Abstract
Necrotizing enterocolitis (NEC) remains a devastating disease that affects preterm infants. Hydrogen sulfide (H2S) donors have been shown to reduce the severity of NEC, but the optimal compound has yet to be identified. We hypothesized that oral H2S-Mesalamine (ATB-429) would improve outcomes in experimental NEC, and its benefits would be dependent on endothelial nitric oxide synthase (eNOS) pathways. NEC was induced in 5-day-old wild-type (WT) and eNOS knockout (eNOSKO) pups by formula feeding and stress. Four groups were studied in both WT and eNOSKO mice: 1) breastfed controls, 2) NEC, 3) NEC + 50 mg/kg mesalamine, and 4) NEC + 130 mg/kg ATB-429. Mesalamine and ATB-429 doses were equimolar. Pups were monitored for sickness scores and perfusion to the gut was measured by Laser Doppler Imaging (LDI). After euthanasia of the pups, intestine and lung were hematoxylin and eosin-stained and scored for injury in a blind fashion. TLR4 expression was quantified by Western blot and IL-6 expression by ELISA. P < 0.05 was significant. Both WT and eNOSKO breastfed controls underwent normal development and demonstrated milder intestinal and pulmonary injury compared with NEC groups. For the WT groups, ATB-429 significantly improved weight gain, reduced clinical sickness score, and improved perfusion compared with the NEC group. In addition, WT ATB-429 pups had a significantly milder intestinal and pulmonary histologic injury when compared with NEC. ATB-429 attenuated the increase in TLR4 and IL-6 expression in the intestine. When the experiment was repeated in eNOSKO pups, ATB-429 offered no benefit in weight gain, sickness scores, perfusion, intestinal injury, pulmonary injury, or decreasing intestinal inflammatory markers. An H2S derivative of mesalamine improves outcomes in experimental NEC. Protective effects appear to be mediated through eNOS. Further research is warranted to explore whether ATB-429 may be an effective oral therapy to combat NEC.
Keywords: endothelial nitric oxide synthase, hydrogen sulfide, necrotizing enterocolitis
INTRODUCTION
Necrotizing enterocolitis (NEC) remains a devastating disease that affects the intestines of premature infants. It develops after initiation of enteral feeds and is characterized by sepsis, intestinal necrosis, and often death (1). Although there has been progress in improving long-term outcomes for the sequelae of prematurity, the morbidity and mortality remain high for NEC, with data showing that 23.5% of all infants with NEC succumb to the disease (2). In addition, infants who require surgery carry a higher risk of mortality, estimated as high as 40.5% in all infants, and 50.9% in extremely low birth weight infants (2). Part of the challenge in improving outcomes in NEC is the elusiveness of the pathophysiology of the disease. NEC is multifactorial, and over the past 40 years, there have been several proposed theories on the cause of the disease (3). The most certain predisposing risk factors to NEC are prematurity and formula feeding (4). Additional proposed components of the pathophysiology of NEC in humans include intestinal dysbiosis (5), ischemia (6), inflammation (7), and aberrant immune Toll-like receptor 4 (TLR4) signaling (8). In animal models utilizing formula feeding and lipopolysaccharide (LPS), the mechanism of injury is largely due to hyperosmolar formula inducing intestinal mucosal hypoxia and LPS reducing intestinal microcirculation (9, 10). In addition, NEC has extraintestinal manifestations including kidney (11), brain (12), and lung injury (13, 14).
Currently, the treatment for NEC is supportive care and includes cessation of enteral feeds, bowel rest, and antibiotics (4). To improve outcomes and prevent infants from needing surgery, there is a need for a targeted medical therapy that directly addresses the underlying pathophysiology and halts the progression of the disease. Hydrogen sulfide (H2S) is an endogenously created gasotransmitter with vasodilatory properties (15). H2S modulates the activity of several proteins by replacing a -SH group on cysteine with an -SSH group, a process known as persulfidation (16). H2S vasodilatory properties may be mediated through endothelial nitric oxide synthase (eNOS). After persulfidation, eNOS dimerizes to produce nitric oxide (NO), which leads to subsequent vasodilation (17, 18). H2S has also been shown to have anti-inflammatory and antiapoptotic properties (19). These beneficial effects have led to many experiments using H2S to treat intestinal conditions in animal studies, including colitis (20), NSAID-induced intestinal injury (21), and ischemia-reperfusion injury (22–24).
Intraperitoneal injections of H2S donors, such as sodium hydrosulfide (NaHS) and sodium sulfide (Na2S), have been shown to attenuate intestinal injury in experimental NEC (25, 26). However, there is concern over the translational application of intraperitoneal administration of H2S, as it has a short half-life and poor safety profile. H2S-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) have emerged as a safer way to deliver H2S orally (27). These donors provide a mechanism for delivering the vasodilatory and anti-inflammatory properties of H2S and also couple it with an additional therapeutic compound with its own independent desirable effects (28). An H2S derivative of mesalamine (ATB-429) has emerged as a particularly potent anti-inflammatory drug and has attenuated the severity of colitis in a mouse model for inflammatory bowel disease (29). The objective of this study was to assess whether ATB-429 would improve outcomes in experimental NEC when compared with traditional mesalamine. We hypothesized that 1) ATB-429 would improve survival, clinical sickness score, mesenteric blood flow, intestinal injury, and decrease inflammation in experimental NEC and that 2) these benefits would be mediated through eNOS-mediated pathways.
METHODS
Murine Necrotizing Enterocolitis Model
A murine experimental NEC model was used by adapting and modifying previously described protocols (30, 31). C57BL/6J mice and B6.129P2-Nos3tm1Unc/J (eNOSKO) mice were purchased from Jackson Laboratories (Bar Harbor, ME). The presence of eNOSKO mutation was validated using PCR (Fig. 1A). All animal research followed ethical guidelines and a protocol approved by an Indiana University Institutional Animal Care and Use Committee. Four groups were studied in both WT and eNOSKO mice: 1) breastfed controls; 2) NEC; 3) NEC + 50 mg/kg oral mesalamine; and 4) NEC + 130 mg/kg oral ATB-429. Mesalamine and ATB-429 doses were equimolar and were chosen based on the literature (29); n = 11–20 mice/group. Animals that died during the experiment were excluded from analysis. Both mesalamine and ATB-429 were donated by Antibe Pharmaceuticals. Pups underwent experimental NEC beginning on postnatal day 5 (P5) as previously described (25). Pups were taken to satellite housing and kept in a temperature- and humidity-controlled incubator. Temperature was kept at 32°C, and humidity was kept at 40%. Breastfed controls remained with dams and breastfed ad lib. Pups received hyperosmolar formula feeds for a total of 300 kcal/kg/day of Esbilac milk replacer (PetAg, Hampshire, IL) fortified with Similac Advance powder (Similac, Columbus, OH) via gavage feeding with a 1.9-French catheter. Formula was mixed daily and the volume given to each pup per feed was weight-based at 0.055 mL/g. Mesalamine and ATB-429 were dissolved in formula and given three times per day. To minimize variance, pups were fed at the same time each day (0800/1200/1600) throughout the experiment. Lipopolysaccharide (LPS) isolated from Escherichia coli O111:B4 (Cat. No. L4391, Sigma Aldrich Company, St. Louis, MO) was given enterally at 8 mg/kg. Pups were also exposed to hypothermia at 4°C for 12 min twice daily by placing pups in a refrigerator, and hypoxia with 5% oxygen for 10 min three times daily to induce stress. Mice underwent 4 days of experimental NEC and were euthanized via cervical decapitation on P9.
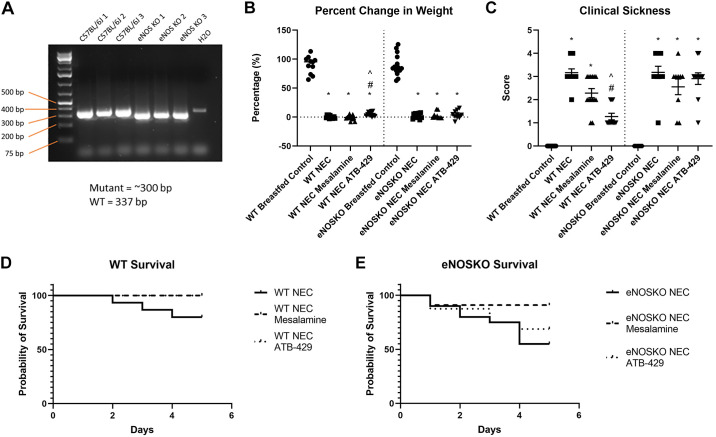
Figure 1.
ATB-429 decreases weight loss, clinical sickness score, and mortality in experimental NEC. Experimental NEC was performed by subjecting neonatal mice to hypoxia, hypothermia, enteral LPS, and formula feeding. Survival and weight loss were monitored, and clinical sickness score was recorded as described in methods. A: mutant eNOSKO mice purchased from Jackson Laboratories were validated using PCR. B: ATB-429 significantly attenuated weight loss in experimental NEC in WT mice but did not affect weight loss in eNOSKO mice. C: ATB-429 improved clinical sickness score in WT mice but had no effect in eNOSKO mice. D: survival curves demonstrating a benefit for both mesalamine and ATB-429 in WT mice, P = 0.0407. E: when the experiment was repeated in eNOSKO mice, there was no survival benefit for mesalamine or ATB-429, P = 0.7787. Statistical analysis for weight loss and clinical sickness was performed using ANOVA with Tukey’s multiple-comparisons test when parametric and Kruskal–Wallis with Dunn’s multiple-comparisons test when nonparametric. Log-rank (Mantel–Cox) test was performed for survival curves. Total n: WT Breastfed Control, n = 11; WT NEC, n = 15; WT NEC Mesalamine, n = 14; WT NEC ATB-429, n = 12; eNOSKO Breastfed Control, n = 15; eNOSKO NEC, n = 20; eNOSKO NEC Mesalamine = 11; eNOSKO NEC ATB-429 = 16. *P < 0.05 vs. Breastfed Control, #P < 0.05 vs. NEC, ^P < 0.05 vs. NEC Mesalamine. eNOSKO, endothelial nitric oxide synthase knockout mice; LPS, lipopolysaccharide; NEC, necrotizing enterocolitis; WT, wild type. n, number of mice.
Weight Gain and Clinical Assessment
Experimental groups were monitored daily for clinical assessment and weight gain. Weight gain was expressed as a percentage change from baseline. Clinical assessment was assessed via a previously established systematic scale that measures pup hydration, activity level, response to touch, and body color (31). The clinical sickness score was measured as the final score before euthanasia minus the baseline score.
Perfusion Analysis
Perfusion to the gut was measured transcutaneously using a Laser Doppler Perfusion Imager (Moor Instruments, Wilmington, DE) as previously described (25). To control for changes in skin thickness and fur development between P5 and P9 between breastfed controls and NEC mice, perfusion of the abdomen was normalized to chest perfusion for each measurement. Mice were placed supine on the morning of P5 after maternal separation and received a baseline perfusion value. After the completion of the NEC experiment, on the morning of P9, repeat perfusion was assessed. Perfusion was expressed as a percentage of baseline perfusion on P5.
Intestine and Lung Histologic Injury Score
Immediately after euthanasia, terminal ileum and the right lower lobe of the lung were harvested. Tissue was placed in tissue cassettes and fixed in paraformaldehyde for 36 h and stored at 4°C for 36 h. Intestine and lung were then dehydrated in 70% ethanol for 24 h. Specimens were paraffin-embedded, cut with a microtome, and stained with hematoxylin and eosin. Intestine and lung were graded blindly by two authors who have previously published in the area of intestinal injury (13, 25, 32). Scores were averaged. Intestinal injury was assessed using a scale published by Zani et al. with scores ranging from 0 to 4: 0 = normal intestine; 1 = mild disarrangement of villus enterocytes with mild villus core separation; 2 = significant disarrangement of villous enterocytes, significant villus-core separation, blunting of villi; 3 = frank sloughing of villi; 4= necrosis. A score of 2 or higher was considered to be NEC, and a score of 3 or higher was considered to be severe NEC.
Lung injury was blindly assessed using a modified scale developed by the American Thoracic Society (ATS) for animal lung injury (33). The scale is composed of six individual scores assessing the presence of neutrophils in the alveolar space, neutrophils in the interstitial space, hyaline membranes, proteinaceous debris, alveolar septal thickening, and red blood cells in the alveolar space. Individual categories were scored from 0 to 2, with a total score of 12 being the maximum possible. A total score of 0 indicated normal lung, whereas a score of 12 indicated severe acute lung injury.
TLR4 and IL-6 Analysis
Immediately after euthanasia, a segment of ileum and lung were snap-frozen and stored at −80°C. Tissue was homogenized in a bullet blender (Next Advance, Troy, NY) in a 4°C cold room in a solution of 400-mL radio-immunoprecipitation assay (RIPA) Buffer (Thermo Fisher Scientific, Waltham, MA) with 1:100 dilution of both protease inhibitor and phosphatase inhibitor (Sigma Aldrich Company, St. Louis, MO). After homogenization, the samples were centrifuged at 12,000 revolutions/min for 5 min. Supernatant was transferred to a fresh 1.5-mL Eppendorf tube and stored at −80°C before assay.
For TLR4 quantification, total protein was first quantified by Bradford assay using a Spectramax M2e Multi-Mode microplate reader spectrophotometer (Molecular Devises, San Jose, CA). After heat denaturation, equal amounts (25 µg) of intestine protein lysates were separated on 4%–15% mini-protean TGX Precast protein gels (Bio-Rad, Hercules, CA) and transferred to PVDF membranes (Millipore, Billerica, MA). The blots were blocked in Intercept (PBS) blocking buffer (Li-Cor, Lincoln, NE) for 1 h and incubated overnight with anti-TLR4 (Cat. No. sc-293072, 1:500), anti-HPRT-1 (Cat. No. sc-376938, 1:500), or anti-β-actin (Cat. No. sc-47778, 1:1,000) at 4°C (Santa Cruz Biotechnology, Santa Cruz, CA). After incubation with IRDye 800CW Goat anti-Mouse IgG secondary antibody (Li-Cor, Lincoln, NE) for 1 h, the membrane was scanned by ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA). The band intensity was quantified using ImageJ software and is expressed as ration of TLR4:HPRT for intestine and TLR4:β-actin for lung.
For IL-6 quantification, total protein was quantified by Bradford assay as detailed above. Murine IL-6 was quantified via ELISA (R&D systems, Bio-Techne Corporation, Minneapolis, MN). Assays were performed at 1:20 dilutions and in duplicate per the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was performed and figures were created using GraphPad Prism 8 (GraphPad Software, San Diego, CA). Ordinal data were reported as median and interquartile range (IQR), and continuous data were reported as mean and standard error (SE). Data were tested for normality and were compared using ANOVA with Tukey’s multiple comparisons test when parametric, and Kruskal–Wallis with Dunn’s multiple-comparisons test when nonparametric. Survival curve analysis was performed using a log-rank (Mantel-Cox) test for trend. P < 0.05 was considered statistically significant.
RESULTS
ATB-429 Improves Clinical Outcomes in Experimental NEC
For the WT pups undergoing experimental NEC, ATB-429 significantly attenuated weight loss when compared with NEC (WT NEC = −0.01 ± 0.76%, WT NEC ATB-429 = +4.94 ± 1.10%, P = 0.0041, Fig. 1B). Mesalamine did not provide any benefit for weight gain (−1.0 ± 1.03%, P = 0.7640). When the experiment was repeated in eNOSKO mice, ATB-429 did not reduce weight loss compared with NEC (eNOSKO NEC = +2.06 ± 1.3%, eNOSKO NEC ATB-429 = +3.95 ± 2.0%, P > 0.999).
For the WT groups undergoing experimental NEC, pups receiving ATB-429 had improved clinical sickness scores compared with NEC pups [WT NEC = 3.0 (3.0 – 3.75), WT NEC ATB-429 = 1.0 (1.0 – 2.0), P < 0.0001, Fig. 1C]. Mesalamine did not significantly reduce clinical sickness score [WT NEC Mesalamine = 2.0 (2.0 – 3.0), P = 0.0608]. In the eNOSKO mice, there was no difference in clinical sickness scores between the three NEC groups [eNOSKO NEC = 3.0 (3.0 – 4.0), eNOSKO NEC Mesalamine = 3.0 (1.5 – 3.0), eNOSKO NEC ATB-429 = 3.0 (3.0 – 3.0), P = 0.2556].
ATB-429 Improves Survival in Experimental NEC
WT pups undergoing experimental NEC had a 20% mortality rate. WT pups receiving mesalamine or ATB-429 all survived the 5-day experiment. Both WT NEC mesalamine and WT NEC ATB-429 had significantly improved survival compared with NEC (P = 0.0407, Fig. 1D). eNOSKO pups undergoing experimental NEC had a 45% mortality rate. eNOSKO NEC pups receiving mesalamine had an 18.1% mortality rate, and eNOSKO NEC pups receiving ATB-429 had a 31.3% mortality rate. There was no difference in survival between the three groups (P = 0.7787, Fig. 1E).
ATB-429 Improves Perfusion in Experimental NEC
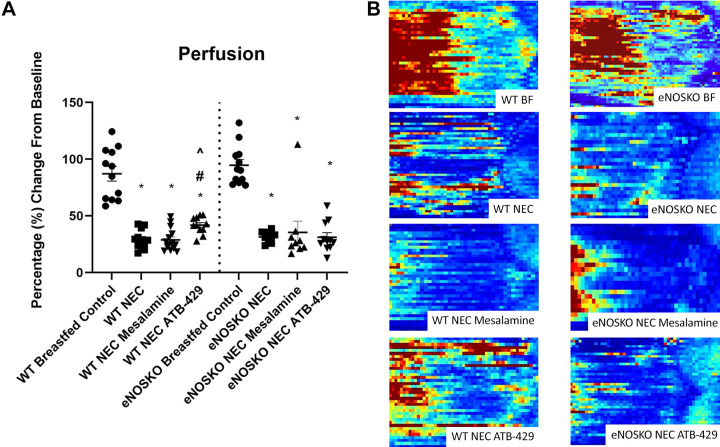
WT pups undergoing experimental NEC receiving ATB-429 had a mean perfusion of 41.86 ± 2.27% compared with 29.27 ± 2.41% in WT NEC pups (P = 0.0043). Mesalamine did not provide a benefit in mesenteric perfusion (WT NEC Mesalamine = 28.78 ± 2.64%, P = 0.9888, Fig. 2A). When the experiment was repeated in eNOSKO pups, ATB-429 did not increase mesenteric perfusion and there was no difference between the three groups (eNOSKO NEC = 31.46 ± 1.68%, eNOSKO NEC Mesalamine = 35.48 ± 9.89%, eNOSKO NEC ATB-429 = 31.17 ± 3.97%, P = 0.4593, Fig. 2A). Representative images of final flux perfusion on P9 are shown in Fig. 2B.
Figure 2.
ATB-429 increases mesenteric perfusion in experimental NEC. Mesenteric perfusion was assessed by Laser Doppler Imaging (LDI) as described in methods. A: ATB-429 significantly increased mesenteric perfusion in WT mice but not in eNOSKO mice. B: representative pictures of each group’s perfusion as measured by Laser Doppler Imaging. Red are areas of high perfusion, and blue are areas of low perfusion. The mouse is oriented with the diaphragm to the left, and pelvis to the right. Statistical analysis was performed using ANOVA with Tukey’s multiple-comparisons test when parametric and Kruskal–Wallis with Dunn’s multiple-comparisons test when nonparametric. *P < 0.05 vs. Breastfed Control, #P < 0.05 vs. NEC, ^P < 0.05 vs. NEC Mesalamine. eNOSKO, endothelial nitric oxide synthase knockout mice; NEC, necrotizing enterocolitis; WT, wild type.
ATB-429 Attenuates Intestinal Injury
Breastfed controls underwent normal development and had healthy intestine compared with pups that underwent experimental NEC. For WT mice, median macroscopic gut score for breastfed controls was 0 (0–0) and for NEC pups was 3 (2–3) (P < 0.0001, Fig. 3B). ATB-429 attenuated the severity of macroscopic gut scores compared with NEC [WT NEC ATB-429 = 1 (1–1), P < 0.0001]. Mesalamine did not provide any benefit [WT NEC Mesalamine = 2 (2–3), P = 0.2688]. When the experiment was repeated in eNOSKO mice, breastfed controls still had normal healthy intestine compared with pups that underwent experimental NEC. Median macroscopic gut score for eNOSKO breastfed controls was 0 (0–0) and for eNOSKO NEC pups was 3 (3–4) (P < 0.0001). Neither mesalamine or ATB-429 provided any benefit in eNOSKO mice compared with NEC [eNOSKO NEC Mesalamine = 3 (2.5–4), P > 0.9999; eNOSKO NEC ATB-429 = 3 (2–3), P = 0.1995].
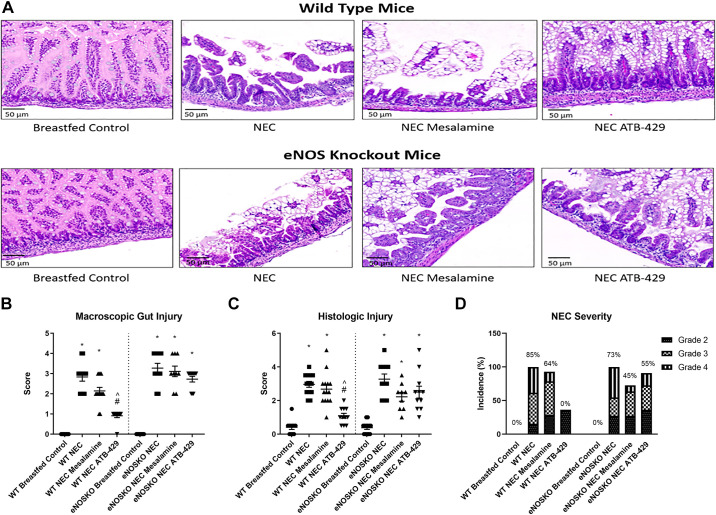
Figure 3.
ATB-429 attenuates intestinal injury in experimental NEC. After euthanasia, intestines were explanted and gross macroscopic gut appearance, histologic injury, and NEC severity were scored as previously published by Zani et al. (31) and as described in methods. Terminal ileum was fixed, paraffin-embedded, sectioned, and stained with hematoxylin and eosin. A: representative photomicrographs of both WT and eNOSKO mice intestinal histology are shown. Breastfed controls have healthy villi and normal architecture. NEC pups have severe injury with sloughing of villi and separation of basement membrane. WT pups that received ATB-429 have intestinal architecture that mirrors breastfed controls. Magnification ×20. B: macroscopic gut score was elevated in NEC animals compared with breastfed controls. ATB-429 significantly reduced macroscopic gut injury in WT mice but not eNOSKO mice. C: intestinal histologic injury was elevated in NEC animals compared with breastfed controls. ATB-429 attenuated histologic injury score in WT mice but not in eNOSKO mice. D: the incidence of severe NEC (greater than grade 2) was 0% in WT mice receiving ATB-429 but increased to 55% in eNOSKO mice. Percentages shown represent total incidence of severe NEC in each group. Statistical analysis was performed using ANOVA with Tukey’s multiple-comparisons test when parametric and Kruskal–Wallis with Dunn’s multiple-comparisons test when nonparametric for macroscopic and histologic gut injury. *P < 0.05 vs. Breastfed Control, #P < 0.05 vs. NEC, ^P < 0.05 vs. NEC Mesalamine. eNOSKO, endothelial nitric oxide synthase knockout mice; NEC, necrotizing enterocolitis; WT, wild type.
Histologic injury results mirrored macroscopic gut score. In WT mice, median histologic injury for breastfed controls was 0.5 (0–0.5) and NEC pups was 3 (2.5–3.5) (P < 0.0001, Fig. 3C). ATB-429 reduced histologic injury compared with NEC [WT NEC ATB-429 = 1 (0.5–1.5), P < 0.0001]. Mesalamine did not significantly reduce histologic injury [WT NEC Mesalamine = 2.5 (2–3), P = 0.9634]. When the experiment was repeated in eNOSKO mice, NEC pups had severe injury compared with breastfed controls [eNOSKO NEC = 3 (2–4), eNOSKO Breastfed Control = 0.5 (0–0.5), P < 0.0001]. ATB-429 did not attenuate histologic injury in eNOSKO mice [eNOSKO NEC ATB-429 = 2.5 (1.5–3), P = 0.1917]. Mesalamine also did not reduce intestinal histologic injury [eNOSKO NEC Mesalamine = 2.5 (1.5–2.75), P = 0.0713]. In addition, ATB-429 reduced the incidence of severe NEC (grade 3 or 4 injury) in WT mice, but not in eNOSKO mice (Fig. 3D). Figure 3A demonstrates H&E photomicrographs of the different groups studied.
ATB-429 Reduces Lung Injury
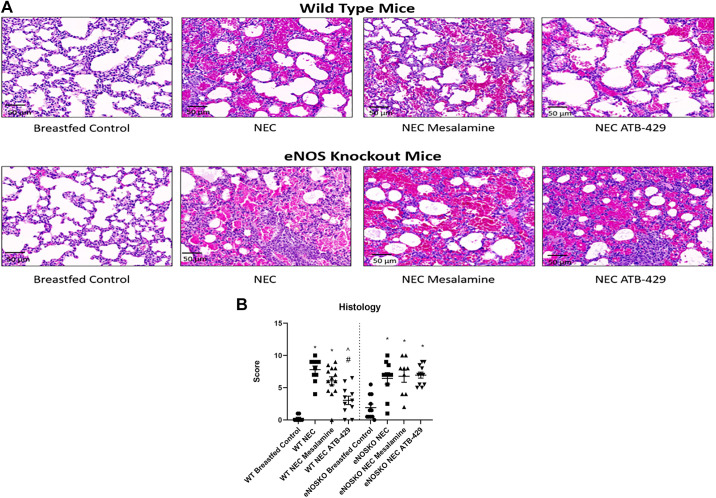
WT breastfed controls had healthy lung tissue without any inflammation or injury with a median injury score of 0 (0–0.75). WT NEC mice had severe lung injury with a median injury score of 8.5 (6.75–9) (P < 0.0001, Fig. 4B). ATB-429 reduced the severity of lung injury when compared with NEC [WT NEC ATB-429 = 3 (1.5–4.5), P = 0.0002]. Mesalamine provided no benefit in reducing the degree of lung injury when compared with NEC [WT NEC Mesalamine = 6.25 (4.5–8), P = 0.2995]. When the experiment was repeated in eNOSKO mice, NEC pups still had severe injury when compared with breastfed controls [eNOSKO NEC = 7 (4.75–8.625), eNOSKO Breastfed Control = 1.5 (0.5–4), P = 0.0063]. However, ATB-429 no longer attenuated lung injury when compared with NEC [eNOSKO NEC ATB-429 = 7 (5.5–8.5), P > 0.9999]. In addition, mesalamine did not reduce lung injury when compared with NEC [eNOSKO NEC Mesalamine = 8 (4–9), P > 0.9999]. Figure 4A demonstrates H&E photomicrographs of the different groups studied.
Figure 4.
ATB-429 reduces histologic lung injury in experimental NEC. At the conclusion of the experiment the right lower lobe of the lung was explanted, sectioned, and stained. Histologic injury was blindly scored using a modified injury scale from the American Thoracic Society as described in methods. A: representative photomicrographs of both WT and eNOSKO mice lung histology. Breastfed controls have thin healthy alveolar septa. NEC pups have severe injury with thickening of alveolar septa, neutrophil infiltrate, hemorrhage, and proteinaceous debris. Magnification ×20. B: experimental NEC caused a significant increase in lung injury in both WT and eNOSKO mice. ATB-429 attenuated the degree of pulmonary injury in WT mice, but not in eNOSKO mice. Statistical analysis was performed using Kruskal–Wallis with Dunn’s multiple-comparisons test. *P < 0.05 vs. Breastfed Control, #P < 0.05 vs. NEC, ^P < 0.05 vs. NEC Mesalamine. eNOSKO, endothelial nitric oxide synthase knockout mice; NEC, necrotizing enterocolitis; WT, wild type.
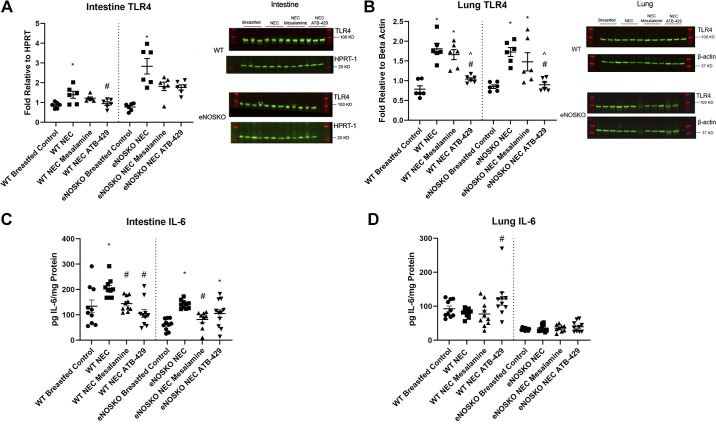
ATB-429 Reduces TLR4 Expression in Both the Intestine and Lung
WT pups undergoing experimental NEC had elevated intestinal TLR4 expression compared with WT breastfed controls (WT NEC = 1.39 ± 0.18, WT Breastfed Control = 0.86 ± 0.06, P = 0.0130, Fig. 5A). ATB-429 reduced the expression of intestinal TLR4 when compared with NEC in WT mice (WT NEC ATB-429 = 0.94 ± 0.09, P = 0.0479). Mesalamine did not affect intestinal TLR4 expression (WT NEC Mesalamine = 1.22 ± 0.06, P = 0.5779). When the experiment was repeated in eNOSKO mice, NEC pups still had elevated intestinal TLR4 expression compared with breastfed controls (eNOSKO NEC = 2.84 ± 0.39, eNOSKO Breastfed Control = 0.77 ± 0.08, P = 0.0012). Neither ATB-429 or mesalamine attenuated intestinal TLR4 expression in eNOSKO mice when compared with NEC (eNOSKO NEC ATB-429 = 1.76 ± 0.13, P = 0.1049; eNOSKO NEC Mesalamine = 1.81 ± 0.22, P = 0.4329).
Figure 5.
ATB-429 alters expression of TLR4 and IL-6 in experimental NEC. Both intestine and lung tissue were homogenized. TLR4 expression was quantified using Western blot, and IL-6 expression was quantified using ELISA techniques as described in methods. A: intestinal TLR4 expression was elevated in NEC mice compared with breastfed controls. ATB-429 attenuated TLR4 expression in WT mice but not eNOSKO mice. Representative blots are shown. B: lung TLR4 expression was elevated in NEC mice compared with breastfed controls. ATB-429 reduced TLR4 expression in both WT and eNOSKO mice. Representative blots are shown. C: IL-6 expression was elevated in the intestine in NEC mice compared with breastfed controls. Both mesalamine and ATB-429 reduced intestinal IL-6 expression in WT mice. ATB-429 failed to reduce IL-6 expression in eNOSKO mice. D: experimental NEC did not cause any alterations in lung IL-6 expression compared with breastfed controls. ATB-429 significantly increased IL-6 expression in the lung in WT mice, but not eNOSKO mice. Statistical analysis was performed using ANOVA with Tukey’s multiple-comparisons test when parametric and Kruskal–Wallis with Dunn’s multiple-comparisons test when nonparametric. *P < 0.05 vs. Breastfed Control, #P < 0.05 vs. NEC, ^P < 0.05 vs. NEC Mesalamine. eNOSKO, endothelial nitric oxide synthase knockout mice; NEC, necrotizing enterocolitis; WT, wild type.
WT pups undergoing experimental NEC had elevated lung TLR4 expression compared with WT breastfed controls (WT NEC = 1.81 ± 0.13, WT Breastfed Control = 0.79 ± 0.09, P < 0.0001, Fig. 5B). NEC pups that received ATB-429 had reduced lung TLR4 expression when compared with NEC (WT NEC ATB-429 = 1.04 ± 0.04, P = 0.0004). Mesalamine did not reduce lung TLR4 expression (WT NEC Mesalamine = 1.66 ± 0.13, P = 0.6035). When the experiment was repeated in eNOSKO mice, NEC pups had elevated lung TLR4 expression when compared with breastfed controls (eNOSKO NEC = 1.73 ± 0.11, eNOSKO Breastfed Control = 0.85 ± 0.04, P = 0.0009). ATB-429 attenuated TLR4 expression in eNOSKO mice (eNOSKO NEC ATB-429 = 0.90 ± 0.07, P = 0.0044), whereas mesalamine did not reduce TLR4 expression (eNOSKO NEC Mesalamine = 1.48 ± 0.23, P = 0.5017).
ATB-429 Affects IL-6 Expression in a Tissue-Dependent Manner
WT pups undergoing experimental NEC had elevated intestinal IL-6 expression compared with breastfed controls (WT NEC = 205.0 pg IL-6/mg protein ± 12.0, WT Breastfed Control = 134.1 pg IL-6/mg protein ± 24.3, P = 0.0225, Fig. 5C). Both ATB-429 and mesalamine reduced IL-6 expression when compared with NEC (WT NEC ATB-429 = 105.3 pg IL-6/mg protein ± 16.6, P < 0.0001; WT NEC Mesalamine = 144.2 pg IL-6/mg protein ± 9.2, P = 0.0072). When the experiment was repeated in eNOSKO mice, NEC pups had elevated IL-6 expression compared with breastfed controls (eNOSKO NEC = 141.4 pg IL-6/mg protein ± 5.2, eNOSKO Breastfed Control = 61.7 pg IL-6/mg protein ± 7.3, P < 0.0001). ATB-429 no longer reduced IL-6 expression when compared with NEC (eNOSKO NEC ATB-429 = 105.2 pg IL-6/mg protein ± 16.0, P = 0.0955). Mesalamine, however, significantly reduced IL-6 expression (eNOSKO NEC Mesalamine = 81.6 pg IL-6/mg protein ± 11.2, P = 0.0056).
Regarding the lung, in WT mice, experimental NEC did not affect IL-6 expression (WT NEC = 80.3 pg IL-6/mg protein ± 3.9, WT Breastfed Control = 92.7 pg IL-6/mg protein ± 8.0, P = 0.1787, Fig. 5D). ATB-429 significantly increased IL-6 expression in the lung when compared with NEC (WT NEC ATB-429 = 121.4 pg IL-6/mg protein ± 18.3, P = 0.0289). Mesalamine did not significantly affect IL-6 expression (WT NEC Mesalamine = 77.2 pg IL-6/mg protein ± 11.4, P = 0.8989). When the experiment was repeated in eNOSKO mice, ATB-429 no longer increased IL-6 expression and there was no difference between any of the four groups (P = 0.9237).
DISCUSSION
Necrotizing enterocolitis remains a devastating disease for the newborns. Currently, there is no targeted medical therapy available to treat NEC. In this study, we observed that an orally administered H2S derivative of mesalamine improved outcomes in experimental NEC. Furthermore, the effects of ATB-429 appear to be mediated through eNOS.
Fiorucci et al. (29) first described the anti-inflammatory properties of ATB-429 in their study assessing the treatment of a mouse model of colitis. The authors assessed both ATB-429 and traditional mesalamine and their ability to attenuate clinical sickness (composed of a scale weighting factors such as diarrhea and weight loss), histologic injury, and cytokine expression. In their study, ATB-429 significantly reduced sickness score, histologic colitis severity, and expression of several cytokines (IL2, TNF-α, IFN-γ). Our study mirrored these results, where we observed ATB-429 attenuating several outcomes in experimental NEC, including mortality, weight loss, clinical sickness score, intestinal histologic injury, and IL-6 expression. Similarly, we also observed a modest benefit from traditional mesalamine in some areas, such as survival and intestinal IL-6 expression, but ATB-429 was significantly more effective. In an additional study, Fiorucci et al. (29) described the pharmacokinetics of ATB-429 by administering the drug and measuring serum H2S concentrations at specific time intervals. They demonstrated that peak H2S concentrations occurred 10 min after administration of the drug, and returned to baseline levels after 60 min (34).
In addition to the pharmacokinetics, it is important to consider the mechanism of action of ATB-429. First, it appears that its beneficial properties require the enzyme eNOS. The role of eNOS and NEC was well demonstrated by showing that submucosal arterioles harvested from intestinal tissue that was resected from infants with NEC failed to vasodilate due to eNOS dysregulation. Lack of eNOS-produced NO left vessels tonically vasoconstricted, possibly due to unopposed endothelin-1 (35). Although ATB-429 improved survival, weight loss, mesenteric blood flow, and intestinal injury in WT mice, it was unable to improve these same outcomes in eNOSKO mice, suggesting it functions through eNOS. Given that it is an H2S releasing compound, these results are unsurprising as it has been previously demonstrated that H2S-induced vasodilation is heavily mediated by eNOS (36). This is most relevant to our findings that ATB-429 improved blood flow as measured by laser doppler imaging in WT mice, but not eNOSKO mice. This has been shown in vitro using pressure myography where mesenteric arteries harvested from WT mice vasodilated in the presence of H2S. However, when the experiment was repeated with mesenteric arteries taken from eNOSKO mice, vasodilation was significantly decreased (37). Our data are also consistent with previously published in vivo studies where H2S donors lose their beneficial properties in eNOKO mice. Intraperitoneal H2S donor NaHS provided significant benefit in blood flow, histologic injury, and cytokine elevation in an ischemia/reperfusion model in WT mice, but not eNOSKO mice (22). In addition, intraperitoneal administration of H2S donor GYY4137 improved outcomes for experimental NEC in WT mice. When the experiment was repeated; however, in eNOSKO mice, GYY4137 provided no benefit (32).
Aside from observing that ATB-429 improved mesenteric perfusion in WT mice, we also observed that it affected TLR4 expression. TLR4 has repeatedly been shown to be elevated in the intestine in both experimental NEC studies and intestinal tissue taken from infants with NEC (8). In our study, we noted similar results with an increase in intestinal TLR4 expression in both WT and eNOSKO NEC mice. ATB-429 attenuated this increase only in WT mice, not in eNOSKO mice, suggesting that ATB-429’s ability to mitigate aberrant TLR4 signaling is affected by the presence of functional eNOS. Similarly, it has been previously demonstrated that TLR4 expression in the lung is elevated in experimental NEC (14). We observed similar results, where both WT and eNOSKO mice had elevated pulmonary TLR4 expression. Interestingly, ATB-429 attenuated TLR4 expression in both WT and eNOSKO mice, suggesting its ability to modulate TLR4 expression in the lung is not reliant on eNOS. However, ATB-429 reduced the severity of pulmonary histologic injury in only WT mice, not eNOSKO mice, suggesting there are likely other mechanisms involved in NEC-induced lung injury other than TLR4 expression.
It has been previously demonstrated that the addition of an H2S moiety to an NSAID decreases its intestinal absorption and systemic bioavailability (38). Indeed, this may be a beneficial property of ATB-429, as it remains in the gut longer and is able to act locally on the intestine to decrease inflammation and improve microcirculation. The poor absorption makes it unlikely that ATB-429 is absorbed and delivered to the lung effectively. Thus, the ability of ATB-429 to attenuate pulmonary injury in experimental NEC is likely an indirect effect by preventing secondary lung injury through decreasing systemic illness, rather than a direct effect by acting locally on alveoli.
We observed that experimental NEC caused a significant elevation in intestinal IL-6 expression. This is consistent with other studies, where human data have shown elevations in IL-6 in infants with NEC (39). Indeed, IL-6 has been studied as a biomarker to predict the need for surgical intervention in infants with NEC (40). ATB-429 blunted the increased expression of IL-6 in WT mice, but not in eNOSKO mice, suggesting some of its anti-inflammatory properties are dependent on eNOS. Paradoxically, ATB-429 increased the expression of IL-6 in the lung, suggesting its activity with IL-6 is tissue-dependent. IL-6 has been shown to have anti-inflammatory properties in the lung in a mouse model for acute lung injury, suggesting its association with increased expression after receiving ATB-429 may be beneficial (41). In addition, IL-6 administration has been explored to treat acute lung injury in influenza, further supporting its potential anti-inflammatory properties in the lung (42). However, it is important to note that experimental NEC did not cause an increase in IL-6 when compared with breastfed control, highlighting it is not a key mediator in the acute lung injury associated with experimental NEC.
LIMITATIONS
This experiment is limited by the nature that it is in animal model. As stated previously, NEC is a complex multifactorial disease and there are several variations of animal models that try to capture the pathophysiology (43). These models have clearly deepened our understanding of the disease, but likely do not fully represent the underlying pathophysiology of the disease process. Second, a component of the pulmonary injury score is alveolar septal thickness. True septal thickness is most accurately measured when the entire lungs are fixed at a pressure of 15–25 cmH2O (33). To standardize our specimens, we routinely remove the right lower lobe for histologic analysis, making it difficult to inflate. Procuring the entire lung and fixing the specimen, whereas under a constant pressure would more reliably assess alveolar wall thickness and may have affected the total lung injury score.
In addition, whereas our data are clear that administration of ATB-429 is associated with increased mesenteric perfusion, decreased intestinal TLR4 expression, and decreased intestinal IL-6 expression, it is not possible to discern the antecedent event from these data. It is possible that ATB-429’s vasodilatory properties resulted in augmenting intestinal blood flow and reducing ischemia, preventing a secondary rise in TLR4 and IL-6 in the intestine. However, it is equally possible that ATB-429’s anti-inflammatory and immune-modulating properties directly inhibited the aberrant increased expression of TLR4 and IL-6, which prevented injury and secondarily prevented ischemia from following. Future temporal studies assessing mesenteric perfusion, the degree of intestinal injury, TLR4 expression, and cytokine elevation and how it varies with time throughout the experiment would better elucidate the mechanism of action of ATB-429.
Although Fiorucci et al. (34) have described the pharmacokinetics of ATB-429 in a previous experiment, there has been no animal study to date that has assessed potential side effects of the drug. Our study did not assess liver and kidney function after administration of ATB-429. Given that this is an experimental therapeutic, there needs to be further investigation into potentially harmful side effects of ATB-429 before use in clinical trials.
PERSPECTIVES AND SIGNIFICANCE
The H2S donor ATB-429 is a novel oral drug for the treatment of experimental NEC. It attenuates the severity of NEC by increasing mesenteric blood flow, reducing intestinal TLR4 and IL-6 expression and also reduces NEC-associated pulmonary injury. Its beneficial properties appear to be mediated through eNOS. Further study is warranted to assess whether ATB-429 could be a safe and effective targeted medical therapy for NEC.
GRANTS
This study was supported by the following grants: National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K08DK113226; to T.A.M.); American College of Surgeons Clowe’s Memorial Research Fund (to T.A.M.); Gerber Foundation (to T.A.M.); Riley Children’s Foundation (to T.A.M.); and the Indiana University Department of Surgery.
DISCLOSURES
T. A. Markel serves as a consultant for Noveome Biotherapeutics and receives consultation fees for this service. The materials presented herein have no relationship with that entity or his role in that company. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
B.D.H. and T.A.M. conceived and designed research; B.D.H., H.L., N.A.D., and W.C.S. performed experiments; B.D.H., C.E.H., H.L., N.A.D., W.C.S., and T.A.M. analyzed data; B.D.H., C.E.H., H.L., N.A.D., and T.A.M. interpreted results of experiments; B.D.H. and C.E.H. prepared figures; B.D.H., C.E.H., and T.A.M. drafted manuscript; B.D.H., C.E.H., H.L., N.A.D., A.R.P., K.M., W.C.S., and T.A.M. edited and revised manuscript; B.D.H., C.E.H., H.L., N.A.D., A.R.P., K.M., W.C.S., and T.A.M. approved final version of manuscript.
REFERENCES
- 1. Tanner SM, Berryhill TF, Ellenburg JL, Jilling T, Cleveland DS, Lorenz RG, Martin CA. Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am J Pathol 185: 4–16, 2015. doi: 10.1016/j.ajpath.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones IH, Hall NJ. Contemporary outcomes for infants with necrotizing enterocolitis—a systematic review. J Pediatr 220: 86–92.e3, 2020. doi: 10.1016/j.jpeds.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 3. Zani A, Pierro A. Necrotizing enterocolitis: controversies and challenges. F1000Res 4: 1373, 2015. doi: 10.12688/f1000research.6888.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, Gregory KE, Kroll JS, McMurtry V, Ferris MJ, Engstrand L, Lilja HE, Hollister EB, Versalovic J, Neu J. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5: 31, 2017. doi: 10.1186/s40168-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Y, Chang KT, Lian DW, Lu H, Roy S, Laksmi NK, Low Y, Krishnaswamy G, Pierro A, Ong CC. The role of ischemia in necrotizing enterocolitis. J Pediatr Surg 51: 1255–1261, 2016. doi: 10.1016/j.jpedsurg.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 7. Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci USA 97: 6043–6048, 2000. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143: 708–718.e5, 2012. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Koike Y, Chi L, Ahmed A, Miyake H, Li B, Lee C, Delgado-Olguín P, Pierro A. Formula feeding and immature gut microcirculation promote intestinal hypoxia, leading to necrotizing enterocolitis. Dis Model Mech 12: dmm040998, 2019. doi: 10.1242/dmm.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, Neal MD, Jia H, Lin J, Ma C, Branca MF, Prindle T, Richardson WM, Ozolek J, Billiar TR, Binion DG, Gladwin MT, Hackam DJ. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci USA 110: 9451–9456, 2013. doi: 10.1073/pnas.1219997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg PM, Tatum R, Ravisankar S, Shekhawat PS, Chen YH. Necrotizing enterocolitis in a mouse model leads to widespread renal inflammation, acute kidney injury, and disruption of renal tight junction proteins. Pediatr Res 78: 527–532, 2015. doi: 10.1038/pr.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biouss G, Antounians L, Li B, O’Connell JS, Seo S, Catania VD, Guadagno J, Rahman A, Zani-Ruttenstock E, Svergun N, Pierro A, Zani A. Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain. J Neuroinflammation 16: 97, 2019. doi: 10.1186/s12974-019-1481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drucker NA, Jensen AR, Te Winkel JP, Ferkowicz MJ, Markel TA. Loss of endothelial nitric oxide synthase exacerbates intestinal and lung injury in experimental necrotizing enterocolitis. J Pediatr Surg 53: 1208–1214, 2018. doi: 10.1016/j.jpedsurg.2018.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jia H, Sodhi CP, Yamaguchi Y, Lu P, Martin LY, Good M, Zhou Q, Sung J, Fulton WB, Nino DF, Prindle T Jr, Ozolek JA, Hackam DJ. Pulmonary epithelial TLR4 activation leads to lung injury in neonatal necrotizing enterocolitis. J Immunol 197: 859–871, 2016. doi: 10.4049/jimmunol.1600618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhatia M. Hydrogen sulfide as a vasodilator. IUBMB Life 57: 603–606, 2005. doi: 10.1080/15216540500217875. [DOI] [PubMed] [Google Scholar]
- 16. Paul BD, Snyder SH. Protein sulfhydration. Methods Enzymol 555: 79–90, 2015. doi: 10.1016/bs.mie.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 17. Altaany Z, Ju Y, Yang G, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal 7: ra87, 2014. doi: 10.1126/scisignal.2005478. [DOI] [PubMed] [Google Scholar]
- 18. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 2012. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zuidema MY, Peyton KJ, Fay WP, Durante W, Korthuis RJ. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of heme oxygenase-1. Am J Physiol Heart Circ Physiol 301: H888–H894, 2011. doi: 10.1152/ajpheart.00432.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 137: 569–578, 2009. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 21. Blackler RW, Motta JP, Manko A, Workentine M, Bercik P, Surette MG, Wallace JL. Hydrogen sulphide protects against NSAID-enteropathy through modulation of bile and the microbiota. Br J Pharmacol 172: 992–1004, 2015. doi: 10.1111/bph.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen AR, Drucker NA, Khaneki S, Ferkowicz MJ, Markel TA. Hydrogen sulfide improves intestinal recovery following ischemia by endothelial nitric oxide-dependent mechanisms. Am J Physiol Gastrointest Liver Physiol 312: G450–G456, 2017. doi: 10.1152/ajpgi.00444.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jensen AR, Drucker NA, Olson KR, Markel TA. Stem cell therapy and hydrogen sulfide: conventional or nonconventional mechanisms of action? Shock 53: 737–743, 2020. doi: 10.1097/SHK.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jensen AR, Drucker NA, Te Winkel JP, Ferkowicz MJ, Markel TA. The route and timing of hydrogen sulfide therapy critically impacts intestinal recovery following ischemia and reperfusion injury. J Pediatr Surg 53: 1111–1117, 2018. doi: 10.1016/j.jpedsurg.2018.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drucker NA, Jensen AR, Ferkowicz M, Markel TA. Hydrogen sulfide provides intestinal protection during a murine model of experimental necrotizing enterocolitis. J Pediatr Surg 53: 1692–1698, 2018. doi: 10.1016/j.jpedsurg.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 26. Li B, Lee C, Martin Z, Li X, Koike Y, Hock A, Zani-Ruttenstock E, Zani A, Pierro A. Intestinal epithelial injury induced by maternal separation is protected by hydrogen sulfide. J Pediatr Surg 52: 40–44, 2017. doi: 10.1016/j.jpedsurg.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 27. Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology 132: 261–271, 2007. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 28. Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med 42: 706–719, 2007. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 29. Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, Santucci L, Cirino G, Wallace JL. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br J Pharmacol 150: 996–1002, 2007. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 177: 3273–3282, 2006. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zani A, Cordischi L, Cananzi M, De Coppi P, Smith VV, Eaton S, Pierro A. Assessment of a neonatal rat model of necrotizing enterocolitis. Eur J Pediatr Surg 18: 423–426, 2008. doi: 10.1055/s-2008-1038951. [DOI] [PubMed] [Google Scholar]
- 32. Drucker NA, Jensen AR, Te Winkel JP, Markel TA. Hydrogen sulfide donor GYY4137 acts through endothelial nitric oxide to protect intestine in murine models of necrotizing enterocolitis and intestinal ischemia. J Surg Res 234: 294–302, 2019. doi: 10.1016/j.jss.2018.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM, Kuebler WM; Acute Lung Injury in Animals Study Group . An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44: 725–738, 2011. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Russo G, Caliendo G, Santagada V, Cirino G, Wallace JL, Fiorucci S. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of post-inflammatory hypersensitivity. J Pharmacol Exp Ther 319: 447–458, 2006. doi: 10.1124/jpet.106.106435. [DOI] [PubMed] [Google Scholar]
- 35. Nowicki PT, Caniano DA, Hammond S, Giannone PJ, Besner GE, Reber KM, Nankervis CA. Endothelial nitric oxide synthase in human intestine resected for necrotizing enterocolitis. J Pediatr 150: 40–45, 2007. doi: 10.1016/j.jpeds.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 36. Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA 109: 9161–9166, 2012. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Te Winkel J, John QE, Hosfield BD, Drucker NA, Das A, Olson KR, Markel TA. Mesenchymal stem cells promote mesenteric vasodilation through hydrogen sulfide and endothelial nitric oxide. Am J Physiol Gastrointest Liver Physiol 317: G441–G446, 2019. doi: 10.1152/ajpgi.00132.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gemici B, Wallace JL. Anti-inflammatory and cytoprotective properties of hydrogen sulfide. Methods Enzymol 555: 169–193, 2015. doi: 10.1016/bs.mie.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 39. Harris MC, Costarino AT Jr, Sullivan JS, Dulkerian S, McCawley L, Corcoran L, Butler S, Kilpatrick L. Cytokine elevations in critically ill infants with sepsis and necrotizing enterocolitis. J Pediatr 124: 105–111, 1994. doi: 10.1016/S0022-3476(94)70264-0. [DOI] [PubMed] [Google Scholar]
- 40. Wisgrill L, Weinhandl A, Unterasinger L, Amann G, Oehler R, Metzelder ML, Berger A, Benkoe TM. Interleukin-6 serum levels predict surgical intervention in infants with necrotizing enterocolitis. J Pediatr Surg 54: 449–454, 2019. doi: 10.1016/j.jpedsurg.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 41. Voiriot G, Razazi K, Amsellem V, Tran Van Nhieu J, Abid S, Adnot S, Mekontso Dessap A, Maitre B. Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury. Respir Res 18: 64, 2017. doi: 10.1186/s12931-017-0553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang ML, Wang CT, Yang SJ, Leu CH, Chen SH, Wu CL, Shiau AL. IL-6 ameliorates acute lung injury in influenza virus infection. Sci Rep 7: 43829, 2017. doi: 10.1038/srep43829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ares GJ, McElroy SJ, Hunter CJ. The science and necessity of using animal models in the study of necrotizing enterocolitis. Semin Pediatr Surg 27: 29–33, 2018. doi: 10.1053/j.sempedsurg.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]