Abstract
Fifty-four Angus-cross steers (297 kg ± 12) were stratified by body weight (BW) to pens (six steers per pen) to determine the effects of supplemental Zn on posttransit growth performance and blood and muscle metabolites. Dietary treatments started 25 d before trucking: control (CON; analyzed 54 mg Zn/kg DM), industry (IND; CON + 70 mg supplemental Zn/kg DM), and supranutritional Zn (SUPZN; CON + 120 mg supplemental Zn/kg DM). Supplemental Zn was bis-glycinate bound Zn (Plexomin Zn; Phytobiotics North America, Cary, NC). On day 0, steers were loaded onto a commercial trailer and transported in 18 h (1,822 km). Individual BW was recorded on days –26, –25, –1, and 0 (pre-transit), 1 (posttransit), 6, 27, and 28. Blood was collected on days –1, 1, 6, and 27. Longissimus thoracis biopsies were collected on days –1, 1, and 28. Daily individual feed disappearance was recorded via GrowSafe bunks. Data were analyzed using Proc Mixed of SAS with fixed effect of diet and steer as the experimental unit (growth performance, blood: n = 18 steers per treatment; muscle: n = 12 steers per treatment). Individual initial BW was used as a covariate in BW analysis. Contrast statements to test linear, quadratic, and Zn effects were used to analyze performance and blood parameters. Repeated measures analysis was used for posttransit DMI recovery and weekly posttransit DMI and Zn intake with the repeated effect of time. MetaboAnalyst 5.0 was utilized for statistical analysis of day 1 (off truck) muscle metabolites. Plasma Zn linearly increased due to Zn on days 1, 6, and 27 (P = 0.01), and off-truck (day 1) serum lactate increased over day –1 by 20%, 0%, and 20% in CON, IND, and SUPZN, respectively (Quadratic: P = 0.01). Muscle lactate tended to increase posttransit in CON and IND (P ≤ 0.07) but not SUPZN. Muscle metabolites relating to amino acid and nitrogen metabolism were increased in all treatments posttransit (P ≤ 0.02), and alanine-glucose cycle metabolites tended to increase in CON and IND (P ≤ 0.07). Steers supplemented with Zn recovered pretransit DMI quicker than CON (by d 2: P = 0.01), while IND had greater overall posttransit DMI than CON with SUPZN intermediate (P = 0.04), and Zn-fed steers had greater ADG posttransit (P = 0.04). Zinc supplementation mitigated muscle or serum lactate increases due to transit and increased posttransit ADG.
Keywords: behavior, cattle, feedlot, muscle, transportation, zinc
Zinc is an essential micronutrient which can increase growth performance and potentially prevent muscle fatigue. Beef steers were supplemented with three concentrations of zinc prior to an 18-h transit event to determine the effects of zinc supplementation on posttransit growth performance and blood and muscle metabolites.
Introduction
All cattle are likely transported at least once in their lives (Gonzalez et al., 2012; Schuetze et al., 2017). Long hours of standing combined with the vibrations of the trailer (Gebresenbet et al., 2011; Morris et al., 2021) can lead to muscle fatigue. Gosker and Schols (2008) described muscle fatigue as the “reversible loss of muscle force due to work over time”. Lactate is a valuable biomarker of muscle fatigue and is created from pyruvate, which is an anaerobic ATP production end product (Van Hall, 2010). Before entering the Cori Cycle pathway to support the energy demands of muscle during exercise (Sharma et al., 2019; Strachecka et al., 2019), lactate can be converted back into pyruvate via the Zn-dependent enzyme lactate dehydrogenase (Price, 1962). As such, a practical solution to improve the transition into the feedlot may be increasing the dietary Zn concentration of calves before long-duration transportation to the feedlot.
Though the current national recommendation for dietary Zn concentration is 30 mg Zn/kg DM (NASEM, 2016), a survey reported that, on average, consulting nutritionists recommend supplementing 109 mg Zn/kg DM in receiving diets, and most nutritionists utilize a blend of organic and inorganic mineral sources (Samuelson et al., 2016). It is typical to assume increased bioavailability of minerals from organic sources. In fact, Zn status has been shown to increase with supplementation of an organic Zn source (Spears et al., 2004; Deters et al., 2021).
The objective of this study was to investigate the effects of increasing concentrations of dietary Zn prior to trucking on the posttransit performance, blood and muscle metabolite profiles, and gene expression of growing beef feedlot steers. The hypothesis for this study was that steers fed supplemental Zn from Plexomin Zn (Phytobiotics North America, Cary, NC) would have improved growth performance and decreased indicators of muscle fatigue, such as blood and muscle such as lactate, compared to steers fed no supplemental Zn.
Materials and Methods
The Iowa State University Institutional Animal Care and Use Committee approved all experimental procedures for this study (IACUC Log Number 20-139).
Animals and experimental design
Fifty-four steers from a single source in Iowa were utilized for this study; the management of the steers prior to their arrival at the Iowa State University Beef Nutrition Research Unit (Ames, IA) is unknown. Steers were housed in partially covered concrete pens with six steers in each pen (7.4 m2 per steer). Pens were equipped with GrowSafe bunks (GrowSafe Systems LTD., Airdrie, Alberta, Canada), and steers were adapted to the single-feeder system over 14 d prior to the start of trial. On day –26, steers were individually weighed. Those bodyweights (BW) were used to stratify steers to treatments to begin the trial (18 steers per treatment). Steers were weighed, given an implant (Component TE-IS; 80 mg trenbolone acetate + 16 mg estradiol; Elanco Animal Health, Greenfield, IN), sorted into trial pens, and began treatment diets on day –25 (initial BW: 297 kg ± 2.8). Treatments were control (CON; basal diet containing 54 mg Zn/kg DM), industry (IND; CON + 70 mg Zn/kg DM), and supranutritional (SUPZN; CON + 120 mg Zn/kg DM) concentrations of zinc. Zinc was supplemented via Plexomin Zn (Phytobiotics North America, Cary, NC). Concentrations for the IND treatment were determined based on the average concentration of Zn in receiving diets, according to a survey of consulting nutritionists (Samuelson et al., 2016). The SUPZN dietary Zn concentrations were based on findings suggesting greater carcass-adjusted final BW and average daily gain (ADG) for steers fed 150 mg Zn/kg DM (Genther-Schroeder et al., 2016). Zinc treatments were mixed into a corn-silage-based total mixed ration (TMR); treatment diets are shown in Table 1.
Table 1.
Nutrient composition of diets fed before and after an 18-h transit event for beef feedlot steers
| CON1 | IND1 | SUPZN1 | |
|---|---|---|---|
| DM, % of feed | 55.1 | 55.1 | 54.2 |
| Ingredient, % DM | |||
| Corn silage | 40.0 | 40.0 | 40.0 |
| Sweet Bran2 | 40.0 | 40.0 | 40.0 |
| Dried distiller’s grains with solubles | 15.0 | 10.0 | 10.0 |
| Microingredient premix3 | 5.0 | 5.0 | 5.0 |
| Zn treatment premix6 | – | 5.0 | 5.0 |
| Analyzed composition4, % DM | |||
| Crude protein | 18.4 | 18.0 | 17.9 |
| NDF | 31.3 | 29.7 | 29.3 |
| Ether extract | 3.92 | 3.86 | 2.90 |
| Calculated NEg7, Mcal/kg DM | 1.30 | 1.30 | 1.30 |
| Analyzed trace mineral5, mg/kg DM | |||
| Cu | 14 | 13 | 14 |
| Fe | 143 | 145 | 141 |
| Mn | 40 | 39 | 39 |
| Zn | 54 | 116 | 162 |
Dietary treatments: CON – no supplemental Zn; IND – 70 mg supplemental Zn/kg DM as Plexomin Zn (Phytobiotics North America, Cary, NC); SUPZN – 120 mg supplemental Zn/kg DM as Plexomin Zn (NASEM, 2016).
Branded wet corn gluten feed (Cargill Corn Milling, Blair, NE).
Vitamin and mineral premix provided per kilogram of the diet DM: 0.15-mg Co (cobalt carbonate), 10-mg Cu (Plexomin Cu; Phytobiotics), 20-mg Mn (Plexomin L-Mn; Phytobiotics), 0.1-mg Se (sodium selenite), 0.5 mg I (calcium iodate), and 2,200 IU vitamin A and 25 IU vitamin E (DSM Nutritional Products, Ames, IA). Provided as a percentage of total diet DM: dried distiller’s grains with solubles (3.05%), limestone (1.50%), salt (0.31%), and Rumensin 90 (0.015%).
From TMR analysis by Dairyland, Inc. (Arcadia, WI).
Analyzed via inductively coupled plasma optical emission spectrometry (ICP Optima 7000 DV, Perkin Elmer, Waltham, MA).
Dried distiller’s grains with solubles used as a carried to deliver 70-mg supplemental Zn/kg DM as Plexomin Zn (Phytobiotics) to IND and 120-mg supplemental Zn/kg DM to SUPZN.
Feedstuff NEg values obtained from the Nutrient Requirements of Beef Cattle.
On day 0, all 54 steers were loaded as groups into five compartments of a commercial livestock trailer (Silverstar PSDLC-402; Wilson Trailer Company, Sioux City, IA) at approximately 1300 h; treatment groups were evenly dispersed throughout trailer compartments (average loading density: 1.96 m2/steer). Steers were transported continuously for 18 h (1,822 km) within the state of Iowa (mostly on state highways); steers had no access to feed or water during transport. No bedding was used in the trailer, and airflow was not restricted as no sides were boarded up. Upon return to the feedlot (~0700 h), steers were immediately weighed off-truck and sorted back into original treatment pens to continue to receive treatment diets for a 28-d posttransit period. Muscle biopsies from the longissimus thoracis were collected on day –1, 1, 6, and 28, relative to transit, using methods described by Pampusch et al. (2003) and Messersmith and Hansen (2021). Steers were allowed feed access prior to muscle biopsies on all collection days to prevent excessive time restricted from feed.
Measurements
Bodyweight, ADG, and gain to feed (G:F) were determined. Steers were individually weighed on the days –26, –25, –1, 0, 1, 6, 27, and 28, relative to transit. Weights were recorded prefeeding except on day 0, which was collected immediately before transit (~1100 h). The d 1 BW was directly after transit. Percent weight loss (shrink) due to transit was calculated using days 0 and 1 BW.
Blood was collected prior to feed access via jugular venipuncture from all steers on days –1, 1, 6, and 27, relative to transit. Blood was kept on ice and transported to a campus laboratory for centrifugation. Serum was allowed to clot at room temperature for 90 min before centrifugation. Plasma and serum were centrifuged for 20 min at 4 °C at 1000 × g. After centrifugation, plasma and serum were aliquoted, and serum was stored at –80 °C and plasma at –20 °C for future analyses. Serum urea nitrogen (SUN), lactate, ferric reducing antioxidant power (FRAP), nonesterified fatty acids (NEFA), and glucose were measured on samples from days –1, 1, and 6. Plasma was used to assess Zn concentrations in samples from each blood collection day. Commercially available kits were used to analyze SUN (Teco Diagnostics, Anaheim, CA; intra-assay CV = 3.68%, interassay CV = 3.40%), L-lactate (Biomedical Research Service Center, Buffalo, NY; intra-assay CV = 4.29%, inter-assay CV = 5.44%), FRAP (#K043-H1, Arbor Assays, Ann Arbor, MI; intra-assay CV = 5.28%, interassay CV = 3.86%), NEFA (FUJIFILM Wako Diagnostics, Lexington, MA; intra-assay CV = 3.53%, interassay CV = 1.40%), and glucose (FUJIFILM Wako Diagnostics; intra-assay CV = 2.15%, interassay CV = 4.19%).
Plasma trace mineral concentrations were determined on days –1, 1, 6, and 27, TMR, and muscle zinc concentrations were determined on day 28 using methods described by Pogge and Hansen (2013) and Messersmith and Hansen (2021). A known trace element serum control (UTAK, Valencia, CA) was used for plasma analysis and bovine liver from the National Institute of Standards and Technology (Gaithersburg, MA) was used for TMR and muscle analysis to verify the accuracy of the inductively coupled optical emission spectrometer (ICP Optima 7000 DV, Perkin Elmer, Waltham, MA). Yttrium was added to each sample and standard as an internal standard.
Muscle samples from d -1 and 1 were used for total metabolite analysis at the W. M. Keck Metabolomics Research Laboratory at Iowa State University (Ames, IA). Approximately 25 mg (wet basis) of powdered muscle tissue was weighed into a 2.0 L microcentrifuge tube, and 10 µL of internal standard (adonitol; 1.0 mg/mL) was added. Cold methanol (0.50 mL) was added, and samples were kept on ice for 10 min. Samples were sonicated in an ice-cold water bath for 10 min before being vortexed for 5 min. Centrifugation at 12,000 × g at 4 °C for 10 min occurred before removing 100 µL of supernatant. Afterward, 0.40 mL of cold chloroform was added, and samples were vortexed for 3 min. Cold HPLC grade water was added (0.34 mL) before samples were again vortexed for 10 min and then sonicated in an ice-cold water bath for 10 min. Samples were then vortexed for 3 min followed by centrifugation for 7 min, as previously described. The polar (top) fraction was then transferred to a GC vial and stored at –80 °C. To derivatize samples for gas chromatography-mass spectrometry (GCMS), polar samples were dried to completion using a speed vac overnight. After drying, 50-µL methoxyamine (dissolved in pyridine at 20 mg/mL) was added before incubating samples for 90 min at 30 °C. Then, 70 µL of bis-trimethyl silyl trifluoroacetamide with 1% trimethylchlorosilane was added for silylation, and samples were incubated at 60 °C for 30 min.
The same instrument (Agilent Technologies Model 6890 GC coupled to Model 5975 MS) was used to run all samples; the retention index calibrator was a hydrocarbon mix (C8-C40). A GC-MS column (Agilent-HP5MSI; 30 m × 250 µM × 0.25 µM) completed sample separation. For the oven program, an initial temperature of 50 °C for 1 min, a 5 °C/min increase to 100 °C, and a 20 °C/min increase to 320 °C, and a final hold for 5 min was used with inlet and interface temperature controlled at 250 °C. The detection mass range was set to 50–600 m/z, and the GC–MS was controlled by the Agilent ChemStation software. The 2017 mass spectral library from the National Institutes of Standards and Technology was used for reference for building the known metabolite library.
Muscle samples from the days –1 and 1 were used to assess quantitative gene expression in all treatments. As previously described by McGill et al. (2016), RNA was isolated from muscle samples using Trizol Reagent (Life Technologies, Carlsbad, CA, USA), and, after RNA isolation, cDNA was created using Superscript III Reverse Transcriptase and random primers (Invitrogen, Waltham, MA, USA) following the manufacturer’s instructions. Gene expression analysis was performed using a 96.96 Dynamic Array Integrated Fluidic Circuit (Fluidigm, San Francisco, CA, USA) as previously described by Suasnavas et al. (2015). Supplemental Table S1 lists the 24 targeted genes used for analysis. Using the 2-ΔΔCt method described by Livak and Schmittgen (2001), two housekeeping genes [eukaryotic translation elongation factor 1 alpha 2 (EEF1A2) and ribosomal protein S9 (RPS9)] were used as a reference for relative gene expression.
Feed ingredient and TMR samples were collected weekly for dry matter (DM) analysis. Subsamples were dried for 48 h in a forced air oven at 70 °C. Dried samples were ground using a 2-mm screen and composited by diet and month. Composites were sent to Dairyland Laboratories (Arcadia, WI) for macronutrient analysis (methods 990.03 and 920.39, AOAC, 1996). Individual DMI was calculated for each steer based on GrowSafe feed disappearance and weekly TMR DM. Due to intermittent data recording issues with the GrowSafe system in the first few weeks of the trial, DMI was not calculated from day –25 to –6. DM intake leading up to the transit event (days –5 to –2) was used as a baseline to determine the percentage of previous intake steers were consuming posttransit. Individual G:F (feed efficiency) was calculated using each steer’s average ADG and DMI for the period.
Video was recorded for all pens via mounted cameras (LaView Security, Industry, CA) as described by Heiderscheit et al. (2022). Videos were randomized and played back on a VLC media player, pausing at timepoints. Two trained observers, blinded to treatments, assessed steer standing behavior from day 1 (when steers arrived back to pens) to dusk on day 5 by counting the number of steers standing in the pen using instantaneous scan sampling every 15 min from dawn (0730 hours) to dusk (1600 hours) from each day. Due to the simplicity of the behavior recorded, the inter- and intra-observer reliability were 1.0. The 15-min interval was chosen because Mitlohner et al. (2001) determined scan sampling at 15-min intervals did not yield different results than continuous sampling. Dawn to dusk observations were used as cattle typically rest overnight with little activity (Kilgour, 2012). Standing behavior disregarded motion (i.e., walking or idle), and laying behavior disregarded posture (i.e., lateral or sternal recumbency).
CowManager tags (Select Sires, Inc., Plain City, OH) were placed on all animals and recorded time spent ruminating, active, and nonactive as described by Heiderscheit et al. (2022). CowManager data from arrival back to the farm on day 1 (~0700 hours) to the beginning of the day 6 weighing event (~0700) were utilized to calculate the area under the curve (AUC) using R. For every 6 h, an AUC value was calculated so that four AUC values were obtained for each behavior in a 24-h period for each steer. This compiling technique decreased the data points equal to zero while maintaining the ability to assess changes in behavior over time each day.
Statistical analysis
Steer was the experimental unit for all analyses. Proc Mixed of SAS 9.4 was used. The fixed effect of treatment was used in all models. Individual initial BW (day –25) was used as a covariate for BW analysis. Proc IML was used to generate coefficients for contrast statements to test the linear and quadratic effects of increasing dietary Zn concentrations and the nonZn supplemented treatment (CON) vs. the Zn supplemented treatments (IND and SUPZN) on performance, CowManager behavior minutes, and trace minerals and metabolites in the blood. The number of steers standing in a pen at each timepoint was used to calculate AUC in R. Repeated measures analysis was used for posttransit DMI recovery, weekly posttransit DMI, weekly posttransit Zn intake, standing behavior AUC, and gene expression with the repeated effect of time; the covariance structure with the lowest Akaike information criterion was used for each parameter. Outliers were determined using Cook’s D; data with a Cook’s D value of 0.5 or greater were removed from the analysis. Significance was determined at P ≤ 0.05, while tendencies were decided at 0.05 < P ≤ 0.10.
For metabolomics, the internal standard adonitol was used to normalize and quantify MS peaks for each metabolite, and MetaboAnalyst 5.0 (Chong et al., 2018; Xia Lab, McGill, CA) was used for statistical analysis of metabolites. Metabolites that were missing >30% of values were removed from the data set. The half minimum method was used to replace any missing values in the metabolites containing ≤30% missing values. Differential abundance classification and a student’s t-test were used to analyze identified metabolites; differences were reported as fold changes (FC ≥ 1.25; P ≤ 0.1). Potential biomarkers were identified using the biomarker analysis feature of MetaboAnalyst. Metabolites with AUC values greater than 0.7 were considered a potential biomarker (Xia et al., 2013).
Results
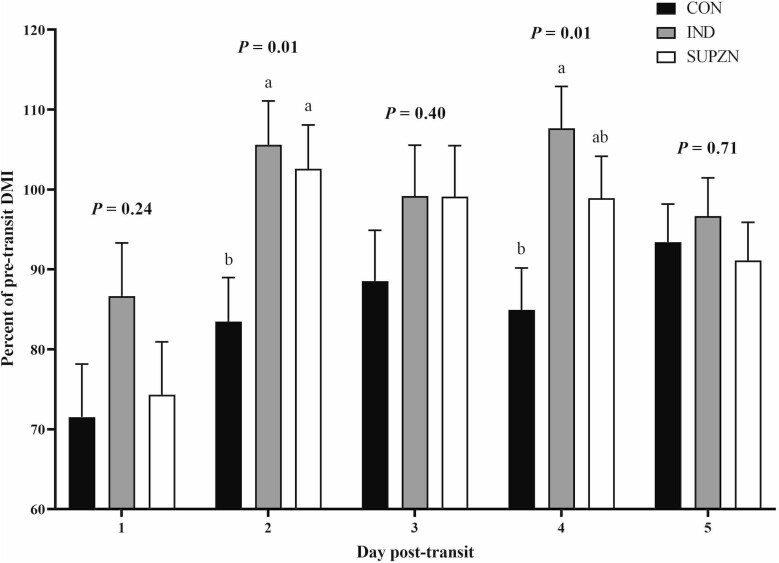
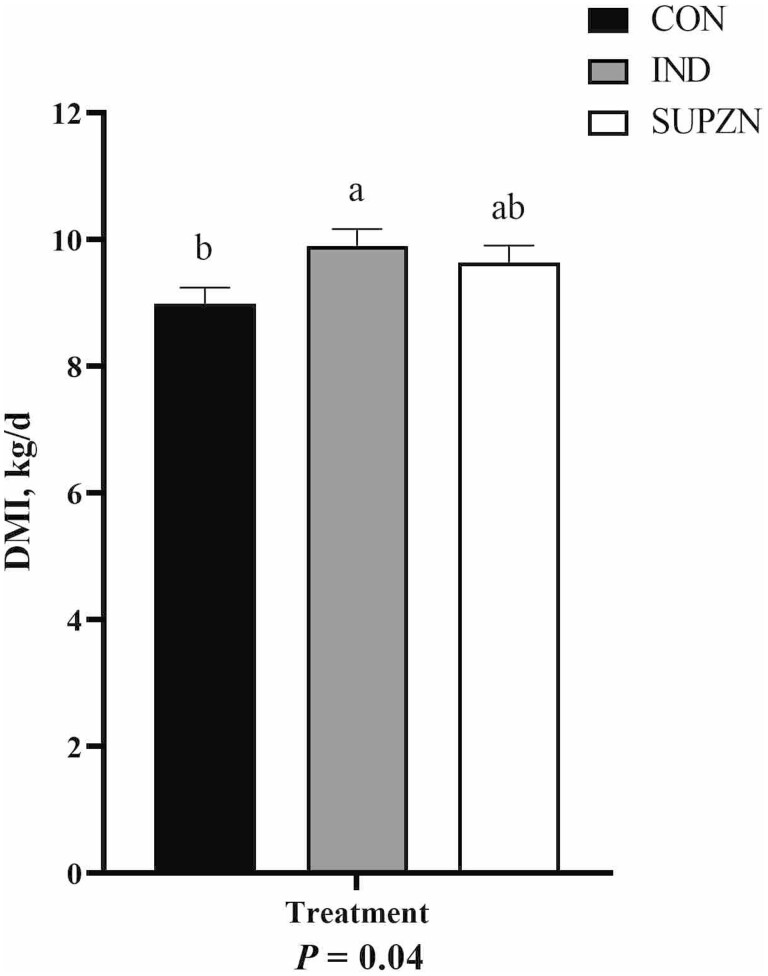
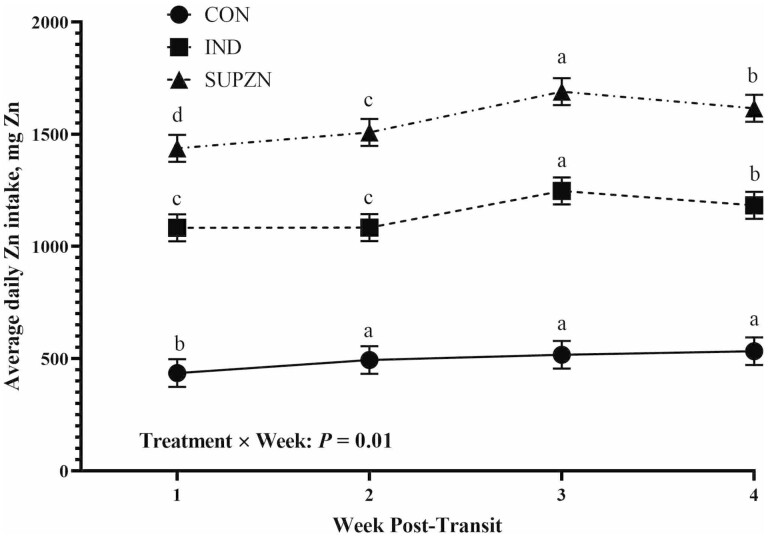
Growth performance data are reported in Table 2. There were no linear effects of increasing dietary Zn concentrations on BW for any timepoint (P ≥ 0.15) and no effects on BW of the Zn vs. No Zn contrast (P ≥ 0.15), though there were tendencies for a quadratic effect on days 0 and 1 with IND having lesser BW than CON and SUPZN (P ≤ 0.07). Treatment did not affect pretransit (days –25 to –1) and early posttransit (days 1 to 6) ADG (P ≥ 0.13). However, from days 6 to 28, ADG linearly increased with increasing dietary Zn (P = 0.02), and steers supplemented with Zn had greater ADG than CON (P = 0.02). There was a tendency (P = 0.10) for posttransit ADG (days 1 to 28) to linearly increase with increasing dietary Zn, and steers supplemented with Zn had greater posttransit (days 1 to 28) ADG than steers with no Zn supplementation (No Zn vs Zn: P = 0.04). There were no differences in off-truck shrink (P ≥ 0.69) or G:F at any timepoint (P ≥ 0.15). DM intake from days –5 to –1 was not different among treatments (P ≥ 0.53). Posttransit (days 1 to 5) DMI recovery is displayed in Figure 1. Treatment did not affect DMI recovery on days 1, 3, or 5 (P ≥ 0.24), but IND and SUPZN had returned to pretransit DMI by day 2 with CON remaining below pretransit intakes (P = 0.01). While there was no treatment by week effect for posttransit DMI (P = 0.94), IND had overall greater daily DMI in the posttransit period than CON while SUPZN was not different from either CON or IND (P = 0.04; Figure 2). There was a week effect for posttransit DMI with intakes increasing in the second half of the feeding period (week 1: 8.7 kg/d, week 2: 9.1 kg/d, week 3: 10.2 kg/d, week 4: 10.0 kg/d, SEM: 0.30; P = 0.01). There was a treatment by week effect on daily Zn intake. Zinc intake increased over time with SUPZN having the greatest Zn intake, IND being intermediate, and CON having the lowest Zn intake. Zinc intake in CON remained similar to weeks 2 and 4, and the greatest Zn intake for IND and SUPZN steers was the third week posttransit (treatment × week: P = 0.01; Figure 3).
Table 2.
Treatment averages of individual body weights (BW), average daily gain (ADG), off-truck shrink percentage, pretrucking dry matter intake (DMI), and feed efficiency (G:F) of feedlot steers fed increasing concentrations of supplemental Zn
| Treatment1 | Contrasts | ||||||
|---|---|---|---|---|---|---|---|
| CON | IND | SUPZN | SEM | Linear | Quadratic | No Zn vs Zn | |
| n = 18 steers | n = 18 steers | n = 18 steers | |||||
| BW2, kg | |||||||
| days –26/25 | 297 | 297 | 297 | 2.8 | 0.91 | 0.99 | 0.93 |
| Day 03 | 359 | 355 | 362 | 2.5 | 0.58 | 0.07 | 0.80 |
| Day 14 | 330 | 327 | 332 | 2.1 | 0.56 | 0.07 | 0.83 |
| Day 6 | 361 | 361 | 363 | 3.0 | 0.78 | 0.78 | 0.89 |
| Days 27/28 | 404 | 412 | 414 | 5.1 | 0.15 | 0.76 | 0.15 |
| ADG, kg/d | |||||||
| Day –25 to –1 | 2.40 | 2.31 | 2.47 | 0.10 | 0.69 | 0.29 | 0.94 |
| Days 1 to 6 | 6.22 | 6.84 | 6.05 | 0.38 | 0.86 | 0.13 | 0.63 |
| Days 6 to 28 | 1.93 | 2.31 | 2.34 | 0.13 | 0.02 | 0.39 | 0.02 |
| Days 1 to 28 | 2.73 | 3.15 | 3.03 | 0.14 | 0.10 | 0.16 | 0.04 |
| Trucking shrink, % | 8.10 | 7.97 | 8.13 | 0.30 | 0.98 | 0.69 | 0.89 |
| DMI5, kg/d | |||||||
| Days –5 to –16 | 9.5 | 9.8 | 9.6 | 0.36 | 0.75 | 0.53 | 0.58 |
| G:F | |||||||
| Days 1 to 66 | 0.765 | 0.751 | 0.647 | 0.039 | 0.15 | 0.40 | 0.32 |
| Days 6 to 286 | 0.215 | 0.238 | 0.231 | 0.015 | 0.41 | 0.46 | 0.29 |
| Days 1 to 286 | 0.319 | 0.329 | 0.304 | 0.021 | 0.66 | 0.47 | 0.91 |
Dietary treatments were: control (CON; no supplemental Zn), industry (IND; 70 mg supplemental Zn/kg DM), and supranutritional Zn (SUPZN; 120 mg supplemental Zn/kg DM); all supplemented Zn provided via Plexomin Zn (Phytobiotics, Cary, NC).
Days –26/25 BW covariate was applied during analysis.
On-truck – BW recorded after feeding and directly before loading onto trailer for 18-h transportation event.
Off-truck – BW recorded directly after unloading prior to feed access.
Outliers removed: DMI (days –5 to –1) – 1 IND; G:F (days 1 to 6) – 1 CON, 1 SUPZN; G:F (days 6 to 28) – 1 SUPZN; G:F (days 1 to 28) – 1 SUPZN
Figure 1.
Recovery of dry matter intake (DMI) after transportation of beef feedlot steers. Pretransit DMI was used to calculate the percent of DMI recovery. CON: no supplemental Zn (n = 18 steers); IND: 70-mg supplemental Zn/kg DM (n = 18 steers); SUPZN: 120 mg supplemental Zn/kg DM (n = 18 steers). All supplemental Zn provided via Plexomin Zn (Phytobiotics, Cary, NC). a,bMeans with different superscripts in the same day are different (P ≤ 0.05).
Figure 2.
Average dry matter intake (DMI) for the 28 d following transportation of beef feedlot steers. CON: no supplemental Zn (n = 18 steers); IND: 70-mg supplemental Zn/kg DM (n = 18 steers); SUPZN: 120 mg supplemental Zn/kg DM (n = 18 steers). All supplemental Zn provided via Plexomin Zn (Phytobiotics, Cary, NC). Treatment × week: P = 0.94. a,bMeans with different superscripts are different (P ≤ 0.05).
Figure 3.
Average daily Zn intake of beef feedlot steers in the 28 d after transportation. CON: no supplemental Zn (n = 18 steers); IND: 70 mg supplemental Zn/kg DM (n = 18 steers); SUPZN: 120 mg supplemental Zn/kg DM (n = 18 steers). All supplemental Zn provided via Plexomin Zn (Phytobiotics, Cary, NC). a,bMeans with different superscripts within treatment are different by week (P = 0.01).
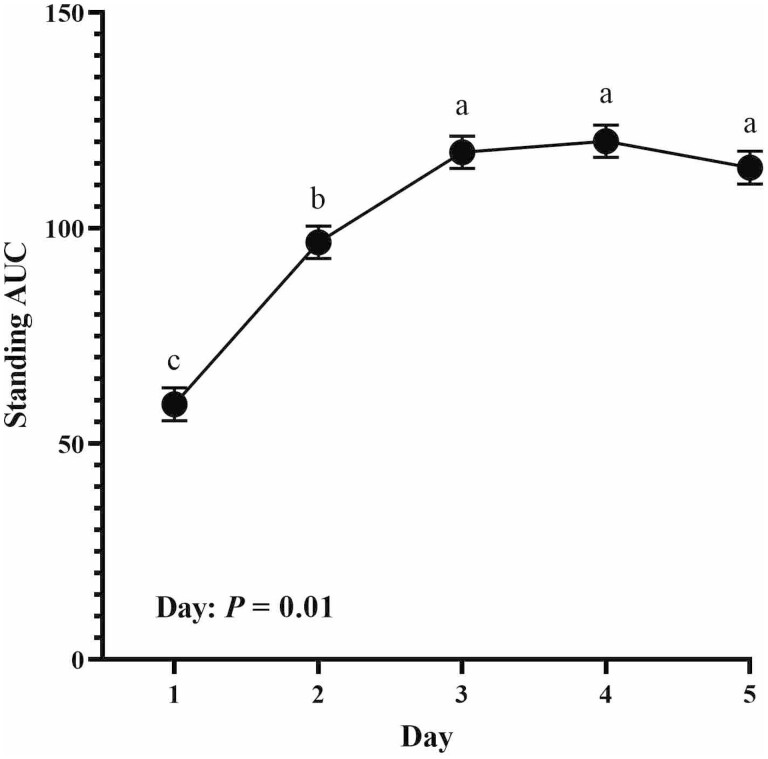
Standing behavior AUC data are displayed in Figure 4. There was no interaction of treatment and day for standing behavior (P = 0.57), nor did Zn treatment affect standing AUC (P = 0.34). However, standing was affected by days posttransit as steers stood less on day 1 than days 3 to 5 with day 2 being intermediate (Day; P = 0.01). Summary statistics of behavior data collected via CowManager tags are reported in Table 3. There was no difference due to treatment in total minutes spent ruminating, active, or nonactive on any day recorded (P ≥ 0.14).
Figure 4.
Standing behavior area under the curve (AUC) for each day after arrival back to the farm from transportation event. Treatment × day: P = 0.57. a,bMeans with different superscripts are different (P ≤ 0.05).
Table 3.
Summary statistics for rumination minutes during the 3 d following transportation for feedlot steers fed varying concentrations of Zn
| Treatment1 | Contrasts | ||||||
|---|---|---|---|---|---|---|---|
| CON | IND | SUPZN | SEM | Linear | Quadratic | No Zn vs. Zn | |
| n = 18 steers | n = 18 steers | n = 18 steers | |||||
| Rumination2 | |||||||
| Total, min/24 h | |||||||
| Day 13 | 309 | 286 | 259 | 23.1 | 0.14 | 0.84 | 0.21 |
| Day 2 | 335 | 325 | 296 | 31.0 | 0.39 | 0.73 | 0.52 |
| Day 3 | 317 | 302 | 281 | 30.8 | 0.42 | 0.88 | 0.50 |
| Active | |||||||
| Total, min/24 h | |||||||
| Day 1 | 460 | 468 | 506 | 20.9 | 0.20 | 0.52 | 0.36 |
| Day 2 | 474 | 515 | 497 | 16.8 | 0.55 | 0.46 | 0.39 |
| Day 3 | 510 | 526 | 518 | 18.0 | 0.83 | 0.76 | 0.75 |
| Nonactive | |||||||
| Total, min/24 h | |||||||
| Day 1 | 604 | 628 | 626 | 20.6 | 0.42 | 0.67 | 0.36 |
| Day 2 | 614 | 585 | 636 | 25.6 | 0.64 | 0.19 | 0.90 |
| Day 3 | 591 | 599 | 627 | 23.8 | 0.32 | 0.66 | 0.47 |
Dietary treatments – control (CON; no supplemental Zn); industry (IND; 70-mg supplemental Zn/kg DM); supranutritional Zn (SUPZN; 120-mg supplemental Zn/kg DM). All supplemental Zn provided through Plexomin Zn (Phytobiotics, Cary, NC).
Rumination, active, and nonactive minutes recorded via CowManager tags (Select Sires Inc., Plain City, OH).
Day 1: started immediately after unloading (0700 hours) and ended 24 h later; day 2: started 24 h after unloading (0700 hours) and ended 24 h later; day 3: started 48 h after unloading (0700 hours) and ended 24 h later.
Blood metabolites are reported in Table 4. There was no difference in lactate on days –1 or 6 (P ≥ 0.18), though there was a quadratic effect of Zn treatment on off-truck (day 1) serum lactate where CON and SUPZN had greater lactate concentrations than IND (Quadratic: P = 0.01) and Zn supplemented steers had less lactate than CON (No Zn vs Zn: P = 0.04). Though there were no differences in serum glucose on day –1 or 1 (P ≥ 0.25), there was a quadratic effect of Zn treatment on serum glucose on day 6 where IND had lesser glucose concentrations than CON or SUPZN (Quadratic: P = 0.03). On day –1, there were linear effects of increasing Zn supplementation where SUN concentrations increased with increasing dietary Zn concentrations (Linear: P = 0.02). Also on day –1, SUN tended to be quadratically affected by Zn supplementation where steers fed SUPZN had the greatest concentration of SUN (Quadratic: P = 0.07). On day 1 (off-truck), there was a tendency for SUPZN to have the greatest concentration of SUN (Quadratic: P = 0.06). On day 6, there was a linear increase in SUN as Zn supplementation increased (P = 0.01), and Zn supplemented steers had greater SUN than CON steers (P = 0.01). There were no differences due to treatment for FRAP or NEFA on any day (P ≥ 0.13).
Table 4.
Blood serum metabolites of beef feedlot steers fed different concentrations of Zn and transported 18 h
| Treatment1 | Contrasts | ||||||
|---|---|---|---|---|---|---|---|
| CON | IND | SUPZN | SEM | Linear | Quadratic | No Zn vs. Zn | |
| n = 18 steers | n = 18 steers | n = 18 steers | |||||
| Lactate, mmol/L | |||||||
| Day –1* | 5.5 | 4.7 | 4.9 | 0.25 | 0.35 | 0.45 | 0.25 |
| Day 1*,5 | 6.6 | 4.7 | 5.9 | 0.26 | 0.27 | 0.01 | 0.04 |
| Day 6*,5 | 4.9 | 4.0 | 4.5 | 0.20 | 0.43 | 0.18 | 0.20 |
| Glucose, mg/dL | |||||||
| Day –1* | 103 | 98 | 100 | 1.5 | 0.38 | 0.39 | 0.25 |
| Day 1* | 99 | 97 | 104 | 1.8 | 0.41 | 0.30 | 0.75 |
| Day 6* | 103 | 94 | 101 | 1.3 | 0.51 | 0.03 | 0.14 |
| FRAP2, µM | |||||||
| Day –1 | 308 | 303 | 317 | 14.6 | 0.71 | 0.60 | 0.90 |
| Day 15 | 278 | 281 | 282 | 13.8 | 0.83 | 0.96 | 0.83 |
| Day 65 | 293 | 279 | 284 | 12.2 | 0.58 | 0.53 | 0.44 |
| SUN3, mg/dL | |||||||
| Day –1 | 10.3 | 10.3 | 11.8 | 0.41 | 0.02 | 0.07 | 0.13 |
| Day 1 | 8.9 | 8.6 | 9.8 | 0.36 | 0.12 | 0.06 | 0.52 |
| Day 6 | 9.5 | 10.5 | 11.3 | 0.42 | 0.01 | 0.86 | 0.01 |
| NEFA4, mEq/L | |||||||
| Day –1* | 196 | 174 | 178 | 6.4 | 0.33 | 0.50 | 0.25 |
| Day 1* | 399 | 478 | 450 | 16.4 | 0.24 | 0.26 | 0.13 |
| Day 6* | 193 | 196 | 203 | 7.4 | 0.66 | 0.87 | 0.74 |
Treatment were no supplemental Zn (CON), 70-mg supplemental Zn/kg DM (IND), and 120-mg supplemental Zn/kg DM (SUPZN). All supplemental Zn provided via Plexomin Zn (Phytobiotics, Cary, NC).
Ferric reducing antioxidant power.
Serum urea nitrogen.
Nonesterified fatty acid.
Outliers removed: Lactate (day 1) – 1 CON, 1 SUPZN; Lactate (day 6) – 1 IND; FRAP (day 1) – 1 CON, 1 SUPZN; FRAP (day 6) – 1 CON, 1 SUPZN.
Back-transformed means are reported.
Plasma and muscle trace mineral concentrations are displayed in Table 5. There were no treatment differences in concentrations of plasma Cu or Fe at any timepoint (P ≥ 0.12). Plasma Zn on day –1 was not linearly or quadratically affected nor affected by supplementation of Zn (P ≥ 0.15). Off-truck (day 1), Zn supplementation increased plasma Zn concentrations compared to CON (P = 0.01). Similarly, there was a linear increase in plasma Zn concentrations on days 6 and 27 as dietary Zn increased (P = 0.01), and Zn-supplemented steers had greater plasma Zn concentrations than CON (P = 0.01). There was a tendency for a linear decrease in day 28 muscle Zn concentrations where SUPZN had lesser muscle Zn than CON or IND (P = 0.09).
Table 5.
Trace mineral concentrations of plasma and muscle by dietary treatment pretransit (day –1), posttransit (day 1), 6 d posttransit (day 6), and 27 d posttransit (day 27) in beef feedlot steers
| Treatment1 | Contrasts | ||||||
|---|---|---|---|---|---|---|---|
| CON | IND | SUPZN | SEM | Linear | Quadratic | No Zn vs. Zn | |
| Plamsa2 | |||||||
| Copper, mg/L | |||||||
| Day –1*,4 | 0.92 | 0.89 | 0.86 | 0.02 | 0.25 | 0.88 | 0.32 |
| Day 1 | 1.08 | 1.04 | 0.99 | 0.04 | 0.12 | 0.84 | 0.19 |
| Day 6* | 1.00 | 0.95 | 0.97 | 0.02 | 0.66 | 0.53 | 0.51 |
| Day 27*,4 | 0.85 | 0.84 | 0.80 | 0.02 | 0.39 | 0.72 | 0.52 |
| Iron, mg/L | |||||||
| Day –1 | 2.06 | 2.05 | 2.08 | 0.10 | 0.89 | 0.85 | 0.96 |
| Day 1 | 1.19 | 1.28 | 1.20 | 0.09 | 0.89 | 0.45 | 0.66 |
| Day 6 | 2.03 | 2.00 | 2.30 | 0.13 | 0.18 | 0.27 | 0.45 |
| Day 27 | 1.93 | 2.03 | 1.97 | 0.09 | 0.71 | 0.46 | 0.52 |
| Zinc, mg/L | |||||||
| Day –14 | 1.08 | 1.09 | 1.15 | 0.04 | 0.15 | 0.54 | 0.29 |
| Day 14 | 1.26 | 1.43 | 1.43 | 0.05 | 0.01 | 0.21 | 0.01 |
| Day 6* | 1.12 | 1.22 | 1.34 | 0.02 | 0.01 | 0.76 | 0.01 |
| Day 27 | 1.10 | 1.20 | 1.25 | 0.04 | 0.01 | 0.77 | 0.01 |
| Muscle3 | |||||||
| Zinc, mg/kg DM | |||||||
| Day 28 | 181 | 182 | 155 | 11.4 | 0.09 | 0.25 | 0.30 |
Treatment were no supplemental Zn (CON), 70-mg supplemental Zn/kg DM (IND), and 120-mg supplemental Zn/kg DM (SUPZN). All supplemental Zn provided via Plexomin Zn (Phytobiotics, Cary, NC). Zinc supplementation started on day –25, relative to trucking.
Steers per treatment: CON, n = 18; IND, n = 18; SUPZN, n = 18.
Steers per treatment: CON, n = 12; IND, n = 12; SUPZN, n = 11.
Outliers removed: Plasma: Copper (day –1) – 1 SUPZN;Copper (day 27) – 1 CON;Zinc (day –1) – 2 SUPZN; Zinc (day 1) – 1 CON, 1 SUPZN.
Back-transformed means are reported.
Steer muscle metabolites are presented in Table 6, and results from biomarker analysis of steer muscle metabolites are shown in Supplemental Table S2. Data are displayed in contrasts between dietary treatments and separated by pre- and posttransit timepoints. Contrasts between timepoints within dietary treatment are also reported. Notably, lactic acid tended to be greater in SUPZN than CON and IND prior to transit (P ≤ 0.06), and muscle glucose tended to be lesser in IND and SUPZN posttransit than CON (P ≤ 0.10). Metabolites related to amino acid and nitrogen metabolism (serine and allothreonine) were greater posttransit in all treatments (P ≤ 0.02). Metabolites involved in the glucose-alanine cycle tended to be greater posttransit in CON (glucose, glutamine, and alanine; P ≤ 0.07) and IND (alanine; P = 0.06).
Table 6.
Muscle metabolites of beef feedlot steers fed different concentrations of Zn and transported for 18 h
| Pretransit3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| IND1 vs. CON | SUPZN vs. CON | IND vs. SUPZN | ||||||
| Name | FC2 | P-value2 | Name | FC | P-value | Name | FC | P-value |
| 5-Oxoproline | 0.64 | 0.03 | Urea | 1.41 | 0.01 | Urea | 0.73 | 0.03 |
| 3-Hydroxybutyric acid | 1.63 | 0.09 | Lactic Acid | 1.29 | 0.03 | L-Alanine | 0.69 | 0.03 |
| Serine | 0.75 | 0.10 | L-Valine | 1.28 | 0.03 | L-Proline | 0.75 | 0.03 |
| 3-Hydroxybutyric acid | 1.89 | 0.03 | L-Valine | 0.77 | 0.05 | |||
| 5-Oxoproline | 0.74 | 0.05 | ||||||
| Lactic Acid | 0.79 | 0.06 | ||||||
| Allothreonine | 0.72 | 0.06 | ||||||
| Malic acid | 0.71 | 0.09 | ||||||
| Butanedioic acid | 0.72 | 0.10 | ||||||
| Posttransit4 | ||||||||
|---|---|---|---|---|---|---|---|---|
| IND vs. CON | SUPZN vs. CON | IND vs. SUPZN | ||||||
| Name | FC | P-value | Name | FC | P-value | Name | FC | P-value |
| L-Phenylalanine | 0.67 | 0.06 | D-Glucose | 0.52 | 0.01 | |||
| D-Glucose | 0.56 | 0.10 | L-Norleucine | 1.32 | 0.04 | |||
| L-Phenylalanine | 0.61 | 0.04 | ||||||
| L-Valine | 1.26 | 0.08 | ||||||
| Posttransit v Pretransit within a single Zn treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| CON | IND | SUPZN | ||||||
| Name | FC | P-value | Name | FC | P-value | Name | FC | P-value |
| p-Toluic acid | 2.38 | 0.01 | Allothreonine | 2.51 | 0.01 | p-Toluic acid | 2.49 | 0.01 |
| D-Glucose | 3.83 | 0.01 | p-Toluic acid | 2.95 | 0.01 | Allothreonine | 2.20 | 0.01 |
| Galactopyranose | 3.03 | 0.01 | Serine | 1.91 | 0.01 | Serine | 1.89 | 0.01 |
| Allothreonine | 2.03 | 0.01 | Butanedioic acid | 1.64 | 0.03 | Malic acid | 0.60 | 0.02 |
| Serine | 1.63 | 0.02 | L-Alanine | 1.57 | 0.06 | Myristic acid | 1.72 | 0.02 |
| L-Phenylalanine | 1.52 | 0.02 | 3-Hydroxybutyric acid | 0.70 | 0.07 | Octanoic acid | 1.67 | 0.07 |
| Hydroxyproline | 1.61 | 0.04 | Lactic acid | 1.47 | 0.07 | Octadecanoic acid | 1.54 | 0.08 |
| L-Glutamine | 3.52 | 0.04 | L-Glutamic acid | 0.71 | 0.08 | |||
| Lactic acid | 1.65 | 0.04 | ||||||
| L-Glutamic acid | 0.69 | 0.06 | ||||||
| L-Alanine | 1.53 | 0.07 | ||||||
| L-Norleucine | 0.75 | 0.09 | ||||||
CON, no supplemental Zn (n = 12 steers); IND, 70-mg supplemental Zn/kg DM (n = 12 steers); SUPZN, 120-mg supplemental Zn/kg DM (n = 11 steers). All supplemental Zn provided through Plexomin Zn (Phytobiotics, Cary, NC).
Identified muscle metabolites were analyzed in MetaboAnalyst 5.0 (Chong et al., 2018; Xia Lab, McGill, CA) using a student’s t-test and differential abundance classification (FC ≥ 1.25; P ≤ 0.1). Differences were reported as fold changes (FC; FC = first contrast parameter AUC/second contrast parameter AUC).
Pretransit samples were collected postfeed access on the day before the 18-h transit event (day –1).
Posttransit samplers were collected the day steers arrived back to the farm after steers had been weighed, had blood drawn, and had access to feed (d 1)
Gene expression data are presented in Table 7. Transit increased the relative expression of metallothionein 2 and succinate dehydrogenase complex flavoprotein subunit A (P ≤ 0.03), and tended to increase the relative expression of interleukin 15 (P = 0.07). Transit decreased the relative expression of adenosine monophosphate deaminase 1, lactate dehydrogenase B, mitogen-activated protein kinase 1, mitogen-activated protein kinase 3, myogenic differentiation 1, pyruvate dehydrogenase phosphatase catalytic subunit 1, solute carrier family 16 member 3, solute carrier family 2 member 4, solute carrier family 30 member 7, and solute carrier family 39 member 8 (P ≤ 0.03), and tended to decrease the relative expression of solute carrier family 39 member 7 (P = 0.07). Zinc effects were minimal, but lactate dehydrogenase B relative expression was greater in IND than SUPZN while CON was not different from either IND or SUPZN (P = 0.05) and myogenic differentiation 1 relative expression was lesser in IND and SUPZN than CON (P = 0.01). The interaction of Treatment and Day tended to affect the relative expression of mitogen-activated protein kinase 1 (P = 0.10) where pretransit CON steers had greater relative expression and all other treatments and days. Likewise, the relative expression of myogenic differentiation 1 tended to be affected by Treatment × Day (P = 0.08) where posttransit SUPZN had lesser relative expression than posttransit CON which had lesser expression than both pretransit SUPZN and pretransit CON; pre- and posttransit IND relative expression was intermediate.
Table 7.
The main effects of transit (day) and dietary zinc treatment on the relative expression of various genes
| Day | Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene3 | Biological function | PRE1 | POST1 | SEM | P-value | CON2 | IND2 | SUPZN2 | SEM | P-value |
| AMPD1 | Energy metabolism | 1.00 | 0.66 | 0.18 | 0.02 | 1.00 | 1.03 | 0.77 | 0.22 | 0.36 |
| IL15 | Cytokine | 1.00 | 1.86 | 0.33 | 0.07 | 1.00 | 0.72 | 0.78 | 0.24 | 0.52 |
| LDHB | Lactate metabolism | 1.00 | 0.67 | 0.17 | 0.02 | 1.00ab | 1.16b | 0.68a | 0.24 | 0.05 |
| MAPK14 | Regulation/phosphorylation | 1.00 | 0.80 | 0.18 | 0.03 | 1.00 | 0.80 | 0.80 | 0.20 | 0.10 |
| MAPK3 | Regulation/phosphorylation | 1.00 | 0.67 | 0.19 | 0.03 | 1.00 | 0.92 | 0.99 | 0.23 | 0.91 |
| MT2A | Zinc binding protein | 1.00 | 2.19 | 0.38 | 0.02 | 1.00 | 0.86 | 0.67 | 0.21 | 0.56 |
| MYOD14 | Muscle cell differentiation | 1.00 | 0.58 | 0.18 | 0.01 | 1.00b | 0.59a | 0.72a | 0.21 | 0.01 |
| NFKB2 | Inflammation | 1.00 | 1.41 | 0.25 | 0.21 | 1.00 | 0.81 | 0.57 | 0.21 | 0.17 |
| PDP1 | Energy metabolism | 1.00 | 0.65 | 0.19 | 0.01 | 1.00 | 1.00 | 0.79 | 0.22 | 0.54 |
| P1K3CG | Immune system | 1.00 | 0.80 | 0.21 | 0.11 | 1.00 | 1.05 | 0.93 | 0.25 | 0.77 |
| SDHA | Energy metabolism | 1.00 | 1.52 | 0.27 | 0.03 | 1.00 | 0.96 | 0.78 | 0.21 | 0.57 |
| SLC16A1 | Lactate transporter | 1.00 | 0.90 | 0.19 | 0.61 | 1.00 | 0.72 | 0.98 | 0.22 | 0.55 |
| SLC16A3 | Lactate transporter | 1.00 | 0.44 | 0.18 | 0.01 | 1.00 | 0.86 | 0.64 | 0.22 | 0.20 |
| SLC2A4 | Glucose transporter | 1.00 | 0.62 | 0.19 | 0.01 | 1.00 | 0.82 | 0.75 | 0.21 | 0.37 |
| SLC30A7 | Zinc transporter | 1.00 | 0.61 | 0.19 | 0.01 | 1.00 | 1.12 | 0.79 | 0.25 | 0.46 |
| SLC39A14 | Zinc transporter | 1.00 | 1.58 | 0.28 | 0.19 | 1.00y | 0.95xy | 0.62x | 0.23 | 0.09 |
| SLC39A7 | Zinc transporter | 1.00 | 0.74 | 0.20 | 0.07 | 1.00 | 0.87 | 1.01 | 0.23 | 0.64 |
| SLC39A8 | Zinc transporter | 1.00 | 0.60 | 0.22 | 0.01 | 1.00 | 0.85 | 1.07 | 0.27 | 0.61 |
| SOD1 | Antioxidant | 1.00 | 1.00 | 0.18 | 0.99 | 1.00 | 1.16 | 1.02 | 0.24 | 0.85 |
Relative quantification to PRE.
Relative quantification to CON.
Gene abbreviations: AMPD1, adenosine monophosphate deaminase 1; IL15, interleukin 15; LDHB, lactate dehydrogenase B; MAPK1, mitogen-activated protein kinase 1; MAPK3, mitogen-activated protein kinase 3; MT2A, metallothionein 2A; MYOD1, myogenic differentiation 1; NFKB2, nuclear factor kappa B subunit 2; PDP1, pyruvate dehydrogenase phosphatase catalytic subunit 1; PIK3CG, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma; SDHA, succinate dehydrogenase complex flavoprotein subunit A; SLC16A1, solute carrier family 16 member 1; SLC16A3, solute carrier family 16 member 3; SLC2A4, solute carrier family 2 member 4; SLC30A7, solute carrier family 30 member 7; SLC39A14, solute carrier family 39 member 14; SLC39A7, solute carrier family 39 member 7; SLC39A8, solute carrier family 39 member 8; SOD1, superoxide dismutase 1.
The interaction of treatment × day tended to affect the relative expression of MAPK1 (CON-PRE: 1, IND-PRE: 0.71, SUPZN-PRE: 0.61, CON-POST: 0.62, IND-POST: 0.56, SUPZN-POST: 0.64, SEM: 0.29; P = 0.10) and MYOD1 (CON-PRE: 1, IND-PRE: 0.46, SUPZN-PRE: 0.80, CON-POST: 0.53, IND-POST: 0.40, SUPZN-POST: 0.34, SEM: 0.30; P = 0.08) but had no effect on any other relative gene expression (P ≥ 0.14).
Discussion
This study investigated the effects of increasing dietary supplemental Zn concentrations on growth performance, DMI recovery, standing behavior, gene expression, and blood and muscle metabolites in beef feedlot steers transported for 18 h. It was hypothesized steers supplemented with Zn would have improved posttransit growth performance and lesser muscle fatigue indicators compared to controls.
Serum lactate increased 20%, 0%, and 20% in CON, IND, and SUPZN, respectively, after 18 h of transportation and was lesser due to Zn supplementation at this time. Lactate is a well-accepted biomarker of muscle fatigue as an increase in lactate indicates an increase in anaerobic glucose metabolism (Van Hall, 2010). This suggests Zn-fed steers were experiencing less muscle fatigue than CON after the transit event. Lactate is cleared from the muscle and taken to the liver, where it is converted back to pyruvate (Sharma et al., 2019; Strachecka et al., 2019) via the Zn-dependent enzyme lactate dehydrogenase B (LDH; Price, 1962). Pyruvate can then enter gluconeogenesis to produce glucose, which could return to the muscle for ATP production. Steers fed IND had greater relative gene expression of LDH B compared to SUPZN steers while CON was not different from IND or SUPZN. More research is needed to clarify a role of Zn in muscle lactate metabolism in response to transit stress.
One of the guiding principles of animal welfare is the Five Freedoms (Brambell, 1965; FAWC, 2009). When viewing the results of this project through the lens of the Five Freedoms, steers supplemented with Zn had improved posttransit DMI recovery (reducing hunger and returning steers to normal behavior patterns) and lesser serum lactate concentration posttransit, indicating reduced muscle fatigue (reduction of physical discomfort and pain/injury). Furthermore, Zn-fed steers had greater posttransit ADG compared to CON; IND had greater overall posttransit DMI than CON, while SUPZN was not different from CON or IND. Genther and Hansen (2014) saw increased DMI recovery after a 20-h transit event in trace mineral adequate steers vs. those that were mildly trace mineral deficient. Based on the results from the current study, Zn appears critical to posttransit steer DMI recovery. The GrowSafe system had data recording issues during a window in the middle of the pretransit period, so this study cannot examine the effects of Zn on DMI pretransit. However, pretransit growth performance was not affected by treatment. This suggests the 54 mg Zn/kg DM in the CON diet was sufficient to support growth prior to the transit stressor. However, despite the differences in DMI recovery, there were no differences due to treatment in behaviors (rumination, active, nonactive) as assessed by CowManager tags. With increased DMI in the Zn treatments, it would be expected that rumination minutes would increase as well (Galvani et al., 2010). However, it may be rumination minutes are not sensitive enough to pick up on treatment differences.
Steers fed SUPZN had greater muscle lactate concentrations than CON and IND pretransit but did not increase due to transit. There was an increase in muscle lactate due to transit in CON and IND, there were, surprisingly, no differences in muscle lactate between treatments posttransit. During prolonged muscle use, such as that occurring during transit, lactate is likely quickly removed from the muscle to prevent a decrease in muscle pH (Metzger and Moss, 1990), resulting in changes in serum lactate as the metabolite is transported to the liver to be converted eventually to glucose. Lactate can be transported via monocarboxylate transport (MCT) proteins (Wilson et al., 1998), particularly MCT1 and MCT4 (also known as solute carrier family 16 member 1 and member 3, respectively). According to Wilson et al. (1998), MCT4 is a major route for lactate to leave glycolytic skeletal muscle. Intriguingly, MCT4 relative expression decreased posttransit in the current study. This may indicate lactate may not have been readily exported from the muscle during transit, though the longissimus thoracis has mixed fiber types and this may have influenced the expression of MCT4; fiber type was not evaluated in the current study. Future studies should include an examination of liver lactate metabolism to further our understanding of important organ cross-talk in transported cattle.
It was hypothesized that increasing dietary Zn concentrations would decrease muscle fatigue and therefore increase standing behavior as Zn supplemented steers would need less time to recover from a transit event. No differences in standing behavior were found, but it should be noted that standing observations were not continuous and some standing behavior may have been missed. Since steers in the current study were preconditioned, they may have been more comfortable laying down to recover unlike unconditioned steers that will have increased standing time (Meléndez et al., 2021) and therefore longer recovery times. Since standing was similar from days 3 to 5, steers likely only needed 2 d to recover from muscle fatigue, indicating the general resiliency of cattle to recover from stress.
Though there were no differences among treatments at any time point for serum glucose, muscle glucose was increased posttransit in CON compared to both Zn treatments. Glucose uptake by the muscle is increased during exercise (Katz et al., 1986; Hargreaves et al., 1992). The rate of glucose uptake by the muscle may be negatively associated with muscle glycogen concentrations (James et al., 1985; Hargreaves et al., 1992) as the muscle seeks energetic substrates. As such, CON may have increased muscle glucose after transportation because of a greater depletion of muscle glycogen than the Zn supplemented steers, though muscle glycogen was not measured in the present study. Similarly, there was a decrease in the relative expression of the glucose importer, solute carrier family 2 member 4 (GLUT4) posttransit. The relative expression of GLUT4 likely decreased because blood insulin concentrations are decreased during exercise (James et al., 1985). Likewise, the posttransit decreased relative expression of GLUT4 may be tied with the decreased relative expression of solute carrier family 30 member 7 and solute carrier family 39 member 7 (Znt7 and ZIP7, respectively). Huang et al. (2012) indicated insulin signaling was impaired in Znt7-knockout mice, and decreased expression of ZIP7 was linked to decreased expression of GLUT4 in C2C12 myoblast cells (Myers et al., 2013).
During prolonged exercise, amino acids are catabolized to provide energy to muscle. Phenylalanine can be utilized as a proxy for protein degradation, as it can be neither synthesized nor degraded in muscle (Henriksson, 1991). In the current study, CON had increased muscle concentrations of phenylalanine posttransit compared to Zn treatments, indicating CON had a greater need for muscle degradation to support energy metabolism during transportation. Serine can be directly deaminated into pyruvate and nitrogen via the zinc-depended enzyme serine dehydratase (Tanaka et al., 2011; Ito et al., 2012). Muscle serine concentrations were increased posttransit in all treatments, indicating the importance of serine to form pyruvate to enter gluconeogenesis and provide energy to the muscle during exercise. Posttransit, alanine was greater in CON and IND. Alanine is the critical amino acid for energy metabolism as it is the most common amino acid released from the muscle and absorbed by other organs (Marliss et al., 1971). The glucose-alanine cycle supports energy metabolism; alanine is created via transamination of nitrogen from catabolized amino acids and pyruvate in the muscle (Felig et al., 1970). Alanine is then transported to the liver where it undergoes deamination to form pyruvate which enters gluconeogenesis, and the nitrogen group enters the urea cycle (Felig et al., 1970). In spite of the increased creation of urea from catabolized amino acids, a lack of feed intake during transit likely explains the decrease in SUN on day 1 in all treatments. This is a similar result to Pettiford et al. (2008) who saw a decrease in blood urea nitrogen in steers transported for 6 h.
In the current study, plasma Zn increased with increasing dietary Zn concentrations, particularly posttransit. It should be noted that all treatments were considered adequate in Zn status at all timepoints based on reference ranges by Kincaid (2000). Though the value of plasma Zn as a status index is not fully understood, changes in plasma Zn may be more useful, reflecting changes in intake, response to stress, or exercise. On day 1, all treatments had increased plasma Zn concentrations due to transit, though the Zn treatments had greater plasma Zn concentrations than CON. Serum/plasma Zn concentrations are increased immediately after exercise (Chu et al., 2016). However, Zn intake and status can affect the increase in plasma Zn concentrations postexercise; men had smaller increases postexercise in plasma Zn concentrations when consuming 3.6 mg Zn/d than when consuming 8.6 or 33.6 mg Zn/d (Lukaski et al., 1984). This may be why the CON steers do not have as great of an increase in plasma Zn posttransit as they had lesser Zn intake per day than IND or SUPZN.
This study utilized a bis-glycinate bound source for all supplemental Zn. Organic sources are generally assumed to be more bioavailable. Spears et al. (2004) noted an increase in liver Zn concentrations in the steers fed Zn glycine complex compared to those fed no Zn, Zn sulfate, or Zn methionine. Likewise, lambs supplemented with the same Zn source fed in the present study had increased plasma Zn concentrations compared to those supplemented with Zn sulfate (Deters et al., 2021). As 77.3% of consulting nutritionists report using blends of inorganic and organic sources in receiving diets (Samuelson et al., 2016), further research should be conducted to determine if similar results are obtained using a blend of inorganic and organic Zn sources and if supplemental concentrations should be altered.
To conclude, the present study utilized metabolomic and gene expression approaches in addition to blood chemistry to elucidate mechanisms and identify potential areas for future study to improve cattle welfare in response to transit. Overall, supplementing greater amounts of Zn improved DMI and ADG of cattle posttransit, suggesting Zn is important to the resiliency and recovery of cattle undergoing long distance transit. Muscle metabolites associated with changes in energy metabolism (lactate, alanine, serine) were increased posttransit, and muscle glucose was greater in CON posttransit than the Zn treatments, potentially indicating greater need for energy substrates in CON muscle. Providing supplemental Zn may be a practical and affordable way to prevent prolonged transit-induced hunger and increased muscle fatigue indicators, improving overall animal welfare.
Supplementary Material
Acknowledgments
We would like to thank Phytobiotics North America (Cary, NC, USA) for partial funding of this study, Deraad Trucking (Colfax, IA, USA) for transporting the steers during the transit event, and the Thornton Lab at Utah State University for all their assistance in assessing gene expression.
Glossary
Abbreviations
- ADG
average daily gain
- AUC
area under the curve
- BW
body weight
- DM
dry matter
- FC
fold change
- FRAP
ferric reducing antioxidant power
- G:F
gain to feed
- GCMS
gas chromatography-mass spectrometry
- LDH
lactate dehydrogenase
- NASEM
National Academies of Sciences, Engineering, and Medicine
- NEFA
nonesterified fatty acid
- NIST
National Institute of Standards and Technology
- SUN
serum urea nitrogen
- TMR
total mixed ration
Contributor Information
Katie J Heiderscheit, Department of Animal Science, Iowa State University, Ames, IA 50011, USA.
Stephanie L Hansen, Department of Animal Science, Iowa State University, Ames, IA 50011, USA.
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Association of Official Analytical Chemists (AOAC). 1996. Official methods of analysis, 16th ed. Rockville, MD, USA: AOAC International. [Google Scholar]
- Brambell, F. W. R. 1965. Report of the technical committee to enquire into the welfare of animals kept under intensive livestock husbandry systems. London: Her Majesty’s Stationary Office. Available from https://edepot.wur.nl/134379. [Google Scholar]
- Chong, J., Soufan O., Li C., Caraus I., Li S., Bourque G., Wishart D. S., and Xia J... 2018. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 46:W486–W494. doi: 10.1093/nar/gky310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, A., Petocz P., and Samman S... 2016. Immediate effects of aerobic exercise on plasma/serum zinc levels: a meta-analysis. Med. Sci. Sports Exerc. 48:726–733. doi: 10.1249/MSS.0000000000000805 [DOI] [PubMed] [Google Scholar]
- Deters, E. L., VanDerWal A. J., VanValin K. R., Beenken A. M., Heiderscheit K. J., Hochmuth K. G., Jackson T. D., Messersmith E. M., McGill J. L., and Hansen S. L... 2021. Effect of bis-glycinate bound zinc or zinc sulfate on zinc metabolism in growing lambs. J. Anim. Sci. 99:1–9. doi: 10.1093/jas/skab252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAWC. 2009. Farm animal welfare in Great Britain: past, present, and future. UK: Farm Animal Welfare Committee. Available from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/319292/Farm_Animal_Welfare_in_Great_Britain_-_Past__Present_and_Future.pdf [Google Scholar]
- Felig, P., Pozefsky T., Marliss E., and Cahill G. F... 1970. Alanine: key role in gluconeogenesis. Science (80-.) 167:1003–1004.https://www.jstor.org/stable/1728239?sid=primo&saml_data=eyJzYW1sVG9rZW4iOiJkYjkwMWFiMi0xZThjLTQzNGUtOGY4ZC01ZTY5M2JjYWM4YTUiLCJpbnN0aXR1dGlvbklkcyI6WyI3YmRlODIyNC04OTQzLTQwY2MtYjA1YS0xZDE3ZjI5NjEzYTIiXX0&seq=1#metadata_info_tab_contents . [DOI] [PubMed] [Google Scholar]
- Galvani, D. B., Pires C. C., Wommer T. P., Oliveira F., and Santos M. F... 2010. Chewing patterns and digestion in sheep submitted to feed restriction. J. Anim. Physiol. Anim. Nutr. (Berl) 94:e366–e373. doi: 10.1111/j.1439-0396.2010.01022.x [DOI] [PubMed] [Google Scholar]
- Gebresenbet, G., Aradom S., Bulitta F. S., and Hjerpe E... 2011. Vibration levels and frequencies on vehicle and animals during transport. Biosyst. Eng. 110:10–19. doi: 10.1016/j.biosystemseng.2011.05.007. [DOI] [Google Scholar]
- Genther-Schroeder, O. N., Branine M. E., and Hansen S. L... 2016. The influence of supplemental Zn-amino acid complex and ractopamine hydrochloride feeding duration on growth performance and carcass characteristics of finishing beef cattle. J. Anim. Sci. 94:4338–4345. doi: 10.2527/jas.2015-0159 [DOI] [PubMed] [Google Scholar]
- Genther, O. N., and Hansen S. L... 2014. Effect of dietary trace mineral supplementation and a multi-element trace mineral injection on shipping response and growth performance of beef cattle. J. Anim. Sci. 92:2522–2530. doi: 10.2527/jas.2013-7426 [DOI] [PubMed] [Google Scholar]
- González, L. A., Schwartzkopf-Genswein K. S., Bryan M., Silasi R., and Brown F... 2012. Benchmarking study of industry practices during commercial long haul transport of cattle in Alberta, Canada. J. Anim. Sci. 90:3606–3617. doi: 10.2527/jas.2011-4770 [DOI] [PubMed] [Google Scholar]
- Gosker, H. R., and Schols A. M. W. J... 2008. Fatigued muscles in COPD but no finishing line in sight. Eur. Respir. J. 31:693–694. doi: 10.1183/09031936.00015308 [DOI] [PubMed] [Google Scholar]
- Hargreaves, M., Meredith I., and Jennings G. L... 1992. Muscle glycogen and glucose update during exercise in humans. Exp. Physiol. 77:641–644. doi: 10.1113/expphysiol.1992.sp003627 [DOI] [PubMed] [Google Scholar]
- Heiderscheit, K. J., Freestone A. D., Beenken A. M., Deters E. L., Peschel J. M., and Hansen S. L... 2022. Long-duration transit and food and water deprivation alter behavioral activities and aggressive interactions at the feed bunk in beef feedlot steers. J. Anim. Sci. 100:1–9. doi: 10.1093/jas/skac060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson, J. 1991. Effect of exercise on amino acid concentrations in skeletal muscle and plasma. J. Exp. Biol. 160:149–165. doi: 10.1242/jeb.160.1.149 [DOI] [PubMed] [Google Scholar]
- Huang, L., Kirschke C. P., Lay Y. E., Levy L. B., Lamirande D. E., and Zhang P. H... 2012. Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. J. Biol. Chem. 287:33883–33896. doi: 10.1074/jbc.M111.309666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T., Koga K., Hemmi H., and Yoshimura T... 2012. Role of zinc ion for catalytic activity in d-serine dehydratase from Saccharomyces cerevisiae. FEBS J. 279:612–624. doi: 10.1111/j.1742-4658.2011.08451.x [DOI] [PubMed] [Google Scholar]
- James, D. E., Kraegen E. W., and Chisholm D. J... 1985. Muscle glucose metabolism in exercising rats: comparison with insulin stimulation. Am. J. Physiol. Endocrinol. Metab. 11:E575–E580. doi: 10.1152/ajpendo.1985.248.5.e575 [DOI] [PubMed] [Google Scholar]
- Katz, A., Broberg S., Sahlin K., and Wahren J... 1986. Leg glucose uptake during maximal dynamic exercise in humans. Am. J. Physiol. Endocrinol. Metab. 251:E65–E70. doi: 10.1152/ajpendo.1986.251.1.e65 [DOI] [PubMed] [Google Scholar]
- Kilgour, R. J. 2012. In pursuit of “normal”: a review of the behaviour of cattle at pasture. Appl. Anim. Behav. Sci. 138:1–11. doi: 10.1016/j.applanim.2011.12.002. [DOI] [Google Scholar]
- Kincaid, R. L. 2000. Assessment of trace mineral status of ruminants: a review. J. Anim. Sci. 77:1–10. doi: 10.2527/jas2000.77E-Suppl1x [DOI] [Google Scholar]
- Livak, K. J., and Schmittgen T. D... 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lukaski, H. C., Bolonchuk W. W., Klevay L. M., Milne D. B., and Sandstead H. H... 1984. Changes in plasma zinc content after exercise in men fed a low-zinc diet. Am. J. Physiol. 247:E88–E93. doi: 10.1152/ajpendo.1984.247.1.E88 [DOI] [PubMed] [Google Scholar]
- Marliss, E. B., Aoki T. T., Pozefsky T., Most A. S., and Cahill G. F... 1971. Muscle and splanchnic glutamine and glutamate metabolism in postabsorptive and starved man. J. Clin. Invest. 50:814–817. doi: 10.1172/JCI106552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill, J. L., Rusk R. A., Guerra-Maupome M., Briggs R. E., and Sacco R. E... 2016. Bovine gamma delta T cells contribute to rxacerbated IL-17 production in response to co-infection with bovine RSV and mannheimia haemolytica. PLoS One 11:1–20. doi: 10.1371/journal.pone.0151083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meléndez, D. M., Marti S., Haley D. B., Schwinghamer T. D., and Schwartzkopf-Genswein K. S... 2021. Effects of conditioning, source, and rest on indicators of stress in beef cattle transported by road. PLoS One 16:1–25. doi: 10.1371/journal.pone.0244854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith, E. M., and Hansen S. L... 2021. Effect of zinc source and implant strategy on performance, carcass characteristics, and trace mineral concentrations in finishing feedlot steers. Transl. Anim. Sci. 5:1–11. doi: 10.1093/tas/txab218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger, J. M., and Moss R. L... 1990. Effects on tension and stiffness due to reduced pH in mammalian fast- and slow-twitch skinned skeletal muscle fibres. J. Physiol. 428:737–750. doi: 10.1113/jphysiol.1990.sp018238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitlohner, F. M., Morrow-Tesch J. L., Wilson S. C., Dailey J. W., and McGlone J. J... 2001. Behavioral sampling techniques for feedlot cattle. J. Anim. Sci. 79:1189–1193. doi: 10.2527/2001.7951189x [DOI] [PubMed] [Google Scholar]
- Morris, B. K., Davis R. B., Brokesh E., Flippo D. K., Houser T. A., Najar-Villarreal F., Turner K. K., Williams J. G., Stelzleni A. M., and Gonzalez J. M... 2021. Measurement of the three-axis vibration, temperature, and relative humidity profiles of commercial transport trailers for pigs. J. Anim. Sci. 99:1–14. doi: 10.1093/jas/skab027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, S. A., Nield A., Chew G. S., and Myers M. A... 2013. The zinc transporter, Slc39a7 (Zip7) is implicated in glycaemic control in skeletal muscle cells. PLoS One 8(11):1–15. doi: 10.1371/journal.pone.0079316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2016. Nutrient requirements of beef cattle, 8th rev, ed. Washington, DC: The National Academies Press. pp.109–139 doi: 10.17226/19014 [DOI] [Google Scholar]
- Pampusch, M. S., Johnson B. J., White M. E., Hathaway M. R., Dunn J. D., Waylan A. T., and Dayton W. R... 2003. Time course of changes in growth factor mRNA levels in muscle of steroid-implanted and nonimplanted steers. J. Anim. Sci. 81:2733–2740. doi: 10.2527/2003.81112733x [DOI] [PubMed] [Google Scholar]
- Pettiford, S. G., Ferguson D. M., Lea J. M., Lee C., Paull D. R., Reed M. T., Hinch G. N., and Fisher A. D... 2008. Effect of loading practices and 6-hour road transport on the physiological responses of yearling cattle. Aust. J. Exp. Agric. 48:1028–1033. doi: 10.1071/ea08051 [DOI] [Google Scholar]
- Pogge, D. J., and Hansen S. L... 2013. Supplemental vitamin C improves marbling in feedlot cattle consuming high sulfur diets. J. Anim. Sci. 91:4303–4314. doi: 10.2527/jas.2012-5638 [DOI] [PubMed] [Google Scholar]
- Price, C. A. 1962. A zinc-dependent lactate dehydrogenase in Euglena gracilis. Biochem. J. 82:61–66. doi: 10.1042/bj0820061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson, K. L., Hubbert M. E., Galyean M. L., and Löest C. A... 2016. Nutritional recommendations of feedlot consulting nutritionists: the 2015 New Mexico state and Texas tech university survey. J. Anim. Sci. 94:2648–2663. doi: 10.2527/jas.2016-0282 [DOI] [PubMed] [Google Scholar]
- Schuetze, S. J., Schwandt E. F., Maghirang R. G., and Thomson D. U... 2017. Review: transportation of commercial finished cattle and animal welfare considerations. Prof. Anim. Sci. 33:509–519. doi: 10.15232/pas.2017-01620 [DOI] [Google Scholar]
- Sharma, A., Oonthonpan L., Sheldon R. D., Rauckhorst A. J., Zhu Z., Tompkins S. C., Cho K., Grzesik W. J., Gray L. R., Scerbo D. A.,. et al. 2019. Impaired skeletal muscle mitochondrial pyruvate uptake rewires glucose metabolism to drive whole-body leanness. Elife 8:1–28. doi: 10.7554/eLife.45873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears, J. W., Schlegel P., Seal M. C., and Lloyd K. E... 2004. Bioavailability of zinc from zinc sulfate and different organic zinc sources and their effects on ruminal volatile fatty acid proportions. Livest. Prod. Sci. 90:211–217. doi: 10.1016/j.livprodsci.2004.05.001 [DOI] [Google Scholar]
- Strachecka, A., Grzybek M., Ptaszynska A. A., Los A., Chobotow J., and Rowinski R... 2019. Comparison of lactate dehydrogenase activity in hive and forager honeybees may indicate delayed onset muscle soreness – preliminary studies. Biochem. 84:435–440. doi: 10.1134/S0006297919040114 [DOI] [PubMed] [Google Scholar]
- Suasnavas, E. A., Heywood S., Ward A., Cox L., O’Grady M., Zhao Y., Gilbert L., and Isom S. C... 2015. Isolation and characterization of trophoblast-derived stem-like cells from peri-implantation porcine embryos. Anim. Reprod. Sci. 154:128–141. doi: 10.1016/j.anireprosci.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Tanaka, H., Senda M., Venugopalan N., Yamamoto A., Senda T., Ishida T., and Horiike K... 2011. Crystal structure of a zinc-dependent d-serine dehydratase from chicken kidney. J. Biol. Chem. 286:27548–27558. doi: 10.1074/jbc.M110.201160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hall, G. 2010. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 199:499–508. doi: 10.1111/j.1748-1716.2010.02122.x [DOI] [PubMed] [Google Scholar]
- Wilson, M. C., Jackson V. N., Heddle C., Price N. T., Pilegaard H., Juel C., Bonen A., Montgomery I., Hutter O. F., and Halestrap A. P... 1998. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J. Biol. Chem. 273:15920–15926. doi: 10.1074/jbc.273.26.15920 [DOI] [PubMed] [Google Scholar]
- Xia, J., Broadhurst D. I., Wilson M., and Wishart D. S... 2013. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics 9:280–299. doi: 10.1007/s11306-012-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.