Pulsed dye laser: what's new in non-vascular lesions? Journal of the European Academy of Dermatology and Venereology 2007;21(7):877-90. [DOI] [PubMed] [Google Scholar]
Köse 2008
- Köse O, Waseem A. Keloids and hypertrophic scars: are they two different sides of the same coin? Dermatologic Surgery 2008;34(3):336-46. [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Lesaffre 2009
- Lesaffre E, Philstrom B, Needleman I, Worthington H. The design and analysis of split-mouth studies: what statisticians and clinicians should know. Statistics in Medicine 2009;28(28):3470-80. [DOI] [PubMed] [Google Scholar]
Li‐Tsang 2005
- Li-Tsang CW, Lau JC, Chan CC. Prevalence of hypertrophic scar formation and its characteristics among Chinese population. Burns 2005;31(5):610-6. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff JM, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Medicine 2009;3(3):123-30. [PMC free article] [PubMed] [Google Scholar]
Mutalik 2005
- Mutalik S. Treatment of keloid and hypertrophic scars. Indian Journal of Dermatology, Venereology, and Leprology 2005;71(1):3-8. [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5.4 (RevMan 5). Version 5.4. Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Schünemann 2021a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Schünemann 2021b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Seifert 2009
- Seifert O, Mrowietz U. Keloid scarring: bench and bedside. Archives of Dermatologic Research 2009;301(4):259-72. [DOI] [PubMed] [Google Scholar]
Vrijman 2011
- Vrijman C, Drooge AM, Limpens J, Bos JD, Van der Veen JP, Spuls PI, et al. Laser and intense pulsed light therapy for the treatment of hypertrophic scars: a systematic review. British Journal of Dermatology 2011;165(5):934-42. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Leszczynski 2015
- Leszczynski R, Da Silva CA, Kuczynski U, Da Silva EM. Laser therapy for treating hypertrophic and keloid scars. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD011642. [DOI: 10.1002/14651858.CD011642] [DOI] [PMC free article] [PubMed] [Google Scholar]


